Abstract
The World Health Organization (WHO) recommends periodic assessment of the therapeutic efficacy of praziquantel (PZQ) to detect reduced efficacy that may arise from drug resistance in schistosomes. In this multi-country study (2014), we assessed the therapeutic efficacy of a single oral dose of PZQ (40 mg/kg) against Schistosoma mansoni (Brazil, Cameroon, Ethiopia, Mali, Madagascar and Tanzania), S. haematobium (Cameroon, Ethiopia, Mali, Tanzania and Zanzibar) and S. japonicum (the Philippines) infections in school-aged children, across a total of 12 different trials. Each trial was performed according to the standardized methodology for evaluating PZQ efficacy as described by the WHO. Overall, therapeutic efficacy, measured as the reduction in arithmetic mean of schistosome egg counts following drug administration (egg reduction rate; ERR), was high for all three schistosome species (S. mansoni: 93.4% (95%CI: 88.8–96.8); S. haematobium: 97.7% (95%CI: 96.5–98.7) and S. japonicum: 90.0% (95%CI: 68.4–99.3). At the trial level, therapeutic efficacy was satisfactory (point estimate ERR ≥90%) for all three Schistosoma species with the exception of S. mansoni in Cameroon where the ERR was 88.5% (95%CI: 79.0–95.1). Furthermore, we observed that in some trials individual drug response could vary significantly (wide 95%CI) and that few non-responsive individuals could significantly impact ERR point estimates. In conclusion, these results do not suggest any established reduced efficacy of the standard PZQ treatment to any of the three schistosome species within these countries. Nevertheless, the substantial degree of variation in individual responses to treatment in some countries underpins the need for future monitoring. The reported ERR values serve as reference values to compare with outcomes of future PZQ efficacy studies to ensure early detection of reduced efficacies that could occur as drug pressure continues increase. Finally, this study highlights that 95%CI should be considered in WHO guidelines to classify the therapeutic efficacy of PZQ.
Keywords: schistosomiasis, Mass drug administration programs, Praziquantel, Drug efficacy, Anthelmintic resistance, Egg reduction rate
Graphical abstract
Highlights
-
•
PZQ efficacy against schistosomes was assessed in school-aged-children in seven countries.
-
•
There was no overall sign of reduced PZQ efficacy against any schistosome species.
-
•
Notable variation in individual responses to treatment does require future monitoring.
-
•
It is recommended to include reporting of the 95%CI in future WHO guidelines.
1. Introduction
Human schistosomiasis is a parasitic disease caused by Schistosoma haematobium (and hybrids therein; causing urogenital schistosomiasis) S. mansoni, S. japonicum, S. guineensis, S. intercalatum and S. mekongi (causing intestinal schistosomiasis). It is estimated that over 229 million people currently require preventive treatment against this disease, the vast majority of whom live in sub-Saharan Africa (WHO, 2020a). In 2017, an estimated 1.4 million disability adjusted life years (DALYs) were lost to schistosomiasis, accounting for 8.3% of the total disease burden attributable to the Neglected Tropical Diseases (NTDs) (DALYs and HALE Collaborators, 2018).
Today, the backbone of schistosomiasis control and/or elimination strategies remains large-scale preventive chemotherapy (PC) deworming programs, during which a single oral dose praziquantel (PZQ; 40 mg/kg) is administered to at-risk populations such as school-aged children (SAC). During the last decades considerable progress has been made towards the control of schistosomiasis, the proportion of the at-risk SAC receiving PZQ increasing from 30 million (26%) in 2012 to 76.2 million (61.3%) in 2018 (WHO, 2014, 2019). The WHO thus set goals for achieving elimination as a public health problem (defined as prevalence of heavy intensity infections below 1% in all sentinel sites) in 88% of endemic countries by 2025 and in all 78 endemic countries by 2030 (WHO, 2020b). However, progress towards these ambitious goals could be threatened by the potential emergence of anthelmintic resistance. For example, Crellen et al. (2016) observed suboptimal PZQ efficacy (reduction in schistosome egg counts following drug administration (ERR) < 90%) in Ugandan SAC following multiple rounds of PZQ administration. In this study, ~16% of SAC that had received 8–9 rounds of PZQ showed a treatment response below 90%, compared to only ~5% in SAC who had received up to 5 rounds of PZQ, underpinning the need to closely monitor drug efficacy during any control program.
In 2013, the Department of NTDs of WHO has published guidelines on how to best monitor drug efficacy against both schistosomiasis and soil-transmitted helminthiasis (WHO, 2013). Over time, it has reached out to its partners to evaluate the efficacy of PZQ against different Schistosoma species in countries with ongoing large-scale deworming programs. The ultimate goal was to estimate the baseline efficacy that can be expected following a single PZQ treatment based on the current WHO guidelines, which can then serve as a reference for future efficacy studies in these countries.
In the present study, we report the data from a WHO-supported, multi-country study in which we assessed the therapeutic efficacy of a single oral dose of PZQ (40 mg/kg) against S. mansoni (Brazil, Cameroon, Ethiopia, Mali, Madagascar and Tanzania), S. haematobium (Cameroon, Ethiopia, Mali, Tanzania and Zanzibar) and S. japonicum (the Philippines) infections in SAC.
2. Materials and methods
2.1. Ethics statement
The study protocol was reviewed and approved by the Institutional Review Board (IRB) of the Faculty of Medicine and Health Sciences of Ghent University, Belgium (Ref. No, 2013/580, B670201318077). The trial protocol was subsequently reviewed and approved by the IRBs associated with each trial site (Brazil: Ethics Committee of Oswaldo Cruz Institute - Fiocruz (Ref. No. CAAE, 18257613.3.0000.5248); Cameroon: Ethics board (Ref. No. 147/CNE/DNM/11); Ethiopia: IRB of Aklilu Lemma Institute of Pathobiology, Addis Ababa University (Ref. No. IRB/22-A/2012/13); Madagascar: National Ethics Committee under the Ministry of Health in Madagascar (Ref. No. 017/MSANP/SG/AMM/CE/2016); Mali: Ethics Board of the National Institute of Research and Public Health (Ref. No. 02/2014/CE-INRSP); Philippines: Research Ethics Board of University of the Philippines Manila (Ref. No. UPM-REB, 2013-243-01); Tanzania: Lake Zone Institutional Review Board (Ref. No MR/53/100/143); Zanzibar: Zanzibar Medical Research and Ethics Committee, (Ref. No. ZAMREC 0003/September/011). Parent(s) or guardians of participants signed an informed consent document to confirm that they understood the purpose and procedures of the study, and that they allowed their child to participate. Participants that were older than 12 years of age were only included if they signed an informed consent document (also referred to as assent for older children; informed consent for parent/guardian) to confirm that they understood the purpose and the procedures of the study, and were willing to participate.
2.2. Trial sites
The current study reports the results of 12 different trials performed in seven endemic countries located in sub-Saharan Africa (Cameroon, Ethiopia, Mali, Madagascar, the United Republic of Tanzania (mainland and Zanzibar)), Asia (Philippines), and Latin America (Brazil). These Schistosoma-endemic countries were selected based on the presence of investigator groups or institutions with extensive experience in the diagnosis, together with historical schistosomiasis control efforts. Table 1 provides an overview of the history of PZQ administration in the different study sites up to the year of the drug efficacy study.
Table 1.
Overview of the praziquantel treatment history up to the year 2014 at each study area included in this multi-country study.
| Country | District/Province | Schistosoma species | Treatment history prior to efficacy trial. |
|---|---|---|---|
| Brazil | Municipality of Malacacheta, state of Minas Gerais | S. mansoni | No widespread PZQ distribution prior to 2014 but area is subjected to successive control campaigns by the Schistosomiasis Surveillance and Control Program since 1997 (Cabello et al., 2016). |
| Cameroon | District of Galim |
S. haematobium | Sporadic treatment before 2009, significant coverage since then. |
| District of Ndikiniméki | S. mansoni | ||
| Ethiopia | Finchaa Sugar Estate, Oromia regional state | S. mansoni | Distribution of PZQ since 2013, maximum 1 round of PZQ prior to the start of the trial. |
| Hasoba area, Afar regional state | S. haematobium | ||
| Madagascar | District of Brickaville in the Atsinanana Region and district of Ihosy in the Ihorombe Region | S. mansoni | Distribution of PZQ since 2008, maximum 7/8 rounds of PZQ prior to the start of the trial (2016). |
| Mali | Dougoulakoro in the district of Kati | S. mansoni and S. haematobium | Distribution of PZQ since 2006, maximum 8 rounds of PZQ prior to the start of the trial. |
| M'Peba in the district of Segou | S. haematobium | ||
| Philippines | Municipalities of Bunawan, Prosperidad, Rosario, San Francisco and Trento in the Province of Agusan del Sur | S. japonicum | Distribution of PZQ since 2008, maximum 6 rounds of PZQ prior to the start of the trial. |
| Tanzania mainland, United Republic of Tanzania | Ukerewe Islands district, Mwanza region, Lake zone. | S. mansoni | Intermittent distribution of PZQ since 2005 (i.e. 2005, 2006, 2008, 2009 and 2013) with a maximum of 5 rounds of PZQ prior to the start of the trial. |
| Bariadi district, Simiyu region, Lake zone. | S. haematobium | Distribution of PZQ only in 2005 and 2006 with a maximum of 2 rounds of PZQ prior to the start of the current trial. | |
| Zanzibar, United Republic of Tanzania | Chake-Chake district, South Pemba | S. haematobium | Intermittent distribution of PZQ since 1986 but continuous distribution from 2010. |
2.3. Trial design
After obtaining informed consent, SAC between the age of 5 and 18 were recruited and asked to provide a fresh stool and/or urine sample. The target was to reach a minimum of 50 complete cases (children who were positive at baseline who also provided a follow-up sample) in each site, as per WHO guidelines (WHO, 2013a). In many trial sites however, a much higher of number of complete cases was obtained. All children providing a stool/urine sample were treated with PZQ 600 mg tablets under direct observation. Each child was given a light snack (e.g. a slice of bread, a biscuit or porridge) before PQZ administration. The number of tablets that was administered to each child depended on their respective weight (measured by a scale) to ensure a minimum treatment dose of 40 mg/kg body weight (15–22.4 kg: 1.5 tablets; 22.5–29.9 kg: 2 tablets; 30–37.4 kg: 2.5 tablets; 37.5–44.9 kg: 3 tablets and 45–59.9 kg: 4 tablets). Children were observed for approximately 4 h following drug administration while they remained at school and continued with their usual activities. They were asked to report any side effect as soon as they occurred. Study participants who reported to have vomited following drug administration were excluded from the analysis.
Fourteen to 21 days after PZQ administration, a second stool and/or a urine sample was collected from those SAC who had tested positive for Schistosoma infections at baseline. During follow-up examination, egg counts were determined in the same way as during baseline. Stool and urine samples were collected between 10 a.m. and 2 p.m. both at baseline and at follow-up. Subjects who were: (i) unable to provide a sample at follow-up; (ii) experienced a severe concurrent medical condition; (iii) had diarrhea at time of the first sampling; (iv) were potentially pregnant or (v) had shown adverse reactions to PZQ in the past were excluded from the study. After follow-up, subjects that were infected with soil-transmitted helminths (Ascaris lumbricoides, Trichuris trichiura and hookworm) at baseline were also treated with a single-oral dose of 400 mg albendazole or 500 mg of mebendazole.
2.4. Parasitological techniques
A single Kato-Katz smear was applied on each stool sample collected during baseline and follow-up screening to determine the S. mansoni or S. japonicum egg counts. The Kato-Katz thick smear was performed as described previously (WHO, 1991). The fecal egg counts (FEC) per Kato-Katz slide were expressed as eggs per gram of stool (EPG) by multiplying the FEC with a factor 24. The presence of S. haematobium eggs in urine samples was determined by urine syringe filtration method (WHO, 1991). The result was expressed as number of eggs per 10 ml of filtered urine.
2.5. Statistical analysis
Treatment efficacy was calculated on the data obtained from complete cases only (i.e., individuals who were positive at baseline and did also provide a follow-up sample), and was reported separately for each of the Schistosoma species (S. mansoni, S. japonicum and S. haematobium). At baseline, the intensity of schistosome infection in the complete cases was classified into low or moderate-to-heavy based on the thresholds proposed by WHO (WHO, 2013b). For S. mansoni and S. japonicum infections this threshold was 100 EPG, while for S. haematobium, ≥50 eggs per 10 ml urine represent a moderate-to-heavy intensity infection (WHO, 2013b).
Efficacy was expressed as ERR, applying the formula: ERR = 100% x (1 – arithmetic mean (egg count at follow-up)/arithmetic mean (egg count at baseline)) (WHO, 2013a). The corresponding 95% confidence intervals (95% CI) were calculated as described by Levecke and colleagues (Levecke et al., 2018). Efficacy classification was based on the WHO thresholds (WHO, 2013a), whereby a point estimate of ERR of ≥90% is considered satisfactory, <80% is considered reduced, and ERR between 80% and 90% is considered doubtful. Individual egg reduction rates (iERRs) were calculated as follows: iERR = 100% x (1-egg count at follow-up/egg count at baseline).
3. Results and discussion
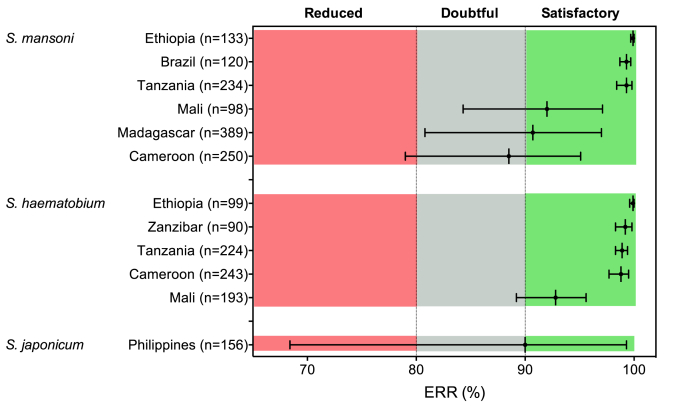
We conducted 12 trials (6 on S. mansoni, 5 on S. haematobium and 1 trial on S. japonicum). The results of the different trials are summarized in Fig. 1 and Supplementary Table S1 and S2. Overall, complete data were available for 2229 individuals, with sample sizes at the trial level ranging from 90 to 381. The study showed that PZQ efficacy against S. mansoni was satisfactory (ERR based on all complete cases = 92.9% (95%CI: 87.6–96.7)) in all trials except for the trial performed in Cameroon, which showed an ERR of 88.5% but with a 95%CI spanning from 79.0% to 95.1%. The efficacy of PZQ to S. haematobium was also satisfactory in all five study sites, with ERR based on all complete cases of 97.4% (95%CI: 96.2–98.3). The efficacy of a single PZQ treatment to S. japonicum was only evaluated in the Philippines and showed to be borderline satisfactory (ERR = 90.0%) with the lower 95% confidence interval spanning into the zone of reduced efficacy (95%CI: 68.4–99.3). Overall, these results appear to be consistent with the findings of previous meta-analyses that found that a single oral dose of PZQ (40 mg/kg) was highly effective against Schistosoma spp. infections without any reports of severe adverse events (Zwang and Olliaro, 2014, 2017).
Fig. 1.
Measured egg reduction rates of a single treatment with 40 mg/kg of praziquantel against Schistosoma spp. The black vertical lines represent the measured egg reduction rate (ERR) for each Schistosoma species in each trial site. The 95% confidence intervals are represented by the error flags. The colored zones represent ERR levels that correspond to a satisfactory (green), doubtful (grey) or reduced (red) drug efficacy of praziquantel 40 mg/kg according to WHO guidelines (WHO, 2013a). The number of complete cases per trial is indicated between brackets. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
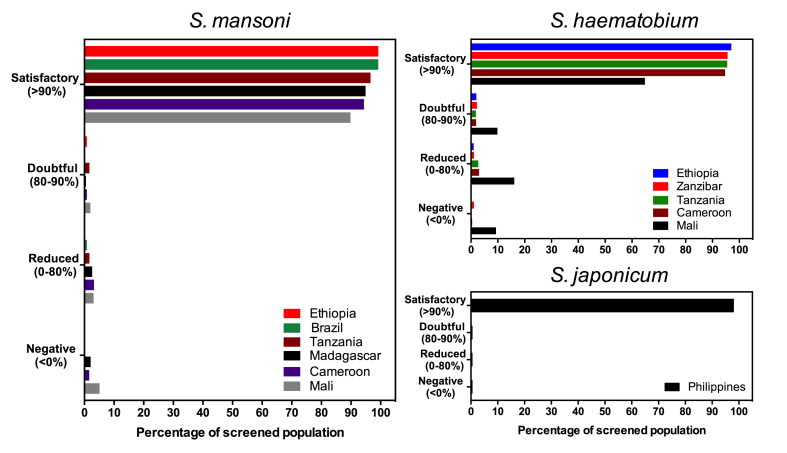
It is of importance to note that there were five trials for which the lower limit of the 95%CI confidence interval stretched within the doubtful (S. mansoni: Madagascar and Mali; S. haematobium: Mali) or even reduced (S. mansoni: Cameroon; S. japonicum: the Philippines) ERR levels. However, when we classify the individual responses (iERRs) to the treatment (Fig. 2), we notice that in the Philippines, in only three (1.9%) out of the 156 S. japonicum cases there was no satisfactory response (iERR >90%). These three cases were thus responsible for the borderline ERR and the large 95%CIs. In the current WHO guidelines (WHO, 2013a), a measure of uncertainty around the calculated ERR is not considered in the interpretation of the results. Yet, such an indicator of uncertainty does carry important additional information on the possible variation in drug efficacy that can be expected. A large measure of uncertainty indicates that the measured efficacy is highly variable and integrating this variation in the interpretation of trial data is important to reach the appropriate conclusions with regards to true drug efficacy. Exploring the possibility to pivot towards more complex models that consider the distribution of individual drug responses (Walker et al., 2016) might also be of substantial value.
Fig. 2.
Distribution of individual egg reduction rates following a single treatment with 40 mg/kg of praziquantel against Schistosoma spp. For each trial, the individual egg reduction rates (iERRs) were calculated and grouped into four different drug efficacy categories. The iERRs were classified into being satisfactory (iERR ≥ 90%), doubtful (90 > iERR ≥ 80), reduced (80 > iERR ≥0) or negative (iERR < 0).
The reasons for the lack of observed drug efficacy in certain individuals is unclear. Although this might be explained by potential emergence of anthelmintic resistance, multiple other factors can affect the observed drug efficacy (WHO, 2013a). It is for example possible that participants do not report having vomited following treatment, leading to the inclusion of individuals that actually did not receive the optimal dose. The occurrence of non-compliers and their impact on reported drug efficacy estimates is a well-established in the field of schistosomiasis and soil-transmitted helminthiasis (Montresor, 2007; Moser et al., 2020; Olliaro et al., 2015; Speich et al., 2015). The implications of having a small proportion of individuals who show reduced drug efficacy for long-term sustainability of PZQ-based PC are still unknown and certainly warrant close longitudinal monitoring. There is an on-going important debate on how to address these outliers or non-responders in the context of drug efficacy studies (Moser et al., 2020).
Overall, our results confirm that, at the time of performing these efficacy trials (2014), there was little reason to doubt the high efficacy of a single oral dose PZQ against schistosome infections in SAC, that an ERR point estimate exceeding 90% should be expected for all schistosome species in future surveys and that any deviation from this expected therapeutic efficacy should be viewed with concern in light of potential development of drug resistance.
In 2016, Crellen and colleagues (Crellen et al., 2016) reported the first indications of reduced PZQ efficacy to S. mansoni in Uganda more than 10 years into the control program (program started in 2003, the efficacy trial was performed in 2014). In our study, Mali had the longest running schistosome PC program (since 2006) and also showed the highest number of individuals with iERRs below 90% for both S. mansoni (10.2%) as S. haematobium (35.2%) (Fig. 2). Further research is required to exclude anthelmintic resistance as possible cause of these findings.
Finally, this study highlights that care should be taken to investigate the impact of apparent non-responsive individuals on overall reported drug efficacy and that including measures of uncertainty around the drug efficacy estimates would be of value to better interpret reported results. Nevertheless, the need for continued vigilance of PZQ efficacy in the large-scale deworming programs era remains, especially while alternative therapies remain absent and studies indicate the possible existence of a negative effect of multiple rounds on PZQ efficacy. Today, WHO and its partners are updating the current WHO manual (WHO, 2013a) to take into account the experiences from drug efficacy trials conducted in the past seven years and to facilitate the interpretation of study results.
Declaration of competing interest
All authors hereby confirm that there is not conflict of interest.
Acknowledgements
Thanks are due to the health and educational services of all trial sites for providing assistance and facilities. Special thanks to the community agents, local authorities, school directors and teachers, but most of all to all participating schoolchildren and their parents.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2020.10.003.
Funding
The studies in Madagascar and Zanzibar were funded by WHO through a grant from Merck KgA, which provided the PZQ. Other studies were WHO funded through WHO Collaborating Center BEL-42 (Ghent University).
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Cabello R., Beck L., Massara C.L., Murta F.L.G., Guimaraes R., Pieri O.S., Schall V.T., Favre T.C. Schistosoma mansoni infection and related knowledge among schoolchildren in an endemic area of Minas Gerais, Brazil, prior to educational actions. Acta Trop. 2016;164:208–215. doi: 10.1016/j.actatropica.2016.09.015. [DOI] [PubMed] [Google Scholar]
- Crellen T., Walker M., Lamberton P.H., Kabatereine N.B., Tukahebwa E.M., Cotton J.A., Webster J.P. Reduced efficacy of praziquantel against Schistosoma mansoni Is associated with multiple rounds of mass drug administration. Clin. Infect. Dis. 2016;63:1151–1159. doi: 10.1093/cid/ciw506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALYs, HALE Collaborators Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1859–1922. doi: 10.1016/S0140-6736(18)32335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levecke B., Kaplan R.M., Thamsborg S.M., Torgerson P.R., Vercruysse J., Dobson R.J. How to improve the standardization and the diagnostic performance of the fecal egg count reduction test? Vet. Parasitol. 2018;253:71–78. doi: 10.1016/j.vetpar.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Montresor A. Arithmetic or geometric means of eggs per gram are not appropriate indicators to estimate the impact of control measures in helminth infections. Trans. R. Soc. Trop. Med. Hyg. 2007;101:773–776. doi: 10.1016/j.trstmh.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser W., Keiser J., Speich B., Sayasone S., Knopp S., Hattendorf J. One mean to rule them all? The arithmetic mean based egg reduction rate can be misleading when estimating anthelminthic drug efficacy in clinical trials. PLoS Neglected Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0008185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olliaro P.L., Vaillant M., Diawara A., Coulibaly J.T., Garba A., Keiser J., King C.H., Knopp S., Landoure A., N'Goran E.K., Raso G., Scherrer A.U., Sousa-Figueiredo J.C., Stete K., Zhou X.N., Utzinger J. Toward measuring Schistosoma response to praziquantel treatment with appropriate descriptors of egg excretion. PLoS Neglected Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speich B., Ali S.M., Ame S.M., Bogoch, Alles R., Huwyler J., Albonico M., Hattendorf J., Utzinger J., Keiser J. Efficacy and safety of albendazole plus ivermectin, albendazole plus mebendazole, albendazole plus oxantel pamoate, and mebendazole alone against Trichuris trichiura and concomitant soil-transmitted helminth infections: a four-arm, randomised controlled trial. Lancet Infect. Dis. 2015;15:277–284. doi: 10.1016/S1473-3099(14)71050-3. [DOI] [PubMed] [Google Scholar]
- Walker M., Mabud T.S., Olliaro P.L., Coulibaly J.T., King C.H., Raso G., Scherrer A.U., Stothard J.R., Sousa-Figueiredo J.C., Stete K., Utzinger J., Basanez M.G. New approaches to measuring anthelminthic drug efficacy: parasitological responses of childhood schistosome infections to treatment with praziquantel. Parasites Vectors. 2016;9:41. doi: 10.1186/s13071-016-1312-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva: 1991. Basic Laboratory Methods in Medical Parasitology. [Google Scholar]
- WHO . World Health Organisation; Geneva, Switserland: 2013. Assessing the Efficacy of Anthelminthic Drugs against Schistosomiasis and Soil-Transmitted Helminthiases. [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2013. Schistosomiasis: Progress Report 2001-2011, Strategic Plan 2012-2020. [Google Scholar]
- WHO . 2014. Schistosomiasis: number of people receiving preventive chemotherapy in 2012; pp. 21–28. Weekly epidemiological record 2. [PubMed] [Google Scholar]
- WHO Schistosomiasis and soil-transmitted helminthiasis: numbers of people treated in 2018. Wkly. Epidemiol. Rec. 2019;50:601–612. [Google Scholar]
- WHO . 2020. Schistosomiasis: Fact Sheet.https://www.who.int/news-room/fact-sheets/detail/schistosomiasis Accessed online 15th. [Google Scholar]
- WHO . World Health Organization; Geneva, Switserland: 2020. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021-2030. [Google Scholar]
- Zwang J., Olliaro P.L. Clinical efficacy and tolerability of praziquantel for intestinal and urinary schistosomiasis-a meta-analysis of comparative and non-comparative clinical trials. PLoS Neglected Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0003286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwang J., Olliaro P. Efficacy and safety of praziquantel 40 mg/kg in preschool-aged and school-aged children: a meta-analysis. Parasites Vectors. 2017;10:47. doi: 10.1186/s13071-016-1958-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.