Abstract
Objective
To evaluate the effect of a new type of automatic glycated hemoglobin analyzer on the separation of abnormal hemoglobin.
Methods
Samples diagnosed as hemoglobin variants by capillary electrophoresis and gene testing were selected, and HbA1c analyzer was used for separation and detection.
Results
A total of 13 hemoglobin variants in 40 samples could be separated from the normal peaks.
Conclusions
The variant mode of hemoglobin HbA1c can identify a variety of hemoglobin variants, and the type of variants can be preliminarily determined according to the retention time and characteristic peak shape of the variants.
Keywords: HbA1c, hemoglobin variant, high‐performance liquid chromatography
(A) Hb New York demonstrating bifurcation of HbA1c peak and higher P4 peak (118.8s) near HbA0(125.9s). (B) There was a high peak at 226.8s, which was significantly different from 230.7s of Hb E, so it could be identified as an abnormal variant Hb G‐Taipei. (C) The content is beyond 5%and retention time(105.0s) is close to the P4 peak(100.7s), which may be considered as the mutant Hb J‐Bangkok. (D) Hb Q‐Thailand demonstrates abnormal peak at 300.1s. (E) HbE is separated thoroughly from HbA2 in 230.7s and reported as an independent name “HbE”. (F) Hb Tamano demonstrating downward slope of HbA0 as hump at 126.9s. (G) Abnormal peak at 237.2s could be considered as Hb Maputo variant. (H) Hb G‐Honolulu presents an abnormal peak at 238.7s. (I) Hb G‐Cousshatta presents an abnormal peak at 224.6s. (J) Hb Ottawa demonstrates abnormal peak at 283.7s. (K) Hb J‐Wenchang‐Wumingdemonstrating upward slope of P3 as hump at 87.8s. (L) Hb J‐Baltimorepresents an independent peak at 100.6s in P4 position. (M) Hb H will lead to HbA1a result get higher due to less positive charge at retention time of 12.18s.

1. INTRODUCTION
Hemoglobin synthesis and functional disease is extremely common throughout the world. Abnormal hemoglobin variant is a variety of forms of hemoglobin whose structures and properties had changed. One or more amino acids are structurally abnormal, mutated, or replaced. More than 1000 naturally occurring human hemoglobin variants have been identified, some of which have no specific clinical manifestations, while others can cause severe clinical symptoms. Study of these abnormal hemoglobin variants has accumulated a lot of clinical data which are of great significance to the practice of hematology.
HbA1c is detected by high‐performance liquid chromatography (HPLC) in some laboratories. This method is based on the difference in the charge of different hemoglobin to achieve separation. HPLC has two main disadvantages. The presence of hemoglobin variants can lead to false increase or decrease in HbA1c. On the other hand, some variants are not easily detected by IE HPLC, depending upon the specific assay method.
2. MATERIALS AND METHODS
2.1. Research Objects
In order to evaluate the separation of abnormal hemoglobin by the variant mode of the new hemoglobin analyzer, whole blood samples with abnormal zones during thalassemia screening were collected by capillary electrophoresis and stored in a 1‐mL cryotube at −80°C refrigerator. The type of abnormal hemoglobin was also identified by genetic diagnosis. All specimens were treated with EDTA anticoagulation.
2.2. Main instruments and reagents
Sebia Capillarys2 fully automatic capillary electrophoresis apparatus and the original reagent kit (Sebia); H9 HbA1c analyzer and reagent kit (Shenzhen Lifotronic Technology Co., Ltd.); Gene diagnostic kit (Yaneng Bioscience Shenzhen Co., Ltd); Hema9600 extender (Zhuhai Heima); ABI3730XL genetic analyzer (ABI).
2.3. Methods
2.3.1. Hemoglobin HbA1c Analysis (Thalassemia Mode)
H9 with thalassemia mode and reagent kit are provided by Shenzhen Lifotronic Technology co., LTD. It uses ion exchange high‐performance liquid chromatography (HPLC) method to elute and identify different types of hemoglobin corresponding to different retention time. It takes only 350 seconds to separate hemoglobin HbA1a, HbA1b, HbF, LA1c, HbA1c, P3, P4, HbA0, HbA2 and common HbE, D, S, and C four categories of variant, forming a series of chromatographic peaks. Then, by calculating and comparing the retention time and area of each peak, the corresponding hemoglobin name and percentage content are automatically obtained.
2.3.2. Detection of Common Types of Thalassemia Genes
Common types of thalassemia genes were detected by Gap‐PCR across the break points (‐SEA, ‐α 3.7, and ‐α 4.2). Reverse dot blotting (PCR‐RDB) method was used to detect common three kinds of the Chinese people lack of alpha to lean gene (α QS, α CS, α WS) and 17 types of beta to lean point mutations (‐28, ‐29, CD17, CD41‐42, CD43, βE, CD71‐72, IVS ‐ Ⅱ‐ 654, ‐ 32, ‐30, CAP, Initiation condon, CD14‐15, CD27‐28, IVS ‐ I ‐ 1, IVS ‐ I ‐ 5, CD31). The above tests are performed according to the instructions of the instrument and kit.
2.3.3. α‐globin Gene Sequencing Analysis
α‐globin gene sequencing was detected by Yaneng Bioscience Shenzhen Co., Ltd. PCR was used to amplify the α2 and α1 globin genes, and the α2 and α1 globin genes were sequenced by Sanger dideoxy chain termination method. Vector net 8.0 software was used to compare the sequencing results and thalassemic anemia in human abnormal hemoglobin library (http://globin.bx.psu.edu/cgi‐bin/hbvar/query_vars3) analysis to find the mutations.
2.3.4. Analysis of Globin Gene Sequence
Bead protein gene sequence analysis and bead protein gene sequencing were detected by Yaneng Bioscience Shenzhen co., Ltd. PCR was used to amplify the globulin gene sequence in 9600extender (Heima, Zhuhai), and the globulin gene sequence was determined by Sanger dideoxy chain termination method on the 3730XL genetic analyzer (ABI). Sequencing results were compared by using Vector NET 8.0, the analysis software of the 3730XL genetic analyzer, and mutation sites were analyzed and found in human abnormal Hb thalassemia library (http://globin.bx.psu.edu).
3. RESULTS
3.1. Interpretation of normal sample by HPLC chromatogram and retention time
The whole process of HbA1a, HbA1b, HbF,LA1c, HbA1c, P3, P4, HbA0, HbA2 and variant including HbE/D/S/C can be separated in 350 s (Table 1; Figure 1).The general peak shape of normal specimens should be smooth and symmetrical without bifurcation, the total area should be in the range of 50 000‐170 000, the baseline should be straight and no drifting, without obvious burr, each peak should be identified correctly, and the peak except HbA0 and HbA1c should be less than 5%.
Table 1.
Retention time and range of different hemoglobin components in normal sample
| Name of the peak | Retention time (s) |
|---|---|
| HbA1a | 13.8 (12.0‐15.0) |
| HbA1b | 21.0 (17.0‐20.0) |
| HbF | 30.1 (27.0‐34.0) |
| LA1c | 38.8 (36.0‐40.0) |
| HbA1c | 54.3 (50.0‐58.0) |
| P3 | 82.0 (78.0‐89.0) |
| P4 | 100.7 (95.0‐110.0) |
| HbA0 | 125.9 (120.0‐135.0) |
| HbA2 | 192.0 (180.0‐200.0) |
| HbE | 230.7 (220.0‐237.9) |
| HbD | 245.3 (238.0‐253.9) |
| HbS | 270.5 (254.0‐289.9) |
| HbC | 310.7 (290.0‐330.0) |
Figure 1.
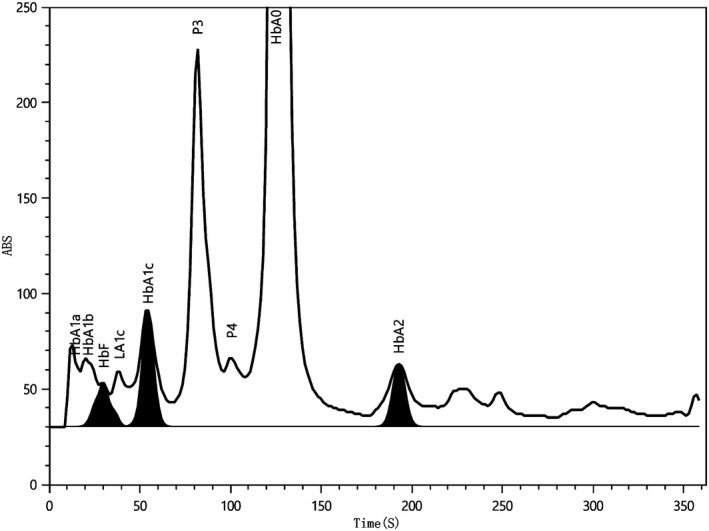
Separation of different hemoglobin components in normal sample
3.2. Electrophoresis and genetic test results of 40 hemoglobin variants
A total of 13 abnormal hemoglobin variants were detected in 40 samples: Hb E, Hb Ottawa, Hb G‐Taipei, Hb Tamano, Hb Maputo, Hb G‐Honolulu, Hb G‐Coushatta, Hb Q‐Thailand, Hb New York, Hb J‐Bangkok, Hb J‐Wenchang‐Wuming, Hb J‐Baltimore, Hb H.
3.3. Chromatographic features of HPLC thalassemia mode
A total of 13 abnormal hemoglobin types in 40 samples could be separated or identified in thalassemia mode. Among them, Hb Tamano, Hb Maputo, and Hb New York had abnormal peak shape, while other abnormal hemoglobin only showed different retention time (Table 2 as below). So visual review is particularly important. H9 system is intelligent and editable for different kinds of cases such as:
any abnormal peak except HbA1c and HbA0 whose result is over 5%;
unknown peak presents;
total area is out of range;
HbF/HbA1c/HbA2 results are out of reportable range;
there is no peak within HbF/HbA1c/HbA2 scope of the identification window.
Table 2.
Retention time and peak shape characteristics of different hemoglobin variants
| Variant Species | Number of cases | Retention time (SD) | Peak shape |
|---|---|---|---|
| Hb E | 5 | 230.7 (0.30) | Normal |
| Hb Ottawa | 2 | 283.7; 283.2 | Normal |
| Hb G‐Taipei | 2 | 226.8; 227.3 | Normal |
| Hb Tamano | 1 | 126.9 | The downward slope of A0 is abnormal |
| Hb Maputo | 1 | 237.2 | The upward slope of A0 is abnormal |
| Hb G‐Honolulu | 2 | 238.7; 238.4 | Normal |
| Hb G‐Coushatta | 1 | 224.6 | Normal |
| Hb Q‐Thailand | 7 | 300.1 (0.30) | Normal |
| Hb New York | 9 | 118.8 (0.08) | HbA1c bifurcation; HbA0 bifurcation; P4 peak rise |
| Hb J‐Bangkok | 3 | 105.0 (0.23) | Normal |
| HbJ‐Wenchang‐Wuming | 2 | 87.8; 88.0 | Characteristic hump on the upward slope of the P3 elution peak |
| Hb J‐Baltimore | 1 | 100.6 | Normal |
| Hb H | 4 | 12.18 (0.15) | Normal |
So it is easy for the operator to identify different types of variants and report results with more confidence.
3.4. Chromotograms from 13 types of variants in H9
These are illustrated in Figure 2.
Figure 2.
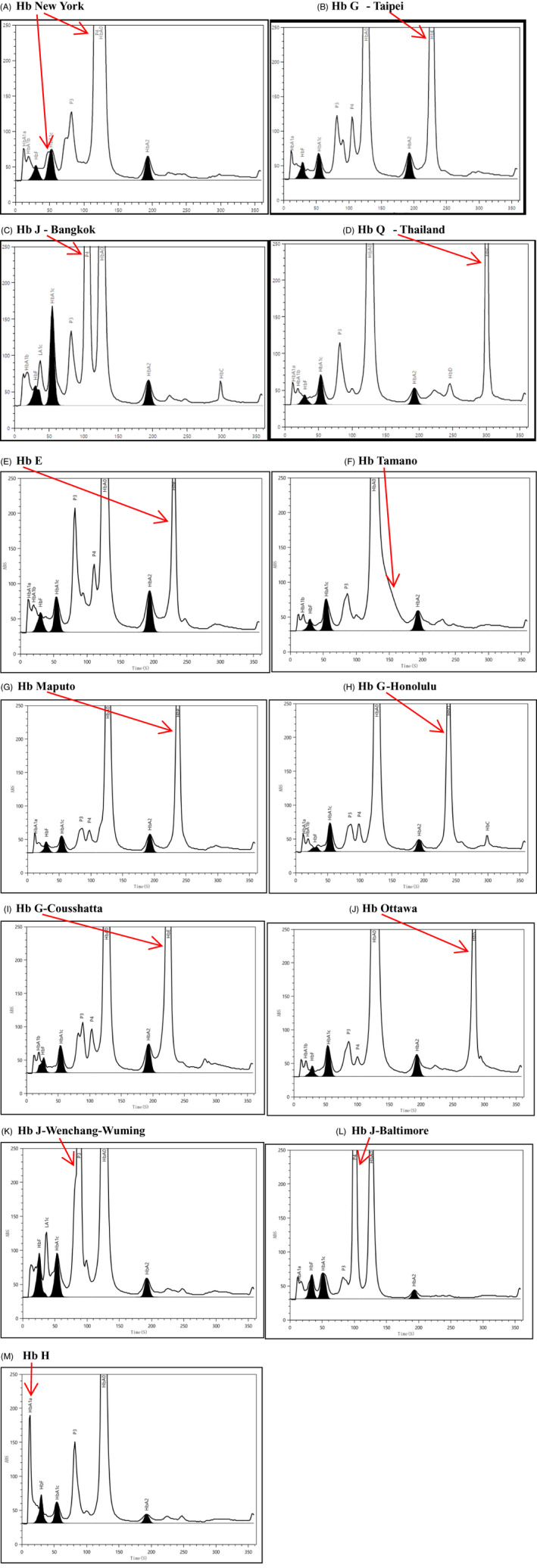
Chromotograms from 13 types of variants in H9. A, Hb New York demonstrating bifurcation of HbA1c peak and higher P4 peak (118.8 s) near HbA0 (125.9 s). B, There was a high peak at 226.8 s, which was significantly different from 230.7 s of Hb E, so it could be identified as an abnormal variant Hb G‐Taipei. C, The content is beyond 5% and retention time (105.0 s) is close to the P4 peak (100.7 s), which may be considered as the mutant Hb J‐Bangkok. D, Hb Q‐Thailand demonstrates abnormal peak at 300.1 s. E, HbE is separated thoroughly from HbA2 in 230.7 s and reported as an independent name “HbE.” F, Hb Tamano demonstrating downward slope of HbA0 as hump at 126.9 s. G, Abnormal peak at 237.2 s could be considered as Hb Maputo variant. H, Hb G‐Honolulu presents an abnormal peak at 238.7 s. I, Hb G‐Coushatta presents an abnormal peak at 224.6 s. J, Hb Ottawa demonstrates abnormal peak at 283.7 s. K, Hb J‐Wenchang‐Wuming demonstrating upward slope of P3 as hump at 87.8s. L, Hb J‐Baltimore presents an independent peak at 100.6 s in P4 position. M, Hb H will lead to HbA1a result get higher due to less positive charge at retention time of 12.18 s
4. DISCUSSION
According to the World Health Organization, about 7% of the world's population is affected by hemoglobin variants. Although some of these variants do not result in any clinical manifestations, the study of hemoglobin variants has important implications for the interpretation of HbA1c results. 1 , 2 Hemoglobin variants may affect erythrocyte life span, leading to HbA1c results inconsistent with clinical conditions and misleading clinical diagnosis. In addition, abnormal hemoglobin that has not been separated may overlap with HbA0 peak or HbA1c peak, resulting in a false increase or decrease in HbA1c result. Hemoglobin variants may also affect the glycosylation of hemoglobin, which in turn affects the outcome of HbA1c. 3 Mutations in certain genes lead to increased affinity between hemoglobin and oxygen, which stimulates the release of erythropoietin by inhibiting the transmission of oxygen tissue, resulting in increased erythropoietin production, which can be manifested as redness and swelling. Mutations in certain genes lead to decreased affinity between hemoglobin and oxygen, and deoxyhemoglobin is clinically manifested as cyanosis. In addition to affecting skin color, these hemoglobin variants have no other harm. However, these clinical manifestations are easily misdiagnosed as pulmonary or cardiogenic diseases, leading to unnecessary examinations and invasive medical intervention for patients. Therefore, clinical recognition of hemoglobin variants is very necessary.
In this study, we used H9 HbA1c analyzer thalassemia mode to get 13 kinds of abnormal hemoglobin separation, in which 12 kinds of good separation according to retention time and normal hemoglobin component are obviously different. The Hb New York separation is inefficient, but still can be distinguished by the peak shape change to judge the existence of the abnormal hemoglobin, general performance for the bifurcation of HbA1c and A0 peaks or P4 increased significantly. Therefore, the thalassemia mode of H9 HbA1c analyzer can be used as a screening device for hemoglobin diseases. At the same time, the type of variant can be preliminarily detected and determined according to the retention time and peak shape characteristics.
In general, thalassemia mode is longer duration and therefore better capable of separating variant peaks than HbA1c mode. Simon Degandt and others’ studies showed that Bio‐Rad D‐100, Menarini HA 8180T, Sebia Capillarys2 Flex Piercing, Tosoh HLC‐723 G8 analyzer can identify four kinds of hemoglobin Hb C, D, E, and S common variant hemoglobin. However, Tosoh HLC‐723 G8 analyzer could not detect some rare hemoglobin variants such as Hb J‐Baltimore in HbA1c mode. 4 Ji et al showed that heterozygous Hb E or homozygous Hb E might affect the detection of HbA1c by HPLC depending upon the specific assay machine. 5 Therefore, in the detection of HbA1c, the recognition of hemoglobin variants is very necessary. It can help the laboratory staff to comprehensively evaluate the effect of hemoglobin variants on the results of HbA1c, and other testing items can be used to evaluate the blood glucose level of patients if necessary.
In many countries, hemoglobin diseases are generally detected by routine neonatal tests such as thalassemia screening. The detection methods of hemoglobin variants in the laboratory can be divided into four categories. The first is the physical method, which can distinguish hemoglobin A, S, C, F, A2, etc., according to the migration rate of abnormal hemoglobin. Electrophoresis and chromatography are commonly used. Secondly, it is oxygenated hemoglobin under a given pressure curve method; the percentage of oxygenated hemoglobin and hemoglobin variant with increased oxygen affinity saturated state in oxygen tension is low, causing oxygenation curve shift to the left, on the contrary, with a low oxygen affinity of hemoglobin variant oxygenation curve moves to the right. This method is difficult to perform and most laboratories do not use it. The third is hemoglobin stability detection; determination of hemoglobin in mechanical stirring, heating, isopropyl alcohol solution, zinc acetate solution, and other conditions of denaturation tendency can also be stained to detect Heinz body after observing abnormal accumulation of globin. 6 , 7 , 8 Fourth, special detection methods, including mass spectrometry and gene sequencing, are used to confirm the presence of hemoglobin variants. 9 , 10 In addition, some laboratories use HPLC for HbA1c detection, so the variant pattern of HbA1c detection can also provide more evidence for the screening of hemoglobin diseases.
Li R, Tang H, Kan L, et al. Evaluation on the separated effect of 13 hemoglobin variants by a new automatic Hba1c analyzer. J Clin Lab Anal. 2020;34:e23446 10.1002/jcla.23446
Contributor Information
Rui Li, Email: 18823211695@139.com.
Xiuming Zhang, Email: zxm0760@163.com.
REFERENCES
- 1. Thom CS, Dickson CF, Gell DA, Weiss MJ Hemoglobin variants: biochemical properties and clinical correlates. Cold Spring Harb Perspect Med. 2013;3:a011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ. 2008;86:480‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ohba Y, Miyaji T, Murakami M, et al. Hb Himeji or beta 140 (H18) Ala––Asp. A slightly unstable hemoglobin with increased beta N‐terminal glycation. Hemoglobin. 1986;10:109‐125. [DOI] [PubMed] [Google Scholar]
- 4. Degandt S, Coens R, Cauwelier B, et al. Evaluation of four hemoglobin separation analyzers for hemoglobinopathy diagnosis. J Clin Lab Anal. 2018;32(1):e22224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ji L, Yu J, Zhou Y, et al. Erroneous HbA1c measurements in the presence of beta‐thalassemia and common Chinese hemoglobin variants. Clin Chem Lab Med. 2015;53:1451‐1458. [DOI] [PubMed] [Google Scholar]
- 6. Asakura T, Adachi K, Shapiro M, et al. Mechanical precipitation of hemoglobin koln. Biochem Biophys Acta. 1975;412:197‐201. [DOI] [PubMed] [Google Scholar]
- 7. Bender JW, Adachi K, Asakura T. Precipitation of oxyhemoglobins A and S by isopropanol. Hemoglobin. 1981;5:463‐474. [DOI] [PubMed] [Google Scholar]
- 8. Carrell RW, Kay R. A simple method for the detection of unstable haemoglobins. Br J Haematol. 1972;23:615‐619. [DOI] [PubMed] [Google Scholar]
- 9. Wajcman H, Moradkhani K. Abnormal haemoglobins: detection & characterization. Ind J Med Res. 2011;134:538‐546. [PMC free article] [PubMed] [Google Scholar]
- 10. Wajcman H, Préhu C, Bardakdjian‐Michau J, et al. Abnormal hemoglobins: laboratory methods. Hemoglobin. 2001;25:169‐181. [DOI] [PubMed] [Google Scholar]


