Abstract
Purpose
To describe a case of pregnancy-related central serous chorioretinopathy (pCSCR) and the utility of monitoring with optical coherence tomography (OCT) and OCT angiography (OCTA).
Observations
A 34-year-old female in her third trimester of pregnancy presented with symptomatic visual disturbances of the right eye. Medical history was otherwise unremarkable. Optical coherence tomography (OCT) disclosed a serous retinal detachment with trace subretinal fibrin and elevation of the retinal pigment epithelium (RPE). OCT angiography (OCTA) demonstrated absence of choroidal neovascular membrane (CNV). It was decided to monitor with weekly OCTs until delivery, after which the patient had full resolution of symptoms and subretinal fluid.
Conclusions and Importance
Pregnancy-related CSCR may be complicated by fibrin deposition and RPE changes suspicious for CNV. This provides evidence of the utility of OCT for monitoring pCSCR progression and of OCTA to non-invasively assess presence of CNV.
Keywords: Pregnancy, Central serous chorioretinopathy, Optical coherence tomography, Optical coherence tomography angiography, Choroidal neovascular membrane
Highlights
-
•
Pregnancy-related central serous chorioretinopathy (pCSCR) can be safely monitored using non-invasive imaging modalities.
-
•
Optical coherence tomography angiography (OCTA) is useful to assess presence of choroidal neovascularization in pCSCR.
-
•
Pregnancy is a risk factor for central serous chorioretinopathy (CSCR).
1. Introduction
Central serous chorioretinopathy (CSCR) is a common posterior segment condition characterized by serous retinal detachments and retinal pigment epithelial (RPE) detachments (PED).1 It occurs in approximately 9.9 and 1.7 per 100,000 males and females, respectively, and is the fourth most common retinopathy affecting vision.2 Several risk factors have been implicated for CSCR development, including stress, topical and systemic corticosteroids, and obstructive sleep apnea.3 The pathophysiology of CSCR is thought to be due to elevated serum cortisol and catecholamines causing leaky RPE tight junctions and/or hypoxia causing a thickened choroid.2
Despite a six-fold higher prevalence rate in males than females,2 pregnancy increases the risk of CSCR in women by an odds-ratio of 7.1.3 Vascular hyperpermeability and hormonal changes in pregnancy may contribute to the development of CSCR.2,3 Pregnancy-associated CSR (pCSCR), although often resolving with delivery, is unique in that up to 90% of eyes with pCSCR present with subretinal fibrin deposition as compared with 20% of idiopathic CSCR. This is important to be aware of because subretinal fibrin can be mistaken for CNV, the latter of which occurs in 5% of CSCR cases.
Studies have utilized OCT angiography (OCTA) to non-invasively detect CNV associated with exudative age-related macular degeneration,4 pathologic myopia,5 and polypoidal choroidal vasculopathy.6 In this report, we present the utility of OCT to monitor structural changes and OCTA to assess for CNV in acute pCSCR.
2. Case report
A 34-year-old female at 32 weeks gestational age presented to retina clinic with a central scotoma in the right eye. She denied any use of systemic or topical steroids, history of sleep apnea, or hypertension. Her medical history was unremarkable except for a miscarriage one year prior. Blood pressure was 111/72 mmHg.
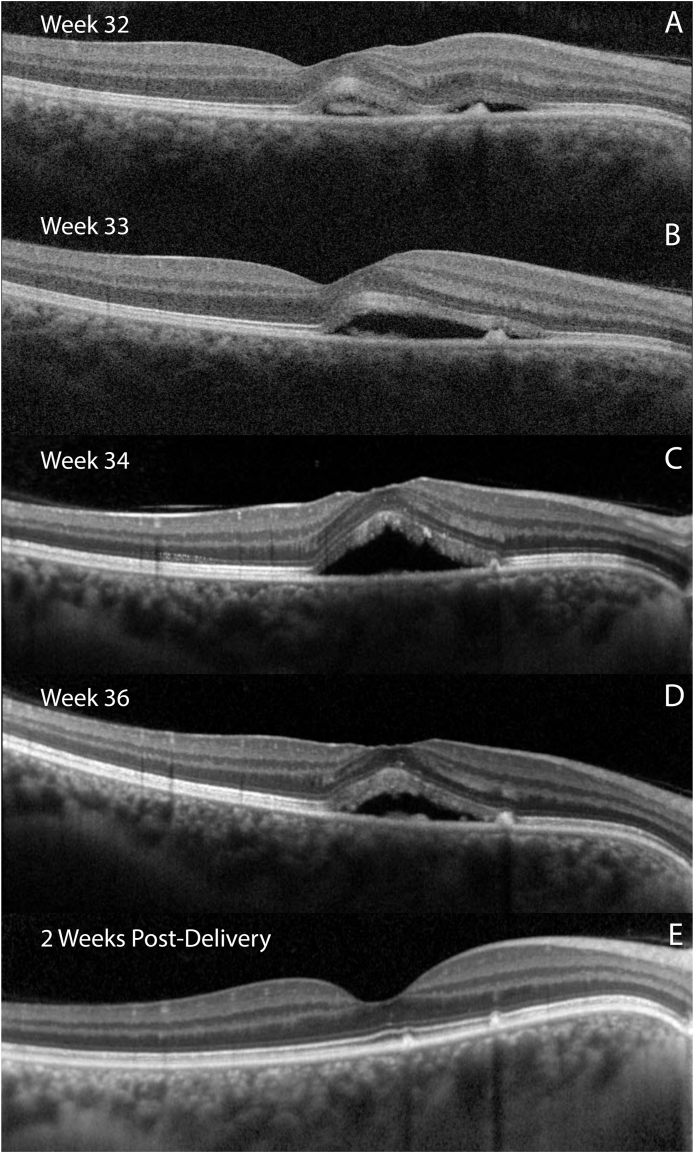
Visual acuities (VA) were 20/30 + 1 and 20/20–2 in the right and left eyes, respectively by Snellen. Intraocular pressures and anterior segment exams were normal. Dilated fundus exam of the right eye disclosed a pigment epithelial detachment in the macula, while that of the left eye was normal. Spectral-domain OCT imaging (SD-OCT) (Spectralis, Heidelberg Engineering GmbH, Heidelberg, Germany) showed a serous neurosensory detachment with subretinal hyperreflective material (Fig. 1). On OCTA (AngioPlex, Carl Zeiss AG, Oberkochen, Germany), choriocapillaris segmentation did not show flow through the subretinal material (Fig. 2), and deeper choroidal segmentation was unremarkable.
Fig. 1.
Panels A, B, C, D: Optical coherence tomography (OCT) B-scans through the fovea of the right eye of the patient, taken at gestational weeks 32, 33, 34, and 36, respectively. Note subfoveal hyperreflective material and nasal retinal pigment epithelial elevation. Panel E: OCT taken one month after delivery showing resolution of subretinal fluid.
Fig. 2.
Panel A: Infrared photo of right macula showing a macular lesion. Panel B: En-face optical coherence tomography angiography (OCTA) scan segmented for the avascular complex (outer nuclear layer through Bruch's membrane) showing irregularity of nasal macular vessels without an obvious choroidal neovascular membrane (CNV). Panels C, D: Horizontal and vertical B-scans, respectively, through an area of retinal pigment epithelial elevation without significant flow signal.
Given the low suspicion of CNV, treatment was deferred; however, because the patient was very bothered by the scotoma and concerned about progression, she elected to be followed weekly. She was counseled on risk factor management such as stress and blood pressure control, as well as the potential treatment options and their risks and benefits. After discussion with her obstetrician, they decided to consider early labor induction if the vision worsened. Subsequent imaging showed an initial increase in SRF followed by a decrease at 37 weeks gestation with VA improving to 20/20–2. The patient underwent scheduled cesarean section at 39 weeks, and OCT showed resolution of SRF one month later.
2.1. Patient consent
The patient consented to publication of the case orally. This report does not contain any personal information that could lead to the identification of the patient.
3. Discussion
Associations between CSCR and pregnancy have been demonstrated in several retrospective studies.2,3,7,8 The general onset of visual symptoms often occurs in the third trimester, the period of greatest hemodynamic changes.7 Parameters in pregnancy associated with pCSCR include hypertension, hypercoagulability, decreased colloidal osmotic pressure, and preeclampsia.7
Retinal toxemia of pregnancy and CSCR have similar risk factors and overlapping clinical presentation.9 In fact, the most extreme cases of pCSCR resemble retinal toxemia of pregnancy with subretinal fluid, hyperpermeable RPE, and thickened choroid. It is therefore reasonable for any pregnant patient presenting with eye findings suggestive of CSCR to be referred for obstetric evaluation for possible preeclampsia.
Acute pCSCR usually resolves spontaneously after delivery, and there are no reports of CNV complicating pCSCR. However, given the high rates of subretinal fibrin deposition in pCSCR that may mimic CNV, ruling out CNV is important. Traditional detection of CNV relies on FA which is relatively invasive and not preferred during pregnancy. OCTA is therefore a good option given its non-invasive nature, including no need for dilation. OCTA detection of CNV in non-pregnancy related CSR has shown up to 100% specificity and sensitivity compared with FA.10
In our patient, despite an initial increase in fluid on OCTs, there was no CNV detected on OCTA, and the patient was safely monitored weekly through delivery. Fibrin threatening the fovea is reasonable to treat by OCT-guided focal laser photocoagulation,8 and photodynamic therapy with verteporfin may be considered but is pregnancy class C.11 In the absence of fibrin in or near the fovea, close monitoring is recommended.
There are no clear obstetric guidelines in this situation, although investigations have noted increased maternal cortisol levels during spontaneous vaginal delivery (SVD) versus cesarean section (CS),12 which could theoretically worsen pCSCR. After discussion with the patient's obstetrician, planned elective CS was chosen to reduce the risk of stress associated with vaginal delivery. Resolution of pCSCR resolution was confirmed on post-natal follow up with OCT.
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Cameron Pole, Stephanie Gaw, and Irena Tsui. The first draft of the manuscript was written by Cameron Pole, MD and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
NIH Funding, R21EY03029501A1; Unrestricted grant by Research to Prevent Blindness given to the Stein Eye Institute.
Intellectual property
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
Research ethics
We further confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Contact with the editorial office
We understand that this author is the sole contact for the Editorial process (including EVISE and direct communications with the office). He/she is responsible for communicating with the other authors, including the Corresponding Author, about progress, submissions of revisions and final approval of proofs.
Declaration of competing interest
The following authors have no financial disclosures: CP, SG, IS.
Acknowledgements
None.
Contributor Information
Cameron Pole, Email: cameron.pole@med.usc.edu.
Stephanie L. Gaw stephanie, Email: gaw@ucsf.edu.
Irena Tsui irena, Email: itsui@jsei.ucla.edu, tsui@gmail.com.
References
- 1.van Rijssen T.J., van Dijk E.H.C., Yzer S. Central serous chorioretinopathy: towards an evidence-based treatment guideline. Prog Retin Eye Res. 2019:100770. doi: 10.1016/j.preteyeres.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Daruich A., Matet A., Dirani A. Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retin Eye Res. 2015;48:82–118. doi: 10.1016/j.preteyeres.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Morikawa M., Cho K., Kojima T. Risk factors for central serous chorioretinopathy in pregnant Japanese women. J Obstet Gynaecol Res. 2017;43(5):866–872. doi: 10.1111/jog.13289. [DOI] [PubMed] [Google Scholar]
- 4.Iafe N.A., Phasukkijwatana N., Sarraf D. Optical coherence tomography angiography of type 1 neovascularization in age-related macular degeneration. Dev Ophthalmol. 2016;56:45–51. doi: 10.1159/000442776. [DOI] [PubMed] [Google Scholar]
- 5.Querques G., Corvi F., Querques L., Souied E.H., Bandello F. Optical coherence tomography angiography of choroidal neovascularization secondary to pathologic myopia. Dev Ophthalmol. 2016;56:101–106. doi: 10.1159/000442800. [DOI] [PubMed] [Google Scholar]
- 6.Srour M., Querques G., Souied E.H. Optical coherence tomography angiography of idiopathic polypoidal choroidal vasculopathy. Dev Ophthalmol. 2016;56:71–76. doi: 10.1159/000442781. [DOI] [PubMed] [Google Scholar]
- 7.Kalogeropoulos D., Sung V.C., Paschopoulos M. The physiologic and pathologic effects of pregnancy on the human visual system. J Obstet Gynaecol. 2019:1–12. doi: 10.1080/01443615.2019.1584891. [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal J.M., Johnson M.W. Management of retinal diseases in pregnant patients. J Ophthalmic Vis Res. 2018;13(1):62–65. doi: 10.4103/jovr.jovr_195_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Day H., Burns J., Bosio P. A case of bilateral serous retinal detachments in severe pre-eclampsia. J Obstet Gynaecol. 2008;28(5):534–535. doi: 10.1080/01443610802234467. [DOI] [PubMed] [Google Scholar]
- 10.Bonini Filho M.A., de Carlo T.E., Ferrara D. Association of choroidal neovascularization and central serous chorioretinopathy with optical coherence tomography angiography. JAMA ophthalmology. 2015;133(8):899–906. doi: 10.1001/jamaophthalmol.2015.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Visudyne (verteporfin for injection) package label. 2005. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021119s022lbl.pdf Published online April 20.
- 12.Shokry E., Marchioro L., Uhl O. Investigation of the impact of birth by cesarean section on fetal and maternal metabolism. Arch Gynecol Obstet. 2019;300(3):589–600. doi: 10.1007/s00404-019-05213-w. [DOI] [PubMed] [Google Scholar]