Abstract
The aim of this study was to determine the effects of live probiotics Lactobacillus reuteri (LR) and Clostridium butyricum (CB) on the expression of genes of innate immune system in broiler chick ileum and cecum. Chicks were administered 500 µl water with or without LR or CB, daily from day 1 to 6 after hatching. The ileum and cecum were collected on day 7 for analysis of gene expression of Toll-like receptors (TLRs), pro- and anti-inflammatory cytokines, and antimicrobial peptides (AMPs) using real-time PCR. The expression of TLR2-1 was upregulated by CB in the ileum and that of TLR5 was upregulated by both LR and CB. Expression of IL-1β and TGFβ2 in the ileum and of TGFβ3 and TGFβ4 in the cecum was upregulated by both LR and CB. The gene expressions of avian β-defensin (AvBD) 1 and cathelicidin (CATH) 3 were upregulated by CB and that of AvBD4 was upregulated by LR in the cecum. However, the expression of CATH2 in the ileum was downregulated by LR. These results suggest that probiotic LR and CB treatments affect a part of the innate defense system in the ileum and cecum by modulating the expression of innate immune molecules including TLRs, pro- and anti-inflammatory cytokines, and AMPs.
Keywords: antimicrobial peptides, chick intestine, cytokines, innate immunity, probiotics, Toll-like receptor
Introduction
Infection by pathogenic microorganisms in the chick gut may cause health impairments and bacterial contamination of their products. The gut-associated lymphoid tissues and lymphocyte functions undergo development during the first few weeks of life (Bar-shira et al., 2003). The innate immune system and maternal antibodies are important in the protection of chicks from infections by microorganisms before the development of lymphoid tissues.
Toll-like receptors (TLRs) recognize microbe-associated molecular patterns (MAMPs) of bacteria and viruses. Ten TLRs have been identified in chickens. The peptidoglycans and lipoproteins of gram-positive bacteria are recognized by TLR2 that form heterodimers with TLR1 (Keestra et al., 2007). Lipopolysaccharides (LPS) of gram-negative bacteria are recognized by TLR4 (St. Paul et al., 2013). The bacterial flagellin is recognized by TLR5 (St. Paul et al., 2013), and bacterial proteases and heat-stable secretory substances are recognized by TLR15 (de Zoete et al., 2011). The ds- and ss-RNA viruses are recognized by TLR3 and 7, respectively (Brownlie and Allan, 2011). TLR21 recognizes unmethylated CpG-oligodeoxynucleotides (ODN) of bacteria and viruses (Keestra et al., 2010). The expression of TLR 1–5, 7, 15, and 21 in the different intestinal segments has been revealed in chicks (Mackinnon et al., 2009), and the stimulation of TLR ligands modulated the expression of innate immune factors, namely antimicrobial peptides and cytokines in the chick intestine (Terada et al., 2020).
Defensins and cathelicidins involved in innate immunity are antimicrobial peptides (AMPs) that exert a broad spectrum of antimicrobial activities against gram-negative and gram-positive bacteria, enveloped viruses, and fungi (Cuperus et al., 2013). Fourteen avian β-defensins (AvBD1–14) and four cathelicidins (CATH1–3, CATH-1B) have been identified in chickens (Lynn et al., 2004; Van Dijk et al., 2005; Xiao et al., 2006; Achanta et al., 2012). The antimicrobial activities of synthesized peptides of several AMPs against bacteria and fungi have been demonstrated (van Dijk et al., 2012; Yacooub et al., 2015; Lee et al., 2016). We reported that the genes of ten AvBDs (AvBD1–8, 10, and 12) were expressed in chick ileum and cecum, and the expression levels of eight AvBDs declined during embryo development and chick growth (Terada et al., 2018). The expression of four CATHs was also detected in chick proventriculus and cecum (Mohammed et al., 2016). Previous studies have shown the presence of AvBD2 and CATH2 in the mucosal leukocytes (van Dijk et al., 2012; Cuperus et al., 2016; Terada et al., 2018), AvBD8 in the luminal epithelium cells and villi (Rengaraj et al., 2018), and AvBD9 in the enteroendocrine cells in the luminal and crypt epithelium in the embryo and chick intestine (Cuperus et al., 2016). The expression of AvBDs and CATHs in the chick intestine was affected by infection with Salmonella, Campylobacter, and Eimeria in in vivo studies (Hong et al., 2012; Shao et al., 2016; Taha-Abdelaziz et al., 2017). IL-1β, a pro-inflammatory cytokine, may play a role in the regulation of AvBDs because expression of AvBDs was upregulated by IL-1β in the ovarian and oviduct tissues (Abdelsalam et al., 2012; Sonoda et al., 2013). Meanwhile, anti-inflammatory cytokines, such as transforming growth factor (TGF) β may play roles in the maintenance of physiological inflammation in the gut, which is the homeostatic balance between tolerance to microbiota and the reactivity to pathogen invasion (Kogut et al., 2018). Thus, it is assumed that the expression of AMPs in the intestinal mucosa is affected by a luminal microorganism complex together with pro-inflammatory and anti-inflammatory cytokines.
In chicken, mice, and piglets, live probiotics have been suggested to induce dynamic changes in the microbiome, and a factor regulating them may be the short-chain fatty acids in the intestinal contents (Wang et al., 2019; Vemuri et al., 2019; Neijat et al., 2019; Cao et al., 2019). Additionally, butyrate has been reported to enhance not only epithelial cell proliferation with the development of villi (Guilloteau et al., 2010; Ahsan et al., 2016) but also the mucosal barrier by tight junctions (Peng et al., 2009; Guilloteau et al., 2010) and induction of host defense peptide expression including AvBDs and CATHs in the chick intestine (Peng et al., 2009; Sunkara et al., 2011, 2014). Probiotic bacteria, such as Lactobacillus and Clostridium butyricum, may play a role in increasing those short-chain fatty acids in the gut. Nii et al. (2020) suggested that the administration of Lactobacillus reuteri induces the growth of ileum villus and the expression of the tight junction-related molecules in the crop and duodenum of chicks. Zhang et al. (2016) reported that an Escherichia coli challenge induces a decrease in TNF-α and IL-4 concentration in chick jejunal mucosa, whereas C. butyricum suppressed that decrease in TNF-α and IL-4. Thus, the probiotics L. reuteri and C. butyricum may have benefits that enhance the immunodefense and mucosal barrier system in the chick intestine. However, the specific effects of these two probiotics on the innate immune system in the chick intestine remain unknown. This knowledge is necessary to consider a strategy for the development of probiotic products for chicks.
The aim of this study was to determine the effects of these live probiotics on the expression levels of factors involved in the innate immune system in the chick intestine. We examined the effects of live commercial probiotics of L. reuteri and C. butyricum on the gene expression levels of TLRs, pro- and anti-inflammatory cytokines, and AMPs in chick ileum and cecum.
Materials and Methods
Treatment of Birds and Tissue Collection
Fertilized eggs (Chunky broiler) obtained from a local hatchery (Fukuda Breeders Co., Okayama, Japan) were incubated in a humidified incubator at 37.5°C. After hatching, 1-day-old male chicks were divided into three groups: L. reuteri (LR), C. butyricum (CB), and Control (Con) (n=7 in each group). They were maintained in a brooding room with a lighting schedule of 23 h light: 1 h dark for 7 days. They were given a commercial starter diet (Nichiwa Sangyo Co. Ltd., Kobe, Japan) and water ad libitum. Chicks in each group were administered an oral gavage with 500 µl water with or without live probiotic materials daily from day 1 to 6 before used on day 7. The solution (500 µl) given in the LR group contained 2×109 CFU of L. reuteri (10 mg FINELACT, Asahi Calpis Wellness Co., Ltd., Tokyo, Japan) and that in the CB group contained 1.3×107 cells of C. butyricum (Ace Bio Product Co., Ltd., Nagano, Japan). Control group chicks were given only deionized water. On day 7, chicks in all groups were euthanized using carbon dioxide, and their ileum and cecum were collected for examination. This study was approved by the Hiroshima University Animal Research Committee (No. C15–16).
RNA Isolation and cDNA Preparation
Total RNA was extracted using Sepasol RNAI Super (Nacalai Tesque, Inc.), as described by the manufacturer's instructions. The extracted total RNA samples were dissolved in TE buffer (10 mM Tris–HCl, pH 8.0, 1 mM EDTA) and stored at −80°C until use. The RNA samples were treated with RQ1 RNase-free DNase mixture (Promega Co., Madison, WI, USA; 1 µg total RNA, 1× DNase buffer, and 1 UDNase in 10 µl) on a programmable thermal controller (PTC-100; MJ Research, Waltham, MA, USA), programmed at 37°C for 30 min and then at 65°C for 10 min with 1URQ1 DNase Stop Solution (Promega Co.). The concentration of RNA in each sample was measured using Nano Drop Lite (Thermo Fisher Scientific., Waltham, MA, USA). The RNA samples were reverse-transcribed using Rever-Tra Ace (Toyobo Co., Ltd., Osaka, Japan) as per manufacturer's instructions. The reaction mixture (10 µl) comprised 0.5 µg total RNA, 1× reverse transcription buffer (Toyobo Co., Ltd.), 1 µM deoxyribonucleotide triphosphate (dNTP) mixture (Toyobo Co., Ltd.), 5 URNase inhibitor (Toyobo Co. Ltd.), 0.25 µg of oligo (dt) 20 (Toyobo Co., Ltd.), and 50U Rever Tra Ace. The reverse transcription was performed at 42°C for 30 min, followed by heat inactivation at 99°C for 5 min using a programmable thermal controller. Finally, the cDNA samples were stored at −20°C until use.
Real-time PCR
Real-time PCR was performed using the Aria Mix Real-time PCR system (Agilent Technologies Japan. Ltd., Tokyo, Japan). The reaction mixture (10 µl) consisted of 1 µl cDNA, 1×Brilliant III SYBR Green QPCR Mix (Agilent Technologies Japan, Ltd.), 0.25 µM of each primer and water. The primer sequences used in this study are shown in Table 1 (Supplementary data), and two different PCR protocols were used. TLR2-1, 4, 5, and 21 were selected as the receptors recognizing bacterial molecular patterns. IL-1β was examined as a pro-inflammatory cytokine. AvBD1, 2, 4, 6, and 7 were selected because their PCR products were detectable. The primers for CATH2 and 3 were designed against specific sequences of these genes, whereas CATH1/3 was designed against the sequence shared by both CATH1 and CATH3 since the two CATHs shared >90% similarity throughout their sequence (Xiao et al., 2006). CATH-B1 was omitted since negligible PCR products were formed. The first protocol was 50 cycles at 95°C for 5 s, and 58°C (TGFβ2 and 4), 60°C (RPS17, TGFβ3, CATH1/3, 2, 3, TLR2-1, 4, 5, and 21) or 62°C (AvBD2, 4, 6, and 7) for 10 s. The second protocol was 50 cycles at 95°C for 5 s, and 55°C (AvBD1) and 72°C for 10 s each. Real-time PCR data were analyzed using the 2–ΔΔct method to calculate the relative level of gene expression in each sample and were expressed as ratios of the RPS17 housekeeping gene (Livak and Schmittgen, 2001). RNA from the control group was used as a standard sample.
Statistical Analysis
The significance of differences in the real-time PCR data between Con and probiotics treatment (LR and CB) groups was examined using the Kruskal-Wallis test followed by the Steel test (Con was compared with the LR and CB groups). Differences were considered significant when the P-value was <0.05.
Results
Figure 1 shows the effects of probiotic treatments on gene expression levels of TLR2-1, 4, 5, and 21 in the ileum and cecum. In the ileum, the expression levels of TLR2-1 in the CB group and TLR5 in the LR and CB groups were significantly higher than that of the Con group (Fig. 1a, e). The expression of the four TLRs in the LR and CB groups were not significantly different from that of the Con group in the cecum (Fig. 1b, d, f, h).
Fig. 1.
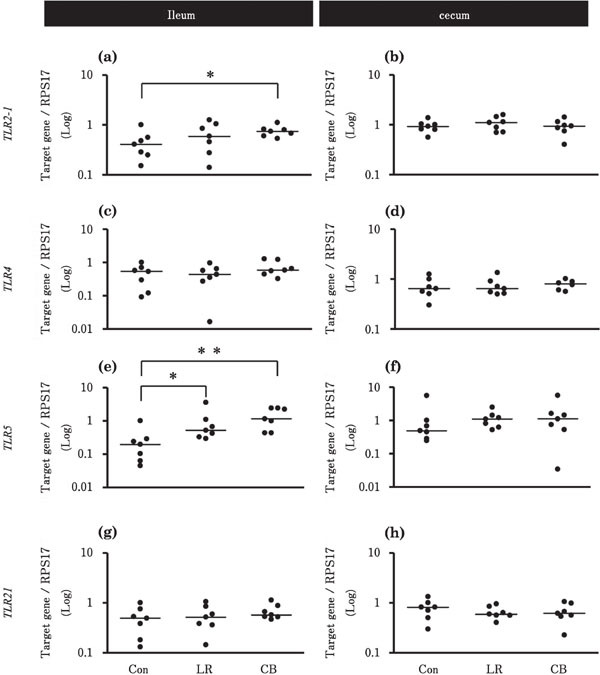
Effects of probiotic treatments on the expression levels of TLRs in the chick ileum and cecum. The values are fold changes in gene expression. Chicks in Con, LR, and CB groups were orally administrated with 500 µl deionized water, 2×109 CFU L. reuteri, and 1.3×107 cells C. butyricum, respectively [n=7 in each group, except for TLR4 data for the cecum in the CB group (n=6). The solid bar represents the median value within each group. Asterisks indicate significant differences between the Con group and each probiotic group, determined by Kruskal-Wallis and Steel tests (* P<0.05, ** P<0.01).
Figure 2 shows the effects of probiotic treatments on the gene expression levels of IL-1β and TGFβ2-4 in the ileum and cecum. In the ileum, the expression levels of IL-1β in the LR and CB groups and that of TGFβ2 in the CB group were higher than that in the Con group (Fig. 2a, c). On the contrary, in the cecum, the expression levels of TGFβ3 in the LR group and that of TGFβ4 in the LR and CB groups were higher than that in the Con group (Fig. 2f, h).
Fig. 2.
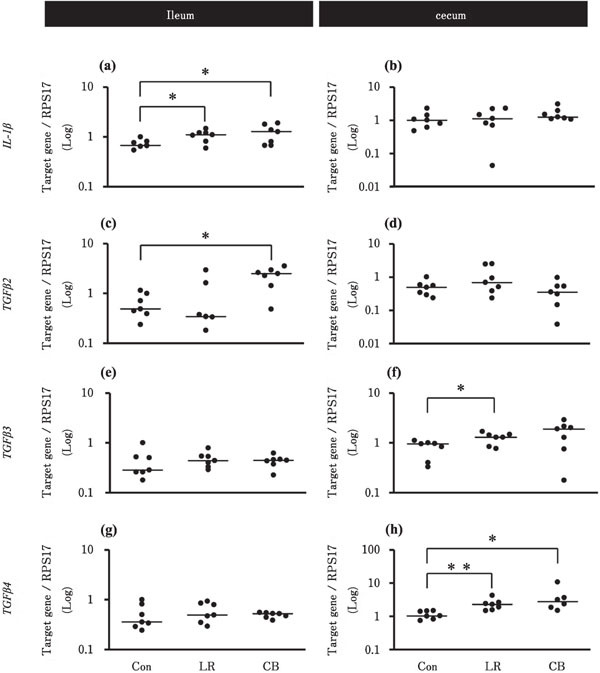
Effects of probiotic treatments on the expression levels of pro- and anti-inflammatory cytokines in the chick ileum and cecum. Chicks in Con, LR, and CB groups were orally administrated with 500 µl deionized water, 2×109 CFU L. reuteri, and 1.3×107 cells C. butyricum, respectively (n=7 in each group). Asterisks indicate significant differences between the Con group and each probiotic group, determined by Kruskal-Wallis and Steel tests (* P<0.05, ** P<0.01). See Fig. 1 for other explanations.
Figure 3 shows the effects of probiotic treatments on the gene expression levels of AvBDs in the ileum and cecum. In the ileum, the expression levels of all the tested AvBDs were not significantly different between Con and probiotics treatment (LR and CB) groups (Fig. 3a, c, e, g, and i). On the contrary, in the cecum, the expression levels of AvBD1 in the CB group and that of AvBD4 in the LR group were significantly higher than that of the Con group (Fig. 3b and f).
Fig. 3.
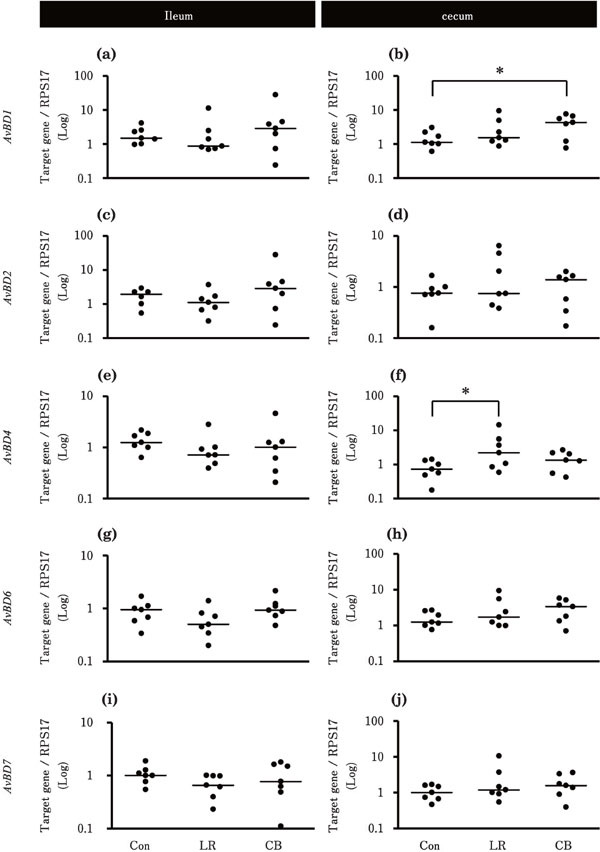
Effects of probiotic treatments on the expression levels of AvBDs in the chick ileum and cecum. Chicks in Con, LR, and CB groups were orally administrated with 500 µl deionized water, 2×109 CFU L. reuteri, and 1.3×107 cells C. butyricum, respectively [n=7 for each group, except for AvBD2 data in the ileum in the Con group (n=6)]. Asterisks indicate significant differences between the Con group and each probiotic group, determined by Kruskal-Wallis and Steel tests (* P<0.05). See Fig. 1 for other explanations.
Figure 4 shows the effects of probiotic treatments on the gene expression of CATHs in the ileum and cecum. In the ileum, the expression levels of CATH2 were significantly lower in the LR group than in the Con group (Fig. 4c). In the cecum, the expression levels of CATH3 were significantly higher in the CB group than that in the Con group (Fig. 4f).
Fig. 4.
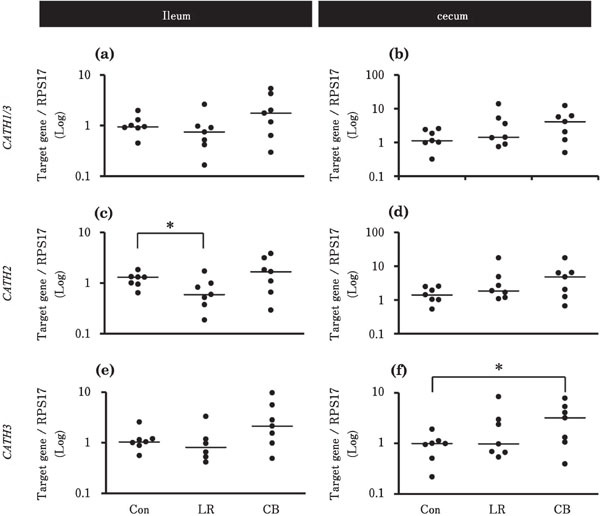
Effects of probiotic treatments on the expression levels of CATHs in the chick ileum and cecum. Chicks in Con, LR, and CB groups were orally administrated with 500 µl deionized water, 2×109 CFU L. reuteri, and 1.3×107 cells C. butyricum, respectively (n=7 in each group). Asterisks indicate significant differences between the Con group and each probiotic group, determined by Kruskal-Wallis and Steel tests (* P<0.05). See Fig. 1 for other explanations.
Discussion
Our study reports that the probiotics LR and CB affect the expression levels of TLRs, cytokines, and AMPs in chick ileum and cecum. The following were the major findings: (1) LR significantly increased TLR5, IL-1β, and CATH2 in the ileum and TGFβ3, TGFβ4, and AvBD4 in the cecum; (2) CB significantly increased TLR2-1, TLR5, IL-1β, and TGFβ2 in the ileum and TGFβ4, AvBD1, and CATH3 in the cecum.
The increase in TLR2-1 and TLR5 transcription may result in enhanced ability to recognize gram-positive bacteria, including probiotic bacteria and bacterial flagellin. Wang et al. (2013) reported that the live probiotic Lactobacillus casei Zhang (LcZ) promotes the transcription of TLR2, whereas heat-killed LcZ increases the transcription of TLR2, 3, 4, and 9 in a murine macrophage cell line. Thus, it is likely that probiotics used in this study modulate the expression of TLRs in the ileum cells as was observed in the mammalian macrophage cell line.
The current results reveal an increase in expression of a pro-inflammatory cytokine (IL-1β) in the ileum and also an increase in anti-inflammatory cytokines (TGFβ2-4) in the ileum and cecum, by LR and CB treatments. We assume that the balanced expression of pro- and anti-inflammatory cytokines were thus maintained in the chicks treated with LR and CB. This would be necessary for regulation of physiological inflammation that is responsible for normal immune functioning of the intestinal mucosa (Crhanova et al., 2011) and maintaining homeostatic balance between tolerance of the microbiota and reactivity to pathogen invasion (Kogut et al., 2018).
Treatment with CB promoted the transcription of AvBD1 and CATH3, and LR treatment enhanced transcription of AvBD4 in the cecum. The pattern molecules in the cell wall of LR and CB may be commonly recognized by TLR2 because both are gram-positive bacteria. However, treatment with LR and CB affected the transcription of different AvBDs and CATHs in the cecum and ileum. Thus, the effects of CB and LR on AMP transcription may be regulated not only through TLRs but also by other factors. Short-chain organic acids produced by them could be one such factor. Organic acids produced by probiotic bacteria are known to lower the luminal pH and inhibit the growth of some pathogens, such as E. coli (Ohland and MacNaughton, 2010). We assume that changes in the luminal microbiome composition may affect the expression of AMPs in the intestine. Furthermore, it was reported that dietary supplementation with butyrate induced AvBD9, AvBD14, and CATHB1 in the jejunum and cecum and reduced the colonization of bacteria following experimental infection with S. enteritidis in chickens. Butyrate also has synergic effects on the induction of AvBD9 by cyclic AMP in chick jejunum explants and macrophages. In humans, it is also reported that butyrate upregulates the expression of cathelicidins LL37 in the luminal epithelium of colonocytes (Schauber et al., 2003). Thus, we suggest that LR and CB modulate the expression of AMPs in the cecum, probably not only through TLR stimulation but also through organic acids produced by them, whereas the effects on AMP expression may be different between LR and CB. Also, in the current study, there were variations in the expression levels of some genes within LR and CB treatment groups; this may be due to the differences in the response of the expression of the immune factors to probiotics among the chicks.
The modulatory effects of LR and CB supplementation on AvBDs and CATHs were found only in the cecum, and probable suppressive effect on CATH2 by LR was observed in the ileum in this study. We reported that supplementation with probiotics in feed (Streptococcus faecalis, Clostridium butyricum, and Bacillus mesentericus) did not affect the AvBD and CATH expression in the chick proventriculus (Mohammed et al., 2016), whereas expression of CATH2 in response to LPS was enhanced in the cecum by probiotic feeding (Mohammed et al., 2016). Treatment of chicks with probiotics (Lactobacillus acidophilus, Bifidobacterium bifidum, and Enterococcus feacalis) suppressed the increase in AvBD and CATH expression induced by Salmonella typhimurium challenge in the cecal tonsil (Akbari et al., 2008). Thus, all our findings suggest that the cecum may be more sensitive in AMP expression to probiotic bacteria than upper segments of the gut. We speculate that the higher sensitivities in the cecum associate with the rich microbiota stock in that segment.
In conclusion, we suggest that probiotic LR and CB treatments affect the expression of innate immune molecules (TLRs, pro- and anti-inflammatory cytokines, and AMPs). The significant increase in the expression of TLRs in the ileum and AMPs, including AvBDs and CATHs, in the cecum by these probiotics, may enhance a part of the innate immunodefense system in these tissues of chicks.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science (No. 17H03904) to YY.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abdelsalam M, Isobe N, Yoshimura Y. Effects of lipopolysaccharide and interleukins on the expression of avian β-defensins in hen ovarian follicular tissue. Poultry Science, 91: 2877-2884. 2012. [DOI] [PubMed] [Google Scholar]
- Achanta M, Sunkara LT, Dai G, Bommineni YR, Jiang W, Zhang G. Tissue expression and developmental regulation of chicken cathelicidin antimicrobial peptides. Journal of Animal Science and Biotechnology, 3: 15-21. 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahsan U, Cengiz Ö, Raza I, Kuter E, Chacher MFA, Iqbal Z, Umar S, Çakir S. Sodium butyrate in chicken nutrition: the dynamics of performance, gut microbiota, gut morphology, and immunity. World's Poultry Science Association, 72: 265-275. 2016. [Google Scholar]
- Akbari MR, Haghighi HR, Chambers JR, Brisbin J, Read LR, Sharif S. Expression of antimicrobial peptides in cecal tonsils of chickens treated with probiotics and infected with Salmonella enterica serovar typhimurium. Clinical and Vaccine Immunology, 15: 1689-1693. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shira E, Sklan D, Friedman A. Establishment of immune competence in the avian GALT during the immediate post-hatch period. Developmental & Comparative Immunology, 27: 147-157. 2003. [DOI] [PubMed] [Google Scholar]
- Brownlie R, Allan B. Avian toll-like receptors. Cell and Tissue Research, 343: 121-130. 2011. [DOI] [PubMed] [Google Scholar]
- Cao G, Tao F, Hu Y, Li Z, Zhang Y, Deng B, Zhan X. Positive effects of a Clostridium butyricum-based compound probiotic on growth performance, immune responses, intestinal morphology, hypothalamic neurotransmitters, and colonic microbiota in weaned piglets. Food & Function, 10: 2926-2934. 2019. [DOI] [PubMed] [Google Scholar]
- Crhanova M, Hradecka H, Faldynova M, Matulova M, Havlickova H, Sisak F, Rychlik I. Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar enteritidis infection. Infection and Immunity, 79: 2755-2763. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curperus T, Coorens M, van Dijk A, Haagsman HP. Avian host defense peptides. Developmental and Comparative Immunology, 41: 352-369. 2013. [DOI] [PubMed] [Google Scholar]
- Cuperus T, van Dijk A, Dwars RM, Haagsman HP. Localization and developmental expression of two chicken host defense peptides: cathelicidin-2 and avian β-defensin 9. Development and Comparative Immunology, 61: 48-59. 2016. [DOI] [PubMed] [Google Scholar]
- de Zoete MR, Bouwman LI, Keestra AM, van Putten JP. Cleavage and activation of a toll-like receptor by microbial proteases. Proceeding of the National Academy Sciences of the United States of America, 108: 4968-4973. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilloteau P, Martin L, Eeckhaut V, Ducatelle R, Zabielski R, Van Immerseel F. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutrition Research Reviews, 23: 366-384. 2010. [DOI] [PubMed] [Google Scholar]
- Hong YH, Song W, Lee SH, Lillehoj HS. Differential gene expression profiles of β-defensins in the crop, intestine, and spleen using a necrotic enteritis model in 2 commercial broiler chicken lines. Poultry Science, 91: 1081-1088. 2012. [DOI] [PubMed] [Google Scholar]
- Keestra AM, de Zoete MR, van Aubel RA, van Putten JP. The central leucine-rich repeat region of chicken TLR16 dictates unique ligand specificity and species-specific interaction with TLR2. Journal of Immunology, 178: 7110-7119. 2007. [DOI] [PubMed] [Google Scholar]
- Keestra AM, de Zoete MR, Bouwman LI, van Putten JP. Chicken TLR21 is an innate CpG DNA receptor distinct from mammalian TLR9. Journal of Immunology, 185: 460-467. 2010. [DOI] [PubMed] [Google Scholar]
- Kogut MH, Genovese KJ, Swaggerty CL, He H, Broom L. Inflammatory phenotypes in the intestine of poultry: not all inflammation is created equal. Poultry Science, 97: 2339-2346. 2018. [DOI] [PubMed] [Google Scholar]
- Lee MO, Jang HJ, Rengaraj D, Yang SY, Han JY, Lamont SJ, Womack JE. Tissue expression and antibacterial activity of host defense peptides in chicken. BMC Veterinary Research, 12: 231 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods, 25: 402-408. 2001. [DOI] [PubMed] [Google Scholar]
- Lynn DJ, Higgs R, Gaines S, Tierney J, James T, Lloyd AT, Fares MA, Mulcahy G, O'Farrelly C. Bioinformatic discovery and initial characterization of nine novel antimicrobial peptide genes in the chicken. Immunogenetics, 56: 170-177. 2004. [DOI] [PubMed] [Google Scholar]
- MacKinnon KM, He H, Nerren JR, Swaggerty CL, Genovese K.J, Kogut MH. Expression profile of toll-like receptors within the gastrointestinal tract of 2-day-old Salmonella enteriditis-infected broiler chickens. Veterinary Microbiology, 137: 313-319. 2009. [DOI] [PubMed] [Google Scholar]
- Mohammed ESI, Isobe N, Yoshimura Y. Effects of probiotics on the expression of cathelicidins in response to stimulation by Salmonella Minnesota lipopolysaccharides in the proventriculus and cecum of broiler chicks. Journal of Poultry Science, 53: 298-304. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neijat M, Habtewold J, Shirley RB, Welsher A, Barton J, Thiery P, Kiariea E. Bacillus subtilis strain DSM 29784 modulates the cecal microbiome, concentration of short-chain fatty acids, and apparent retention of dietary components in shaver white chickens during grower, developer, and laying phases. Applied and Environmental Microbiology, 85: e00402-19. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nii T, Jirapat J, Isobe N, Yoshimura T. Effects of oral administration of Lactobacillus reuteri on mucosal barrier function in the digestive tract of broiler chicks. Journal of Poultry Science, 57: 67-76. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohland CL, Macnaughton WK. Probiotic bacteria and intestinal epithelial barrier function. American journal of physiology. Gastrointestinal and liver physiology, 298: G807-819. 2010. [DOI] [PubMed] [Google Scholar]
- Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate Enhances the Intestinal Barrier by Facilitating Tight Junction Assembly via Activation of AMP-Activated Protein Kinase in Caco-2 Cell Monolayers. Journal of Nutrition, 139: 1619-1625. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengaraj D, Truong AD, Lillehoj HS, Han JH, Hong YH. Expression and regulation of avian beta-defensin 8 protein in immune tissues and cell lines of chickens. Asian-Australasian Journal of Animal Science, 31: 1516-1524. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Wang Z, Tian X, Guo Y, Zhang H. Yeast β-D-glucans induced antimicrobial peptide expressions agonist Salmonella infection in broiler chicken. International Journal of Biological Macromolecules, 85: 573-584. 2016. [DOI] [PubMed] [Google Scholar]
- Sonoda Y, Abdel Mageed AM, Isobe N, Yoshimura Y. Induction of avian β-defensins by CpG oligodeoxynucleotides and proinflammatory cytokines in hen vaginal cells in vitro. Reproduction, 145: 621-631. 2013. [DOI] [PubMed] [Google Scholar]
- St Paul M, Bribin JT, Abdul-Careem MF, Sharif S. Immunostimulatory properties of Toll-like receptor ligands in chickens. Veterinary Immunology and Immunopathology, 152: 191-199. 2013. [DOI] [PubMed] [Google Scholar]
- Schauber J, Svanholm C, Termén S, Iffland K, Menzel T, Scheppach W, Melcher R, Agerberth B, Lührs H, Gudmundsson GH. Expression of the cathelicidin LL-37 is modulated by short chain fatty acids in colonocytes: relevance of signalling pathways. Gut, 52: 735-741. 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkara LT, Achanta M, Schreiber NB, Bommineni YR, Dai G, Jiang W, Lamont S, Lillehoj HS, Beker A, Teeter RG, Zhang G. Butyrate enhances disease resistance of chickens by inducing antimicrobial host defense peptide gene expression. PLOS ONE, 6: e27225 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkara LT, Zeng X, Curtis AR, Zhang G. Cyclic AMP synergizes with butyrate in promoting β-defensin 9 expression in chickens. Molecular Immunology, 57: 171-180. 2014. [DOI] [PubMed] [Google Scholar]
- Taha-Abdelaziz K, Alkie TN, Hodgins DC, Yitbareka A, Shojadoosta B, Sharifa S. Gene expression profiling of chicken cecal tonsils and ileum following oral exposure to soluble and PLGA-encapsulated CpG-ODN, and lysate of Campylobacter jejuni. Veterinary Microbiology, 212: 67-74. 2017. [DOI] [PubMed] [Google Scholar]
- Terada T, Nii T, Isobe N, Yoshimura Y. Changes in the expression of avian β-defensins (AvBDs) and proinflammatory cytokines and localization of AvBD2 in the intestine of broiler embryos and chicks during growth. Journal of Poultry Science, 55: 280-287. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada T, Nii T, Isobe N, Yoshimura Y. Effects of Toll-like receptor ligands on the expression of proinflammatory cytokines and avian β-defensins in cultured chick intestine. Journal of Poultry Science, 57: 210-222. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk A, Veldhuizen EJ, van Asten AJ, Haagsman HP. CMAP27, a novel chicken cathelicidin-like antimicrobial protein. Veterinary Immunology and Immunopathology, 106: 321-327. 2005. [DOI] [PubMed] [Google Scholar]
- van Dijk A, Herrebout M, Tersteeg-Zijderveld MHG, Tjeerdsmavan Bokhoven JLM, Bleumink-Pluym N, Janman AJM, Veldhuizen EJA, Haagsman HP. Compylobacter jejuni is highly susceptible to killing by chicken host defense peptide cathelicidin-2 and suppresses intestinal cathelicidin-2 expression in young broilers. Veterinary Microbiology, 160: 347-354. 2012. [DOI] [PubMed] [Google Scholar]
- Vemuri R, Gundamaraju R, Shinde T, Perera AP, Basheer W, Southam B, Gondalia SV, Karpe AV, Beale DJ, Tristram S, Ahuja KDK, Ball M, Martoni CJ, Eri R. Lactobacillus acidophilus DDS-1 modulates intestinal-specific microbiota, short-chain fatty acid and immunological profiles in aging mice. Nutrients, 11: 1297 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xie J, Wang N, Li Y, Sun X, Zhang Y, Zhang H. Lactobacillus casei Zhang modulate cytokine and Toll-like receptor expression and beneficially regulate poly I:C-induced immune responses in RAW264.7 macrophages. Microbiology and Immunology, 57: 54-62. 2013. [DOI] [PubMed] [Google Scholar]
- Wang YN, Meng XC, Dong YF, Zhao XH, Qian JM, Wang HY, Li JN. Effects of probiotics and prebiotics on intestinal microbiota in mice with acute colitis based on 16S rRNA gene sequencing. Chinese Medical Journal, 5: 1833-1842. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Cai Y, Bommineni YR, Fernando SC, Prakash O, Gilliland SE, Zhang G. Identification and functional characterization of three chicken cathelicidins with potent antimicrobial activity. Journal of Biology and Chemistry, 281: 2858-2867. 2006. [DOI] [PubMed] [Google Scholar]
- Yacoub HA, Elazzazy AM, Abuzinadah OA, Al-Hejin AM, Mahmoud MM, Harakeh SM. Antimicrobial activities of chicken β-defensin (4 and 10) peptides against pathogenic bacteria and fungi. Frontiers in Cellular and Infection Microbiology, 5: 36 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang L, Zhan X, Zeng X, Zhou L, Cao G, Chen A, Yang C. Effects of dietary supplementation of probiotic, Clostridium butyricum, on growth performance, immune response, intestinal barrier function, and digestive enzyme activity in broiler chickens challenged with Escherichia coli K88. Journal of Animal Science and Biotechnology, 7: 3 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]


