Abstract
Blastocystis sp. is a group of anaerobic protozoa parasitizing the gastrointestinal tract of humans and a broad variety of animals. Evidences of Blastocystis parasites resistance development to antiprotozoal drugs urge the exploration of new therapeutics. Antiprotozoal potential of Salvadora persica, a medicinal plant traditionally used for oral hygiene, was evaluated in vitro against Blastocystis sp. human isolates. Until now, no study has described the effect of S. persica extracts on this parasitic protozoa. Blastocystis sp. positive stool samples collected from patients with gastrointestinal complaints and asymptomatic individuals diagnosed by microscopy were furthermore cultured in vitro and characterized by PCR and multiplex-PCR using sequence-tagged-site primers to determine their subtypes. Out of 21 Blastocystis sp. isolates, five were determined as ST1, 14 as ST3, and two as ST5 subtypes. Antiprotozoal activity of untreated and heat-treated S. persica roots aqueous extracts was evaluated in vitro by serial dilutions on three Blastocystis sp. subtypes; ST1, ST3, and ST5 isolated from symptomatic patients. A significant killing activity was observed with both, untreated and heat-treated aqueous extracts of S. persica at minimal concentration of 2.5 μl/ml compared to parasites’ growth controls (P < 0.05). Maximal antiprotozoal effect was reached at a concentration of 20 µl/ml of S. persica aqueous extract. Means of growth inhibition effect obtained with untreated and heat-treated extracts at 40 µl/ml against the three subtypes of Blastocystis sp. were 80% (SD 2.3) and 82% (SD 1.1), respectively. No significant difference was observed in the inhibitory effect of S. persica extracts between the three Blastocystis sp. subtypes. Aqueous extract of S. persica roots contains therefore heat-stable components with significant antiprotozoal activity against Blastocystis sp. subtypes ST1, ST3, and ST5 in vitro. Further investigations are required to determine and characterize the active antiprotozoal components of S. persica roots and their evaluation in vivo.
Keywords: Blastocystis sp., Salvadora persica, STS sub-typing, Aqueous extract, Anti-protozoal activity
Introduction
Blastocystis sp. is a group of anaerobic protozoan parasites infecting the lower gastrointestinal tract of a wide variety of hosts including humans (Tan 2004). Its mode of transmission is not fully understood, however it is confirmed that the infection occurs after ingestion of a cyst form (Stenzel and Boreham 1996). Human infections have been recorded worldwide with wide-ranging prevalence, rising to levels as high as 60% in population groups with poor levels of hygiene or consumption of contaminated water and food and contact with animals (Pegelow et al. 1997; Tan 2008).
Blastocystis sp. infections have been reported in patients with various symptoms including weight loss, nausea, vomiting, abdominal pain, and diarrhea (Cirioni et al. 1999; Rossignol et al. 2005; Tasova et al. 2000), but Blastocystis parasites are also found in fecal samples from asymptomatic and apparently healthy persons (Dogruman et al. 2008; Leder et al. 2005; Mohamed et al. 2017). Accordingly, Blastocystis sp. pathogenicity remains arguable. Many reports suggested a direct relation between the density of Blastocystis sp. infection and pathogenesis (Giacometti et al. 1999; Kain et al. 1987; Sheehan et al. 1986; Zaki et al. 1991). Pathogenesis has also been directly related to the genotype and subtype of the infecting Blastocystis sp. parasites (Clark 1997; Hameed et al. 2011). It has been postulated that proteases activity in some Blastocystis sp. subtypes represents a significant virulence factor involved in protein and neutralizing mucosal antibodies degradation, and evasion from host immunity in vivo (Abdel-Hameed and Hassanin 2011; Puthia et al. 2005). Nevertheless, no virulence factor genes have been completely characterized (Tan 2008). Latest studies showed a large diversity in Blastocystis sp. subtypes based on the small subunit ribosomal DNA (SSU rDNA) gene sequences. PCR and multiplex-PCR methods using SSU rDNA sequence tagged-site (STS) primers are universally applied to determine the subtypes of Blastocystis sp. isolates (Stensvold et al. 2007; Yoshikawa et al. 1998). Among all known Blastocystis sp. subtypes, only nine (ST1-ST9) were isolated from humans so far (Parkar et al. 2010).
There are strong evidences of resistance development in Blastocystis sp. parasites against antiprotozoal drugs in use, namely metronidazole as first line and cotrimoxazole as second line therapy (Haresh et al. 1999; Moghaddam et al. 2005; Rajamanikam et al. 2019; Wu et al. 2014). Hence, is becoming necessary to investigate new treatments for Blastocystis sp. infections, especially against virulent subtypes. Various medicinal plants have been explored as alternative sources to synthetic anti-protozoal drugs against Blastocystis sp. parasites; such as Brucea javanica (Yang et al. 1996), Artemisia judaica (Mokhtar et al. 2019), Quercus infectoria (Sawangjaroen and Sawangjaroen 2005), and Nigella sativa (Wakil 2007). Yet, no previous studies had evaluated the antiprotozoal activity of Salvadora persica (Salvadoraceae) plant against Blastocystis sp. parasites. S. persica is a perennial tree or shrub with a large geographic distribution including wide regions of Asia and Africa that has been traditionally used in many countries for its diverse biological activities (Sher et al. 2010). Sticks harvested from S. persica roots, twigs, and stems are popularly used as tooth brushing sticks and for oral hygiene (Hattab 1997). Reports from previous investigations showed that it has important anti-bacterial, anti-fungal, and anti-inflammatory action (Abeer et al. 2011; Noumi et al. 2010; Sofrata et al. 2008). In the present study we explored in vitro susceptibility of Blastocystis sp. isolates from symptomatic patients to S. persica components collected in aqueous extract.
Materials and methods
Samples collection, examination, and in vitro cultures
This study included fecal specimens received for analysis in the central laboratory of a tertiary health care center of Makkah city in 2019 between January and June. A part of samples were from patients with gastrointestinal complaints and another part from asymptomatic persons who underwent a regular or mandatory health exam. All samples were examined by direct microscopy for parasites. Samples from patients with GIT symptoms, if negative by direct wet mount, were further examined after formalin ethyl acetate concentration. Blastocystis sp. parasites were recognized by morphological features as vacuolar, granular, ameboid, or cystic and with very variable sizes (Mohamed et al. 2017). Two parts of about 0.5 g of each Blastocystis sp. positive sample were inoculated in two separate 15 ml tubes containing 3 ml of culture media consisting of Dulbecco’s modified Eagle medium (DMEM) (Thermo Fisher Scientific, USA) containing 15% inactivated horse serum (Gibco), 12 mg/ml ampicillin, and 4 mg/ml streptomycin. The prepared DMEM media was sterilized by filtration.
Subtyping of Blastocystis sp. isolates
First, genomic DNAs were extracted from parasites subcultures using QIAmp DNA extraction kit (QIAmp, QIAGEN Inc, Germany) following the manufacturer’s instructions. Molecular subtyping of Blastocystis isolates was performed by PCR and multiplex-PCR using sequence-tagged site (STS) primers under the conditions reported by (Chandrasekaran et al. 2014; Yoshikawa et al. 2004) (Table 1). PCR reactions were carried out in duplicate for each isolate’s genomic DNA and each primer set as described by Bakri et al. 2019. PCR and multiplex-PCR PCR products were separated in 1.5% agarose gels, stained with ethidium bromide, and photographed.
Table 1.
SSUrDNA-based primer pairs for sequence-tagged-site PCR for Blastocystis sp. subtyping (Chandrasekaran et al. 2014; Yoshikawa et al. 2004)
| Blastocystis subtype | Primer sets | GenBank accession nos. | Primer sequences (5′-3′) | Product size (bp) |
|---|---|---|---|---|
| ST 1 | SB83 | AF166086 |
F: GAAGGACTCTCTGACGATGA R:GTCCAAATGAAAGGCAGC |
351 |
| ST 2 | SB155 | AF166087 |
F:ATCAGCCTACAATCTCCTC R: ATCGCCACTTCTCCAAT |
650 |
| ST 3 | SB227 | AF166088 |
F:TAGGATTTGGTGTTTGGAGA R:TTAGAAGTGAAGGAGATGGAAG |
526 |
| SB228 | AF166089 |
F: GACTCCAGAAACTCGCAGAC R: TCTTGTTTCCCCAGTTATCC |
473 | |
| SB229 | AF166090 |
F: CACTGTGTCGTCATTGTTTTG R: AGGGCTGCATAATAGAGTGG |
631 | |
| ST4 | SB332 | AF166091 |
F: GCATCCAGACTACTATCAACATT R:CCATTTTCAGACAACCACTTA |
338 |
| ST5 | SB340 | AY048752 |
F: TGTTCTTGTGTCTTCTCAGCTC R:TTCTTTCACACTCCCGTCAT |
704 |
| ST6 | SB336 | AY048751 |
F:GTGGGTAGAGGAAGGAAAACA R:AGAACAAGTCGATGAAGTGAGAT |
317 |
| ST7 | SB337 | AY048750 |
F: GTCTTTCCCTGTCTATTCTTGCA R:AATTCGGTCTGCTTCTTCTG |
487 |
Preparation of Salvadora persica roots extracts
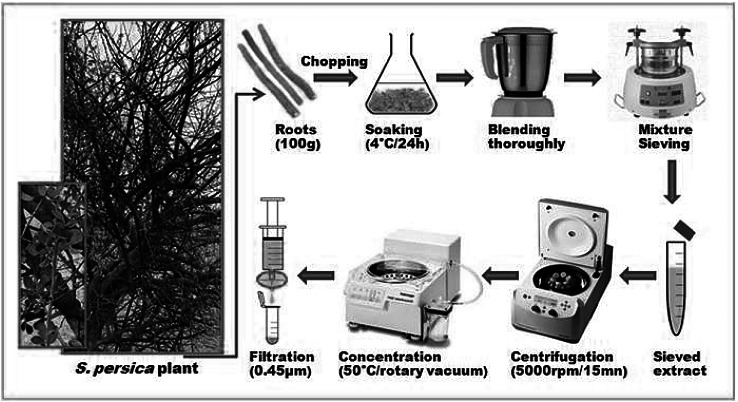
Freshly harvested roots of S. persica were kindly provided by an expert botanist after a field tour to its natural habitats in Jazan area in the south west region of Saudi Arabia. Immediately, 100 g of fresh roots were chopped in tiny pieces and soaked into 300 ml of distilled water under agitation at 4 °C for 24 h. The mixture was thoroughly blended, sieved under pressure, and centrifuged at 5000 rpm for 15 mn. The supernatant was then concentrated in a rotary vacuum evaporator at 50 °C to a final volume of 100 ml, filtered through 0.45 µm pore-size syringe filters (Durapore PVDF membrane, Millipore), and stored at 4 °C for posterior use (Fig. 1). Each µl in the final extract solution was corresponding then to the aqueous material present in 1 µg of fresh roots. Heat-treatment of extracts was performed at 100 °C for 10 mn before use.
Fig. 1.
Representation of the process of Salvadora persica roots aqueous extract preparation
In vitro antiprotozoal activity assays
Antiprotozoal activity of untreated and heat-treated aqueous extract of S. persica roots was evaluated on three Blastocystis sp. isolates of different genotypes, molecularly characterized as ST1, ST3, and ST5 subtypes. Untreated and heat-treated aqueous extracts were tested using serial dilutions from 40 to 2.5 µl/ml of culture media. Each assay was carried out in triplicate in 3 ml culture media seeded with 105 parasites/ml and incubated at 37 °C for 48 h in presence of a gas pack (BD gas pack-Becton, USA) to create an anaerobic environment as described by (Zaman and Zaki 1996). Metronidazole was used at a concentration of 0.2 mg/ml as antiprotozoal control. Three cultures without additions were also used in parallel as parasites growth controls. After 48 h incubation time, parasites were precipitated by centrifugation at 500 rpm/5 min, 2.5 ml of supernatant were discarded, and the sediment was fully resuspended in the remaining 0.5 ml of media. Trypan blue (Sigma-Aldrich Corp. USA) was then supplemented at a concentration of 0.4% as viability indicator before counting living parasites in haemocytometer chambers (Improved Neubauer, Hausser Scientific).
Statistical analysis
Statistical analysis of data was performed using Chi square test considering P values < 0.05 as statistically significant. A Persons correlation study was conducted. Statistical analysis was done using SPSS software version 21.
Results
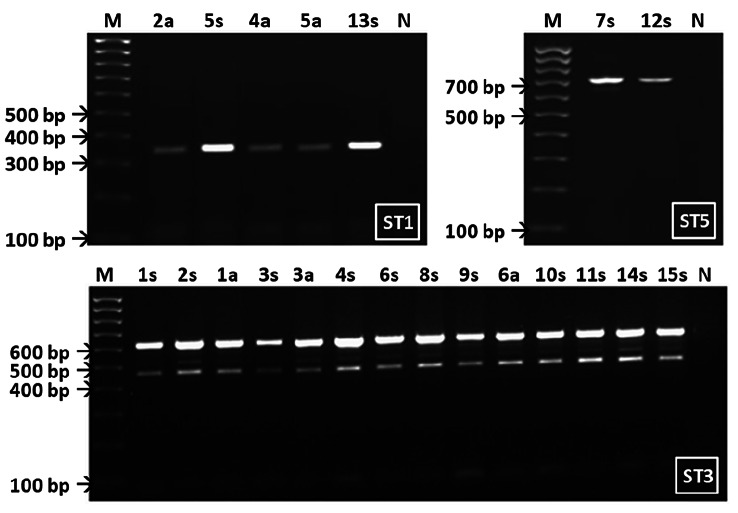
During the period between January and June of 2019, microscopic examination in a tertiary health care center of 643 fecal samples revealed 27 Blastocystis sp. positive ones. In vitro cultures of positive stools were successful for 21 specimens, among which 15 (isolates 1s–15s) were from patients with gastrointestinal complaints and 6 (isolates 1a–6a) from asymptomatic and apparently healthy individuals. Molecular subtyping of the 21 isolates by specific sequence-tagged-site (STS) primers identified five isolates as ST1, 14 as ST3, and two as ST5 subtypes (Fig. 2).
Fig. 2.
Analytical gels of sequence-tagged-site PCR products of Blastocystis sp. ST1, ST3, ST5 subtypes collected from symptomatic patients (N°s) and asymptomatic individuals (N°a). Negative controls (lanes N) and 100 bp molecular size marker (lanes M) were separated in parallel
Susceptibility assays to S. persica aqueous extract were carried out on the three isolates labeled 5s, 1s, and 7s representing the three different subtypes ST1, ST3, and ST5. A significant killing activity was observed with both, untreated and heat-treated S. persica aqueous extracts starting from the minimal concentration of 2.5 μl/ml compared to growth control cultures (P < 0.05). Maximal antiprotozoal effect was reached at a concentration of 20 μl/ml. Blastocystis sp. Means of growth inhibition effect obtained with untreated and heat-treated extracts at 40 µl/ml against the three subtypes of Blastocystis sp. were 80% (SD 2.3) and 82% (SD 1.1), respectively. No significant difference of killing effects of both, untreated and heat-treated S. persica extract, were noticed between Blastocystis sp. subtypes ST1, ST3 and ST5 tested in this study (Fig. 3).
Fig. 3.
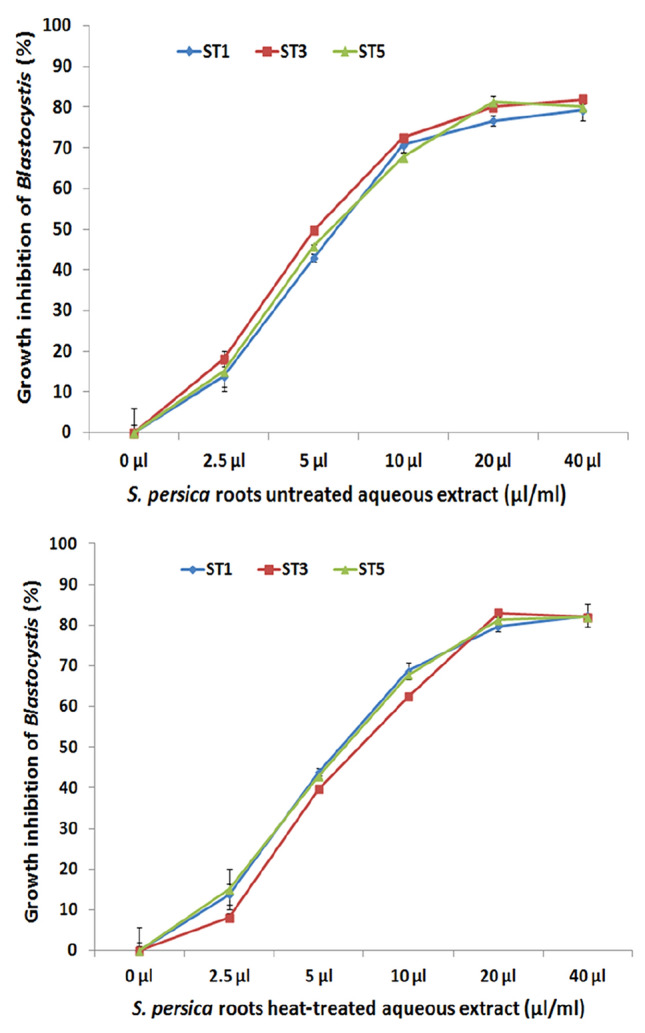
In vitro antiparasitic activity of untreated (left) and heat-treated aqueous extracts of S. persica roots serially diluted (40–2.5 µl/ml) against Blastocystis sp. subtypes ST1, ST3 and ST5 collected from symptomatic patients
To explore the antiprotozoal effect of both untreated and heat-treated extracts on each Blastocystis sp. subtypes (ST1, ST3 and ST5) tested in this study, Pearson’s correlation coefficient was determined between the concentration (volume) of each extract and parasites counts (growth inhibition) of each Blastocystis sp. isolate. A statistically significant association was observed between concentrations of both extracts and the counts of parasites after 48 h incubation for each of the three Blastocystis sp. (Table 2).
Table 2.
Correlation coefficient between concentrations of untreated and heat-treated aqueous extract of Salvadora persica roots and Blastocystis sp. subtypes ST1, ST3 and ST5 parasites’ counts during in vitro susceptibility assays
| S. persica aqueous extract | Blastocystis sp. subtypes | Pearson correlation coefficient (r) and P value |
|---|---|---|
| Untreated | ST1 | r = -0.804 P < 0.001 |
| ST3 | r = -0.809 P < 0.001 | |
| ST5 | r = -0.828 P < 0.001 | |
| Heat-treated | ST1 | r = -0.799 P < 0.001 |
| ST3 | r = -0.800 P < 0.001 | |
| ST5 | r = -0.816 P < 0.001 |
Discussion
Salvadora persica has been used by humans since ancient times as a safe medicinal plant, particularly for oral hygiene (Hattab 1997). Since past several years, therapeutic potential of different parts of this plant has been investigated against several pathogens including bacteria, fungi, and parasites (Sher et al. 2010). Nevertheless, until now, no study has described the effect of S. persica extracts on Blastocystis sp., a very common gastrointestinal protozoan parasite existing under different subtypes with controversial degrees of pathogenecity (Tan 2008). The aim of the present study was to explore in vitro antiprotozoal potential of S. persica against the different subtypes of Blastocystis sp. found in Saudi patients with gastrointestinal complaints. Among 21 Blastocystis sp. isolates collected in a Saudi tertiary health care center and successfully cultivated in vitro, five were identified as ST1, 14 as ST3, and two as ST5 subtypes. Worldwide, nine genetic subtypes of Blastocystis sp. have been isolated from humans and the four most common ones are ST1, ST2, ST3 and ST4. ST5 subtype has rarely been described in humans, is rather a common zoonotic subtype (Tan 2008). Among the 21 isolated parasites, two ST1, eleven ST3 and two ST5 were collected from symptomatic patients. Antiprotozoal effect of S. persica roots extract was evaluated against the three Blastocystis sp. subtypes (ST1, ST3 and ST5) isolated from symptomatic patients in this study for a wider applicability.
Salvadora persica roots are traditionally used as sticks for teeth brushing; a practice during which sticks are partly chewed and the chewed extract is naturally swallowed with the saliva without harmful consequences on the gastrointestinal tract (Khoory 1983; Wu et al. 2001). Thus, we investigated the aqueous extract of S. persica roots as a potential anti-Blastocystis sp. natural remedy against ST1, ST3 and ST5 isolated strains. Different types of S. persica extracts have been investigated for their antibacterial, antifungal, and antiparasitic potential. Ethanol and hexane extracts of S. persica were found to exhibit significant antimicrobial activity against Gram-positive bacteria species most commonly associated with dental caries such as Streptococcus mutans, S. sanguis and S. salivarius (Balto et al. 2017). Methanolic extract inhibited growth of both Gram positive and negative bacterial strains, although it was more effective on Gram positive bacteria than Gram negative ones (Amir et al. 2014). Aqueous extract of S. persica has also shown to have significant antibacterial properties against several bacterial strains including E. coli, Streptococcus pyogenes, methicillin-resistant Staphylococcus aureus (MRSA), Acinetobacter baumannii, and Stenotrophomonas maltophilia (Al-Ayed et al. 2016). Antifungal effect of methanol, ethyl acetate, and acetone extracts of S. persica were confirmed against different Candida species, with ethyl acetate extract being the most potent one (Noumi et al. 2010). Aqueous extract of S. persica roots has also shown in vitro high fungicidal effect against isolated Candida species (Al-Bagieh et al. 1994), and Aspergillus niger, A. flavus, and A. fumigatus pathogenic species (Saddiq and Alkinani 2019).
In our study, S. persica aqueous extract showed significant concentration-dependent in vitro antiprotozoal activity against Blastocystis sp. ST1, ST3 and ST5 subtypes reaching 80% (SD 2.3) parasites growth inhibition mean at 40 µl/ml after 48 h incubation. Antiprotozoal effects of different extracts of S. persica were reported against other human parasites; stems and leaves extracts showed anti-plasmodial activity against Plasmodium falciparum NF54 strain at 0.6 mg/ml and 0.7 mg/ml, respectively (Ali et al. 2002). A significant in vitro killing activity of S. persica extracts against erythrocytic schizonts of P. falciparum, intracellular amastigotes of Leishmania infantum and Trypanosoma cruzi, and free trypomastigotes of T. brucei have been reported (Al-Musayeib et al. 2012). Roots extract of S. persica has also been reported to have effective anticoccidial activity against Eimeria paillata induced infection in mice jejunum (Dkhil et al. 2019). Antihelmintic activity of S. persica roots extract has been proved in vitro against protoscolices from hydatid cysts of Echinococcus granulosus at a concentration of 50 mg/ml, killing 81.4% then 100% of protoscolices after 10 and 20 min, respectively (Abdel-Baki et al. 2016).
Heat treatment at 100 °C for 10 min of the aqueous extract did not affect its killing effect on the three tested subtypes of Blastocystis sp. showing 82% (SD 1.1) parasites growth inhibition mean at 40 µl/ml after 48 h incubation. These findings reveal for the first time that the S. persica roots extract have a potential anti-Blastocystis property which is stable even in extreme temperature conditions. Results of this study open an opportunity to further investigate the prevalence of Blastocystis sp. infections among regular users of S. persica as natural toothbrushes and also on the potential of S. persica roots extract mixed food in controlling Blastocystis sp. infections among farm animals.
Conclusion
Aqueous extract of S. persica roots contains heat-stable components with significant antiprotozoal activity against Blastocystis sp. in vitro. S. persica roots aqueous extract showed similar growth inhibition effect on parasites of three different subtypes; ST1, ST3, and ST5 collected from patients with gastrointestinal complaints. Further investigations are required to determine and characterize the active antiprotozoal components of S. persica roots and their evaluation in vivo.
Acknowledgements
The authors would like to thank medical staff of Diagnostic Parasitology Unite at Al-noor hospital, Makkah city, for their valuable contribution in the identification and collection of samples for this study.
Author contributions
MAE-B coordinated the study and wrote the first draft of the manuscript. AA and RTM performed in vitro cultures and susceptibility assays. MAEL-M shared in microscopic examination and genotyping experiments, RAB and SAA shared in molecular experiments and statistical analysis. All authors read and approved the final manuscript.
Funding
This work was financially supported by Umm-Alqura University, Makkah city, Saudi Arabia through the Grant Number (ISR43409049).
Compliance with Ethical standards
Conflict of interest
Authors have declared that no conflict of interest exist.
Ethical approval
Ethical permission for this research study and informed consent form were officially agreed by Medical Research Ethics Committee of Umm-Alqura University, Saudi Arabia (Reference Number #43409049).
Informed consent
All participants whose samples were included in this study had given their consent and signed the informed consent form.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdel-Baki AA, Almalki E, Mansour L, Al-Quarishy S. In vitro scolicidal effects of Salvadora persica root extract against protoscolices of Echinococcus granulosus. Korean J Parasitol. 2016;54:61–66. doi: 10.3347/kjp.2016.54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Hameed DM, Hassanin OM. Proteaese activity of Blastocystis hominis subtype3 in symptomatic and asymptomatic patients. Parasitol Res. 2011;109:321–327. doi: 10.1007/s00436-011-2259-x. [DOI] [PubMed] [Google Scholar]
- Abeer YI, El-Gengaihi SE, Motawea HM, Sleem AM. Anti-inflammatory activity of Salvadora persica L. against carrageenan induced paw oedema in rat relevant to inflammatory cytokines. Not Sci Biol. 2011;3:22–28. doi: 10.15835/nsb.3.4.6378. [DOI] [Google Scholar]
- Al-Ayed MS, Asaad AM, Qureshi MA, Attia HG, AlMarrani AH. Antibacterial activity of Salvadora persica L. (Miswak) extracts against multidrug resistant bacterial clinical isolates. Alternat Med: eCAM. 2016;2016:7083964. doi: 10.1155/2016/7083964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Bagieh NH, Idowu A, Salako NO. Effect of aqueous extract of miswak on the in vitro growth of Candida albicans. Microbios. 1994;80:107–113. [PubMed] [Google Scholar]
- Ali H, Konig GM, Khalid SA, Wright AD, Kaminsky R. Evaluation of selected Sudanese medicinal plants for their in vitro activity against hemoflagellates, selected bacteria, HIV-1-RT and tyrosine kinase inhibitory, and for cytotoxicity. J Ethnopharmacol. 2002;83:219–228. doi: 10.1016/s0378-8741(02)00245-3. [DOI] [PubMed] [Google Scholar]
- Al-Musayeib NM, Mothana RA, Al-Massarani S, Matheeussen A, Cos P, Maes L. Study of the in vitro antiplasmodial, antileishmanial and antitrypanosomal activities of medicinal plants from Saudi Arabia. Molecules. 2012;17:11379–11390. doi: 10.3390/molecules171011379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir ARG, Afsaneh R, Seied Hosein MS, et al. Inhibitory activity of Salvadora persica extracts against oral bacterial strains associated with periodontitis: an in vitro study. J Oral Biol Craniofac Res. 2014;4(1):19–23. doi: 10.1016/j.jobcr.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakri RA, Mohamed RT, Alharthi A, Hushlul OM, El-Shehry SA, El-Bali MA. In vitro antiparasitic activity of camel milk against Blastocystis sp. Int J Trop Dis Health. 2019;34:1–9. doi: 10.9734/IJTDH/2018/45844. [DOI] [Google Scholar]
- Balto H, Al-Sanie I, Al-Beshri S, Aldrees A. Effectiveness of Salvadora persica extracts against common oral pathogens. Saudi Dent J. 2017;29:1–6. doi: 10.1016/j.sdentj.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran H, Govind SK, Panchadcharam C, Bathmanaban P, Raman K, Thergarajan G. High lipid storage in vacoular forms of subtype 6 Blastocystis sp. in ostrich. Parasite Vector. 2014;7:469. doi: 10.1186/s13071-014-0469-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirioni O, Giacometti A, Drenaggi D, Ancarani F, Scalise G. Prevalence and clinical relevance of Blastocystis hominis in diverse patient cohorts. Eur J Epidemiol. 1999;15:389–393. doi: 10.1023/a:1007551218671. [DOI] [PubMed] [Google Scholar]
- Clark CG. Extensive genetic diversity in Blastocystis hominis. Mol Biochem Parasitol. 1997;87:79–83. doi: 10.1016/s0166-6851(97)00046-7. [DOI] [PubMed] [Google Scholar]
- Dkhil MA, Thagfan FA, Hassan AS, Al-Shaebi EM, Abdel-Gaber R, Al-Quraishy S. Anthelmintic, anticoccidial and antioxidant activity of Salvadora persica root extracts. Saudi J Biol Sci. 2019;26:1223–1226. doi: 10.1016/j.sjbs.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogruman F, Dagci H, Yoshikawa H, Kurt O, Demirel M. A possible link between subtype 2 and asymptomatic infections of Blastocystis hominis. Parasitol Res. 2008;103:685–689. doi: 10.1007/s00436-008-1031-3. [DOI] [PubMed] [Google Scholar]
- Giacometti A, Cirioni O, Fiorentini A, Fortuna M, Scalise G. Irritable bowel syndrome in patients with Blastocystis hominis infection. Eur J Clin Microbiol Infect Dis. 1999;18:436–439. doi: 10.1007/s100960050314. [DOI] [PubMed] [Google Scholar]
- Hameed DM, Hassanin OM, Zuel-Fakkar NM. Association of Blastocystis hominis genetic subtypes with urticaria. Parasitol Res. 2011;108:553–560. doi: 10.1007/s00436-010-2097-2. [DOI] [PubMed] [Google Scholar]
- Haresh K, Suresh K, Khairul AA, Saminathan S. Isolate resistance of Blastocystis hominis to metronidazole. Trop Med Int Health. 1999;4:274–277. doi: 10.1046/j.1365-3156.1999.00398.x. [DOI] [PubMed] [Google Scholar]
- Hattab F. Meswak: the natural toothbrush. J Clin Dent. 1997;8:125–129. [PubMed] [Google Scholar]
- Kain KC, Noble MA, Freeman HJ, Barteluk RL. Epidemiology and clinical features associated with Blastocystis hominis infection. Diagn Microbiol Infect Dis. 1987;8:235–244. doi: 10.1016/0732-8893(87)90055-1. [DOI] [PubMed] [Google Scholar]
- Khoory T. The use of chewing sticks in preventive oral hygiene. Clin Prev Dent. 1983;5:11–14. [PubMed] [Google Scholar]
- Leder K, Hellard ME, Sinclair MI, Fairley CK, Wolfe R. No correlation between clinical symptoms and Blastocystis hominis in immunocompetent individuals. J Gastroenterol Hepatol. 2005;20:1390–1394. doi: 10.1111/j.1440-1746.2005.03868.x. [DOI] [PubMed] [Google Scholar]
- Moghaddam DD, Ghadirian E, Azami M. Blastocystis hominis and the evaluation of efficacy of metronidazole and trimethoprim/sulfamethoxazole. Parasitol Res. 2005;96:273–275. doi: 10.1007/s00436-005-1363-1. [DOI] [PubMed] [Google Scholar]
- Mohamed RT, El-Bali MA, Mohamed AA, et al. Subtyping of Blastocystis sp. isolated from symptomatic and asymptomatic individuals in Makkah, Saudi Arabia. Parasite Vector. 2017;10:174. doi: 10.1186/s13071-017-2114-2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokhtar AB, Ahmed SA, Eltamany EE, Karanis P. Anti-Blastocystis activity in vitro of Egyptian Herbal extracts (Family: Asteraceae) with emphasis on Artemisia judaica. Int J Environ Res Public Health. 2019 doi: 10.3390/ijerph16091555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noumi E, Snoussi M, Hajlaoui H, Valentin E, Bakhrouf A. Antifungal properties of Salvadora persica and Juglans regia L. extracts against oral Candida strains. Eur J Clin Microbiol Infect Dis. 2010;29:81–88. doi: 10.1007/s10096-009-0824-3. [DOI] [PubMed] [Google Scholar]
- Parkar U, Traub RJ, Vitali S, Elliot A, Levecke B, Robertson I, Geurden T, Steele J, Drake B, Thompson RC. Molecular characterization of Blastocystis isolates from zoo animals and their animal-keepers. Vet Parasitol. 2010;169:8–17. doi: 10.1016/j.vetpar.2009.12.032. [DOI] [PubMed] [Google Scholar]
- Pegelow K, Gross R, Pietrzik K, Lukito W, Richards AL, Fryauff DJ. Parasitological and nutritional situation of school children in the Sukaraja district, West Java, Indonesia. Southeast Asian J Trop Med Public Health. 1997;28:173–190. [PubMed] [Google Scholar]
- Puthia MK, Vaithilingam A, Lu J, Tan KS. Degradation of human secretory immunoglobulin A by Blastocystis. Parasitol Res. 2005;97:386–389. doi: 10.1007/s00436-005-1461-0. [DOI] [PubMed] [Google Scholar]
- Rajamanikam A, Hooi HS, Kudva M, Samudi C, Kumar S. Resistance towards metronidazole in Blastocystis sp.: a pathogenic consequence. PLoS ONE. 2019;14:542. doi: 10.1371/journal.pone.0212542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol JF, Kabil SM, Said M, Samir H, Younis AM. Effect of nitazoxanide in persistent diarrhea and enteritis associated with Blastocystis hominis. Clin Gastroenterol Hepato. 2005;3:987–991. doi: 10.1016/s1542-3565(05)00427-1. [DOI] [PubMed] [Google Scholar]
- Saddiq AA, Alkinani MH. Fungicidal impact of Salvadora Persica L. (Miswak) extract on growth of foodborne pathogens, Aspergillus species. Dose Response. 2019;17:1559325819876218. doi: 10.1177/1559325819876218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawangjaroen N, Sawangjaroen K. The effects of extracts from anti-diarrheic Thai medicinal plants on the in vitro growth of the intestinal protozoa parasite: Blastocystis hominis. J Ethnopharmacol. 2005;98:67–72. doi: 10.1016/j.jep.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Sheehan DJ, Raucher BG, McKitrick JC. Association of Blastocystis hominis with signs and symptoms of human disease. J Clin Microbiol. 1986;24:548–550. doi: 10.1128/JCM.24.4.548-550.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher H, Al-Yemeni MN, Masrahi YS, Shah AH. Ethnomedicinal and ethnoecological evaluation of Salvadora persica L.: a threatened medicinal plant in Arabian Peninsula. J Med Plants Res. 2010;4:1209–1215. [Google Scholar]
- Sofrata AH, Claesson RL, Lingstrom PK, Gustafsson AK. Strong antibacterial effect of miswak against oral microorganisms associated with periodontitis and caries. J Periodontol. 2008;79:1474–1479. doi: 10.1902/jop.2008.070506. [DOI] [PubMed] [Google Scholar]
- Stensvold CR, Suresh GK, Tan KS. Terminology for Blastocystis subtypes-a consensus. Trends Parasitol. 2007;23:93–96. doi: 10.1016/j.pt.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Stenzel DJ, Boreham PF. Blastocystis hominis revisited. Clin Microbiol Rev. 1996;9:563–584. doi: 10.1128/CMR.9.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KS. Blastocystis in humans and animals: new insights using modern methodologies. Vet Parasitol. 2004;126:121–144. doi: 10.1016/j.vetpar.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Tan KS. New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin Microbiol Rev. 2008;21:639–665. doi: 10.1128/CMR.00022-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasova Y, Sahin B, Koltas S, Paydas S. Clinical significance and frequency of Blastocystis hominis in Turkish patients with hematological malignancy. Acta Med Okayama. 2000;54:133–136. doi: 10.18926/AMO/32298. [DOI] [PubMed] [Google Scholar]
- Wakil SE. Evaluation of the in vitro effect of Nigella sativa aqueous extract on Blastocystis hominis isolates. J Egypt Soc Parasitol. 2007;37:801–813. [PubMed] [Google Scholar]
- Wu CD, Darout IA, Skaug N. Chewing sticks: timeless natural toothbrushes for oral cleansing. J Periodontal Res. 2001;36:275–284. doi: 10.1034/j.1600-0765.2001.360502.x. [DOI] [PubMed] [Google Scholar]
- Wu Z, Mirza H, Tan KS. Intra-subtype variation in enteroadhesion accounts for differences in epithelial barrier disruption and is associated with metronidazole resistance in Blastocystis subtype-7. PLoS Negl Trop Dis. 2014;8:e2885. doi: 10.1371/journal.pntd.0002885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LQ, Singh M, Yap EH, Ng GC, Xu HX, Sim KY. In vitro response of Blastocystis hominis against traditional Chinese medicine. J Ethnopharmacol. 1996;55:35–42. doi: 10.1016/s0378-8741(96)01471-7. [DOI] [PubMed] [Google Scholar]
- Yoshikawa H, Nagano I, Wu Z, Yap EH, Singh M, Takahashi Y. Genomic polymorphism among Blastocystis hominis strains and development of subtype-specific diagnostic primers. Mol Cell Probes. 1998;12:153–159. doi: 10.1006/mcpr.1998.0161. [DOI] [PubMed] [Google Scholar]
- Yoshikawa H, Wu Z, Kimata I, et al. Polymerase chain reaction-based genotype classification among human Blastocystis hominis populations isolated from different countries. Parasitol Res. 2004;92:22–29. doi: 10.1007/s00436-003-0995-2. [DOI] [PubMed] [Google Scholar]
- Zaki M, Daoud AS, Pugh RN, Al-Ali F, Al-Mutairi G, Al-Saleh Q. Clinical report of Blastocystis hominis infection in children. J Trop Med Hygiene. 1991;94:118–122. [PubMed] [Google Scholar]
- Zaman V, Zaki M. Resistance of Blastocystis hominis cysts to metronidazole. Trop Med Int Health (TM & IH) 1996;1:677–678. doi: 10.1111/j.1365-3156.1996.tb00094.x. [DOI] [PubMed] [Google Scholar]