Abstract
Introduction
Chronic kidney disease (CKD) affects approximately 13% of the world’s population and will lead to dialysis or kidney transplantation. Unfortunately, clinically available drugs for CKD show limited efficacy and toxic extrarenal side effects. Hence, there is a need to develop targeted delivery systems with enhanced kidney specificity that can also be combined with a patient-compliant administration route for such patients that need extended treatment. Towards this goal, kidney-targeted nanoparticles administered through transdermal microneedles (KNP/MN) is explored in this study.
Methods
A KNP/MN patch was developed by incorporating folate-conjugated micelle nanoparticles into polyvinyl alcohol MN patches. Rhodamine B (RhB) was encapsulated into KNP as a model drug and evaluated for biocompatibility and binding with human renal epithelial cells. For MN, skin penetration efficiency was assessed using a Parafilm model, and penetration was imaged via scanning electron microscopy. In vivo, KNP/MN patches were applied on the backs of C57BL/6 wild type mice and biodistribution, organ morphology, and kidney function assessed.
Results
KNP showed high biocompatibility and folate-dependent binding in vitro, validating KNP’s targeting to folate receptors in vitro. Upon transdermal administration in vivo, KNP/MN patches dissolved within 30 min. At varying time points up to 48 h post-KNP/MN administration, higher accumulation of KNP was found in kidneys compared with MN that consisted of the non-targeting, control-NP. Histological evaluation demonstrated no signs of tissue damage, and kidney function markers, serum blood urea nitrogen and urine creatinine, were found to be within normal ranges, indicating preservation of kidney health.
Conclusions
Our studies show potential of KNP/MN patches as a non-invasive, self-administrable platform to direct therapies to the kidneys.
Electronic supplementary material
The online version of this article (10.1007/s12195-020-00622-3) contains supplementary material, which is available to authorized users.
Keywords: Chronic kidney disease, Kidney-targeting, Nanoparticles, Folate, Microneedles
Introduction
Chronic kidney disease (CKD) is recognized as a major public health concern worldwide, affecting ~ 15% of American adults (37 million) and ~ 13.4% of the world’s population.22,23,42,47,52 Specifically, polycystic kidney disease (PKD) is the fourth leading cause of CKD, characterized by massive kidney enlargement and nephron architecture distortion due to cyst formation. PKD can ultimately progress to end stage renal disease (ESRD), where dialysis and kidney replacement remains the only possibilities for maintaining a patient’s long-term survival.51,52 Treatments for PKD, such as everolimus and sirolimus,34,56 bardoxolone methyl,8,15 and paricalcitol,16,43 have been historically disappointing as many of these potential drug candidates failed in clinical trials owing to their safety issues and lack of efficacy. In 2018, tolvaptan, a small molecule drug for PKD, received approval from the Food and Drug Administration (FDA) as the first drug to inhibit disease progression. However, tolvaptan has been projected to delay ESRD only by 5 years after 18 years of treatment and found to have off-target side effects that adversely affects the patient’s quality of life including hepatotoxicity, high urine production, and constant thirst.4
Several kidney-specific delivery systems have been previously described to selectively deliver drugs to the kidneys including macromolecules such as chitosan,62 enzymes such as lysozyme,63 prodrugs,32 and nanoparticles (NP).64 Specifically, self-assembling NP based on polyethylene glycol (PEG)-phospholipid amphiphiles have been found to be advantageous for kidney-targeted drug delivery applications due to their ability to pass through the fenestrations of the glomerular filtration barrier for enhanced kidney accumulation7,11,12,58,59 and excellent biocompatibility.9,13,53 The hydrophobic core can be used to load lipophilic drugs while the hydrophilic corona containing the PEG moiety can be used to couple targeting ligands such as folate. Folate (FA) has been proposed as a ligand for kidney-targeting due to the high expression of folate receptors on renal proximal tubule cells.49,50 Previously, Wang et al. found folate-conjugated diethylene triamine penta-acetic acid (DTPA) to be largely distributed in the kidneys of tumor-bearing mice compared with its rapid clearance from folate receptor-negative tissues.57 Moreover, Weimbs et al. showed that folate-rapamycin conjugates can efficiently target polycystic kidneys and inhibit renal cyst growth in PKD mouse models compared to the free drug.17,26 Thus, decorating NP with folate has the potential to achieve active targeting and enhance kidney drug delivery for applications in CKD.
Despite its promise, most NP are designed for intravenous administration, which is not ideal for patients with chronic diseases. Transmucosal delivery including nasal, oral, ocular, and sublingual administration present alternatives to conventional, parenteral methods, but also show unique limitations including limited penetration through mucous, low retention due to the fast recycling time of mucosal layers, and drug degradation in gastric pH.1 Although oral delivery is favorable for self-administration for patients with chronic diseases, up to 90% of the drug taken orally can get eliminated via first pass metabolism in the liver, causing off-target side effects as mentioned with PKD drugs.18,45
Addressing the limitations of intravenous and oral routes, in this study, we designed a transdermal MN patch composed of an FDA-approved polymer, polyvinyl alcohol (PVA), to deliver kidney-targeting nanoparticles (KNP). MN patches allow for painless penetration into the skin and enhanced drug delivery past the stratum corneum,24 the outermost layer and the main barrier to delivering drugs through the skin, and is combined with the convenience of a self-administrated patch.25,30 Dissolvable MN break down in the skin interstitial fluid to release the encapsulated payload,29 and have already been reported to enhance delivery of drugs, vaccines, and proteins.18,31,33,39
To take advantage of these properties, rhodamine B (RhB)-encapsulated KNP comprising of folate-functionalized DSPE-PEG2000 (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-amino(polyethylene glycol)]-2000) was loaded into PVA MN. After administration on the backs of C57BL/6 wildtype mice, in vivo biodistribution and half-life of KNP was evaluated for up to 48 h. Finally, kidney function and overall safety was measured by urine creatinine, blood urea nitrogen levels, and histological analyses.
Materials and Methods
Fabrication of MN Patches
The dissolving, transdermal MN patch was fabricated using polyvinyl alcohol (PVA) via a micro-molding technique. Briefly, silicone molds were purchased from Blueacre Technology Ltd. consisting of a 21 × 21 microneedle array with 600 μm needle height, 300 μm base width, and 50 μm interspacing at the base, in a mold area of 0.5 cm2. First, an aqueous blend of PVA (10% w/v) was prepared, deposited in a silicone mold, followed by 20 min of vacuum and centrifugation at 300 rpm for 1 min to compact the PVA solution into the MN cavities. Then, an additional solution of PVA was deposited into the mold and the vacuum-centrifugation step was repeated 4–5 times. Finally, a backing layer consisting of 15% PVA solution was poured onto the mold and air-dried for 48 h at room temperature. The patch was then carefully peeled off from the silicone mold and stored at room temperature for further studies.
Characterization of MN Patches
The morphology of MN patches was characterized using scanning electron microscope (SEM, JEOL JSM-7001-SEM, JEOL USA, Inc.) after sputter coating with platinum/palladium. The dissolution properties were evaluated by fabricating RhB-loaded MN patches (3 mg RhB/30 mg PVA) and placing individual MN patches in 10 mL phosphate buffered saline (PBS, pH 7.4) for 1–10 min at 37 °C, followed by fluorescence measurements using a microplate reader at an excitation/emission wavelength of 546 nm and 568 nm. In addition, the degrading MN patches were imaged using light microscopy at 10 seconds, 1, 2, 5, and 10 mins (Nikon Eclipse TS100, Minato, Tokyo, Japan). The MN insertion efficiency was evaluated using a previously reported Parafilm M® insertion model,27 where varying number of Parafilm M® sheets were stretched, layered to form ~1 mm thickness, and the MN patch was inserted with 5 min manual thumb pressure. After insertion, sheets were imaged using SEM and the % of MN to penetrate each layer was calculated.
Synthesis of Kidney-Targeted Nanoparticles (KNP)
KNP with low and high folate content referred as 20F-KNP and 40F-KNP were assembled by mixing DSPE-PEG2000-folate (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)]-2000-folate) (Avanti Polar Lipids, Alabaster, AL, USA) and DSPE-PEG2000-methoxy amphiphiles at 20:80 or 40:60 molar ratios in methanol and evaporating the solution under nitrogen to form a thin film. Residual methanol was removed by drying the film under vacuum overnight, followed by hydration at 80 °C for 30 min in PBS or water and then allowed to cool to room temperature. Control-NP (non-targeting) were prepared consisting of only DSPE-PEG2000-methoxy.
The NP morphology was examined using transmission electron microscopy (TEM, JEOL JEM-2100F, JEOL, Ltd., Tokyo, Japan) using negative staining. Briefly, 2% uranyl acetate solution was added to 100 μM NP aqueous solution on a carbon grid for 2 min, excess solution was removed with filter paper followed by air-drying and imaging. Hydrodynamic diameter measurements were taken at 90° and 637 nm using a Wyatt DynaPro Mobius (Wyatt, Santa Barbara, CA, USA) and zeta potential measurements via Zetasizer Nano ZS (Malvern, Worcestershire, UK). Samples (100 μM) were prepared with PBS or water, filtered through Puradisc 0.22-μm polyvinylidene fluoride membrane filters (PVDF, GE Healthcare Life Sciences, Pittsburgh, PA, USA), and then analyzed.
Synthesis of RhB-Loaded NP
RhB was encapsulated into the hydrophobic core of the NP and used as a model hydrophobic drug. In brief, RhB and NP was mixed in a 10:1 weight ratio in methanol (1000 µM NP), dried to form a thin film, and then hydrated with PBS or water. Resulting products were then filtered through a 0.22-μm hydrophobic PTFE syringe filter (Sigma Aldrich, St. Louis, MO, USA) to remove the unloaded dye. The resultant RhB-loaded NP solution was lyophilized and stored at − 80 °C. The concentration of RhB loading was quantified by measuring the fluorescence at λexcitation = 495 nm, λemission = 519 nm using a fluorescence spectrophotometer and loading calculated using the following equation:
Loading capacity for non-targeting NP, 20FA-KNP, and 40FA-KNP was then calculated and compared with a standard curve.
In Vitro Biocompatibility and NP Binding
Biocompatibility and cellular binding of NP was evaluated using human primary renal proximal tubule epithelial cells (RPTEC, PCS-400-010™) as well as renal epithelial cells derived from an autosomal dominant polycystic kidney disease (ADPKD) patient (WT 9-12, ATCC, Manass, Virginia, USA).64 RPTEC were cultured in renal epithelial cell basal medium supplemented with renal epithelial cell growth kit at 37 °C in a humidified incubator under 5% CO2. WT 9-12 cells were expanded in DMEM containing L-glutamine (200 mg/L) supplemented with (10% v/v) FBS, penicillin (100 U/mL), and streptomycin (100 U/mL), and maintained in a humidified incubator at 37 °C and 5% CO2. In vitro biocompatibility was performed with a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) cell proliferation colorimetric assay (Biovision, Milpitas, CA, USA). RPTEC and WT 9-12 (5,000 cells/well, 96-well plate) were treated with 1–100 μM of NP for 24 h followed by MTS reagent addition and absorbance measured at 420 nm using a microplate reader. Viability was normalized to PBS-treated controls (n = 6).
To assess NP binding, non-targeting NP, 20FA-KNP, and 40FA-KNP were synthesized using DSPE-PEG2000-methoxy: DSPE-PEG2000-folate: DSPE-PEG2000-FITC monomers at 0:90:10, 70:20:10 and 50:40:10 molar ratios, respectively. Cells were incubated with 50 μM NP samples for 1 or 2 h, followed by fluorescence measurement at an excitation and emission spectrum peak wavelengths of 495 nm and 519 nm, respectively. In addition, 20FA-KNP and 40FA-KNP targeting was also studied by a competition assay, where cells were pre-incubated with 5 mM folate (i.e. 100-fold excess) for 1 h prior to NP addition (n = 6).
Synthesis and Characterization of KNP/MN Patch
To prepare the KNP/MN patch, aqueous solutions of PVA and 40FA-KNP at 1:10 w/w ratio was mixed, followed by deposition in the silicone mold, vacuum-centrifugation for 4-5 times (as mentioned), addition of a backing layer (PVA, 15% w/w), and air drying for 48 h. Control (non-targeting) NP/MN patches were also fabricated. Each MN patch consisted of 30 mg PVA with 3 mg RhB-loaded KNP (0.24 mg RhB, 1000 µM KNP). Furthermore, to evaluate the stability of KNP upon MN dissolution, KNP/MN were dissolved in PBS for 10 min at RT and the NP size was analyzed using DLS (n = 4).
In Vivo Application of Transdermal KNP/MN Patch
The MN patch-mediated transdermal delivery of KNP was evaluated in male and female, 7 week old C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME, USA). MN patches consisted of RhB-loaded 40FA-KNP or RhB-loaded non-targeting NP at 1:10 w/w% NP to PVA in PBS, as described above. Before MN application, animals were anaesthetized (2–4% isoflurane) and fur from the back was removed using a hair removal cream. Patches were applied to the skin using firm thumb pressure for 5 min, and further secured for 30 min with Micropore™ tape (3M, Saint Paul, MN, USA) before removal. To assess biodistribution, animals were euthanized after 24 and 48 h (n = 4), and ex vivo optical imaging was performed of excised organs (brain, heart, lungs, kidney, liver, spleen, intestines, bladder and skin) using AMI1000 (Spectral Instruments Imaging, Tucson, AZ, USA).
At the final time point, blood as well as urine was collected to investigate kidney function, measured by urine creatinine (Crystal Chem, Elk Grove Village, IL, USA) and serum blood urea nitrogen (BUN) (Bioo Scientific, Austin, TX, USA) through commercially available kits. Organ morphology was assessed after embedding and freezing organs in Optimal Cutting Temperature (OCT) compound, and staining tissue sections (5–10 µm) obtained using a cryostat (Leica CM3050S, Leica, Wetzlar, Germany) with hematoxylin & eosin (H&E), followed by imaging using a light microscope (Nikon Eclipse TS100, Minato, Tokyo, Japan). For comparison, additional groups of animals were injected intraperitonially (IP) with 100 μL of RhB-loaded NP solution in PBS (0.24 mg RhB, 1000 µM NP) as well as 100 μL PBS only.
To assess half-life, blood samples (≤ 100 μL) were collected through the retro-orbital sinus at 0.5, 1, 2, 3, 6, 12 and 24 h (n = 4) in male and female, 7 week old C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME, USA). All animal procedures followed NIH guidelines for the care and use of laboratory animals and were approved by the University of Southern California’s Institutional Animal Care and Use Committee (Los Angeles, CA, USA).
Statistical Analysis
Quantification data are given as mean ± standard deviation. All statistical analyses were performed using GraphPad Prism 8 (San Diego, CA, USA). A student’s t test or analysis of variance (ANOVA) was used with a Tukey’s test for post-hoc analysis to determine statistical significance and p ≤ 0.05 was considered to be significant.
Results and Discussion
MN Patch Characterization
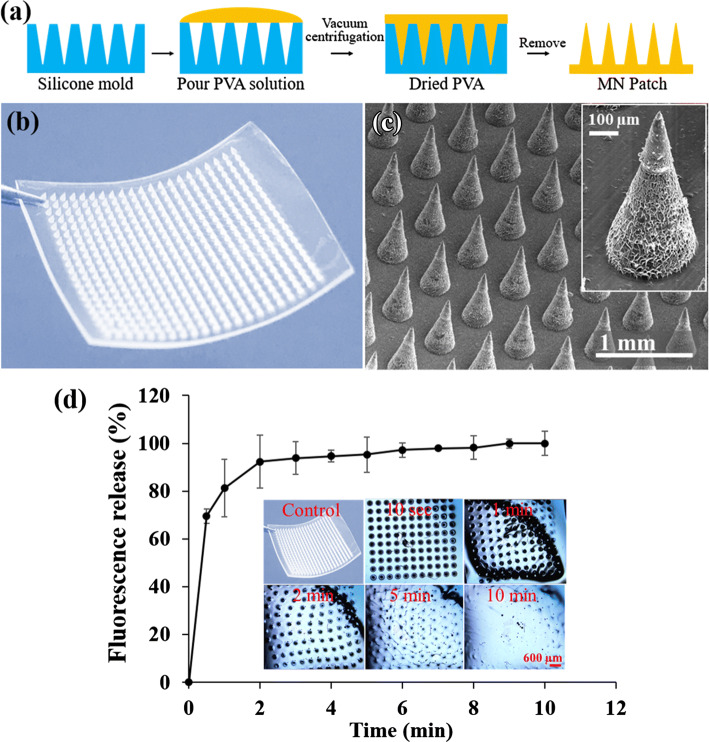
The design of a minimally-invasive, transdermal patch for in vivo applications should consider the use of a biocompatible material that can insert into the skin while rapidly dissolving upon interaction with the interstitial fluid to deliver the payload.30,40 Guided by these design criteria, in this study, the transdermal dissolving MN patch was microfabricated with the FDA-approved polymer, PVA, as shown in Figs. 1a and 1b. The 12 mm by 12 mm patch consisted of a uniformly arranged 21 × 21 MN array (Fig. 1c), in which each MN is conical in shape with a tip diameter of 5 µm, a base diameter of 300 µm, and a height of 600 µm. These dimensions will allow for penetration of the stratum corneum (10–15 µm thickness) and passage through the viable epidermis (50–100 µm thickness, avascular) and the vascularized papillary dermis (100–200 µm thickness), which is vital for reaching systemic circulation for drug delivery.20
Figure 1.
(a) Fabrication of dissolvable MN patch using micromolding technique. (b) Photograph of dissolvable MN patch. (c) SEM images of the MN array (inset: single MN). The MN patch consist of a 21 × 21 needle array, and each MN has a 300 μm base width to prevent significant tissue damage and 600 μm length for penetration into the dermis. (d) Dissolution efficiency and RhB release profile from the MN patch in PBS (10 mL, pH 7.4) for up to 10 min at 37 °C. Each data point represents the mean RhB fluorescence intensity at 556 nm excitation and 580 nm emission spectrum peak wavelength. Inset of d shows the light microscopic images of MN patch captured at different time points.
To assess MN dissolution, the PVA MN patch was placed in PBS for 1–10 min (Fig. 1d). PVA MN were found to be highly water soluble, as dissolution was evident within ~30 s and the needles completely dissolved by 10 min, consistent with other PVA studies.5,6,56 RhB-loaded PVA MN released ~ 90% RhB over 2 min (Fig. 1d), which further confirms its rapid dissolution and its potential to release drugs and NP upon insertion into the skin. However, the PVA MN dissolution rate in the skin of living models will likely vary depending on the presence of skin interstitial fluid (ISF). A previous study showed the collection of ~ 1 µL ISF within 20 min using a MN patch in pig cadaver skin and living human subjects.49 Hence, the MN dissolution rate will likely be slower in vivo compared to those obtained in PBS and will be further optimized in future studies.
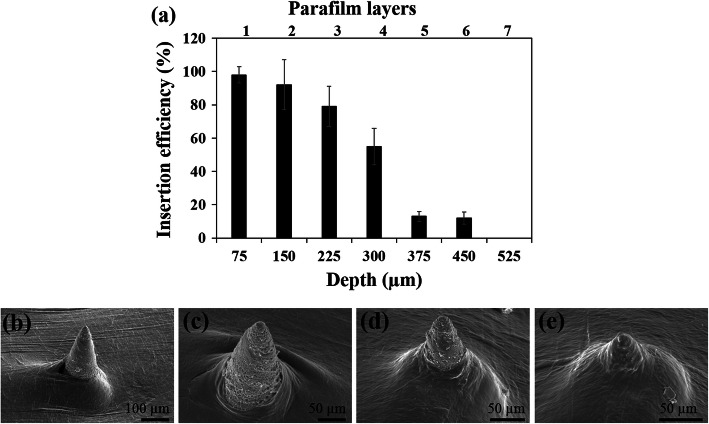
Next, in order to test PVA MN could penetrate the skin, PVA MN patches were first evaluated using a previously established Parafilm M® skin model,27 in which varying number of Parafilm M® sheets are stretched, layered, and the MN patch is inserted. Figure 2 shows the puncture efficiency in each Parafilm M® layer up to 7 layers (Fig. 2a) and the corresponding SEM images (Figs. 2b–2e) showing MN were capable of piercing up to 6 layers of Parafilm M®. The average thickness of a single Parafilm M® layer in our study is 75 ± 10 μm, which indicates that the MN inserted up to 450 ± 21 μm of the total 600 μm height, or ~ 80% of MN height, which is greater than similar methyl vinyl ether-based MN which was previously reported to insert up to 60%.27,36
Figure 2.
(a) The insertion efficiency generated in each Parafilm M® layer upon MN patch insertion. SEM images of MN insertion efficiency in a Parafilm M® model with varying layers: (b) 2 layers, (c) 4 layers, (d) 6 layers, and (e) 8 layers.
KNP Characterization
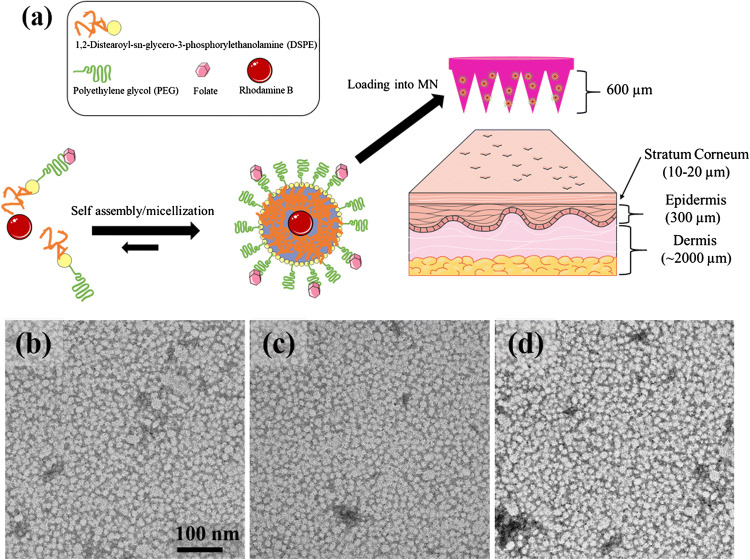
Towards delivering renal-targeting carriers through transdermal MN administration, KNP consisting of self-assembling micelle nanoparticles with 20:80 or 40:60 molar ratios of DSPE-PEG2000-folate amphiphiles and DSPE-PEG2000-methoxy were developed and loaded into MN (Fig. 3).10,26 Figures 3b–3d show TEM micrographs of non-targeting (control) NP, 20FA-KNP (low folate content), and 40FA-KNP (high folate content). The TEM images confirmed monodispersed, spherically shaped NP with an average hydrodynamic size of 16.3 ± 1.06, 10.6 ± 0.2 and 12.1 ± 0.84 nm and neutral zeta potentials of − 0.0 2± 0.03, − 0.04 ± 0.14 and − 1.83 ± 0.04 mV for non-targeting NP, 20FA-KNP and 40FA-KNP, respectively (Table 1). NP that are <15 nm in diameter have been reported to bypass the reticuloendothelial system and allow for passage through the glomerular filtration barrier via kidney filtration for enhanced kidney bioavailability.37,41 Hence, our KNP was confirmed to have favorable physicochemical properties for kidney accumulation.28
Figure 3.
(a) Schematic representation of micelle self-assembly and micelle loading into MN. Portions were adapted and reprinted from Servier Medical Art, under creative commons license. TEM micrographs of (b) control (non-targeting) NP and KNP with (c) low folate content (20FA-KNP) and (d) high folate content (40FA-KNP).
Table 1.
DLS and zeta potential measurements for non-targeting and kidney-targeting NP (20FA-KNP and 40FA-KNP).
| Nanoparticles | Diameter (nm) | Zeta potential (mV) |
|---|---|---|
| 20FA-KNP | 12.1 ± 0.84 | − 0.04 ± 0.14 |
| 40FA-KNP | 10.6 ± 0.2 | − 1.83 ± 0.04 |
| Control NP | 16.3 ± 1.06 | − 0.02 ± 0.03 |
Drug Loading and NP Stability
As a proof-of-concept, RhB was chosen as a model hydrophobic drug, given tolvaptan, the only FDA-approved PKD therapy, is also hydrophobic and similar in molecular weight (448.9 g/mol for tolvaptan, 443.6 g/mol for RhB).47 In addition, RhB has also been reported to be highly stable in vivo and is widely used in a variety of murine models.38,55,65 RhB was loaded into the hydrophobic core of the NP, followed by lyophilization and rehydration. The RhB loading efficiency into the NP was found to be approximately 80.00 ± 5.61 % for all NP formulations (control NP, 20FA-KNP, and 40FA-KNP). In addition, when RhB-loaded NP were lyophilized and rehydrated in water, the RhB amount detected was found to be 95.00 ± 2.17 %, suggesting high dye recovery and the potential for prolonged shelf-life of lyophilized drug-loaded NP.
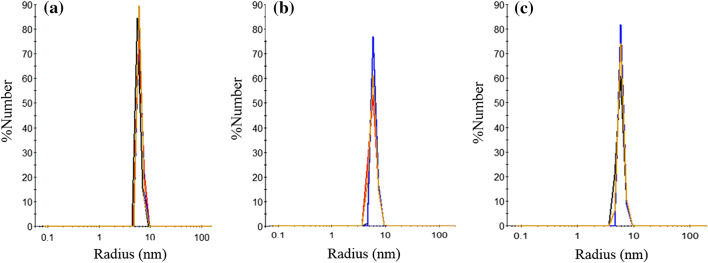
To test NP stability in the PVA MN upon dissolution, combined NP/MN were dissolved in PBS for 10 min. Figure 4 shows particle size of KNP in the MN polymer/water solution after dissolution. The average hydrodynamic size via DLS was observed to be similar to KNP formed in PBS (8.0 ± 1.3, 9.2 ± 1.3 nm and 10.1 ± 2.1 nm for non-targeting NP, 20FA-KNP and 40FA-KNP, respectively), which indicates NP recovery upon PVA MN dissolution and their feasibility for transdermal release.
Figure 4.
DLS measurements of particle size distributions of (a) non-targeting NP, (b) 20FA-KNP and (c) 40FA-KNP in the MN polymer solution shows recovery of KNP (n = 4).
KNP Biocompatibility and Targeting
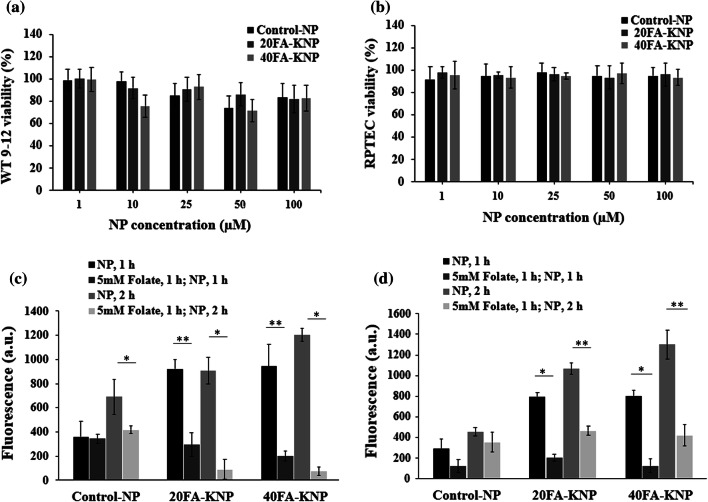
Figures 5a and 5b shows the in vitro biocompatibility of non-targeting NP, 20FA-KNP, and 40FA-KNP at a concentration of 1–100 µM on RPTEC and WT 9-12 over the course of 24 h at 37 °C. Over 90% of cells were viable upon treatment with all groups of NP and were comparable to the PBS-treated control group in RPTEC cells, while for WT 9-12 cells, starting at 10 µM some cytotoxicity was observed. Nonetheless, at the highest concentration of 100 µM, WT 9-12 cells showed 80% viability, which is consistent with previous reports of micelle NP biocompatibility.2,3,8,9,44,60,61 To assess the targeting of KNP to kidney cells, RPTEC and WT 9-12 cells were treated with 50 µM NP. After 1 h, approximately 40% higher binding of KNP was found compared with the non-targeting NP (Figs. 5c and 5d). Notably, the KNP with high folate content demonstrated higher binding (non-targeting NP < 20FA-KNP < 40FA-KNP; RPTEC: 296.8 ± 91, 795.3 ± 41.7, 802.8 ± 53.2 a.u. and 455.6 ± 40.2, 1067.9 ± 52.6, 1301.6 ± 139.7 a.u. for non-targeting NP, 20FA-KNP, 40FA-KNP at 1 or 2 h, respectively; WT 9-12: 359.5 ± 130.2, 920.5 ± 79.7, 942.9 ± 180 a.u. and 690.6 ± 146.3, 908.1 ± 109.3, 1203 ± 52.5 a.u. for non-targeting NP, 20FA-KNP, 40FA-KNP treated at 1 and 2 h, respectively). Moreover, a competitive inhibition assay was performed to further confirm targeting by folate. Both cell types were pre-treated with or without excess free folate (5 mM) in the medium for 1 h before NP incubation for an additional 1 h. As expected, the KNP binding was suppressed after folate addition (123.3 ± 62.1, 236.9 ± 34.1, 127 ± 63.8 a.u. and 356.2 ± 95.9, 464.9 ± 44.3, 421.6 ± 1.5.3 a.u. for RPTEC cells; 343.3 ± 38.7, 294.3 ± 98.2, 200.8 ± 39.4 a.u. and 413 ± 31.1, 90.4 ± 80.9, 76.2 ± 33.3 a.u. for WT 9-12 cells treated with non-targeting NP, 20FA-KNP, 40FA-KNP at 1 and 2 h, respectively). Although some effects on binding of control NP were seen upon folate pre-treatment, with the exception of the 1 h time point on RPTEC cells, these differences were not statistically significant. Taken together, this supports the ability of KNP to specifically target kidney cells without compromising biocompatibility.
Figure 5.
In vitro cell viability studied by MTT assay of (a) RPTEC and (b) WT 9–12. Cells were treated with 1–100 µM of non-targeting NP, 20FA-KNP and 40FA-KNP for 24 h. % viability normalized by PBS-treated controls. Cellular binding of RhB-loaded non-targeting NP, 20FA-KNP, and 40FA-KNP in (c) RPTEC and (d) WT 9–12. Cells were treated with 50 µM NP for 1 and 2 h with or without excess free (5 mM) folate (n = 6).
In Vivo Application of Transdermal KNP/MN Patch
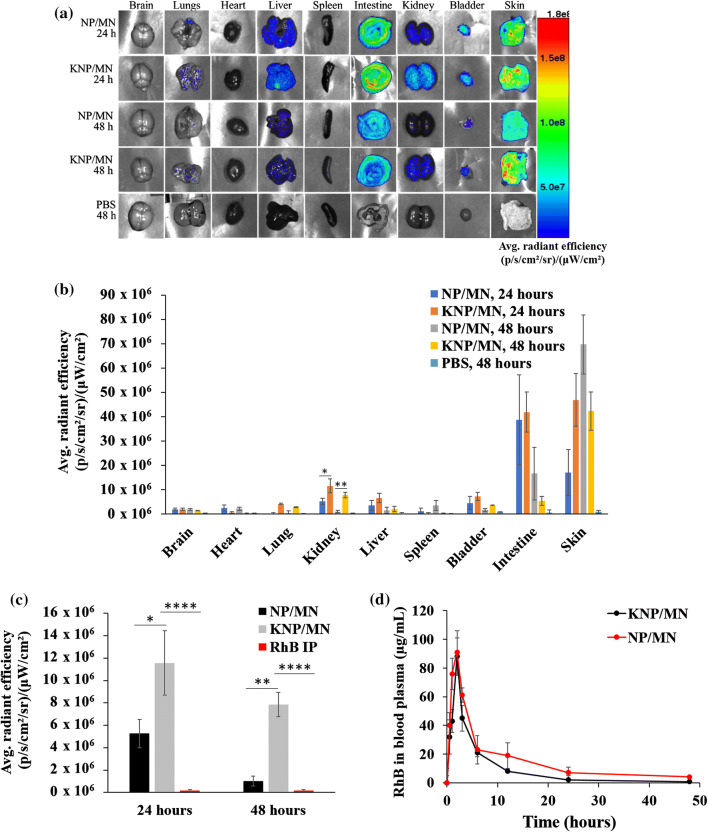
The in vivo feasibility of KNP/MN patch-mediated transdermal delivery was investigated in healthy C57BL/6J mice (Fig. 6, Fig. S1). For this study, the transdermal patch was incorporated with 40FA-KNP, since an enhanced binding of 40FA-KNP in vitro was evident in Figs. 5c and 5d. Figure 6 shows the biodistribution of KNP/MN and control NP/MN 24 and 48 h post-MN application or PBS-treated mice via IP injection. Ex vivo imaging showed both control NP and KNP accumulated mainly in the kidneys, liver, intestines, and skin (Fig. 6a). Specifically, quantitative comparison between ex vivo kidney fluorescence levels showed an average of 2.2 times higher accumulation of KNP compared to non-targeting NP after 24 h, and an average of 7.7 times higher accumulation of KNP compared to non-targeting NP after 48 h (Fig. 6b). Compared to a RhB control administered via IP injection, KM/MN administration showed RhB maintenance in the kidney for up to 48 h, whereas the dye is cleared before 24 h when administered IP (Fig. 6c, S2). Since fluorescence is observed in the intestines, a major pathway of clearance of both KNP and NP is excretion through feces, which is an important aspect to limiting nanomaterial accumulation for chronic diseases that may require multiple dosing.
Figure 6.
Biodistribution study of KNP, NP, and PBS after 24 and 48 h in C57BL/6 mice. (a) Ex vivo organ imaging post-MN application shows accumulation of KNP mostly in the kidney, liver, intestines, and skin. (b) Quantitative measurements of fluorescence levels in the brain, heart, lung, kidney, liver, spleen, bladder, intestines, and skin post-MN application at 24 and 48 h and PBS-treated mice via IP injection at 48 h. (c) Quantitative comparison of kidney fluorescence levels showed an average of 2.2 times higher accumulation of KNP compared to non-targeting NP after 24 h, and an average of 7.7 times higher accumulation of KNP compared to non-targeting NP after 48 h. (d) The half-life of RhB was found to be 90 ± 12 min for KNP/MN, and 120 ± 10 min for NP/MN (n = 4).
Figure 6d shows the RhB levels in the blood plasma after application of KNP/MN and NP/MN patch evaluated at different time points. An increase in the RhB blood level was observed as early as 2 h post-transdermal administration of the KNP/MN patch and the half-life of KNP and NP in blood was calculated to be 90 ± 12 min and 120 ± 10 min, respectively, which is similar to other reported half-lives of DSPE-PEG2000 nanoparticle systems.21,35 These results indicate that the dissolution of PVA MN allowed RhB-loaded KNP release into systemic circulation via the skin, to ultimately access and accumulate into the kidneys.
Organ Morphology and Kidney Function
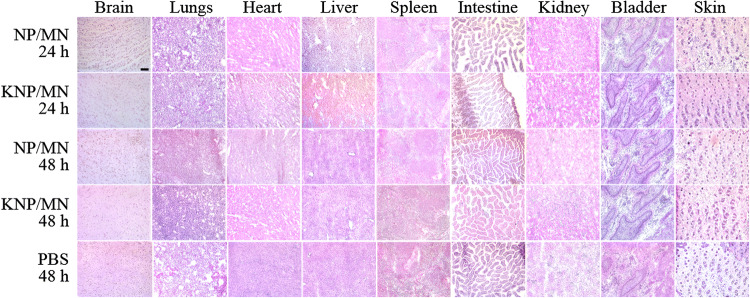
Histopathological evaluations of organs (brain, heart, lung, kidney, liver, spleen, intestines, bladder and skin) after transdermal application of KNP/MN or NP/MN patch for 24 and 48 h is shown in Fig. 7. All groups demonstrated a lack of morphological changes or cellular or tissue damage, compared with the PBS control group. These results were in line with other DSPE-PEG2000 based NP systems.13,14,60 In addition, no evidence of necrosis was found in histological skin sections, confirming biocompatibility of MN. To further ensure safety, kidney function was assessed by measuring serum BUN and urine creatinine levels (Table 2). All groups showed BUN and urine creatinine were within the range of healthy C57BL/6 mice (25.0–75.0 mg/dL and 4.7 ± 3. mg/dL, respectively) and are not statistically significant, which indicates the preservation of kidney health.19,46 In kidney disease, creatinine levels in urine are found to be abnormally low since it is not being removed from blood at a physiological rate.19 As KNP/MN patches do not alter kidney function, our KNP/MN system has potential as a safe platform for future therapeutic studies.
Figure 7.
H & E staining of organs after 24 and 48 h after KNP/MN, NP/MN, and PBS administration displayed no morphological changes. Scale bar: 100 µm.
Table 2.
Kidney function markers (serum BUN and urine creatinine) levels in healthy C57BL/6 after 24 and 48 h post-administration of KNP/MN, NP/MN, and PBS.
| Sample | Blood urea nitrogen (mg/dL) | Creatinine (mg/dL) |
|---|---|---|
| KNP/MN patch, 24 h | 46.7 ± 12.4 | 7.1 ± 2.1 |
| KNP/MN patch, 48 h | 38.3 ± 8.6 | 9.0 ± 1.5 |
| Control NP/MN patch, 24 h | 32.5± 14.1 | 8.3 ± 1.1 |
| Control NP/MN patch, 48 h | 26.4 ± 7.1 | 6.0 ± 2.7 |
| PBS (IP), 48 h | 21.9 ± 10.1 | 4.1 ± 3.8 |
Conclusion
In summary, we have developed a minimally invasive combinatorial platform for application in kidney diseases by integrating transdermal MN patch technology with KNP, which can facilitate delivery of biologics specifically to the kidneys, thereby limiting systemic side effects. Specifically, our KNP is <15 nm which offers kidney accumulation via passage through the glomerular filtration barrier and is decorated with folate, which can target folate receptors highly expressed on renal epithelial cells. Future investigation of this platform will incorporate ADPKD drugs and will test therapeutic efficacy in diseased animal models.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors would like to acknowledge the financial support from the Women in Science and Engineering (WiSE), Gabilan Assistant Professorship, L. K. Whittier Foundation, the National Heart, Lung, and Blood Institute (NHLBI, R00HL124279), and NIH New Innovator Award (DP2-DK121328) awarded to EJC. TEM images were taken with the aid of the USC Center of Excellence in Nano Imaging.
Conflict of interest
Nirmalya Tripathy, Jonathan Wang, Madelynn Tung, Claire Conway, and Eun Ji Chung have no conflicts of interest to disclose.
Animal Studies
All animal studies followed NIH guidelines for the care and use of laboratory animals and were conducted and approved by the University of Southern California’s Institutional Animal Care and Use Committee (Los Angeles, CA, USA).
Human Studies
No human studies were carried out by the authors for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abhang P, et al. Transmucosal drug delivery-an overview. Drug Deliv. Lett. 2014 doi: 10.2174/22103031113039990011. [DOI] [Google Scholar]
- 2.Au-Poon C, Au-Sarkar M, Au-Chung EJ. Synthesis of monocyte-targeting peptide amphiphile micelles for imaging of atherosclerosis. JoVE. 2017;129:e56625. doi: 10.3791/56625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black KA, et al. Biocompatibility and characterization of a peptide amphiphile hydrogel for applications in peripheral nerve regeneration. Tissue Eng Part A. 2015;21(7–8):1333–1342. doi: 10.1089/ten.tea.2014.0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blair HA. Tolvaptan: a review in autosomal dominant polycystic kidney disease. Drugs. 2019;79(3):303–313. doi: 10.1007/s40265-019-1056-1. [DOI] [PubMed] [Google Scholar]
- 5.Brough C, et al. Use of polyvinyl alcohol as a solubility enhancing polymer for poorly water-soluble drug delivery (part 2) AAPS PharmSciTech. 2016;17(1):180–190. doi: 10.1208/s12249-016-0490-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brough C, et al. Use of polyvinyl alcohol as a solubility-enhancing polymer for poorly water soluble drug delivery (part 1) AAPS PharmSciTech. 2016;17(1):167–179. doi: 10.1208/s12249-015-0458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chin MP, et al. Risk factors for heart failure in patients with type 2 diabetes mellitus and stage 4 chronic kidney disease treated with bardoxolone methyl. J. Cardiac Fail. 2014;20(12):953–958. doi: 10.1016/j.cardfail.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Chin DD, et al. Hydroxyapatite-binding micelles for the detection of vascular calcification in atherosclerosis. J. Mater. Chem. B. 2019;7(41):6449–6457. doi: 10.1039/c9tb01918a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chin DD, et al. Collagenase-cleavable peptide amphiphile micelles as a novel theranostic strategy in atherosclerosis. Adv. Ther. 2020;3:1900196. doi: 10.1002/adtp.201900196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu H, et al. Detecting functional and accessible folate receptor expression in cancer and polycystic kidneys. Mol. Pharm. 2019;16(9):3985–3995. doi: 10.1021/acs.molpharmaceut.9b00624. [DOI] [PubMed] [Google Scholar]
- 11.Chung EJ. Targeting and therapeutic peptides in nanomedicine for atherosclerosis. Exp. Biol. Med. (Maywood, N.J.) 2016;241(9):891–898. doi: 10.1177/1535370216640940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung EJ. Nanoparticle Strategies for Biomedical Applications: Reviews from the University of Southern California Viterbi School of Engineering. SLAS TECHNOLOGY: Transl. Life Sci. Innov. 2019;24(2):135–136. doi: 10.1177/2472630318816665. [DOI] [PubMed] [Google Scholar]
- 13.Chung EJ, et al. Fibrin-binding, peptide amphiphile micelles for targeting glioblastoma. Biomaterials. 2014;35(4):1249–1256. doi: 10.1016/j.biomaterials.2013.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung EJ, et al. In vivo biodistribution and clearance of peptide amphiphile micelles. Nanomedicine: Nanotechnol. Biol. Med. 2015;11(2):479–487. doi: 10.1016/j.nano.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 15.de Zeeuw D, et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N. Engl. J. Med. 2013;369(26):2492–2503. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delanaye P, et al. Paricalcitol for reduction of albuminuria in diabetes. The Lancet. 2011;377(9766):635. doi: 10.1016/S0140-6736(11)60222-5. [DOI] [PubMed] [Google Scholar]
- 17.Dong Y, et al. Folate-conjugated nanodiamond for tumor-targeted drug delivery. RSC Adv. 2015;5(101):82711–82716. [Google Scholar]
- 18.Donnelly RF, Raj Singh TR, Woolfson AD. Microneedle-based drug delivery systems: microfabrication, drug delivery, and safety. Drug Deliv. 2010;17(4):187–207. doi: 10.3109/10717541003667798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunn SR, et al. Utility of endogenous creatinine clearance as a measure of renal function in mice. Kidney International. 2004;65(5):1959–1967. doi: 10.1111/j.1523-1755.2004.00600.x. [DOI] [PubMed] [Google Scholar]
- 20.Flaten GE, et al. In vitro skin models as a tool in optimization of drug formulation. Eur. J. Pharm. Sci. 2015;75:10–24. doi: 10.1016/j.ejps.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 21.Gill KK, Kaddoumi A, Nazzal S. PEG–lipid micelles as drug carriers: physiochemical attributes, formulation principles and biological implication. Journal of Drug Targeting. 2015;23(3):222–231. doi: 10.3109/1061186X.2014.997735. [DOI] [PubMed] [Google Scholar]
- 22.Health, N.I.o., USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States., M.N.I.o.H. Bethesda, National Institute of Diabetes and Digestive and Kidney Diseases, Editor. 2018.
- 23.Hill NR, et al. Global prevalence of chronic kidney disease—a systematic review and meta-analysis. PLoS ONE. 2016;11(7):e0158765–e0158765. doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ita K. Transdermal delivery of drugs with microneedles-potential and challenges. Pharmaceutics. 2015;7(3):90–105. doi: 10.3390/pharmaceutics7030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito Y, et al. Two-layered dissolving microneedles formulated with intermediate-acting insulin. Int. J. Pharm. 2012;436(1):387–393. doi: 10.1016/j.ijpharm.2012.06.047. [DOI] [PubMed] [Google Scholar]
- 26.Kipp KR, et al. Comparison of folate-conjugated rapamycin versus unconjugated rapamycin in an orthologous mouse model of polycystic kidney disease. Am. J. Physiol.-Renal Physiol. 2018;315(2):F395–F405. doi: 10.1152/ajprenal.00057.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larrañeta E, et al. A proposed model membrane and test method for microneedle insertion studies. Int. J. Pharm. 2014;472(1):65–73. doi: 10.1016/j.ijpharm.2014.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lasagna-Reeves C, et al. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochem. Biophys. Res. Commun. 2010;393(4):649–655. doi: 10.1016/j.bbrc.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 29.Lau S, et al. Multilayered pyramidal dissolving microneedle patches with flexible pedestals for improving effective drug delivery. J. Controlled Rel. 2017;265:113–119. doi: 10.1016/j.jconrel.2016.08.031. [DOI] [PubMed] [Google Scholar]
- 30.Lee JW, Park J-H, Prausnitz MR. Dissolving microneedles for transdermal drug delivery. Biomaterials. 2008;29(13):2113–2124. doi: 10.1016/j.biomaterials.2007.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li W, et al. Rapidly separable microneedle patch for the sustained release of a contraceptive. Nature Biomedical Engineering. 2019;3(3):220–229. doi: 10.1038/s41551-018-0337-4. [DOI] [PubMed] [Google Scholar]
- 32.Lin Y, et al. Targeted drug delivery to renal proximal tubule epithelial cells mediated by 2-glucosamine. J. Controlled Rel. 2013;167(2):148–156. doi: 10.1016/j.jconrel.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Liu S, et al. The development and characteristics of novel microneedle arrays fabricated from hyaluronic acid, and their application in the transdermal delivery of insulin. J. Controlled Rel. 2012;161(3):933–941. doi: 10.1016/j.jconrel.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 34.Liu YM, Shao YQ, He Q. Sirolimus for treatment of autosomal-dominant polycystic kidney disease: a meta-analysis of randomized controlled trials. Transpl. Proc. 2014;46(1):66–74. doi: 10.1016/j.transproceed.2013.10.040. [DOI] [PubMed] [Google Scholar]
- 35.Lukyanov AN, et al. Polyethylene glycol-diacyllipid micelles demonstrate increased accumulation in subcutaneous tumors in mice. Pharm. Res. 2002;19(10):1424–1429. doi: 10.1023/a:1020488012264. [DOI] [PubMed] [Google Scholar]
- 36.Lutton REM, et al. A novel scalable manufacturing process for the production of hydrogel-forming microneedle arrays. Int. J. Pharm. 2015;494(1):417–429. doi: 10.1016/j.ijpharm.2015.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maeda H, et al. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Controlled Rel. 2000;65(1):271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 38.Mascari TM, Foil LD. Evaluation of rhodamine B as an orally delivered biomarker for rodents and a feed-through transtadial biomarker for phlebotomine sand flies (Diptera: Psychodidae) J. Med. Entomol. 2009;46(5):1131–1137. doi: 10.1603/033.046.0521. [DOI] [PubMed] [Google Scholar]
- 39.Matsuo K, et al. A low-invasive and effective transcutaneous immunization system using a novel dissolving microneedle array for soluble and particulate antigens. J. Controlled Rel. 2012;161(1):10–17. doi: 10.1016/j.jconrel.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 40.Moga KA, et al. Rapidly-dissolvable microneedle patches via a highly scalable and reproducible soft lithography approach. Adv. Mater. 2013;25(36):5060–5066. doi: 10.1002/adma.201300526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moretton MA, et al. Molecular implications in the nanoencapsulation of the anti-tuberculosis drug rifampicin within flower-like polymeric micelles. Colloids Surf. B: Biointerfaces. 2010;79(2):467–479. doi: 10.1016/j.colsurfb.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 42.Murphy D, et al. Trends in prevalence of chronic kidney disease in the United States. Ann. Internal Med. 2016;165(7):473–481. doi: 10.7326/M16-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perez-Gomez MV, et al. Horizon 2020 in diabetic kidney disease: the clinical trial pipeline for add-on therapies on top of renin angiotensin system blockade. J. Clin. Med. 2015;4(6):1325–1347. doi: 10.3390/jcm4061325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poon C, et al. Hybrid, metal oxide-peptide amphiphile micelles for molecular magnetic resonance imaging of atherosclerosis. J. Nanobiotechnol. 2018;16(1):92. doi: 10.1186/s12951-018-0420-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prausnitz MR, Mitragotri S, Langer R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004;3(2):115–124. doi: 10.1038/nrd1304. [DOI] [PubMed] [Google Scholar]
- 46.Rodrigues WF, Miguel CB, Napimoga MH, Oliveira CJF. Lazo-Chica JE (2014) Establishing standards for studying renal function in mice through measurements of body size-adjusted creatinine and urea levels. Biometr. Biosec. 2014;872827:8. doi: 10.1155/2014/872827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rogers ES. Iris, fundamentals of chemistry: solubility. Wisconsin: Department of Chemistry, University of Wisconsin; 2000. [Google Scholar]
- 48.Saigusa T, Bell PD. Molecular pathways and therapies in autosomal-dominant polycystic kidney disease. Physiology. 2015;30(3):195–207. doi: 10.1152/physiol.00032.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samant PP, Prausnitz MR. Mechanisms of sampling interstitial fluid from skin using a microneedle patch. Proceedings of the National Academy of Sciences. 2018;115(18):4583. doi: 10.1073/pnas.1716772115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sandoval RM, et al. Uptake and trafficking of fluorescent conjugates of folic acid in intact kidney determined using intravital two-photon microscopy. Am. J. Physiol.-Cell Physiol. 2004;287(2):C517–C526. doi: 10.1152/ajpcell.00006.2004. [DOI] [PubMed] [Google Scholar]
- 51.Shi H, et al. Folate-dactolisib conjugates for targeting tubular cells in polycystic kidneys. J. Controlled Rel. 2019;293:113–125. doi: 10.1016/j.jconrel.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 52.Stenvinkel P. Chronic kidney disease: a public health priority and harbinger of premature cardiovascular disease. J. Internal Med. 2010;268(5):456–467. doi: 10.1111/j.1365-2796.2010.02269.x. [DOI] [PubMed] [Google Scholar]
- 53.Torres VE, Harris PC. Strategies targeting cAMP signaling in the treatment of polycystic kidney disease. J. Am. Soc. Nephrol. 2014;25(1):18. doi: 10.1681/ASN.2013040398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trac NT, Chung EJ. Peptide-based targeting of immunosuppressive cells in cancer. Bioactive Mater. 2020;5(1):92–101. doi: 10.1016/j.bioactmat.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ueda Y, et al. In vivo imaging of T cell lymphoma infiltration process at the colon. Sci. Rep. 2018;8(1):3978. doi: 10.1038/s41598-018-22399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vora LK, et al. Novel nanosuspension-based dissolving microneedle arrays for transdermal delivery of a hydrophobic drug. J. Interdiscip. Nanomed. 2018;3(2):89–101. doi: 10.1002/jin2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walz G, et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2010;363(9):830–840. doi: 10.1056/NEJMoa1003491. [DOI] [PubMed] [Google Scholar]
- 58.Wang S, et al. Design and synthesis of [111In]DTPA−folate for use as a tumor-targeted radiopharmaceutical. Bioconjugate Chem. 1997;8(5):673–679. doi: 10.1021/bc9701297. [DOI] [PubMed] [Google Scholar]
- 59.Wang J, Masehi-Lano JJ, Chung EJ. Peptide and antibody ligands for renal targeting: nanomedicine strategies for kidney disease. Biomater. Sci.. 2017;5(8):1450–1459. doi: 10.1039/c7bm00271h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang J, et al. Design and in vivo characterization of kidney-targeting multimodal micelles for renal drug delivery. Nano Res. 2018;11(10):5584–5595. [Google Scholar]
- 61.Yoo SP, et al. Gadolinium-functionalized peptide amphiphile micelles for multimodal imaging of atherosclerotic lesions. ACS Omega. 2016;1(5):996–1003. doi: 10.1021/acsomega.6b00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yuan Z-X, et al. Specific renal uptake of randomly 50% n-acetylated low molecular weight chitosan. Mol. Pharm. 2009;6(1):305–314. doi: 10.1021/mp800078a. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Z, et al. The targeting of 14-succinate triptolide-lysozyme conjugate to proximal renal tubular epithelial cells. Biomaterials. 2009;30(7):1372–1381. doi: 10.1016/j.biomaterials.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 64.Zhou P, Sun X, Zhang Z. Kidney–targeted drug delivery systems. Acta Pharmaceutica Sin. B. 2014;4(1):37–42. doi: 10.1016/j.apsb.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu Y-H, et al. Incorporation of a rhodamine B conjugated polymer for nanoparticle trafficking both in vitro and in vivo. Biomater. Sci. 2019;7(5):1933–1939. doi: 10.1039/c9bm00032a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.