Abstract
Background
Oral glucose tolerance test (OGTT) performed at 24‐28 weeks gestation is the current recommended method to the diagnosis of gestational diabetes mellitus (GDM). Many recent studies investigating HbA1c in detecting GDM yield different results. There are no published data on HbA1c in the diagnosis of GDM in Sub‐Saharan countries including Sudan.
Methods
A cross‐sectional study was carried out at the antenatal care of Saad Abuelela Maternity Hospital, Khartoum, Sudan during the period from February to November 2018 to assess the reliability of HbA1c in the diagnosis of GDM. GDM was diagnosed according to the International Association of Diabetes and Pregnancy Study Groups using a 75‐g oral glucose tolerance test.
Results
Three hundred and forty‐eight women were enrolled. The mean (SD) of the age, gravidity, and gestational age of the enrolled women were 27.8 (5.6) years, 2.36 (2.2) and 26.26 (2.43) weeks, respectively. Sixty‐eight women (19.5%) had GDM. A poor productively for HbA1c in diagnosis GDM was shown (AUC = 0.62, 95% CI = 0.55‐0.69). At HbA1c level of 4.150%, the sensitivity and specificity of the diagnosis for GDM were 76.51% and 37.85%, respectively. At HbA1c level of 5.850%, the sensitivity and specificity of the diagnosis for GDM were 13.24% and 91.43%, respectively. While there was no significant (Spearman) correlation between fasting blood glucose and HbA1c, there were significant correlations between HbA1c and OGTT 1 and 2 hours of OGTT.
Conclusion
In this study, HbA1c has a poor reliability, insufficient sensitivity or specificity for use to diagnose GDM.
Keywords: gestational diabetes, glucose tolerance test, hemoglobin A1c, pregnancy, Sudan
1. INTRODUCTION
Gestational diabetes mellitus (GDM) is defined when glucose intolerance resulting in different severity level of hyperglycemia is discovered during gestation/pregnancy. 1 GDM is one of the common public health problems worldwide, and its prevalence is expected to increase dramatically. 2 , 3 GDM is one of the leading causes of adverse maternal and fetal outcomes such as hypertensive disorders of pregnancy, increased cesarean delivery rate, fetal overgrowth, 4 type 2 diabetes, cardiovascular diseases in later life in mothers, and increased risk for macrosomia. 5
Oral glucose tolerance test (OGTT) performed at 24‐28 weeks of gestation is the current recommended test to diagnose GDM. However, it necessitates fasting for 10 hours, waiting for at least two hours, require labor and repeated venipunctures. HA1c is the measurement of glycated hemoglobin which is used routinely as an indicator of blood glucose control in the prior 3 months. It may be the way for earlier identification of women at risk of GDM. Currently, international guidelines “(American Diabetic Association and the International Expert Committee on Diabetes)” recommend the use of HA1c for the diagnosis of diabetes rather than the measurement of fasting or postprandial plasma glucose in non‐pregnant population. 6 Moreover, HbA1c measurement if it is performed in early pregnancy could be of value in diagnosing preexisting diabetes. 7 , 8 , 9 A number of studies have demonstrated elevated levels of HbA1c in women with GDM. 10 , 11 , 12 , 13 , 14 Moreover, elevated levels of HbA1c during pregnancy were associated with adverse neonatal outcome. 4 , 15 Recent studies have reported various levels of reliability/accuracy of HbA1c in diagnosing GDM. While some studies have shown a poor reliability, 10 , 16 , 17 , 18 others have shown a good or excellent reliability of HbA1c in diagnosing GDM. 12 , 14 , 19 , 20 , 21
There was a paucity of published data on HbA1c for the diagnosis of GDM in Sub‐Saharan Africa. A recent meta‐analysis has shown a high prevalence of GDM in Africa 22 ; hence, there is a need to assess HbA1c for the diagnosis of GDM. The aim of the current study was to determine the reliability/accuracy of HbA1c for the diagnosis of GDM among Sudanese women.
2. METHODS
A cross‐sectional study was carried out at the antenatal care of Saad Abuelela Maternity Hospital, Khartoum, Sudan during the period from February to November 2018. After signing an informed consent form, sequential pregnant Sudanese women with singleton pregnancy, who were ≥18 years old, have been in good health (not suffering from any disease), attended the antenatal clinic (between 24 and 28 weeks of gestational age) and consuming a normal diet (without any restriction) were enrolled in this study. Smoker, women with chronic diseases such as severe anemia (hemoglobin < 7 g/dL), hypertension, type 1 or type 2 diabetes, renal disease, thyroid disease and liver disease, or taking chronic medication were excluded. The details of the age, parity, gestational age, education, residence, history of diabetes, history of miscarriage, and history of intrauterine fetal death were collected using a questionnaire. Then women's weight and height were recorded and were used to compute body mass index (BMI) as weight in kg/(height in m)2. A 75‐gram oral glucose tolerance test was performed following overnight fasting (for 10 hours). Two mL sample was collected in fluoride vacutainer in fasting state followed by 75 g oral glucose load and 1 and 2 hours postprandial samples. A sample of 2 mL of blood was collected in ethylenediaminetetraacetic acid vacutainer for assessment of glycosylated hemoglobin. The diagnosis of GDM in this study was based on the International Association of Diabetes and Pregnancy Study Groups (IADPSG) recommendations “fasting blood glucose (FBG) ≥ 92 mg/dL or 1‐hour blood glucose ≥ 180 mg/dL and/or 2‐hour blood glucose ≥ 153 mg/dL, after 75‐g oral glucose load”. 23 Glucose oxidase method was used to measure glucose level following the manufacturer's instructions (Shino‐Test Corp.). An Ichroma machine (Republic of Korea) was used to measure HbA1c levels.
The sample size of 348 women was calculated to obtain the desired sensitivity (90%), and specificity (70%) for the prevalence (15%) of GDM among the screened women. This sample would provide 80% power to detect type I error (ie, P‐value < .05), with the assumption that complete data or enough samples might not be available for 10% of the women. 24
2.1. Statistics
Data were entered in a computer using SPSS (version 20) for Windows for data analysis. Normality of distribution of continuous data was assessed, and mean (standard deviation) or median (interquartile) was used to express the normally distributed and abnormally distributed variables, respectively. t Test and non‐parametric test (Mann‐Whiney U) were used to compare normally distributed and abnormally distributed data between the women with GDM and women with no GDM, respectively. A X 2 test was used to compare the proportions between the two groups. Reliability tests (sensitivity, specificity) and cutoff values for HbA1c were performed by the receiver operating characteristic (ROC) curve and the area under the curve (AUC). 25 The agreement between HbA1c levels and the values of the GTT (1 and 2 hours) were assessed using Bland‐Altman plot. Spearman correlations between OGTT and HbA1c were performed. A two sided. A P‐value < .05 was considered statistically significant.
2.2. Ethics
This study was approved by the Research Ethical Committee of the Department of Obstetrics and Gynecology, Faculty of Medicine, University of Khartoum, Sudan (#2018, 08).
3. RESULTS
The mean (SD) age, gravidity, and gestational age of the enrolled women (348) were 27.8 (5.6) years, 2.36 (2.2), and 26.26 (2.43) weeks, respectively. Fifty‐two (14.9%) of 348 women were rural residents, 101(29.02) were housewives, and 41(11.78%) women had an education level less or equal to secondary level. Eighty (22.98%) and 180 (51.72) women had a history of miscarriage and had a family history of diabetes mellitus, respectively. The mean (SD) hemoglobin level was 11.2 (0.9) g/dL, and 95 (27.3%) women were anemic (hemoglobin < 11.0 g/dL).
The median (interquartile) range of BMI was 26.92 (24.41‐30.80) kg/m2. The median (interquartile) range of fasting blood glucose, I hour OGTT, 2 hours OGTT, and Hb A1c were 70.0 (63.0‐77.0) mg/dL, 133.0 (114.0‐153.0) mg/dL, 118.0 (100.0‐139.0) mg/dL, and 4.6 (3.8‐5.2) %, respectively.
Sixty‐eight women (19.5%) had GDM as defined above. The median (interquartile) range of fasting blood glucose [75.5 (68.0‐91.0) mg/dL vs 68.0 (62.0‐76.0) mg/dL, P < .001], I− hour OGTT [163.0 (145.2‐179.5) mg/dL vs 126.0 (111.0‐142.7) mg/dL, P < .001], 2‐hours OGTT [162.0 (153.2‐176.7) mg/dL vs 111.0 (96.2‐128.7) mg/dL, P < .001] and HbA1c [4.8 (4.2‐5.6)% vs 4.6 (3.5‐5.1)%, P = .001] were significantly higher in women with GDM.
Receiver operating characteristic curve analysis and calculation of the AUC for HbA1c were performed for prediction of GDM. A poor GDM productively for HbA1c was shown (AUC = 0.62, 95% CI = 0.55‐0.69), P = .001). At HbA1c level of 4.150%, the sensitivity, specificity, positive predictive value, and negative predictive value were 76.51%, 37.85%, 23.0%, and 86.90%, respectively, in the diagnosis for GDM. At HbA1c level of 5.850%, the sensitivity, specificity positive predictive value, and negative predictive were 13.24%, 91.43%, 27.2%, and 81.32%, respectively, Table 1, Figure 1.
TABLE 1.
Sensitivity and specificity for all values of HbA1c
| Hemoglobin A1c | Sensitivity % | 95% CI | Specificity % | 95% CI | Likelihood ratio |
|---|---|---|---|---|---|
| 2.150 | 100 | 94.72%‐100.0% | 1.786 | 0.5823%‐4.118% | 1.018 |
| 2.350 | 100 | 94.72%‐100.0% | 2.143 | 0.7904%‐4.605% | 1.022 |
| 2.450 | 100.00 | 94.72%‐100.0% | 2.857 | 1.241%‐5.552% | 1.029 |
| 2.600 | 100.0 | 94.72%‐100.0% | 4.286 | 2.234%‐7.367% | 1.045 |
| 2.750 | 98.53 | 92.08%‐99.96% | 6.071 | 3.576%‐9.543% | 1.049 |
| 2.850 | 97.06 | 89.78%‐99.64% | 10.36 | 7.047%‐14.54% | 1.083 |
| 2.950 | 95.59 | 87.64%‐99.08% | 12.14 | 8.558%‐16.55% | 1.088 |
| 3.050 | 94.12 | 85.62%‐98.37% | 15.36 | 11.34%‐20.12% | 1.112 |
| 3.150 | 94.12 | 85.62%‐98.37% | 16.43 | 12.29%‐21.30% | 1.126 |
| 3.250 | 92.65 | 83.67%‐97.57% | 20.36 | 15.80%‐25.56% | 1.163 |
| 3.350 | 92.65 | 83.67%‐97.57% | 21.43 | 16.77%‐26.70% | 1.179 |
| 3.450 | 92.65 | 83.67%‐97.57% | 22.14 | 17.42%‐27.47% | 1.19 |
| 3.550 | 92.65 | 83.67%‐97.57% | 26.43 | 21.36%‐32.00% | 1.259 |
| 3.650 | 91.18 | 81.78%‐96.69% | 27.14 | 22.02%‐32.75% | 1.251 |
| 3.750 | 91.18 | 81.78%‐96.69% | 28.21 | 23.02%‐33.88% | 1.27 |
| 3.850 | 86.76 | 76.36%‐93.77% | 29.29 | 24.02%‐34.99% | 1.227 |
| 3.950 | 85.29 | 74.61%‐92.72% | 31.43 | 26.03%‐37.22% | 1.244 |
| 4.050 | 77.94 | 66.24%‐87.10% | 34.29 | 28.74%‐40.17% | 1.186 |
| 4.150 | 76.47 | 64.62%‐85.91% | 37.86 | 32.15%‐43.82% | 1.231 |
| 4.250 | 73.53 | 61.43%‐83.50% | 40.00 | 34.22%‐46.00% | 1.225 |
| 4.350 | 70.59 | 58.29%‐81.02% | 42.14 | 36.29%‐48.16% | 1.22 |
| 4.450 | 69.12 | 56.74%‐79.76% | 44.64 | 38.73%‐50.67% | 1.249 |
| 4.510 | 64.71 | 52.17%‐75.92% | 47.5 | 41.53%‐53.53% | 1.232 |
| 4.560 | 63.24 | 50.67%‐74.61% | 48.93 | 42.93%‐54.95% | 1.238 |
| 4.650 | 63.24 | 50.67%‐74.61% | 54.29 | 48.25%‐60.23% | 1.383 |
| 4.750 | 60.29 | 47.70%‐71.96% | 59.64 | 53.64%‐65.44% | 1.494 |
| 4.850 | 50.00 | 37.62%‐62.38% | 62.86 | 56.91%‐68.53% | 1.346 |
| 4.950 | 47.06 | 34.83%‐59.55% | 67.5 | 61.67%‐72.95% | 1.448 |
| 5.050 | 45.59 | 33.45%‐58.12% | 70.71 | 65.01%‐75.98% | 1.557 |
| 5.150 | 42.65 | 30.72%‐55.23% | 76.43 | 71.01%‐81.28% | 1.809 |
| 5.250 | 36.76 | 25.39%‐49.33% | 80.00 | 74.83%‐84.52% | 1.838 |
| 5.350 | 35.29 | 24.08%‐47.83% | 83.57 | 78.70%‐87.71% | 2.148 |
| 5.450 | 32.35 | 21.51%‐44.79% | 85.71 | 81.06%‐89.59% | 2.265 |
| 5.550 | 26.47 | 16.50%‐38.57% | 86.79 | 82.25%‐90.52% | 2.003 |
| 5.650 | 20.59 | 11.74%‐32.12% | 88.21 | 83.85%‐91.75% | 1.747 |
| 5.750 | 17.65 | 9.465%‐28.80% | 90.00 | 85.87%‐93.25% | 1.765 |
| 5.850 | 13.24 | 6.235%‐23.64% | 91.43 | 87.52%‐94.43% | 1.544 |
| 5.950 | 11.76 | 5.218%‐21.87% | 93.57 | 90.03%‐96.15% | 1.83 |
| 6.050 | 10.29 | 4.240%‐20.07% | 93.93 | 90.46%‐96.42% | 1.696 |
FIGURE 1.
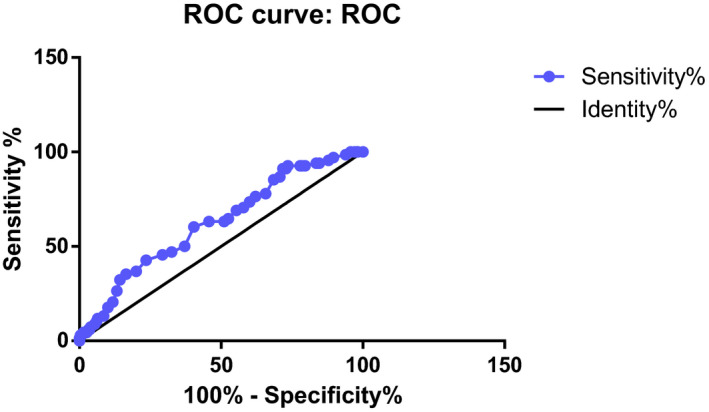
ROC curve analysis and calculation of the AUC for HbA1c
While there was no significant (Spearman) correlation between fasting blood glucose and HbA1c, there were significant correlations between HbA1c and OGTT 1 and 2 hours of OGTT. Likewise, while there was no significant correlation between fasting blood glucose and hemoglobin, there were significant correlations between hemoglobin and OGTT 1 and 2 hours of OGTT. There was no correlation between hemoglobin and HbA1, Table 2.
TABLE 2.
Spearman correlations between OGTT, HbA1c, and hemoglobin
| Variable | Fasting blood glucose |
OGTT 1 h, mg/dL |
OGTT 2 h, md/dL |
HBA1C% | Hemoglobin, gm/dL |
|---|---|---|---|---|---|
| r | r | r | r | r | |
| P | P | P | P | P | |
| Fasting blood glucose | .318 | .265 | .101 | .06 | |
| <.001 | <.001 | .060 | .397 | ||
| OGTT 1 h, mg/dL | .703 | .168 | .198 | ||
| <.001 | .002 | <.001 | |||
| OGTT 2 h, md/dL | .230 | .148 | |||
| <.001 | .006 | ||||
| HBA1C% | −.030 | ||||
| .579 |
Abbreviations: HbA1c, glycosylated hemoglobin; OGTT, oral glucose tolerance test.
Bland‐Altman correlations between OGTT and HbA1c are shown in Figures 2 and 3. The HbA1c distribution by GDM status is shown in Figure 4.
FIGURE 2.
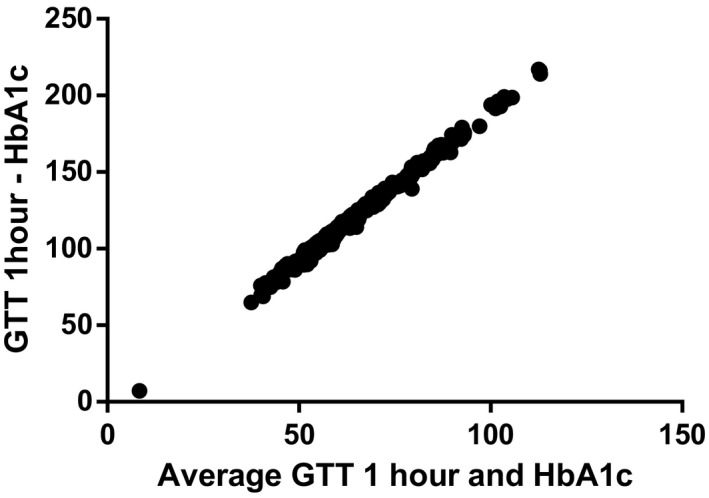
Bland‐Altman correlations between oral glucose tolerance test 1 h and HbA1c
FIGURE 3.
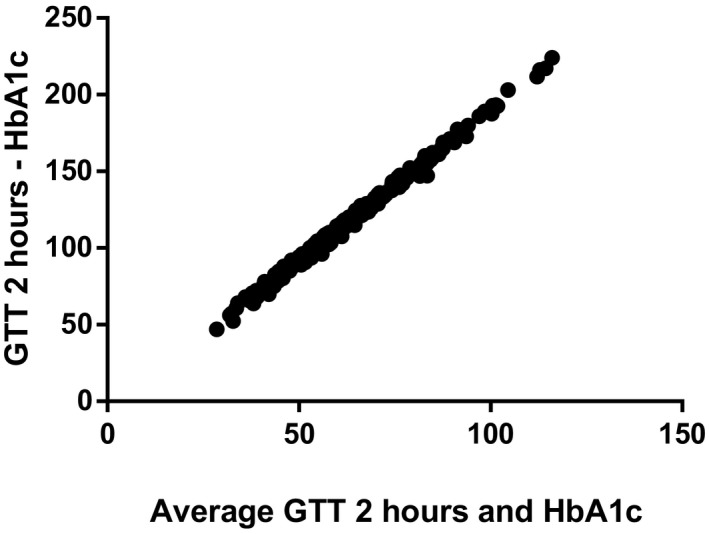
Bland‐Altman correlations between oral glucose tolerance test 2 h and HbA1c
FIGURE 4.
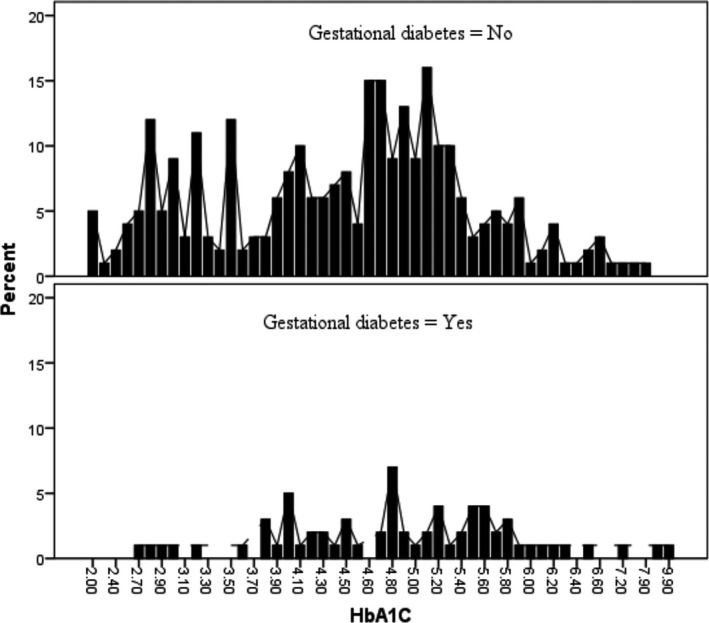
HbA1c distributions by gestational diabetes mellitus status
4. DISCUSSION
In the current study, the median HbA1c level was significantly higher in women with GDM compared with women who had no GDM (4.8% vs 4.6%, P = .001). These findings are in agreement with the previous studies which reported a significantly higher HbA1c values in women with GDM compared with HbA1c values in women without GDM. 10 , 11 , 12 , 13 , 14
The current study has shown a poor reliability of HbA1c for the diagnosis of GDM (AUC = 0.62). This finding was similar to the previous studies findings 10 , 16 , 17 , 18 , 19 which reported that HbA1c values cannot replace OGTT for the diagnosis of GDM. On the other hand, several previous studies have shown a high efficiency with a good/excellent reliability of HbA1c for the diagnosis of GDM in which the AUC values ranged from 0.805 to 0.937. 12 , 14 , 21 Interestingly, in a recent meta‐analysis enrolling 6406 pregnant women in eight studies, a good level of diagnostic accuracy (AUC = 0.825) of HbA1c for the diagnosis of GDM has been reported. 20
In our study, at HbA1c level of 5.850% the sensitivity and specificity for the diagnosis of GDM was 13.24% and 91.43%, respectively. Recently, Patcharaporn et al 13 have reported a 17.1% and 100.0% sensitivity and specificity, respectively, for HbA1c for the diagnosis of GDM at 5.8% cutoff point. A 26.4% sensitivity and 94.9% specificity were reported when HbA1c of 5.8% was used as a cutoff point. 20 A previous study suggested the use of HbA1c at 5.95% as a cutoff point to confirm the diagnosis of GDM in women in India (28.6% and 97.2% for sensitivity and specificity, respectively). 26 Recently, Dubey et al 14 have shown that by using a WHO 75 g OGTT criteria for the diagnosis of GDM, HbA1c at a cutoff ≥ 5.45% has the higher sensitivity (84.3%) and specificity (81.8%). Various levels of sensitivity and specificity (50.3% and 83.7%; 24.7% and 95.5% for the cutoffs of 5.4% and 5.7%, respectively) of HbA1 for the diagnosis GDM have been reported in the recent meta‐analysis. 20 It is worth mentioning that HbA1c level might have different and varied values according to the gestational age. 27 , 28 , 29
In the current study, while there was no correlation of HbA1c and fasting blood, significant positive correlations were found between of HbA1c with 1 and 2 hours. A previous study has reported significant correlations of HbA1c with fasting blood and 2 hours postprandial blood glucose. 14
Our results and the results of the later studies should be compared cautiously because different studies used different diagnostic criteria for GDM. 30 , 31 Moreover, the high rate of anemia (27.3%) in our study might explain the poor reliability of HbA1c for the diagnosis of the GDM. O'Connor et al 26 have suggested that anemia was one of the explanations for the lower HbA1c levels among pregnant women. HbA1c reference ranges may vary according to gestational age, ethnicity, genetic difference, and exposure to different risk factors. 32 , 33 , 34 It is worth mentioning that HbA1c assay during pregnancy was recently reported to be not cost effective as a screening method for GDM. 35
5. CONCLUSION
In this study, HbA1c has a poor reliability, insufficient sensitivity, and specificity to diagnose GDM.
5.1. Limitation of the study
Various other factors that could have effects on the HbA1c and GDM such as or ferritin, iron levels, and hepcidin were not assessed in our study. 36 , 37 We failed to follow‐up these women so as to access the maternal and perinatal outcomes in relation to HbA1c.
ACKNOWLEDGMENTS
The authors wish to thank the enrolled women.
Rayis DA, Ahmed ABA, Sharif ME, ElSouli A, Adam I. Reliability of glycosylated hemoglobin in the diagnosis of gestational diabetes mellitus. J Clin Lab Anal. 2020;34:e23435 10.1002/jcla.23435
REFERENCES
- 1. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop‐Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(Suppl. 2), S251‐S260. [DOI] [PubMed] [Google Scholar]
- 2. Association AD, Bantle JP, Wylie‐Rosett J, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31(Suppl. 1):S61‐S78. [DOI] [PubMed] [Google Scholar]
- 3. Dabelea D, Snell‐Bergeon JK, Hartsfield CL, et al. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care. 2005;28(3):579‐584. [DOI] [PubMed] [Google Scholar]
- 4. HAPO Study Cooperative Research Group , Metzger BE, Lowe LP, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991‐2002. [DOI] [PubMed] [Google Scholar]
- 5. Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep. 2016;16(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. International Expert Committee TIE . International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327‐1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hughes RCE, Moore MP, Gullam JE, Mohamed K, Rowan J. An early pregnancy HbA1c≥5.9% (41mmol/mol) is optimal for detecting diabetes and identifies women at increased risk of adverse pregnancy outcomes. Diabetes Care. 2014;37(11).2953–2959. 10.2337/dc14-1312 [DOI] [PubMed] [Google Scholar]
- 8. Gold AE, Reilly R, Little J, Walker JD. The effect of glycemic control in the pre‐conception period and early pregnancy on birth weight in women with IDDM. Diabetes Care. 1998;21(4):535‐538. [DOI] [PubMed] [Google Scholar]
- 9. Kerssen A, de Valk HW, Visser GHA. Sibling birthweight as a predictor of macrosomia in women with type 1 diabetes. Diabetologia. 2005;48(9):1743‐1748. [DOI] [PubMed] [Google Scholar]
- 10. Renz PB, Cavagnolli G, Weinert LS, Silveiro SP, Camargo JL. HbA1c test as a tool in the diagnosis of gestational diabetes mellitus. PLoS One. 2015;10(8):e0135989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khalafallah A, Phuah E, Al‐Barazan AM, et al. Glycosylated haemoglobin for screening and diagnosis of gestational diabetes mellitus. BMJ Open. 2016;6(4):e011059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kwon SS, Kwon JY, Park YW, Kim YH, Lim JB. HbA1c for diagnosis and prognosis of gestational diabetes mellitus. Diabetes Res Clin Pract. 2015;110(1):38‐43. [DOI] [PubMed] [Google Scholar]
- 13. Siricharoenthai P, Phupong V. Diagnostic accuracy of HbA1c in detecting gestational diabetes mellitus. J Matern Neonatal Med. 2019:1‐4. PMID: 30691324. [DOI] [PubMed] [Google Scholar]
- 14. Dubey D, Kunwar S, Gupta U. Mid‐trimester glycosylated hemoglobin levels (HbA1c) and its correlation with oral glucose tolerance test (World Health Organization 1999). J Obstet Gynaecol Res. 2019;45(4):817‐823. [DOI] [PubMed] [Google Scholar]
- 15. Evers IM, De Valk HW, Mol BW, Ter Braak EW, Visser GHA. Macrosomia despite good glycaemic control in Type I diabetic pregnancy; results of a nationwide study in The Netherlands. Diabetologia. 2002;45(11):1484‐1489. [DOI] [PubMed] [Google Scholar]
- 16. Sevket O, Sevket A, Ozel A, Dansuk R, Kelekci S. The use of HbA1c as an aid in the diagnosis of gestational diabetes mellitus. J Obstet Gynaecol (Lahore). 2014;34(8):690‐692. [DOI] [PubMed] [Google Scholar]
- 17. Maesa J‐M, Fernandez‐Riejos P, Gonzalez‐Rodriguez C, Sanchez‐Margalet V. Screening for gestational diabetes mellitus by measuring glycated hemoglobin can reduce the use of the glucose challenge test. Ann Lab Med. 2019;39(6):524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ye M, Liu Y, Cao X, et al. The utility of HbA1c for screening gestational diabetes mellitus and its relationship with adverse pregnancy outcomes. Diabetes Res Clin Pract. 2016;1(114):43‐49. [DOI] [PubMed] [Google Scholar]
- 19. Rajput R, YogeshYadav RM, Nanda S. Utility of HbA 1c for diagnosis of gestational diabetes mellitus. Diabetes Res Clin Pract. 2012;98(1):104‐107. [DOI] [PubMed] [Google Scholar]
- 20. Renz PB, Chume FC, Timm JRT, Pimentel AL, Camargo JL. Diagnostic accuracy of glycated hemoglobin for gestational diabetes mellitus: a systematic review and meta‐analysis. Clin Chem Lab Med. 2019;57(10):1435‐1449. [DOI] [PubMed] [Google Scholar]
- 21. Ryu AJ, Moon HJ, Na JO, et al. The usefulness of the glycosylated hemoglobin level for the diagnosis of gestational diabetes mellitus in the Korean population. Diabetes Metab J. 2015;39(6):507‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mwanri AW, Kinabo J, Ramaiya K, Feskens EJM. Gestational diabetes mellitus in sub‐Saharan Africa: systematic review and metaregression on prevalence and risk factors. Trop Med Int Health. 2015;20(8):983‐1002. [DOI] [PubMed] [Google Scholar]
- 23. International Association of Diabetes and Pregnancy Study Groups Consensus Panel , Metzger BE, Gabbe SG, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bujang MA, Adnan TH. Requirements for minimum sample size for sensitivity and specificity analysis. J Clin Diagn Res. 2016;10(10):YE01‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harper R, Reeves B. Reporting of precision of estimates for diagnostic accuracy: a review. BMJ. 1999;318(7194):1322‐1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O'Connor C, O'Shea PM, Owens LA, et al. Trimester‐specific reference intervals for haemoglobin A1c (HbA1c) in pregnancy. Clin Chem Lab Med. 2011;50(5):905‐909. [DOI] [PubMed] [Google Scholar]
- 27. Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27(5):1200‐1201. [DOI] [PubMed] [Google Scholar]
- 28. Radder JK, van Roosmalen J. HbA1c in healthy, pregnant women. Neth J Med. 2005;63(7):256‐259. [PubMed] [Google Scholar]
- 29. American Diabetes Association AD . Standards of medical care in diabetes–2014. Diabetes Care. 2014;37(Suppl. 1), S14‐S80. [DOI] [PubMed] [Google Scholar]
- 30. Macaulay S, Ngobeni M, Dunger DB, Norris SA. The prevalence of gestational diabetes mellitus amongst black South African women is a public health concern. Diabetes Res Clin Pract. 2018;139:278‐287. [DOI] [PubMed] [Google Scholar]
- 31. WHO . Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization Guideline. Diabetes Res Clin Pract. 2014;103(3):341‐363. [DOI] [PubMed] [Google Scholar]
- 32. Law GR, Gilthorpe MS, Secher AL, et al. Translating HbA1c measurements into estimated average glucose values in pregnant women with diabetes. Diabetologia. 2017;60(4):618‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bleyer AJ, Hire D, Russell GB, et al. Ethnic variation in the correlation between random serum glucose concentration and glycated haemoglobin: Original Article: Metabolism. Diabet Med. 2009;26(2):128‐133. [DOI] [PubMed] [Google Scholar]
- 34. Grimsby JL, Porneala BC, Vassy JL, et al. Race‐ethnic differences in the association of genetic loci with HbA1c levels and mortality in U.S. adults: the third National Health and Nutrition Examination Survey (NHANES III). BMC Med Genet. 2012;27(13).30 10.1186/1471-2350-13-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walker AR, Caughey AB. Positivity thresholds of HbA1c assay as a screening test for diabetes mellitus in the first trimester in high‐risk populations. J Matern Neonatal Med. 2020;9:1‐5. 10.1080/14767058.2020.1716213 [DOI] [PubMed] [Google Scholar]
- 36. Iqbal S, Ekmekcioglu C. Maternal and neonatal outcomes related to iron supplementation or iron status: a summary of meta‐analyses. J Matern Neonatal Med. 2017;1‐13. [DOI] [PubMed] [Google Scholar]
- 37. Fernández‐Cao JC, Aranda N, Ribot B, Tous M, Arija V. Elevated iron status and risk of gestational diabetes mellitus: a systematic review and meta‐analysis. Matern Child Nutr. 2017;13(4):e12400. [DOI] [PMC free article] [PubMed] [Google Scholar]


