Abstract
Background
Dysfunction of the corticostriatal network has been implicated in the pathophysiology of schizophrenia, but findings are inconsistent within and across imaging modalities. We used multimodal neuroimaging to analyze functional and structural connectivity in the corticostriatal network in people with schizophrenia and unaffected first-degree relatives.
Methods
We collected resting-state functional magnetic resonance imaging and diffusion tensor imaging scans from people with schizophrenia (n = 47), relatives (n = 30) and controls (n = 49). We compared seed-based functional and structural connectivity across groups within striatal subdivisions defined a priori.
Results
Compared with controls, people with schizophrenia had altered connectivity between the subdivisions and brain regions in the frontal and temporal cortices and thalamus; relatives showed different connectivity between the subdivisions and the right anterior cingulate cortex (ACC) and the left precuneus. Post-hoc t tests revealed that people with schizophrenia had decreased functional connectivity in the ventral loop (ventral striatum–right ACC) and dorsal loop (executive striatum–right ACC and sensorimotor striatum–right ACC), accompanied by decreased structural connectivity; relatives had reduced functional connectivity in the ventral loop and the dorsal loop (right executive striatum–right ACC) and no significant difference in structural connectivity compared with the other groups. Functional connectivity among people with schizophrenia in the bilateral ventral striatum–right ACC was correlated with positive symptom severity.
Limitations
The number of relatives included was moderate. Striatal subdivisions were defined based on a relatively low threshold, and structural connectivity was measured based on fractional anisotropy alone.
Conclusion
Our findings provide insight into the role of hypoconnectivity of the ventral corticostriatal system in people with schizophrenia.
Introduction
Schizophrenia is a severe, disabling, highly heritable psychiatric disorder of unknown etiology.1,2 Elevated striatal activity3,4 and a dysfunctional prefrontal cortex5–7 are among the most prominent abnormalities observed in people with schizophrenia.8 The striatum, where antipsychotic drugs mainly act,9,10 interacts with cortical areas involved in neurocognitive domains, such as affect and cognitive control.11–13 Neuroimaging studies have revealed that schizophrenia is associated with aberrant connectivity of the corticostriatal network,14–16 and that corticostriatal functional connectivity can predict response to antipsychotic drug treatment,17 implicating the corticostriatal network in the pathophysiology of schizophrenia.
The striatum is a complex structure comprising the caudate, putamen and nucleus accumbens, and it has various functions and roles.18,19 The corticostriatal network has a highly topographic pattern along the striatum.20 According to projections from different cortical areas to the striatum, the striatum itself can be divided into the ventral, associative and sensorimotor areas. The ventral striatum receives projections from the limbic cortex, including the orbitofrontal cortex (OFC), the ventromedial prefrontal cortex, the anterior cingulate cortex (ACC) and other limbic areas.18,21,22 The ventral circuitry is critically involved in emotion-processing and reward-based learning,23 and dysfunction of the ventral circuit has long been hypothesized to underlie psychotic symptoms. Functional MRI (fMRI) studies have reported impaired prefrontal–ventral striatum functional coupling in people with schizophrenia during reward processing,23 executive processing16 and at rest.15 Furthermore, similar ventral frontostriatal dysfunction during reward processing has also been seen in unaffected relatives of people with schizophrenia.24,25 High-resolution MRI studies based on a rodent model of schizophrenia have found impaired limbic corticostriatal structures.26 As well, increased ventral striatal CB1 receptor binding has been related to negative symptoms in drug-free people with schizophrenia.27 Convergent evidence implicates compromised structural integrity of the ventral prefrontal–striatum pathway in people with schizophrenia28 and unaffected siblings of people with schizophrenia.29 These findings suggest that altered connectivity between the ventral striatum and cortical regions may represent a risk phenotype in people with schizophrenia.
In addition to the focus on the ventral system, it has also been reported that not only does increased intrinsic activity in the ventral striatum correspond to psychosis, but activity in the dorsal striatum (the associative and sensorimotor striatum) is also correlated with the disease state.30 The dorsal striatum has multiple reciprocal channels of communication with the dorsal prefrontal cortex through the hippocampus,31 and it is highly involved in executive and other higher cognitive processes.32 Studies with high-resolution tomographic imaging have revealed altered dopamine in the dorsal striatum in unmedicated people with schizophrenia33 and in people with prodromal signs of psychosis.34 Resting-state fMRI studies have found abnormalities in functional coupling between the dorsal caudate and the dorsolateral prefrontal cortex in people with first-episode schizophrenia, unaffected first-degree relatives35 and people with an at-risk mental state for psychosis.36 Meta-analyses have shown that dopaminergic dysfunction is greater in dorsal than in ventral subdivisions of the striatum in people with schizophrenia.37 A diffusion tensor imaging (DTI) study suggested that people with chronic schizophrenia have fewer fibre connections and reduced fractional anisotropy in the striatal associative loop.38 People with first-episode schizophrenia have been shown to have abnormal white matter microstructure in tracts connecting the prefrontal cortex and the associative striatum, and such abnormality is significantly associated with executive dysfunction.14 A voxel-wise study of DTI data has shown that patients with adolescent-onset schizophrenia have abnormal connectivity between the dorsal striatum and the ACC.39 Additionally, functional connectivity between the dorsal caudate and the dorsolateral prefrontal cortex, as well as glutamate levels in the associative striatum, are modulated by pharmacologic intervention.40,41 Collectively, these findings indicate that disruptions in the corticostriatal circuitry are implicated in the pathophysiology of schizophrenia. However, most existing studies have examined structural or functional brain connectivity alone, and were not equipped to characterize both structural and functional corticostriatal circuitry.
In the present study, we adopted multimodal neuroimaging to measure the functional and structural connectivity of the corticostriatal circuitry in people with schizophrenia and their unaffected first-degree relatives. Because first-degree relatives share an average of 50% of the genes of people with schizophrenia, including schizophrenia risk genes,42,43 and because they also have a 10-fold higher risk of developing schizophrenia,44 studies of unaffected relatives may help identify brain-connectivity patterns that are predictive of schizophrenia. We hypothesized that corticostriatal connectivity would be aberrant in people with schizophrenia, and that such aberrance would appear in their unaffected first-degree relatives as well.
Methods
Participants
This study included 47 people with schizophrenia, 30 unaffected first-degree relatives (12 siblings, 10 offspring and 8 parents), and 49 nonclinical controls. The schizophrenia group included 10 probands of the relatives. All participants provided written informed consent to participate. The protocol was approved by the ethics committee of Beijing Hui-Long-Guan Hospital (Beijing, China). The people with schizophrenia were evaluated using the Positive and Negative Syndrome Scale (PANSS) for symptom severity.45 We also assessed cognitive function in all participants using the digit span, digit symbol coding and verbal fluency tests46,47 to evaluate working memory, information-processing ability and executive functioning, which have been reported to be impaired in people with schizophrenia.48,49 Participant inclusion and exclusion criteria and medication details can be found in Appendix 1, available at jpn.ca/190015-a1.
Data acquisition and processing
We collected imaging data using a Siemens Magnetom Trio 3.0 T imaging system with a standard head coil at the Peking University Third Hospital. We acquired high-resolution structural T1 imaging data with the following parameters: matrix size 256 × 256; 192 contiguous axial slices, slice thickness 1 mm, voxel resolution 1 × 1 × 1 mm3, flip angle 7°, echo time 3.44 ms, repetition time 2530 ms, inversion time 1100 ms. We used a gradient-recalled echo-planar imaging sequence (repetition time 2000 ms, echo time 30 ms, flip angle 90°) to collect resting-state fMRI scans with a matrix size of 64 × 64, a field of view of 220 × 220 mm2, and a slice thickness of 4 mm (no gap), yielding resting-state fMRI scans with 240 time points at a voxel size of 3.4 × 3.4 × 4.0 mm3. During data acquisition, participants were instructed to close their eyes, relax and remain awake. We collected DTI data with the following parameters: repetition time 7000 ms, echo time 92 ms, field of view 256 × 256 mm2, b0 image and 64 gradient directions at b = 1000 s/mm2, matrix size 128 × 128, voxel size 2.0 × 2.0 × 3.0 mm3, number of slices 50. All scans were checked for artifacts, structural abnormalities and pathologies by a qualified neuroradiologist. Participants were excluded if their head motion involved translation greater than 2 mm or rotation greater than 2°, or if mean frame-wise displacement was greater than 0.5 mm (13 people with schizophrenia, 1 relative and 1 control participant were excluded). Preprocessing steps are outlined in Appendix 1.
Definition of seed regions of interest
We identified 3 subdivisions of the striatum — the ventral striatum (VST), executive striatum (EST) and sensorimotor striatum (SMST) — using the Oxford–GSK–Imanova Striatal Connectivity Atlas; each voxel had a probability above 25%.50,51 In particular, the ventral striatum is equivalent to the limbic striatum, comprising the anterior ventral caudate and putamen, the nucleus accumbens and the ventral postcommissural putamen, which contributes to 20 ± 7% of the total striatal volume. The executive striatum includes the rostral striatum and extends postcommissurally. The sensorimotor striatum includes the dorsal tier of rostral striatum, the dorsal putamen, the caudate to the anterior commissure and the postcommissural striatum.
Functional connectivity measurement
We measured functional connectivity using a seed-based, whole-brain, voxel-wise functional connectivity analysis approach. We extracted mean resting-state fMRI time courses from each seed region. Then, we computed the Pearson correlation coefficient between each voxel’s time course and the mean time courses for each of the striatal subdivisions for every participant. Then, we applied Fisher r-to-z transformation to the functional connectivity maps, yielding 6 maps for each participant at a spatial resolution of 2 × 2 × 2 mm3 in the Montreal Neurological Institute space.
Structural connectivity measure
We performed probabilistic tractography using the FSL (http://fsl.fmrib.ox.ac.uk/fsl) suite. We conducted fibre tracking from the 6 subdivisions of the striatum to each voxel in the whole brain. We adopted the default parameters of the fibre tracking (5000 samples, maximum number of steps 2000, step length 0.5 mm, curvature threshold 0.2). We identified the seeds in the native space of each participant’s DTI scan using the inverse deform field transformation obtained for coregistering multimodal scans. We computed the structural connectivity strength of each connection as the weighted mean fractional anisotropy value of the probabilistic fibres from the striatum subdivisions to other voxels.52 Finally, we spatially normalized the single-participant images to the Montreal Neurological Institute space using DARTEL in SPM8 (www.fil.ion.ucl.ac.uk/spm/software/spm8/), resampled to 2 × 2 × 2 mm3 during normalization, yielding 6 whole-brain structural connectivity maps for each participant.
Statistical analysis
Behavioural data analysis
We compared demographic data (age and education) and cognitive testing results for each domain across groups using 1-way analysis of variance, followed by least significant difference–Student Newman Keuls post hoc contrast analyses. The χ2 tests showed that sex distribution was equal across groups (p > 0.05), but age differed across groups. We used age as a covariate in an analysis of covariance.
Multimodal connectivity analysis
We used a 2-sample Hotelling T2 test to jointly examine group voxel-wise differences in the functional connectivity and structural connectivity maps. For each voxel, we concatenated 6 functional connectivity measures and 6 structural connectivity measures of the subdivisional striatum as an input to the test. We identified brain regions with statistically significant group differences using permutation tests (n = 10 000, pFWE < 0.001, cluster size > 100). We then applied post hoc t tests to functional connectivity and structural connectivity measures of connections that had significant differences to investigate modality-specific differences.
Corticostriatal connectivity patterns and statistical group differences
We used statistical nonparametric mapping (SnPM13; http://warwick.ac.uk/snpm) for separate group analyses of functional connectivity and structural connectivity maps. We applied pseudo voxel-level 1-sample t tests to functional and structural connectivity maps within each group to identify brain regions with statistically significant functional or structural connectivity measures (1000 permutations; pFWE < 0.05; cluster size > 10). Then, we used pseudo voxel-level 2-sample t tests to compare functional or structural connectivity maps between groups (1000 permutations, pFWE < 0.05, cluster size > 10) in brain regions with statistically significant functional or structural connectivity measures identified by the pseudo 1-sample t tests.
Correlation analysis of brain connectivity and clinical measures
We performed a correlation analysis in people with schizophrenia between functional and structural connectivity measures and a set of quantitative measures, including clinical symptoms, cognitive tests and chlorpromazine-equivalent doses of antipsychotic medication. Specifically, we focused on functional and structural connectivity measures between the subdivisional striatum and brain regions with significant group differences identified in the multimodal connectivity analysis. For each specific symptom score using PANSS, we used a linear regression model to regress out other scores except for the total score before computing its correlations with the connectivity measures. We used sex, age, education and duration of illness as covariates in the correlation analysis between connectivity and PANSS. We set statistical significance at p < 0.0021 (Bonferroni correction for multiple comparisons, p = 0.05/24 = 0.0021).
Results
Demographic and clinical characteristics
Demographic and clinical information for all participants is presented in Table 1. The groups did not differ by sex or education level. Relatives were older than people with schizophrenia and controls (F2,123 = 18.04, p < 0.05). We included age as a covariate in all statistical analyses.
Table 1.
Participant demographic and clinical features*
| Post hoc, p value | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Characteristic | Schizophrenia (n = 47) | Relatives (n = 30) | Controls (n = 49) | F2,123 / χ2 | p value | Schizoprenia v. control | Schizoprenia v. relatives | Relatives v. control |
| Age, yr | 28.11 ± 8.14 | 32.97 ± 7.86 | 26.24 ± 5.96 | 18.04 | 0.001 | 0.64 | 0.015 | < 0.001 |
| Sex, male/female | 18/29 | 11/19 | 25/24 | 2.20 | 0.33 | 0.21 | 0.89 | 0.21 |
| Education level, yr | 12.91 ± 3.01 | 12.07 ± 3.40 | 14.02 ± 4.56 | 2.99 | 0.05 | 0.39 | 0.92 | 0.06 |
| Age at onset, yr | 25.61 ± 8.09 | — | — | — | — | — | — | — |
| Length of illness, yr | 2.88 ± 2.10 | — | — | — | — | — | — | — |
| PANSS score | ||||||||
| Total | 77.41 ± 6.94 | — | — | — | — | — | — | — |
| Positive | 26.07 ± 3.04 | — | — | — | — | — | — | — |
| Negative | 15.78 ± 2.61 | — | — | — | — | — | — | — |
| General | 35.61 ± 3.86 | — | — | — | — | — | — | — |
| Drug dose, mg/d† | 235.88 ± 93.71 | — | — | — | — | — | — | — |
| Digit span score | 13.79 ± 2.90 | 13.90 ± 2.72 | 16.24 ± 2.64 | 11.46 | 0.002 | < 0.001 | 0.86 | < 0.001 |
| Symbol coding score | 45.81 ± 14.73 | 57.80 ± 12.82 | 68.33 ± 10.26 | 37.81 | < 0.001 | < 0.001 | < 0.001 | 0.001 |
| Verbal fluency score | 17.70 ± 5.35 | 19.60 ± 5.15 | 23.10 ± 4.25 | 14.92 | 0.007 | < 0.001 | 0.008 | 0.30 |
| Frame-wise displacement, mm | 0.13 ± 0.06 | 0.13 ± 0.07 | 0.14 ± 0.07 | 0.12 | 0.09 | 0.63 | 0.86 | 0.82 |
PANSS = Positive and Negative Syndrome Scale.
Findings are mean ± standard deviation unless otherwise indicated.
Chlorpromazine equivalent.
We found significant differences across groups in test scores for digit span (F2,123 = 11.46, p = 0.002), digit symbol coding (F2,123 = 37.81, p < 0.001) and verbal fluency (F2,123 = 14.92, p = 0.007). People with schizophrenia performed significantly worse than controls on all cognitive tests. Relatives had lower scores than controls on the digit symbol coding and digit span tests, but results on the verbal fluency test did not differ. People with schizophrenia also showed significantly lower scores than relatives on the digit symbol coding and verbal fluency tests, but no difference on the digit span test.
Corticostriatal connectivity
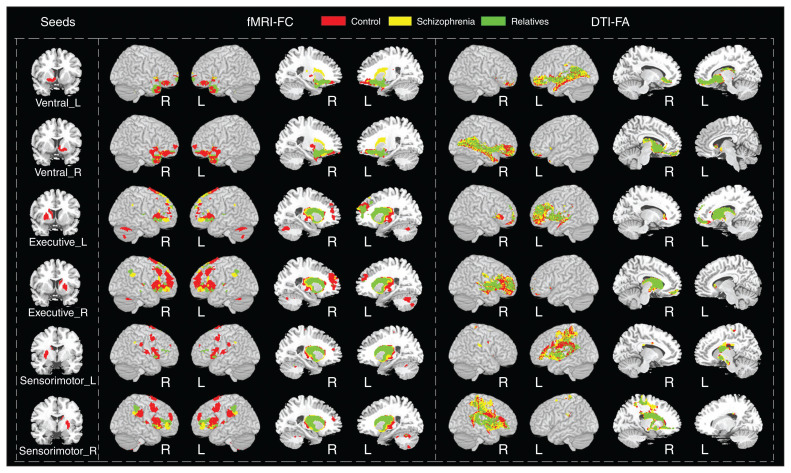
Subdivisions of the striatum had connections to distinct, largely nonoverlapping cortical regions (Fig. 1). The brain regions connected with the VST included the ACC, OFC, thalamus and bilateral hippocampi; the brain regions connected with the EST included the middle and inferior frontal gyri, occipital cortex, ACC and middle cingulate cortex; the brain regions connected with the SMST included the supplementary motor cortex, precuneus, calcarine sulcus, superior parietal gyrus and insula. The corticostriatal connectivity patterns were slightly different in people with schizophrenia, relatives and controls.
Fig. 1.
Brain regions with statistically significant functional and structural connections to the bilateral ventral striatum, executive striatum and sensorimotor striatum in controls, people with schizophrenia and unaffected relatives. Significance was set at pFWE < 0.05, cluster size > 10. The corticostriatal connectivity patterns were slightly different for controls, people with schizophrenia and unaffected relatives, displayed in red, yellow and green, respectively. DTI = diffusion tensor imaging; FA = fractional anisotropy; FC = functional connectivity; fMRI = functional magnetic resonance imaging; FWE = family-wise error; L = left hemisphere; R = right hemisphere.
Group differences in multimodal connectivity
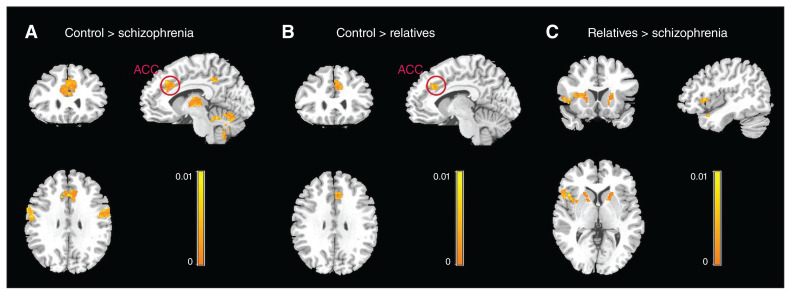
Results of the Hotelling T2 test revealed that multimodal subdivisional striatal connectivity patterns were statistically different across groups (Fig. 2 and Table 2). Compared with controls, people with schizophrenia showed altered connectivity between the striatal subdivisions and several cortical regions, including the right OFC, the left superior frontal gyrus, the bilateral superior temporal gyri, the right precentral gyrus, the right ACC and the thalamus (Fig. 2A); relatives showed different connectivity between the striatal subdivisions and the right ACC and the left precuneus (Fig. 2B). Specifically, both people with schizophrenia and relatives showed altered connectivity between the striatal subdivisions and the right ACC, which we selected as a region of interest (a union of regions that were significantly different in their multimodal connectivity measures between people with schizophrenia and controls, and between relatives and controls) for a post hoc test to delineate how the groups under study differed in their functional connectivity and structural connectivity patterns. We also observed significant differences in connectivity between the right ACC and the putamen in people with schizophrenia and relatives (Fig. 2C).
Fig. 2.
Brain regions with significantly different structural and functional connectivity measures between groups. (A) Differences between people with schizophrenia and controls. (B) Differences between unaffected first-degree relatives of people with schizophrenia and controls. (C) Differences between relatives and people with schizophrenia. Results are displayed at pFWE < 0.01, cluster size > 100. In particular, the anterior cingulate cortex (ACC) was common in people with schizophrenia and unaffected relatives; the striatal connectivity patterns were significantly different from controls. FWE = family-wise error.
Table 2.
Multimodal analysis — regions of significant differences among groups
| Region of difference (BA) | Side | Cluster size, voxels | MNI coordinates, x, y, z |
|---|---|---|---|
| Schizophrenia < controls | |||
| Orbitofrontal cortex (47/11) | Right | 133 | 38, 42, −14 |
| Anterior cingulate cortex (32/24) | Right | 372 | 0, 24, 18 |
| Anterior cingulate cortex (31) | Right | 363 | 8, −44, 38 |
| Superior frontal gyrus (10) | Left | 270 | −26, 56, 2 |
| Precentral gyrus (6) | Right | 247 | 60, 2, 16 |
| Superior temporal gyrus (22) | Left | 422 | −66, −10, 8 |
| Superior temporal gyrus (43) | Right | 116 | 50, −6, 2 |
| Thalamus | Left | 278 | −20, −26, 0 |
| Thalamus | Right | 320 | 18, −26, 0 |
| Cerebellum posterior lobe | Left | 1068 | −14, −80, −48 |
| Cerebellar tonsil | Right | 120 | 38, −54, −46 |
| Relatives < controls | |||
| Anterior cingulate cortex (32) | Right | 124 | 2, 22, 30 |
| Precuneus | Left | 187 | −6, −78, 38 |
| Relatives > schizophrenia | |||
| Temporal pole | Left | 111 | −30, 8, −26 |
| Temporal pole | Right | 103 | 38, 22, −38 |
| Putamen | Left | 413 | −14, 10, −2 |
| Putamen | Right | 129 | 16, 6, −4 |
BA = Brodmann area; MNI = Montreal Neurological Institute.
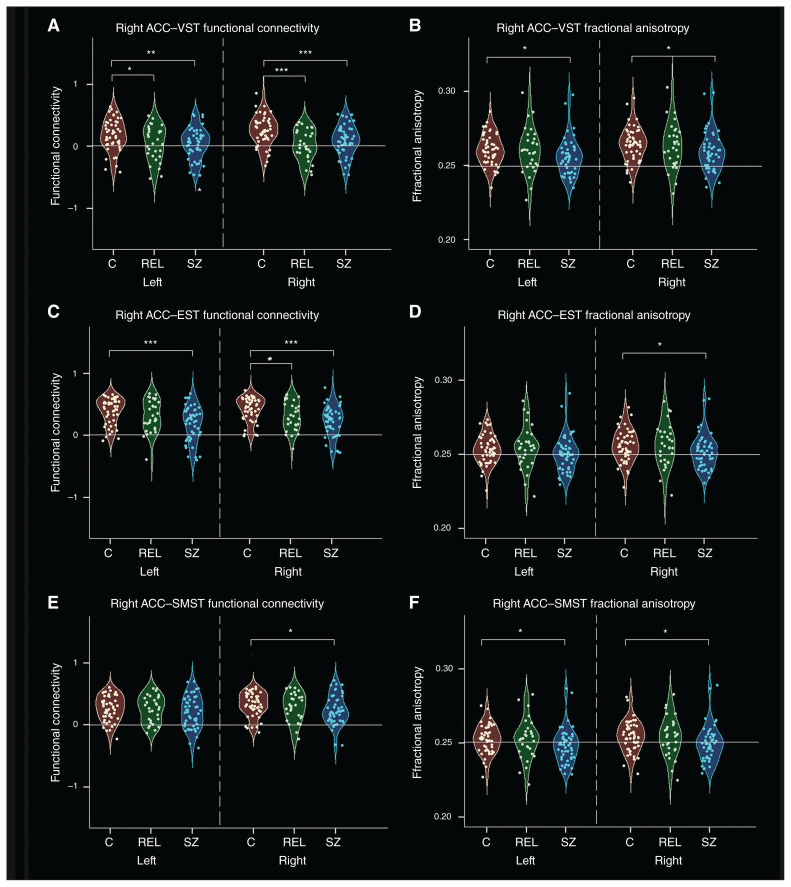
Post hoc t tests revealed that the connectivity pattern between the right ACC and the subdivisional striatum was different across groups. As shown in Figure 3, people with schizophrenia showed weaker functional connectivity in the ventral loop and the dorsal loop, including between the bilateral VST and right ACC, between the bilateral EST and right ACC, and between the right SMST and right ACC. Relatives had reduced functional connectivity in the ventral loop and the executive loop, including functional connectivity between the bilateral VST and right ACC, and between the right EST and right ACC. We observed no significant differences in the functional connectivity of right ACC–striatal subdivisions between people with schizophrenia and relatives.
Fig. 3.
Functional connectivity and structural connectivity (fractional anisotropy) between the striatum and the right anterior cingulate cortex (ACC) in controls, people with schizophrenia and unaffected relatives. (A) Scatter plots of functional connectivity between the right ACC and the left ventral striatum (VST; limbic striatum). (B) Scatter plots of fractional anisotropy between the right ACC and the VST. (C) Scatter plots of functional connectivity between the right ACC and the executive striatum (EST). (D) Scatter plots of fractional anisotropy between the right ACC and the EST. (E) Scatter plots of functional connectivity between the right ACC and the sensorimotor striatum (SMST). (F) Scatter plots of fractional anisotropy between the right ACC and the SMST. Asterisks indicate significant group differences: *p < 0.05; **p < 0.005; ***p < 0.001. C = controls; REL = relatives; SZ = schizophrenia.
People with schizophrenia showed weaker structural connectivity than controls between the bilateral VST and right ACC, between the right EST and right ACC, and between the bilateral SMST and right ACC. Relatives had no significant differences in structural connectivity compared to controls or people with schizophrenia (Fig. 3).
Statistical group comparisons of functional and structural connectivity maps
We also separately examined group differences in the whole-brain voxel-wise functional connectivity and structural connectivity maps. Significant group differences are presented in Appendix 1. Compared with controls, people with schizophrenia and relatives had weaker functional connectivity between the VST and cortical areas of the OFC (Brodmann areas [BA] 11 and 47) and the ACC (BA32), and people with schizophrenia had weaker functional connectivity than relatives (Appendix 1, Fig. S1). People with schizophrenia also had weaker functional connectivity in the dorsal loops (sensorimotor and executive loops; Appendix 1, Tables S2 and S3 and Fig. S1). Compared with controls, people with schizophrenia had weaker structural connectivity between the bilateral EST and the superior frontal gyrus, and between the bilateral SMST and the inferior parietal lobule; they had stronger structural connectivity between the SMST and the precentral gyrus (Appendix 1, Fig. S2). Relatives had stronger structural connectivity between the VST and the orbitofrontal cortex, the right EST–right superior frontal gyrus, and the right EST–middle frontal gyrus, orbital part, than controls.
Correlation analysis of brain connectivity and clinical measures
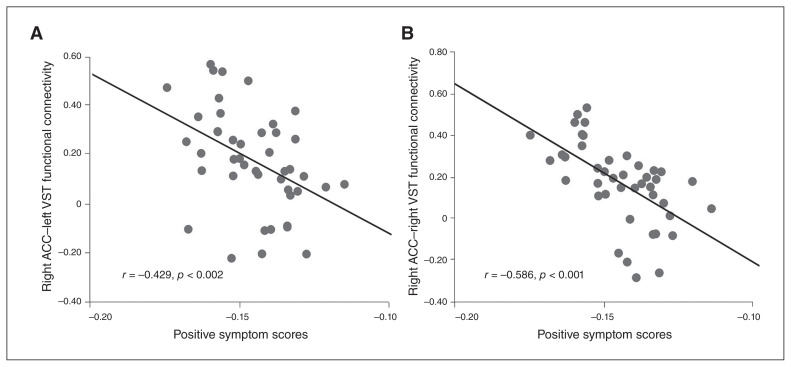
We correlated the functional connectivity measures between the bilateral VST and the ACC with PANSS positive scores (right: r = −0.586, p = < 0.001; left: r = −0.429, p < 0.002), and these correlations survived Bonferroni correction for multiple comparisons (Fig. 4). The structural connectivity between the EST and the ACC was marginally correlated with PANSS total score (r = −0.276, p = 0.033). Connectivity measures were not significantly correlated with cognitive tests or antipsychotic medication dosage.
Fig. 4.
Correlations between positive symptom severity and decreased connectivity of the ventral corticostriatal circuitry in people with schizophrenia. (A) Scatter plot of the association between positive symptom scores and connectivity between the left ventral striatum (VST; limbic striatum) and the right anterior cingulate cortex (ACC). (B) Scatter plot of the association between positive symptom scores and the connectivity between the right VST (limbic striatum) and the right ACC.
Discussion
In the present study, we examined corticostriatal connectivity in people with schizophrenia and their unaffected first-degree relatives using multimodal fMRI and DTI. Our imaging data analysis revealed that each subdivisional striatum (3 per hemisphere) connected to distinct, largely nonoverlapping cortex regions, similar to existing findings.31,53,54 We found that the connectivity between many cortical areas (e.g., the OFC and ACC) and the striatum was altered in people with schizophrenia; relatives showed reduced connectivity between the striatum and the right ACC and the left precuneus. Specifically, we observed aberrant functional connectivity of the ventral corticostriatal circuitry in both people with schizophrenia and relatives; however we detected structural abnormalities only in people with schizophrenia. In addition, the abnormal connectivity between the VST and the right ACC was correlated with positive symptom severity in people with schizophrenia. These results, in conjunction with subregional striatum shape abnormalities found in patients with childhood-onset schizophrenia in a longitudinal study,55 demonstrate the potential of the striatum as an endophenotype for schizophrenia.
The role of the corticostriatal circuit in schizophrenia
Consistent with the long-standing and well-accepted view of corticostriatal dysfunction in schizophrenia,13 we observed decreased corticostriatal connectivity according to both functional and structural connectivity measures in people with schizophrenia. Hypoactivity in the corticostriatal system and decreased functional coupling during working memory have been reported in fMRI studies of schizophrenia5,56 and proactive inhibition tasks.57 Findings from resting-state fMRI studies have also demonstrated altered corticostriatal circuitry in people with chronic schizophrenia58 and in people with schizophrenia with auditory/verbal hallucinations.59 The widespread dysregulation of corticostriatal dynamics identified in our study was similar to functional dysconnectivity patterns in the corticostriatal circuitry found in people with first-episode psychosis.35 However, a recent study of first-episode, treatment-naïve people with schizophrenia observed decreased functional connectivity in the ventral loop, but not in the sensorimotor or associative loops of the frontostriatal circuitry,60 but no significant difference in functional connectivity of the striatum between healthy people and people with psychosis.40 These inconsistent findings might be caused by various factors, including differences in the samples under study (such as sample size, illness stage or medication use) and as different diagnostic groups (e.g., psychotic disorder or schizophrenia-spectrum disorders).
Several studies have demonstrated that functional connectivity of the striatum is correlated with treatment outcomes. In particular, effective treatment of psychotic symptoms was associated with increased connectivity between the striatum and frontal and limbic regions40; baseline striatal functional connectivity was predictive of response to antipsychotic drug treatment17; and longer duration of untreated psychosis was correlated with worse response to treatment, as well as with overall decreased functional connectivity in corticostriatal circuits.61
We also found that people with schizophrenia showed reduced structural connectivity between the striatum and the right ACC, but we observed no significant structural connectivity difference between relatives and controls. These findings might reflect the fact that structural changes are more stable and show slightly progressive alterations over the course of disease.62 Human brain development is characterized by progressive remodelling of the brain’s structural and functional architecture, including the proliferation and differentiation of neurons, the pruning of cell processes and the formation of synapses. Our results support the concept of a neurodevelopmental defect in corticostriatal circuits in schizophrenia63,64 and may help to explain the dysfunction of dopaminergic regulation in schizophrenia psychopathology. Studies using DTI have identified abnormalities in the ventral and dorsal white matter tracts. In particular, reduced frontostriatal white matter integrity has been reported in people with schizophrenia and unaffected siblings,29 and reduced structural connectivity in the associative loop of corticostriatal white matter tracts has been reported in people with first-episode schizophrenia14 and chronic schizophrenia.38 The striatum relays and modulates communication between subcortical regions and the cortex, and plays a major role in various neurocognitive domains, including motor and cognitive control, motivation and emotional processing,13 the dysfunction of which may account for the wide array of clinical and cognitive symptoms observed in schizophrenia. However, in the present study we found no correlations between functional or structural connectivity of the corticostriatal network and results on cognitive tests. Structural and functional connectivity abnormalities might be the result of poor white matter integrity contributing to disturbances in functional connectivity in the adjacent grey matter. Our findings of hypoconnectivity in the corticostriatal circuit suggest a closely linked functional–structural system and add new evidence to the neural mechanisms underlying the psychopathology of schizophrenia. However, we found no correlations between connectivity and antipsychotic medication dosage.
Dysfunction of the ventral corticostriatal circuit
We also observed changes in functional connections of the ventral corticostriatal circuit in relatives. Existing studies have revealed that people at ultra-high risk for schizophrenia have weaker ventral striatal activation during reward-processing than healthy people65; hyperconnectivity of the ventral frontostriatal circuitry is an endophenotype for psychosis35; and risk for psychosis is mediated by a complex interplay of alterations in both the dorsal and ventral corticostriatal systems.36 A meta-analysis has also revealed significant bilateral VST hypoactivation during reward-processing in psychosis.66 Together, these results indicate that dysconnectivity in ventral circuits could be detected early, even in people without clinical symptoms.
We identified no significant group differences in structural connectivity between the right ACC and the subdivisional striatum in relatives, although a previous DTI study revealed reduced ventral frontostriatal white matter integrity in unaffected siblings.29 Such a discrepancy might have been caused by the fact that our sample included siblings, offspring and parents.
Symptom-related corticostriatal dysconnectivity
We also found that functional connectivity of the ventral corticostriatal circuit was correlated with positive symptom severity in the patient group, suggesting that this pathway may play an important role in the formation of psychotic symptoms. Similarly, ventral striatal activation was reported to be positively correlated with positive symptoms in people with schizotypal personality traits and early psychosis.67 Moreover, vulnerabilities in the ventral circuits mediate the transition from genetic liability to the emergence of psychotic symptoms, such as increased dopamine levels and striatal dopamine synthesis capacity,68 as well as altered glutamatergic–dopaminergic interactions.69 Alterations in the dopaminergic reward system, predominantly in the ventral frontostriatal networks, constitute core characteristics of schizophrenia. Meanwhile, psychosis could be correlated with abnormal assignment of salience to internal and external stimuli.70,71 It has been reported that clinical outcomes are related to longitudinal changes in ventral striatum function during salience processing in people at ultra-high risk for psychosis.72 The ACC, a region connected with the salience network, has been associated with a variety of salience attribution functions.73 We demonstrated that the decreased ventral striatum–ACC connectivity associated with positive symptoms was implicated in the pathogenesis of disease, and this may help establish biomarkers that can be used to follow the effects of treatment. Further confirmation of this potential mechanism in future studies is needed.
Alternative network analysis methods and future studies
Individualized functional connectivity pattern analysis methods, such as personalized intrinsic network topography algorithms,74 might help better identify individually specific functional connectivity patterns than the group-wise, image registration–based method we used in the present study. It remains unclear what caused the aberrant corticostriatal connectivity in people with schizophrenia and relatives. Myelin-forming oligodendrocytes might be causally involved in aberrant corticostriatal connectivity.75 Furthermore, neuroinflammation could contribute to structural and functional dysconnectivity, because it is associated with white matter pathology in people with schizophrenia and even in the first episode of psychosis.76 Further investigation is needed to elucidate what causes the aberrant brain connectivity in schizophrenia. Finally, machine-learning tools may help derive individualized scores to quantify aberrant brain-connectivity patterns.77–81
Limitations
The present study had several limitations. First, the sample size of the relatives was moderate, so that group could not be divided into subgroups of parents and siblings. However, their differences in neuroanatomy might be subtle.82 Furthermore, the familial structures of the people with schizophrenia and the relatives were not explicitly accounted for in all analyses. Second, we defined the subdivisions of the striatum using the Oxford–GSK–Imanova Striatal Connectivity Atlas50,51 based on a relatively low threshold of 25%. However, subdivisions defined based on a higher threshold of 50% could make the findings more specific for certain striatal subregions. Third, we characterized the structural connectivity of each connected path using the mean of fractional anisotropy values of voxels on the connected path under study. However, the number of streamlines, mean tract length and the average of mean diffusivity values of voxels on the connected path could also be used to characterize structural connectivity. Therefore, the present study provides results for only one specific aspect of structural connectivity. Furthermore, DTI is not equipped to describe fibre directionality in brain regions with 2 or more fibre populations that have different orientations, although the probabilistic fibre-tracking method we adopted was robust to the crossing-fibre problem.83,84
Conclusion
Our findings demonstrated that people with schizophrenia showed abnormalities in functional and structural connectivity of the corticostriatal system. Impairment in the ventral system was specifically associated with unaffected relatives who are at higher genetic risk for schizophrenia, indicating that measuring ventral corticostriatal circuit function could serve as a biological marker for schizophrenia.
Acknowledgements
This work was supported in part by the National Basic Research Program of China (no. 2015CB856400), the National Natural Science Foundation of China (no. 81501158), and an NIH grant (EB022573).
Footnotes
Competing interests: None declared.
Contributors: P. Li, L. Lu and Y. Fan designed the study. P. Li, R.-J. Zhao, L. Shi, H.-Q. Sun and Z. Ding acquired the data, which P. Li, R.-X. Jing, X. Lin and Y. Fan analysed. P. Li, R.-X. Jing and Y. Fan wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.Ban TA. Neuropsychopharmacology and the genetics of schizophrenia: a history of the diagnosis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:753–62. doi: 10.1016/j.pnpbp.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–92. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- 3.Simpson EH, Kellendonk C, Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 2010;65:585–96. doi: 10.1016/j.neuron.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69:776–86. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon JH, Minzenberg MJ, Raouf S, et al. Impaired prefrontal-basal ganglia functional connectivity and substantia nigra hyperactivity in schizophrenia. Biol Psychiatry. 2013;74:122–9. doi: 10.1016/j.biopsych.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guerrero-Pedraza A, McKenna PJ, Gomar JJ, et al. First-episode psychosis is characterized by failure of deactivation but not by hypo- or hyperfrontality. Psychol Med. 2012;42:73–84. doi: 10.1017/S0033291711001073. [DOI] [PubMed] [Google Scholar]
- 7.Tu PC, Lee YC, Chen YS, et al. Schizophrenia and the brain’s control network: aberrant within- and between-network connectivity of the frontoparietal network in schizophrenia. Schizophr Res. 2013;147:339–47. doi: 10.1016/j.schres.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Heckers S, Konradi C. Substantia nigra hyperactivity in schizophrenia. Biol Psychiatry. 2013;74:82–3. doi: 10.1016/j.biopsych.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Seeman P, Lee T. Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science. 1975;188:1217–9. doi: 10.1126/science.1145194. [DOI] [PubMed] [Google Scholar]
- 10.Kapur S, Mamo D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1081–90. doi: 10.1016/j.pnpbp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Wiesendanger M. Motor functions of the basal ganglia. Appl Neurophysiol. 1986;49:269–77. doi: 10.1159/000100156. [DOI] [PubMed] [Google Scholar]
- 12.MacLean PD. Cerebral evolution and emotional processes: new findings on the striatal complex. Ann N Y Acad Sci. 1972;193:137–49. doi: 10.1111/j.1749-6632.1972.tb27830.x. [DOI] [PubMed] [Google Scholar]
- 13.Shepherd GM. Corticostriatal connectivity and its role in disease. Nat Rev Neurosci. 2013;14:278–91. doi: 10.1038/nrn3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quan M, Lee SH, Kubicki M, et al. White matter tract abnormalities between rostral middle frontal gyrus, inferior frontal gyrus and striatum in first-episode schizophrenia. Schizophr Res. 2013;145:1–10. doi: 10.1016/j.schres.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lui S, Li T, Deng W, et al. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry. 2010;67:783–92. doi: 10.1001/archgenpsychiatry.2010.84. [DOI] [PubMed] [Google Scholar]
- 16.Quide Y, Morris RW, Shepherd AM, et al. Task-related frontostriatal functional connectivity during working memory performance in schizophrenia. Schizophr Res. 2013;150:468–75. doi: 10.1016/j.schres.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Sarpal DK, Argyelan M, Robinson DG, et al. Baseline striatal functional connectivity as a predictor of response to antipsychotic drug treatment. Am J Psychiatry. 2016;173:69–77. doi: 10.1176/appi.ajp.2015.14121571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 19.Verstynen TD, Badre D, Jarbo K, et al. Microstructural organizational patterns in the human corticostriatal system. J Neurophysiol. 2012;107:2984–95. doi: 10.1152/jn.00995.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–30. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Sesack SR, Carr DB. Selective prefrontal cortex inputs to dopamine cells: implications for schizophrenia. Physiol Behav. 2002;77:513–7. doi: 10.1016/s0031-9384(02)00931-9. [DOI] [PubMed] [Google Scholar]
- 22.Mathai A, Smith Y. The corticostriatal and corticosubthalamic pathways: two entries, one target. So what? Front Syst Neurosci. 2011;5:64. doi: 10.3389/fnsys.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris RW, Vercammen A, Lenroot R, et al. Disambiguating ventral striatum fMRI-related BOLD signal during reward prediction in schizophrenia. Mol Psychiatry. 2012;17:235, 280–9. doi: 10.1038/mp.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Leeuw M, Kahn RS, Vink M. Fronto-striatal dysfunction during reward processing in unaffected siblings of schizophrenia patients. Schizophr Bull. 2015;41:94–103. doi: 10.1093/schbul/sbu153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grimm O, Heinz A, Walter H, et al. Striatal response to reward anticipation: evidence for a systems-level intermediate phenotype for schizophrenia. JAMA Psychiatry. 2014;71:531–9. doi: 10.1001/jamapsychiatry.2014.9. [DOI] [PubMed] [Google Scholar]
- 26.Barnes SA, Sawiak SJ, Caprioli D, et al. Impaired limbic corticostriatal structure and sustained visual attention in a rodent model of schizophrenia. Int J Neuropsychopharmacol. 2014;18:pyu010. doi: 10.1093/ijnp/pyu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ceccarini J, De Hert M, Van Winkel R, et al. Increased ventral striatal CB1 receptor binding is related to negative symptoms in drug-free patients with schizophrenia. Neuroimage. 2013;79:304–12. doi: 10.1016/j.neuroimage.2013.04.052. [DOI] [PubMed] [Google Scholar]
- 28.Bracht T, Horn H, Strik W, et al. White matter pathway organization of the reward system is related to positive and negative symptoms in schizophrenia. Schizophr Res. 2014;153:136–42. doi: 10.1016/j.schres.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 29.De Leeuw M, Bohlken MM, Mandl RC, et al. Reduced frontostriatal white matter integrity in schizophrenia patients and unaffected siblings: a DTI study. NPJ Schizophr. 2015;1:15001. doi: 10.1038/npjschz.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorg C, Manoliu A, Neufang S, et al. Increased intrinsic brain activity in the striatum reflects symptom dimensions in schizophrenia. Schizophr Bull. 2013;39:387–95. doi: 10.1093/schbul/sbr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pantelis C, Barnes TR, Nelson HE, et al. Frontal-striatal cognitive deficits in patients with chronic schizophrenia. Brain. 1997;120:1823–43. doi: 10.1093/brain/120.10.1823. [DOI] [PubMed] [Google Scholar]
- 33.Kegeles LS, Abi-Dargham A, Frankle WG, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67:231–9. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- 34.Howes OD, Montgomery AJ, Asselin MC, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- 35.Fornito A, Harrison BJ, Goodby E, et al. Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiatry. 2013;70:1143–51. doi: 10.1001/jamapsychiatry.2013.1976. [DOI] [PubMed] [Google Scholar]
- 36.Dandash O, Fornito A, Lee J, et al. Altered striatal functional connectivity in subjects with an at-risk mental state for psychosis. Schizophr Bull. 2014;40:904–13. doi: 10.1093/schbul/sbt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCutcheon R, Beck K, Jauhar S, et al. Defining the locus of dopaminergic dysfunction in schizophrenia: a meta-analysis and test of the mesolimbic hypothesis. Schizophr Bull. 2018;44:1301–1311. doi: 10.1093/schbul/sbx180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levitt JJ, Nestor PG, Levin L, et al. Reduced structural connectivity in frontostriatal white matter tracts in the associative loop in schizophrenia. Am J Psychiatry. 2017;174:1102–11. doi: 10.1176/appi.ajp.2017.16091046. [DOI] [PubMed] [Google Scholar]
- 39.James A, Joyce E, Lunn D, et al. Abnormal frontostriatal connectivity in adolescent-onset schizophrenia and its relationship to cognitive functioning. Eur Psychiatry. 2016;35:32–8. doi: 10.1016/j.eurpsy.2016.01.2426. [DOI] [PubMed] [Google Scholar]
- 40.Sarpal DK, Robinson DG, Lencz T, et al. Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry. 2015;72:5–13. doi: 10.1001/jamapsychiatry.2014.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De la Fuente-Sandoval C, Leon-Ortiz P, Azcarraga M, et al. Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA Psychiatry. 2013;70:1057–66. doi: 10.1001/jamapsychiatry.2013.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 43.Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818–27. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- 44.Gejman PV, Sanders AR, Kendler KS. Genetics of schizophrenia: new findings and challenges. Annu Rev Genomics Hum Genet. 2011;12:121–44. doi: 10.1146/annurev-genom-082410-101459. [DOI] [PubMed] [Google Scholar]
- 45.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 46.Dickinson D, Iannone VN, Gold JM. Factor structure of the Wechsler Adult Intelligence Scale-III in schizophrenia. Assessment. 2002;9:171–80. doi: 10.1177/10791102009002008. [DOI] [PubMed] [Google Scholar]
- 47.Henry JD, Crawford JR. A meta-analytic review of verbal fluency deficits in schizophrenia relative to other neurocognitive deficits. Cogn Neuropsychiatry. 2005;10:1–33. doi: 10.1080/13546800344000309. [DOI] [PubMed] [Google Scholar]
- 48.Fioravanti M, Carlone O, Vitale B, et al. A meta-analysis of cognitive deficits in adults with a diagnosis of schizophrenia. Neuropsychol Rev. 2005;15:73–95. doi: 10.1007/s11065-005-6254-9. [DOI] [PubMed] [Google Scholar]
- 49.Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64:532–42. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- 50.Tziortzi AC, Haber SN, Searle GE, et al. Connectivity-based functional analysis of dopamine release in the striatum using diffusion-weighted MRI and positron emission tomography. Cereb Cortex. 2014;24:1165–77. doi: 10.1093/cercor/bhs397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johansen-Berg H, Behrens TE, Sillery E, et al. Functional-anatomical validation and individual variation of diffusion tractography-based segmentation of the human thalamus. Cereb Cortex. 2005;15:31–9. doi: 10.1093/cercor/bhh105. [DOI] [PubMed] [Google Scholar]
- 52.van den Heuvel MP, Sporns O. Rich-club organization of the human connectome. J Neurosci. 2011;31:15775–86. doi: 10.1523/JNEUROSCI.3539-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Di Martino A, Scheres A, Margulies DS, et al. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18:2735–47. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- 54.Jung WH, Jang JH, Park JW, et al. Unravelling the intrinsic functional organization of the human striatum: a parcellation and connectivity study based on resting-state FMRI. PLoS One. 2014;9:e106768. doi: 10.1371/journal.pone.0106768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chakravarty MM, Rapoport JL, Giedd JN, et al. Striatal shape abnormalities as novel neurodevelopmental endophenotypes in schizophrenia: a longitudinal study. Hum Brain Mapp. 2015;36:1458–69. doi: 10.1002/hbm.22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wagshal D, Knowlton BJ, Suthana NA, et al. Evidence for corticostriatal dysfunction during cognitive skill learning in adolescent siblings of patients with childhood-onset schizophrenia. Schizophr Bull. 2014;40:1030–9. doi: 10.1093/schbul/sbt147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zandbelt BB, van Buuren M, Kahn RS, et al. Reduced proactive inhibition in schizophrenia is related to corticostriatal dysfunction and poor working memory. Biol Psychiatry. 2011;70:1151–8. doi: 10.1016/j.biopsych.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 58.Tu PC, Hsieh JC, Li CT, et al. Corticostriatal disconnection within the cingulo-opercular network in schizophrenia revealed by intrinsic functional connectivity analysis: a resting fMRI study. Neuroimage. 2012;59:238–47. doi: 10.1016/j.neuroimage.2011.07.086. [DOI] [PubMed] [Google Scholar]
- 59.Hoffman RE, Fernandez T, Pittman B, et al. Elevated functional connectivity along a corticostriatal loop and the mechanism of auditory/verbal hallucinations in patients with schizophrenia. Biol Psychiatry. 2011;69:407–14. doi: 10.1016/j.biopsych.2010.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin P, Wang X, Zhang B, et al. Functional dysconnectivity of the limbic loop of frontostriatal circuits in first-episode, treatment-naive schizophrenia. Hum Brain Mapp. 2018;39:747–57. doi: 10.1002/hbm.23879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sarpal DK, Robinson DG, Fales C, et al. Relationship between duration of untreated psychosis and intrinsic corticostriatal connectivity in patients with early phase schizophrenia. Neuropsychopharmacology. 2017;42:2214–21. doi: 10.1038/npp.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chan RC, Di X, McAlonan GM, et al. Brain anatomical abnormalities in high-risk individuals, first-episode, and chronic schizophrenia: an activation likelihood estimation meta-analysis of illness progression. Schizophr Bull. 2011;37:177–88. doi: 10.1093/schbul/sbp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009;7:e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Di Martino A, Kelly C, Grzadzinski R, et al. Aberrant striatal functional connectivity in children with autism. Biol Psychiatry. 2011;69:847–56. doi: 10.1016/j.biopsych.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Juckel G, Friedel E, Koslowski M, et al. Ventral striatal activation during reward processing in subjects with ultra-high risk for schizophrenia. Neuropsychobiology. 2012;66:50–6. doi: 10.1159/000337130. [DOI] [PubMed] [Google Scholar]
- 66.Radua J, Schmidt A, Borgwardt S, et al. Ventral striatal activation during reward processing in psychosis: a neurofunctional meta-analysis. JAMA Psychiatry. 2015;72:1243–51. doi: 10.1001/jamapsychiatry.2015.2196. [DOI] [PubMed] [Google Scholar]
- 67.Kirschner M, Hager OM, Muff L, et al. Ventral striatal dysfunction and symptom expression in individuals with schizotypal personality traits and early psychosis. Schizophr Bull. 2018;44:147–57. doi: 10.1093/schbul/sbw142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Delgado MR. Reward-related responses in the human striatum. Ann N Y Acad Sci. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- 69.Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- 71.Jensen J, Willeit M, Zipursky RB, et al. The formation of abnormal associations in schizophrenia: neural and behavioral evidence. Neuropsychopharmacology. 2008;33:473–9. doi: 10.1038/sj.npp.1301437. [DOI] [PubMed] [Google Scholar]
- 72.Schmidt A, Antoniades M, Allen P, et al. Longitudinal alterations in motivational salience processing in ultra-high-risk subjects for psychosis. Psychol Med. 2017;47:243–54. doi: 10.1017/S0033291716002439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Orliac F, Naveau M, Joliot M, et al. Links among resting-state default-mode network, salience network, and symptomatology in schizophrenia. Schizophr Res. 2013;148:74–80. doi: 10.1016/j.schres.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 74.Dickie EW, Ameis SH, Shahab S, et al. Personalized intrinsic network topography mapping and functional connectivity deficits in autism spectrum disorder. Biol Psychiatry. 2018;84:278–86. doi: 10.1016/j.biopsych.2018.02.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nave K-A, Ehrenreich H. Myelination and oligodendrocyte functions in psychiatric diseases. JAMA Psychiatry. 2014;71:582–4. doi: 10.1001/jamapsychiatry.2014.189. [DOI] [PubMed] [Google Scholar]
- 76.Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res. 2015;161:102–12. doi: 10.1016/j.schres.2014.04.041. [DOI] [PubMed] [Google Scholar]
- 77.Fan Y, Gur RE, Gur RC, et al. Unaffected family members and schizophrenia patients share brain structure patterns: a high-dimensional pattern classification study. Biol Psychiatry. 2008;63:118–24. doi: 10.1016/j.biopsych.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fan Y, Liu Y, Wu H, et al. Discriminant analysis of functional connectivity patterns on Grassmann manifold. Neuroimage. 2011;56:2058–67. doi: 10.1016/j.neuroimage.2011.03.051. [DOI] [PubMed] [Google Scholar]
- 79.Li P, Jing RX, Zhao RJ, et al. Electroconvulsive therapy-induced brain functional connectivity predicts therapeutic efficacy in patients with schizophrenia: a multivariate pattern recognition study. NPJ Schizophr. 2017;3:21. doi: 10.1038/s41537-017-0023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jing R, Li P, Ding Z, et al. Machine learning identifies unaffected first-degree relatives with functional network patterns and cognitive impairment similar to those of schizophrenia patients. Hum Brain Mapp. 2019;40:3930–9. doi: 10.1002/hbm.24678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rozycki M, Satterthwaite TD, Koutsouleris N, et al. multisite machine learning analysis provides a robust structural imaging signature of schizophrenia detectable across diverse patient populations and within individuals. Schizophr Bull. 2018;44:1035–44. doi: 10.1093/schbul/sbx137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oertel-Knochel V, Knochel C, Matura S, et al. Cortical-basal ganglia imbalance in schizophrenia patients and unaffected first-degree relatives. Schizophr Res. 2012;138:120–7. doi: 10.1016/j.schres.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 83.Behrens TE, Woolrich MW, Jenkinson M, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–88. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- 84.Behrens TEJ, Berg HJ, Jbabdi S, et al. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34:144–55. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]