Abstract
The early atherosclerotic lesions develop by the accumulation of arterial foam cells derived mainly from cholesterol-loaded macrophages. Therefore, cholesterol and cholesteryl ester transfer protein (CETP) have been considered as causative in atherosclerosis. Moreover, recent studies indicate the role of trimethylamine N-oxide (TMAO) in development of cardiovascular disease (CVD). The current study aimed to investigate the association between TMAO and CETP polymorphisms (rs12720922 and rs247616), previously identified as a genetic determinant of circulating CETP, in a population of coronary artery disease (CAD) patients (n = 394) and control subjects (n = 153). We also considered age, sex, trimethylamine (TMA) levels and glomerular filtration rate (GFR) as other factors that can potentially play a role in this complex picture. We found no association of TMAO with genetically determined CETP in a population of CAD patients and control subjects. Moreover, we noticed no differences between CAD patients and control subjects in plasma TMAO levels. On the contrary, lower levels of TMA in CAD patients respect to controls were observed. Our results indicated a significant correlation between GFR and TMAO, but not TMA. The debate whether TMAO can be a harmful, diagnostic or protective marker in CVD needs to be continued.
Subject terms: Risk factors, Biomarkers
Introduction
Despite significant progress in prevention and treatment strategies of coronary artery disease (CAD), cardiovascular events still constitute the leading cause of mortality and morbidity in the modern world1. CAD is characterized by atherosclerosis progressively narrowing the epicardial coronary arteries and impairing myocardial blood flow. The early atherosclerotic lesions develop by the accumulation of arterial foam cells mainly derived from cholesterol-loaded macrophages2. Therefore, cholesterol metabolism has been considered as causative in atherosclerosis3.
The pathogenesis and potential treatment of the atherosclerotic lesions have been studied using numerous animal models, such as a mouse4. However, related to cholesterol metabolism, resistance to atherosclerosis is the major limitation of mouse models5. The absence of cholesteryl ester transfer protein (CETP) in mice causes lower plasma cholesterol levels, with high-density lipoprotein (HDL) as the major circulating lipoprotein6,7. Thus, genetic modifications, such as low-density lipoprotein (LDL) receptor deficient (LDLR−/−) and apolipoprotein E knockout (ApoE−/−), have been applied to induce hypercholesterolemia in mice8–12. Using a knockout mouse model, trimethylamine N-oxide (TMAO) has been indicated as the key pro-atherogenic compound13. High blood TMAO levels activate macrophage influx of cholesterol which leads to foam cell formation and ultimately atherosclerotic lesions14. TMAO is produced by the hepatic flavin monooxygenases (FMOs), mainly FMO3, converting trimethylamine (TMA) as a substrate15,16. TMA is a waste product of gut microbes, which utilize choline or carnitine as a carbon fuel source. Hence, a link between gut microbes and atherosclerosis has been proposed13,17,18. However, in ApoE-/- mice transfected with human CETP, an increase in plasma TMAO was associated with a significantly reduced area of aortic lesions19. Nevertheless, recent clinical studies have shown a positive correlation between elevated plasma TMAO and an increased risk for major adverse cardiovascular events defined as death, myocardial infarction, or stroke20,21.
According to the current dogma, CETP decreases HDL-cholesterol and increases low-density lipoprotein LDL-cholesterol. Remarkably, genome-wide association studies followed by a Mendelian randomization22 have shown that some independent genetic variants (in particular rs12720922 and rs247616), located in the CETP gene, largely determine CETP concentration. Per-allele increase in serum CETP was 0.32 µg/mL for rs247616-C and 0.35 µg/mL for rs12720922-A22. Moreover, these CETP SNPs have been causally associated with lower concentrations of HDL components, while no associations with LDL components have been measured23. This demonstrates that rs12720922 and rs247616 are makers able to predict HDL-cholesterol levels, and corroborates the hypothesis that CETP can mediate cardiovascular risk by affecting HDL-cholesterol levels. Thus, in accordance with previous evidence on mice model, it can be hypothesized that the different genetic background determining the CETP concentration might modulate the association between TMAO and CVD risk.
Therefore, the aim of the current study was to investigate the association between TMAO and CETP polymorphisms (rs12720922 and rs247616), previously identified as genetic determinants of circulating CETP and HDL levels22,23, in a population of CAD patients and control subjects with no self-reported medical history of cardiovascular disease (CVD).
Results
Descriptive statistics
Among all the 547 enrolled subjects, 358 were male (65.4%), and 189 were female (34.6%). The control group was composed of 153 individuals, while 394 patients suffered from CAD. Descriptive statistics for the analysed variables are displayed in Table 1.
Table 1.
Characteristics of the study participants.
| Control n = 153 |
CAD n = 394 |
p | |
|---|---|---|---|
| Age in years | 64.3 ± 8.1 | 66.4 ± 11.7 | 0.016 |
| Female | 64 (41.8) | 125 (31.7) | 0.028 |
| BMI in kg/m2 | 27.8 ± 4.1 | 28.8 ± 4.5 | 0.030 |
| Glomerular filtration rate (GFR) | 92.1 ± 31.0 | 86.6 ± 34.7 | 0.079 |
| Stable angina | 0 | 196 (49.7) | |
| Acute coronary syndrome | 0 | 198 (50.3) | |
| STEMI | 0 | 44 (11.2) | |
| NSTEMI | 0 | 111 (28.2) | |
| UA | 0 | 43 (10.9) | |
| Hypertension | 63 (41.2) | 301 (76.4) | 0.001 |
| Diabetes mellitus | 21 (13.7) | 118 (29.9) | 0.001 |
| Current or past smokers | 59 (38.6) | 196 (49.7) | 0.022 |
Data are shown as mean ± standard deviation or number (%).
BMI body mass index, GFR glomerular filtration rate, STEMI ST-elevation myocardial infarction, NSTEMI non-ST-elevation myocardial infarction, UA unstable angina.
TMAO and TMA in CAD patients and controls
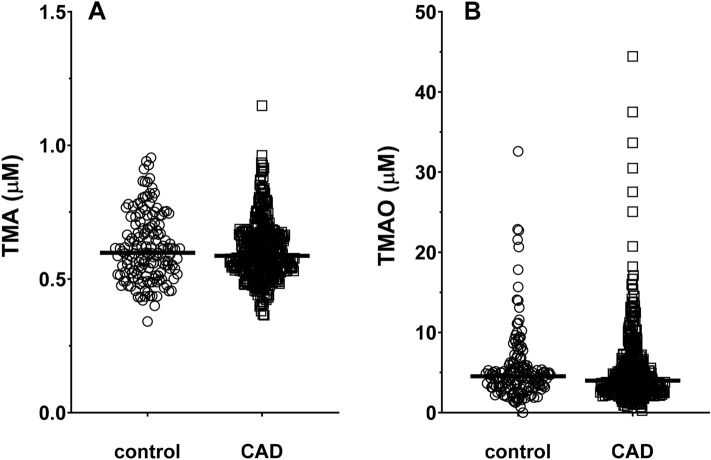
No differences were noted in row values of plasma TMA between controls 0.62 ± 0.13 μM (mean ± SD) and CAD patients 0.60 ± 0.11 μM (Fig. 1A). However, Generalized Linear Model (GLM) analysis, including adjustments for glomerular filtration rate (GFR), age, body mass index (BMI) and sex, identified a significant difference between the two groups for TMA (expected marginal means ± SD: controls = 0.63 ± 0.01 μM; CAD patients = 0.60 ± 0.01 μM; p = 0.004). TMAO was not significantly different between controls and CAD patients (Fig. 1B), regardless of the row values (p = 0.712) or in the analysis adjusted for the covariates (p = 0.251).
Figure 1.
Plasma TMA (A) and TMAO (B) concentrations in controls (n = 153) and CAD (n= 394) patients. Scatter dot plot with lines as median values.
Genotyping
Genotype and minor allele frequencies of the selected polymorphisms are reported in Table 2. All the polymorphisms were in Hardy–Weinberg Equilibrium (HWE) (p > 0.05) and minor allele frequencies (MAF) at both rs12720922 and rs247616 SNPs were consistent with Northern Europe reference population data (Table 2).
Table 2.
Genotypic data in the analysed population.
|
CETP rs247616 n (%) |
CETP rs12720922 n (%) |
|||
|---|---|---|---|---|
| Genotype frequency | CC | 238 (43.5) | AA | 17 (3.1) |
| CT | 249 (45.5) | AG | 171 (31.3) | |
| TT | 60 (11.0) | GG | 359 (65.6) | |
| HWE (P) | 0.182 | 0.384 | ||
| MAF current study population | 0.337 | 0.187 | ||
| MAF Estonian population (dbSNP) | 0.317 | 0.189 | ||
| MAF European population (gnomAD–Genomes) | 0.319 | 0.179 | ||
MAF minor allele frequency.
CETP SNPs are directly associated with HDL-cholesterol levels
Since most of the CAD patients were treated with statins (commonly used as primary or secondary prevention measurement), we relied on a Mendelian randomization-based approach to study the impact of CETP and HDL-cholesterol on TMA and TMAO. Despite the potential interference of statins treatment, rs12720922 and rs247616 CETP SNPs were significantly associated with HDL-cholesterol levels in the total population (Supplementary Fig. S1 online). Conversely, these polymorphisms were not associated with LDL-cholesterol or total cholesterol levels. This evidence suggests that rs12720922 and rs247616 SNPs can selectively predict HDL-cholesterol even in presence of statin treatment. However, since the risk of unpredictable effects due to the statin treatment cannot be excluded (Supplementary Table S1 online), we confirmed the usage of the Mendelian randomization-based approach for the subsequent analysis and did not consider the raw data on lipid profile.
CETP SNPs are not directly associated with CAD
Chi-square analysis revealed that genotypes were not differently distributed among controls or CAD patients, thus neither CETP rs247616 (p = 0.426) nor rs12720922 (p = 0.488) appear to be directly associated with CVD considering a codominant model. Moreover, no associations were detected using additive models; similarly, no differences in the distribution of alleles between the two classes were detected for any of the analysed SNP (Table 3).
Table 3.
Differences in genotypic and allelic distributions between controls and CAD patients.
| CAD n (%) | Control n (%) | versus | p | |
|---|---|---|---|---|
| rs247616 | ||||
| CC | 178 (45.2) | 60 (39.2) | CT + TT | 0.208 |
| CT | 173 (43.9) | 76 (49.7) | CC | 0.192 |
| TT | 43 (10.9) | 17 (11.1) | CC | 0.623 |
| CC + CT | 351 (89.1) | 136 (88.9) | TT | 0.947 |
| C | 529 (67.1) | 196 (64.1) | T | 0.334 |
| T | 259 (32.9) | 110 (35.9) | C | 0.334 |
| rs12720922 | ||||
| AA | 12 (3.0) | 5 (3.3) | AG + GG | 0.893 |
| AG | 129 (32.8) | 42 (27.4) | AA | 0.662 |
| GG | 253 (64.2) | 106 (69.3) | AA | 0.992 |
| AA + AG | 141 (35.8) | 47 (30.7) | GG | 0.263 |
| A | 153 (19.4) | 52 (17.0) | G | 0.357 |
| G | 635 (80.6) | 254 (83.0) | A | 0.357 |
Effects of different CETP genotypes on TMAO, TMA and TMAO/TMA
CETP rs12720922 genotype was associated with TMAO levels (p = 0.008) and TMAO/TMA ratio (p = 0.018) (GLM analysis; sex, age and GFR as covariates; Fig. 2); conversely, it was not linked to TMA levels (p = 0.159). Accordingly, the recessive model resulted in the best fitting, displaying the lowest Akaike’s information criterion (AIC) and Bayesian information criterion (BIC) values both for both rs12720922 (AIC = 3775.1; BIC = 3809.6) and rs247616 (AIC = 3781.2; BIC = 3811.4). Indeed, with respect to rs12720922-AG/GG, rs12720922-AA displayed higher TMAO values (p = 0.004) and higher TMAO/TMA ratio (p = 0.020).
Figure 2.
Effect of rs12720922 genotype on plasma TMAO concentrations (A) and TMAO/TMA ratio (B) in controls and CAD patients. Scatter dot plot with lines as median values. *p < 0.05, **p < 0.01.
On the contrary, CETP rs247616 was not associated with TMAO, TMA, or TMAO/TMA levels (sex, age and GFR as covariates).
TMAO, TMA in CVD; CETP genetic background association
GLM analysis showed a different association between TMAO or TMAO/TMA levels and health status (controls vs CAD patients) depending on the rs247616 genotype. In particular, the rs247616-CC individuals belonging to the control group displayed lower TMAO levels than the carriers of the same genotype in the CAD group. On the other hand, T carriers, that had higher TMAO values in controls, exhibited reduced TMAO levels in the CAD group (P = 0.049) (Supplementary Fig. S2A online). This evidence preliminarily suggested that the increase of TMAO in CAD is typical of those individuals that carry the rs247616-CC risk genotype (associated to genetically determined higher CETP and lower HDL levels), but is not generalizable to the entire population. A similar effect was observed for TMAO/TMA ratio, which was different in the control or CAD group depending on the rs247616 genotype (p = 0.046) (Supplementary Fig. S2B online). No significant TMA variations between the control or CAD group were measured neither in dependence on the rs12720922 (p = 0.903) nor the rs247616 (p = 0.569) genotype.
Haplotype association with CVD, TMAO and TMA
Analysis of haplotypes revealed that it was not possible to demonstrate a cumulative effect of the SNPs from data collected in this study. Indeed, distribution of haplotypes in CAD patients was not different in comparison to controls (p = 0.19) (Table 4).
Table 4.
Haplotype frequencies estimation (n = 547) in the total population, in controls and CAD groups.
| rs247616 | rs12720922 | Total | Controls | CAD | Cumulative frequency |
|---|---|---|---|---|---|
| C | G | 0.4829 | 0.4706 | 0.4883 | 0.4829 |
| T | G | 0.3297 | 0.3595 | 0.3175 | 0.8126 |
| C | A | 0.1798 | 0.1699 | 0.183 | 0.9924 |
| T | A | 0.0076 | 0 | 0.0112 | 1 |
Moreover, there was not a significant association between haplotypes and TMAO (global haplotype association, p = 0.45) nor TMA levels (global haplotype association, p = 0.16) (Table 5).
Table 5.
Haplotype association with TMAO and TMA in the total population.
| rs247616 | rs12720922 | Frequency | Difference (95% CI) | p | |
|---|---|---|---|---|---|
| (A) Haplotype association with TMAO (n = 547, adjusted by sex + age + BMI + GFR) | |||||
| 1 | C | G | 0.4831 | 0.00 | – |
| 2 | T | G | 0.3295 | 0.13 (− 0.51–0.76) | 0.69 |
| 3 | C | A | 0.1796 | 0.31 (− 0.46–1.08) | 0.43 |
| rare | * | * | 0.0078 | − 1.37 (− 4.91–2.17) | 0.45 |
| Global haplotype association p-value 0.45 | |||||
| (B) Haplotype association with TMA (n = 547, adjusted by sex + age + BMI + GFR) | |||||
| 1 | C | G | 0.4829 | 0.00 | – |
| 2 | T | G | 0.3297 | 0.02 (0–0.03) | 0.039 |
| 3 | C | A | 0.1798 | 0.02 (0–0.03) | 0.059 |
| rare | * | * | 0.0076 | 0.06 (− 0.03–0.16) | 0.200 |
| Global haplotype association p-value 0.16 | |||||
Other markers
TMAO significantly correlated with GFR (Spearman coefficient = − 0.289; p = 0.001) and age (Spearman coefficient = 0.196; p = 0.000). TMA was associated with GFR (Spearman coefficient = − 0.104; p = 0.015) as well as BMI (Spearman coefficient = − 0.146; p = 0.001).
Discussion
In this study, we found no association between TMAO levels and genetically determined CETP in a population of CAD patients and control subjects. Moreover, we noticed no differences between CAD patients and control subjects in plasma TMAO levels.
In particular, we investigated two SNPs, rs247616 and rs12720922, as largely determining CETP concentration22. An increase in genetically determined serum CETP concentration has been previously associated with decreased total cholesterol concentration and HDL-cholesterol concentration22, with CETP as an important determinant of HDL-cholesterol, but not affecting LDL-cholesterol concentration and composition23. This evidence was essential in the design of this study since direct measurement of HDL- and LDL-cholesterol were not reliable markers in the recruited population, because most of the CAD patients were treated with statins (commonly used as primary or secondary prevention measurement). Results on CETP rs247616 genotyping were similar to those previously shown in the Polish population24. Despite the comparability in CETP rs247616 genotype and the higher number of subjects recruited, we were not able to observe significant differences on the rs247616 genotypes distribution between CAD patients and control groups. Similarly, no significant differences were observed for the rs12720922 genotype, revealing that the risk-alleles were not differently distributed between controls or CAD patients. Thus, we failed to find an association between the HDL-cholesterol increasing genotypes of CETP to CVD. It must be noted that genetic mechanisms raising plasma HDL-cholesterol do not decrease the risk of myocardial infarction25, and only SNPs affecting LDL-cholesterol levels or both, LDL-cholesterol and HDL-cholesterol levels, influence CVD risk26.
Moreover, data collected in the current study did not support the hypothesis that TMAO is directly associated with CVD. We observed similar plasma TMAO levels in patients with confirmed angiographically CAD and control subjects with no medical history of CVD, and plasma TMAO concentration were coherent with values previously measured in the general population27. Moreover, no significant pure associations between the CETP genotypes and TMAO metabolism has been found. Nevertheless, some aspects of the CETP genotype can be mentioned. Firstly, higher TMAO levels have been measured in the rs12720922-AA carriers, which are the subjects with genetically elevated circulating CETP and lower HDL-cholesterol levels. On the contrary, rs12720922-G carriers displayed similar levels of TMAO in both groups. However, it must be noticed that the group of s12720922-AA carriers in CAD patients is limited to a very small number of subjects (n = 12), which is 3.0% of examined CAD population. Secondly, preliminary evidence suggested that the association between high TMAO and CAD is peculiar of the rs247616-CC risk genotype (which is associated to higher CETP and lower HDL levels), but is not generalizable to the entire population. Thus, the involvement of CETP in CAD seems to be more complex than initially hypothesized24, and the association between TMAO and CAD might be not as strong as previously suggested28,29. In fact, despite previously reported the pro-atherogenic effect of TMAO13, recent studies did not observe a positive correlation between plasma TMAO concentrations and atherosclerosis development30,31.
Previous evidence suggested an important implication of HDL metabolism in modulating the association between TMAO and atherosclerosis. Firstly, since the production of TMAO is dependent on liver FMO315, genetic variants of FMO3 have been implicated in a number of diseases32 and TMA/FMO3/TMAO has been identified as a key pathway16,33. In particular, expression of FMO3 modifications in LDLR−/− mice alters circulating and hepatic lipid levels16. Moreover, knockdown of FMO3 reorganizes whole body cholesterol balance by regulation of reverse cholesterol transport33. Moreover, in humans, FMO3 is significantly associated with age, gender, and genotype34. Indeed, several cofounding factors that mediates the association between TMAO and atherosclerosis has been identified. We have not determined FMO3 genotype, but differences in TMA/TMAO ratio due to differences in the amount and activity of FMO3 might be present in our population16, 35. For this reason, both age and gender were a priori selected as covariates in statistical analyses. Another aspect to consider is that CVD and kidney disease (KD) are closely interrelated36 and diminished renal function is strongly associated with morbidity and mortality in heart failure patients37. In ApoE−/− mice model of atherosclerosis, the hypercholesterolemia led to early renal dysfunction that can progress into chronic KD38. In chronic KD, TMAO elimination from the body fails, causing the elevation of its plasma concentration39. Therefore, higher plasma TMAO in humans was suggested as a marker of kidney damage40. Since plasma TMAO has been inversely correlated with GFR41, some studies suggest that GFR can be a cofounder in this association42–44. Moreover, in the end-stage KD patients, not only TMAO but also plasma TMA is elevated39. Thus, we also added GFR as a covariate in the analysis investigating the relationship between TMA/TMAO levels and CVD, so we can exclude that GFR could be responsible for the observed results.
Finally, it is worthy of note that chronic, low-dose oral TMAO treatment showed a reduction in diastolic pressure and cardiac fibrosis in spontaneously hypertensive rats45. Since TMAO stabilize proteins against various environmental stress factors, including high hydrostatic pressure46, TMAO has been suggested as a result rather than a cause of CVD29. Thus, not TMAO, but TMA has been suggested as implicated in CVD47. In our results, marginally lower levels of TMA in CAD patients respect to controls were observed. Therefore, the microbial origin of TMA is of great interest. Indeed, a major role is played by the microbiome in regulating health and well-being48, and dysbiosis of the gut microbiota has been measured in stroke and transient ischemic attack patients whose blood TMAO levels were decreased49.
In conclusion, the studied polymorphisms had no direct roles in the development of CVD in the studied Polish population. Moreover, we observed no differences between CAD patients and control subjects in plasma TMAO levels, TMAO which can be affected by intra-individual variation50. The debate whether TMAO can be a harmful, diagnostic or protective marker in CVD28,29,32 has to be continued.
Materials and methods
Participants
CAD patients were consecutively recruited in one hospital with angiographically confirmed CAD or with angina referred to elective or urgent coronary angiography as inclusion criteria. The diagnosis of ST-segment elevation myocardial infarction (STEMI) and non-ST-segment elevation myocardial infarction (NSTEMI) was established according to the Third Universal Definition of Myocardial Infarction, and unstable angina (UA) was diagnosed according to the 2015 ESC guidelines for the management of NSTE-ACS351,52. Control subjects were recruited in the same region amongst the subjects without a self-reported medical history of CVD. The study was approved by the Regional Bioethical Committee (RBC) in Gdansk (KB-27/16 and KB 32–17). All methods were carried out in accordance with relevant guidelines and regulations approved by RBC. Informed consent was obtained from all subjects.
Samples collection
Venous blood samples were collected in EDTA-containing tubes. The plasma samples were prepared by centrifugation at 1300×g for 10 min at 18–25 °C, and were kept frozen at − 80 °C for later TMA and TMAO analysis.
TMA and TMAO analyses
Plasma TMA and TMAO were determined by the Ultra-Performance Liquid Chromatography (UHPLC) tandem mass spectrometry method, based on the methods described previously53,54. UHPLC separation was performer on an XBridge HILIC 3.5 μm (3.0 mm × 50 mm) column on a NEXERA Shimadzu UHPLC system coupled with QT4500 SCIEX. Trimethyl-d9-amine HCl (d9-TMA) was used as an internal standard. The 3 μM of d9-TMA working solution of internal standard (ISWS) was prepared in methanol/acetonitrile (15:85) and 0.1% formic acid (v/v). Calibration samples, QC and plasma samples were prepared by addition 100 μl of cold ISWS to 50 μl of each sample type. All samples were vortexed and kept on ice for 15 min for protein precipitation. Centrifuged samples (14,000 rpm, 4 °C, for 20 min.) were divided into two parts: without dilution which were used for analysis of TMA concentration and diluted (5:95 of ISWS) for analysis of TMAO. The mobile phase was 70% of acetonitrile with 0.1% formic acid (v/v) and 30% of 15 mmol/L ammonium formate with 0.1% formic acid (v/v) at a flow rate of 0.4/min. The mass spectrometer was operated in multiple-reaction monitoring (MRM)-positive electrospray ionization (ESI+). MRM parameters are included in Supplementary Table S2. Mass spectrometer optimized settings were as follows: IonSpray Voltage = 5.5 kV, source temperature = 300 °C, collision gas = 8, curyine gas = 30.0. Calibration curve range was from 0.3 to 30 μM and from 0.1 to 30 μM TMAO and TMA respectively. The limits of quantification (LOQ) were 0.3 μM and 0.1 μM for TMAO and TMA respectively.
DNA extraction and genotyping
Genomic DNA was extracted from blood using the kit for genomic DNA purification (A&A Biotechnology, Gdynia, Poland) and it was quantified by NanoDrop 2000 (Thermo Scientific, MA, USA) CETP rs12720922 and rs247616 were assessed in real-time PCR by TaqMan assays (Thermo Fisher Scientific, MA, USA), according to the manufacturer instructions.
Statistical analysis
The sample size was calculated through a power analysis performed by G*Power. The effect size of TMAO variation in CAD patients respect to controls was calculated from the study of Tang and colleagues18, which has been identified as a high-quality study in the meta-analysis from Qi and colleagues55. The calculated effect size is 1.158; thus, to have a power of 0.95, the minimum sample size is 34 subjects (see Supplementary Fig. S3 online).
Power analysis has been performed using G*Power software56. The Shapiro–Wilk test was used for the analysis of the normality of data distribution. Spearman correlation, Chi-square test, Kruskal–Wallis test and Generalized Linear Model (GLM) were used to test correlations and significant differences among analysed variables. Hardy–Weinberg equilibrium was calculated for all the Single Nucleotide Polymorphisms (SNPs) analysed. The best fitting model of the association was determined using the Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) provided by SNPStats. The model with the lowest AIC and BIC values was considered the best fitting model. Haplotype frequencies estimation and global haplotype association were calculated using SNPstats57. If not differently specified, statistical analyses were performed using the SPSS package for Windows, v.20.0 (SPSS Inc, Chicago, IL).
Supplementary information
Acknowledgements
The excellent laboratory assistance of Vanessa Smerilli is acknowledged.
Abbreviations
- CAD
Coronary artery disease
- CVD
Cardiovascular disease
- TMA
Trimethylamine
- TMAO
Trimethylamine N-oxide
- CETP
Cholesteryl ester transfer protein
- GFR
Glomerular filtration rate
Author contributions
R.O., R.G. conceived the study and directed the project; J.S., A.S., L.L. obtained the samples and clinical details; L.B., I.P.-M., A.R. performed samples analysis; L.B. performed the statistical analysis; L.L., L.K., R.G., R.O. interpreted the results; R.O., L.B., L.L., L.K. participated in drafting the article or revising it critically for important intellectual content; all authors reviewed the manuscript.
Funding
This work was supported by RG’s Institutional research fund-Unicam (FPA000033) and the Ministry of Science and Higher Education Poland (Grant no DIR/WK/2017/01).
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Laura Bordoni, Email: laura.bordoni@unicam.it.
Robert A. Olek, Email: robert.olek@aol.com
Supplementary information
is available for this paper at 10.1038/s41598-020-75633-1.
References
- 1.Prince MJ, et al. The burden of disease in older people and implications for health policy and practice. Lancet. 2015;385:549–562. doi: 10.1016/S0140-6736(14)61347-7. [DOI] [PubMed] [Google Scholar]
- 2.Yurdagul A, Jr, Finney AC, Woolard MD, Orr AW. The arterial microenvironment: the where and why of atherosclerosis. Biochem. J. 2016;473:1281–1295. doi: 10.1042/BJ20150844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daniels TF, Killinger KM, Michal JJ, Wright RW, Jr, Jiang Z. Lipoproteins, cholesterol homeostasis and cardiac health. Int. J. Biol. Sci. 2009;5:474–488. doi: 10.7150/ijbs.5.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emini Veseli B, et al. Animal models of atherosclerosis. Eur. J. Pharmacol. 2017;816:3–13. doi: 10.1016/j.ejphar.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Paigen B, Morrow A, Brandon C, Mitchell D, Holmes P. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis. 1985;57:65–73. doi: 10.1016/0021-9150(85)90138-8. [DOI] [PubMed] [Google Scholar]
- 6.Vergeer M, Holleboom AG, Kastelein JJ, Kuivenhoven JA. The HDL hypothesis: does high-density lipoprotein protect from atherosclerosis? J. Lipid Res. 2010;51:2058–2073. doi: 10.1194/jlr.R001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lo Sasso G, et al. The Apoe(-/-) mouse model: a suitable model to study cardiovascular and respiratory diseases in the context of cigarette smoke exposure and harm reduction. J. Transl. Med. 2016;14:146. doi: 10.1186/s12967-016-0901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh V, Tiwari RL, Dikshit M, Barthwal MK. Models to study atherosclerosis: a mechanistic insight. Curr. Vasc. Pharmacol. 2009;7:75–109. doi: 10.2174/157016109787354097. [DOI] [PubMed] [Google Scholar]
- 9.Getz GS, Reardon CA. Animal models of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012;32:1104–1115. doi: 10.1161/ATVBAHA.111.237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 11.Ishibashi S, et al. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J. Clin. Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veniant MM, Withycombe S, Young SG. Lipoprotein size and atherosclerosis susceptibility in Apoe(-/-) and Ldlr(-/-) mice. Arterioscler Thromb. Vasc. Biol. 2001;21:1567–1570. doi: 10.1161/hq1001.097780. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett BJ, et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17:49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang DH, et al. Isoform specificity of trimethylamine N-oxygenation by human flavin-containing monooxygenase (FMO) and P450 enzymes: selective catalysis by FMO3. Biochem. Pharmacol. 1998;56:1005–1012. doi: 10.1016/s0006-2952(98)00218-4. [DOI] [PubMed] [Google Scholar]
- 16.Shih DM, et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J. Lipid. Res. 2015;56:22–37. doi: 10.1194/jlr.M051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koeth RA, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang WH, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins HL, et al. L-Carnitine intake and high trimethylamine N-oxide plasma levels correlate with low aortic lesions in ApoE(-/-) transgenic mice expressing CETP. Atherosclerosis. 2016;244:29–37. doi: 10.1016/j.atherosclerosis.2015.10.108. [DOI] [PubMed] [Google Scholar]
- 20.Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta-analysis of prospective studies. J. Am. Heart Assoc. 2017 doi: 10.1161/JAHA.116.004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roncal C, et al. Trimethylamine-N-oxide (TMAO) predicts cardiovascular mortality in peripheral artery disease. Sci. Rep. 2019;9:15580. doi: 10.1038/s41598-019-52082-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blauw LL, et al. CETP (cholesteryl ester transfer protein) concentration: a genome-wide association study followed by mendelian randomization on coronary artery disease. Circ. Genom. Precis. Med. 2018;11:e002034. doi: 10.1161/CIRCGEN.117.002034. [DOI] [PubMed] [Google Scholar]
- 23.Blauw LL, et al. Mendelian randomization reveals unexpected effects of CETP on the lipoprotein profile. Eur. J. Hum. Genet. 2019;27:422–431. doi: 10.1038/s41431-018-0301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwanicka J, et al. Relationship between CETP gene polymorphisms with coronary artery disease in Polish population. Mol. Biol. Rep. 2018;45:1929–1935. doi: 10.1007/s11033-018-4342-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voight BF, et al. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet. 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bustami J, et al. Cholesteryl ester transfer protein (CETP) I405V polymorphism and cardiovascular disease in eastern European Caucasians: a cross-sectional study. BMC Geriatr. 2016;16:144. doi: 10.1186/s12877-016-0318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gessner A, et al. Trimethylamine-N-oxide (TMAO) determined by LC-MS/MS: distribution and correlates in the population-based PopGen cohort. Clin. Chem. Lab. Med. 2020;58:733–740. doi: 10.1515/cclm-2019-1146. [DOI] [PubMed] [Google Scholar]
- 28.Cho CE, Caudill MA. Trimethylamine-N-oxide: friend, foe, or simply caught in the cross-fire? Trends Endocrinol. Metab. 2017;28:121–130. doi: 10.1016/j.tem.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Nowinski A, Ufnal M. Trimethylamine N-oxide: a harmful, protective or diagnostic marker in lifestyle diseases? Nutrition. 2018;46:7–12. doi: 10.1016/j.nut.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Lindskog Jonsson A, et al. Impact of gut microbiota and diet on the development of atherosclerosis in apoe(-/-) mice. Arterioscler. Thromb. Vasc. Biol. 2018;38:2318–2326. doi: 10.1161/ATVBAHA.118.311233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aldana-Hernandez P, et al. Dietary choline or trimethylamine n-oxide supplementation does not influence atherosclerosis development in ldlr-/- and apoe-/- male mice. J. Nutr. 2020;150:249–255. doi: 10.1093/jn/nxz214. [DOI] [PubMed] [Google Scholar]
- 32.Phillips IR, Shephard EA. Flavin-containing monooxygenase 3 (FMO3): genetic variants and their consequences for drug metabolism and disease. Xenobiotica. 2020;50:19–33. doi: 10.1080/00498254.2019.1643515. [DOI] [PubMed] [Google Scholar]
- 33.Warrier M, et al. The TMAO-generating enzyme flavin monooxygenase 3 is a central regulator of cholesterol balance. Cell Rep. 2015;10:326–338. doi: 10.1016/j.celrep.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu M, et al. Genetic and nongenetic factors associated with protein abundance of flavin-containing monooxygenase 3 in human liver. J. Pharmacol. Exp. Ther. 2017;363:265–274. doi: 10.1124/jpet.117.243113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Obeid R, et al. Plasma trimethylamine N-oxide concentration is associated with choline, phospholipids, and methyl metabolism. Am. J. Clin. Nutr. 2016;103:703–711. doi: 10.3945/ajcn.115.121269. [DOI] [PubMed] [Google Scholar]
- 36.Gansevoort RT, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382:339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 37.Damman K, et al. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur. Heart J. 2014;35:455–469. doi: 10.1093/eurheartj/eht386. [DOI] [PubMed] [Google Scholar]
- 38.Balarini CM, et al. Hypercholesterolemia promotes early renal dysfunction in apolipoprotein E-deficient mice. Lipids Health. Dis. 2011;10:220. doi: 10.1186/1476-511X-10-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bain MA, Faull R, Fornasini G, Milne RW, Evans AM. Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol. Dial. Transplant. 2006;21:1300–1304. doi: 10.1093/ndt/gfk056. [DOI] [PubMed] [Google Scholar]
- 40.Hauet T, et al. Citrate, acetate and renal medullary osmolyte excretion in urine as predictor of renal changes after cold ischaemia and transplantation. Clin. Chem. Lab. Med. 2000;38:1093–1098. doi: 10.1515/CCLM.2000.162. [DOI] [PubMed] [Google Scholar]
- 41.Missailidis C, et al. Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS ONE. 2016;11:e0141738. doi: 10.1371/journal.pone.0141738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mueller DM, et al. Plasma levels of trimethylamine-N-oxide are confounded by impaired kidney function and poor metabolic control. Atherosclerosis. 2015;243:638–644. doi: 10.1016/j.atherosclerosis.2015.10.091. [DOI] [PubMed] [Google Scholar]
- 43.Mafune A, et al. Associations among serum trimethylamine-N-oxide (TMAO) levels, kidney function and infarcted coronary artery number in patients undergoing cardiovascular surgery: a cross-sectional study. Clin. Exp. Nephrol. 2016;20:731–739. doi: 10.1007/s10157-015-1207-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gruppen EG, et al. TMAO is associated with mortality: impact of modestly impaired renal function. Sci. Rep. 2017;7:13781. doi: 10.1038/s41598-017-13739-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huc T, et al. Chronic, low-dose TMAO treatment reduces diastolic dysfunction and heart fibrosis in hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2018;315:H1805–H1820. doi: 10.1152/ajpheart.00536.2018. [DOI] [PubMed] [Google Scholar]
- 46.Baskakov I, Bolen DW. Forcing thermodynamically unfolded proteins to fold. J. Biol. Chem. 1998;273:4831–4834. doi: 10.1074/jbc.273.9.4831. [DOI] [PubMed] [Google Scholar]
- 47.Jaworska K, Bielinska K, Gawrys-Kopczynska M, Ufnal M. TMA (trimethylamine), but not its oxide TMAO (trimethylamine-oxide), exerts haemodynamic effects: implications for interpretation of cardiovascular actions of gut microbiome. Cardiovasc. Res. 2019;115:1948–1949. doi: 10.1093/cvr/cvz231. [DOI] [PubMed] [Google Scholar]
- 48.Tang WH, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ. Res. 2017;120:1183–1196. doi: 10.1161/CIRCRESAHA.117.309715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yin J, et al. Dysbiosis of gut microbiota with reduced trimethylamine-N-oxide level in patients with large-artery atherosclerotic stroke or transient ischemic attack. J. Am. Heart Assoc. 2015 doi: 10.1161/JAHA.115.002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuhn T, et al. Intra-individual variation of plasma trimethylamine-N-oxide (TMAO), betaine and choline over 1 year. Clin. Chem. Lab. Med. 2017;55:261–268. doi: 10.1515/cclm-2016-0374. [DOI] [PubMed] [Google Scholar]
- 51.Thygesen K, et al. Third universal definition of myocardial infarction. Glob. Heart. 2012;7:275–295. doi: 10.1016/j.gheart.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Roffi M, et al. [2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the european society of cardiology (ESC)] G. Ital. Cardiol. 2016;17:831–872. doi: 10.1714/2464.25804. [DOI] [PubMed] [Google Scholar]
- 53.Grinberga S, et al. Determination of trimethylamine-N-oxide in combination with L-carnitine and gamma-butyrobetaine in human plasma by UPLC/MS/MS. Biomed. Chromatogr. 2015;29:1670–1674. doi: 10.1002/bmc.3477. [DOI] [PubMed] [Google Scholar]
- 54.Awwad HM, Geisel J, Obeid R. Determination of trimethylamine, trimethylamine N-oxide, and taurine in human plasma and urine by UHPLC-MS/MS technique. J. Chromatogr. B. 2016;1038:12–18. doi: 10.1016/j.jchromb.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 55.Qi J, et al. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: a systematic review and meta-analysis of 11 prospective cohort studies. J. Cell Mol. Med. 2018;22:185–194. doi: 10.1111/jcmm.13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 57.Sole X, Guino E, Valls J, Iniesta R, Moreno V. SNPStats: a web tool for the analysis of association studies. Bioinformatics. 2006;22:1928–1929. doi: 10.1093/bioinformatics/btl268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.