Abstract
Background
Abnormal microRNAs (miRNAs) expression is closely related to the development and poor prognosis of pancreatic ductal adenocarcinoma (PDAC). We aimed to elucidate the invasive mechanism and clinical significance of miR‐10b in PDAC.
Methods
The RNA sequence data of pancreatic cancer were extracted from the TCGA database. R packages were performed to analyze the differential expression of RNAs. TargetScan, picTar, and miRanda were used to predict the target gene of miRNA. The expression level of the selected candidate was tested by western blot and RT‐PCR in PDAC cells and tissues. Scrape and Transwell assays were determined the effect of candidate molecules on cell migration and invasion. The gain of function and loss of function was achieved by co‐culture with mimics and vector. Luciferase reporters were generated based on the psiCHECK2 vector. The relative luciferase activity was measured with the Dual‐Luciferase Reporter Assay System and Infinate M200 PRO microplate reader.
Results
Based on the TCGA data and bioinformatics analysis, we obtained seven differentially expressed miRNAs. Both TCGA data and our center clinical date indicated that miR‐10b was contributed to the poor survival of PDAC. Based on the target gene prediction database, we found that E2F7 was a target mRNA of miR‐10b. In subsequent experiments in molecular biology, miR‐10b expression was downregulated in PDAC cells and tissues, while E2F7 was upregulated. Scrape and Transwell assay indicated that miR‐10b could inhibit the invasion and migration of PDAC. MiR‐10b was confirmed to be by the E2F7 targeting site by dual‐luciferase report. Moreover, rescue experiments prove that miR‐10b could inhibit the invasion and migration of PDAC cells by regulating E2F7 expression.
Conclusion
Our results suggest that miR‐10b could inhibit the progression of PDAC by regulating E2F7 expression and acts as an independent prognostic risk factor for PDAC.
Keywords: E2F7, invasion and metastasis, miR‐10b, pancreatic ductal adenocarcinoma, prognosis
Acquired the miRNA sequencing dataset and corresponding clinical records of PDAC from TCGA. Differentially expressed miRNA analysis and survival analysis indicated miR‐10b was PDAC progression associated miRNA. And E2F7 was a potential target of miR‐10b. And miR‐10b may inhibit PDAC cell migration and invasion by targeting E2F7.
1. INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is a highly invasive malignancy of the digestive system with an overall 5‐year survival rate of no more than 5%. 1 The elderly are susceptible to PDAC, and the incidence of PDAC increases with age. In the United States, the PDAC diagnosis rate for people under 60 is 13%. 2 The early diagnosis rate of PDAC is low, and it prone to invasion and metastasis. 3 At present, only 20% of patients are resectable PDAC, and the recurrence rate is 80%. 4 However, so far, the mechanism of PDAC invasion and metastasis is still not precise. It needs a series of studies in this field to provide potential markers.
With the completion of the Human Genome Project and the parallel development of Next Generation Sequencing (NGS), Human transcriptional analysis has found that over 98% of genes do not encode proteins but are non‐coding RNAs (ncRNAs). There is evidence that ncRNAs play a critical role in regulating the expression of oncogenes or suppressor genes. 5 MicroRNA (miRNA) is a common ncRNA with a length of about 19‐25 nucleotides, which regulates cell proliferation, migration, and other phenotypes by modifying target genes. 6 MiRNAs can regulate the biological function of target genes by binding to 3′‐untranslated regions (3′‐UTR) to inhibit translation or regulate degradation. 7 Wang et al reported that miR‐30a could modulate gemcitabine chemoresistance of PDAC through the SNAI1/IRS1/AKT pathway. 8 Hu et al revealed that, as an onco‐miRNA, miR‐361‐3p could cooperate with DUSP2 and ERK to participate in epithelial‐to‐mesenchymal transition (EMT) of PDAC. 9 Besides, exosomal miRNAs can be used as a tool for intercellular information communication, affecting the malignant phenotype of PDAC, and as a potential marker for molecular diagnosis. 10
To avoid data bias and heterogeneity, we obtained a normal and tumor sample of PDAC from The Cancer Genome Atlas (TCGA) database. The clinical data of the patients in our center were used for additional verification. We found that miR‐10b was low expressed in PDAC and confirmed the role of miR‐10b in PDAC prognosis. According to the sequence information of miR‐10b, we speculated that E2F Transcription Factor 7 (E2F7) might be the target gene of miR‐10b. E2F transcription factor family includes eight members (E2F1‐E2F8), E2F1‐E2F3 play roles in transcriptional activation. The functions of E2F4‐E2F8 are not the same. Moreover, we demonstrated miR‐10b might modulate the invasion of PDAC via E2F7.
2. MATERIALS AND METHODS
2.1. Data download and processing
The miRNA sequencing dataset and corresponding clinical records of PDAC were downloaded from TCGA (TCGA‐PAAD, https://portal.gdc.cancer.gov/). MiRNA sequence data were obtained from Illumina HiSeq_miRNASeq sequencing platforms. R language was used to analyze the miRNA data.
The RNA expression profiles raw data were downloaded by "RTCGA.miRNASeq" package in the R platform. A whole of 183 samples includes 178 PDAC tissues and five normal tissue samples. The survival data were obtained by "RTCGA. Clinical". Package "survival" was used for survival analysis.
2.2. Screening for differentially expressed miRNAs
Package "edgeR," "limma," "deseq2" were used to identify the differentially expressed miRNA (DEMis). The intersection of the three data was taken as the "real" difference expressed DEMis. P < .05 and |logFC| ≥ 1 were set as the cutoff criteria. “Ggplot2” performed data visualization.
2.3. Tissue samples and cell culture
The tissue samples and clinical data of 180 patients undergoing radical surgery were collected in Shengjing Hospital of China medical university from 2012 to 2015. Patients signed informed consent before surgery. And we recorded the disease‐free survival (DFS) and overall survival (OS) time. The study was permitted by the Ethics Committee, Shengjing hospital.
HPDE6‐C7 (human normal pancreatic epithelial cell), ASPC‐1, T3M4, BxPc‐3, Panc‐1 and Miapaca‐2 (human pancreatic cancer cell lines) were obtained from China Medical University. Cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS; Invitrogen), 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen) at 37°C under 5% CO2 and 1% O2. All experiments were repeated three times independently.
2.4. Real‐time reverse transcription‐polymerase chain reaction (Real‐Time PCR)
Total RNA was isolated with TRIzol (Invitrogen) cell separation reagent according to the manufacturer's instructions from cells and tissue. Promega cDNA core kit (Promega) was used to generate complementary DNA from 500 ng of total RNA. SYBR Master Mixture (Takara Bio, Inc) was used to perform to real‐time PCR (LightCycler 480; Roche AG). Each sample was analyzed three times. U6 worked as a loading control. Fold changes of mRNA expression in different cells were determined by 2−△△CT normalization. Each sample was analyzed in triplicate. The specific primer sequence of miR‐10b is as followed: Sense: 5′‐TACCCTGTAGAACCGAATTTGTG‐3′ and antisense: 5′‐CAGTGCGTGTCGTGGAGT‐3′; the specific primer sequence of E2F7 is as followed: Sense: 5′‐CTTCTACTCTTGGTGCTCTC‐3′ and antisense: 5′‐ GGAACTGGTGACTGATGTAA‐3′. The primer sequence of U6 is as followed: Sense: 5′‐CTTCGGCAGCACATATACT‐3′ and antisense: 5′‐AAAATATGGAACGCTTCACG‐3′.
2.5. Western blot analysis
10%‐15% SDS polyacrylamide gel and PVDF membranes were used to separate and transfer protein (20 μg) from cells. TBS‐Tween buffer (20 mmol/L Tris‐HCl, 5% nonfat milk, 150 mmol/L NaCl, and 0.05% Tween‐20, pH 7.5) was used to block membranes for 1 hour at 21°C after blotting. Then the members were incubated with primary antibodies overnight at 4°C (E2F7, 1:100, Boster Biological Technology and β‐actin, 1:4,000, Santa Cruz). Finally, members were incubated with a secondary antibody (1:5,000, Santa Cruz). β‐actin acted as a control. The gray value of proteins was measured by ImageJ (NIH). Averages of three independent data were presented as the final result.
2.6. Lentivirus vector system, plasmids, and cell transfection
Vector of E2F7 and miR‐10b mimics were obtained from GeneChem. The viruses and Polybrene reagent (Abbott Laboratories) were used to infect cells. GC cells were cultured for 72 hours in medium containing puromycin for cell screening.
2.7. Scrape motility and transwell invasion assays
Scrape motility assay was used to evaluate cell migration. PDAC cells were plated into culture inserts (Ibidi). Incubation after 24 hours, the inserts were removed. Inverted microscope (XDS‐100; Shanghai Caikon Optical Instrument Co., Ltd.) was used to capture the wound monolayers images at 0 and 24 hours post wounding.
Transwell assay was performed to determine cell invasion. Transwell upper chambers coated with gelatin were used to plate PDAC cells. The lower chambers with 600 μL FBS (30%; Costar). Methanol, hematoxylin, and eosin were used to fix and stain cells after incubation for 24 hours (Sigma‐Aldrich). Remove the upper chambers. The cells on the surface of the lower chambers were migrated cells that were counted and captured by a microscope at 100× magnification in five fields. The average cell number of the per field represented the migrated cells.
2.8. Luciferase reporter assay
Luciferase reporters were generated based on the psiCHECK2 vector (Promega). The complete 3′UTR of Homo E2F7 mRNA, which included the predicted miR‐10b binding sites, was PCR amplified and cloned into the psiCHECK2 vector. According to the manufacturer's guidelines, luciferase reporter genes were co‐transfected with miR‐10b mimics and miR‐NC into ASPC‐1 cells using Lipofectamine 2000. Dual‐Luciferase Reporter Assay System (Promega) and Infinate M200 PRO microplate reader (Tecan) were performed to measure the relative luciferase activity.
2.9. Statistical analysis
Statistical analysis was performed by Statistical Package for Social Science (SPSS; IBM) version 23.0. All data are presented as means ± SD (standard deviation). The comparisons of data were conducted by Student's t test when homogeneity of variance was satisfied between groups; otherwise, the Wilcoxon signed‐rank test would be used. Multiple groups were compared using one‐way analysis of variance (ANOVA). The chi‐square test tested the relationship between clinicopathological parameters and miRNA. Drawn the Kaplan‐Meier survival curves with survival data. Log‐rank test performed univariate survival analysis. Cox proportional hazard model evaluated the clinical significance of miRNA P < .05 were considered as statistical significance.
3. RESULTS
3.1. Differentially expressed miRNAs in PDAC
TCGA‐PAAD miRNA sequence dataset includes 178 PDAC samples and five para‐carcinoma tissues. EdgeR, limma, and deseq2 were applied to identify the DEMis (Foldchange ≥ 2, P < .05).
For limma, 24 uDEMis and 16 dDEMis (Figure 1A,B) were identified. For deseq2, 5 uDEMis and 8 dDEMis (Figure S1A,C) were recognized. For edgeR, 3 uDEMis and 18 dDEMis (Figure S1B,D) were identified.
FIGURE 1.
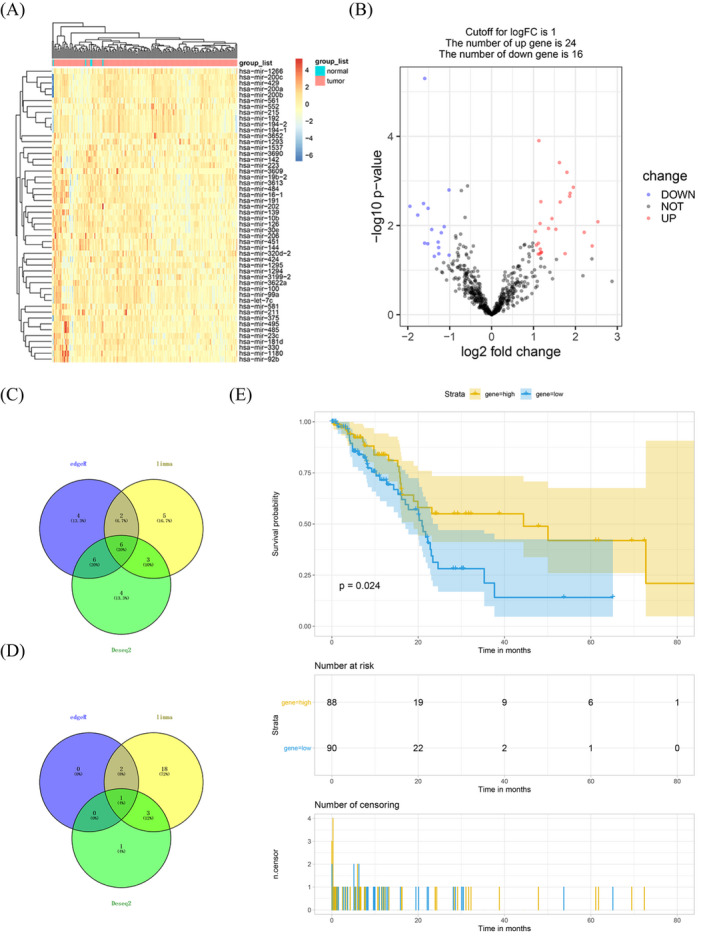
Differentially expressed miRNAs in pancreatic ductal adenocarcinoma. A, Heat maps of the expression levels of the top 50 differentially expressed miRNAs screened by limma. B, Volcano plots of the expression levels of differentially expressed miRNAs by limma. C and D, Venn diagram showed all downregulated and upregulated miRNAs by the three R package. E, Kaplan‐Meier analyzed the correlation between miR‐10b expression levels and the overall survival of patients in The Cancer Genome Atlas
The Venny showed the intersection of dDEMis: miR‐10b, miR‐139, miR‐142, miR‐144, miR‐206, and miR‐451 (Figure 1C) and uDEMis: miR‐100 (Figure 1D). Survival analysis showed that the expression of miR‐10b was firmly related to the prognosis of PDAC (Figure 1E). The appearance of the other six miRNAs was not associated with the prediction of PDAC (Figure S2).
3.2. MiR‐10b was weak expressed and indicated a poor prognosis
To further verify the expression of miR‐10b in PDAC, we analyzed the expression of miR‐10b in PDAC tissues and cells. The RT‐PCR results revealed that the appearance of miR‐10b was weak expressed in both ASPC‐1 and T3M4 compared with it in HPDE6‐C7 (Figure 2A). Consistent results were also obtained in 180 cases of PDAC tissue and paired para‐cancer specimens (Figure 2B). To explore the relationship between miR‐10b and patient clinicopathological data, we grouped patients according to the median of miR‐10b expression. 88 (48.89%) patients were assigned to the high‐expression group, 92 (51.11%) patients were assigned to the low‐expression group. The statistical results showed that the expression of miR‐10b is closely related to Tumor differentiation (χ2 = 15.49, P < .01) and TNM stage (χ2 = 13.13, P < .01). Besides, we also found that there might be a correlation between miR‐10b and gender, but the P‐value is more significant than .05. It is worthwhile to expand the sample size in the future (Table 1).
FIGURE 2.
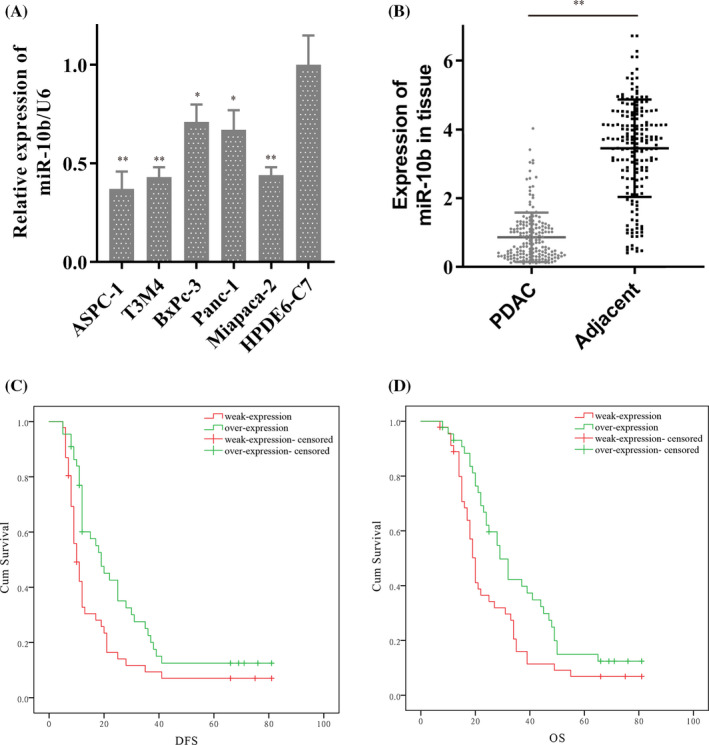
MiR‐10b was low expressed in pancreatic ductal adenocarcinoma (PDAC) and contributed to the poor survival of PDAC. A, MiR‐10b was weak expressed in ASPC‐1, and T3M4 compare to it in HPDE6‐C7 by RT‐PCR. B, MiR‐10b was overexpressed in PDAC tissue. C and D, The survival curves showed the expression of miR‐10b contributed to disease‐free survival (DFS) and overall survival (OS) in 180 patients. Data are shown as mean ± SD, n = 3. The Student's t test assesses statistical data significance. *P < .05, **P < .01
TABLE 1.
MiR‐10b expressions and clinicopathologic characteristics
| Characteristics | miR‐10b | P | χ2 | |
|---|---|---|---|---|
| Weak (%) | High (%) | |||
| 92 | 88 | |||
| Age | ||||
| ≥60 | 42 (45.7) | 46 (52.3) | .37 | 0.53 |
| <60 | 50 (54.3) | 42 (47.7) | ||
| Gender | ||||
| Male | 48 (52.2) | 58 (65.9) | .06 | 3.51 |
| Female | 44 (47.8) | 30 (34.1) | ||
| BMI (kg/m2) | ||||
| ≤25 | 66 (71.7) | 68 (77.3) | .40 | 0.72 |
| >25 | 26 (28.3) | 20 (22.7) | ||
| Diabetes mellitus | ||||
| No | 56 (60.9) | 53 (60.2) | .93 | 0.00 |
| Yes | 36 (39.1) | 35 (39.8) | ||
| Tumor size (cm) | ||||
| ≥2 | 42 (45.7) | 34 (38.6) | .34 | 0.91 |
| <2 | 50 (54.3) | 54 (61.4) | ||
| Location | ||||
| Head | 49 (53.3) | 43 (48.8) | .83 | 0.37 |
| Middle | 16 (17.4) | 16 (18.2) | ||
| Tail | 27 (29.3) | 29 (33.0) | ||
| Tumor differentiation | ||||
| Moderate and high | 12 (13.0) | 34 (38.6) | .00 | 15.49 |
| Poor | 80 (87.0) | 54 (61.4) | ||
| Vessel invasion | ||||
| Yes | 38 (41.3) | 26 (29.5) | .10 | 2.71 |
| No | 54 (58.7) | 62 (70.5) | ||
| Positive resection margin | ||||
| Yes | 14 (15.2) | 14 (15.9) | .90 | 0.16 |
| No | 78 (84.8) | 74 (84.1) | ||
| TNM stage | ||||
| I | 14 (15.2) | 22 (25.0) | .00 | 13.13 |
| II | 18 (19.6) | 32 (36.4) | ||
| III | 58 (63.0) | 32 (36.4) | ||
| IV | 2 (2.2) | 2 (2.3) | ||
In this study, a total of 180 patients were investigated, and 10 were lost to follow‐up. There were 154 deaths, with a mortality rate of 85.56%. The maximum survival time was 81 months, and the minimum was 7 months. Patients with low miR‐10b expression had poor prognosis: DFS (13.47 vs 27.69, P < .01), OS (23.53 vs 36.34, P < .01) (Table 2; Figure 2C,D). Subsequent multivariate analysis showed that miR‐10b, like TNM and tumor differentiation, was an independent prognostic risk factor for PDAC both in DFS and OS. (miR‐10b, DFS: P = .04, HR = 1.70, 95% CI: 1.09‐2.79; OS: P = .04, HR = 1.94, 95% CI: 1.25‐2.98; Table 3).
TABLE 2.
Patients characteristics and univariate analysis (n = 180)
| Characteristics | N (%) | DFS | OS | ||||
|---|---|---|---|---|---|---|---|
| Months | P | F | Months | P | F | ||
| Age | |||||||
| ≥60 | 88 | 20.01 | .20 | 1.65 | 29.74 | .28 | 1.16 |
| <60 | 92 | 24.53 | 33.31 | ||||
| Gender | |||||||
| Male | 106 | 19.50 | .34 | 0.93 | 29.43 | .33 | 0.97 |
| Female | 74 | 25.01 | 33.31 | ||||
| BMI (kg/m2) | |||||||
| ≤25 | 134 | 22.45 | .95 | 0.00 | 32.53 | .98 | 0.00 |
| >25 | 46 | 21.61 | 31.17 | ||||
| Diabetes mellitus | |||||||
| No | 109 | 23.66 | .65 | 0.21 | 32.60 | .48 | 0.50 |
| Yes | 71 | 19.94 | 29.47 | ||||
| Tumor size (cm) | |||||||
| ≥2 | 76 | 15.32 | .00 | 17.84 | 25.40 | .00 | 14.15 |
| <2 | 104 | 27.65 | 36.30 | ||||
| Location | |||||||
| Head | 92 | 21.77 | .82 | 0.41 | 30.69 | .70 | 0.72 |
| Middle | 32 | 21.21 | 31.13 | ||||
| Tail | 56 | 24.11 | 33.35 | ||||
| Tumor differentiation | |||||||
| Moderate and high | 46 | 35.56 | .00 | 17.59 | 43.58 | .00 | 15.59 |
| Poor | 134 | 18.17 | 27.81 | ||||
| Vessel invasion | |||||||
| Yes | 64 | 17.68 | .00 | 14.41 | 23.09 | .03 | 5.08 |
| No | 116 | 27.19 | 35.29 | ||||
| Positive resection margin | |||||||
| Yes | 28 | 11.00 | .00 | 9.00 | 20.36 | .00 | 8.61 |
| No | 152 | 24.57 | 33.73 | ||||
| TNM stage | |||||||
| I | 36 | 44.24 | .00 | 92.34 | 54.77 | .00 | 95.55 |
| II | 50 | 31.13 | 42.27 | ||||
| III | 90 | 9.52 | 12.87 | ||||
| IV | 4 | 5.00 | 8.50 | ||||
| miR‐10b | |||||||
| Weak expression | 92 | 13.47 | .00 | 22.91 | 23.53 | .00 | 18.46 |
| High expression | 88 | 27.69 | 36.34 | ||||
P < .05 were considered as statistical significance.
TABLE 3.
Multivariate analysis of significant prognostic factors for survival in patients with PDAC
| DFS | OS | |||||
|---|---|---|---|---|---|---|
| P | HR | 95% CI | P | HR | 95% CI | |
| Tumor size | .44 | 0.87 | 0.62‐1.23 | .94 | 0.99 | 0.70‐1.40 |
| Tumor differentiation | .00 | 1.98 | 1.26‐3.11 | .04 | 1.95 | 1.24‐3.01 |
| Vessel invasion | .25 | 0.79 | 0.52‐1.18 | .67 | 0.92 | 0.61‐1.37 |
| Positive resection margin | .84 | 1.06 | 0.64‐1.74 | .94 | 1.02 | 0.62‐1.68 |
| TNM stage | .00 | 6.48 | 4.39‐9.57 | .00 | 7.61 | 5.05‐11.48 |
| miR‐10b | .04 | 1.70 | 1.09‐2.79 | .04 | 1.94 | 1.25‐2.98 |
3.3. E2F7 is a potential target of miR‐10b
To further explore the molecular function of miR‐10b, TargetScan, picTar, and miRanda were used to predict the downstream target genes of miR‐10b. The result indicated the E2F7 might be a potential downstream gene of miR‐10b. Then we detected the expression of E2F7 in PDAC cells. Both the RT‐PCR and western blot revealed that E2F7 was overexpressed in PDAC cells than in HPDE6‐C7 (Figure 3A,B). Then we transfected the mimics of miR‐10b into the PDAC cells to upregulate the expression of miR‐10b (Figure 3C). Subsequently, we examined the expression of E2F7 protein in PDAC, which had overexpressed miR‐10b. The western blot indicated with the appearance of miR‐10b increased, the expression of E2F7 decreased sharply (Figure 3D).
FIGURE 3.
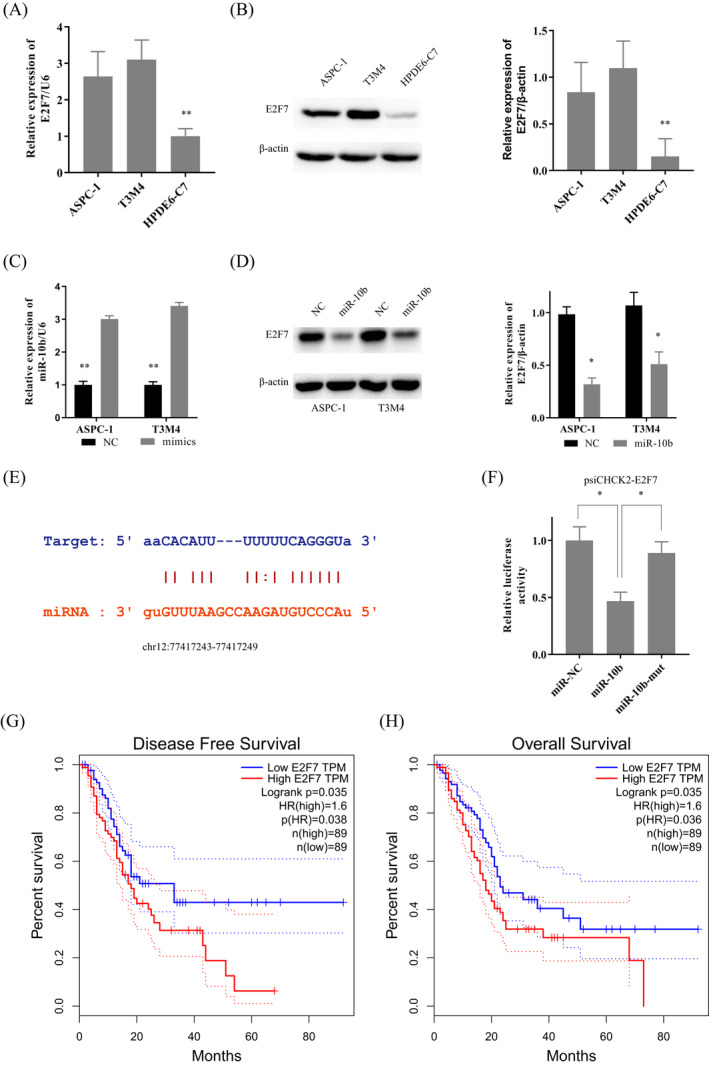
E2F7 was overexpressed in pancreatic ductal adenocarcinoma (PDAC) and is a target of miR‐10b. A and B, E2F7 was overexpressed in ASPC‐1, and T3M4 compare to it in HPDE6‐C7 by RT‐PCR and western‐blot. C, PDAC cells were transfected with the mimics of miR‐145. D, With the transfection of miR‐10b mimics, the protein level of E2F7 was decreased. E, The binding sites between miR‐10b and the E2F7 transcript. F, Luciferase activities were measured in ASPC‐1 cells co‐transfected with luciferase reporter containing E2F7 and the mimics of miR‐10b or mutant. G, H, The overexpression of E2F7 contributed to the poor DFS and OS of GC patients. Data are presented as the relative ratio of renilla luciferase activity and firefly luciferase activity. Data are shown as mean ± SD, n = 3. The Student's t test assesses statistical data significance. *P < .05, **P < .01
According to the predicted binding site (chr12:77417243‐77417249) by TargetScan (Figure 3E), we tried to show a direct correlation between E2F7 and miR‐10b. We then transfected the luciferase reporter plasmid psiCHECK2‐E2F7 into ASPC‐1 cells to test the effects of miR‐10b on E2F7 expression. The overexpressed miR‐10b decreased the luciferase activity of psiCHECK2‐ E2F7, but not miR‐10b‐mut (Figure 3F). Based on this, it could be concluded that miR‐10b could bind to 3′‐UTR of E2F7, thus inhibiting the protein expression of E2F7. TCGA data showed that the overexpression of E2F7 predicted poor DFS and OS in PDAC (Figure 3G,H).
3.4. MiR‐10b affected the invasion of PDAC by targeting E2F7
Transwell and Scrape assay were applied to evaluate the invasion and migration ability of miR‐10b and E2F7. The mimics of miR‐10b was transfected into PDAC cells. With the overexpression of miR‐10b, the fewer cells were passing through the membrane (Figure 4A), and the migration distance of the cells was decreased (Figure 4B). Base on this, we transfected mimics of miR‐10b and the vector of E2F7 into PDAC cells together.
FIGURE 4.
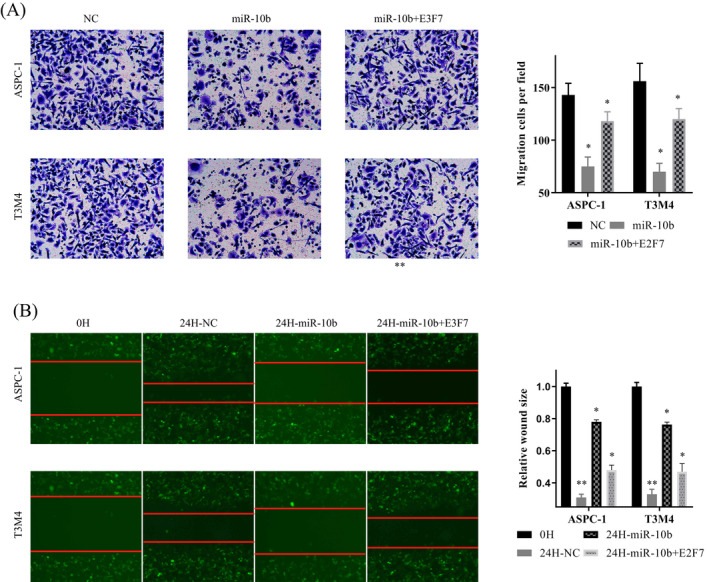
MiR‐10b reduced the invasive and migration capacity of pancreatic ductal adenocarcinoma (PDAC) cells. A, Transwell assays were used to evaluate the involvement of miR‐10b for the invasion in mi‐10b overexpressed and mi‐10b +E2F7 co‐overexpressed PDAC cells. B, Scrape assays were used to evaluate the involvement of miR‐10b for migration in mi‐10b overexpressed and mi‐10b +E2F7 co‐overexpressed PDAC cells. In all figures, 200× magnification was used
With the upregulated of E2F7, the number of cells was increased, and the inhibitory effect of miR‐10b on cell invasion was decreased (Figure 4A). Similarly, we got consistent results in scrape assay: miR‐10b slowed down the cell migration, E2F7 could counteract this effect (Figure 4B). It proved that miR‐10b could inhibit cell invasion and migration by regulated E2F7.
4. DISCUSSION
Pancreatic ductal adenocarcinoma accounts for about 90% of pancreatic malignancies, with a high mortality rate. 11 And the invasion and metastasis of PDAC is the essential cause of unresectable and reduced survival rate. 12 Therefore, it is urgent to elucidate the molecular mechanism of invasion and metastasis of PDAC and provide evidence for novel therapeutic targets. With the development of NGS technology and molecular biology, the crucial role of ncRNA in gene expression regulation was gradually realized. 13 In this study, we downloaded the miRNA transcriptome data from TCGA‐PAAD. We attempted to explore the target molecules closely related to the development of PDAC through a series of bioinformatics analyses.
To accurately obtain the differential expression miRNA, three analysis modes were used to analyze the transcriptional data. Seven differential expression miRNAs were obtained through bioinformatics analysis. Among them, the miR‐10b, which was low expressed in cancer tissues. Both of the clinical data of TCGA and our center were exhibited that miR‐10b was a poor prognostic factor of PDAC. Mir‐10b is a tumor‐associated miRNA in many types of cancer. It could be enriched in exosomes of hepatocellular carcinoma cells and was a potential marker for the diagnosis of early‐stage hepatocellular carcinoma. 14 Hypoxia could induce the expression of miR‐10b‐3p and promote the growth and metastasis of esophageal squamous cell carcinoma by targeting TSGA10. 15 It could also improve the invasion of PDAC through suppressed TIP30 and increased EGF. 16 Besides, miR‐10b could inhibit the expression of HOXD10 to effect the neoadjuvant therapy response of PDAC and contributed to the survival of PDAC. 17 Moreover, a number of studies had suggested that exosomal miR‐10b might be the diagnostic molecule of PDAC. 18 , 19 However, Iorio MV et al reported that miR‐10b was downregulated in primary breast tumors compared with normal breast tissue. 20 Based on these contradictory conclusions, we aimed to explore the functions and significance of miR‐10b in PDAC.
The subsequent cell experiments demonstrated that miR‐10b expression was reduced in tumor cells, which was consistent with the data of TCGA. Moreover, overexpressed miR‐10b could inhibit cell invasion and migration. And this was done by the action of miR‐10b on 3′‐UTR of E2F7. The E2F7 gene is located at chromosome 12q21.2, contains 14 exons, a member of E2F transcription factors. E2F7 plays an essential role in the regulation of the cell cycle. 21 Besides, it also contributed to the invasion of rectal adenocarcinoma cells, 22 , 23 esophageal cancer, 24 papillary thyroid cancer, 25 and lung cancers. 26 , 27 Raman et al established a 5‐gene model (one of which was E2F7) to evaluate the prognosis of PDAC. 28 In our study, we observed that E2F7 was overexpressed in PDAC cells. And the protein expression of E2F7 was decreased significantly when we promoted the expression of miR‐10b. Subsequent luciferase reports identified the direct binding sites between E2F7 and miR‐10b. Not surprisingly, Transwell and scrape assay confirmed that the overexpressed miR‐10b could inhibit the invasion and migration of PDAC. And E2F7 could rescue this anti‐cancer effect. It was proved that the biological role of miR‐10b was realized through E2F7.
5. CONCLUSION
Our study demonstrates that miR‐10b may inhibit PDAC cell migration and invasion by targeting E2F7 and that low expressed of miR‐10b predicts poor prognosis for PDAC. This was the first study to prove the interaction between miR‐10b and E2F7 and to exhibit the role of miR‐10b in PDAC. It is hoped that this study can be helpful to the study of the invasion mechanism of PDAC and provide references for the diagnosis and treatment of PDAC.
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
AUTHOR CONTRIBUTIONS
Xu Cui performed the majority of experiments and analyzed the data and drafted the manuscript; Qi Xiang‐xiu provided critical revision of the manuscript for important intellectual content.
Ethics statement
The study was reviewed and approved by the Faculty of Science Ethics Committee at ShengJing Affiliated Hospital of China Medical University.
Informed consent
Informed consent was obtained from patients before surgery at the ShengJing Hospital of China Medical University.
Supporting information
Fig S1
Fig S2
Xu C, Qi X. MiR‐10b inhibits migration and invasion of pancreatic ductal adenocarcinoma via regulating E2F7. J Clin Lab Anal. 2020;34:e23442 10.1002/jcla.23442
Funding information
Liaoning S&T Project (20180550971).
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7‐34. [DOI] [PubMed] [Google Scholar]
- 2. Higuera O, Ghanem I, Nasimi R, et al. Management of pancreatic cancer in the elderly. World J Gastroenterol. 2016;22(2):764‐775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jurcak N, Zheng L. Signaling in the microenvironment of pancreatic cancer: transmitting along the nerve. Pharmacol Ther. 2019;200:126‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Furukawa K, Shiba H, Hamura R, et al. Prognostic factors in patients with recurrent pancreatic cancer: a multicenter database analysis. Anticancer Res. 2020;40(1):293‐298. [DOI] [PubMed] [Google Scholar]
- 5. Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29(4):452‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li Y, Liu Y, Fan J, et al. Validation and bioinformatic analysis of propofol‐induced differentially expressed microRNAs in primary cultured neural stem cells. Gene. 2018;664:90‐100. [DOI] [PubMed] [Google Scholar]
- 7. Griffiths‐Jones S, Grocock RJ, van Dongen S, et al. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34(90001):D140‐D144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang T, Chen G, Ma X, et al. MiR‐30a regulates cancer cell response to chemotherapy through SNAI1/IRS1/AKT pathway. Cell Death Dis. 2019;10(3):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu J, Li LE, Chen H, et al. MiR‐361‐3p regulates ERK1/2‐induced EMT via DUSP2 mRNA degradation in pancreatic ductal adenocarcinoma. Cell Death Dis. 2018;9(8):807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li Z, Tao Y, Wang X, et al. Tumor‐secreted exosomal miR‐222 promotes tumor progression via regulating P27 expression and re‐localization in pancreatic cancer. Cell Physiol Biochem. 2018;51(2):610‐629. [DOI] [PubMed] [Google Scholar]
- 11. Khorana AA, Mangu PB, Katz MHG. Potentially curable pancreatic cancer: american society of clinical oncology clinical practice guideline update summary. J Oncol Pract. 2017;13(6):388‐391. [DOI] [PubMed] [Google Scholar]
- 12. Versteijne E, Suker M, Groothuis K, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the dutch randomized phase III PREOPANC trial. J Clin Oncol. 2020;38(16):1763‐1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anastasiadou E, Jacob LS, Slack FJ. Non‐coding RNA networks in cancer. Nat Rev Cancer. 2018;18(1):5‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cho HJ, Eun JW, Baek GO, et al. Exosomal microRNA, miR‐10b‐5p, as a potential diagnostic biomarker for early‐stage hepatocellular carcinoma. J Clin Med. 2020;9(1):281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Q, Zhang J, Fu Z, et al. Hypoxia‐induced microRNA‐10b‐3p promotes esophageal squamous cell carcinoma growth and metastasis by targeting TSGA10. Aging. 2019;11(22):10374‐10384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ouyang H, Gore J, Deitz S, et al. microRNA‐10b enhances pancreatic cancer cell invasion by suppressing TIP30 expression and promoting EGF and TGF‐beta actions. Oncogene. 2014;33(38):4664‐4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Preis M, Gardner TB, Gordon SR, et al. MicroRNA‐10b expression correlates with response to neoadjuvant therapy and survival in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2011;17(17):5812‐5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lai X, Wang MU, McElyea SD, et al. A microRNA signature in circulating exosomes is superior to exosomal glypican‐1 levels for diagnosing pancreatic cancer. Cancer Lett. 2017;393:86‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duell EJ, Lujan‐Barroso L, Sala N, et al. Plasma microRNAs as biomarkers of pancreatic cancer risk in a prospective cohort study. Int J Cancer. 2017;141(5):905‐915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iorio MV, Ferracin M, Liu C‐G, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65(16):7065‐7070. [DOI] [PubMed] [Google Scholar]
- 21. Di Stefano L, Jensen MR, Helin K. E2F7, a novel E2F featuring DP‐independent repression of a subset of E2F‐regulated genes. EMBO J. 2003;22(23):6289‐6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wan P, Bai X, Yang C, et al. miR‐129‐5p inhibits proliferation, migration, and invasion in rectal adenocarcinoma cells through targeting E2F7. J Cell Physiol. 2020;235(7‐8):5689‐5701. [DOI] [PubMed] [Google Scholar]
- 23. Liu W, Song Y, Zhang C, et al. The protective role of all‐transretinoic acid (ATRA) against colorectal cancer development is achieved via increasing miR‐3666 expression and decreasing E2F7 expression. Biomed Pharmacother. 2018;104:94‐101. [DOI] [PubMed] [Google Scholar]
- 24. Lu T, Wang R, Cai H, et al. Long non‐coding RNA DLEU2 promotes the progression of esophageal cancer through miR‐30e‐5p/E2F7 axis. Biomed Pharmacother. 2020;123:109650. [DOI] [PubMed] [Google Scholar]
- 25. Guo H, Zhang L. MicroRNA‐30a suppresses papillary thyroid cancer cell proliferation, migration and invasion by directly targeting E2F7. Exp Ther Med. 2019;18(1):209‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liang R, Xiao G, Wang M, et al. SNHG6 functions as a competing endogenous RNA to regulate E2F7 expression by sponging miR‐26a‐5p in lung adenocarcinoma. Biomed Pharmacother. 2018;107:1434‐1446. [DOI] [PubMed] [Google Scholar]
- 27. Wang C, Li S, Xu J, et al. microRNA‐935 is reduced in non‐small cell lung cancer tissue, is linked to poor outcome, and acts on signal transduction mediator E2F7 and the AKT pathway. Br J Biomed Sci. 2019;76(1):17‐23. [DOI] [PubMed] [Google Scholar]
- 28. Raman P, Maddipati R, Lim KH, et al. Pancreatic cancer survival analysis defines a signature that predicts outcome. PLoS ONE. 2018;13(8):e0201751. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2


