Abstract
Female and male very elderly intensive patients (VIPs) might differ in characteristics and outcomes. We aimed to compare female versus male VIPs in a large, multinational collective of VIPs with regards to outcome and predictors of mortality. In total, 7555 patients were included in this analysis, 3973 (53%) male and 3582 (47%) female patients. The primary endpoint was 30-day-mortality. Baseline characteristics, data on management and geriatric scores including frailty assessed by Clinical Frailty Scale (CFS) were documented. Two propensity scores (for being male) were obtained for consecutive matching, score 1 for baseline characteristics and score 2 for baseline characteristics and ICU management. Male VIPs were younger (83 ± 5 vs. 84 ± 5; p < 0.001), less often frail (CFS > 4; 38% versus 49%; p < 0.001) but evidenced higher SOFA (7 ± 6 versus 6 ± 6 points; p < 0.001) scores. After propensity score matching, no differences in baseline characteristics could be observed. In the paired analysis, the mortality in male VIPs was higher (mean difference 3.34% 95%CI 0.92–5.76%; p = 0.007) compared to females. In both multivariable logistic regression models correcting for propensity score 1 (aOR 1.15 95%CI 1.03–1.27; p = 0.007) and propensity score 2 (aOR 1.15 95%CI 1.04–1.27; p = 0.007) male sex was independently associated with higher odds for 30-day-mortality. Of note, male gender was not associated with ICU mortality (OR 1.08 95%CI 0.98–1.19; p = 0.14). Outcomes of elderly intensive care patients evidenced independent sex differences. Male sex was associated with adverse 30-day-mortality but not ICU-mortality. Further research to identify potential sex-specific risk factors after ICU discharge is warranted.
Trial registration: NCT03134807 and NCT03370692; Registered on May 1, 2017 https://clinicaltrials.gov/ct2/show/NCT03370692.
Subject terms: Health care, Medical research
Introduction
Patients 80 years of age and older, who are admitted to the intensive care unit (ICU) consume a large proportion of health care resources and yet continue to suffer from high mortality1–3. Detailed knowledge of these very elderly intensive patients (VIPs) could help to perform better risk stratification and ultimately guide clinicians in whom to admit or whom not to admit to the ICU. The Clinical Frailty Scale (CFS), evaluating frailty through a simple clinical assessment, has been shown to adequately risk-stratify such elderly patients4,5.
For several medical conditions, including acute myocardial infarction, gender outcome disparities have been reported6. However, some studies investigated gender differences in ICU patients, and have found distinct differences7,8. Male and female intensive care patients differ with regards to baseline characteristics, risk distribution and admission diagnoses and these differences may influence outcomes9,10. Male sex was linked to adverse outcomes in a sub-set of VIPs with sepsis10,11. On the other hand, female sex was reported to be independently associated with the decision to withdraw or withhold intensive care12. Recently, the FROG-ICU evaluated survival in critically ill patients and reported a trend towards higher survival in elderly women compared to male patients13.
We, therefore, aimed to compare male versus female VIPs with regards to the distribution of risk factors, potential differences in management, and outcome as well as predictors of mortality with special emphasis on frailty. The main goal with this study using data from two recent large, multinational studies of VIPs was to compare male and female patients with regards to crude unadjusted und adjusted baseline characteristics and outcomes4,14,15.
Methods
Study subjects
VIP1 and VIP2 were prospective, multicenter studies, registered on ClinicalTrials.gov (ID: NTC03134807, NCT03370692). Both studies included very old intensive care patients (VIPs), defined as patients admitted to an ICU and being aged 80 years or older. These patients have been analyzed in other contexts and methods and results have been published previously4,5,16. In summary, for VIP1, each participating ICU could include either consecutive patients during three months or the first 20 consecutive patients fulfilling the inclusion criteria (all patients 80 of age or older). Data were collected between October 2016 and February 2017. For VIP2, VIPs were included from May 2018 to May 2019. All methods were carried out in accordance with relevant guidelines and regulations. All experimental protocols were approved by the local institutional and/or licensing committees. Informed consent was obtained from all subjects if not omitted by the ethics vote.
In this post-hoc analysis of these two prospective trials, all patients admitted acutely (non-electively) with complete data on age, gender, clinical frailty score (CFS) frailty score and sequential organ failure assessment (SOFA) score and 30-day-mortality were included (Supplemental Fig. 1). Elective patients from VIP1 were specifically excluded as they significantly differ from acutely admitted patients in risk distribution and outcomes as previously shown17. The primary endpoint of this study was 30-day-mortality. Frailty was assessed by CFS and the respective visual and simple description which were used with permission18–20. For the patients of the VIP2 trial Katz activities of daily living (Katz ADL) with ADL score ≤ 4 defining disability and Short form of Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE), with IQCODE ≥ 3.5 defining cognitive decline were assessed18–20.
Statistical analysis
Continuous data points are expressed as mean ± standard deviation (SD) or median ± interquartile range depending on distribution. Differences between independent groups were calculated using student’s T-test or Mann Whitney U-test accordingly. Categorical data are expressed as numbers (percentage). Chi-square test was applied to calculate differences between groups and McNemar’s test for paired survival data.
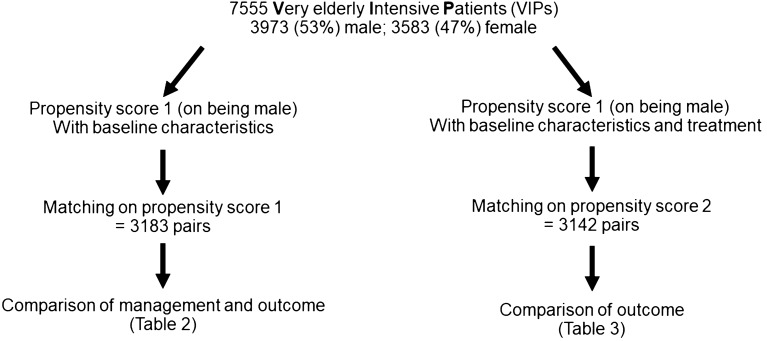
Two propensity scores for being male were calculated (Fig. 1). Propensity score 1 included age (per year), CFS score (per point), SOFA score (per point), location (Western Europe, Eastern Europe, Non-European) and admission diagnosis (respiratory failure, circulatory failure, combined respiratory and circulatory failure, sepsis, multi-trauma with and without head injury, isolated head injury, intoxication, cerebral injury without trauma, emergency surgery, other). Propensity score 2 included all items of propensity score 1 plus the non-baseline variables use of vasoactive drugs, of renal replacement therapy, of intubation, of non-invasive ventilation (NIV) as well as the decision to limit life-sustaining treatment (withdrawal and/or withholding). Two matched cohorts, matching males 1:1 to females, for (1) propensity score 1 and (2) propensity score 2 were obtained using “nearest neighbor” matching, the maximum allowed distance was a Δ in propensity score 1 or 2 of 0.001. The matching significantly reduced differences in baseline characteristics and management.
Figure 1.
Flow chart of the propensity-score matching process.
Sensitivity analysis, analyzing only patients without treatment restrictions and European patients, was performed. Univariable and multivariable logistic regression analysis was performed to assess associations with treatment withdrawal and mortality. Odds ratios (OR) and adjusted odds ratios (aOR) with respective 95% confidence intervals (CI) were calculated. Two multivariable logistic regression models were built for the total cohort, (1) using propensity score 1 and (2) using propensity score 2 as covariable. For the sub-group analysis assessing associations of parameters with 30-day-mortality in male and female patients, variables with a p value < 0.10 in the univariable analysis were included in the multivariable model, then a backward elimination was performed, the elimination criterion was 0.10. All tests were two-sided, and a p value of < 0.05 was considered statistically significant. SPSS version 23.0 (IBM, USA) and MedCalc Statistical Software version 19.1.3 (MedCalc Software bv, Ostend, Belgium; https://www.medcalc.org; 2019) were used for all statistical analyses.
Ethics approval and consent to participate
A study protocol was provided to participating centers. Every participating center obtained ethics approval according to local legislation. A copy of the ethics approval was sent to the study coordinator before start of the study.
Consent for publication
Written informed consent was obtained of all included subjects, except for patients from VIP2 of sites where study inclusion was explicitly granted without written informed consent.
Results
Study population
In total, 7555 patients were included in this analysis, 3973 (53%) male and 3582 (47%) female patients. Admission diagnoses and baseline characteristics are presented in Table 1. Male patients were younger compared to female patients, with fewer male patients being over 90 years of age (6% vs. 8%; p < 0.001). Male patients were less often frail (CFS > 4; 38% vs. 49%; p < 0.001) and less often suffered from disability (ADL ≤ 4; 25% vs. 31%; p < 0.001), and cognitive decline (IQCODE ≥ 3.5; 29% vs. 36%; p < 0.001).
Table 1.
Baseline characteristics in the total cohort, male versus female VIPs.
| Male | Female | p value | |
|---|---|---|---|
| n = 3973 | n = 3582 | ||
| Age | |||
| Median (± IQR) | 83 (5) | 84 (5) | < 0.001 |
| Age > 90 n (%) | 227 (5.7%) | 288 (8%) | < 0.001 |
| Frailty score—CFS | |||
| Median (± IQR) | 4 (2) | 4 (3) | < 0.001 |
| Frailty (CFS > 4) n (%) | 1519 (38%) | 1754 (49%) | < 0.001 |
| ADL | |||
| Median (± IQR) | 6 (1) | 6 (2) | < 0.001 |
| Disablitiy (ADL ≤ 4) | 446 (25%) | 489 (31%) | < 0.001 |
| IQCODE | |||
| Median (± IQR) | 3.2 (0.6) | 3.3 (0.8) | 0.001 |
| Cognitive decline (IQCODE ≥ 3.5) | 455 (29%) | 486 (36%) | < 0.001 |
| median (± IQR) | 7 (6) | 6 (6) | < 0.001 |
| ICU length of stay (hours) | |||
| median (± IQR) | 89 (154) | 72 (131) | < 0.001 |
| Treatment withdraw and/or withold (%) | 1235 (35) | 1342 (34) | 0.53 |
| NIV n (%) | 933 (25%) | 873 (24%) | 0.54 |
| Intubation n (%) | 2108 (53%) | 1728 (48%) | < 0.001 |
| Renal replacement therapy n (%) | 530 (13%) | 296 (8%) | < 0.001 |
| Vasoactive drugs n (%) | 2397 (60%) | 2038 (57%) | 0.003 |
| Admission diagnosis | |||
| Respiratory failure | 928 (23%) | 889 (25%) | < 0.001 |
| Circulatory failure | 577 (15%) | 490 (14%) | |
| Combined circulatory and respiratory failure | 493 (12%) | 395 (11%) | |
| Sepsis | 555 (14%) | 451 (13%) | |
| Multitrauma w/o head injury | 82 (2%) | 58 (2%) | |
| Trauma with head injury | 74 (2%) | 57 (2%) | |
| Head injury | 100 (3%) | 83 (2%) | |
| Intoxication | 12 (< 1%) | 24 (1%) | |
| Cerebral injury (non-traumatic) | 231 (6%) | 248 (7%) | |
| Emergency surgery | 442 (6%) | 464 (13%) | |
| Other | 479 (12%) | 423 (12%) | |
CFS Clinical Frailty Scale, SOFA Sequential Organ Failure Assessment, ADL Activity of Daily Life measured with the Katz Index, IQCODE Informant Questionnaire on COgnitive Decline in the Elderly, ICU intensive care unit, NIV non-invasive ventilation, SD standard deviation.
Rates of non-invasive ventilation usage (NIV; 25% vs. 24%; p = 0.29) did not differ between male and female patients. Rates of intubation (53% vs. 48%; p < 0.001), renal replacement therapy (13% vs. 8%; p < 0.001) and vasoactive drugs (60% vs. 57%; p = 0.003) were higher in male patients compared to females.
Organ failures as assessed by SOFA score was higher in male patients (7 ± 6 vs. 6 ± 6 points; p < 0.001) and the length of ICU stay was longer (89 ± 154 vs. 72 ± 131 h; p < 0.001).
The rates of life-sustainment limitation were similar (35% vs. 34%; p = 0.53). In multivariable logistic regression model, after correction for propensity score 1, male gender was not independently associated with any treatment limitation (aOR 0.92 95%CI 0.83–1.03; p = 0.14).
Survival analysis in the total cohort
In univariable analysis in the unbalanced total cohort, 30-day-mortality was higher (43% vs. 39%; OR 1.18 95%CI 1.08–1.30; p < 0.001) in male patients compared to female patients. In multivariable logistic regression models correcting for propensity score 1 (aOR 1.15 95%CI 1.03–1.27; p = 0.007) as well as propensity score 2 (aOR 1.15 95%CI 1.04–1.27; p = 0.007) male gender was independently associated with higher odds for 30-day-mortality. Also, after adjustment for propensity score 2 and length of ICU stay, male sex (aOR 1.13 95%CI 1.03–1.24; p = 0.01) remained independently associated with higher odds for 30-day-mortality.
In sensitivity analysis excluding patients with treatment limitation, after correction for propensity score 1 male gender was independently associated with mortality (aOR 1.19 95%CI 1.04–1.38; p = 0.02) and remained so in trend after correction for propensity score 2 (aOR 1.15 95%CI 0.996–1.326; p = 0.056). In sensitivity analysis excluding non-European countries, male gender was independently associated with higher rates of 30-day-mortality after correction for propensity score 1 (aOR 1.14 95%CI 1.03–1.26; p = 0.01) and propensity score 2 (aOR 1.14 95%CI 1.03–1.27; p = 0.01). Of note, male gender was not associated with ICU mortality (OR 1.08 95%CI 0.98–1.19; p = 0.14).
Matched-cohort 1
Baseline characteristics of the matched-cohort 1 (matched on propensity score 1, which included only baseline variables, see Fig. 1) are given in Table 2. Risk parameters were evenly distributed between male and female patients, but rates of renal replacement therapy were higher (13% vs. 9%; p < 0.001) in males as were lengths of ICU stay.
Table 2.
Baseline characteristics in the matched cohort 1, male versus female VIPs.
| Male | Female | p value | |
|---|---|---|---|
| n = 3183 | n = 3183 | ||
| Age | |||
| Median (± IQR) | 84 (6) | 84 (6) | 0.91 |
| Age > 90 n (%) | 207 (7%) | 195 (6%) | 0.57 |
| Frailty score—CFS | |||
| Median (± IQR) | 4 (3) | 4 (3) | 0.94 |
| Frailty (CFS > 4) n (%) | 1379 (43%) | 1409 (44%) | 0.46 |
| ADL | |||
| Median (± IQR) | 6 (2) | 6 (2) | 0.40 |
| Disablitiy (ADL ≤ 4) | 400 (28%) | 375 (27%) | 0.56 |
| IQCODE | |||
| Median (± IQR) | 3.3 (0.7) | 3.3 (0.7) | 0.92 |
| Cognitive decline (IQCODE ≥ 3.5) | 404 (32%) | 404 (33%) | 0.49 |
| SOFA score | |||
| Median (± IQR) | 7 (6) | 6 (6) | 0.19 |
| ICU length of stay (hours) | |||
| Median (± IQR) | 86 (151) | 72 (132) | < 0.001 |
| Treatment withdraw and/or withold (%) | 1054 (33%) | 1111 (35%) | 0.15 |
| NIV n (%) | 789 (25%) | 784 (25%) | 0.88 |
| Intubation n (%) | 1623 (51%) | 1559 (49%) | 0.10 |
| Renal replacement therapy n (%) | 397 (13%) | 277 (9%) | < 0.001 |
| Vasoactive drugs n (%) | 1846 (58%) | 1850 (58%) | 0.92 |
| Admission diagnosis | |||
| Respiratory failure | 783 (25%) | 786 (25%) | 0.99 |
| Circulatory failure | 445 (14%) | 449 (14%) | |
| Combined circulatory and respiratory failure | 353 (11%) | 356 (11%) | |
| Sepsis | 413 (13%) | 410 (13%) | |
| Multitrauma w/o head injury | 63 (2%) | 55 (2%) | |
| Trauma with head injury | 52 (2%) | 55 (2%) | |
| Head Injury | 78 (3%) | 74 (2%) | |
| Intoxication | 8 (< 1%) | 14 (< 1%) | |
| Cerebral injury (non-traumatic) | 203 (6%) | 199 (6%) | |
| Emergency surgery | 393 (12%) | 391 (12%) | |
| Other | 392 (12%) | 391 (12%) | |
CFS Clinical Frailty Scale, SOFA Sequential Organ Failure Assessment, ADL Activity of Daily Life measured with the Katz Index, IQCODE Informant Questionnaire on COgnitive Decline in the Elderly, ICU intensive care unit, NIV non-invasive ventilation, SD standard deviation.
In the paired analysis, the mortality in male VIPs was higher (mean difference 3.33% 95%CI 0.92–5.74%; p = 0.007) compared to females. In univariable logistic regression, male gender was associated with higher odds for 30-day-mortality (42% vs. 38%; OR 1.15 95%CI 1.04–1.27; p = 0.007). Again, male gender was not (OR 1.02 95%CI 0.92–1.14; p = 0.69) associated with intra-ICU mortality in this matched cohort.
Matched-cohort 2
Table 3 shows baseline characteristics of matched-cohort 2 (matched on propensity score 2, which includes baseline variables and information on organ support as well as treatment limitations, see Fig. 1). Again, male patients evidenced longer ICU stays (p < 0.001).
Table 3.
Baseline characteristics in the matched cohort 2, male versus female VIPs.
| Male | Female | p value | |
|---|---|---|---|
| n = 3142 | n = 3142 | ||
| Age | |||
| Mean (± SD) | 84 (5) | 84 (6) | 0.61 |
| Age > 90 n (%) | 213 (7%) | 207 (7%) | 0.80 |
| Frailty score—CFS | |||
| Mean (± SD) | 4 (3) | 4 (3) | 0.60 |
| Frailty (CFS > 4) n (%) | 1355 (43%) | 1406 (45%) | 0.20 |
| ADL | |||
| Mean (± SD) | 6 (2) | 6 (2) | 0.40 |
| Disablitiy (ADL ≤ 4) | 390 (27%) | 366 (27%) | 0.73 |
| IQCODE | |||
| Mean (± SD) | 3.2 (0.7) | 3.3 (0.7) | 0.41 |
| Cognitive decline (IQCODE ≥ 3.5) | 392 (32%) | 390 (33%) | 0.38 |
| SOFA score | |||
| Mean (± SD) | 7 (6) | 6 (6) | 0.48 |
| ICU length of stay (hours) | |||
| Mean (± SD) | 78 (136) | 72 (133) | 0.007 |
| Treatment withdraw and/or withold (%) | 1077 (34%) | 1080 (34%) | 0.96 |
| NIV n (%) | 779 (25%) | 792 (25%) | 0.73 |
| Intubation n (%) | 1566 (50%) | 1548 (49%) | 0.67 |
| Renal replacement therapy n (%) | 287 (9%) | 285 (9%) | 0.93 |
| Vasoactive drugs n (%) | 1825 (58%) | 1819 (58%) | 0.90 |
| Admission diagnosis | |||
| Respiratory failure | 766 (24%) | 781 (25%) | |
| Circulatory failure | 453 (14%) | 441 (14%) | |
| Combined circulatory and respiratory failure | 361 (12%) | 362 (12%) | |
| Sepsis | 406 (13%) | 418 (13%) | |
| Multitrauma w/o head injury | 53 (2%) | 57 (2%) | |
| Trauma with head injury | 51 (2%) | 50 (2%) | |
| Head injury | 79 (3%) | 80 (3%) | |
| Intoxication | 7 (< 1%) | 7 (< 1%) | |
| Cerebral injury (non-traumatic) | 200 (6%) | 195 (6%) | |
| Emergency surgery | 397 (13%) | 375 (12%) | |
| Other | 369 (12%) | 376 (12%) | |
| Other | 369 (12%) | 376 (12%) | |
CFS Clinical Frailty Scale, SOFA sequential organ failure assessment, ADL Activity of Daily Life measured with the Katz Index, IQCODE Informant Questionnaire on COgnitive Decline in the Elderly, ICU intensive care unit, NIV Non-invasive ventilation, SD standard deviation.
Again, in the paired analysis, the mortality in male VIPs was higher (mean difference 3.34% 95%CI 0.92–5.76%; p = 0.007) compared to females. In univariable logistic regression, male gender was associated with higher odds for 30-day-mortality (42% vs. 39%; aOR 1.15 95%CI 1.04–1.27; p = 0.007). Again, gender was not associated with ICU mortality (OR 1.02 95%CI 0.92–1.14; p = 0.67) in this matched cohort.
Sub-group analysis of female and male patients
The presence of frailty (CFS > 4) was associated with increased 30-day-mortality in male patients (OR 1.73 95%CI 1.52–1.97; p < 0.001) and remained so in multivariable logistic regression (Table 4a).
Table 4.
Associations of relevant factors with 30-day mortality in (a) male patients and (b) female patients.
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p value | aOR | 95%CI | p value | |
| a | ||||||
| Male patients | ||||||
| Age (per year) | 1.04 | 1.02–1.05 | < 0.001 | 1.03 | 1.01–1.05 | 0.02 |
| SOFA (per point) | 1.18 | 1.16–1.20 | < 0.001 | 1.11 | 1.08–1.13 | < 0.001 |
| Frailty (per CFS point) | 1.21 | 1.16–1.25 | < 0.001 | 1.13 | 1.09–1.18 | < 0.001 |
| Vasoactive drug (yes vs. no) | 2.44 | 2.13–2.79 | < 0.001 | 0.98 | 0.81–1.19 | 0.87 |
| Intubation (yes vs. no) | 2.75 | 2.41–3.14 | < 0.001 | 2.34 | 1.97–2.78 | < 0.001 |
| Renal replacement therapy (yes vs. no) | 2.05 | 1.71–2.47 | < 0.001 | 1.58 | 1.27–1.98 | 0.001 |
| Treatment withdrawal or withholding (yes vs. no) | 9.02 | 7.74–10.51 | < 0.001 | 8.99 | 7.62–10.61 | < 0.001 |
| b | ||||||
| Female patients | ||||||
| Age (per year) | 1.02 | 1.002–1.038 | 0.03 | 1.02 | 0.99–1.04 | 0.17 |
| SOFA (per point) | 1.22 | 1.19–1.14 | < 0.001 | 1.14 | 1.11–1.17 | < 0.001 |
| Frailty (per CFS point) | 1.22 | 1.17–1.27 | < 0.001 | 1.16 | 1.10–1.21 | < 0.001 |
| Vasoactive drug (yes/no) | 3.09 | 2.67–3.57 | < 0.001 | 1.27 | 1.03–1.55 | 0.02 |
| Intubation (yes/no) | 3.63 | 3.15–4.18 | < 0.001 | 2.53 | 2.08–3.07 | < 0.001 |
| Renal replacement therapy (yes/no) | 3.52 | 2.73–4.52 | < 0.001 | 3.34 | 1.74–3.15 | < 0.001 |
| Treatment withdrawal or withholding (yes/no) | 6.95 | 5.96–8.10 | < 0.001 | 8.15 | 6.83–9.72 | < 0.001 |
OR odds ratio, aOR adjusted OR, SOFA Sequential Organ Failure Assessment, CFS Clinical Frailty Scale.
In female patients frailty (CFS > 4) was associated with 30-day mortality in univariable analysis (OR 1.65 95%CI 1.44–1.89; p < 0.001) and remained so after correction in multivariable logistic regression (Table 4b).
Furthermore, one-point increases of CFS, as well as SOFA, were independently associated with increased odds for 30-day-mortality in multivariable logistic regression in male (Table 4a) as well as in female (Table 4b) VIPs.
Discussion
In this post-hoc analysis of a large group of VIPs included in two international ICU prospective studies, differences in the distribution of baseline characteristics and risk factors between male and female patients could be found. Further, male sex was associated with increased 30-day-mortality in VIPs and remained so after propensity-score adjustments for both baseline characteristics alone and baseline characteristics as well as in-ICU variables. However, sex was not associated with ICU-mortality, neither in the total cohort nor in the adjusted matched cohorts.
Frailty assessed by CFS was independently associated with 30-day-mortality both in male and female patients after adjustment for baseline risk factors. Therefore, CFS could safely be integrated in guiding pre-ICU triage as well as intra-ICU triage both in male and female patients.
Male and female patients differed with regards to baseline characteristics, management, and outcomes. Male VIPs in this cohort were younger and evidenced lower rates of frailty, disability and cognitive impairment. On the other hand, male patients were clinically sicker as expressed by higher SOFA scores. Consequently, unadjusted 30-day-mortality was higher in male compared to female VIPs. After adjustment for baseline characteristics, except for rates of renal replacement therapy, there were no differences in the management of organ support between male and female patients. Of note, there are recent data indicating higher susceptibility of kidney to injury in male epithelial cells as compared to female21. Importantly, the rates of treatment limitation did not differ between male and female VIPs, nor after adjustment in multivariable analysis in the total cohort neither in the propensity-matched cohorts.
However, after matching and adjustment for both baseline characteristics alone as well as baseline characteristics plus ICU management, male gender was still independently associated with increased 30-day-mortality in this analysis. Further, male gender remained independently associated with increased 30-day-mortality in a sensitivity analysis excluding patients with treatment limitations. Importantly, these results confirm observed trends in a recent sub-study of the FROG-ICU study: Hollinger et al. reported increased survival rates in moderately elderly women compared to men, whereas in the overall cohort consisting of more than 2000 critically ill patients no sex-related differences in outcomes could be found13. These findings, relating male gender to adverse outcomes, are consistent with previous studies reporting adverse outcomes in male septic VIPs10. On the other hand, this trend in gender difference was not observed for illness-adjusted mortality in a large Austrian cohort study on 25,998 patients without age-restriction22. Therefore, the observed sex differences in mortality could be age-dependent.
Several factors could contribute to this finding. Certainly, bias and lack of data need to be considered, although extensive adjustment for baseline characteristics as well as treatment management was performed using propensity scores. However, importantly, only adjustment to available and known covariables is possible. First, this study lacks extensive data on comorbidities, which probably influence management and outcome23. However, adjustments for frailty, which is associated with the amount and extent of comorbidities, were performed. Second, further sensitivity analysis and adjustment on both macro- microcirculatory parameters could have improved our understanding of this cohort as men have a shorter life expectancy and die at a younger age: Their bodies are more worn at a same age which could be underestimated in categorical datasets, like SOFA and CFS: continuous data (like biomarkers) could pick up this difference24. Especially biomarkers such as lactate concentration might help to further explain the findings—on the other hand, male sex was independently associated with increased mortality after correction for baseline variables including SOFA score which integrates clinical findings and laboratory values25. Also, other important biomarkers such as serum levels of albumin and blood urine nitrogen could contribute to the observed sex specific differences in outcome, but were not available for this dataset, which is a limitation26. Third, this cohort of VIPs was not designed to evaluate gender-related differences and, therefore, this analysis remains of retrospective and thesis-generating character per se. Fourth, other potential confounders, such as smoking status or socioeconomic data are lacking, which is another limitation27. Fifth, we observed a sex-specific difference in 30-day-mortality, but not in ICU mortality. We speculate, that this could be due to sex-specific differences in management and treatment after discharge from ICU. However, we do not have any data available to support this notion, which, therefore, remains speculative. As the overall event rate increases from ICU mortality to the 30-day-mortality increases, sex-specific outcome differences could be present at ICU discharge, but our dataset be underpowered to detect these differences. Still, to our knowledge, this study constitutes the largest cohort of VIPs reporting gender-related outcomes. Therefore, we think that this strong signal towards adverse outcomes in male VIPs must be taken seriously.
Several biological and non-biological factors could influence gender-related outcomes. Males and females are known to differ in genetic, endocrine, and immunological factors13,28. Sex-specific treatment algorithms and ICU management could contribute to minimizing the observed gender disparities in VIPs. Further, male and female patients could differ in post-ICU discharge factors. Socioeconomic factors beyond the scope of this study could influence outcomes29. Males and females are known to differ in their readiness to assume risk and especially after an ICU stay, gender-specific complications such as falls might in part explain observed distinct outcomes30,31. This notion is supported by the finding that although mortality was consistently higher in males, ICU-mortality was similar between males and females, both in the unadjusted and adjusted cohorts. The benefit of intensive care in VIP is controversial in general 32. VIPs are known to suffer from high mortality after surviving the initial ICUstay32. Based on our data, male patients might be particularly prone to die after ICU discharge as ICU mortality was similar between genders, but 30-day-mortality independently associated with male gender. This finding could have several implications. First, male gender could be interpreted as an independent risk factor and influence management decisions. Second, if male VIPs are admitted to ICU and survive, post-ICU management could be particularly important in male patients. Specific geriatric ICUs and discharge to specialist geriatric wards, as well as close interdisciplinary collaboration with social workers and integration of the patient’s family, could further improve outcomes in both genders, but especially males. Therefore, not only gender-specific ICU treatment but also post-ICU management could help to improve outcomes in general and reduce observed gender disparities in VIPs.
Conclusion
Outcomes of elderly intensive care patients evidenced independent sex differences. Male sex was associated with adverse 30-day-mortality but not ICU-mortality. Further research to identify potential sex-specific risk factors after ICU discharge is warranted.
Supplementary information
Acknowledgements
The full list of members of the VIP2 study group can be found in the accompanying Supplementary Information file.
Author contributions
B.W. and R.R.B and CJ and H.F. and B.G and D.D.L wrote the first draft of the main manuscript text and prepared the Figures and Tables. All authors are involved in some work of the manuscript. All authors provided critical revision of the manuscript..
Funding
Open Access funding enabled and organized by Projekt DEAL. No (industry) sponsorship has been received for this investigator-initiated study.
Data availbility
No additional data available. All data relevant for this study will be given by the authors upon specific request.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: In the original version of this Article, the VIP2 Study Group was incorrectly listed as an author in the author list. All members of the VIP2 Study Group are listed in the accompanying Supplementary Information file.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/5/2021
A Correction to this paper has been published: 10.1038/s41598-021-93448-6
Supplementary information
is available for this paper at 10.1038/s41598-020-74910-3.
References
- 1.Boumendil A, Somme D, Garrouste-Orgeas M, Guidet B. Should elderly patients be admitted to the intensive care unit? Intensive Care Med. 2007;33:1252. doi: 10.1007/s00134-007-0621-3. [DOI] [PubMed] [Google Scholar]
- 2.Guidet B, de Lange DW, Flaatten H. Should this elderly patient be admitted to the ICU? Intensive Care Med. 2018 doi: 10.1007/s00134-018-5054-7. [DOI] [PubMed] [Google Scholar]
- 3.Flaatten H, Garrouste-Orgeas M. The very old ICU patient: a never-ending story. Intensive Care Med. 2015;41:1996–1998. doi: 10.1007/s00134-015-4052-2. [DOI] [PubMed] [Google Scholar]
- 4.Flaatten H, et al. The impact of frailty on ICU and 30-day mortality and the level of care in very elderly patients (>/= 80 years) Intensive Care Med. 2017;43:1820–1828. doi: 10.1007/s00134-017-4940-8. [DOI] [PubMed] [Google Scholar]
- 5.Muessig JM, et al. Clinical Frailty Scale (CFS) reliably stratifies octogenarians in German ICUs: a multicentre prospective cohort study. BMC Geriatr. 2018;18:162. doi: 10.1186/s12877-018-0847-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiele F, et al. Propensity score-matched analysis of effects of clinical characteristics and treatment on gender difference in outcomes after acute myocardial infarction. Am. J. Cardiol. 2011;108:789–798. doi: 10.1016/j.amjcard.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 7.Schoeneberg C, et al. Gender-specific differences in severely injured patients between 2002 and 2011: data analysis with matched-pair analysis. Crit. Care. 2013;17:R277. doi: 10.1186/cc13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park J, et al. A nationwide analysis of intensive care unit admissions, 2009–2014—The Korean ICU National Data (KIND) study. J. Crit. Care. 2018;44:24–30. doi: 10.1016/j.jcrc.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Tibullo L, Esquinas A. Outcomes difference in non-invasive ventilation in ‘very old’ patients with acute respiratory failure: occult gender effect? Emerg. Med. J. 2019;36:514. doi: 10.1136/emermed-2019-208692. [DOI] [PubMed] [Google Scholar]
- 10.Cilloniz C, et al. Risk and prognostic factors in very old patients with sepsis secondary to community-acquired pneumonia. J. Clin. Med. 2019 doi: 10.3390/jcm8070961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 12.Block L, et al. Age, SAPS 3 and female sex are associated with decisions to withdraw or withhold intensive care. Acta Anaesthesiol. Scand. 2019;63:1210–1215. doi: 10.1111/aas.13411. [DOI] [PubMed] [Google Scholar]
- 13.Hollinger A, et al. Gender and survival of critically ill patients: results from the FROG-ICU study. Ann. Intensive Care. 2019;9:43. doi: 10.1186/s13613-019-0514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guidet B, et al. The contribution of frailty, cognition, activity of daily life and comorbidities on outcome in acutely admitted patients over 80 years in European ICUs: the VIP2 study. Intensive Care Med. 2019 doi: 10.1007/s00134-019-05853-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Agostino RB., Jr Propensity scores in cardiovascular research. Circulation. 2007;115:2340–2343. doi: 10.1161/CIRCULATIONAHA.105.594952. [DOI] [PubMed] [Google Scholar]
- 16.Guidet B, et al. Withholding or withdrawing of life-sustaining therapy in older adults (>/= 80 years) admitted to the intensive care unit. Intensive Care Med. 2018;44:1027–1038. doi: 10.1007/s00134-018-5196-7. [DOI] [PubMed] [Google Scholar]
- 17.Jung C, et al. A comparison of very old patients admitted to intensive care unit after acute versus elective surgery or intervention. J. Crit. Care. 2019;52:141–148. doi: 10.1016/j.jcrc.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 18.Rockwood K, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol. Med. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 20.Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J. Am. Geriatr. Soc. 1983;31:721–727. doi: 10.1111/j.1532-5415.1983.tb03391.x. [DOI] [PubMed] [Google Scholar]
- 21.Seppi T, et al. Sex differences in renal proximal tubular cell homeostasis. J. Am. Soc. Nephrol. 2016;27:3051–3062. doi: 10.1681/ASN.2015080886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valentin A, Jordan B, Lang T, Hiesmayr M, Metnitz PG. Gender-related differences in intensive care: a multiple-center cohort study of therapeutic interventions and outcome in critically ill patients. Crit. Care Med. 2003;31:1901–1907. doi: 10.1097/01.CCM.0000069347.78151.50. [DOI] [PubMed] [Google Scholar]
- 23.Nathanson BH, et al. Do elderly patients fare well in the ICU? Chest. 2011;139:825–831. doi: 10.1378/chest.10-1233. [DOI] [PubMed] [Google Scholar]
- 24.Jung C. Assessment of microcirculation in cardiogenic shock. Curr. Opin. Crit. Care. 2019;25:410–416. doi: 10.1097/MCC.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 25.Masyuk M, et al. Prognostic relevance of serum lactate kinetics in critically ill patients. Intensive Care Med. 2019;45:55–61. doi: 10.1007/s00134-018-5475-3. [DOI] [PubMed] [Google Scholar]
- 26.Pan SW, et al. Synergistic impact of low serum albumin on intensive care unit admission and high blood urea nitrogen during intensive care unit stay on post-intensive care unit mortality in critically ill elderly patients requiring mechanical ventilation. Geriatr. Gerontol. Int. 2013;13:107–115. doi: 10.1111/j.1447-0594.2012.00869.x. [DOI] [PubMed] [Google Scholar]
- 27.Thomson B, et al. Association of childhood smoking and adult mortality: prospective study of 120 000 Cuban adults. Lancet Glob. Health. 2020;8:e850–e857. doi: 10.1016/S2214-109X(20)30221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bastian K, et al. Association of social deprivation with 1-year outcome of ICU survivors: results from the FROG-ICU study. Intensive Care Med. 2018;44:2025–2037. doi: 10.1007/s00134-018-5412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ski CF, King-Shier KM, Thompson DR. Gender, socioeconomic and ethnic/racial disparities in cardiovascular disease: a time for change. Int. J. Cardiol. 2014;170:255–257. doi: 10.1016/j.ijcard.2013.10.082. [DOI] [PubMed] [Google Scholar]
- 30.Reniers RL, Murphy L, Lin A, Bartolome SP, Wood SJ. Risk perception and risk-taking behaviour during adolescence: the influence of personality and gender. PLoS ONE. 2016;11:e0153842. doi: 10.1371/journal.pone.0153842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng MH, Chang SF. Frailty as a risk factor for falls among community dwelling people: evidence from a meta-analysis. J. Nurs. Scholarsh. 2017;49:529–536. doi: 10.1111/jnu.12322. [DOI] [PubMed] [Google Scholar]
- 32.Leblanc G, Boumendil A, Guidet B. Ten things to know about critically ill elderly patients. Intensive Care Med. 2017;43:217–219. doi: 10.1007/s00134-016-4477-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No additional data available. All data relevant for this study will be given by the authors upon specific request.