Abstract
A study using pair-feeding technique was conducted to determine whether heat exposure directly or indirectly (via reduced feed intake) increases intestinal mucosal damage and permeability to endotoxin in broiler chickens. Male broiler chickens (Ross 308), 27-d-old, were subjected to one of the three treatments (n=8): 1) thermo-neutral conditions (24°C) with ad libitum feed intake, 2) heat stress conditions (33°C) with ad libitum feed intake, or 3) pair-feeding under thermo-neutral conditions, with the feed intake identical to that of heat-stressed chickens. Using these groups, two experiments were performed to evaluate temporal changes in the intestinal morphology in response to each treatment. In experiment 1, chickens were sacrificed after 24 h of exposure to the treatment conditions, while in experiment 2, chickens were sacrificed after 12 or 72 h of exposure to the treatment conditions. In experiment 1, exposure to heat stress conditions for 24 h significantly decreased both the villus height to crypt depth ratio and number of proliferating cell nuclear antigen (PCNA)-positive cells in the duodenum and increased the plasma endotoxin concentration. These findings were not observed in pair-fed chickens. In experiment 2, intestinal integrity and function were unaffected by 12 h of heat stress. On the other hand, chickens exposed to heat stress for 72 h exhibited significantly damaged intestinal morphology in the duodenum as well as increased plasma endotoxin concentration; these negative effects were not observed in pair-fed chickens. These findings suggest that the intestinal morphology and permeability changes observed in chickens that are heat-stressed for 24–72 h are due to the heat stress conditions and not due to reduced feed intake.
Keywords: broiler chicken, heat stress, intestinal morphology, intestinal permeability, pair-fed
Introduction
Heat stress is an important concern in poultry production worldwide, the negative effects of which are likely to be further aggravated if global warming continues as predicted. In addition, genetic selection for rapid skeletal muscle growth might increase the susceptibility of chickens (especially meat-type chickens) to heat stress, as enhanced lean tissue accretion is accompanied by increased metabolic heat production. Therefore, defining the biology and mechanisms underlying poor performance due to heat stress is critical to developing approaches to ameliorate current poultry production issues.
Heat exposure markedly decreases bodyweight gain and ad libitum feed intake in chickens (Bottje and Harrison, 1985). In line with these findings, chickens exposed to heat stress showed intestinal morphological damage and increased intestinal permeability to endotoxin (Quinteiro-Filho et al., 2010; Song et al., 2013; Varasteh et al., 2015). It was hypothesized that these intestinal dysfunctions might contribute in part to reduced growth performance during the summer months. However, to the authors' knowledge, virtually no data were available to confirm whether heat exposure directly or indirectly (via reduced feed intake) compromises intestinal integrity and barrier functions in broiler chickens subjected to heat stress. Numerous animal and cell culture models have been used to examine the etiology of heat-induced intestinal damage (Hales et al., 1979; Prosser et al., 2004; Dokladny et al., 2006). Heat-stressed animals redistribute blood to the periphery to maximize radiant heat dissipation, while vasoconstriction occurs in the gastrointestinal tract to reprioritize blood flow (Lambert, 2009). Consequently, the reduced blood and nutrient flow to the intestinal epithelium compromises the integrity of the intestinal barrier (Yan et al., 2006). On the other hand, feed restriction and fasting also affect the structure of the intestinal mucosa because feed ingredients contain compounds that directly stimulate growth of this tissue. Feed restriction may induce atrophy of the intestinal mucosa in mammals (Song et al., 2009) and birds (Palo et al., 1995; Fischer et al., 2007; Silva et al., 2009). As such, fasting, even for a limited period, may interfere with intestinal epithelial cell synthesis and negatively affect the bird's growth. Despite using all available data to comprehend heat-stressed responses, it is still not known whether heat exposure directly or indirectly (via reducing feed intake), causes morphological and functional damages in the small intestine in broiler chickens.
Therefore, experiments using a pair-feeding technique were conducted to determine whether heat exposure directly or indirectly (via reduced feed intake) increases intestinal mucosal damage and permeability to endotoxin in broiler chickens. Our study shows that heat stress directly impairs intestinal morphology and increases intestinal permeability to endotoxin in chickens exposed to heat stress for 24–72 h. These data might contribute to the development of approaches to prevent reduction of growth performance under heat stress conditions.
Material and Methods
Animals and Experimental Design
The Animal Care and Use Committee of the Graduate School of Agricultural Science, Tohoku University, approved all procedures, and every effort was made to minimize pain or discomfort to the animals. Male broiler chickens (Ross 308 strain) were obtained from a commercial hatchery (Economic Federation of Agricultural Cooperatives Hatchery, Miyagi, Japan) at 0 day of age for both experiments. They were housed in electrically heated batteries under continuous light for 11 days, and provided with access to water and corn-soybean diet (crude protein, 22%; metabolizable energy content, 3100 kcal/kg) ad libitum. At 11 days of age, the birds were moved to individual cages. At 27 days of age, the chickens were subjected to one of the three treatments: (1) thermo-neutral conditions (TN: 24°C, humidity 55×5%) with ad libitum feed intake, (2) heat stress conditions (HS: 33°C, humidity 55×5%) with ad libitum feed intake, or (3) pair-feeding (PF) under TN conditions to mirror the feed intake of HS chickens. In order to avoid long-term starvation in the PF group, the same amount of feed consumed in HS group for the previous interval was provided to the PF group, at 6 h intervals. On the first day of heat exposure, the temperature was increased by 1°C per hour from 24°C to 33°C, to prevent rapid physiological changes. The body weights and feed intake of chickens over the experimental period were recorded. Blood samples were collected from the wing vein into a heparinized syringe, centrifuged at 700×g for 10 min at 4°C, and the plasma was stored at −80°C until analyzed. After 24 h (experiment 1), and 12 or 72 h (experiment 2) of heat exposure, chickens from each group (n=8) were sacrificed via exsanguination. The small intestine was immediately harvested and divided into three parts: the duodenum (from the pylorus to the distal point of entry of the bile ducts), jejunum (Meckel's diverticulum marked the endpoint of the jejunum), and ileum (the ileocecal junction marked the end of the ileum). One centimeter length of intestinal tissue from the midpoint of the duodenum and the midpoint of the ileum were collected from each of the chickens and stored in 10% formalin neutral buffer solution prior to morphological analysis.
Analysis of Intestinal Morphology
Villus height and crypt depth as well as the villus height to crypt depth ratio were measured for evaluation of intestinal morphology indicative of gut health in animals, as suggested by Pluske et al. (1996a, 1996b) and Xu et al. (2003). Formalin-fixed intestinal tissue was dehydrated and embedded in paraffin. Four micron-thick sections were cut on a microtome and then stained with hematoxylin-eosin. Villus height and crypt depth from 10 randomly selected villi and associated crypts on two sections per chicken were measured using a microscope. The ratio of villus height to crypt depth was used as an index of the maturity and functional capacity of enterocytes (Hampson, 1986).
Immunohistochemistry Analysis of Proliferating Cell Nuclear Antigen (PCNA)
PCNA, known as DNA-polymerase delta auxiliary protein, is a 36-kb nonhistone nuclear protein that functions as cofactor for DNA polymerase delta (Linden et al., 1992). PCNA was applied to visualize proliferation activity in the crypt region. Paraffin-embedded intestinal tissues sections (4 µm thick) were deparaffinized in xylene, rehydrated in graded ethanol, and rinsed with distilled water. Endogenous peroxidases were neutralized with hydrogen peroxide (3%) for 15 min followed by rinsing with distilled water for 5 min. Antigen retrieval was achieved by incubating slides in 10 mM citric acid buffer solution, pH 6.0, in a steamer at 90°C for 60 min. Nonspecific immunoglobulin was blocked by incubating the slides with 5% casein for 60 min, before application of the primary antibody. Immunohistochemical staining was performed using the Histofine Simple Stain system (MAX-PO (MULTI), Nichirei Biosciences Inc., Tokyo, Japan). Slides were incubated for 18 h at 4°C with polyclonal rabbit anti-PCNA antibodies (catalogue number sc-7907, Santa Cruz Biotechnology, Dallas, TX, USA) at a dilution of 1:200. Sections were then incubated with Histofine Simple Stain MAX-PO for 30 min and developed with diaminobenzidine. Next, sections were lightly counterstained with hematoxylin, dehydrated, and mounted, as described by Uni et al. (1998). A microscope was used to visualize the stained cells, with the percentage of proliferating cells calculated from the number of enterocytes per crypt and the number of PCNA-stained cells. Calculations were made from two randomly selected sections per chicken.
Determination of Plasma Endotoxin Concentrations
Plasma endotoxin concentrations were measured as one of the markers of intestinal permeability, since changes in the intestinal morphology (such as decreases in villus height and/or villus height to crypt depth ratio), indicative of damage to the intestinal epithelium are supposed to contribute largely to the increased permeability to endotoxin (Lambert et al., 2002). Plasma was separated and stored at −80°C until assayed. Endotoxin (lipopolysaccharide, LPS) concentrations were determined using a chromogenic Limulus amoebocytelysate (LAL) end-point assay (QCL-1000, Lonza Group Ltd., Basel, Switzerland). Plasma samples used for LPS determination were stored in LPS-free tubes to prevent the loss of endotoxin to the plastic tube walls.
Statistical Analysis
Statistical analysis was performed separately for each experiment. Data were first analyzed using a general linear model analysis of variance procedure, and means were compared using the Tukey-Kramer multiple comparison test (P<0.05). All data are expressed in the form of mean×standard error (SE, n=8 per measurement).
Results
Experiment 1
Feed intake of the HS group was found to significantly decrease (P<0.05) compared to that of the TN group (Fig. 1A). Compared to the TN group, the body weight gain of both the HS and PF groups were markedly decreased in line with the reduced feed intake (Fig. 1B), but there were no differences between the HS and PF groups. Feed efficiency of HS group, but not the PF group, was significantly decreased compared to the TN group (Fig. 1C). The villus height of the duodenum in the HS group showed a tendency to decrease (P<0.07) as compared to the TN group, but the same was not observed in the PF group (data not shown). No difference in the crypt depth was observed among any of the groups (data not shown). The villus height to crypt depth ratio in the duodenum was significantly decreased in the HS group compared to the TN group, but not in the PF group (Fig. 1D). No significant differences in the villus height, crypt depth, and villus height to crypt depth ratio were observed among the groups in relation to ileal tissue (Fig. 1D). PCNA staining of duodenal tissue showed the presence of dividing cells, with most of the cells in the crypt undergoing proliferation (Fig. 1E left). The percentage of PCNA-positive cells in the three regions of the crypt was significantly reduced in the HS group compared to the TN group, but not in the PF group (Fig. 1E right). Plasma endotoxin concentrations were significantly higher in the HS group compared to the TN group, while no difference was observed between the TN and PF groups (Fig. 1F).
Fig. 1.
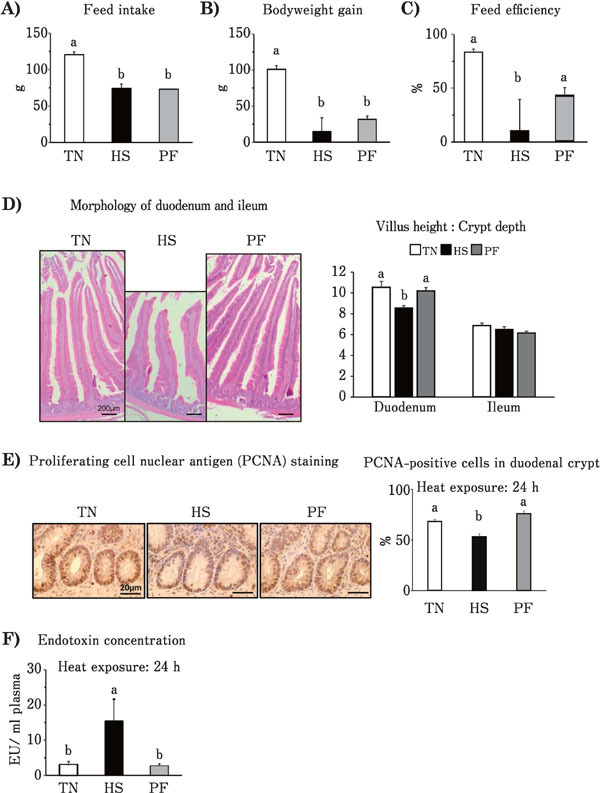
Effects of ad libitum feed intake in thermo-neutral conditions (TN, 24°C), ad libitum feed intake in heat stress (HS, 33°C) conditions and pair-feeding in TN conditions (PF) for 24 h on: feed intake (A), body weight gain (B), feed efficiency (C), villus height to crypt depth ratio in duodenum and ileum (D), ratio of proliferating cell nuclear antigen (PCNA)-positive cells to overall cell number in the duodenal crypt (E) and plasma endotoxin concentration (F) of broiler chickens. Values are means±SE, n=8 per group. a, b P<0.05 for each treatment; values with different letters are statistically different.
Experiment 2
Compared to the respective TN groups, feed intake was significantly decreased in HS-treated chickens at 12 and 72 h (Fig. 2A). In line with this, bodyweight gains following 12 and 72 h of heat exposure were significantly decreased too (Fig. 2B), while no differences were observed between the HS and PF groups for the two treatment periods. After 12 h heat exposure, feed efficiency of HS group was significantly decreased compared to that of the TN group, while the PF group was less affected than the HS group (Fig. 2C left). After 72 h heat exposure, no differences in feed efficiency were observed among groups (Fig. 2C right). After 12 h of heat exposure, no significant differences in the villus height to crypt depth ratio in duodenal or ileum tissues (Fig. 2D left), in the percentage of PCNA-positive cells in the duodenal crypts (Fig. 2E left) and in the plasma endotoxin concentration (Fig. 2F left) were observed among the groups. After 72 h of heat exposure, the villus height to crypt depth ratio in duodenal tissue from the HS group was significantly lower than that of the TN group. However, there was no difference in the ratio in duodenal tissue between the PF and TN groups, and in the ratio in ileum tissue (Fig. 2D right) and percentage of PCNA-positive cells in the duodenal crypts (Fig. 2E right) among all the groups. The plasma endotoxin concentration in the HS group after 72 h was significantly greater than that of the TN group, though there was no difference in the concentration between the PF and TN groups (Fig. 2F right).
Fig. 2.
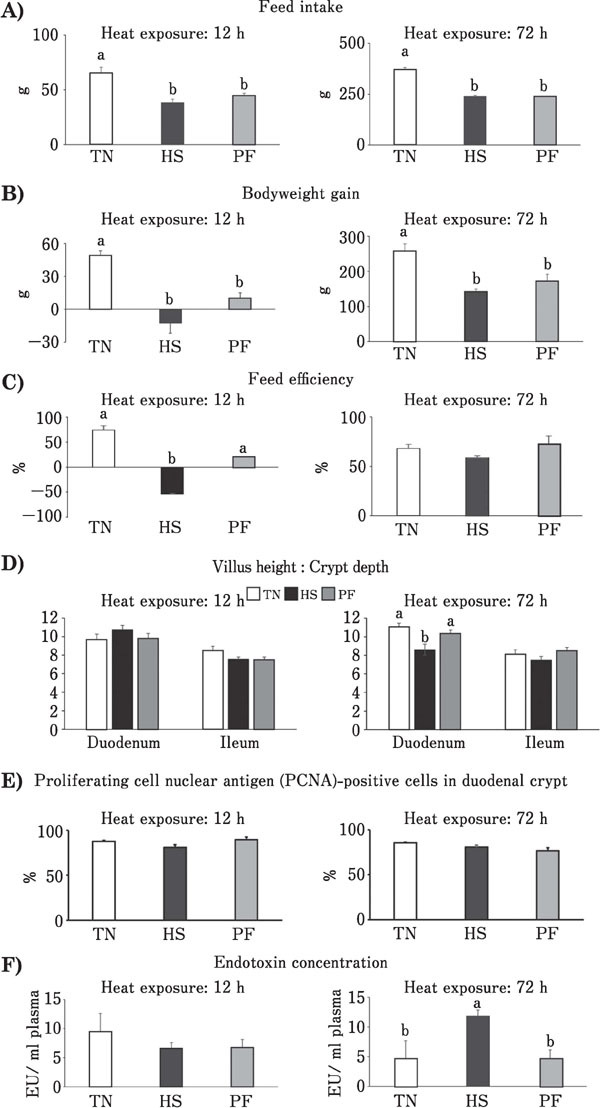
Effects of ad libitum feed intake in thermo-neutral conditions (TN, 24°C), ad libitum feed intake in heat stress (HS, 33°C) conditions and pair-feeding in TN conditions (PF) for 12 or 72 h on: feed intake (A), body weight gain (B), feed efficiency (C), villus height to crypt depth ratio in duodenum and ileum (D), ratio of proliferating cell nuclear antigen (PCNA)-positive cells to overall cell number in the duodenal crypt (E) and plasma endotoxin concentration (F) of broiler chickens. Values are means±SE, n=8 per group. a, b P<0.05 for each treatment; values with different letters are statistically different.
Discussion
Heat stress adversely affects intestinal morphology and increases intestinal permeability in many animal species (Hall et al., 2001; Dokladny et al., 2006; Yang et al., 2007; Lambert, 2009). However, it is not clear whether these alterations are caused directly by heat exposure or indirectly as a consequence of reduced feed intake. The objectives of this study, therefore, were to determine if heat exposure directly or indirectly (via reduced feed intake) increases intestinal mucosal damage and permeability to endotoxin in broiler chickens. In both the experiments with chickens i) after 24 h exposure, and ii) 12 and 72 h exposure, the feed intake of HS chickens was markedly reduced, while that of PF chickens was reduced by design to mirror that of the HS group. In line with the reduced feed intake, both HS and PF chickens showed significant reductions in bodyweight gain and feed efficiency compared to the TN chickens, with the reductions in HS chickens being more severe than those observed in PF chickens. These results indicate that the poorer growth performance of HS chickens might be caused not only by a reduction in feed intake, but also by the changes in feed intake-independent responses to heat exposure.
It is well known that intestinal damage is a primary response to heat stress, including compromised intestinal integrity and barrier function (Quinteiro-Filho et al., 2010; Song et al., 2013; Varasteh et al., 2015). Therefore, in this study we evaluated morphological and functional damages to the small intestine in TN, HS, and PF chickens. For evaluation of intestinal morphological damages, we measured villus height and crypt depth as well as the villus height to crypt depth ratio, which are useful indicators of gut health in animals (Pluske et al., 1996a, 1996b; Xu et al., 2003). Shortened height of intestinal villi indicates damage to the intestinal epithelium, while increased ratio of villus height to crypt depth directly correlates with increased epithelial turnover (Fan et al., 1997). Furthermore, we also measured the number of PCNA-positive cells to know if cellular responses, proliferation, or turnover are included in heat-induced damages; the mucosal epithelia regeneration is sensitive to stresses, inducing cellular necrosis and desquamation, especially at the tip of intestinal villus (Lambert et al., 2002). For evaluation of functional damages, we measured plasma endotoxin levels because intestinal permeability to endotoxin is influenced by damage to the epithelium, such as epithelial sloughing and necrosis (Lambert et al., 2002).
In our study, the intestinal integrity and function were unaffected by 12 h of heat stress, suggesting that heat exposure for 12 h was not long enough to induce intestinal damage. In contrast, exposure to heat for 24 h significantly decreased both, villus height to crypt depth ratio and the number of PCNA-positive cells in the duodenum as well as increased plasma endotoxin concentrations, whereas these changes in the duodenum and plasma were not observed in PF chickens subjected to feed restriction. Therefore, these results demonstrate that heat stress directly affected intestinal morphology and permeability in chickens exposed to heat stress for 24 h. Similar changes in villus height to crypt depth ratio, the number of PCNA-positive cells and plasma endotoxin concentrations were observed in chickens exposed to heat stress or feed restriction for 72 h, except that the number of PCNA-positive cells in the duodenum crypt did not decrease in the HS group. This means that after 72 h of heat exposure, the proliferation activity in the duodenum was not diminished, suggesting a self-recovery mechanism in the intestines of chickens under heat stress as a means of adaptation (Abdelqader et al., 2016).
In conclusion, our results indicate that heat stress directly impairs intestinal morphology and increases intestinal permeability to endotoxin in chickens exposed to heat stress for 24–72 h. Based on these observations, short-term feed restriction (up to 72 h) was slightly disruptive to intestinal integrity and function in chickens, and heat-induced stress responses (hypoxia and oxidative stress) contributed largely to intestinal dysfunction. Hence, our study suggests that feed supplementation with antioxidants, which could alleviate hypoxia and/or oxidative stress, is a feasible strategy to prevent or improve damage to intestinal integrity in heat-stressed chickens. Considering that the intestinal integrity and function were unaffected by 12 h of heat exposure, feeding antioxidant supplements before 12 h may be more effective against heat stress. These data might contribute to the development of dietary approaches for poultry to prevent performance reduction under heat exposure conditions.
Acknowledgments
This study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI program (grant nos. 25850182/16H06205 [M.K.] and 15H04582 [M.T.]), by the JSPS Research Fellowship for Young Scientists (No. 13J06451; F.N.), and by the JSPS Core-to-Core Advanced Research Networks Program under the theme “Establishment of international agricultural immunology research-core for a quantum improvement in food safety”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abdelqader A, Rahman A, Fataftah A. Effect of dietary butyric acid on performance, intestinal morphology, microflora composition and intestinal recovery of heat-stressed broilers. Livestock Science, 183: 78-83. 2016. [Google Scholar]
- Bottje WG, Harrison PC. Effect of carbonated water on growth performance of cockerels subjected to constant and cyclic heat stress temperatures. Poultry Science, 64: 1285-1292. 1985. [DOI] [PubMed] [Google Scholar]
- Dokladny K, Moseley PL, Ma TY. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. American Journal of Physiology, 290: G204-G212. 2006. [DOI] [PubMed] [Google Scholar]
- Fan YK, Croom J, Christensen VL, Black BL, Bird AR, Daniel LR, Mcbride BW, Eisen EJ. Jejunal glucose uptake and oxygen consumption in turkey poults selected for rapid growth. Poultry Science, 76: 1738-1745. 1997. [DOI] [PubMed] [Google Scholar]
- Fischer da Silva AV, Borges SA, Maiorka AI, Givisiez PEN, Rocha CV, Macari M. Ornithine decarboxylase expression in the small intestine of broilers submitted to feed restriction and glutamine supplementation. Brazilian Journal of Poultry Science, 9: 111-115. 2007. [Google Scholar]
- Hales JR, Rowell LB, King RB. Regional distribution of blood flow in awake heat stressed baboons. American Journal of Physiology, 237: H705-712. 1979. [DOI] [PubMed] [Google Scholar]
- Hall DM, Buettner GR, Oberley LW, Xu L, Matthes RD, Gisolfi CV. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. American Journal of Physiology, 280: H509-H521. 2001. [DOI] [PubMed] [Google Scholar]
- Hampson DJ. Alterations in piglet small intestinal structure at weaning. Research in Veterinary Science, 40: 32-40. 1986. [PubMed] [Google Scholar]
- Lambert GP. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. Journal of Animal Science, 87: E101-E108. 2009. [DOI] [PubMed] [Google Scholar]
- Lambert GP, Gisolfi CV, Berg DJ, Moseley PL, Oberley LW, Kregel KC. Molecular biology of thermoregulation selected contribution: Hyperthermia-induced intestinal permeability and the role of oxidative and nitrosative stress. Journal of Applied Physiology, 92: 1750-1761. 2002. [DOI] [PubMed] [Google Scholar]
- Linden MD, Torres FX, Kubus J, Zarbo RJ. Clinical application of morphologic and immunocytochemical assessments of cell proliferation. American Journal of Clinical Pathology, 97: S4-S13. 1992. [PubMed] [Google Scholar]
- Palo PE, Sell JL, Piquer FJ, Vilaseea L, Soto-Salanova MF. Effect of early nutrient restriction on broiler chickens: Performance and digestive enzyme activities. Poultry Science, 74: 1470-1483. 1995. [DOI] [PubMed] [Google Scholar]
- Pluske JR, Williams IH, Aherne FX. Maintenance of villus height and crypt depth in piglets by providing continuous nutrition after weaning. Animal Science, 62: 131-144. 1996. a. [Google Scholar]
- Pluske JR, Williams IH, Aherne FX. Villus height and crypt depth in piglets in response to increases in the intake of cows' milk after weaning. Animal Science, 62: 145-1581. 1996. b. [Google Scholar]
- Prosser C, Stelwagen K, Cummins R, Guerin P, Gill N, Milne C. Reduction in heat-induced gastrointestinal hyperpermeability in rats by bovine colostrum and goat milk powders. Journal of Applied Physiology, 96: 650-654. 2004. [DOI] [PubMed] [Google Scholar]
- Quinteiro-Filho WM, Ribeiro A, Ferraz-de-Paula V, Pinheiro ML, Sakai M, Sá LR, Ferreira AJ, Palermo-Neto J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poultry Science, 89: 1905-14. 2010. [DOI] [PubMed] [Google Scholar]
- Silva AV, Maiorka A, Borges SA, Santin E, Boleli IC, Macari M. Surface area of the tip of the enterocytes in small intestine mucosa of broilers submitted to early feed restriction and supplemented with glutamine. International Journal of Poultry Science, 6: 31-35. 2009. [Google Scholar]
- Song J, Wolf SE, Wu XW, Finnerty CC, Gauglitz GG, Herndon DN, Jeschke MG. Starvation-induced proximal gut mucosal atrophy diminished with aging. Journal of parenteral and enteral nutrition, 33: 411-6. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Xiao K, Ke YL, Jiao LF, Hu CH, Diao QY, Zou XT, et al. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poultry Science, 93: 581-588. 2013. [DOI] [PubMed] [Google Scholar]
- Uni Z, Platin R, Sklan D. Cell proliferation in chicken intestinal epithelium occurs both in the crypt and along the villus. Journal of Comparative Physiology B, 168: 241-7. 1998. [DOI] [PubMed] [Google Scholar]
- Varasteh S, Braber S, Akbari P, Garssen J, Fink-Gremmels J. Differences in susceptibility to heat stress along the chicken intestine and the protective effects of galacto-oligosaccharides. PloS one, 10: e0138975 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZR, Hu CH, Xia MS, Zhan XA, Wang MQ. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poultry Science, 82: 1030-1036. 2003. [DOI] [PubMed] [Google Scholar]
- Yan Y, Zhao Y, Wang H, Fan M. Pathophysiological factors underlying heatstroke. Medical Hypotheses, 67: 609-617. 2006. [DOI] [PubMed] [Google Scholar]
- Yang PC, He SH, Zheng PY. Investigation into the signal transduction pathway via which heat stress impairs intestinal epithelial barrier function. Journal of Gastroenterology and Hepatology, 22: 1823-1831. 2007. [DOI] [PubMed] [Google Scholar]


