Abstract
Cannabinoids have been found to be effective in controlling seizures and the highly purified form of cannabinoid derived for Cannabis sativa . Cannabidiol (CBD) is now approved for Lennox–Gastaut syndrome (LGS) and Dravet syndrome. CBD was used in a 9-year-old boy with LGS (unknown etiology) with very good results. The electroencephalography (EEG) response was very dramatic with near normalization of EEG background and complete control of seizures. The effect of CBD on EEG with such an improvement has not been described previously. Also, this adds to evidence that early intervention in LGS with CBD might be more helpful and improve outcomes.
Keywords: cannabinoid, EEG, Lennox-Gastaut syndrome
Introduction
Cannabinoids (CBs) are the new class of drugs that have been found to be effective for epilepsy. The mechanism of action is not clear, though the CBs are known to act on CB (cannabinoid) receptors as an antagonist at both CB1R and CB2R and have no psychoactive properties.
CBs are derived from Cannabis sativa plant and more than 100 CBs are known to exist. 1 A purified form of CB, cannabidiol(CBD) (Epidiolex), was approved for Lennox–Gastaut syndrome (LGS) and Dravet syndrome by FDA (US Food and Drug Administration, 2018). In children 2 to 18 years of age with Dravet syndrome, there was significant reduction in convulsive seizures (tonic, clonic, tonic–clonic, or atonic) frequency compared with placebo. There were three patients who became seizure free on CBD. 2 There was significant improvement in the overall condition as rated on Caregiver Global Impression of Change. Similar results were seen with CBD in LGS patients, aged 2 to 55 years with improvement in overall condition 3
No studies have been published that document the effect of CBDs on electroencephalography (EEG) yet. It is not yet known whether CBD changes EEG and how it improves as the seizures get better in those who respond to the treatment with reduction in seizures.
Case Presentation
The child presented with absence seizures at the age of 9 years. The EEG captured absence seizures with typical 3 Hz spike and wave-generalized discharges. Parents refused treatment with medications and a self-formulated ketogenic diet initiated by parents helped control his seizures partially. A year later (at the age of 10 years), he had his first generalized tonic–clonic seizure (GTCS). He was started on levetiracetam (100 mg/kg/d) and ketogenic diet was initiated. He never achieved ketosis on the diet and his seizures remained uncontrolled with infrequent GTCS and daily absence seizure. He was admitted with status epilepticus, twice. Valproic acid loading dose caused agitation and confusion and could not be continued. Phenytoin (8 mg/kg/d) controlled his GTCS with levetiracetam 1000 mg bid (60 mg/kg/d, since higher doses did not help control seizures). He also got 5-day course of high-dose steroids during this admission and ketogenic diet was discontinued. He was still having some absence seizures and Epidiolex (CBD) was added. Maintaining his phenytoin levels at around 25 mg/L while CBD was been titrated up to target dose of 5 mg/kg/d helped control his absence seizures. He started having drop attacks at the age of 10.5 years and made some gurgling sounds followed by suddenly dropping limp to ground. By increasing the dose of CBD (350 mg/d, 11 mg/kg/d) and continuing phenytoin 150 mg twice daily (9 mg/kg/d), (levetiracetam was discontinued on parent’s insistence), he became seizure free in May 2019.
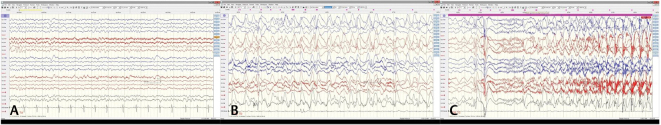
His initial EEG showed age-appropriate backgrounds and generalized 3 Hz spike and wave discharges (EEG report only available) that transitioned into typical LGS pattern and slow background rhythms with slow 2 to 3 Hz generalized spike and wave discharges in Dec 2018 ( Fig. 1 ) when he started having increased frequency of GTCS. The pattern persisted despite relatively well-controlled seizures in Jan 2019 ( Fig. 2 ). In July 2019 ( Fig. 3 ), his EEG returned back to near normal background rhythms and continues to be normal with infrequent generalized epileptiform discharges. Magnetic resonance imaging brain was normal and the expanded genetic epilepsy panel (Invitae) did not detect any mutations in the 146 genes tested.
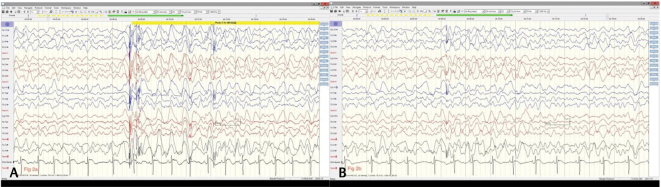
Fig. 1.
( A ) Electroencephalography showing slow background rhythms at 7 Hz with well-preserved anteroposterior gradient (Dec ’18) before start of increased frequency of seizures. ( B ) Sleep augmented generalized spike and wave discharges with anterior leads showing rhythmic slow 1–2 Hz delta activity. ( C ) Typical tonic seizure arising in sleep (Dec ’18).
Fig. 2.
( A ) Generalized slowing with generalized spike and wave generalized discharges during awake state prior to initiation of cannabidiol. The slowing persists during sleep ( B ) and there is absence of typical sleep architecture diagnostic of Lennox–Gastaut syndrome (along with clinical features).
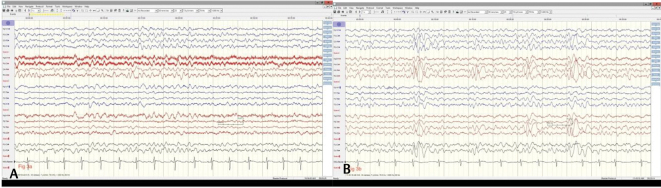
Fig. 3.
( A ) Background rhythms have returned to 7 Hz frequency with well-developed anteroposterior gradient during awake state (Dec ’19) and during drowsy state ( B ) there is appearance of vertex waves (July ’19) after cannabidiol was initiated in Feb ’19 and seizures were fully controlled.
He has not had any seizures on combination of phenytoin and CBD and he is doing well in school now, currently in regular classes and is learning at age-appropriate levels.
Discussion
The child presented with typical absence seizures characteristic of absence epilepsy of childhood and EEG pattern of 3 Hz spike and wave generalized discharges. He started having GTCS after approximately a year of uncontrolled absence seizures and rapidly deteriorated with change in EEG pattern characteristic of LGS. He also started having atonic seizures despite appropriate medication management.
D failed ketogenic diet, levetiracetam, and valproic acid. Seizure control improved but the EEG did not improve on combination of levetiracetam and phenytoin. When CBD was added, he had complete control of his seizures and his EEG improved with only mild slowing of background rhythms. There was significant decrease in the epileptiform discharges during sleep and awake state. It has always been thought that in epileptic encephalopathies, the seizures themselves are partly responsible for encephalopathy 4 . Early intervention is believed to improve outcome and in our patient that may have been the case. Also, it is well known that those who have later onset of LGS have a better chance of normal or better cognitive outcome as well as seizure control. Those with unknown or genetic etiology are known to have better outcomes too.
There is little data on EEG outcomes in children with LGS. A few studies have looked at the EEG characteristics in patients with LGS who responded to ketogenic diet. 5 The EEG patterns improves as these children respond with reduction in seizures and even normalize in a small number. EEG response has been found to be predictor of response to treatment with the diet. Effect of CBD on EEG is not yet clear and no data yet exists. It remains unclear which patients respond to CBD and what are the genetic characteristics of responders or nonresponders. LGS itself is a clinical syndrome with a very wide heterogeneity of underlying etiology. It is not clear which genetic variation is more favorable to better outcomes and in our child, we did not find any mutation that has been described before. In our patient, the EEG improved after achieving seizure control.
In conclusion, CBD improves EEG patterns besides improving seizure control as our case demonstrates. Early intervention besides age of onset of LGS might influence outcome. However, larger studies and analysis of EEG in patients with unknown/genetic etiology of LGS will be needed to confirm the finding. As genetic testing becomes more easily accessible, we will have a better understanding of underlying mechanism of action of CBD and which patients it might be most helpful with.
Footnotes
Conflict of Interest None declared.
References
- 1.Radwan M M, ElSohly M A, El-Alfy A T et al. Isolation and pharmacological evaluation of minor cannabinoids from high-potency Cannabis sativa. J Nat Prod. 2015;78(06):1271–1276. doi: 10.1021/acs.jnatprod.5b00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devinsky O, Cross J H, Wright S. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;377(07):699–700. doi: 10.1056/NEJMc1708349. [DOI] [PubMed] [Google Scholar]
- 3.Sekar K, Pack A.Epidiolex as adjunct therapy for treatment of refractory epilepsy: a comprehensive review with a focus on adverse effects[version 1; peer review: 3 approved]F1000 Res 20198F1000 Faculty Rev-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalser J, Cross J H. The epileptic encephalopathy jungle - from Dr West to the concepts of aetiology-related and developmental encephalopathies. Curr Opin Neurol. 2018;31(02):216–222. doi: 10.1097/WCO.0000000000000535. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Wang Y, Zhou Y, Zhang L, Yu L, Zhou S. Therapeutic effects of the ketogenic diet in children with Lennox-Gastaut syndrome. Epilepsy Res. 2016;128:176–180. doi: 10.1016/j.eplepsyres.2016.11.003. [DOI] [PubMed] [Google Scholar]