Abstract
Oleanolic acid (OA) is reported to possess antihypertensive activity via the regulation of lipid metabolism; however, the mechanisms underlying lipid regulation by OA are yet to be fully elucidated. The aim of the present study was to evaluate the mechanisms via which OA regulates lipid metabolism in spontaneously hypertensive rats (SHRs) via ultra-performance liquid chromatography-quadrupole/Orbitrap-mass spectrometry (MS)-based lipidomics analysis. SHRs were treated with OA (1.08 mg/kg) for 4 weeks. The liver tissues were excised, homogenized in dichloromethane and centrifuged, and subsequently the supernatant layer was collected and concentrated under vacuum to dryness. The dichloromethane extract was subjected to MS analysis and database searching, and comparison of standards was performed to identify potential biomarkers. Partial least squares-discriminant analysis performed on the liver lipidome revealed a total of 14 endogenous metabolites that were significantly changed in the SHR model group (SH group) compared with Wistar Kyoto rats [normal control (NC group)], including glycerophospholipids, sphingolipids and glycerides. Heatmaps revealed that the liver lipid profiles in the OA group were clustered more closely compared with those observed in the NC group, indicating that the antihypertensive effect of OA was mediated via regulation of liver lipid metabolites. It was observed that the protein levels of secretory phospholipase A2 (sPLA2) and fatty acid synthase (FAS) were increased in the SH group compared with the NC group. In addition, the levels of lysophosphatidylcholine and triglycerides in the liver were elevated, whereas the levels of low-density lipoprotein cholesterol and high-density lipoprotein cholesterol were reduced in the SH group. Upon treatment with OA, the mRNA and protein levels of PLA2 and FAS were observed to be downregulated. Collectively, the present study indicated that the antihypertensive activity of OA was mediated via downregulation of sPLA2 and FAS in SHRs, and that treatment with OA resulted in significant improvements in blood pressure and associated abnormalities in the lipid metabolites.
Keywords: oleanolic acid, lipid profiles, spontaneous hypertensive rat, lipidomics, phospholipase A2, fatty acid synthase
Introduction
Oleanolic acid (OA), a triterpenoid, is found in a variety of foods and herbs (1). OA has been reported to exhibit a myriad of biological activities: It may function as an antioxidant, an anti-inflammatory (2,3), an antimicrobial (4), hepatoprotective (5) and chemopreventive (6) agent, and it also possesses immunomodulatory (7), antiarrhythmic and cardiotonic (8) activities. A number of studies have shown that OA has a blood pressure-lowering effect (9,10). Bachhav et al (11) reported that OA prevents hypertension via antioxidant and nitric oxide (NO)-releasing actions. Oral administration of OA for 9 weeks has shown antihypertensive effects in spontaneously hypertensive rats (SHRs) (12) and Dahl salt-sensitive rats (12,13), leading to a decrease in the mean arterial blood pressure, increased urinary Na+ output, a reduction in malondialdehyde, a marker of lipid peroxidation, and increased superoxide dismutase and glutathione peroxidase activity in the liver, heart and kidney (12). Oral administration of OA has also exhibited antihypertensive effects during pressure overload-induced cardiac remodeling in mice (14), and in renovascular hypertensive rats (15) and an insulin-resistant rat model of hypertension (13). Madlala et al (16) reported that OA resulted in the relaxation of aortic rings and mesenteric arteries that were pre-contracted with phenylephrine or a KCl-enriched solution. It was observed that endothelium denudation and indomethacin partially inhibited the relaxation, whereas a NO synthase inhibitor, N-ω-nitro-L-arginine, did not result in any inhibitory effects. It was suggested that the activity of OA may be mediated via endothelium-dependent and -independent mechanisms (16).
Spontaneous hypertension is one of the most common systemic metabolic diseases, and its causes include genetic, environmental and behavioral factors (17,18). Research attention has focused on investigating the role of lipids in hyper-tension (19). In a previous study, the levels of lipid metabolites were observed to be elevated, whereas sphinganine levels were lower, in SHRs compared with Wistar Kyoto (WKY) rats (20). Additionally, in another previous study, it was found that the sphingomyelin (SM) (d18:0/14:0) level was increased in SHRs compared with in WKY rats; the increased SM content was reported to reduce endothelial membrane fluidity (21). Studies have also demonstrated the involvement of sphingolipids in the regulation of vascular tone (22,23). It was shown that sphingo-lipids regulate NO and endothelium-derived hyperpolarizing factor (EDHF)-mediated relaxation responses in various types of blood vessels (24). Graessler et al (25) found that, compared with healthy individuals, the levels of phosphatidylcholine (PC) and phosphatidylethanolamine (PE) lipid species, including PC (O-36:4), PC (O-38:4), PE (O-38:5), PE (O-38:6) and PE (O-40:5), were lower in patients with hypertension. Liu et al (26) reported reduced levels of PC (16:1/14:1) in SHRs compared with WKY rats, whereas higher levels of lysoPC (22:5), ceramide (Cer) and dihydroceramide were observed in the SHRs compared with the WKY rats.
Hu et al (27) reported that PC and triglycerides (TGs) possess important roles in blood lipid metabolism. As an important proinflammatory mediator, phospholipase A2 (PLA2) functions to hydrolyse PCs into lysoPCs (28). LysoPC exerts influence on numerous processes in various cell types, and may have an important role in inflammatory diseases (29). Inflammation itself is also hypothesized to initiate and maintain hypertensive episodes (30). As the accumulation of diglycerides (DGs) triggers activation of protein kinases C, it ultimately contributes to insulin resistance (31,32). DGs regulate hypertension via mechanisms such as increased renal sodium reabsorption, sympathetic nervous system activation, transmembrane ion transport alteration and hypertrophy of vascular smooth muscle (33). Additionally, associations of TGs with both systolic blood pressure (SBP) and diastolic blood pressure (DBP) have been reported in a community-dwelling sample of Japanese adults (34). Due to the bidirectional association that has been identified between hypertension and endothelial dysfunction (35), an increase in TG levels may result in hypertension. For patients with hyper-tension, Hu et al (27) reported a positive association between the degree of hypertension and levels of TGs. In their study, the authors commented on the relatively higher levels of TGs in the livers of patients with hypertension compared with those of healthy individuals.
Numerous studies have suggested that lipid-lowering strategies, and particularly statins, could influence blood pressure control (36,37). The use of lipid-lowering measures can significantly improve blood pressure control in subjects with both hypercholesterolemia and hypertension; the amelioration of elevated blood pressure appears to be enhanced in subjects treated with statins (36). Valsartan displays remarkable anti-inflammatory efficacy in patients with hypertension that possess an elevated inflammatory burden; total cholesterol (CHOL) and low-density lipoprotein cholesterol (LDL-C) levels were significantly reduced following valsartan therapy (37). Both statins and fibrates have been shown to reduce blood pressure in clinical trials and exhibit protective effects on arterial wall structure (38,39). These effects on blood pressure may account for some of the clinical effects of lipid-lowering drugs on cardiovascular risk (40). Thus, lipid-lowering measures may provide an additional method to treat patients with hypertension.
The main organ involved in lipid metabolism is the liver (29). An excessive accumulation of TGs, free fatty acids (FFAs), CHOL, PC, PE, Cer and SM is the main cause underlying lipid metabolic disorders (41-43). These components are primarily produced and stored in liver cells (44,45). One of the main characteristics of hypertension is dysregulation of lipid metabolism (25,46). Previous studies have shown that sphingolipids (24) and phospholipids (47) exert significant effects on hypertension, among which Cer, the precursor of sphingosine-1-phosphate, was found to induce antiproliferative and proapoptotic effects (48). Additionally, sphingolipids have been shown to be involved in processes associated with vascular tone regulation, including the regulation of NO and EDHF responses in various types of blood vessels (48-50).
LysoPC is produced via hydrolysis of membrane PC by PLA2 (51). PCs have been reported to be the most abundant phospholipids in LDL particles (52). The most important families under the category of PLA2s are secreted (s)PLA2s and cytosolic PLA2s (53). sPLA2 is reported to promote vascular inflammation, leading to coronary artery disease (54). Increased sPLA2 levels were reported to be associated with coronary artery diseases (55). Additionally, the hydrolysis of PC by sPLA2 results in the formation of lysoPC, and lysoPC has been reported to exert proatherogenic and proinflammatory effects on arterial wall cells, including the upregulation of adhesive molecules, monocyte chemoattractant protein-1 and growth factors, cell proliferation, cell migration, apoptosis, activation of protein kinase C and inhibition of endothelium-dependent relaxation (56,57). It is an intracellular messenger and is found as a major phospholipid component in chemically modified LDL (58). LysoPC is present in oxidized LDLs that are significantly associated with inflammation and cardiovascular diseases (59), and performs crucial roles in a myriad of biological activities. For example, in endothelial cells, lysoPC has been shown to stimulate the transcription of adhesion molecules and growth factors (60). In fatty liver, fatty acid synthase (FAS) is associated with the accumulation of TGs (61). Elevated hepatic FAS activity and fatty liver are observed in ob/ob mice (62), although a mechanistic link between the two findings has yet to be established. Subjects who are overweight and have metabolic syndrome exhibiting obesity, inflammation and hypertension are found to have altered levels of FAS activity/expression, which highlights the association between FAS, and hypertension pathogenesis and metabolic dysfunction (63,64). In addition, decreased levels of FAS mRNA were identified in the adipose tissue of hypertensive individuals (65). Both FAS and PLA2 are also independent risk factors for hypertension and metabolic dysfunction (66,67).
Lipidomics is an important branch of metabolomics, and its primary aim is to characterize lipids, as well as lipid metabolic pathways and networks associated with biological systems (68). The advances that have been achieved in mass spectrometry (MS) as a technique has rendered the analysis of lipidomics more accurate and efficient. Lipidomics has been developed as an efficient analytical method to be applied in biomedical sciences (69). The molecules of a given sample are ionized and the first spectrometer (designated MS) separates these ions by their mass-to-charge ratio (m/z) (70). Ions of a particular m/z in MS are selected for further MS to split them into smaller fragments (MS/MS) (71). Analysis of MS/MS fragments can improve the accuracy of small molecule identification (70).
As glycerophospholipids, sphingolipids and glycerides have been shown to be involved in blood pressure regulation, it was hypothesized that changes in the content of glycerophospholipids, sphingolipids and glycerides in the livers of SHRs may result in altered blood pressure. OA may regulate blood pressure by regulating glycerophospholipids, sphingolipids and glycerides in the livers of SHRs, and the inhibition of FAS and PLA2 by OA may result in regulation of cholesterol levels in the liver and serum lipid metabolism in SHRs. The present study aimed to test these hypotheses.
Materials and methods
Chemicals and reagents
Lipid internal standard [(PE) (17:1/12:0)] and other lipid standards [PC (17:0/14:1), lysoPC (18:0), Cer (18:1/18:0), phosphatidylglycerol (PG) (17:0/20:4) and SM (18:1/12:0)] were purchased from Avanti Polar Lipids, Inc. The chemicals (ammonium formate, methanol, dichloromethane, isopropanol and acetonitrile) were of high-performance liquid chromatography (HPLC)-MS grade and obtained from Thermo Fisher Scientific, Inc. OA (purity >98%), and DNase/RNase-free water were purchased from Beijing Solarbio Science & Technology Co., Ltd. SYBR Premix Ex Taq™ II (Tli RNaseH Plus), PrimeScript™ RT reagent kit with gDNA Eraser (Perfect Real Time) and TRIzol® Total RNA Extraction kit were purchased from Takara Bio, Inc.
Animal experiment and study design
Animal handling was performed according to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (72) and the experimental protocol was approved by the Animal Care and Ethics Committee of Shandong University of Traditional Chinese Medicine (approval no. SDUTCM2018120301). A total of 20 male SHRs and 10 male WKY rats (age, 4 weeks; weight, 200-220 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. The SHRs were randomly and equally divided into two groups (n=10/group): The disease control group (SH group) and the OA treatment group (OA group). The WKY rats served as the normal control group (NC group). The animals were housed in an air-conditioned room (25°C, 55% humidity and a 12:12-h light/dark cycle). The animals were provided certified standard diet and tap water ad libitum. OA was suspended in normal saline to prepare the required concentrations for oral administration. Following a protocol in a previous study (10), the OA group received 1.08 mg/kg OA daily for 4 weeks. All animals received ≤2 ml of the suspension. The NC and SH group received 2 ml normal saline. The blood pressure of each rat was monitored every 7th day using a non-invasive blood pressure analysis system (Softron BP-98A; Beijing Softron Biotechnology Co., Ltd.).
At 12 h after the last treatment, the rats were anesthetized with sodium pentobarbital (40 mg/kg, intraperitoneal) (73) and blood samples were collected into normal vacuum tubes via cardiac puncture. The serum was collected by centrifuging the blood at 2,300 × g at 4°C for 10 min (74). The liver tissues were also excised under anesthetic conditions, and the serum and liver tissues were stored at -80°C prior to further experimentation.
Measurement of biochemical parameters
Serum CHOL, LDL-C, high density lipoprotein cholesterol (HDL-C) and TGs were analyzed using an automatic blood chemistry analyzer (BS-400; Mindray Medical International Ltd.).
Lipidomics analysis
Preparation of lipid standards
Lipid internal standard, PE (17:1/12:0), was used for adjustment of possible inter- and intra-assay variances (internal standardization). The lipid standards [PC (17:0-14:1), lysoPC (18:0), Cer (18:1/18:0) and SM (18:1/12:0)] were used for lipid family assignment (external standardization). Stock solutions of internal standard and lipid standards were prepared by dissolving accurately weighed amounts in 1 ml isopropanol:methanol (2:1 v/v).
Sample preparation and lipid extraction
Lipids were extracted from the liver samples using a modified version of the Folch method (75). Lipids from liver samples were extracted as previously described (21); specific details are presented in Data S1.
Ultra-performance (UP) LC-electrospray ionization (ESI)-MS/MS analysis
The lipid extracts were subjected to MS/MS using a UPLC system (UltiMate 3000; Thermo Fisher Scientific, Inc.) coupled to an ESI-quadrupole/Orbitrap mass spectrometer (Q Exactive™; Thermo Fisher Scientific, Inc.) in both positive and negative ionization modes. The HPLC analysis conditions and the MS parameters were optimized in our previous study (Data S1) (20).
Data processing and biomarker identification
The data were processed as described previously (20,21). The multivariate statistical analysis package SimcaP 14.1 (Umetrics AB; Sartorius Stedim Biotech AS) was used for principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) (20). Various metabolites were identified from the PCA and PLS-DA loading plots. Differential expression of lipid biomarkers among the NC, SH and OA groups was identified on the basis of variable importance in projection (VIP) values (calculated using SimcaP 14.1 software), and this analysis was followed by performing one-way ANOVA to determine statistical significance, as well as the calculation of fold change (FC) in biomarker expression using Mass Profiler Professional software (v12.6.1; Agilent Technologies, Inc.). Lipid biomarkers with VIP >1, P<0.01 and FC >2 were considered to be differentially regulated in the SH group compared with the NC group.
The metabolites were identified based on molecular ion peaks [(M+H)+ or (M-H)−] and MS/MS ions, in addition to comparing the retention times with the metabolites in the human metabolome database (HMDB; http://www.hmdb.ca), LIPID MAPS Lipidomics Gateway (http://www.lipidmaps.org), METLIN (https://metlin.scripps.edu) and Kyoto Encyclopedia of Genes and Genomes (KEGG; http://www.genome.jp/kegg) databases. The pathway analysis was performed using MetaboAnalyst 4.0 software (http://www.metaboanalyst.ca). Library searches were performed using a maximum mass deviation of 5 ppm. In addition to comparing the mass fragment patterns with those reported in these databases (76), the mass fragment patterns of the lipid metabolites are presented in Figs. S1 and S2, and the m/z values of the metabolites are presented in Table I.
Table I.
Effects of OA on potential lipid biomarkers associated with hypertension in liver.
| Number | tR, min | VIP value | Formula | Metabolite | Adduct ion | m/z | Fold change
|
Pathway | |
|---|---|---|---|---|---|---|---|---|---|
| SH/NCa | OA/SHa | ||||||||
| 1 | 10.34 | 1.24 | C34H67NO3 | GluCer (d18:1/25:0) | [M-H]− | 825.70577 | 2.11 | 0.23 | Sphingolipid metabolism |
| 2 | 10.49 | 1.38 | C39H68O5 | DG (36:4) | [M+H]+ | 616.50624 | 2.34 | 0.42 | Glycerolipid metabolism |
| 3 | 10.47 | 1.24 | C50H95NO13 | LacCer (d18:1/20:0) | [M+H]+ | 917.68544 | 0.36 | 1.96 | Sphingolipid metabolism |
| 4 | 11.93 | 1.78 | C55H105NO13 | LacCer (d18:1/25:0) | [M+H]+ | 987.75350 | 0.29 | 2.28 | Sphingolipid metabolism |
| 5 | 9.21 | 1.51 | C26H52NO7P | LysoPC (18:1) | [M-H]− | 521.34950 | 4.34 | 0.60 | Glycerophospholipid metabolism |
| 6 | 11.63 | 1.10 | C43H85O8P | PA (40:0) | [M-H]− | 759.5954 | 2.51 | 0.43 | Glycerophospholipid metabolism |
| 7 | 10.14 | 1.52 | C40H76NO8P | PC (32:2) | [M-H]− | 729.53166 | 2.19 | 0.32 | Glycerophospholipid metabolism |
| 8 | 10.49 | 1.32 | C42H80NO8P | PC (34:2) | [M-H]− | 757.56290 | 2.54 | 0.32 | Glycerophospholipid metabolism |
| 9 | 9.52 | 1.61 | C42H76NO8P | PC (34:4) | [M-H]− | 753.53070 | 2.05 | 0.43 | Glycerophospholipid metabolism |
| 10 | 10.22 | 1.37 | C37H74NO8P | PE (32:0) | [M-H]− | 691.51380 | 0.15 | 2.78 | Glycerophospholipid metabolism |
| 11 | 13.73 | 1.01 | C45H93N2O6P | SM (d18:0/22:0) | [M-H]− | 788.67284 | 0.30 | 1.42 | Sphingolipid metabolism |
| 12 | 16.40 | 1.52 | C59H92O6 | TG (56:11) | [M+H]+ | 896.68605 | 2.10 | 0.40 | Glycerolipid metabolism |
| 13 | 15.83 | 1.60 | C61H92O6 | TG (58:13) | [M+H] + | 920.68623 | 2.94 | 0.33 | Glycerolipid metabolism |
| 14 | 11.16 | 1.31 | C60H111NO18 | TriCer (d18:1/24:1) | [M-H]− | 1133.7749 | 0.12 | 8.01 | Sphingolipid metabolism |
P<0.01. NC, negative control; SH, spontaneously hypertensive; OA, oleanolic acid; tR, retention time; VIP, variable importance in projection; LacCer, lactosylceramide; TriCer, trihexosylceramide; GluCer, glucosylceramide; SM, sphingomyelin; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PA, phosphatidic acid; lysoPC, lysophosphatidylcholine; DG, diglyceride; TG, triglyceride.
The effect of OA on differentially regulated markers in SHRs was determined by performing one-way ANOVA and determining the FC values. Biomarkers with P<0.01 between SH group and OA group were designated as having been affected by treatment with OA. Box plots were produced using GraphPad Prism v5.01 (GraphPad Software, Inc.) using the log2 normalized abundance intensity values for each lipid metabolite.
Receiver operating characteristic (ROC) curve analysis and biomarker selection
ROC curve analysis is considered to be a viable method for determining the clinical utility of biomarkers in metabolomics studies (77,78). ROC curves were constructed using SPSS 22.0 (IBM Corp.). The area under the curve (AUC) is used to evaluate the sensitivity and specificity of biomarkers. In the present analysis, an AUC value >0.7 indicated that the lipids may be effective diagnostic biomarkers (79).
Pathway impact analysis (PIA)
PIA was performed to deter-mine potential metabolic pathways and networks influenced by hypertension by using a web-based tool (Metaboanalyst 4.0; https://www.metaboanalyst.ca/) to perform Metabolomics Pathway Analysis (MetPA) (80). The differential expression of lipid species in the OA group were consequently analyzed by MetPA as previously described (81). The impact value threshold was set to 0.01, and pathways with an impact value above this threshold were removed via filtration (80). The false discovery rate was calculated to reduce the risk of a false positive using an adjusted P<0.05 based on the Benjamini-Hochberg method (82).
Reverse transcription-quantitative PCR (RT-qPCR) assay
The remaining liver tissues were homogenized in liquid nitrogen, and total RNA was extracted (n=3/group) with TRIzol reagent according to the manufacturer's protocol. The concentration and purity of RNA were measured using an Ultra-Micro UV Visible Spectrophotometer (Quawell Q-5000; Quawell Technology, Inc.). RNA samples (100 ng) were dissolved in 25 µl DNase/RNase-free water and stored at -80°C prior to further experimentation. The PrimeScript RT reagent kit was used to synthesize first-strand cDNAs at 42°C for 15 min. The qPCR primers used in the present study (obtained from Shanghai Shenggong Co., Ltd.) were presented in Table II. qPCR was performed using SYBR Premix Ex Taq II (Tli RNaseH Plus) and an Applied CFX Connect Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.). The PCR cycling conditions were 95°C for 30 sec, followed by 45 cycles of 95°C for 30 sec and 60°C for 34 sec (10). The housekeeping gene, β-actin, was used as a reference gene for normalization of target gene expression, and the relative expression of genes was determined using the 2−ΔΔCq method (83). To determine the differences in the relative expression of genes in the SH group compared with the NC group, the NC group served for calibration purposes, and the SH group was the experimental test group. Alternatively, to determine the effects of OA in SHRs (comparing the relative expression of genes in the OA group with those in the SH group), the SH group served for calibration purposes, and the OA group served as the experimental test groups.
Table II.
Primer sequences.
| Gene | Sequence (5′-3′) |
|---|---|
| sPLA2 | F: TGTCGATATGGAAAGGCACCAA |
| R: TAGCAGACGTCCAACTGGTTAC | |
| FAS | F: GGTAGGCTTGGTGAACTGTCTC |
| R: TCTAACTGGAAGTGACGGAAGG | |
| β-actin | F: CATCTATGAGGGTTACGCGCT |
| R: ATTTCCCTCTCAGCTGTGGTG |
sPLA2, secretory phospholipase A2; FAS, fatty acid synthase.
Western blot assay for FAS and sPLA2 proteins
Liver tissues were homogenized using RIPA lysis buffer (Beyotime Institute of Biotechnology), and the total extracted proteins were harvested and quantified using BCA assay analysis (Beyotime Institute of Biotechnology) (84). Samples (20 µg protein) were analyzed using SDS-PAGE (12% gels). Gel electrophoresis was performed at 120 V for 2 h, followed by transfer onto a polyvinylidene fluoride membrane (0.45 mm) at 100 V for 1 h. The membranes were blocked in 5% non-fat milk in TBS-0.1% Tween 20 buffer for 1 h at room temperature. The membranes were then probed with rabbit anti-FAS monoclonal antibody (1:1,500; cat. no. ab22759; Abcam) or rabbit anti-sPLA2 polyclonal antibody (1:1,500; cat. no. ab23705; Abcam) at 4°C overnight, followed by horse-radish peroxidase-conjugated goat anti-rabbit secondary antibody (1:1,000; cat. no. SE134; Beijing Solarbio Science & Technology Co., Ltd.) for 2 h at room temperature. The protein bands were developed using an UltraSignal ECL kit (4A Biotech Co., Ltd.) and normalized against β-actin (1:1,500; cat. no. K006153P; Beijing Solarbio Science & Technology Co., Ltd.). Band intensities were quantified using ImageJ (v1.51; National Institutes of Health) (85).
Statistical analysis
All data are reported as the mean ± SD. Data were analyzed using SPSS 22.0. One-way ANOVA was used to analyze three groups, followed by Bonferroni post hoc test. A mixed two-way ANOVA was performed to analyze the changes in blood pressure over time within and between groups groups; Bonferroni post hoc test was used for multiple comparisons. The confidence interval (CI) was set to 95%, and P<0.05 was considered to indicate a statistically significant difference.
Results
OA reverses the elevated SBP and DBP observed in SHRs
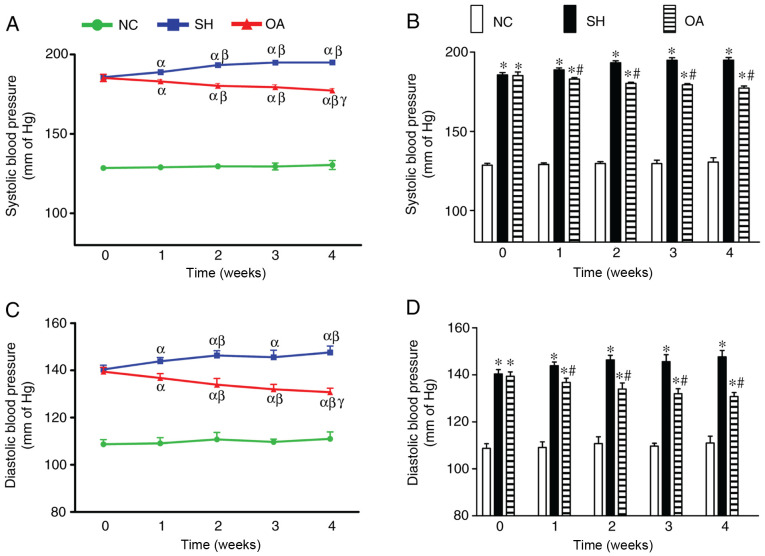
The effects of OA on SBP and DBP are shown in Fig. 1. In the NC group, no significant changes in SBP were identified throughout the duration of the study, and the mean SBP throughout the experiment period was 129.44±1.90 mmHg. The SH group, however, had significantly higher SBP compared with the NC group throughout the treatment period. In addition, a significant increase in SBP was observed over time throughout the experimental period. The mean SBP in the SH group during week 0 was 185.67±1.43 mmHg, which continued to increase over the time, reaching a peak of 194.93±1.65 mmHg at week 4. Treatment of SHR rats with OA, however, led to a significant reversal in the elevated SBP compared with the SH group between weeks 1 and 4.
Figure 1.
Effects of OA on SBP and DBP in SH rats. (A) Comparison of SBP across weeks in the NC, SH and OA groups. αP<0.05 vs. week 0; βP<0.05 vs. week 1; γP<0.05 vs. week 2. (B) Changes in SBP between the NC, SH and OA groups. *P<0.01 vs. NC; #P<0.01 vs. SH. (C) Comparison of DBP across weeks in the NC, SH and OA groups. αP<0.05 vs. week 0; βP<0.05 vs. week 1; γP<0.05 vs. week 2. (D) Changes in DBP between the NC, SH and OA groups. *P<0.01 vs. NC; #P<0.01 vs. SH. Data are presented as the mean ± SD; n=10/group. NC, negative control; SH, spontaneously hypertensive; OA, oleanolic acid; SBP, systolic blood pressure; DBP, diastolic blood pressure.
In the NC group, there were no notable changes in DBP throughout the duration of the experiment, and the mean DBP throughout the experimental period was 109.83±2.46 mmHg. The SH group exhibited significantly higher DBP compared with the NC group throughout the treatment period. In addition, there was a significant increase in DBP up to week 2, after which no further increases in DBP were noted. The mean DBP in the SH group at week 0 was 140.37±1.84 mmHg, which increased to 146.33±1.94 mmHg at week 2. OA treatment led to a significant decrease in DBP in the SHRs across the 4 weeks.
Analysis of biochemical markers
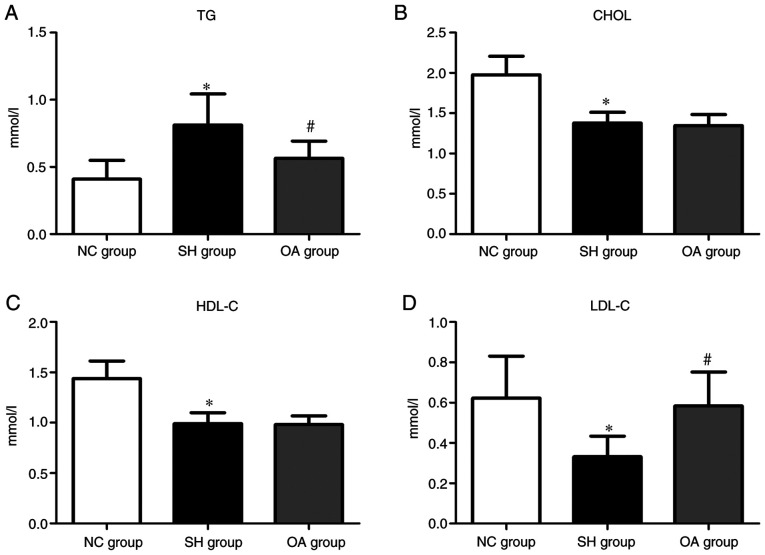
The lipid profiles of serum TGs, CHOL, HDL-C and LDL-C in the NC, SH and OA groups are presented in Fig. 2. The serum levels of CHOL, HDL-C and LDL-C were significantly lower in the SH group compared with the NC group, and TGs were significantly elevated in the SH group compared with the NC group (P<0.01). In the OA group, the TG level was reduced compared with the SH group (P<0.01); conversely, LDL-C was increased compared with the SH group (P<0.01). However, there were no significant differences in CHOL and HDL-C levels between the SH and OA groups. Collectively, these results demonstrated that SHRs exhibited typical pathological features as reported in a previous study (86), and that OA regulated the serum levels of TGs and LDL-C.
Figure 2.
Levels of serum biochemical markers among the NC, SH and OA groups. Levels of (A) TG, (B) CHOL, (C) HDL-C and (D) LDL-C. Data are presented as the mean ± SD; n=10/group. *P<0.01 vs. NC group; #P<0.01 vs. SH group. NC, normal control; SH, spontaneously hypertensive; OA, oleanolic acid; TG, triglyceride; CHOL, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Lipidomics analysis of liver homogenates
The possible mechanism of action of OA was subsequently determined using lipidomics analysis of liver homogenates using UPLC-Q-Orbitrap/MS. Total ion chromatograms of lipid extracts from liver homogenates revealed a good degree of separation (Fig. S1). Instrument stability and analytical repeatability were determined to confirm that the inherent differences between groups were truly the source of significant differences in liver metabolites in LC-MS, and to eliminate possible interference from instrumental drift. The study included an analysis of quality control (QC) samples during the analytical run.
The instrument and method reproducibility were deter-mined using one QC sample for every 6 test samples. The relative standard deviation (RSD) of the QC sample intensity and retention time in positive ion mode were 2.09-4.73 and 0-0.12%, respectively, and in negative ion mode, the RSD of the QC sample intensity and retention time were 0.62-7.04 and 0-0.12%, respectively (Table SI). The deviation variation of all QC samples was further determined via PCA for method validation. The results revealed that 12 QC samples in the positive ion mode, and 6 QC samples in the negative ion mode, fell within 2 SDs with a 95% CI (Fig. S3). QC samples, together with test samples, were further analyzed using the PCA and PLS-DA method (Fig. S4). A number of QC samples were closely clustered in score plots. These data demonstrated that the analytical method was precise, reproducible and suitable for the metabolomics study.
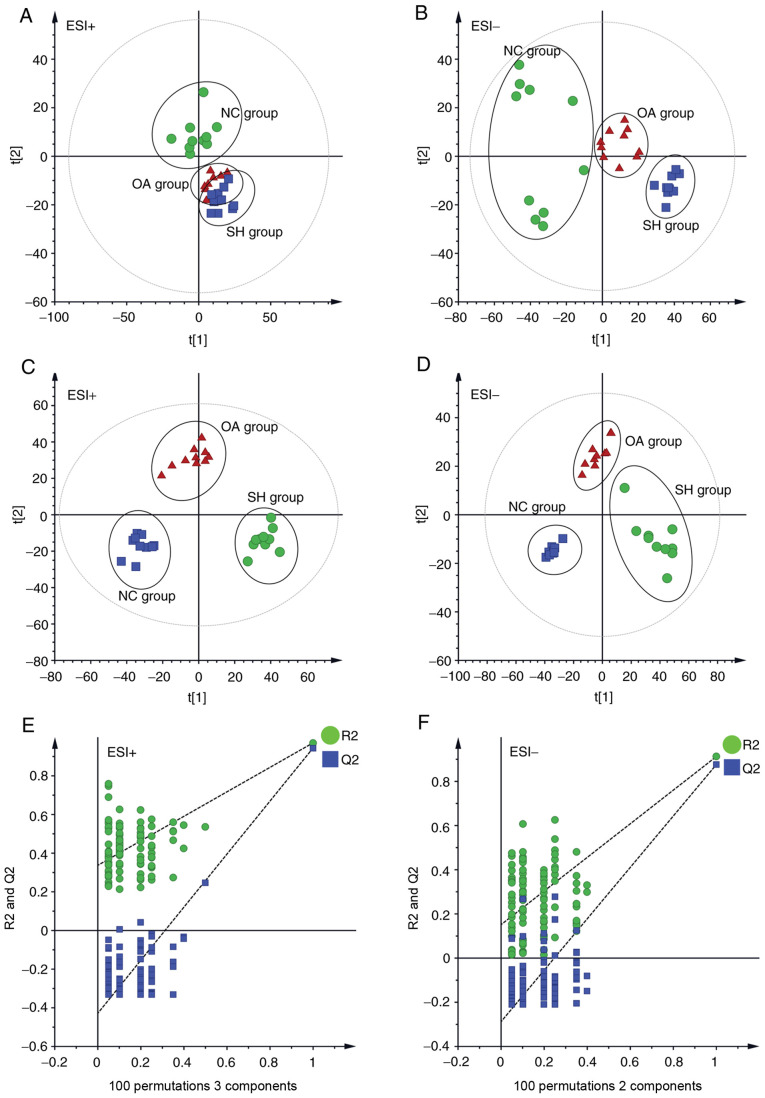
PCA was performed to determine the differential regulation of lipid species in the three groups (NC, SH and OA), and the resulting data are presented as score plots in Fig. 3A (positive ionization mode), whereas Fig. 3B (negative ionization mode) represents the sample distribution in multivariate space. As shown in Fig. 3A and B, the lipidomic profiles in positive and negative ionization modes of the NC and SH groups were clearly separated, revealing the perturbations of lipid profiles in the SH group. The R2X predictive ability values of the PCA models were 0.509 and 0.506 in positive and negative modes, respectively, suggesting that the data were statistically reliable.
Figure 3.
Multivariate data analysis of liver lipidomics. (A) PCA score plot of the NC, SH and OA groups in the ESI+ mode (R2=0.927). (B) PCA score plot of the NC, SH and OA groups in the ESI- mode (R2=0.839). (C) PLS-DA score plot of the NC, SH and OA groups in the ESI+ mode (R2X=0.509, R2Y=0.971, Q2=0.937). (D) PLS-DA score plot of the NC, SH and OA groups in the ESI- mode (R2x=0.506, R2y=0.915, Q2=0.889). (E) 100 permutation tests of the PLS-DA model in the ESI+ mode (R2=0.337 and Q2=−0.428). (F) 100 permutation tests of the PLS-DA model in ESI- mode (R2=0.151 and Q2=−0.288). NC, normal control; SH, spontaneously hypertensive; OA, oleanolic acid; PCA, principal component analysis; PLS-DA, partial least squares discriminant analysis; ESI, electrospray ionization.
PLS-DA analysis was subsequently performed to further assess the effect of OA treatment on lipid profiles in SHRs. In PLS-DA analysis (Fig. 3C and D), the SH and NC groups were clearly separated, which was consistent with the findings of the PCA analysis. The lipidomics profile of the OA group was distinct compared with that of the SH group, suggesting that the dysregulation of lipids in SHRs was ameliorated following treatment with OA. Distinct separation of the NC, SH and OA groups was observed in the PLS-DA score plots. The distribution obtained suggested that OA treatment led to a partial recovery of the hypertension status. A permutation test was subsequently performed to test the overfitting of PLS-DA after modelling the data. A hundred permutation tests generated intercepts of R2=0.337 and Q2=−0.428 in positive mode, and R2=0.151 and Q2=-0.288 in negative mode (Fig. 3E and F), which demonstrated that the PLS-DA models were robust without overfitting.
From the VIP values, it was concluded that the contributions of the features for the model were employed to select the potential biomarkers. Metabolite candidates with VIP >1, P<0.01 and FC >2 were noted as potential biomarkers. Based on the threshold, 14 lipid species in liver that were potentially associated with the influence exerted by OA upon hypertension in SHRs were identified (Table I).
Liver homogenates were used to identify the differentially expressed lipid species according to the score plots. In the positive ionization mode, six lipid species [one PC, two TGs, one DG and two lactosylceramides (LacCers)], and in the negative ionization mode, seven lipid species [one lysoPC, two PCs, one PE, one phosphatidic acid (PA), one SM and one trihexosylceramide (TriCer)] were detected (Table I). DGs, TGs, lysoPC, PC, PE, Cer, SM and PA were present as either [M+H]+ or [M-H]− adducts. Lipid species were identified on the basis of: i) Pseudomolecular ion masses ([M+H]+ or [M-H]−); ii) MS/MS product ion analysis; and/or iii) comparison with authentic standards or information in databases, including HMDB, LIPID MAPS Lipidomics Gateway, METLIN and KEGG databases. The differentially regulated lipid species are shown in Table I, and the identification results of lipid species according to HPLC-ESI-MS/MS are shown in Fig. S5 and Table SII. MS and MS/MS data of these reference standards and QC samples were obtained by collision-induced dissociation.
Lipid metabolite analysis
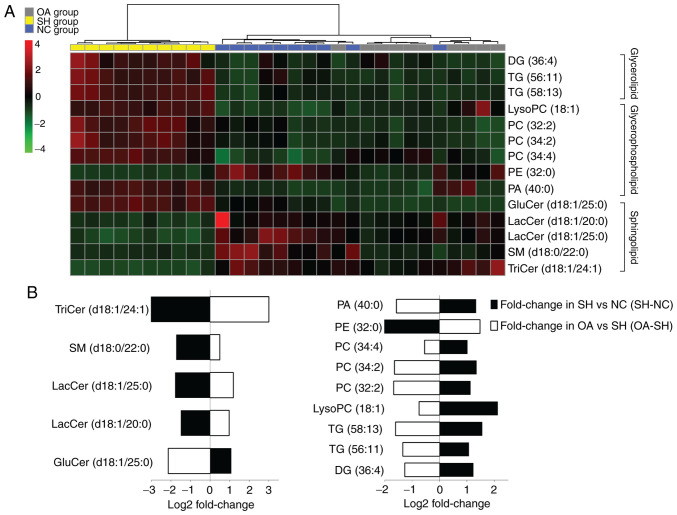
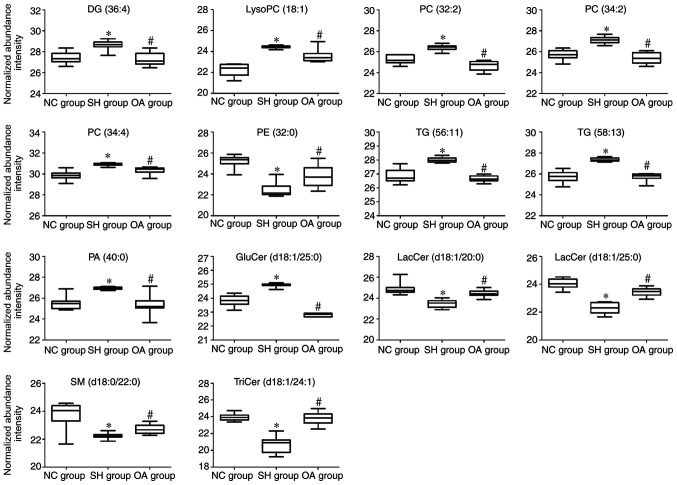
Selected biomarker data were analyzed using clustering heatmaps to determine the associations and differences between samples (Fig. 4). In the SH group, the concentrations of LacCer (d18:1/20:0, d18:1/25:0), glucosylceramide (GluCer) (d18:1/25:0), SM (d18:0/22:0), TriCer (d18:1/24:1), PE (32:0) and PA (40:0) were significantly decreased, whereas those of lysoPC (18:1), PCs (32:2, 34:2, 34:4), TGs (56:11, 58:13) and DG (36:4) were increased (Table I).
Figure 4.
Lipidomics profiling of the 14 identified lipid species. (A) Heatmap showing the hierarchical clustering of the liver lipid species in the NC, SH and OA groups, colored by abundance intensity; the identified lipid species are represented by each line on the graph. The scale from −4 to +4 is colored from green through to red representing low to high abundance, respectively. (B) Fold changes in liver lipid metabolite levels in the NC and OA groups (n=10/group) compared with the SH group. The log2 ratio for signals of each lipid species were calculated and normalized to the abundance intensity. Groups were analyzed using one-way ANOVA. NC, normal control; SH, spontaneously hypertensive; OA, oleanolic acid; LacCer, lactosylceramide; TriCer, trihexosylceramide; GluCer, glucosylceramide; SM, sphingomyelin; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PA, phosphatidic acid; lysoPC, lysophosphatidylcholine; DG, diglyceride; TG, triglyceride.
To further understand the metabolic differences between the NC, SH and OA groups, the lipid data were analyzed using a heatmap. The identified lipids clearly distinguished the metabolic profile of the SH group, and were regarded as potential biomarkers (Fig. 4A). Additional analysis of lipidomics data using one-way ANOVA revealed that the significantly dysregulated lipid classes between the NC, SH and OA groups were sphingolipids, glycerolipids and glycerophospholipids (Fig. 4B). In liver samples obtained at 4 weeks after treatment, the total levels of lysoPC (18:1), PCs (32:2, 34:2, 34:4), TGs (56:11, 58:13) and DG (36:4) were decreased compared with the SH group, whereas the levels of LacCer (d18:1/20:0), LacCer (d18:1/25:0), GluCer (d18:1/25:0) and SM (d18:0/22:0) were increased compared with the SH group.
ROC curve analysis and biomarker selection
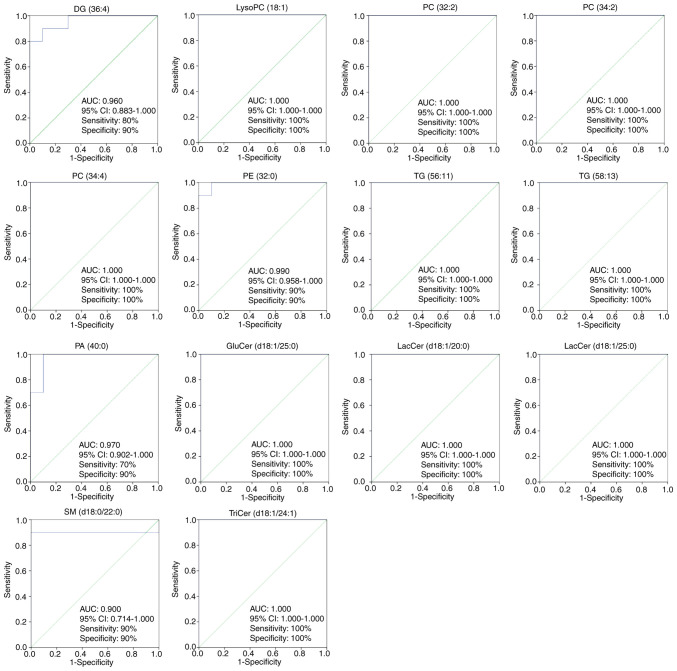
In the present analysis, ROC curves were used to screen markers by examining the AUC values of the biomarkers in the SH and OA groups (87). ROC curves were performed to further identify biomarkers of antihypertensive effects of OA. The 14 lipid species shown in Fig. 5 with AUC values ≥0.900, sensitivity >70% and specificity >90% (95% CI) were considered as potential biomarkers of the lipid-regulating effects of OA. These 14 lipid species were DG (36:4), lysoPC (18:1), PC (32:2), PC (34:2), PC (34:4), PE (32:0), TG (56:11), TG (58:13), PA (40:0), GluCer (d18:1/25:0), LacCer (d18:1/20:0), LacCer (d18:1/25:0), SM (d18:0/22:0) and TriCer (d18:1/24:1). Fig. 6 presents the differences in the levels of the key lipid biomarkers between the NC, SH and OA groups. Collectively, these findings indicated that sphingolipids, glycerides and glycerophospholipids represent potential biomarkers of the lipid-regulating effects of OA.
Figure 5.
Partial least squares discriminant analysis-based receiver operator characteristic curves of the 14 lipid species used for the selection of biomarkers for the antihypertensive effects of oleanolic acid. The corresponding AUC, 95% CI, specificities, and sensitivities are presented. LacCer, lactosylceramide; TriCer, trihexosylceramide; GluCer, glucosylceramide; SM, sphingomyelin; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PA, phosphatidic acid; lysoPC, lysophosphatidylcholine; DG, diglyceride; TG, triglyceride; AUC, area under the curve; CI, confidence interval.
Figure 6.
Box plots representing changes in lipid biomarker levels between the NC, SH and OA groups. *P<0.01 vs. NC group; #P<0.01 vs. OA group. The y-axis shows the log2 normalized abundance intensity. NC, normal control; SH, spontaneously hypertensive; OA, oleanolic acid; LacCer, lactosylceramide; TriCer, trihexosylceramide; GluCer, glucosylceramide; SM, sphingomyelin; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PA, phosphatidic acid; lysoPC, lysophosphatidylcholine; DG, diglyceride; TG, triglyceride.
PIA
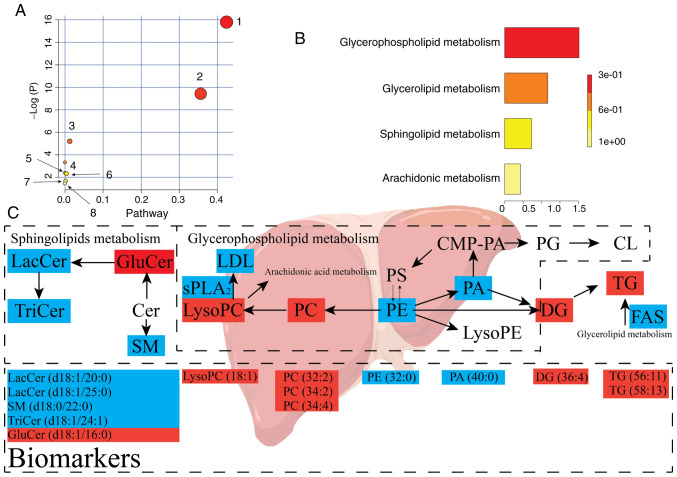
PIA revealed dysregulation of eight pathways, including 'glycerophospholipid metabolism', 'sphingolipid metabolism', 'glycerolipid metabolism', 'linoleic acid metabolism', 'α-linolenic acid metabolism', 'glycosylphosphatidylinositol-anchor biosynthesis', 'phosphatidylinositol signaling system' and 'arachidonic acid metabolism'. PIA of the eight pathways above is presented in Fig. 7A and Table SIII. Fig. 7 presents that the pathways that were responsive to hypertension were glycerophospholipid metabolism, sphingolipid metabolism and glycerolipid metabolism. The eight pathways were regulated by OA treatment, and may act as targets for OA against hypertension. Additionally, eight metabolic pathways were determined to be dysregulated in IPA based on the quantitative enrichment analysis algorithm of MetPA (Fig. 7B) and overview of the integrated metabolic pathway (Fig. 7C). The false discovery rate was calculated to reduce the risk of a false positive using an adjusted P<0.05 based on the Benjamini-Hochberg method (88).
Figure 7.
Lipid metabolic pathway analysis of the identified differential lipid species. (A) Analysis of liver lipid metabolic pathways of the identified differential lipid species in SH rats upon treatment with oleanolic acid. The pathways are numbered as follows: 1, sphingolipid metabolism; 2, glycerophospholipid metabolism; 3, glycerolipid metabolism; and 4, linoleic acid metabolism; 5, α-linolenic acid metabolism; 6, glycosylphosphatidylinositol-anchor biosynthesis; 7, phosphatidylinositol signaling system; 8, arachidonic acid metabolism. (B) Quantitative enrichment analysis performed using metabolite set enrichment analysis. (C) Lipid metabolic changes in the development of hypertension. Upregulated metabolites are shown in red, whereas metabolites shown in blue represent downregulated metabolites in the SH group compared with in the NC group (n=10/group). The treatment with OA (OA group) reversed both the up- and down- regulated lipid species. NC, normal control; SH, spontaneously hypertensive; LacCer, lactosylceramide; TriCer, trihexosylceramide; GluCer, glucosylceramide; SM, sphingomyelin; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PA, phosphatidic acid; PG, phosphatidylglycerol; PS, phosphatidylserine; lysoPC, lysophosphatidylcholine; lysoPE, lysophosphatidylethanolamine; CMP-PA, cytidine monophosphate-phosphatidic acid; CL, cardiolipin; DG, diglyceride; TG, triglyceride; LDL, low-density lipoprotein; sPLA2, secretory phospholipase A2; FAS, fatty acid synthase.
Expression of FAS and sPLA2 in the livers of SHRs
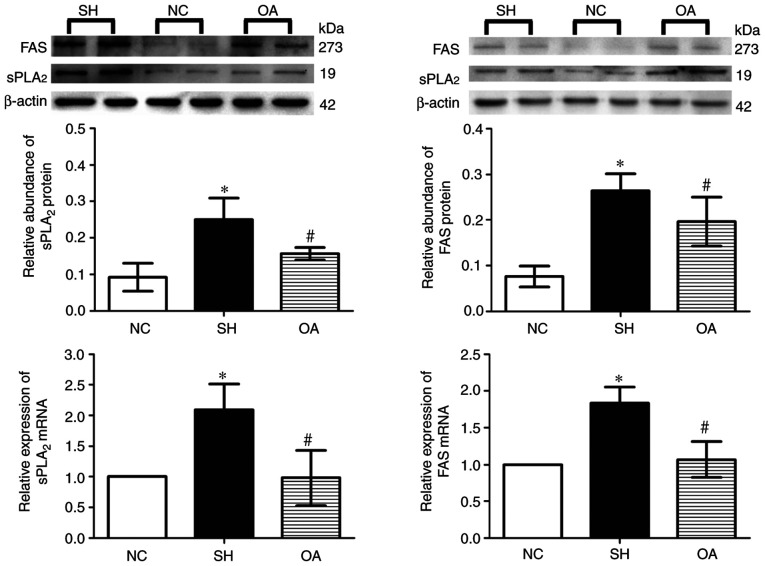
As presented in Fig. 8, Compared with normal group, the expression levels of FAS and sPLA2 mRNA were significantly increased in the SH group (P<0.05). These mRNA levels were significantly reduced by OA compared with the SH group (P<0.05). Furthermore, in SH group the protein expression levels of sPLA2 and FAS in the liver were increased (P<0.05) compared with those in the NC group. OA treatment resulted in a significant decrease in sPLA2 and FAS protein levels (P<0.05) compared with those in the SH group.
Figure 8.
Effect of OA on the expression levels of sPLA2 and FAS mRNA and protein in rat livers. Data are presented as the mean ± SD. NC, normal control; SH, spontaneously hypertensive; OA, oleanolic acid; sPLA2, secretory phospholipase A2; FAS, fatty acid synthase. *P<0.05 vs. NC; #P<0.05 vs. SH.
Discussion
Hypertension has been epidemiologically proven to be a risk factor for stroke and cardiovascular diseases (86). SHRs are known to possess lipid metabolism abnormalities, as well as exhibiting spontaneous hypertension (89). In the present study, it was demonstrated that SBP and DBP in SHR rats increased significantly with age compared with the WKY group. In SHRs, LDL-C, HDL-C and CHOL levels were significantly lower, whereas there was a significantly higher level of TGs.
Lipidomics analysis yields data concerning numerous variables that are difficult to analyze according to conventional methods. PCA is the dominant method of lipidomics analysis (90). PLS-DA is one of the methods that may effectively identify abnormal endogenous substances and drug treatment targets. Using lipidomics in conjunction with MS/MS analysis also helps to improve the accuracy of lipid marker identification. In the present study, lipidomics analysis was conducted, which highlighted that glycerophospholipid metabolism and sphingolipid metabolism may serve as pathways that could be involved in the long-term mechanisms underlying the spontaneously hypertensive response. In addition, using lipidomics data, it was possible to identify 14 endogenous metabolites as potential determinants in the response to hypertension in rats. The analyses of the candidate lipid pathways further indicated the influence of OA on the SHRs, highlighting that treatment with OA resulted in significant amelioration of hypertension and associated abnormalities in lipid metabolism.
In the present study, five sphingolipid biomarkers were selected based on univariate or multivariate statistical analysis and MS/MS analysis. These were the main biomarkers associated with the antihypertensive effects of OA, and they have previously been identified in the SHR liver (91). Changes in the levels of these sphingolipids, including significant decreases in LacCer (d18:1/20:0), LacCer (d18:1/25:0), GluCer (d18:1/25:0), SM (d18:0/22:0) and TriCer (d18:1/24:1), were observed in SHRs. These results suggested that OA treatment led to a reversal of the original alterations. A previous study showed an association between hypertension and marked alterations in vascular sphingolipid biology, including elevated Cer levels and signaling, which thereby contribute to an increased vascular tone (92). Additionally, in the present study, OA has been shown to exert marked regulatory effects on sphingolipids. Following OA treatment, serum biochemical marker analyses revealed that the levels of total accumulated TG were reduced. Therefore, it is proposed that OA has antihypertensive activity, and is able to regulate lipid metabolism disorders.
Four biomarker candidates have been identified that may account for the effects of antihypertensive drugs; effects of these biomarkers on the drugs captopril and valsartan in SHRs have been identified (93-95). The emulsifying properties of PC reduce the deposition of TGs and cholesterol on blood vessel walls (96). Kulkarni et al (97) suggested that a decrease in the level of PC (34:4) may be an important cause of hypertension. In the present study, the levels of PC (32:2), PC (34:2) and PC (34:4) in SH group were shown to be significantly increased compared with NC group.
LysoPCs are important biomarkers associated with effects observed in SHRs (98). LysoPCs, major lipid constituents of oxidized LDL, are generated via hydrolysis of the lipid components of oxidized LDL by lipoprotein-associated PLA2 (99,100). In the present study, the levels of lysoPC (18:1) were increased in the SH group, but were significantly reduced in the OA group; by contrast, lysoPC (22:6) levels were decreased in SHRs and increased in the OA group. A previous study demonstrated that long-chain polyunsaturated fatty acids are more beneficial for cardiovascular disease, including lowering blood pressure (101), suggesting that the reduced lysoPC (18:1) levels in the SH group may be attributable to alterations in lysoPC and PC metabolism, leading to abnormal fatty acid metabolism.
According to a study conducted by Hu et al (102), TGs exhibit lipotoxic effects. An accumulation of TGs in the blood plasma was identified in patients suffering from hypertension (27). Another study revealed that antihypertensive drugs currently administered to patients with hypertension only exhibit moderate effects in terms of modification of lipid levels (21). Kulkarni et al (97) indicated that DG (16:0/22:5), DG (16:0/22:6) and PE (40:6) are closely linked with hereditary hypertension. Hu et al (27) showed that the levels of plasma TGs (C48, C50, C52, C54 and C56) trended upwards in patients with hypertension; it was found that the levels of TGs (C53, C54, C56, C58, C60 and C66) in the livers of patients with hypertension were elevated compared with normal subjects. In the present study, it was found that the levels of DG (36:4), TG (56:11) and TG (58:13) in the SH group were significantly increased compared with the NC group, suggesting that hypertension is associated with the accumulation of DGs and TGs in the liver. OA regulated the metabolism of DGs and TGs in hypertensive rats.
sPLA2 activity results in the promotion of foam cell formation, increased proinflammatory bioactive lipid levels and decreased HDL levels, and is an independent marker of cardiovascular disease; inhibition of sPLA2 in SHRs has been shown to modify LDL-C and HDL-C levels via the hydrolysis of phospholipids (103). Therefore, in the present study, various liver lipids and serum biochemical markers were measured. OA treatment was shown to decrease TG levels in the OA group compared with the SH group. Compared with the SH group, the LDL-C levels were shown to increase in the OA group, although no significant effects of OA on HDL-C and CHOL levels were observed.
According to the data obtained in the present study, LDL-C and HDL-C synthesis were both inhibited, and cholesterol accumulation was shifted towards TGs; in the SH group, both FAS and sPLA2 were upregulated, resulting in accumulation of TGs and cholesterol. The present study showed that OA downregulated the mRNA and protein levels of FAS and sPLA2 in SHRs. The enzyme sPLA2 catalyzes the conversion of PC into lysoPC (104). FAS is involved in de novo lipogenesis by converting acetyl-coenzyme A into palmitate, which is subsequently esterified into TGs in the liver (105).
In conclusion, in the present study, liver samples of the NC, SH and OA groups were analyzed via lipidomics using UPLC-ESI-MS/MS. Disorders in liver lipid metabolism in SHRs were subsequently identified. Analyses implicated 14 biomarkers and three metabolic pathways (glycerophospholipid metabolism, sphingolipid metabolism and glycerolipid metabolism) in these processes. All 14 biomarkers were found to be regulated by OA. The SHRs exhibited significant increases in the levels of TGs in the liver, and this was associated with changes in the liver concentrations of glycerophospholipids, sphingolipids and DGs. The changes may reflect disorders in terms of both the biosynthesis and metabolism of glycerophospholipids and sphingolipids in SHRs. Treatment with OA, however, was shown to result in marked improvements in terms of blood pressure and the associated abnormalities in the lipid metabolites.
Supplementary Data
Acknowledgments
We would like to thank the Experimental Centre of Shandong University of Traditional Chinese Medicine for their assistance with this study. We would also like to thank Dr Lili-Gong and Dr Wenqing Yang for the maintenance of the instruments used in this study, as well as Ms. Ruixue Yu and Ms. Ana Liu for their technical support.
Funding
This study was supported by foundation from the National Natural Science Foundation of China (grant no. 81774173), Youth Innovation and Technology Program for the Universities of Shandong Province (grant no. 2019KJM005), Key Technology Research and Development Program of Shandong Province (grant no. 2018GSF119007), Natural Science Foundation of Shandong Province (Major Basic Research Projects; grant no. ZR2018ZC1157) and University Science and Technology Program of Shandong Province (grant no. J17KZ004).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YL and HJ designed the study. SZ, YL and DQ performed experiments. SZ, YL, ZT, XW, DQ, HJ and YL analyzed the data. YL supervised the design and data interpretation. The manuscript was drafted by SZ, YL, DQ and HJ, and edited by SZ. All authors discussed the results and commented on the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Animal Care and Ethics Committee of Shandong University of Traditional Chinese Medicine (approval no. SDUTCM2018120301).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Liu J. Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol. 1995;49:57–68. doi: 10.1016/0378-8741(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 2.Somova LO, Nadar A, Rammanan P, Shode FO. Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine. 2003;10:115–121. doi: 10.1078/094471103321659807. [DOI] [PubMed] [Google Scholar]
- 3.Tsai SJ, Yin MC. Antioxidative and anti-inflammatory protection of oleanolic acid and ursolic acid in PC12 cells. J Food Sci. 2008;73:H174–H178. doi: 10.1111/j.1750-3841.2008.00864.x. [DOI] [PubMed] [Google Scholar]
- 4.Jesus JA, Lago JH, Laurenti MD, Yamamoto ES, Passero LF. Antimicrobial activity of oleanolic and ursolic acids: An update. Evid Based Complement Alternat Med. 2015;2015:620472. doi: 10.1155/2015/620472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rohilla S, Bhatt DC. Significance of hepatoprotective liver specific targeted drug delivery: A review on novel herbal and formulation approaches in the management of hepatotoxicity. Curr Drug Targets. 2018;19:1519–1549. doi: 10.2174/1389450119666180104113601. [DOI] [PubMed] [Google Scholar]
- 6.Potočnjak I, Šimić L, Vukelić I, Domitrović R. Oleanolic acid attenuates cisplatin-induced nephrotoxicity in mice and chemo-sensitizes human cervical cancer cells to cisplatin cytotoxicity. Food Chem Toxicol. 2019;132:110676. doi: 10.1016/j.fct.2019.110676. [DOI] [PubMed] [Google Scholar]
- 7.Raphael TJ, Kuttan G. Effect of naturally occurring triterpenoids glycyrrhizic acid, ursolic acid, oleanolic acid and nomilin on the immune system. Phytomedicine. 2003;10:483–489. doi: 10.1078/094471103322331421. [DOI] [PubMed] [Google Scholar]
- 8.Somova LI, Shode FO, Mipando M. Cardiotonic and antidys-rhythmic effects of oleanolic and ursolic acids, methyl maslinate and uvaol. Phytomedicine. 2004;11:121–129. doi: 10.1078/0944-7113-00329. [DOI] [PubMed] [Google Scholar]
- 9.Bachhav SS, Bhutada MS, Patil SP, Sharma KS, Patil SD. Oleanolic acid prevents increase in blood pressure and nephrotoxicity in nitric oxide dependent type of hypertension in rats. Pharmacognosy Res. 2014;7:385–392. doi: 10.4103/0974-8490.159575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu R, Yang W, Qi D, Gong L, Li C, Li Y, Jiang H. Targeted neurotransmitter metabolomics profiling of oleanolic acid in the treatment of spontaneously hypertensive rats. RSC Adv. 2019;9:23276–23288. doi: 10.1039/C9RA02377A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bachhav SS, Patil SD, Bhutada MS, Surana SJ. Oleanolic acid prevents glucocorticoid-induced hypertension in rats. Phyther Res. 2011;25:1435–1439. doi: 10.1002/ptr.3431. [DOI] [PubMed] [Google Scholar]
- 12.Madlala HP, Van Heerden FR, Mubagwa K, Musabayane CT. Changes in renal function and oxidative status associated with the hypotensive effects of oleanolic acid and related synthetic derivatives in experimental animals. PLoS One. 2015;10:e0128192. doi: 10.1371/journal.pone.0128192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Somova LI, Shode FO, Ramnanan P, Nadar A. Antihypertensive, antiatherosclerotic and antioxidant activity of triterpenoids isolated from Olea europaea, subspecies africana leaves. J Ethnopharmacol. 2003;84:299–305. doi: 10.1016/S0378-8741(02)00332-X. [DOI] [PubMed] [Google Scholar]
- 14.Liao HH, Zhang N, Feng H, Zhang N, Ma ZG, Yang Z, Yuan Y, Bian ZY, Tang QZ. Oleanolic acid alleviated pressure overload-induced cardiac remodeling. Mol Cell Biochem. 2015;409:145–154. doi: 10.1007/s11010-015-2520-1. [DOI] [PubMed] [Google Scholar]
- 15.Ahn YM, Choi YH, Yoon JJ, Lee YJ, Cho KW, Kang DG, Lee HS. Oleanolic acid modulates the renin-angiotensin system and cardiac natriuretic hormone concomitantly with volume and pressure balance in rats. Eur J Pharmacol. 2017;809:231–241. doi: 10.1016/j.ejphar.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 16.Madlala HP, Metzinger T, Van Heerden FR, Musabayane CT, Mubagwa K, Dessy C. Vascular endothelium-dependent and independent actions of oleanolic acid and its synthetic oleanane derivatives as possible mechanisms for hypotensive effects. PLoS One. 2016;11:e0147395. doi: 10.1371/journal.pone.0147395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marteau JB, Zaiou M, Siest G, Visvikis-Siest S. Genetic determinants of blood pressure regulation. J Hypertens. 2005;23:2127–2143. doi: 10.1097/01.hjh.0000186024.12364.2e. [DOI] [PubMed] [Google Scholar]
- 18.Bacon SL, Sherwood A, Hinderliter A, Blumenthal JA. Effects of exercise, diet and weight loss on high blood pressure. Sports Med. 2004;34:307–316. doi: 10.2165/00007256-200434050-00003. [DOI] [PubMed] [Google Scholar]
- 19.Hinterwirth H, Stegemann C, Mayr M. Lipidomics Quest for molecular lipid biomarkers in cardiovascular disease. Circ Cardiovasc Genet. 2014;7:941–954. doi: 10.1161/CIRCGENETICS.114.000550. [DOI] [PubMed] [Google Scholar]
- 20.Tian Y, Jiang F, Li Y, Jiang H, Chu Y, Zhu L, Guo W. Evaluation of the anti-hypertensive effect of Tengfu Jiangya tablet by combination of UPLC-Q-exactive-MS-based metabolomics and iTRAQ-based proteomics technology. Biomed Pharmacother. 2018;100:324–334. doi: 10.1016/j.biopha.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 21.Tian Z, Zhang S, Wang H, Chen Z, Sun M, Sun L, Gong L, Li Y, Jiang H. Intervention of uncaria and its components on liver lipid metabolism in spontaneously hypertensive rats. Front Pharmacol. 2020;11:910. doi: 10.3389/fphar.2020.00910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerage D, Brindley DN, Hemmings DG. Review: Novel insights into the regulation of vascular tone by sphingosine 1-phosphate. Placenta. 2014;35(Suppl):S86–S92. doi: 10.1016/j.placenta.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Cogolludo A, Villamor E, Perez-Vizcaino F, Moreno L. Ceramide and regulation of vascular tone. Int J Mol Sci. 2019;20:411. doi: 10.3390/ijms20020411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spijkers LJ, van den Akker RF, Janssen BJ, Debets JJ, De Mey JG, Stroes ES, van den Born BJ, Wijesinghe DS, Chalfant CE, MacAleese L, et al. Hypertension is associated with marked alterations in sphingolipid biology: A potential role for ceramide. PLoS One. 2011;6:e21817. doi: 10.1371/journal.pone.0021817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graessler J, Schwudke D, Schwarz PE, Herzog R, Shevchenko A, Bornstein SR. Top-down lipidomics reveals ether lipid deficiency in blood plasma of hypertensive patients. PLoS One. 2009;4:e6261. doi: 10.1371/journal.pone.0006261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu A, Chu YJ, Wang X, Yu R, Jiang H, Li Y, Zhou H, Gong LL, Yang WQ, Ju J. Serum metabolomics study based on LC-MS and antihypertensive effect of uncaria on spontaneously hypertensive rats. Evidence-based Complement Altern Med. 2018;2018:9281946. doi: 10.1155/2018/9281946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu C, Kong H, Qu F, Li Y, Yu Z, Gao P, Peng S, Xu G. Application of plasma lipidomics in studying the response of patients with essential hypertension to antihypertensive drug therapy. Mol Biosyst. 2011;7:3271–3279. doi: 10.1039/c1mb05342f. [DOI] [PubMed] [Google Scholar]
- 28.Pyttel S, Zschörnig K, Nimptsch A, Paasch U, Schiller J. Enhanced lysophosphatidylcholine and sphingomyelin contents are characteristic of spermatozoa from obese men-A MALDI mass spectrometric study. Chem Phys Lipids. 2012;165:861–865. doi: 10.1016/j.chemphyslip.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Law SH, Chan ML, Marathe GK, Parveen F, Chen CH, Ke LY. An updated review of lysophosphatidylcholine metabolism in human diseases. Int J Mol Sci. 2019;20:1149. doi: 10.3390/ijms20051149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun GY, Shelat PB, Jensen MB, He Y, Sun AY, Simonyi A. Phospholipases A2 and inflammatory responses in the central nervous system. Neuromolecular Med. 2010;12:133–148. doi: 10.1007/s12017-009-8092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lara-Castro C, Garvey WT. Intracellular lipid accumulation in liver and muscle and the insulin resistance syndrome. Endocrinol Metab Clin North Am. 2008;37:841–856. doi: 10.1016/j.ecl.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Erion DM, Shulman GI. Diacylglycerol-mediated insulin resistance. Nat Med. 2010;16:400–402. doi: 10.1038/nm0410-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ke C, Zhu X, Zhang Y, Shen Y. Metabolomic characterization of hypertension and dyslipidemia. Metabolomics. 2018;14:117. doi: 10.1007/s11306-018-1408-y. [DOI] [PubMed] [Google Scholar]
- 34.Kawamoto R, Tabara Y, Kohara K, Kusunoki T, Abe M, Miki T. Interaction between serum uric acid and triglycerides in relation to prehypertension in community-dwelling Japanese adults. Clin Exp Hypertens. 2014;36:64–69. doi: 10.3109/10641963.2013.789043. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu Y, Sato S, Koyamatsu J, Yamanashi H, Nagayoshi M, Kadota K, Kawashiri SY, Inoue K, Nagata Y, Maeda T. Platelets and circulating CD34-positive cells as an indicator of the activity of the vicious cycle between hypertension and endothelial dysfunction in elderly Japanese men. Atherosclerosis. 2017;259:26–31. doi: 10.1016/j.atherosclerosis.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 36.Borghi C, Dormi A, Veronesi M, Sangiorgi Z, Gaddi A, Brisighella Heart Study Working Party Association between different lipid-lowering treatment strategies and blood pressure control in the Brisighella heart study. Am Heart J. 2004;148:285–292. doi: 10.1016/j.ahj.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Kintscher U, Marx N, Martus P, Stoppelhaar M, Schimkus J, Schneider A, Walcher D, Kümmel A, Winkler R, Kappert K, et al. Effect of high-dose valsartan on inflammatory and lipid parameters in patients with Type 2 diabetes and hypertension. Diabetes Res Clin Pract. 2010;89:209–215. doi: 10.1016/j.diabres.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 38.Sarkar K, Sinha AK, Mehta JL. The role of statins in endothelial dysfunction in hypertension. Curr Opin Cardiol. 2006;21:316–321. doi: 10.1097/01.hco.0000231401.87232.71. [DOI] [PubMed] [Google Scholar]
- 39.Jacobson TA, Zimmerman FH. Fibrates in combination with statins in the management of dyslipidemia. J Clin Hypertens. 2006;8:35–43. doi: 10.1111/j.1524-6175.2005.05278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wierzbicki AS. Lipid lowering: Another method of reducing blood pressure? J Hum Hypertens. 2002;16:753–760. doi: 10.1038/sj.jhh.1001483. [DOI] [PubMed] [Google Scholar]
- 41.Kwong E, Li Y, Hylemon PB, Zhou H. Bile acids and sphin-gosine-1-phosphate receptor 2 in hepatic lipid metabolism. Acta Pharm Sin B. 2015;5:151–157. doi: 10.1016/j.apsb.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen H, Chen L, Liu D, Chen DQ, Vaziri ND, Yu XY, Zhang L, Su W, Bai X, Zhao YY. Combined clinical phenotype and lipidomic analysis reveals the impact of chronic kidney disease on lipid metabolism. J Proteome Res. 2017;16:1566–1578. doi: 10.1021/acs.jproteome.6b00956. [DOI] [PubMed] [Google Scholar]
- 43.Walther A, Cannistraci CV, Simons K, Durán C, Gerl MJ, Wehrli S, Kirschbaum C. Lipidomics in major depressive disorder. Front Psychiatry. 2018;9:459. doi: 10.3389/fpsyt.2018.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhuang X, Deng ZB, Mu J, Zhang L, Yan J, Miller D, Feng W, McClain CJ, Zhang HG. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J Extracell Vesicles. 2015;4:28713. doi: 10.3402/jev.v4.28713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen P, Leray V, Diez M, Serisier S, Le Bloc'h J, Siliart B, Dumon H. Liver lipid metabolism. J Anim Physiol Anim Nutr (Berl) 2008;92:272–283. doi: 10.1111/j.1439-0396.2007.00752.x. [DOI] [PubMed] [Google Scholar]
- 46.Xie J, Jiang HQ, Li YL, Nie L, Zhou HL, Yang WQ. Study on the intervention effects of pinggan prescription () on spontaneously hypertensive rats based on metabonomic and pharmacodynamic methods. Chin J Integr Med. 2019;25:348–353. doi: 10.1007/s11655-015-2126-1. [DOI] [PubMed] [Google Scholar]
- 47.Biernacki M, Ambrożewicz E, Gęgotek A, Toczek M, Skrzydlewska E. Long-term administration of fatty acid amide hydrolase inhibitor (URB597) to rats with spontaneous hypertension disturbs liver redox balance and phospholipid metabolism. Adv Med Sci. 2019;64:15–23. doi: 10.1016/j.advms.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Bourbon NA, Sandirasegarane L, Kester M. Ceramide-induced inhibition of Akt is mediated through protein kinase Czeta: Implications for growth arrest. J Biol Chem. 2002;277:3286–3292. doi: 10.1074/jbc.M110541200. [DOI] [PubMed] [Google Scholar]
- 49.Mulders ACM, Mathy MJ, Meyer zu Heringdorf D, ter Braak M, Hajji N, Olthof DC, Michel MC, Alewijnse AE, Peters SL. Activation of sphingosine kinase by muscarinic receptors enhances NO-mediated and attenuates EDHF-mediated vasorelaxation. Basic Res Cardiol. 2009;104:50–59. doi: 10.1007/s00395-008-0744-x. [DOI] [PubMed] [Google Scholar]
- 50.Mulders ACM, Hendriks-Balk MC, Mathy MJ, Michel MC, Alewijnse AE, Peters SLM. Sphingosine kinase-dependent activation of endothelial nitric oxide synthase by angiotensin II. Arterioscler Thromb Vasc Biol. 2006;26:2043–2048. doi: 10.1161/01.ATV.0000237569.95046.b9. [DOI] [PubMed] [Google Scholar]
- 51.Brown WJ, Chambers K, Doody A. Phospholipase A2 (PLA2) enzymes in membrane trafficking: Mediators of membrane shape and function. Traffic. 2003;4:214–221. doi: 10.1034/j.1600-0854.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- 52.Cole LK, Vance JE, Vance DE. Phosphatidylcholine biosynthesis and lipoprotein metabolism. Biochim Biophys Acta. 2012;1821:754–761. doi: 10.1016/j.bbalip.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 53.Benrezzouk R, Terencio MC, Ferrándiz ML, San Feliciano A, Gordaliza M, Miguel del Corral JM, de la Puente ML, Alcaraz MJ. Inhibition of human sPLA2 and 5-lipoxygenase activities by two neoclerodane diterpenoids. Life Sci. 1999;64:PL205–PL211. doi: 10.1016/S0024-3205(99)00119-8. [DOI] [PubMed] [Google Scholar]
- 54.Mallat Z, Lambeau G, Tedgui A. Lipoprotein-associated and secreted phospholipases A2 in cardiovascular disease: Roles as biological effectors and biomarkers. Circulation. 2010;122:2183–2200. doi: 10.1161/CIRCULATIONAHA.110.936393. [DOI] [PubMed] [Google Scholar]
- 55.Boekholdt SM, Keller TT, Wareham NJ, Luben R, Bingham SA, Day NE, Sandhu MS, Jukema JW, Kastelein JJ, Hack CE, Khaw KT. Serum levels of type II secretory phospholipase A2 and the risk of future coronary artery disease in apparently healthy men and women: The EPIC-Norfolk prospective population study. Arterioscler Thromb Vasc Biol. 2005;25:839–846. doi: 10.1161/01.ATV.0000157933.19424.b7. [DOI] [PubMed] [Google Scholar]
- 56.Hurt-Camejo E, Camejo G, Peilot H, Oörni K, Kovanen P. Phospholipase A(2) in vascular disease. Circ Res. 2001;89:298–304. doi: 10.1161/hh1601.095598. [DOI] [PubMed] [Google Scholar]
- 57.Rosengren B, Peilot H, Umaerus M, Jönsson-Rylander AC, Mattsson-Hultén L, Hallberg C, Cronet P, Rodriguez-Lee M, Hurt-Camejo E. Secretory phospholipase A2 group V: Lesion distribution, activation by arterial proteoglycans, and induction in aorta by a Western diet. Arterioscler Thromb Vasc Biol. 2006;26:1579–1585. doi: 10.1161/01.ATV.0000221231.56617.67. [DOI] [PubMed] [Google Scholar]
- 58.Sonoki K, Iwase M, Sasaki N, Ohdo S, Higuchi S, Takata Y, Iida M. Secretory PLA2 inhibitor indoxam suppresses LDL modification and associated inflammatory responses in TNFalpha-stimulated human endothelial cells. Br J Pharmacol. 2008;153:1399–1408. doi: 10.1038/bjp.2008.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guardiola M, Exeter HJ, Perret C, Folkersen L, Van't Hooft F, Eriksson P, Franco-Cereceda A, Paulsson-Berne G, Palmen J, Li K, et al. PLA2G10 gene variants, sPLA2 activity, and coronary heart disease risk. Circ Cardiovasc Genet. 2015;8:356–362. doi: 10.1161/CIRCGENETICS.114.000633. [DOI] [PubMed] [Google Scholar]
- 60.Kume N, Gimbrone MA., Jr Lysophosphatidylcholine transcriptionally induces growth factor gene expression in cultured human endothelial cells. J Clin Invest. 1994;93:907–911. doi: 10.1172/JCI117047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jensen-Urstad APL, Semenkovich CF. Fatty acid synthase and liver triglyceride metabolism: Housekeeper or messenger? Biochim Biophys Acta. 2012;1821:747–753. doi: 10.1016/j.bbalip.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iizuka K, Miller B, Uyeda K. Deficiency of carbohydrate-activated transcription factor ChREBP prevents obesity and improves plasma glucose control in leptin-deficient (ob/ob) mice. Am J Physiol Endocrinol Metab. 2006;291:E358–E364. doi: 10.1152/ajpendo.00027.2006. [DOI] [PubMed] [Google Scholar]
- 63.Scott CL. Diagnosis, prevention, and intervention for the meta-bolic syndrome. Am J Cardiol. 2003;92:35i–42i. doi: 10.1016/S0002-9149(03)00507-1. [DOI] [PubMed] [Google Scholar]
- 64.Berndt J, Kovacs P, Ruschke K, Klöting N, Fasshauer M, Schön MR, Körner A, Stumvoll M, Blüher M. Fatty acid synthase gene expression in human adipose tissue: Association with obesity and type 2 diabetes. Diabetologia. 2007;50:1472–1480. doi: 10.1007/s00125-007-0689-x. [DOI] [PubMed] [Google Scholar]
- 65.Mayas MD, Ortega FJ, Macías-González M, Bernal R, Gómez-Huelgas R, Fernández-Real JM, Tinahones FJ. Inverse relation between FASN expression in human adipose tissue and the insulin resistance level. Nutr Metab (Lond) 2010;7:3. doi: 10.1186/1743-7075-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suzuki T, Muramatsu T, Morioka K, Goda T, Mochizuki K. ChREBP binding and histone modifications modulate hepatic expression of the Fasn gene in a metabolic syndrome rat model. Nutrition. 2015;31:877–883. doi: 10.1016/j.nut.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 67.Nedvedova I, Kolar D, Neckar J, Kalous M, Pravenec M, Šilhavý J, Korenkova V, Kolar F, Zurmanova JM. Cardioprotective regimen of adaptation to chronic hypoxia diversely alters myocardial gene expression in SHR and SHR-mtBN conplastic rat strains. Front Endocrinol (Lausanne) 2019;9:809. doi: 10.3389/fendo.2018.00809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.German JB, Gillies LA, Smilowitz JT, Zivkovic AM, Watkins SM. Lipidomics and lipid profiling in metabolomics. Curr Opin Lipidol. 2007;18:66–71. doi: 10.1097/MOL.0b013e328012d911. [DOI] [PubMed] [Google Scholar]
- 69.Yang K, Han X. Lipidomics: Techniques, applications, and outcomes related to biomedical sciences. Trends Biochem Sci. 2016;41:954–969. doi: 10.1016/j.tibs.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lapthorn C, Pullen F, Chowdhry BZ. Ion mobility spec-trometrymass spectrometry (IMS-MS) of small molecules: Separating and assigning structures to ions. Mass Spectrom Rev. 2013;32:43–71. doi: 10.1002/mas.21349. [DOI] [PubMed] [Google Scholar]
- 71.Martano G, Leone M, D'Oro P, Matafora V, Cattaneo A, Masseroli M, Bachi A. SMfinder: Small molecules finder for metabolomics and lipidomics analysis. Anal Chem. 2020;92:8874–8882. doi: 10.1021/acs.analchem.0c00585. [DOI] [PubMed] [Google Scholar]
- 72.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals . Guide for the Care and Use of Laboratory Animals. 8th edition. National Academies Press; Washington, DC: 2011. [Google Scholar]
- 73.Jones KE, Bennett DJ. Motor axon excitability measures in the rat tail are the same awake or anaesthetized using sodium pentobarbital. doi: 10.1101/651927. [DOI]
- 74.Ma N, Yang Y, Liu X, Kong X, Li S, Qin Z, Jiao Z, Li J. UPLC-Q-TOF/MS-based metabonomic studies on the intervention effects of aspirin eugenol ester in atherosclerosis hamsters. Sci Rep. 2017;7:10544. doi: 10.1038/s41598-017-11422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iverson SJ, Lang SLC, Cooper MH. Comparison of the bligh and dyer and folch methods for total lipid determination in a broad range of marine tissue. Lipids. 2001;36:1283–1287. doi: 10.1007/s11745-001-0843-0. [DOI] [PubMed] [Google Scholar]
- 76.Milne S, Ivanova P, Forrester J, Alex Brown H. Lipidomics: An analysis of cellular lipids by ESI-MS. Methods. 2006;39:92–103. doi: 10.1016/j.ymeth.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 77.Søreide K. Receiver-operating characteristic curve analysis in diagnostic, prognostic and predictive biomarker research. J Clin Pathol. 2009;62:1–5. doi: 10.1136/jcp.2008.061010. [DOI] [PubMed] [Google Scholar]
- 78.Blaise BJ, Gouel-Chéron A, Floccard B, Monneret G, Allaouchiche B. Metabolic phenotyping of traumatized patients reveals a susceptibility to sepsis. Anal Chem. 2013;85:10850–10855. doi: 10.1021/ac402235q. [DOI] [PubMed] [Google Scholar]
- 79.Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev Vet Med. 2000;45:23–41. doi: 10.1016/S0167-5877(00)00115-X. [DOI] [PubMed] [Google Scholar]
- 80.Miao H, Zhao YH, Vaziri ND, Tang DD, Chen H, Chen H, Khazaeli M, Tarbiat-Boldaji M, Hatami L, Zhao YY. Lipidomics biomarkers of diet-induced hyperlipidemia and its treatment with poria cocos. J Agric Food Chem. 2016;64:969–979. doi: 10.1021/acs.jafc.5b05350. [DOI] [PubMed] [Google Scholar]
- 81.Xia J, Mandal R, Sinelnikov IV, Broadhurst D, Wishart DS. MetaboAnalyst 2.0-a comprehensive server for metabolomic data analysis. Nucleic Acids Res. 2012;40(Web Server Issue):W127–W133. doi: 10.1093/nar/gks374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- 83.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 84.Zor T, Selinger Z. Linearization of the Bradford protein assay increases its sensitivity: Theoretical and experimental studies. Anal Biochem. 1996;236:302–308. doi: 10.1006/abio.1996.0171. [DOI] [PubMed] [Google Scholar]
- 85.Yang C, Yuan W, Yang X, Li P, Wang J, Han J, Tao J, Li P, Yang H, Lv Q, Zhang W. Circular RNA circ-ITCH inhibits bladder cancer progression by sponging miR-17/miR-224 and regulating p21, PTEN expression. Mol Cancer. 2018;17:19. doi: 10.1186/s12943-018-0771-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang R, Inagawa H, Kazumura K, Tsuchiya H, Miwa T, Morishita N, Uchibori S, Hanashiro J, Masaki T, Kobara H, Soma GI. Evaluation of a hypertensive rat model using peripheral blood neutrophil activity, phagocytic activity and oxidized LDL evaluation. Anticancer Res. 2018;38:4289–4294. doi: 10.21873/anticanres.12726. [DOI] [PubMed] [Google Scholar]
- 87.Yin J, Xie J, Guo X, Ju L, Li Y, Zhang Y. Plasma metabolic profiling analysis of cyclophosphamide-induced cardiotoxicity using metabolomics coupled with UPLC/Q-TOF-MS and ROC curve. J Chromatogr B Anal Technol Biomed Life Sci. 2016;1033-1034:428–435. doi: 10.1016/j.jchromb.2016.08.042. [DOI] [PubMed] [Google Scholar]
- 88.Kind T, Cho E, Park TD, Deng N, Liu Z, Lee T, Fiehn O, Kim J. Interstitial cystitis-associated urinary metabolites identified by mass-spectrometry based metabolomics analysis. Sci Rep. 2016;6:39227. doi: 10.1038/srep39227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Girard A, Madani S, Boukortt F, Cherkaoui-Malki M, Belleville J, Prost J. Fructose-enriched diet modifies antioxidant status and lipid metabolism in spontaneously hypertensive rats. Nutrition. 2006;22:758–766. doi: 10.1016/j.nut.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 90.Dutta M, Joshi M, Srivastava S, Lodh I, Chakravarty B, Chaudhury K. A metabonomics approach as a means for identification of potential biomarkers for early diagnosis of endometriosis. Mol Biosyst. 2012;8:3281–3287. doi: 10.1039/c2mb25353d. [DOI] [PubMed] [Google Scholar]
- 91.Jiang H, Shen Z, Chu Y, Li Y, Li J, Wang X, Yang W, Zhang X, Ju J, Xu J, Yang C. Serum metabolomics research of the anti-hypertensive effects of Tengfu Jiangya tablet on spontaneously hypertensive rats. J Chromatogr B Anal Technol Biomed Life Sci. 2015;1002:210–217. doi: 10.1016/j.jchromb.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 92.Fenger M, Linneberg A, Jeppesen J. Network-based analysis of the sphingolipid metabolism in hypertension. Front Genet. 2015;6:84. doi: 10.3389/fgene.2015.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Penna C, Tullio F, Moro F, Folino A, Merlino A, Pagliaro P. Effects of a protocol of ischemic postconditioning and/or capto-pril in hearts of normotensive and hypertensive rats. Basic Res Cardiol. 2010;105:181–192. doi: 10.1007/s00395-009-0075-6. [DOI] [PubMed] [Google Scholar]
- 94.Lin CH, Lee SY, Zhang CC, Du YF, Hung HC, Wu HT, Ou HY. Fenretinide inhibits macrophage inflammatory media-tors and controls hypertension in spontaneously hypertensive rats via the peroxisome proliferator-activated receptor gamma pathway. Drug Des Devel Ther. 2016;10:3591–3597. doi: 10.2147/DDDT.S114879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jiang H, Nie L, Li Y, Xie J. Application of ultra-performance liquid chromatography coupled with mass spectrometry to metabonomic study on spontaneously hypertensive rats and intervention effects of Ping Gan prescription. J Sep Sci. 2012;35:483–489. doi: 10.1002/jssc.201100769. [DOI] [PubMed] [Google Scholar]
- 96.Ye X, Kong W, Zafar MI, Chen LL. Serum triglycerides as a risk factor for cardiovascular diseases in type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Cardiovasc Diabetol. 2019;18:48. doi: 10.1186/s12933-019-0851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kulkarni H, Meikle PJ, Mamtani M, Weir JM, Barlow CK, Jowett JB, Bellis C, Dyer TD, Johnson MP, Rainwater DL, et al. Plasma lipidomic profile signature of hypertension in mexican american families: Specific role of diacylglycerols. Hypertension. 2013;62:621–626. doi: 10.1161/HYPERTENSIONAHA.113.01396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tokumura A, Fujimoto H, Yoshimoto O, Nishioka Y, Miyake M, Fukuzawa K. Production of lysophosphatidic acid by lysophospholipase D in incubated plasma of spontaneously hypertensive rats and Wistar Kyoto rats. Life Sci. 1999;65:245–253. doi: 10.1016/S0024-3205(99)00243-X. [DOI] [PubMed] [Google Scholar]
- 99.Kim J, Choi JN, Choi JH, Cha YS, Muthaiya MJ, Lee CH. Effect of fermented soybean product (Cheonggukjang) intake on metabolic parameters in mice fed a high-fat diet. Mol Nutr Food Res. 2013;57:1886–1891. doi: 10.1002/mnfr.201200700. [DOI] [PubMed] [Google Scholar]
- 100.Murugesan G, Fox PL. Role of lysophosphatidylcholine in the inhibition of endothelial cell motility by oxidized low density lipoprotein. J Clin Invest. 1996;97:2736–2744. doi: 10.1172/JCI118728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu JC, Conklin SM, Manuck SB, Yao JK, Muldoon MF. Long-chain omega-3 fatty acids and blood pressure. Am J Hypertens. 2011;24:1121–1126. doi: 10.1038/ajh.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hu C, Hoene M, Zhao X, Häring HU, Schleicher E, Lehmann R, Han X, Xu G, Weigert C. Lipidomics analysis reveals efficient storage of hepatic triacylglycerides enriched in unsaturated fatty acids after one bout of exercise in mice. PLoS One. 2010;5:e13318. doi: 10.1371/journal.pone.0013318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ivandic B, Castellani LW, Wang XP, Qiao JH, Mehrabian M, Navab M, Fogelman AM, Grass DS, Swanson ME, de Beer MC, et al. Role of group II secretory phospholipase A2 in atherosclerosis: 1. Increased atherogenesis and altered lipoproteins in transgenic mice expressing group IIa phospholipase A2. Arterioscler Thromb Vasc Biol. 1999;19:1284–1290. doi: 10.1161/01.ATV.19.5.1284. [DOI] [PubMed] [Google Scholar]
- 104.Dutra FL, Vieira DP, Coelho FS, Adade CM, Atella GC, Silva Neto MAC, Lopes AH. Lysophosphatidylcholine triggers cell differentiation in the protozoan parasite herpetomonas samuelpessoai through the CK2 pathway. Acta Parasitol. 2020;65:108–117. doi: 10.2478/s11686-019-00135-8. [DOI] [PubMed] [Google Scholar]
- 105.Singh N, Shafiq M, Jagavelu K, Hanif K. Involvement of fatty acid synthase in right ventricle dysfunction in pulmonary hypertension. Exp Cell Res. 2019;383:111569. doi: 10.1016/j.yexcr.2019.111569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.