Abstract
Background
Tissue ischemia contributes to necrosis and infection. While angiogenic cell therapies have emerged as a promising strategy against ischemia, current approaches to cell therapies face multiple hurdles. Recent advances in nuclear reprogramming could potentially overcome some of these limitations. However, under severely ischemic conditions necrosis could outpace reprogramming-based repair. As such, adjunctive measures are required to maintain a minimum level of tissue viability/activity for optimal response to restorative interventions.
Methods
Here we explored the combined use of polymerized hemoglobin (PolyHb)-based oxygen nanocarriers with Tissue Nano-Transfection (TNT)-driven restoration to develop tissue preservation/repair strategies that could potentially be used as a first line of care. Random-pattern cutaneous flaps were created in a mouse model of ischemic injury. PolyHbs with high and low oxygen affinity were synthesized and injected into the tissue flap at various timepoints of ischemic injury. The degree of tissue preservation was evaluated in terms of perfusion, oxygenation, and resulting necrosis. TNT was then used to deploy reprogramming-based vasculogenic cell therapies to the flaps via nanochannels. Reprogramming/repair outcomes were evaluated in terms of vascularity and necrosis.
Results
Flaps treated with PolyHbs exhibited a gradual decrease in necrosis as a function of time-to-intervention, with low oxygen affinity PolyHb showing the best outcomes. TNT-based intervention of the flap in combination with PolyHb successfully curtailed advanced necrosis compared to flaps treated with only PolyHb or TNT alone.
Conclusions
These results indicate that PolyHb and TNT technologies could potentially be synergistically deployed and used as early intervention measures to combat severe tissue ischemia.
Electronic supplementary material
The online version of this article (10.1007/s12195-020-00621-4) contains supplementary material, which is available to authorized users.
Keywords: Ischemia, Hemoglobin-Based Oxygen Carrier, Oxygen Transport, Polymerized Hemoglobin, Tissue Nano-Transfection, Reprogramming
Introduction
Occlusive vascular lesions can cause tissue ischemia with devastating sequela (e.g., ulcerations, tissue necrosis, non-healing wounds, gangrene, amputations, stroke, myocardial infarction, etc.) depending on the severity and location of the insult.2,46,51,57 Upwards of 50% of patients with occlusive vascular disease and lesions may not be candidates for conventional treatment options (e.g., surgical bypass or endovascular revascularization), especially those predisposed to chronic tissue ischemia (e.g., diabetic patients), where the underlying vascular and microvascular damage is highly diffuse in nature, often unreconstructible, and is compounded by the existence of multiple other comorbidities.14,39,51,59 Given these challenges, novel strategies are desperately needed to more effectively address tissue ischemia.
A number of preclinical cell-based therapy studies have shown promising results for the treatment of ischemic disorders.6,22,45,48,50,67 Multiple reports have investigated the use of bone marrow mesenchymal stem cells or mononuclear cells for this purpose.45,51,69 However, while clinical trials have shown that such cell pools can contribute to improved outcomes44 the derivation of these cells from autologous sources poses major obstacles, including limited cell availability and/or impaired neovascular potential, especially in cases where ischemia is accompanied by underlying conditions such as diabetes or other co-morbid conditions.26,41,66,72,77 Moreover, current approaches to vascular cell therapies face additional hurdles, such as the need for cumbersome and highly immunogenic and/or carcinogenic ex vivo pre-processing steps (e.g., viral infection, induced pluripotency, expansion, differentiation).23,49 Recent advances in direct cellular reprogramming in vivo have opened up the possibility for the development of patient-specific cell therapies, which are based on more readily available cell sources (e.g., skin fibroblasts), do not require ex vivo cell pre-processing, and avoid some of the potential issues associated with pluripotent cellular states.12,13,58,75 Current approaches to direct reprogramming, however, are fraught with caveats, including safety concerns associated with the use of viral transfection.38,55 And although transfection with adeno-associated viruses (AAV) has been shown to be less pathogenic than other viral vectors, AAV-host interactions/immunity are still a major concern.24,28,40,53,71
To overcome this limitation, we recently developed a non-viral approach to drive direct reprogramming-based vascular cell therapies, in vivo, via tissue nano-transfection (TNT).34 TNT uses solid-state (e.g., Silicon) nanochannels to create small openings on the cell membranes via electroporation. The electrical stimulation is also used to electrophoretically deliver reprogramming genes into tissues in a fast (~ 100 ms), efficient, and benign manner. Compared to standard bulk electroporation, nanochannel-based poration has been shown to minimize electric field-driven damage to the cells, and lead to enhanced transfection efficiencies.34 Previously we reported that TNT-mediated delivery of Etv2, Foxc2, and Fli1 (EFF) plasmid genes into skin tissue can modulate the direct conversion of skin cells into induced endothelial cells (iECs), which can drive de novo formation of induced vasculature (iVas) and counteract tissue ischemia in mice.34EFF-transfected fibroblasts were shown to exhibit ~ 17% conversion efficacies into iECs within the first 7 days.34 However, while promising, in cases of severe tissue ischemia, a hurdle common to all vascular cell therapies is the time-to-effect (e.g., several hours to days), with the progression of ischemia-driven necrosis often outpacing or hindering the cellular and molecular processes that underlie and cross all vascular cell therapies, including gene transfection/expression, phenotypic conversion, vasculogenesis, etc., which all require development over a period of several hours to days.33,34 Therefore, there is a clear need for adjunctive bridge therapies that can curtail tissue degeneration until vascular cell therapies can take effect.
Naturally occurring oxygen (O2) nanocarriers such as hemoglobin (Hb) could potentially be used to provide “bridge oxygenation” as an adjunctive measure to support cellular therapies under conditions of severe ischemia. Degradable and non-degradable O2 nanocarriers have been devised to mimic the O2 storage and transport function of red blood cells (RBCs),64 and have been suggested to be of benefit in multiple conditions, including trauma, hemorrhage, sickle cell disease, etc.9,47 The difference between degradable Hb nanocarriers (i.e., Hb-based O2 carriers or HBOCs) and non-degradable perfluorocarbons (PFCs) is that in HBOCs O2 is covalently bound to the heme prosthetic group in the heme-binding pocket of the α/β globin chain.64 Hence, HBOCs possess greater O2 storage capacity and get saturated at lower pO2 compared to PFCs.25,65 Previous studies with commercial HBOCs, however, have shown adverse side-effects following systemic administration due to the small size (~ 200 kDa) of these HBOCs.4,8,15–17 Nevertheless, polymerization of Hb with difunctional cross-linkers has emerged as a promising and simple-to-implement strategy to address the size issue and prolong the half-life of HBOCs.68 Recent studies by us show that Hb can be polymerized at varying cross-link density to regulate the size of the polymerized hemoglobin (PolyHb) molecule.10,11,62 The presence of chemical cross-links in PolyHbs is also beneficial since it prevents Hb unfolding and release of the heme group, which can contribute to the formation of toxic reactive oxygen species that also decrease the O2-carrying capacity of HBOCs.3,5,70
Here we studied localized delivery of bovine PolyHb as an adjunctive bridge measure to support TNT-driven vascular cell therapies under conditions of severe ischemia. Our results indicate that early intervention with PolyHb following the ischemic insult was key to maintaining a minimum level of tissue viability, which was likely crucial to enabling restorative TNT-driven reprogramming gene expression and phenotypic conversion. Delayed intervention (6–8 hours) with PolyHb following the ischemic insult, on the other hand, led to rapid progression of tissue necrosis. These results suggest that PolyHb-based O2 nanocarriers and TNT-driven cellular therapies could potentially synergize to achieve improved functional outcomes in terms of ischemic tissue repair/preservation.
Methods
Random-Pattern Ischemic Skin Flap
C57BL/6 (7–8-week-old, Jackson Laboratories) male mice were used in this study. Mice were dosed with 1–3% isoflurane for surgery, treatments, and imaging. The dorsal areas were depilated 24 hours prior to the surgical procedure. The dorsal skin of the mice was sanitized using betadine followed by 70% alcohol. Skin flaps (2 cm × 1 cm) were created/elevated by making two 2 cm long parallel incisions (full thickness) with a 1 cm gap (Fig. S1). The bottom portion of the flap was also incised from the surrounding skin to create a free-hanging flap, perpendicular to the longitudinal incision. The edges of the skin flap were cauterized and a silicon gasket (~ 2.1 cm × 1.1 cm) was placed under the flap. The gasket was sutured to the adjacent skin using 6-0 suture (Henry Schein). Treatments (e.g., injection of PolyHb solutions vs. phosphate buffered saline or PBS, and TNT) were applied to the skin post-flap elevation (e.g., 0–8 hours) towards the proximal and distal ends of the flap. For PolyHb or PBS injections, a total of 50 µL were delivered at each location. Laser speckle imaging of tissue perfusion was collected immediately after flap elevation and at various timepoints post-surgery. Necrotic tissue areas were calculated as a percentage of the total flap area using Image J software (National Institutes of Health, Bethesda, MD).
Polymerized Bovine Hemoglobin Synthesis and Characterization
Sodium citrate-anticoagulated whole blood was collected aseptically from adult cattle and purchased from Quad Five (Ryegate, MT). Bovine RBCs were separated and washed by centrifugation with 0.9% saline and lysed with 3.75 mM phosphate buffer. Tangential flow filtration (TFF) cartridges with molecular weight cutoffs (MWCOs) of 500 kDa and 50 kDa were then used to purify and concentrate bovine hemoglobin (bHb) as described in the literature.61 Initially, 30 g of bHb at 20.0 mg/ml was placed into an airtight, amber-tinted reactor vessel with continuous stirring. To produce tense quaternary state polymerized bovine Hb (i.e., T-state PolyHb), the bHb solution was completely deoxygenated via continuous recirculation through the liquid side of a 3M MiniModule gas/liquid exchange membrane (Maplewood, MN) with countercurrent flow of pure nitrogen (N2) gas on the gas side of the membrane. The solution pO2 was measured using a RapidLab 248 Blood Gas Analyzer (Siemens USA, Malvern, PA). When the solution pO2 was lower than 20.0 mmHg, 300 mg of sodium dithionite dissolved in N2 purged PBS at pH 7.4 was added to the bHb solution via a needleless valve and allowed to mix for 15–20 minutes. To ensure complete deoxygenation of the bHb solution, four additional 1 mL bolus injections of 50.0 mg sodium dithionite in N2 purged PBS at pH 7.4 were delivered after the initial bolus injection. The polymerization of bHb in the T-state was not initiated until the pO2 in solution attained a value of 0.0 mmHg. Deoxygenated bHb was maintained throughout the T-state polymerization process by continuous purging of the reactor headspace with N2. The same reactor vessel configuration was used to synthesize relaxed quaternary state PolyHb (i.e., R-state PolyHb) via complete oxygenation of bHb. Instead of flowing pure nitrogen on the gas side of the gas/liquid exchange membrane, pure O2 was used. Polymerization was not initiated until the pO2 was above 745 mmHg. Oxygenation was maintained throughout the R-state polymerization process by continuous purging of the reactor headspace with O2. Both T- and R-state PolyHb were prepared using glutaraldehyde as the crosslinking agent. T-state PolyHb was polymerized at a crosslinker:bHb molar ratio of 35:1, while R-state PolyHb was polymerized at a 30:1 molar ratio. Glutaraldehyde was added dropwise to the bHb solution at a rate of 2 mL/min for a total volume of 50.0 mL, and allowed to react for 2 hours at 37 °C. To quench the polymerization reaction, a bolus addition of NaCNBH3 was introduced into the reactor vessel to reduce free aldehydes and Schiff bases, and mixed for 30 minutes as the reactor temperature was cooled to ambient temperature. NaCNBH3 was prepared at a 7:1 molar ratio of NaCNBH3 to glutaraldehyde in PBS pH 7.4. The reactor was then placed in a refrigerator and maintained at 4 °C overnight. Both R- and T-state PolyHb were then sterile filtered using a 0.2 µm TFF module. T- and R-state PolyHb were consequently buffer exchanged with a modified lactated Ringer’s buffer pH 7.4 via diafiltration on a 500 kDa TFF module. At least 12 diafiltration cycles were required to render the waste permeate essentially void of free Hb. The O2 equilibrium curve (OEC) of bHb, T- and R-state PolyHb was measured using a Hemox analyzer (TCS Instruments, Southampton, PA) at 37 °C. To quantify the oxygen binding affinity (i.e., P50, partial pressure of oxygen at which Hb is half saturated with O2) and cooperativity coefficient (n), the OEC was fit to the Hill equation to regress the parameters as described before.7 The hydrodynamic diameter of bHb and PolyHb was measured using a Malvern Zetasizer Nano ZS (Worcestershire, United Kingdom) as described before.80 The molecular weight (MW) distribution of bHb and PolyHb was estimated via HPLC-SEC on an Acclaim SEC 1000 column (ThermoFisher Scientific, Waltham, MA), which required pre-exclusion of PolyHb molecules > 200 nm in diameter. To represent the size of PolyHb molecules, the polymer order (N) was defined as M = 2N, where N is an integer ranging from 0 to 5, and M is the total number of individual bHb molecules polymerized to yield one PolyHb molecule. Finally, the metHb level of bHb and PolyHb was measured using the cyanomethemoglobin assay.
Tissue Nano-Transfection
TNT devices were fabricated as described by Gallego-Perez et al.34 Briefly, projection lithography followed by deep reactive ion etching (DRIE) was used to drill ~ 500 nm diameter nanochannels (~ 10–15 µm deep) through a 200 µm thick double-side polished silicon (Si) wafer. Contact photolithography and DRIE were then used to etch the backside of the wafers to create fluidic access to the nanochannels. Finally, a ~ 50 nm-thick insulating layer of silicon nitride was deposited on the surface of the processed silicon. The wafers were then diced into ~ 1 cm2 dies, and plasmid reservoirs were created for each die by affixing a plastic ring to the backside of the silicon. TNT interventions were conducted immediately after PBS or PolyHb injections. For this, the plasmid reservoir of the TNT device was filled with a 1:1:1 mixture of Etv2, Foxc2, and Fli1 in PBS. Plasmid details can be found in Table 1. The TNT chip was then pressed against the skin flap, with the nanochannel outlets facing the skin surface, and a pulsed electric field (i.e., 10 pulses, 250 V amplitude, 10 ms duration) was applied across an intradermally inserted electrode (positive lead) and the plasmid reservoir (negative lead), to nanoporate the skin surface and electrophoretically drive the plasmids into the skin flap via the nanochannels.34
Table 1.
List of plasmids used in TNT experiments.
| Name | Catalog number | Antibiotic resistance | Vector | Size (kb) | Vendor |
|---|---|---|---|---|---|
| Etv2 | MR216258 | Kanamycin | PCMV6 | 5.9 | Origene |
| Fli1 | MR225907 | 6.2 | |||
| Foxc2 | MR221977 | 6.4 |
Histological Analyses
The skin flap tissue was harvested and embedded in OCTTM, and sectioned and processed for immunostaining (i.e., with anti-pimonidazole or anti-vWF) or hematoxylin-eosin staining. For hypoxia characterization, pimonidazole was injected into the flapped tissue 2 hours prior to collection. Cell nuclei were stained with DAPI.
Data Analysis
All statistical analyses were run in Sigma Plot 14 and graphs created on GraphPad Prism 8. A total of three or more independent replicates were used for each experiment.
Results
Establishment of the Tissue Ischemia Model
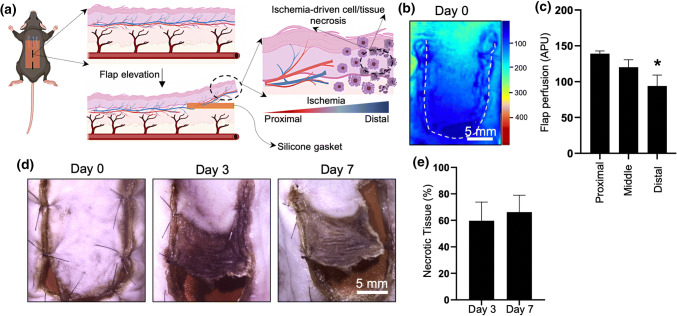
We created a 2 cm × 1 cm random-pattern dorsal skin flap in 7–8-week-old C57BL/6 mice to induce tissue ischemia and evaluate the impact of the combined use of PolyHb and vasculogenic TNT on tissue repair/rescue (Fig. 1a). Laser speckle imaging (LSI) immediately after flap elevation showed a gradual and marked reduction in tissue perfusion towards the distal portion of the flap compared to the proximal cephalad attachment (Figs. 1b and 1c). As expected, decreased tissue perfusion led to a significant increase in tissue necrosis (Fig. 1d), with average necrotic areas ranging between ~ 60 and 66% of the ischemic skin flap at days 3 and 7, respectively (Fig. 1e).
Figure 1.
(a) Schematic diagram illustrating a caudally-based random pattern flap model for tissue ischemia. (b) LSI imaging of flap perfusion at t = 0 days (c) showing a clear impact on skin perfusion as a function of location along the flap (*p<0.05 compared to the top/perfused location) (APU: arbitrary perfusion units). (d, e) Progression of tissue necrosis as a function of time.
PolyHb Synthesis and Characterization
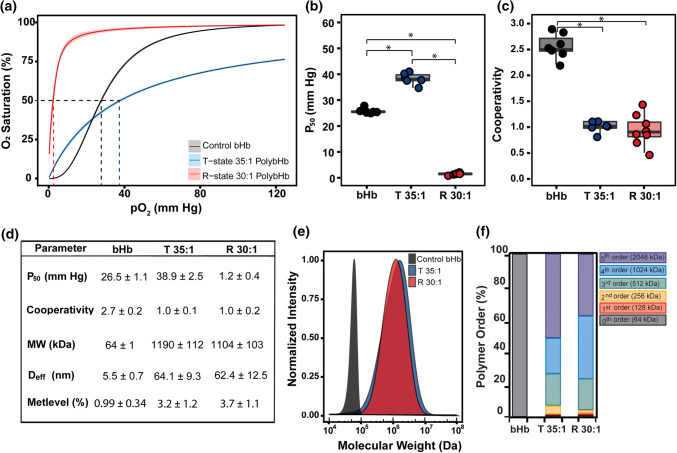
We first investigated the effect of the quaternary state of bovine Hb (bHb) on PolyHb oxygen affinity (P50) and cooperativity coefficient. Figure 2a shows the O2 equilibrium curves (OECs) of monomeric bHb, and tense (T) and relaxed (R) state PolyHb. Fitting the OEC to the Hill equation yielded a P50 of 38.9 ± 2.5 mmHg for T-state PolyHb (35:1) and 1.2 ± 0.4 for R-state PolyHb (30:1), indicating their ability to deliver O2 under vastly different pO2 conditions. The P50 of T-state PolyHb was comparable to previously reported values for commercial T-state PolyHbs.18,56 Figure 2b shows clear differences between the P50 of bHb and PolyHb at both quaternary states. Compared to R-state PolyHb, the higher P50 of T-state PolyHb indicates that oxygen will be released more easily at the same pO2. The cooperativity coefficient (Fig. 2c) showed no significant differences between T- and R-state PolyHb. Both T- and R-state PolyHb molecules display no cooperativity (~ 1.0), which is comparable with previously reported values for commercial PolyHbs.18 Figure 2e clearly shows the impact of cross-linking/polymerization on the size distribution of bHb vs. T- and R-state PolyHb. The average molecular weight (MW) of either T- or R-state PolyHb (~ 1,000 kDa) was substantially higher compared to many commercial PolyHbs, which range in size between 150 and 250 kDa.36,52 Interestingly, T- and R-state PolyHb showed similar size distribution, even when cross-linked at slightly different cross-linker:bHb molar ratios. This finding was consistent with hydrodynamic diameter measurements (Fig. 2d), which averaged ~ 64.1 nm and 62.4 nm for T- and R-state PolyHb, respectively. The methemoglobin (metHb) levels of both T- and R-state PolyHb 30:1 were below 5% (Fig. 2d), which are consistent with, and/or below previously reported values for commercial HBOCs.18,56 We also found that the PolyHbs in this study displayed higher metHb levels compared to free bHb (0.99 ± 0.34%), which could be attributed to Hb autoxidation during the long TFF processing period. On the other hand, analyses of the polymer order (Fig. 2f) for both T- and R-state PolyHb show only trace amounts (<1%) of free bHb in solution, which is important considering that previous in vivo transfusion studies showed that elevated levels (e.g., ~ 38%) of free Hb can lead to adverse side-effects.37,52,60,68,73 Figure 2f, however, showed minor differences between the polymer order composition for T- vs. R-state PolyHb, with T-state PolyHb containing a higher fraction of 2nd (256 kDa) and 5th order (2,048 kDa) polymers compared to R-state PolyHb.
Figure 2.
(a) Comparison of O2 equilibrium curves for control bovine hemoglobin (bHb), T-state PolybHb (35:1), and R-state PolybHb (30:1). Curves represent the fit to the original O2-bHb/PolybHb equilibrium data using the Hill equation. The shaded regions of the same color correspond to 95% confidence interval of the original experimental data for all batches of bHb, T-State PolybHb 35:1, and R-State PolybHb 30:1. (b) Effect of cross-linker:bHb molar ratio on P50 and (c) cooperativity coefficient (*p<0.05). (d) Summary of biophysical properties of bHb, T-state PolybHb 35:1 and R-state PolybHb 30:1. (e) Estimated molecular weight (MW) distribution of bHb, T-state PolybHb 35:1, and R-state PolybHb 30:1. The MW distribution was estimated by HPLC-SEC on an Acclaim SEC 1000 column which required pre-exclusion of PolybHb molecules > 200 nm in diameter. The absorbance at 280 nm was normalized against the maximum intensity. (f) Polymer order composition of bHb, T-state PolybHb 35:1, and R-state PolybHb 30:1.
PolyHb-Based Treatment of Ischemic Skin Flaps
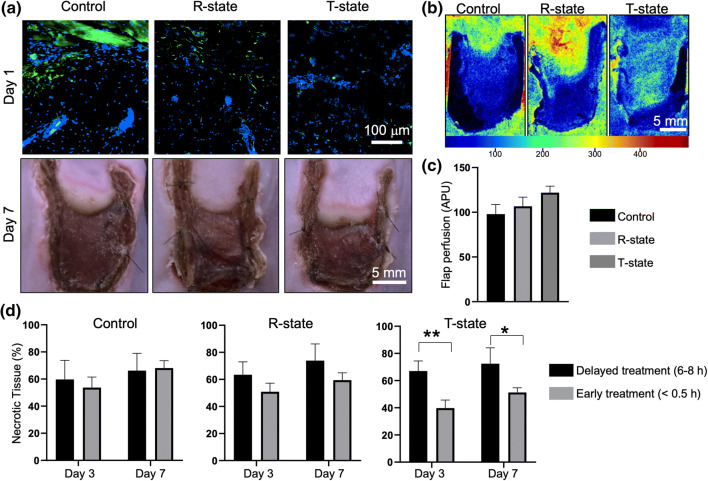
To evaluate whether PolyHb can be used to provide bridge oxygenation to ischemic tissue, we proceeded to inject the skin flaps with T- or R-state PolyHb, either immediately following flap elevation, or ~ 6–8 hours after. Flaps injected with phosphate buffer saline (PBS) served as controls. Histological analyses of tissue hypoxia with pimonidazole (Fig. 3a, top) showed a clear difference between flaps treated with both T- or R-state PolyHb compared to controls, with control flaps displaying increased pimonidazole staining (i.e., increased hypoxia) followed by R-state- and T-state PolyHb-treated flaps. By day 7, however, all treatment groups displayed comparable levels of tissue hypoxia (Fig. S2), suggesting that the effect of PolyHb injection was temporary. LSI analyses showed that the perfusion levels by day 7 were also similar across all treatment groups (Figs. 3b and 3c), indicating that a one-time treatment of ischemic tissue with PolyHb-based oxygen nanocarriers is not likely to impact vascularization. Interestingly, while all treatment groups showed visible signs of tissue necrosis by day 7 at the macro- (Fig. 3a, bottom) and micro-scale (Fig. S3), our results indicate that early injection (i.e., immediately after flap elevation) of T-state PolyHb was conducive to reduced flap necrosis compared to delayed (i.e., ~ 6–8 hours after flap elevation) intervention (Fig. 3d), with average necrotic areas dropping from ~ 67 to ~ 39% at day 3, and from ~ 72 to ~ 51% at day 7, when contrasting the 6-hour vs. the 1-hour T-state PolyHb delivery timepoint. Delayed intervention led to similar levels of necrosis across all treatment groups, thus suggesting that under this ischemia model, key cellular and molecular changes upstream of necrosis likely occur within the first hours following the ischemic insult. Similarly, no significant differences were noted in necrotic tissue areas when comparing early or delayed injection of PBS or R-state PolyHb, indicating that the timing of the injection procedure itself does not have an impact on necrosis. Differences between early intervention with T-state vs. R-state PolyHb possibly stem from the fact that T-state PolyHb has a tendency to release O2 more readily compared to R-state PolyHb (Fig. 2), presumably indicating that T-state PolyHb was more likely to bridge tissue oxygenation during the early stages of ischemia, as the pO2 decreases gradually over time.
Figure 3.
(a) Pimonidazole staining of tissue hypoxia (at t = 1 day, top) and corresponding photographs of skin flaps (at t = 7 days, bottom) that had been immediately injected with PBS (control, left), R- (middle), and T-state (right) PolyHb. (b) LSI imaging and (c) quantification of perfusion for each group at t = 7 days (APU: arbitrary perfusion units). No significant changes in perfusion were seen as a function of treatment. (d) Quantification of necrotic tissue progression as a function of treatment type and timing (*p = 0.06; **p = 0.03).
Combined Use of PolyHb and TNT
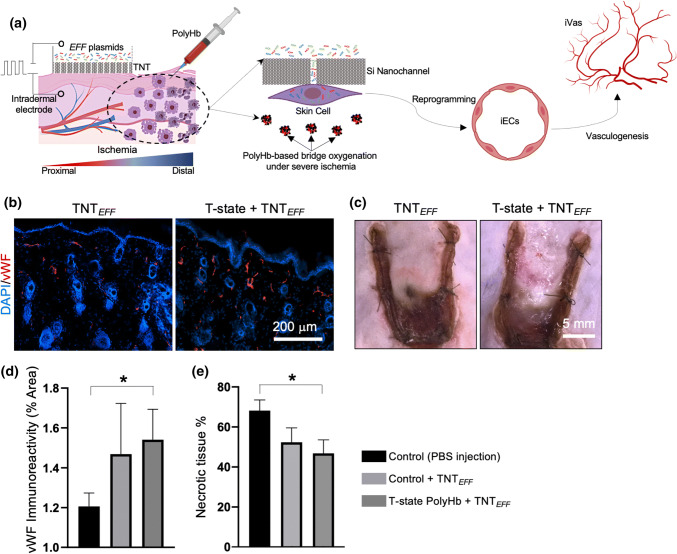
Once we established that T-state PolyHb is able to reduce the extent of tissue necrosis when administered soon after the ischemic insult, we proceeded to evaluate the ability of TNT-based vascular cell therapies to further stem necrosis when applied in combination with early PolyHb intervention (Fig. 4a). TNT is a non-viral technology that could be used to drive reprogramming-based cell therapies in vivo via nanochannel-mediated delivery of reprogramming genes to tissues.1,34,54 Recently we reported that TNT-based co-delivery of Etv2, Foxc2, and Fli1 (EFF) can be used to drive vascular cell therapies, in vivo, by promoting direct conversions of skin cells into induced endothelial cells in a fast and efficient manner (Fig. S4).34 When applied as a preventive measure (i.e., 24 hours pre-ischemic insult), TNT of EFF successfully hindered necrosis progression in a random-pattern skin flap model in mice.34 In the current study, however, we found that while post-operative intervention of the flapped skin with TNT of EFF trended towards improved outcomes (Figs. 4b and 4e), there was no statistically significant difference in necrotic tissue areas when comparing against untreated control flaps (i.e., ~ 52 vs. 68% necrotic tissue coverage), suggesting that ischemia-driven tissue necrosis under this particular model and mode of intervention (i.e., post-ischemic insult) has a tendency to outpace reprogramming-based vascular rescue. TNT-based delivery of EFF alone led to relatively similar outcomes compared to early intervention with T-state PolyHb (i.e., 52 vs. 51% necrotic tissue coverage). Nevertheless, when TNT of EFF was applied post-operatively in combination with an early injection of T-state PolyHb, TNT of EFF successfully curtailed the progression of tissue necrosis compared to control untreated flaps (i.e., ~ 46 vs. 68% necrotic tissue coverage) (Fig. 4e). These observations suggest that “priming” the ischemic flap with T-state PolyHb was presumably more conducive to a “healthier” tissue niche, in which TNT-driven reprogramming gene expression, phenotypic conversion, and vasculogenesis have a better chance to succeed.
Figure 4.
(a) Schematic diagram illustrating the synergy between PolyHb-based oxygen nanocarriers and vasculogenic TNT for the rescue of severely ischemic tissue. T-state PolyHb provides bridge oxygenation under severe ischemia to prevent rapid progression of necrosis and allow the implementation of TNT-based vascular therapies. TNT-driven delivery of EFF has been shown to lead to the conversion of somatic cells into induced endothelial cells (iECs), which can orchestrate the induction of new vascular tissue (iVas), in vivo, that can counteract ischemia and necrosis. TNT is implemented by applying a pulsed electric field across a Si-based nanochanneled membrane directly interfaced with the skin surface, which creates small openings on the cell membranes, and electrophoretically drives EFF plasmids into the cells to subsequently trigger iEC-directed reprogramming. (b) Immunofluorescence of vascular marker vWF and (c) photographs of flapped skin (day 7) treated with TNT alone (left) and TNT in combination with T-state PolyHb. Quantification of (d) vWF immunoreactivity and (e) necrotic tissue area for control flaps (i.e., injected with PBS), flaps injected with PBS and treated with TNT, and flaps injected with T-state PolyHb and treated with TNT (*p<0.05).
Discussion
Depending on the severity and location, tissue ischemia can lead to adverse events and complications. While traditional surgical interventions including bypasses and endovascular revascularization procedures are effective at relieving vascular occlusions in large vessels such as the coronary arteries in the setting of coronary artery disease (CAD), or the aortoiliac, femoropopliteal, and tibiopedal segments in the setting of peripheral artery disease (PAD). However, a significant number of affected patients are not candidates for these procedures because of diffuse unreconstructible anatomic disease, or prohibitive medical comorbidities. Furthermore, there are currently no effective strategies to address tissue ischemia and downstream complications in the vascular beds distal to the large vessels (i.e., at the capillary and microvascular level). Novel treatment options are thus still needed for many of these conditions.
Vascular cell therapy has emerged as a promising alternative strategy for the treatment of tissue ischemia. And while autologous vascular progenitor-based cell therapies face numerous limitations (e.g., limited and/or functionally impaired cell sources), reprogramming-based cell therapies such as TNT could potentially overcome these hurdles and offer a more viable solution for tissue ischemia. Nevertheless, a caveat common to all cell therapy approaches is that ischemia-driven necrosis or tissue malfunction could progress significantly faster than the corrective therapeutic measure. As such, adjunctive strategies that can maintain a minimum level of tissue viability/activity may be key to enabling successful implementation of vascular cell therapies for ischemic disorders.
In this study, we explored the use of an oxygen nanocarrier based on polymerized bovine hemoglobin (PolyHb) as an adjunctive measure to TNT-driven vascular cell therapies in a mouse model of tissue ischemia. Unlike traditional tissue engineering and cell-based therapy approaches that combine the use of biomaterials/scaffolds and single cells or cell clusters,29–32,42,43,63,74 TNT represents a scaffold-free approach to engineer tissues, in vivo, via non-viral nanochannel-mediated gene delivery/manipulation and cellular reprogramming.34 While numerous nanotechnology-based approaches have been developed for non-viral gene delivery/manipulation in dissociated cell populations ex vivo.19–21,27,33,35,76,79 TNT was uniquely designed to facilitate deployment to solid tissues both in vivo and ex vivo.
Our study results indicate that a one-time injection of T-state PolyHb to ischemic tissue had the ability to reduce to some extent the progression of tissue necrosis when delivered shortly after the ischemic insult. Delayed interventions, on the other hand, did not have a significant impact on necrosis progression for any PolyHb formulation. These observations suggest that under this model of ischemia, providing bridge oxygenation early at the onset of ischemia rendering is key to slowing down the progression of the cellular and molecular processes that underlie necrosis. Moreover, the lower O2 affinity of T-state PolyHb compared to R-state PolyHb appears to be crucial to facilitate the release/transfer of sufficient amounts of O2 to increasingly hypoxic tissue during all stages of ischemia progression.
Post-operative TNT based delivery of EFF to the flapped/ischemic tissue did not result in a statistically significant reduction of necrosis by day 7 compared to control untreated flaps. This finding suggests that while the skin may still be viable at the time of the TNT intervention, under this ischemia model and mode of delivery, tissue necrosis appears to progress at a much faster pace compared to TNT-driven gene expression, phenotypic reprogramming, and vasculogenesis. Nevertheless, co-delivery of PolyHb- and TNT-based therapies to the ischemic flap successfully hindered tissue necrosis compared to control untreated flaps, indicating that providing bridge oxygenation to the ischemic tissue via PolyHb may be a viable strategy to slow down necrosis and enable the implementation of vascular cell therapies for improved long-term outcomes. Previous studies have suggested that reduced O2 tensions may facilitate phenotypic conversion under certain reprogramming models (e.g., induced pluripotent stem cells).78 Therefore, future studies could potentially be focused on devising PolyHb-based strategies to better control O2 tension during ischemia progression, to attain specific values that can maximize the efficacy of reprogramming-based vascular cell therapies without compromising tissue viability.
Altogether, our studies suggest that co-implementation of bridge oxygenation approaches based on PolyHb O2 nanocarriers along with TNT-driven vascular cell therapies could offer a viable approach to address different types of chronic tissue ischemia. Such an approach could be applicable to a wide variety of clinical settings, including but not limited to no-option critical limb ischemia, chronic wound management, tissue flaps, or pelvic ischemia or buttock claudication after loss of internal iliac or pelvic circulation. Given the worldwide prevalence of conditions including coronary artery disease, peripheral artery disease, chronic wounds, and other states of tissue ischemia including shock of all etiologies, the potential to enhance outcomes is significant indeed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (DOCX 19439 kb)
Acknowledgments
Funding was partly provided by NINDS/NIH (R21NS099869), NIBIB/NIH (DP2EB028110) NHLBI/NIH (R01HL126945), NIBIB/NIH (R01EB021926), and NHLBI/NIH (R01HL138116). Some of the illustrations were created with BioRender.com.
Conflict of interest
Ludmila Diaz-Starokozheva, Devleena Das, Xiangming Gu, Jordan Moore, Luke R. Lemmerman, Ian Valerio, Heather M. Powell, Natalia Higuita-Castro, Michael R. Go, Andre F. Palmer, and Daniel Gallego-Perez declare that they have no conflicts of interest.
Ethical Approval
All animal studies were performed in accordance with protocols approved by the Laboratory Animal Care and Use Committee of The Ohio State University. No human studies were carried out by the authors of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abbasi J. Nanochip turns skin into a bioreactor. JAMA. 2017;318:898–898. doi: 10.1001/jama.2017.12097. [DOI] [PubMed] [Google Scholar]
- 2.Adler AI, Boyko EJ, Ahroni JH, Smith DG. Lower-extremity amputation in diabetes. The independent effects of peripheral vascular disease, sensory neuropathy, and foot ulcers. Diabetes Care. 1999;22:1029–1035. doi: 10.2337/diacare.22.7.1029. [DOI] [PubMed] [Google Scholar]
- 3.Alayash AI. Hemoglobin-based blood substitutes and the hazards of blood radicals. Free Radic. Res. 2000;33:341–348. doi: 10.1080/10715760000300881. [DOI] [PubMed] [Google Scholar]
- 4.Alayash AI. Blood substitutes: why haven’t we been more successful? Trends Biotechnol. 2014;32:177–185. doi: 10.1016/j.tibtech.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alayash AI, Patel RP, Cashon RE. Redox reactions of hemoglobin and myoglobin: biological and toxicological implications. Antioxid. Redox Signal. 2001;3:313–327. doi: 10.1089/152308601300185250. [DOI] [PubMed] [Google Scholar]
- 6.Al-Khaldi A, Al-Sabti H, Galipeau J, Lachapelle K. Therapeutic angiogenesis using autologous bone marrow stromal cells: improved blood flow in a chronic limb ischemia model. Ann. Thorac. Surg. 2003;75:204–209. doi: 10.1016/S0003-4975(02)04291-1. [DOI] [PubMed] [Google Scholar]
- 7.Arifin DR, Palmer AF. Determination of size distribution and encapsulation efficiency of liposome-encapsulated hemoglobin blood substitutes using asymmetric flow field-flow fractionation coupled with multi-angle static light scattering. Biotechnol. Prog. 2003;19:1798–1811. doi: 10.1021/bp034120x. [DOI] [PubMed] [Google Scholar]
- 8.Baek JH, et al. Extracellular Hb enhances cardiac toxicity in endotoxemic guinea pigs: protective role of haptoglobin. Toxins (Basel) 2014;6:1244–1259. doi: 10.3390/toxins6041244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baudin-Creuza V, et al. Octamers and nanoparticles as hemoglobin based blood substitutes. BBA Proteins Proteomics. 2008;1784:1448–1453. doi: 10.1016/j.bbapap.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Belcher DA, Cuddington C, Martindale E, Pires IS, Palmer AF. Controlled polymerization and ultrafiltration increases the consistency of polymerized hemoglobin for use as an oxygen carrier. Bioconjug. Chem. 2019;31(3):605–621. doi: 10.1021/acs.bioconjchem.9b00766. [DOI] [PubMed] [Google Scholar]
- 11.Belcher DA, et al. Mixtures of tense and relaxed state polymerized human hemoglobin regulate oxygen affinity and tissue construct oxygenation. PLoS ONE. 2017;12(10):e0185988. doi: 10.1371/journal.pone.0185988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat. Rev. Cancer. 2011;11:268–277. doi: 10.1038/nrc3034. [DOI] [PubMed] [Google Scholar]
- 13.Bongso A, Fong CY, Gauthaman K. Taking stem cells to the clinic: major challenges. J. Cell. Biochem. 2008;105:1352–1360. doi: 10.1002/jcb.21957. [DOI] [PubMed] [Google Scholar]
- 14.Brewster L, et al. Expansion and angiogenic potential of mesenchymal stem cells from patients with critical limb ischemia. J. Vasc. Surg. 2016 doi: 10.1016/j.jvs.2015.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buehler PW, et al. Free hemoglobin induction of pulmonary vascular disease: evidence for an inflammatory mechanism. Am. J. Physiol. Lung Cell Mol. Physiol. 2012;303:L312–326. doi: 10.1152/ajplung.00074.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butt OI, Buehler PW, D’Agnillo F. Differential induction of renal heme oxygenase and ferritin in ascorbate and nonascorbate producing species transfused with modified cell-free hemoglobin. Antioxid. Redox Signal. 2010;12:199–208. doi: 10.1089/ars.2009.2798. [DOI] [PubMed] [Google Scholar]
- 17.Butt OI, Buehler PW, D’Agnillo F. Blood-brain barrier disruption and oxidative stress in guinea pig after systemic exposure to modified cell-free hemoglobin. Am. J. Pathol. 2011;178:1316–1328. doi: 10.1016/j.ajpath.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carmichael FL, et al. A phase I study of oxidized raffinose cross-linked human hemoglobin. Crit. Care Med. 2000;28:2283–2292. doi: 10.1097/00003246-200007000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Chang L, et al. 3D nanochannel electroporation for high-throughput cell transfection with high uniformity and dosage control. Nanoscale. 2015 doi: 10.1039/c5nr03187g. [DOI] [PubMed] [Google Scholar]
- 20.Chang L, et al. Dielectrophoresis-assisted 3D nanoelectroporation for non-viral cell transfection in adoptive immunotherapy. Lab Chip. 2015;15:3147–3153. doi: 10.1039/c5lc00553a. [DOI] [PubMed] [Google Scholar]
- 21.Chang L, et al. Controllable large-scale transfection of primary mammalian cardiomyocytes on a nanochannel array platform. Small. 2016 doi: 10.1002/smll.201601465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho SW, et al. Improvement of postnatal neovascularization by human embryonic stem cell derived endothelial-like cell transplantation in a mouse model of hindlimb ischemia. Circulation. 2007;116:2409–2419. doi: 10.1161/CIRCULATIONAHA.106.687038. [DOI] [PubMed] [Google Scholar]
- 23.Cunningham JJ, Ulbright TM, Pera MF, Looijenga LH. Lessons from human teratomas to guide development of safe stem cell therapies. Nat. Biotechnol. 2012;30:849–857. doi: 10.1038/nbt.2329. [DOI] [PubMed] [Google Scholar]
- 24.Daya S, Berns KI. Gene therapy using adeno-associated virus vectors. Clin. Microbiol. Rev. 2008;21:583–593. doi: 10.1128/cmr.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dominguez de Villota ED, Ruiz Carmona MT, Rubio JJ, de Andres S. Equality of the in vivo and in vitro oxygen-binding capacity of haemoglobin in patients with severe respiratory disease. Br. J. Anaesth. 1981;53:1325–1328. doi: 10.1093/bja/53.12.1325. [DOI] [PubMed] [Google Scholar]
- 26.El-Ftesi S, Chang EI, Longaker MT, Gurtner GC. Aging and diabetes impair the neovascular potential of adipose-derived stromal cells. Plasticity Reconstr. Surg. 2009;123:475–485. doi: 10.1097/PRS.0b013e3181954d08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fei Z, et al. Gene delivery to cultured embryonic stem cells using nanofiber-based sandwich electroporation. Anal. Chem. 2013;85:1401–1407. doi: 10.1021/ac302140p. [DOI] [PubMed] [Google Scholar]
- 28.Ferrand M, Da Rocha S, Corre G, Galy A, Boisgerault F. Serotype-specific binding properties and nanoparticle characteristics contribute to the immunogenicity of rAAV1 vectors. Mol. Ther. 2015;23:1022–1033. doi: 10.1038/mt.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallego D, Ferrell N, Sun Y, Hansford DJ. Multilayer micromolding of degradable polymer tissue engineering scaffolds. Mater. Sci. Eng. C Biomimetic Supramol. Syst. 2008;28:353–358. doi: 10.1016/j.msec.2007.04.021. [DOI] [Google Scholar]
- 30.Gallego-Perez D, et al. High throughput assembly of spatially controlled 3D cell clusters on a micro/nanoplatform. Lab Chip. 2010;10:775–782. doi: 10.1039/b919475d. [DOI] [PubMed] [Google Scholar]
- 31.Gallego-Perez D, et al. Portland cement for bone tissue engineering: effects of processing and metakaolin blends. J. Biomed. Mater. Res. B Appl. Biomater. 2011;98B:308–315. doi: 10.1002/jbm.b.31853. [DOI] [PubMed] [Google Scholar]
- 32.Gallego-Perez D, et al. Micro/nanoscale technologies for the development of hormone-expressing islet-like cell clusters. Biomed. Microdevices. 2012;14:779–789. doi: 10.1007/s10544-012-9657-4. [DOI] [PubMed] [Google Scholar]
- 33.Gallego-Perez D, et al. Deterministic transfection drives efficient nonviral reprogramming and uncovers reprogramming barriers. Nanomedicine. 2016;12:399–409. doi: 10.1016/j.nano.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallego-Perez D, et al. Topical tissue nano-transfection mediates non-viral stroma reprogramming and rescue. Nat. Nanotechnol. 2017;12:974. doi: 10.1038/nnano.2017.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao K, et al. Design of a microchannel-nanochannel-microchannel array based nanoelectroporation system for precise gene transfection. Small. 2014;10:1015–1023. doi: 10.1002/smll.201300116. [DOI] [PubMed] [Google Scholar]
- 36.Gould SA, Moss GS. Clinical development of human polymerized hemoglobin as a blood substitute. World J. Surg. 1996;20:1200–1207. doi: 10.1007/s002689900183. [DOI] [PubMed] [Google Scholar]
- 37.Gould SA, et al. The first randomized trial of human polymerized hemoglobin as a blood substitute in acute trauma and emergent surgery. J. Am. Coll. Surg. 1998;187:113–120. doi: 10.1016/S1072-7515(98)00095-7. [DOI] [PubMed] [Google Scholar]
- 38.Grande A, et al. Environmental impact on direct neuronal reprogramming in vivo in the adult brain. Nat. Commun. 2013;4:2373. doi: 10.1038/ncomms3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graziani L, et al. Vascular involvement in diabetic subjects with ischemic foot ulcer: a new morphologic categorization of disease severity. Eur. J. Vasc. Endovasc. Surg. 2007;33:453–460. doi: 10.1016/j.ejvs.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 40.Greenberg B, et al. Prevalence of AAV1 neutralizing antibodies and consequences for a clinical trial of gene transfer for advanced heart failure. Gene Ther. 2016;23:313. doi: 10.1038/gt.2015.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herberts CA, Kwa MS, Hermsen HP. Risk factors in the development of stem cell therapy. J. Transl. Med. 2011;9:29. doi: 10.1186/1479-5876-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Higuita-Castro N, et al. Reinforced Portland cement porous scaffolds for load-bearing bone tissue engineering applications. J. Biomed. Mater. Res. B Appl. Biomater. 2012;100B:501–507. doi: 10.1002/jbm.b.31976. [DOI] [PubMed] [Google Scholar]
- 43.Holfinger SJ, et al. Pancreatic epithelial cells form islet-like clusters in the absence of directed migration. Cell Mol. Bioeng. 2015;8:496–506. doi: 10.1007/s12195-015-0396-5. [DOI] [Google Scholar]
- 44.Humpert PM, et al. Locally applied mononuclear bone marrow cells restore angiogenesis and promote wound healing in a type 2 diabetic patient. Exp. Clin. Endocrinol. Diabetes. 2005;113:538–540. doi: 10.1055/s-2005-872886. [DOI] [PubMed] [Google Scholar]
- 45.Iwase T, et al. Comparison of angiogenic potency between mesenchymal stem cells and mononuclear cells in a rat model of hindlimb ischemia. Cardiovasc. Res. 2005;66:543–551. doi: 10.1016/j.cardiores.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Johannesson A, et al. Incidence of lower-limb amputation in the diabetic and nondiabetic general population: a 10-year population-based cohort study of initial unilateral and contralateral amputations and reamputations. Diabetes Care. 2009;32:275–280. doi: 10.2337/dc08-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawaguchi AT. Artificial oxygen carrier as therapeutics rather than blood substitute for transfusion. Artif. Organs. 2017;41:312–315. doi: 10.1111/aor.12917. [DOI] [PubMed] [Google Scholar]
- 48.Kinoshita M, et al. Long-term clinical outcome after intramuscular transplantation of granulocyte colony stimulating factor-mobilized CD34 positive cells in patients with critical limb ischemia. Atherosclerosis. 2012;224:440–445. doi: 10.1016/j.atherosclerosis.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 49.Lee AS, Tang C, Rao MS, Weissman IL, Wu JC. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat. Med. 2013;19:998–1004. doi: 10.1038/nm.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lian Q, et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121:1113–1123. doi: 10.1161/CIRCULATIONAHA.109.898312. [DOI] [PubMed] [Google Scholar]
- 51.Lu D, et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res. Clin. Pract. 2011;92:26–36. doi: 10.1016/j.diabres.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 52.Marret E, et al. The effects of a polymerized bovine-derived hemoglobin solution in a rabbit model of arterial thrombosis and bleeding. Anesth. Analg. 2004;98:604–610. doi: 10.1213/01.ANE.0000099366.73625.DD. [DOI] [PubMed] [Google Scholar]
- 53.Mays LE, Wilson JM. The complex and evolving story of T cell activation to AAV vector-encoded transgene products. Mol. Ther. 2011;19:16–27. doi: 10.1038/mt.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller MA. Nanotransfection brings progress that’s more than skin-deep. Sci. Transl. Med. 2017 doi: 10.1126/scitranslmed.aao4216. [DOI] [Google Scholar]
- 55.Morita R, et al. ETS transcription factor ETV2 directly converts human fibroblasts into functional endothelial cells. Proc. Natl Acad. Sci. U.S.A. 2015;112:160–165. doi: 10.1073/pnas.1413234112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Napolitano LM. Hemoglobin-based oxygen carriers: first, second or third generation? Human or bovine? Where are we now? Crit. Care Clin. 2009;25:279–301. doi: 10.1016/j.ccc.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 57.Newton KM, et al. The use of automated data to identify complications and comorbidities of diabetes: a validation study. J. Clin. Epidemiol. 1999;52:199–207. doi: 10.1016/S0895-4356(98)00161-9. [DOI] [PubMed] [Google Scholar]
- 58.Okano H, et al. Steps toward safe cell therapy using induced pluripotent stem cells. Circ. Res. 2013;112:523–533. doi: 10.1161/CIRCRESAHA.111.256149. [DOI] [PubMed] [Google Scholar]
- 59.O’Loughlin A, McIntosh C, Dinneen SF, O’Brien T. Review paper: Basic concepts to novel therapies: a review of the diabetic foot. Int. J. Low Extrem. Wounds. 2010;9:90–102. doi: 10.1177/1534734610371600. [DOI] [PubMed] [Google Scholar]
- 60.Olson JS, et al. No scavenging and the hypertensive effect of hemoglobin-based blood substitutes. Free Radic. Bio Med. 2004;36:685–697. doi: 10.1016/j.freeradbiomed.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 61.Palmer AF, Sun G, Harris DR. Tangential flow filtration of hemoglobin. Biotechnol. Prog. 2009;25:189–199. doi: 10.1002/btpr.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Palmer AF, Sun G, Harris DR. The quaternary structure of tetrameric hemoglobin regulates the oxygen affinity of polymerized hemoglobin. Biotechnol. Prog. 2009;25:1803–1809. doi: 10.1002/btpr.265. [DOI] [PubMed] [Google Scholar]
- 63.Pelaez-Vargas A, et al. Effects of density of anisotropic microstamped silica thin films on guided bone tissue regeneration—in vitro study. J. Biomed. Mater. Res. B Appl. Biomater. 2013;101:762–769. doi: 10.1002/jbm.b.32879. [DOI] [PubMed] [Google Scholar]
- 64.Riess JG. Oxygen carriers (“blood substitutes”)—raison d’etre, chemistry, and some physiology. Chem. Rev. 2001;101:2797–2920. doi: 10.1021/cr970143c. [DOI] [PubMed] [Google Scholar]
- 65.Riess JG. Perfluorocarbon-based oxygen delivery. Artif. Cell Blood Substit. Immobil. Biotechnol. 2006;34:567–580. doi: 10.1080/10731190600973824. [DOI] [PubMed] [Google Scholar]
- 66.Rodrigues M, et al. Progenitor cell dysfunctions underlie some diabetic complications. Am. J. Pathol. 2015;185:2607–2618. doi: 10.1016/j.ajpath.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rufaihah AJ, et al. Endothelial cells derived from human iPSCS increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscler. Thromb. Vasc. Biol. 2011;31:e72–79. doi: 10.1161/ATVBAHA.111.230938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sakai H, et al. Molecular dimensions of Hb-based O(2) carriers determine constriction of resistance arteries and hypertension. Am. J. Phys. Heart Circ. Physiol. 2000;279:H908–915. doi: 10.1152/ajpheart.2000.279.3.H908. [DOI] [PubMed] [Google Scholar]
- 69.Shibata T, et al. Transplantation of bone marrow-derived mesenchymal stem cells improves diabetic polyneuropathy in rats. Diabetes. 2008;57:3099–3107. doi: 10.2337/db08-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simons M, et al. Comparison of the oxidative reactivity of recombinant fetal and adult human hemoglobin: implications for the design of hemoglobin-based oxygen carriers. Biosci. Rep. 2018;38(4):BSR20180370. doi: 10.1042/BSR20180370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sudres M, et al. MyD88 signaling in B cells regulates the production of Th1-dependent antibodies to AAV. Mol. Ther. 2012;20:1571–1581. doi: 10.1038/mt.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tamarat R, et al. Impairment in ischemia-induced neovascularization in diabetes: bone marrow mononuclear cell dysfunction and therapeutic potential of placenta growth factor treatment. Am. J. Pathol. 2004;164:457–466. doi: 10.1016/S0002-9440(10)63136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsai AG, et al. Dissociation of local nitric oxide concentration and vasoconstriction in the presence of cell-free hemoglobin oxygen carriers. Blood. 2006;108:3603–3610. doi: 10.1182/blood-2006-02-005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Veysi M, Daniel G-P, Tyler N, John JL, Derek JH. Controlled neuronal cell patterning and guided neurite growth on micropatterned nanofiber platforms. J. Micromech. Microeng. 2015;25:125001. doi: 10.1088/0960-1317/25/12/125001. [DOI] [Google Scholar]
- 75.Vierbuchen T, Wernig M. Direct lineage conversions: unnatural but useful? Nat. Biotechnol. 2011;29:892–907. doi: 10.1038/nbt.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu Y, et al. Surface-mediated nucleic acid delivery by lipoplexes prepared in microwell arrays. Small. 2013;9:2358–2367. doi: 10.1002/smll.201202258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiao-dong K, et al. Endothelial progenitor cells with Alzheimer’s disease. Chin. Med. J. 2011;124:901–906. [PubMed] [Google Scholar]
- 78.Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell. 2009;5:237–241. doi: 10.1016/j.stem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 79.Zhao X, et al. Nanochannel electroporation as a platform for living cell interrogation in acute myeloid leukemia. Adv. Sci. 2015;2:1500111. doi: 10.1002/advs.201500111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou Y. Synthesis and Biophysical Characterization of Polymerized Hemoglobin Dispersions of Varying Size and Oxygen Affinity as Potential Oxygen Carriers for Use in Transfusion Medicine. Columbus: The Ohio State University; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (DOCX 19439 kb)