Abstract
Introduction
Mesothelin (MSLN) is overexpressed in several tumors including ovarian cancer and is the target of current trials. There is limited and conflicting data on MSLN prognostic impact in ovarian cancer.
Methods
We performed a retrospective study on patients with high-grade serous ovarian cancer, analyzing MSLN expression by immunohistochemistry and examining the correlation of its expression to overall and progression-free survival. Correlations of expression of MSLN, CD8, and macrophage markers in different tumor compartments were also investigated.
Results
Positive MSLN expression was detected in 55.1% of primary tumors and 51.5% of the metastases. MSLN expression was not correlated with survival. We observed a significant positive correlation (r = 0.34, p = 0.01) between MSLN expression in the metastatic site and CD11c expression in total tumor area and perivascular area in the primary tumor.
Conclusion
Our results show that MSLN expression does not correlate with clinical outcome. The impact of the correlation between MSLN and CD11c+ cells on immunotherapy outcome should be further explored.
Electronic Supplementary Material
The online version of this article (10.1007/s12325-020-01520-w) contains supplementary material, which is available to authorized users.
Keywords: Immunohistochemistry, Mesothelin, Ovarian cancer
Key Summary Points
| Why carry out this study? |
| Mesothelin (MSLN) is overexpressed in several tumors including ovarian cancer and is the target of current trials. |
| There is limited and conflicting data on MSLN prognostic impact in ovarian cancer. |
| This study evaluated MSLN expression in patients with high-grade serous ovarian cancer and its association level with clinical parameters. |
| What was learned from the study? |
| Our data showed that MSLN expression did not correlate with clinical outcome (OS or PFS), and there was a positive correlation between MSLN expression in the metastatic site and CD11c expression in total tumor area and perivascular area in the primary tumor. |
| These results confirms that MSLN expression does not correlate with clinical outcome impact. |
| The correlation between MSLN and CD11c+ cells should be further explored. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13014242.
Introduction
Ovarian cancer is the most lethal gynecological malignancy [1] and high-grade serous (HGS) ovarian cancer is the most common among the subtypes. For the vast majority of patients, the disease is diagnosed at an advanced stage (60–70% in stage III–IV), and even though a majority of patients respond to primary platinum-containing chemotherapy regimens, a high percentage of them relapse and ultimately die from the disease. Hence, new therapeutic approaches and development of novel drugs are needed.
Mesothelin (MSLN) is a glycosylphosphatidylinositol (GPI)-anchored membrane glycoprotein. Its exact biological function remains unknown. A soluble form of MSLN (is generated by proteolytic cleavage or alternative splicing) is detectable in the sera of patients with tumors. While MSLN may be non-essential in normal cells, it plays a role in promoting tumor cell proliferation and chemoresistance [2]. MSLN is currently explored in early trials as an antigen for target therapy [3]. In patients with ovarian cancer, conflicting results have been published, showing that MSLN tumor expression correlated negatively [4–6], positively [7], or not [8, 9] with survival. High MSLN expression was shown in one report to correlate with serous epithelial ovarian cancer, but not other histological types such as endometroid, clear cell, or mucinous [4], while others have shown that higher MSLN expression in endometrioid compared to serous type [8].
A soluble form of MSLN (containing the GPI anchor) was suggested to bind to the mannose receptor CD206 [10] and play a role in macrophage polarization. Macrophage status in clinical samples is usually analyzed by detection of the CD68 marker. CD11c and CD80, associated with the M1-like phenotype [11], and CD163 associated with the M2-like phenotype [12] are markers that could provide a more specific detection of macrophage subtypes.
In this study, we analyzed MSLN expression in patients with HGS ovarian cancer, determined the association level of tumor MSLN expression with relevant clinical parameters, and explored the correlation of MSLN expression with the expression of CD8, CD11c, CD80, and CD163 immunomarkers.
Methods
Patients and Methods
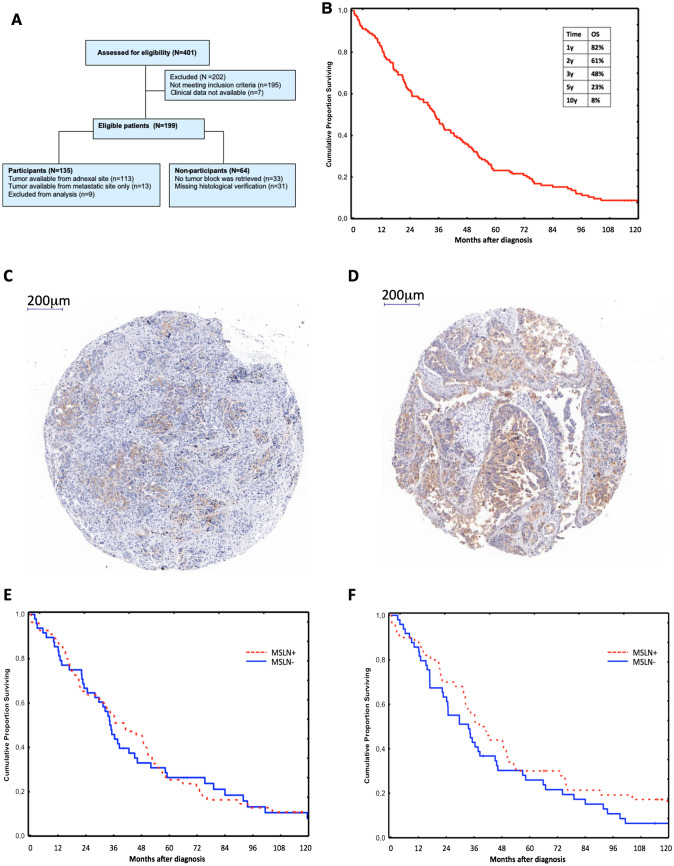
All patients diagnosed with ovarian, fallopian tube, or primary peritoneal carcinoma, or carcinoma of undesignated primary site, in Stockholm County between 2002 and 2006 were identified using the National Swedish Cancer Registry. Of the 401 patients screened, 135 fulfilled the study inclusion criteria (Fig. 1a). The study was approved by the Regional Ethics Committee (Dnr 2012/539-31/1). Platinum free interval (PFI) was defined as the time from the date of last course of platinum to progression, recurrence, or death of any cause (whichever came first). For eligibility criteria, definitions of survival endpoints, tissue microarray (TMA), immunohistochemistry IHC, MSLN expression evaluation, image analysis, and scoring detailed method, see the electronic supplementary material.
Fig. 1.
MSLN detection in patients with HGS ovarian cancer. a Flowchart of the study population. b Overall survival of 135 patients with HGS ovarian cancer. MSLN detection by IHC in TMA, showing c MSLN positive 50%, and d MSLN positive 90%. Kaplan–Meier 10-year overall survival for patients with HGS ovarian cancer based on MSLN expression in e adnexal site (n = 103), and f metastatic site (n = 99)
Statistical Analysis
Statistical differences in overall survival (OS) and in progression-free survival (PFS) were estimated using log rank tests. Correlations between markers and MSLN expression were determined with the Pearson correlation matrix. Calculations were made with the Statistica 13 software (TIBCO Software Inc.).
Results
Patients
Among the 135 patients, adnexal (primary) tumor tissue was available in 113 cases, with paired metastatic tissue in 89 cases. For 13 patients, only tumors from the metastatic site (omentum) were available (Fig. 1a). Clinical data is summarized in Table 1. Median age at diagnosis was 64 years, patients had mostly stage IIIC disease (73%), and most underwent primary debulking surgery (79%). Macroscopic radical surgery was obtained in 28% of the patients. The 5- and 10-year survival rate was 23% and 8%, respectively (Fig. 1b).
Table 1.
Patient characteristics
| Characteristic | Patient cohort N = 126 |
|---|---|
| Median age at diagnosis, years (range) | 64 (36.5–84.2) |
| Diagnosis | |
| Ovarian cancer | 88 (69.8%) |
| Fallopian tube cancer | 13 (10.3%) |
| Peritoneal cancer | 22 (17.5%) |
| Undesignated site | 3 (2.4%) |
| Missing | 0 |
| FIGO stage | |
| IIC | 2 (1.6%) |
| IIIA | 1 (0.8%) |
| IIIB | 6 (4.0%) |
| IIIC | 92 (73.0%) |
| IV | 25 (19.8%) |
| Missing | 0 |
| Type of surgery | |
| Primary surgery | 99 (78.6%) |
| Delayed primary/interval | 18 (14.3%) |
| No surgery | 9 (7.1%) |
| Missing | 0 |
| Macroscopic residual disease after surgery | |
| Absent | 33 (28.2%) |
| Present | 84 (71.8%) |
| Missing | 0 |
| Chemotherapy first line | |
| Platinum based | 116 (92.0%) |
| No platinum | 1 (0.8%) |
| No chemo | 8 (6.3%) |
| Missing | 1 (0.8%) |
| Response at EOT | |
| CR | 69 (59.0%) |
| PR | 26 (22.2%) |
| SD | 3 (2.6%) |
| PD | 16 (13.7%) |
| Missing | 3 (2.6%) |
| Survival | |
| Alive with no evidence of disease | 4 (3.2%) |
| Alive with evidence of disease | 5 (4.0%) |
| Dead from ovarian cancer | 111 (88.1%) |
| Dead from other causes | 3 (2.4%) |
| Lost at follow-up | 3 (2.4%) |
| Median follow-up | 36.4 months (0.4–171.9) |
| Missing | 0 |
| Time from EOT to recurrence/progression | |
| ≥ 6 months (platinum sensitive) | 70 (60.3%) |
| < 6 months (platinum resistant) | 46 (39.7%) |
| Missing | 0 |
FIGO International Federation of Gynecology and Obstetrics, NACT neoadjuvant chemotherapy, EOT end of treatment (including patients that received platinum-based therapy), CR complete response, PR partial response, SD stable disease, PD progressive disease
MSLN Expression in Adnexa and Metastatic Site
MSLN expression was analyzed in primary adnexal site (n = 107) and/or metastatic site (n = 101), examples are shown in Fig. 1c, d. MSLN-positive tumor cells were detected in 55.1% of the primary adnexal tumors and 51.5% of metastases. In 74.5% of the patients where MSLN-positivity was detected in the primary tumor, the paired metastatic site was also MSLN positive. In 78.4% of the patients where the primary tumor was MSLN negative, the paired metastatic site was also MSLN negative. A positive correlation (r = 0.6157) between MSLN expression level in the primary adnexal site and metastatic site was observed (Supplementary Fig. 1). MSLN expression did not correlate with clinicopathological parameters (Supplementary Table 1).
OS and PFS
Positive MSLN expression in the adnexal site showed no significant correlation with OS when compared to negative MSLN (median OS 44 months versus 34 months, log rank p = 0.61, Fig. 1e). Positive MSLN expression in the metastatic site showed a non-significant trend for longer OS when compared to negative MSLN expression (median OS 40 months versus 34 months, log rank p = 0.19, Fig. 1f). No correlation between MSLN expression in adnexal site and PFS was found (log rank p = 0.4) (Supplementary Fig. 2).
MSLN Expression Related to Immunomarkers
We analyzed the correlation between MSLN expression and CD11c, CD80, CD163, and CD8 expression. MSLN expression and CD11c expression were analyzed in adnexal site and in biopsies from the metastatic site, whereas only adnexal tumors were included in the analysis of CD8 and additional macrophage-related markers (CD80 and CD163). MSLN expression in the metastatic site significantly correlated with CD11c expression in the perivascular area (PVA1, r = 0.34, p = 0.011) and total tumor area (r = 0.28, p = 0.043) in the primary adnexal site (Table 2). No other statistically significant correlation was found between MSLN expression and the other immunomarkers (Table 2).
Table 2.
Correlation between MSLN expression and immune markers
| CD11c | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PVA1 | PVA2 | tot. tum | epith | stroma | tot. tum | PVA1 | stroma | epith | ||
| Adnexal site | Metastatic sitea | |||||||||
|
MSLN adn |
r | 0.108 | 0.060 | − 0.008 | 0.056 | 0.058 | 0.074 | 0.036 | − 0.048 | − 0.113 |
| p | 0.434 | 0.662 | 0.954 | 0.686 | 0.676 | 0.54 | 0.762 | 0.659 | 0.302 | |
|
MSLN met |
r | 0.344* | 0.246 | 0.276* | 0.166 | 0.170 | − 0.123 | − 0.051 | 0.008 | 0.046 |
| p | 0.011 | 0.072 | 0.043 | 0.229 | 0.218 | 0.303 | 0.67 | 0.938 | 0.674 | |
| CD80 | CD163 | CD8 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PVA1 | PVA2 | tot. tum | stroma | epith | PVA1 | PVA2 | tot. tum | stroma | epith | epith | stroma | stroma 2–3 | |
|
MSLN adn |
− 0.009 | 0.012 | − 0.131 | − 0.121 | − 0.030 | − 0.080 | − 0.130 | − 0.136 | − 0.109 | 0.008 | 0.160 | 0.050 | 0.100 |
| 0.947 | 0.927 | 0.343 | 0.382 | 0.826 | 0.563 | 0.347 | 0.324 | 0.430 | 0.952 | 0.247 | 0.719 | 0.471 | |
|
MSLN met |
0.188 | 0.103 | 0.006 | − 0.043 | 0.049 | 0.055 | 0.006 | − 0.033 | − 0.002 | 0.112 | 0.095 | 0.078 | 0.099 |
| 0.173 | 0.459 | 0.962 | 0.755 | 0.725 | 0.688 | 0.962 | 0.811 | 0.987 | 0.417 | 0.494 | 0.571 | 0.476 | |
adn adnexal site, met metastatic site, tot. tum total tumor area, epith epithelial, PVA1 perivascular area 1 density (area of 15 µm closest to the CD34-positive region), PVA2 perivascular area 2 density (area of 15 µm PV-A1 surrounding PV-A1 area)
*Correlation with p < 0.05
aThe majority of metastatic site was the omentum
Discussion
MSLN expression in HGS ovarian cancer in our cohort (55.1% in adnexal site) is in line with previous findings [7, 9] of MSLN in HGS ovarian cancer detected by IHC. Our analyses on a selected cohort of only patients with advanced HGS ovarian cancer show that MSLN expression does not correlate with clinical outcome (OS or PFS). While Köbel et al. showed that MSLN expression was not associated with disease-specific survival [9], Yen et al. reported that patients with diffuse MSLN expression (based on staining score) had a longer survival as compared to patients with no or low MSLN expression [7]. Only a limited number of reports have analyzed MSLN expression and survival in patients with ovarian cancer. Okla et al. reported that tumoral MSLN levels (determined by qPCR) did not correlate with survival in patients with epithelial ovarian cancer [8]. Hanaoka et al. showed in patients with epithelial ovarian carcinoma, with no distinction between high or low grade, that high MSLN expression (determined by IHC) associated with shorter PFS and OS [4]. Cheng et al. analyzed MSLN expression in patients with mixed types of epithelial ovarian carcinoma and showed that the OS of patients with high MSLN expression (determined by RT-PCR) was shorter as compared to patients with low MSLN expression [5]. Notably, when MSLN expression was analyzed in relation to OS, solely in the subgroup of patients with HGS, no statistical difference was observed (p = 0.055) between patients with high and low expression. This report also showed that MSLN expression was higher in chemoresistant patients as compared to chemosensitive patients. In our cohort, platinum resistance after 6 (p = 0.66) or 12 (p = 0.68) months was not correlated with MSLN expression in the adnexal site (Supplementary Fig. 3). Finally, to the best of our knowledge only one report, that by Yildiz et al., revealed a positive association between high MSLN expression by IHC and poor prognosis in patients with advanced serous ovarian cancer [6].
The engagement of soluble MSLN (via GPI anchor) to CD206 may impact macrophages polarization [10]. Increased tumor MSLN expression may correlate with increased soluble forms of MSLN (as shown previously [8]) which in turn could bind to macrophages and impact their differentiation. Our data revealed a positive correlation between MSLN expression in the metastatic site and CD11c in the primary site, in total tumor area and perivascular subcompartment. Further mechanistic studies are warranted to elucidate the biological mechanism underlying this association, and the potential impact of MSLN on immune cells. However, these results might suggest a possible role of MSLN-positive cells in promoting the differentiation of macrophages towards a M1 phenotype or, alternatively, imply a stimulating activity of M1-like macrophages towards MSLN expression on tumor cells.
Our study has some limitations. It would be of interest to quantify MSLN in patients with ovarian cancer other than HGS and assess correlation or lack of with clinical parameters. We could not measure soluble MSLN and assess whether there is a correlation between soluble MSLN levels, MSLN expression (detected by IHC), and clinical outcome.
Conclusion
This study confirms that in patients with HGS ovarian cancer, MSLN expression does not predict clinical outcome. Our data show a correlation between MSLN expression and the presence of a CD11c-positive immune infiltrate that needs to be further analyzed and explored for its possible impact on the outcome of immune-related therapies.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the participants of the study.
Funding
Isabelle Magalhaes was supported by Cancerfonden (19 0002 FE) and Clas Groschinskys Minnesfond (M 18224), Sara Corvigno was supported by SSGO-ROCHE, Mats Remberger was supported by the Swedish Research Council (2017-00355), and Jonas Mattsson was supported by Cancerfonden (19 0359 Pj 01 H9, Radiumhemmet (181201), and the Mix private donation. The journal’s Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Isabelle Magalhaes, Daria Glaessgen, Jonas Mattsson and Hanna Dahlstrand conceived and designed the study. Josefin Fernebro, Daria Glaessgen, Sara Corvigno and Hanna Dahlstrand provided materials and patient data. Isabelle Magalhaes, Josefin Fernebro, Sulaf Abd Own, Sara Corvigno, Mats Remberger and Hanna Dahlstrand did the data analysis and interpretation. Isabelle Magalhaes, Josefin Fernebro, Sara Corvigno, Jonas Mattsson and Hanna Dahlstrand wrote the paper, and all authors approved the final version of the paper.
Disclosures
None of the authors (Isabelle Magalhaes, Josefin Fernebro, Sulaf Abd Own, Daria Glaessgen, Sara Corvigno, Mats Remberger, Jonas Mattsson and Hanna Dahlstrand) have anything to disclose.
Compliance with Ethics Guidelines
The study was approved by the Regional Ethics Committee (Dnr 2012/539-31/1).
Data Availability
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Footnotes
Isabelle Magalhaes and Josefin Fernebro contributed equally to this work as joint first authors.
Jonas Mattsson and Hanna Dahlstrand also contributed equally to this work.
References
- 1.Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.Hilliard TS. The impact of mesothelin in the ovarian cancer tumor microenvironment. Cancers (Basel) 2018 doi: 10.3390/cancers10090277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lv J, Li P. Mesothelin as a biomarker for targeted therapy. Biomark Res. 2019;7:18. doi: 10.1186/s40364-019-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanaoka T, Hasegawa K, Kato T, et al. Correlation between tumor mesothelin expression and serum mesothelin in patients with epithelial ovarian carcinoma: a potential noninvasive biomarker for mesothelin-targeted therapy. Mol Diagn Ther. 2017;21(2):187–198. doi: 10.1007/s40291-017-0255-2. [DOI] [PubMed] [Google Scholar]
- 5.Cheng WF, Huang CY, Chang MC, et al. High mesothelin correlates with chemoresistance and poor survival in epithelial ovarian carcinoma. Br J Cancer. 2009;100(7):1144–1153. doi: 10.1038/sj.bjc.6604964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yildiz Y, Kabadayi G, Yigit S, et al. High expression of mesothelin in advanced serous ovarian cancer is associated with poor prognosis. J BUON. 2019;24(4):1549–1554. [PubMed] [Google Scholar]
- 7.Yen MJ, Hsu CY, Mao TL, et al. Diffuse mesothelin expression correlates with prolonged patient survival in ovarian serous carcinoma. Clin Cancer Res. 2006;12(3 Pt 1):827–831. doi: 10.1158/1078-0432.CCR-05-1397. [DOI] [PubMed] [Google Scholar]
- 8.Okla K, Surowka J, Fraszczak K, et al. Assessment of the clinicopathological relevance of mesothelin level in plasma, peritoneal fluid, and tumor tissue of epithelial ovarian cancer patients. Tumour Biol. 2018;40(10):1010428318804937. doi: 10.1177/1010428318804937. [DOI] [PubMed] [Google Scholar]
- 9.Kobel M, Kalloger SE, Boyd N, et al. Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med. 2008;5(12):e232. doi: 10.1371/journal.pmed.0050232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dangaj DAK, Mookerjee A, Zhao A, et al. Mannose receptor (MR) engagement by mesothelin GPI anchor polarizes tumor-associated macrophages and is blocked by anti-MR human recombinant antibody. PLoS One. 2011 doi: 10.1371/journal.pone.0028386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertani FR, Mozetic P, Fioramonti M, et al. Classification of M1/M2-polarized human macrophages by label-free hyperspectral reflectance confocal microscopy and multivariate analysis. Sci Rep. 2017;7(1):8965. doi: 10.1038/s41598-017-08121-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.