Abstract
Background
Volumetric tissue-engineered constructs are limited in development due to the dependence on well-formed vascular networks. Scaffold pore size and the mechanical properties of the matrix dictates cell attachment, proliferation and successive tissue morphogenesis. We hypothesize scaffold pore architecture also controls stromal-vessel interactions during morphogenesis.
Methods
The interaction between mesenchymal stem cells (MSCs) seeded on hydroxyapatite scaffolds of 450, 340, and 250 μm pores and microvascular fragments (MVFs) seeded within 20 mg/mL fibrin hydrogels that were cast into the cell-seeded scaffolds, was assessed in vitro over 21 days and compared to the fibrin hydrogels without scaffold but containing both MSCs and MVFs. mRNA sequencing was performed across all groups and a computational mechanics model was developed to validate architecture effects on predicting vascularization driven by stiffer matrix behavior at scaffold surfaces compared to the pore interior.
Results
Lectin staining of decalcified scaffolds showed continued vessel growth, branching and network formation at 14 days. The fibrin gel provides no resistance to spread-out capillary networks formation, with greater vessel loops within the 450 μm pores and vessels bridging across 250 μm pores. Vessel growth in the scaffolds was observed to be stimulated by hypoxia and successive angiogenic signaling. Fibrin gels showed linear fold increase in VEGF expression and no change in BMP2. Within scaffolds, there was multiple fold increase in VEGF between days 7 and 14 and early multiple fold increases in BMP2 between days 3 and 7, relative to fibrin. There was evidence of yap/taz based hippo signaling and mechanotransduction in the scaffold groups. The vessel growth models determined by computational modeling matched the trends observed experimentally.
Conclusion
The differing nature of hypoxia signaling between scaffold systems and mechano-transduction sensing matrix mechanics were primarily responsible for differences in osteogenic cell and microvessel growth. The computational model implicated scaffold architecture in dictating branching morphology and strain in the hydrogel within pores in dictating vessel lengths.
Electronic supplementary material
The online version of this article (10.1007/s12195-020-00648-7) contains supplementary material, which is available to authorized users.
Keywords: Vascularization, Stroma, Scaffold, Capillaries, Bone
Introduction
Every year millions of bone grafting procedures are performed in order to treat patients who have suffered from injury or disease that led to massive volumetric damage to bone tissue.7 Substantial research has focused on designing biomaterial scaffolds that promote bone regeneration to allow the patient to heal fully without the need for donor tissue from either autologous or allogeneic sources. Currently, a major limitation in the volumetric regeneration of skeletal tissue is lack of vascularization that the newly-formed bone tissue requires.26 This problem is significant, since a patent vascular supply is essential in order to carry out basic physiological transport such as delivery of nutrients to cells and removal of metabolic waste.61 Without the proper vascularization of bone, degeneration of the newly-formed tissue may lead to loss of function and necrosis, ultimately resulting in graft failure.
Highly porous, yet mechanically tough, synthetic scaffolds have been developed using a wide range of polymeric and ceramic materials and combinations there-of in an attempt to promote regeneration of the bone tissue11,15,22,27 Hydroxyapatite (HA), a synthetic calcium phosphate similar to the mineral constituent of bone tissue, has been widely shown to be osteoconductive23–25 and encourage bone regeneration within porous architectures2 or around microparticles15in vitro. Interconnected porosity is desired to overcome tissue infiltration limitations, but highly porous structures have poor mechanical strength and hence poor outcomes, in terms of the functional support required. While vascularization of bone tissue engineering scaffolds is an area of significant interest34,62,68 material-or cell-based approaches have had limited success in concurrent regeneration of functional bone and vasculature. Feng et al.3 reported that ceramic scaffolds with pore sizes < 400 μm limit vascular infiltration.3 Scaffolds have often been seeded with osteogenic cells to improve bone regeneration outcomes, most commonly mesenchymal stem cells (MSCs), derived from the bone marrow, because they are capable of differentiation along an osteogenic lineage,4,56 but also because they exert modulatory effects on host tissue by secretion of trophic factors.58 The presence of bone stromal cells within porous scaffolds might have a three-fold impact on vascularization: (1) increased cell numbers would result in hypoxic conditions within scaffolds, which are known to induce angiogenic signaling,20,46 (2) paracrine signaling may induce vessel growth and (3) potential modification of the local extracellular matrix to either locally support or impede blood vessel growth. For these reasons, to improve bone graft survival and regenerative outcomes, pre-vascularization of synthetic bone graft substitutes is currently under extensive investigation35,65,74,78 These studies are often limited because suitable in vitro and in silico approaches to simulate vascularized tissue development are neither sufficiently advanced nor validated to be used as stringent screening tools for the optimization of novel biomaterial scaffolds.
Recent studies have shown a possible solution to pre-vascularization in the form of adipose tissue-derived microvascular fragments (MVFs) which have been shown to support the growth of angiogenic tissue in several models.38,59 MVFs are a heterogenous mixture of arterioles, capillaries and venules, filtered to between 40 and 400 µm in length that benefit from including the tubular vessel structure, as well as associated stem cells resident in the microvasculature.54 These fragments have also shown retention of regenerative potential when placed within synthetic scaffolds to sustain proliferation of cells and secretion of growth factors vital to tissue regeneration8,36,63 Fibrin based hydrogels have been previously shown to promote the rapid growth and network formation of MVFs, both in vitro and in vitro9,48,66,70 Additionally, large amounts of microvascular fragments can be garnered from adipose tissue of the patient. Solely harvesting from patient adipose tissue could lead to the use of minimal amount of orthotopic tissue from the patient to increase regeneration of musculoskeletal tissue while also decreasing the chance of subsequent rejection of the pre-vascularized grafts as is the case with allografts or xenografts. For this strategy, the time dependence relied on by angiogenesis (vascular ingrowth from the host) is circumvented since blood vessels or vascular networks within implants need to form connections among themselves and inosculate with the host vasculature for long term perfusion and successful engraftment to be possible.37 Fibrin hydrogels also mimic the in situ blood clot (fracture hematoma) that forms at the bone defect site after injury, in that they include the entangled fibrin mesh, but not the inflammatory cytokines from the platelet plug in vitro.32 Fibrin hydrogels and composites thereof have been previously investigated to support bone regeneration in conjunction with biologic or growth factor delivery strategies.29,53
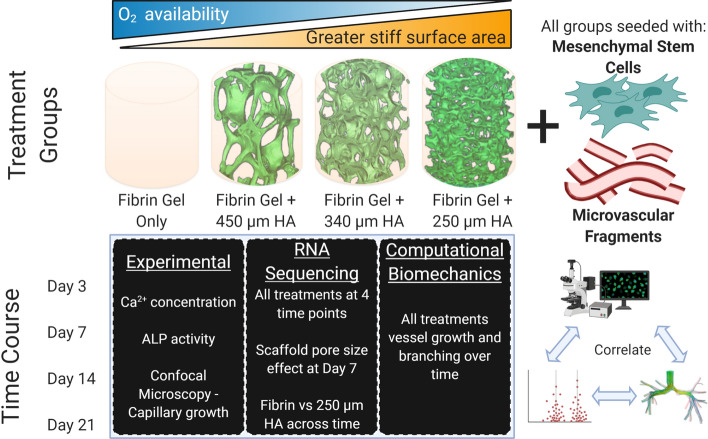
Many biomaterials have been developed for use as vascularized grafts; their limited success, however, necessitates further development, which is impeded by a poor understanding of the specific design parameters which can be optimized to induce enhanced vascularization. The interplay of biochemical, biological and fluid flow variables in the development of vasculature has been extensively studied, but the impact of the mechanics of the tissue extracellular matrix (ECM) and the temporal dynamics of the resistivity (or lack thereof) of the ECM to tissue ingrowth have been largely ignored. This is especially the case for vascular development within tissue regenerative matrices where small caliber capillary networks are necessary. In order to address these challenges, the current study attempts to reconcile three distinct metrics: (1) dynamics of multi-tissue development within biomaterial scaffolds, (2) the underlying cell-signaling pathways, and (3) understanding the impact of matrix mechanics as an independent variable to predict morphological development of vascular networks. The concurrent attachment, proliferation and commitment to bone-specific phenotype of bone-marrow-derived mesenchymal stem cells (MSCs) and growth, branching and capillary morphogenesis of MVFs in fibrin hydrogel filled-porous hydroxyapatite (HA) scaffolds is evaluated in vitro over 3 weeks (schematic in Fig. 1). The temporal dynamics within the multi-lineage culture system are evaluated in an attempt to understand their impact on vascularized tissue development. Furthermore, a computational mechanics model is developed to model blood vessel growth within highly porous synthetic biomaterials taking into account local matrix stiffness gradients—i.e. a fibrin or entangled protein hydrogel within the pore of a stiff, porous scaffold is softer near the center of the pore and stiffest at the scaffold surface. The overall hypothesis of this study is that the pore size of scaffolds thus impacts how vessel networks and osteoid like tissue form within biomaterial scaffolds over time.
Figure 1.
Experimental design. The experiment is based on MSCs seeded on HA scaffolds (450, 340 and 250 µm pore sizes) which are then infiltrated and filled with fibrin hydrogels containing MVFs. The control group is a fibrin hydrogel containing MVFs, with MSCs seeded at the base (mimicking an infinite pore). These scaffolds are evaluated over 21 days of in vitro culture (at day 3, 7, 14 and 21), with multiple experimental analyses (left box), RNA sequencing analysis and 2 sub-analyses (middle box) and compared to a computational biomechanics model (right box).
Methods
HA Scaffold Preparation
Calcium phosphate scaffolds were synthesized via a template coating method using polyurethane foams of varying pore sizes (45, 60, and 80 pores per inch), as described previously.24 Briefly, a hydroxyapatite (HA) (CaP Biomaterials LLC, East Troy, WI) based slurry was prepared using 5% high molecular weight polyvinyl alcohol, 5% carboxymethyl cellulose, 3% ammonium polyacrylate dispersant, and 10% N,N-dimethylformamide (as binders to help the stabilization and sintering of the scaffold). The slurry was coated over polyurethane foams (8 mm diameter and 2 mm height), and pore sizes of 45, 60, and 80 pores per inch which, will be referred to as 450, 340 and 250 µm pore size scaffolds, respectively, based on their architectural characterization.23,24 Samples were allowed to dry followed by sintering in a furnace (Zircar Zirconia, Florida, NY) to a peak value of 1240 °C for 20 h. The sintered scaffolds were coated for a second time with the HA based slurry, dried overnight, and sintered again. All chemical supplies were purchased from Sigma Aldrich (St. Louis, MO) unless otherwise specified.
Scaffold Characterization
Scanning electron microscopy (SEM) evaluation was performed at 40X magnification to measure the strut thickness and strut length (25 measurements per scaffold, n = 4 scaffolds/pore size) and quantify pore size. All microscopy was performed on an JEOL 6610 (JEOL Ltd., Tokyo, Japan) at 20 kV. One hundred independent struts were measured across four scaffolds per architecture of interest to the present study, and averaged to determine strut length as an estimate of pore interconnection/opening size.
Porosity was determined using an AccuPyc II Helium pycnometer (Micromeritics, Norcross, GA) to determine the skeletal volume of each scaffold (n = 6 scaffolds per architecture). Based on measurements of the average scaffold diameter and height, the envelope volume of each scaffold was calculated. Using the ratio of skeletal volume to envelope volume, porosity was determined using the following equation:
| 1 |
Scaffold permeability was measured using a custom flow apparatus consisting of a fluid reservoir that fed into a sample chamber opened to the atmosphere. The pressure head was thus equal to the height of the liquid column above the sample holder. Using Darcy’s Law, permeability was determined as:
| 2 |
where k is the permeability, m is the mass flow rate, µ is the fluid viscosity, L is the specimen length, Acs is the mean cross sectional area of the specimen, ρ is the fluid density, and ΔP is the pressure drop across the scaffold. Distilled water was used for the permeability measurements. Each scaffold was equilibrated by repeatedly running water through it for 5 min prior to testing. Permeability was then computed by measuring the volume of effluent water collected over four different lengths of time through each scaffold. All measurements for porosity and permeability were performed in triplicate for 6 scaffolds per architecture of interest to the present study.
Each scaffold was scanned using micro computed tomography (µCT), as previously reported.24 Briefly, scanning was performed at 100 kV and 100 µA source settings with no filter on a SkyScan 1076 (Bruker Skyscan, Belgium) at 8.87 μm spatial resolution. Morphometric analysis was carried out on CT images using CTanalyzer vs.1.4 (Skyscan) for 6 scaffolds per architecture. In order to determine trabecular bone organization, 9 traditional histomorphometric parameters were computed for each scaffold tested over its 3-D volume. The 9 parameters computed were bone volume ratio (BV/TV), bone surface to total volume ratio (BS/TV), bone surface density (BS/BV), trabecular pattern factor (Tb.Pf), structural model index (SMI), trabecular thickness (Tb.Th), trabecular number (Tb.N), trabecular spacing (Tb.Sp.), and degree of anisotropy (DA).
Rat Bone Marrow-MSC and Adipose MVF Isolation and Culture
Bone-marrow-derived mesenchymal stem cells (MSCs) were isolated from the femurs of wild type male Lewis rats (Charles River Laboratories, Wilmington, MA) using a modified version of a protocol.40 Briefly, the rats were euthanized using CO2 asphyxiation. The femurs were excised and remaining soft tissue was removed. The ends of each femur were scored using a scalpel and cut with a rongeur. The bone marrow of the femur was flushed out using cell culture medium consisting of α-MEM (Gibco, Carlsbad, CA), supplemented with 15% FBS (ATCC, Manassas, VA), 1% PSA (Gibco), and Mycozap (Lonza, Houston, TX) delivered through an 18-gauge needle. The femur was flushed until the bone appeared white. The collected flushed bone-marrow media was transferred to a tissue culture T25 flask (Corning, Corning, NY) and the collected MSCs were allowed to adhere for 24 h under standard culture conditions. At that time, the supernatant media and nonadherent cells were aspirated and the adherent cells were rinsed with 37 °C PBS 3 times. Fresh cell culture media was then added and the adherent cells cultured until confluency. During this time, the supernatant medium was changed every 3–4 days with alternating full and half media changes. MSCs were used for the experiment at passage 5.
Microvascular fragments (MVFs) were isolated from the inguinal, subcutaneous, and epidydimal fat pads of wild type male Lewis rats (Charles River Laboratories).48,55 Briefly, the fat pads were minced with scissors and subjected to digestion using collagenase (Worthington Biochemical Corporation, Lakewood, NJ) at 37 °C with agitation or 8–20 min. The digested fat was filtered and washed through 500 µm and 37 µm filters to remove debris and prevent contamination. MVFs were collected and immediately seeded in fibrin gels either directly (control group) or within the three architectures of HA scaffolds of interest to the present study.
In Vitro Study Design and Analyses
MSCs at passage 5 were seeded onto HA scaffolds at a density of 200,000 cells/scaffold58 and were allowed to adhere for 24 h under standard cell culture conditions. For the control group, MSCs were seeded onto tissue-culture 48 well plates and were cultured in MSC growth media. HA scaffolds preseeded with MSCs, were then infiltrated with MVFs and fibrin gels57 at a MVF density of 20,000 MVF/mL. A total gel volume of 125 µL was created by combining fibrinogen from bovine plasma (20 mg/mL) (Type I-S, 65–85% protein, Sigma Aldrich) and thrombin (MilliporeSigma, Burlington, MA) at a ratio of 2–5, respectively with the ~ 2500 MVFs. HA scaffolds seeded with MSCs were placed in non-tissue culture treated plates, the media aspirated and the fibrin-MVF gel was infiltrated into the pores of the HA by pipetting. For the control group, the 125 µL of fibrin containing MVFs was cast above the culture of MSCs in the well plate. For all groups, the fibrin gel was allowed to completely gel before the addition of co-culture media. Co-culture media was composed half of vessel growth promoting media and half osteogenic induction media. Vessel growth media consisted of 80% DMEM (Gibco), 20% FBS (ATCC), and 1% PSA (Gibco). Osteogenic media consisted of 96% DMEM (Gibco), 3% FBS (ATCC), 1% PSA, 50 µg/mL L-ascorbic acid, 10 mM ß-glycerophsophate, and 10 nM dexamethasone. Mycozap (Lonza) was added 1 mL per 500 mL media. To decrease fibrin contraction, 6-aminocaproic acid was added at a concentration of 1 mg/1 mL of media. Media was used within 7 days due to the instability of certain components in the osteogenic induction media.
Media samples were collected at 1, 3, 7, 10, 14, 17 and 21 days. The supernatant media was collected for alkaline phosphatase (ALP; n = 5 replicates) and calcium colorimetric (Ca2+; n = 5 replicates) analyses according to manufacturer’ instructions (Sigma Aldrich, St. Louis, MO). Biological samples for imaging and RNA sequencing were stopped after 3, 7, 14 and 21 days of culture. Biological samples were fixed with Trizol, homogenized for RNA extraction (n = 5 or 6 replicates), and then frozen. RNA was extracted using RNAEasy Kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol. RNA concentration and quality was evaluated using a Take3 Micro-Volume Plate (Biotek, Winooski, VT) and a spectrophotometer. RNA samples of adequate quality for sequencing, at > 50 ng/µL concentration and 3 biological replicates per group per time point, were used to prepare sequencing libraries according to the manufacturer’s RNA sample protocol (Illumina, San Diego, CA). These prepared libraries were then barcoded, pooled, and sequenced in an Illumina HiSeq 3000 to generate 50 bp single end reads with sequencing depths of 45 million reads on average. Reads were aligned using a TopHat2 aligner mapped to Rattus norvegicus rn6 genome build.30 HTSeq was used to transform alignments to raw read counts per gene.1
Immunohistochemistry (IHC)
Biological samples of in vitro culture from each group tested and time point (n = 3 replicates/group/time point) were fixed using 4% formaldehyde and permeabilized with 0.5% Triton X-100, stained with Rhodamine-labeled Griffonia (Banderaea) Simplicifolia Lectin I (GS-Lectin, Vector Labs, Burlingame, CA), which is a carbohydrate- binding protein that stains the vessel wall. The cultured scaffold samples then underwent a decalcification procedure to dissolve the HA content of each scaffold. Scaffolds were decalcified by immersing in 4% formic acid and with gentle agitation at 25 °C for 1–2 days, until the HA scaffold dissolved as observed by reduction in each sample opacity. After decalcification, the fixed biological samples were re-stained using GS-lectin and were imaged using a TCS SP8 confocal microscope system (Leica Camera, Wetzlar, Germany). Samples were imaged at 10x magnification with a z-stack projection; at least 3 regions of interests (ROIs) were selected per group. The size of each ROI was 780 × 780 µm. The total length of vascular fragments within each ROI were calculated by traced centerline length measurement using ImageJ (U.S. National Institutes of Health, Bethesda, MD)64 and tabulated for comparison between groups.
Experimental Hypotheses Testing
Based on the differences between samples observed in the vascular development, two additional experimental hypotheses were evaluated: (1) since the 250 µm and fibrin gels demonstrated similar vessel patterns, differences in gene expression between those two groups of the RNASeq was modeled across time, and (2) to identify specific differences between the scaffold groups based on pore size, since the only differences in the RNASeq analysis were observed at Day 7, a specific comparison between the scaffolds (450 vs. 340 vs. 250 µm) was conducted based on a sub-analysis of the same RNASeq data at day 7 to highlight significant differences. Pathway enrichment analysis was performed using the clusterProfiler package in R to assess the biological significance of these significantly altered genes.
Finite Element Modeling of Blood Vessel Growth
The µCT scans of the scaffolds (as described in Sect. 2.2) were resized by a factor of four in Ctan (Bruker) with a resulting resolution at 35.08 µm per image. Scans were imported as image stacks into ImageJ and a region of interest (ROI) of a size of 50 × 50 × 50 pixels was selected. The selected region consisted of two components: the scaffold and the negative space which was representative of the fibrin gel loaded with MVFs. The final size of the representative region modeled, was a cube with 1.754 mm side. The respective control was a fibrin gel of a size of 1.754 mm (which represented an infinitely large pore). Each representative volume was then imported to Mimics (Materialise NV, Leuven, Belgium) for volumetric meshing.
Volumetric tetrahedral meshes were formed within Mimics for the control and scaffolds of each pore size. Within Mimics, a series of refinements were performed including correction of planar holes, flipping inverted elements (so that element normal were congruent), and shell reduction with volumetric conservation (to provide a more accurate mesh). Refined meshes were subsequently imported into Abaqus (Dassault Systems, Johnston, RI) to convert these meshes into geometric meshes. Conversion was accomplished by running a python script and allowing for the conversion of the exported tetrahedral mesh into an 8-node hexahedral mesh.
Each hexahedral mesh was imported into AngioFe, a plugin of FEBio (University of Utah, Salt Lake City, UT) to run simulations.17 Meshes were imported to PreView (University of Utah, Salt Lake City, UT) and boundary conditions, time steps, and material properties were assigned.44 The following values were used for material parameters: (a) per single sprout as 3.72 µPa, sprout stress range (b) as 1/250 µm−1, sprout stress width (cosine exponent) (N) as 2, microvessel constitutive mode-modulus () as 3.452 kPa, ECM constitutive model-stiffness of ground matrix () as 34.52 Pa, ECM constitutive model-nonlinear fiber stiffness () as 345.2 Pa, and ECM constitutive model-viscoelastic time constant () as 1.08 s.16,17,33 Rigid constraints were applied along nodes to simulate the interface between the gel and scaffold appropriately. In order to mimic the contact between the fibrin gel and HA scaffold, the interior free surface nodes were constrained in all directions to represent gel attachment to the scaffold surface. The exterior nodes were left unconstrained to represent free surfaces of the gel. Time stepping parameters were set at 20 steps with a step size of 0.1. The material properties of the model were a combination of microvessel (a neo-Hookean material)45 and extracellular matrix materials (ellipsoidal fiber diameter neo-Hookean).50 Seeding of 20 MVFs within the construct was performed using a random number generator for stochastic simulation. The resulting analyses were run using SHAMU, a high performance computing cluster; the simulations were run in batches within a parallel environment. In order to determine how pore size effected rate of growth, angiogenesis was simulated over 7 days in terms of time stepping (in terms of simulated vessel growth rate). The results of the runs were visualized in PostView and total vessel length and number of blood vessel branches was extracted from each run. Each architecture (450, 340 and 250 µm pore size and fibrin) were between 30 (for 450 µm pore size) to 11 times (for fibrin hydrogels) with random seeds to allow the final blood vessel lengths and branching to converge with a confidence of > 90%. In addition, three independent scaffolds (of the same pore size) regions of interest were modeled and evaluated to ensure that the local geometric choice was not a confounding variable.
Statistical Analyses, Gene Set Analyses and Differential Expression
Statistical analyses (for calcium colorimetric assay, ALP, and blood vessel length) was performed in SigmaPlot 13 (SyStat, San Jose, CA) via the two-way ANOVA design and using Tukey testing post hoc. Statistical significance was designated at p < 0.05.
Statistical analysis of differential expression was performed using DESeq243 package on the R platform,73 starting from the read counts for each gene in each sample. The scaffold (four different types: Fibrin, 450 µm, 340 µm, 250 µm) and duration (four different lengths of in vitro culture: day 3, 7, 14 and 21) were treated as independent categorical variables in the design matrix. Log-fold change values were adjusted using apeglm80 method to estimate shrinkage of effect size. P-values were calculated using the Wald test. For the specific gene trend panels, significant differences on normalized counts were conducted using two-way ANOVA with tukey’s test used to determine post hoc significance (p < 0.05).
For more detailed interrogations, the following comparisons were made: (i) 250 µm vs. fibrin across time, (ii) comparison between the scaffolds (450 vs. 340 vs. 250 µm) at day 7 of in vitro culture. Significant hits (adjusted p value < 0.05) were extracted and arranged by log fold change. Gene ontology (GO) gene sets, specifically C2 GO Biological Processes genesets and KEGG pathway genesets, were downloaded from the MSigDB database.42 Gene set over representation and enrichment analyses, and visualizations were performed using clusterProfiler79 and WebGestalt.41
Results
Scaffold Characterization
SEM evaluation revealed an open, porous interconnected structure (Fig. 2a) of the HA scaffolds that potentially allows for tissue ingrowth. Scaffold porosity was 91.2 ± 0.8% for the 450 μm, 88.4 ± 1.5% for the 340 μm, and 86.6 ± 2.6% for the 250 μm pore size scaffolds, respectively (Fig. 2b). The porosity was of the three scaffold types tested in the present study was similar. The permeability of the scaffolds was measured at 9.4 ± 5.9 x 10−10 m2, 5.0 ± 3.3 x 10−10 m2 and 2.4 ± 1.9 x 10−10 m2 for the 450, 340, and 250 μm pore size scaffold respectively (Fig. 2c). The permeability was significantly reduced when the pore size was reduced from 450 to 34 to 250 μm (p < 0.05, each group from the other). The measured structural indices from SEM (strut thickness and strut separation) and µCT (9 histomorphometric parameters) are listed in Table 1. The nomenclature for the scaffolds, namely 450, 340, and 250 μm instead of 45, 60 and 80 pores per inch of the template foam, respectively, was chosen from the strut spacing measurements, which correspond to the opening sizes between pores.
Figure 2.
HA scaffold architectures and porosity. (a) SEM images of 450, 340, and 250 µm pore sized HA scaffolds indicating measurement of strut length and thickness. (b) Porosity measured by helium pycnometry for each pore size indicating no significant differences (n = 6/group). (c) Fluid permeability of each pore size indicated that the permeability of each of the larger pore sizes was significantly greater than each of the smaller (*, ** indicates significance at p < 0.05 from other groups, n = 6/group).
Table 1.
Architectural characterization of the hydroxyapatite scaffolds.
| 450 µm | 340 µm | 250 µm | ||
|---|---|---|---|---|
| Strut spacing | 424.98 ± 178.9 | 359.85 ± 69.6 | 211.71 ± 43.5 | µm |
| Strut thickness | 842.33 ± 14.3 | 95.90 ± 24.2 | 64.07 ± 19.5 | µm |
| Scaffold vol/tissue vol | 11.32 ± 0.9 | 16.06 ± 2.8 | 21.05 ± 3.8 | % |
| Scaffold surf/scaffold vol | 31.25 ± 1.1 | 33.86 ± 6.3 | 38.38 ± 4.6 | 1/mm |
| Scaffold surf/tissue vol | 3.53 ± 0.2 | 5.42 ± 1.5 | 7.94 ± 0.4 | 1/mm |
| Trabecular pattern factor | − 10.44 ± 1.4 | − 2.73 ± 4.3 | 4.98 ± 2.9 | 1/mm |
| Structural model index | − 0.85 ± 0.2 | 0.22 ± 0.8 | 1.15 ± 0.23 | |
| Trabecular thickness | 98.96 ± 3.0 | 100.84 ± 11.6 | 96.11 ± 11.56 | µm |
| Trabecular number | 1.14 ± 0.1 | 1.59 ± 0.2 | 2.18 ± 0.1 | 1/mm |
| Trabecular separation | 842.33 ± 14.3 | 526.10 ± 80.9 | 316.88 ± 9.9 | µm |
| Degree of anisotropy | 1.21 ± 0.0 | 1.22 ± 0.1 | 1.24 ± 0.1 |
Strut thickness and spacing are calculated from scanning electron microscopy, all other metrics are from microcomputed tomography analysis
n = 6 scaffolds per architecture minimum
Vol volume, Surf surface area
Calcium and ALP Content in the Supernatant Media
The calcium (Ca2+) concentration levels of all groups are shown in Fig. 3a. For all groups tested, the in Ca2+ levels peaked at day 3 (24.1 ± 3 nmol/µL) but subsequently decreased after day 3 of the study. The Ca2+ levels were significantly (p < 0.001) lower by Day 14 from Day 3 (p < 0.001). Overall, samples with 450 µm pores had the lowest media Ca2+ levels throughout the duration of 21 days (significantly lower than 340 µm pores, p = 0.048 and the 250 µm pores, p = 0.028, and lower than but not significantly different compared to fibrin at p = 0.389).
Figure 3.
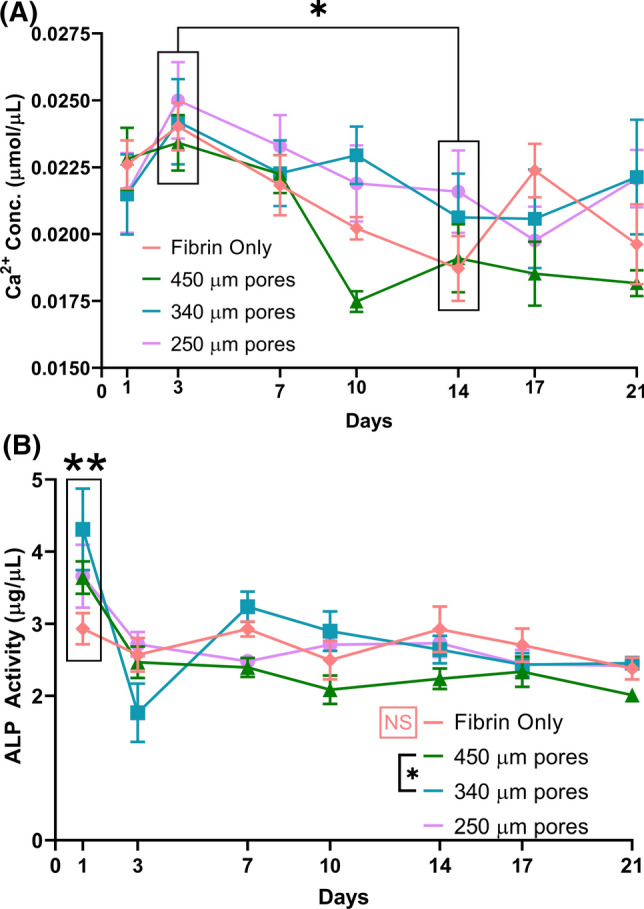
Media Ca2+ and ALP expression. (a) Calcium concentrations. Media Ca2+ was significantly higher at day 3 compared to day 14 (*, significant at p < 0.001, main effect across groups, n = 5/group/time point). (b) ALP activity. Significantly greater ALP in the 340 µm group compared to the 450 µm (*p = 0.026, main effect across time). Significantly greater ALP was observed in all groups at Day 1 (**, main effect, p < 0.001). There was no significance between timepoints within fibrin only group (orange NS, p > 0.67). n = 5/group/time point.
Alkaline phosphatase activity (ALP) (Fig. 3b) peaked (3.87 ± 1.0 µg/mL across HA groups) at Day 1 and was statistically significant (p < 0.001) from other durations of in vitro culture, as a main effect independent of scaffold group. No substantial increase was observed in ALP in the fibrin group at Day 1 (2.93 ± 0.5 µg/mL, compared to 2.70 ± 0.6 µg/mL for all other days, p > 0.668). After day 3, ALP activity decreased and leveled off for the remainder of the study for all scaffolds (2.47 ± 0.6 µg/mL across HA groups on all other days, no significant difference between any group at any time point, Day 7 p > 0.085, Day 10 p > 0.08, Day 14 p > 0.183, Day 17 p > 0.7, Day 21 p > 0.6). ALP expression was significantly different (p < 0.05) between samples with 450 µm pores and 340 µm pores (p < 0.05) across all timepoints and, at day 1, expression was different between samples with 340 µm pores and fibrin (p < 0.001) and at Day 3 between the scaffolds with 340 µm pores and 250 µm pores (p = 0.027). Significant (p < 0.05) difference, in the ALP expression was obtained at day 1 for both the 450 and 340 µm pore scaffolds within their respective groups. Scaffolds within the fibrin only were similar for all timepoints. Detailed statistical analyses for Calcium and ALP content are available in the supplemental data.
Osteogenic Differentiation and Mechanotransductive Signaling
The principal components analysis from the RNASeq study indicated that there was a clear distinction between the response of the HA scaffold groups, compared to the fibrin control over 21 days (Fig. 4a). No distinctions were seen along the principal component axes between the different pore sized scaffold groups themselves at any given time point. On day 3 and day 7 groups, all materials tested were significantly different from each other and from the Day 14 and Day 21 groups. The latter time points overlap to the extent of being indistinguishable (Fig. 4a). The correlation heatmap suggested that the trends in gene expression were similar for the results obtained when the cells were cultured in the 450 μm, 340 μm and 250 μm HA scaffold and distinctly different from the fibrin group over 21 days (Fig. 4b).
Figure 4.

Differences between the scaffolds and fibrin over time. (a) PCA of the RNA Seq analysis for the various conditions tested within the experimental design (4 scaffold groups x 4 time points), showing a clear separation between the fibrin (circled with orange dashed lines) and the HA scaffold based groups (circled with blue dashed lines). Temporal trends were similar in all groups, with Day 3 and Day 7 (circled separately) being functionally distinct compared to Day 14 and 21 samples (combined within circles) (n = 3 per scaffold per duration). (b) Correlation heatmap of the various conditions tested confirming the PCA analysis. Hierarchical clustering was performed across samples along the Y-axis. The first 3 column blocks of scaffold, show the HA scaffold correlations being very similar and distinctly different from the last column block (fibrin).
Expression of genes pertinent to osteogenesis highlighted differences between MSC differentiation on the fibrin and the scaffold groups (Fig. 5). Expression (normalized counts) of genes encoding BMP2 and Osteopontin (spp1) were all much higher when the cells were cultured in the HA scaffold groups at early time points compared to those cultured in the Fibrin group, but the expression levels of cells in all the groups match for all times after Day 7. In the case of bmp2, expression in the 450 µm group was higher than all other groups, p < 0.001 and expression in the 250 and 340 µm groups was higher than fibrin at p < 0.014 at Day 3, while expression in the 340 µm group was higher than all other groups at Day 7, p < 0.01. In the case of spp1, expression was significantly different between all groups except the 450 and 340 µm groups at Day 3, p < 0.01 and significantly different between all groups except 250 and 450 µm groups at Day 7, p < 0.001. Since calcium signaling was identified as a pathway that had multiple genes with significant differential expression, the gene encoding calmodulin was investigated and showed that its expression when cells were cultured in the fibrin group was consistently higher, significantly different from all scaffold groups (main effect, p < 0.001) but no significant differences were found between the scaffold groups (except between 340 vs. 250 µm, p = 0.039). Yap/Taz have been implicated in the HIPPO mechanotransduction pathway and while the trends for these genes over time were the same in all scaffold groups, Taz was consistently lower in scaffolds compared to fibrin (significant for each scaffold vs. fibrin, p > 0.032) and the expression level of Yap1 was consistently higher in scaffolds compared to fibrin (significant for each scaffold vs. fibrin, p < 0.011). Wide variations over time (days 3 and 7 significantly higher than days 14 and 21, p < 0.009) and no difference between groups (p > 0.698) was observed in tgfb1 expression. The gene encoding osteonectin, sparc also exhibited no differences between scaffold groups, but Day 3 expression was much higher than all other time points across groups (p < 0.011). The opposite trend was observed where runx2 and col1a1 expression was initially low, (runx2 Day 3 was significantly lower than all other time points p < 0.001, while col1a1 Days 3 and 7 were significantly lower than later time points, p < 0.006) and then increased towards the end of the study, indicating continuing promotion of osteoid formation (Fig. 5).
Figure 5.
Osteogenic gene evaluation. (a–f) The graphs depict average normalized counts (across 3 samples) of indicated gene regulators of osteogenic differentiation across the four time points from Day 3 to Day 21 for the different experimental treatments. (g, h) The graphs depicting average normalized counts (across 3 samples) of early osteogenic gene markers (col1a1 and runx2) across the four time points, plotted from Day 21 to Day 3 to enable visualization. (All significant differences in supplementary data table, n = 3 per scaffold per duration).
Vascular Growth Within the Hydrogel and HA Scaffolds
Growth of the MVFs in fibrin gel and HA scaffolds were evaluated by confocal imaging using a tagged GS-Lectin to stain the vessel wall (representative images in Fig. 6a). The results revealed that the MVFs were dispersed and short (28-35 µm in size) at day 3, but had grown significantly in length by day 7. The lowest vessel length measurement on day 7 (74 μm) was in the 250-μm-pore size scaffold while the longest vessel fragments (140 μm) were found in fibrin-only group. The 450-μm and 340-μm scaffolds showed a trend of supporting mild vessel growth from day 7 to day 14 and 21, while the fibrin group similarly showed relatively little change in vessel length between day 7 and day 21 (Fig. 6b). The 250-μm scaffolds demonstrated steady increase in vessel length from day 3 to day 7 to day 14 before vessels stabilized in length for the final 7 days of observation. Interestingly, the MVFs in the 450-μm scaffold exhibited looping, structures of larger tubes, with smaller branches growing from them, in the morpohology of and on the same scale as individual pores of the 450-μm size. This effect was evident at day 7 and prominent at day 14. Both the fibrin and 250-μm size scaffolds showed long branched vessels forming from the MVFs, but explicit evidence of network anastomoses between vessels was limited in both groups. These distinct phenotypes are schematically represented in Fig. 6c. The 340-μm pore size scaffolds showed a mixture between some vessel fragments looping within pores and some branching across pore interconnections by days 14 and 21. At day 21, MVF growth exhibited a delineation of tubular structure. It was also observed that individual cells (GS-lectin stained) were migrating away from the fragments by the 21 day observation time point.
Figure 6.
MVF growth over time. (a) Microvascular fragment growth was tracked after 3, 7, 14 and 21 days in the fibrin hydrogels and the decalcified 250 µm, 340 µm and 450 µm pore size scaffolds. Confocal IF imaging of samples stained with GFP conjugated GS-Lectin. The most distinctly different morphologies were observed after 14 days of culture (Scale bar 500 µm). (b) All groups showed increase in length measured from day 3 to day 14 followed by stabilization of length to day 21 (** indicates day 3 significantly lower than other times, p < 0.001 and * indicates fibrin significantly different from 340 µm group at Day 7, p = 0.011). 3 independent biological measures with at least 4 independent regions were measured at each time point for each group. (c) Schematic showing the morphology of vessels in the fibrin hydrogel and 250 µm pores being similar while the morphology in the 450 µm pores was distinctly different.
Vascularization and Hypoxia Signaling
Evaluation of genes of importance to in angiogenesis and blood vessel network organization highlighted differences between the fibrin and the scaffold groups tested in the present study (Fig. 7). The expression (normalized counts) of genes encoding Angiopoietin 2 was higher for the fibrin group compared to the scaffolds (p < 0.001, main effect). However, the trend was reversed for Angiopoietin 1, serpine1 (the gene encoding plasminogen activator inhibitor), camk2 (gene encoding calmodulin dependent protein kinase) and tek (gene encoding the Angiopoietin 1 receptor) were much higher in the scafviafold groups compared to the Fibrin group (main effect, p < 0.001). This shows that vessel stabilization in the scaffolds followed stabilization angpt1 rather than angpt2. The angpt2 gene was highly expressed both early (day 3) and late (days 14 and 21) in the Fibrin group compared to all the HA groups tested (p < 0.001). There were singificant differences in hif1a and vegfa expression between the 450 µm and 250 µm scaffolds vs. Fibrin (p < 0.001) but not between the 340 µm scaffolds vs. Fibrin (p > 0.087), the hypoxic response was much more pronounced in terms of significantly higher hif1a expression in all the scaffold groups compare to respective results obtained from the Fibrin group. This outcome was supported not just by changes at the single gene expression level, but across the HIF-1 signaling pathway, which revealed that several genes (encoding IL-6, HIF1α, camk2, serpine1) indicating either sustained or continuous hypoxia were significantly upregulated across HA scaffold groups compared to Fibrin, while genes in the intermittent hypoxia pathway (encoding NOX, PLCγ, PKC) were significantly upregulated in the Fibrin group over the scaffolds (data not shown). The upregulation of angiopoietins was observed differentially, but was upregulated across the protein class in all groups. This outcome supported the blood vessel development observed in Fig. 6a across all groups over time.
Figure 7.
Angiogenic gene evaluation. (a–e) The graphs depict average normalized counts (across 3 samples) of indicated gene regulators of angiogenesis across the four time points from Day 3 to Day 21 for the different experimental treatments. (f–h) The graphs depicting average normalized counts (across 3 samples) of gene markers of angiogenesis (angpt1, vegfa and serpine) across the four time points, plotted from Day 21 to Day 3 to enable visualization. (All significant differences in supplementary data table, n = 3 per scaffold per duration).
Differences between Scaffolds with small pores and Fibrin hydrogel
The morphology of the vessels in the 250-µm pore size scaffolds exhibited similar morphology in the fibrin hydrogel, especially after 14 days of culture (Fig. 6c). A heat map comparison of genes involved in osteogenesis between the two groups over time shows that BMP2 wass upregulated early (day 3 and day 7) in the 250-µm scaffolds compared to Fibrin (Fig. 8a). The early markers of osteogenic commitment (runx2, col1a1) were upregulated at day 14 and 21 in the scaffold group, while they were upregulated in the fibrin group at day 21 only. Similarly, spp1 (that encodes osteopontin) was upregulated in 250 µm scaffolds at day 3, ahead of upregulation in fibrin at day 7.
Figure 8.
Impact of porous hydroxyapatite scaffold. (a) Hierarchical clustering of normalized expression counts of a subset of osteogenesis genes across samples. The samples are color coded by time points (upper bar) and scaffold type (lower bar) at the top of the heatmap (All significant differences in supplementary data table, n = 3 per scaffold per duration). (b) Pathway enrichment analysis of the significantly altered genes highlights the most significantly altered pathways (blue: downregulated or yellow: upregulated) between 250 μm vs. Fibrin. The x-axis plot the significance of overrepresentation). (c) The angiogenesis pathway was a significant hit in geneset enrichment analysis of 250 μm vs. Fibrin samples across time. The leading-edge graph illustrates the positions of significantly altered genes in our dataset within Angiogenesis dataset in the context of all genes ordered by fold change gene expression. There were distinct subsets of genes (shown by clustering of black bars in the center heatmap band) in the angiogenesis pathway differentially upregulated in 250 μm scaffolds and in Fibrin.
In terms of significantly altered gene sets, it was observed that both hypoxia and mechanotransduction were significantly upregulated in the scaffold compared to fibrin at a significant level of overrepresentation. Cell adhesion molecule pathways, chemokine and calcium signaling were down-regulated in the scaffold group compared to fibrin over 21 days (Fig. 8b). In terms of the angiogenic response, the gene-set enrichment analysis of 250-μm scaffolds vs. Fibrin samples across 21 days indicated many more genes involved in angiogenesis in terms of rank ordered set of fold change in gene expression are upregulated in Fibrin compared to 250-μm scaffolds (Fig. 8c).
Vascular Morphogenesis in Scaffolds with Different Pore Sizes
Another distinction of interest was the separation in the principal component analysis evaluating the different scaffold pore sizes alone (250-, 340-, and 450-µm) at day 7, providing evidence that there were significant differences in the 340-µm scaffold at the gene level compared to the other two groups (Fig. 9a). Differential gene expression analysis indicated that 1570 of the 1990 genes in the 450- vs. 340-µm comparison were common to the 2422 significant genes identified between the 250 vs. 340 µm scaffold groups (Fig. 9b). This observation was also supported by the heatmap of significantly altered genes across scaffold groups at day 7 showing consistencies across the 250- and 450-µm pore size scaffold with stark contrast to the 340-µm pore size scaffold (Fig. 9c). Interestingly, in addition to pathways implicated in cancer (which are often common to fenestrated vasculature development and stromal signaling during the proliferative phase of regeneration), the hypoxia signaling pathway (Hif1α) and the mechanotransduction pathway (Hippo signaling) were identified as significantly over-represented (Fig. 9d).
Figure 9.
Effect of pore size at Day 7. (a) Principal Component Analysis illustrating the separation of the different scaffold groups at Day 7 once fibrin condition was removed. The 340 µm group was significantly different from the other two. (b) Differential gene expression was evaluated for 450 µm vs. 340 and 250 vs. 340 µm scaffold groups and the venn diagram illustrates the common and unique genes within each hit list. (c) Heatmaps of significantly altered genes among the three groups corroborating the difference in 340 µm (center 3 columns) from the other two groups (3 columns on the right and left). (d) The common genes from the venn diagram were used for pathway analysis. The volcano bubble plot illustrates pathways significantly overrepresented in the common hits. The x-axis represents the enrichment score and y-axis plots adjusted p-values of the enrichment.
Computational Studies of Matrix Mechanics on Vascularization
Figure 10 describes the results from the computational biomechanics modeling which simulated vessel growth in fibrin gels within scaffolds constrained at the scaffold-gel interface, resulting in a strain field in the gel which affected vessel growth. During the seven day AngioFE simulation, the 20 randomly seeded MVFs were observed to grow over a period of seven days between the control, 250-µm, 340-µm, and 450-µm pore (Fig. 10a) and achieving significantly greater lengths at Day 7. The fibrin control exhibited the greatest increase in vessel length followed by the 250-µm pore (90603 ± 2578 µm and 77533 ± 4583 µm, respectively) (Fig. 10b). The total vessel length in the fibrin was twice that of the 450-µm pore (48706 ± 3195 µm). The 250-µm pore displayed the greatest number of branches (204 ± 10) after 7 days and the control displayed no statistical difference compared to the 340 µm pore (115 ± 2 and 115 ± 6, respectively after 7 days) (Fig. 10c). Across all groups it was observed that the differences in total vessel length over 21 days tracked were proportional to the branching over time within a group. None of the architectures tested exhibited significant anastomoses over time (data not shown; the highest number recorded was 3, most simulations resulted in 0 anastomoses). The total number of simulations needed to achieve convergence for the total vessel length and number of branches for the control, 450-µm pores, 340-µm pores and 250-µm pores was: 11, 30, 18 and 18, respectively (data not shown, not significant at p = 0.1).
Figure 10.
Computational vessel growth modeling. (a) 3D models of the pore spaces filled with hydrogel were created for the fibrin control as well as the 250 µm, 340 µm and 450 µm pore size scaffolds and vessel growth was simulated over multiple iterations and regions of interest using the AngioFE plugin over a period of 7 days. Distinct differences in (b) Total vessel length and (c) Number of branch points were observed between the groups over 7 days, without significant variations within a given group (variations might have resulted from region of interest selection or random seeding, n = 7 per architecture per time point.)
Discussion
Supporting Osteogenic Differentiation and Matrix Deposition
The fluid conductance of a bone scaffold was suggested to be critical to the revascularization58 and in vitro success of graft integration by promoting cell and vessel invasion into the scaffolds. In the bone fracture healing cascade, this is followed by bone ingrowth, long term remodeling, and tissue regeneration.60 In a rabbit model,28 it was suggested that a minimum threshold conductance of 1.5 × 10−10 m3s−1Pa−1 was necessary and that revascularization and formation of osteoid and fibrous tissue were not achieved below the conductance threshold value. Based on their permeability (equivalent to conductance ranging between 8.82 × 10−10 and 49.95 × 10−10 m3s−1Pa−1) the HA scaffolds investigated in this study were all well above the threshold values, suggesting the ability of these scaffolds to promote and sustain bone in-growth and regeneration. This is supported by the patent growth of cells within the scaffolds in addition to MVFs until day 21 in vitro without dynamic perfusion and despite increasing metabolic demand. While continuous hypoxia is upregulated in the scaffolds, pro-angiogenic signaling was also activated, indicating a system continuing toward homeostasis (Fig. 5).
In human bone, permeability has been implicated in the complex interplay between the remodeling mechanical responses of cortical and cancellous bone via the poroelastic models,10 suggesting that response to fluid shear and the mechano-transduction of pertinent signals were through fluid–solid coupling. The permeability of the scaffolds used in this study, especially the 340-μm pore size scaffolds, was comparable to the permeability of human bone in the femur.51 Permeability was also identified as the critical factor affecting nutrient and calcium transport.31
In terms of the temporal sequence of osteogenesis, the scaffolds benefit from early upregulation of BMP2 (unlike tgfb1 which was high across all groups and fibrin gels at day 3) calmodulin and osteopontin, and later RUNX2 and Collagen type I, which likely resulted in local remodeling supporting further MSC commitment to the osteogenic lineage. This is likely due to secretion from the MVFs, which being freshly isolated might “behave” like a local injured vascular bed supporting both MSC recruitment and niche specific differentiation. Several publications suggest that the wound-site vasculature, especially within a fibrin blood clot, (which the hydrogel in the biphasic system of the present study represents) are a prime source of BMP247,77 The RUNX2 upregulation may then be explained as an MSC response to the early mitogenic and strong differentiation signal of BMP2 (Figs. 3c and 6a). This outcome is further supported by lack of early ALP activity observed in the Fibrin only group which does not seem to promote a wound healing response that is bone niche specific, like the strong attachment to hydroxyapatite surfaces promotes in MSCs.14,49 In terms of the other temporal markers of osteogenic maturation, published reports have noted that expression of non-collagenous maturation-linked proteins (such as Osteopontin (spp1)) was linked to mature osteoblasts resident near mature bone mineral76 (which is what an HA scaffold approximates), while in other reporte, it was also expressed prior to initiation of an osteogenic response in post- marrow ablation treated animals.71 The early expression of spp1 seen in all groups might thus be linked to some form of dysfunction in anticipated signaling such as dysfunction in the inflammatory response seen in vitro post ablation (Figs. 3c and 6a).
Supporting Blood Vessel Growth and Patterning
Edgar et al.19 have recently noted that the local microenvironment, specifically the mechanical interaction between MVFs and their surrounding extracellular matrix, regulates the rate of neo-vessel growth and branching. The present study as well, provided evidence that over similar volumes, the nature of blood vessel growth and branching was very different between the different scaffold architectures because the boundary constraint of the hydrogels not only being unable to separate from the scaffold surface, but also having steeper stiffness gradients closer to the scaffold surface. The sparse strain field of the 450 µm pore size is likely responsible for the vessels growing within the pores preferentially to find lower matrix stiffness, while due to the lower stiffness regions being relatively more abundant in the pore interconnections rather than the pore interior (smaller pores, greater interconnection density per unit volume) of the 250-µm pore size (Fig. 4). This is also supported by the computational mechanics model where there is early branch formation in the 450-µm pore size scaffolds but, since both the seed vessel and daughter branches stay confined to a single pore, their successive branching is inhibited. Counterintuitively, as the 250-µm pore size scaffolds have MVFs initially lengthening across pores, the future daughter branches also resolve across pores in the computational model (Fig. 8a and 8c), resulting in greater branching and, hence, greater total length over time.
The predictive value of the AngioFE model to simulate MVF growth (Fig. 8) with experimental validation (Fig. 4) is meaningful. Biomaterial scaffold architectures vary based on material, manufacturing process, and desired pore sizes. A tool which allows rapid in silico prediction of vascularization will be essential for narrowing down scaffold design variables for further translation. Such a tool also allows for comparison of architectures from various biomaterials scaffolds using a consistent benchmark to explain whether differences in experimental outcomes have a structural basis. Although many models delve into the biological and biochemical signaling processes behind vascularization, the mechanical forces in the matrix and their specific effects on angiogenesis, and stromal morphogenesis are poorly understood.
Tissue stromal cells secrete paracrine factors and, thus, play a critical role in stimulating angiogenesis. The fact that MVF function is independent of tissue of origin, (despite significant differences in stiffness of those tissues52), indicates that vasculature itself may not be inherently tissue-committed: non-vascular cells may assign tissue-specific function to vascular beds. The local distribution of stromal cells is also capable of establishing local chemical gradients and, thus, overcome limited diffusion by globally dense matrices.21 In the current study, higher levels of pro-angiogenic genes, vegfa, angpt1 and hif1a, were expressed within the HA scaffolds that plausibly had more osteogenically committed MSCs (Fig. 5a). Additionally, recent reports indicate that tissue stromal cells induce/promote neovascular invasion across tissue interfaces.12 This might be critical in biphasic porous scaffolds filled with hydrogels as well, where stromal cells might establish either the chemical or mechanical signaling stimulus, which initiates/affects vascularization across pore spaces.
Temporal Changes in Hypoxia and Mechanotransduction Towards Multi-tissue Engineering
Both of the pathways implicated as central to the morphogenesis in this study across the different forms of evaluation; namely hypoxia and mechanotransduction, not only happen to play out in a temporally varying manner across the groups; but also are involved in subsequent downstream signaling regulating both vessel and osteogenic development. The YAP/TAZ (Hippo) signaling was implicated in both developmental angiogenesis (activated by VEGF75) as well in tissue regeneration to regulate vessel network size control during angiogenesis.6 In cases of high mesenchymal density, the Hippo pathway also regulates morphogenetic changes by cytoskeletal regulation.13,72 The pathway has also been implicated in recent reports of angio and osteogenesis in soft materials subjected to pressure in bone defects.59 In the case of the HA scaffolds, where the scaffold surface encourages high cell density locally, (as indicated by the high metabolic load and hypoxia), the upregulated Hippo pathway likely induced an osteogenic phenotype rather than the traditional endochondral preference because of the local presence of vasculature (Fig. 3) based on temporal trends in osteogenic genes. This is supported by literature reports that the hippo signaling is central to healing in vascularized bone healing models where VEGF is involved (like in all the HA groups tested in this study) and there is another factor supporting an osteogenic phenotype (like matrix stiffness5 or BMP239). Thus, scaffold stiffness and properties similar to that of bone mineral seem to play a critical role in promoting an osteogenic phenotype in addition to vasculature in our biphasic model.
Interestingly, the big differences in terms of the pore size effect of the HA scaffolds was limited to the 7 day time point (Fig. 7). Additionally, the critical difference in terms of the poorer/mistimed angiogenic response in the 340-µm pore size scaffold (Fig. 5a) seems to be driven by a difference in the hypoxia signaling pathway. This is not surprising because adequate levels of HIF1α are thought to be indispensable for blood vessel invasion, and avoiding energetic distress as metabolic demand increases drastically during phases of high MSC density during bone formation.67 Thus, the hypoxia signaling is not only pro-angiogenic, but also pro-survival69 for the two tissue types supported in this study.
Confounding factors in the study include the inherent limitation of in vitro cell culture with non-homogenous populations. This is further exacerbated by the fact that MVFs are essentially micro-tissues with cells of multiple lineages, including some which have shown significant plasticity and regenerative potential.48 Even in the current study, cells (that stained positive for GS-Lectin) were observed to come off the MVF networks indicating that the MSCs might not be the only cells providing stromal/matrix support in the current model. Additionally, because of this distinctly heterogenous, time-variant cell population; while signaling pathways that are significantly different between groups and conditions can be identified using RNA sequencing, it is not feasible to use these techniques to ascertain causality of actors. Similarly, within the currently developed computational model for vessel growth and morphology, very specific growth rates and matrix properties are considered to model the fibrin gel18 used within the scaffolds. While these models are the first attempt to specifically model geometric constraints in addition to mechanical boundary variations,33 they can potentially be more accurate if the time variant stiffness of the gels can also be ascertained and incorporated into the model. The significance of these results remains, however, the implication of the hypoxic signaling variance, early initiation of endogenous chemokine signaling, and sustained mechano-transduction pathways as critical players in how cells take cues from the architecture of composite biomaterial scaffolds to dictate stromal and blood vessel growth.
Conclusions
The architecture of the pores critically determines the rate of growth and the nature of branching of vascular sprouts, while stromal cells promote hypoxia signaling and secrete chemokines to promote this outcome. Scaffold materials impact stromal cell adherence and mechano-transduction; in the presence of blood vessels brings about early osteogenic commitment on hydroxyapatite. The computational mechanics model implicated local matrix strain fields in dictating lengths of blood vessel growth and implicated scaffold architecture in dictating branching morphology. The mesenchymal stromal cell—microvascular fragment combination is a viable in vitro model for investigating vascularized tissue morphogenesis in co-cultures.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This research was supported in part by the National Science Foundation CAREER Award (CBET#1847103), an Image Based Biomedical Modeling Fellowship and UTSA Research Funds (GREAT) to TG, funds from the Lutcher Brown Endowment to RB, the USAA Foundation to JLO, NIH SC1DK122578 support to CR NIH GM060655 and 1S10OD021805-01 (RISE training program) support to FMA and CP, UTSA College of Engineering support to EJ, UTSA Graduate School support to GC and SM, CPRIT Award RP160732 and NIH NCATS UL1TR002645 support to YC, and AACR AstraZeneca START grant (18-40-12-GORT) support to AG.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Anders S, Pyl PT, Huber W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31(2):166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appleford MR, Oh S, Oh N, Ong JL. In vivo study on hydroxyapatite scaffolds with trabecular architecture for bone repair. J. Biomed. Mater. Res. A. 2009;89(4):1019–1027. doi: 10.1002/jbm.a.32049. [DOI] [PubMed] [Google Scholar]
- 3.Bai F, Zhang J, Wang Z, Lu J, Chang J, Liu J, Meng G, Dong X. The effect of pore size on tissue ingrowth and neovascularization in porous bioceramics of controlled architecture in vivo. Biomed. Mater. 2011;6(1):015007. doi: 10.1088/1748-6041/6/1/015007. [DOI] [PubMed] [Google Scholar]
- 4.Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25(6):1384–1392. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 5.Barreto S, Gonzalez-Vazquez A, Cameron AR, Cavanagh B, Murray DJ, O’Brien FJ. Identification of the mechanisms by which age alters the mechanosensitivity of mesenchymal stromal cells on substrates of differing stiffness: implications for osteogenesis and angiogenesis. Acta Biomater. 2017;53:59–69. doi: 10.1016/j.actbio.2017.02.031. [DOI] [PubMed] [Google Scholar]
- 6.Boopathy GT, Hong W. Role of hippo pathway-Yap/Taz signaling in angiogenesis. Front. Cell Dev. Biol. 2019;7:49. doi: 10.3389/fcell.2019.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campana V, Milano G, Pagano E, Barba M, Cicione C, Salonna G, Lattanzi W, Logroscino G. Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J. Mater. Sci. 2014;25(10):2445–2461. doi: 10.1007/s10856-014-5240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang CC, Hoying JB. Directed three-dimensional growth of microvascular cells and isolated microvessel fragments. Cell Transplant. 2006;15(6):533–540. doi: 10.3727/000000006783981693. [DOI] [PubMed] [Google Scholar]
- 9.Chang CC, Nunes SS, Sibole SC, Krishnan L, Williams SK, Weiss JA, Hoying JB. Angiogenesis in a microvascular construct for transplantation depends on the method of chamber circulation. Tissue Eng. Part A. 2010;16(3):795–805. doi: 10.1089/ten.tea.2009.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowin SC. Bone Poroelasticity. In: Cowin SC, editor. Bone Mechanics Handbook. Boca Raton: CRC Press; 2001. pp. 231–251. [Google Scholar]
- 11.Dadsetan M, Guda T, Runge MB, Mijares D, LeGeros RZ, LeGeros JP, Silliman DT, Lu L, Wenke JC, Baer PRB. Effect of calcium phosphate coating and rhBMP-2 on bone regeneration in rabbit calvaria using poly (propylene fumarate) scaffolds. Acta Biomater. 2015;18:9–20. doi: 10.1016/j.actbio.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 12.Dale J, Krishnan L, Aliaj K, Beare J, Weiss J, Hoying J. Stromal cells promote neovascular invasion across tissue interfaces. The FASEB Journal. 2017;31(1 Supplement):682.1. doi: 10.3389/fphys.2020.01026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deel MD, Li JJ, Crose LE, Linardic CM. A review: molecular aberrations within hippo signaling in bone and soft-tissue sarcomas. Front. Oncol. 2015;5:190. doi: 10.3389/fonc.2015.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dulgar-Tulloch A, Bizios R, Siegel R. Human mesenchymal stem cell adhesion and proliferation in response to ceramic chemistry and nanoscale topography. J. Biomed. Mater. Res. A. 2009;90(2):586–594. doi: 10.1002/jbm.a.32116. [DOI] [PubMed] [Google Scholar]
- 15.Dumas JE, Prieto EM, Zienkiewicz KJ, Guda T, Wenke JC, Bible J, Holt GE, Guelcher SA. Balancing the rates of new bone formation and polymer degradation enhances healing of weight-bearing allograft/polyurethane composites in rabbit femoral defects. Tissue Eng. A. 2014;20(1–2):115–129. doi: 10.1089/ten.tea.2012.0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edgar LT, Hoying JB, Utzinger U, Underwood CJ, Krishnan L, Baggett BK, Maas SA, Guilkey JE, Weiss JA. Mechanical interaction of angiogenic microvessels with the extracellular matrix. J. Biomech. Eng. 2014;136(2):021001-021001-15. doi: 10.1115/1.4026471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edgar LT, Maas SA, Guilkey JE, Weiss JA. A coupled model of neovessel growth and matrix mechanics describes and predicts angiogenesis in vitro. Biomech. Model. Mechanobiol. 2015;14(4):767–782. doi: 10.1007/s10237-014-0635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edgar LT, Sibole SC, Underwood CJ, Guilkey JE, Weiss JA. A computational model of in vitro angiogenesis based on extracellular matrix fibre orientation. Comput. Methods Biomech. Biomed. Eng. 2013;16(7):790–801. doi: 10.1080/10255842.2012.662678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edgar LT, Underwood CJ, Guilkey JE, Hoying JB, Weiss JA. Extracellular matrix density regulates the rate of neovessel growth and branching in sprouting angiogenesis. PLoS ONE. 2014;9(1):e85178. doi: 10.1371/journal.pone.0085178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Germain, S., C. Monnot, L. Muller, and A. Eichmann. Hypoxia-driven angiogenesis: role of tip cells and extracellular matrix scaffolding. Curr. Opin. Hematol. 17(3), 2010 [DOI] [PubMed]
- 21.Ghajar CM, Chen X, Harris JW, Suresh V, Hughes CC, Jeon NL, Putnam AJ, George SC. The effect of matrix density on the regulation of 3-D capillary morphogenesis. Biophys. J . 2008;94(5):1930–1941. doi: 10.1529/biophysj.107.120774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guda T, Appleford M, Oh S, Ong JL. A cellular perspective to bioceramic scaffolds for bone tissue engineering: the state of the art. Curr. Top. Med. Chem. 2008;8(4):290–299. doi: 10.2174/156802608783790956. [DOI] [PubMed] [Google Scholar]
- 23.Guda T, Walker J, Pollot B, Appleford M, Oh S, Ong J, Wenke J. In vivo performance of bilayer hydroxyapatite scaffolds for bone tissue regeneration in the rabbit radius. J. Mater. Sci. 2011;22(3):647–656. doi: 10.1007/s10856-011-4241-7. [DOI] [PubMed] [Google Scholar]
- 24.Guda T, Walker JA, Singleton B, Hernandez J, Oh DS, Appleford MR, Ong JL, Wenke JC. Hydroxyapatite scaffold pore architecture effects in large bone defects in vivo. J. Biomater. Appl. 2014;28(7):1016–1027. doi: 10.1177/0885328213491790. [DOI] [PubMed] [Google Scholar]
- 25.Guda T, Walker JA, Singleton BM, Hernandez JW, Son JS, Kim SG, Oh DS, Appleford MR, Ong JL, Wenke JC. Guided bone regeneration in long-bone defects with a structural hydroxyapatite graft and collagen membrane. Tissue Eng. A. 2013;19(17–18):1879–1888. doi: 10.1089/ten.tea.2012.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirche C, Xiong L, Heffinger C, Munzberg M, Fischer S, Kneser U, Kremer T. Vascularized versus non-vascularized bone grafts in the treatment of scaphoid non-union. J Orthop Surg (Hong Kong) 2017;25(1):2309499016684291. doi: 10.1177/2309499016684291. [DOI] [PubMed] [Google Scholar]
- 27.Hollister SJ. Porous scaffold design for tissue engineering. Nat. Mater. 2005;4(7):518–524. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 28.Hui PW, Leung PC, Sher A. Fluid conductance of cancellous bone graft as a predictor for graft-host interface healing. J. Biomech. 1996;29(1):123–132. doi: 10.1016/0021-9290(95)00010-0. [DOI] [PubMed] [Google Scholar]
- 29.Kang S-W, Kim J-S, Park K-S, Cha B-H, Shim J-H, Kim JY, Cho D-W, Rhie J-W, Lee S-H. Surface modification with fibrin/hyaluronic acid hydrogel on solid-free form-based scaffolds followed by BMP-2 loading to enhance bone regeneration. Bone. 2011;48(2):298–306. doi: 10.1016/j.bone.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 30.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14(4):R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knothe Tate ML. Interstitial fluid flow. In: Cowin SC, editor. Bone Mechanics Handbook. Boca Raton: CRC Press; 2001. pp. 221–229. [Google Scholar]
- 32.Kolar P, Schmidt-Bleek K, Schell H, Gaber T, Toben D, Schmidmaier G, Perka C, Buttgereit F, Duda GN. The early fracture hematoma and its potential role in fracture healing. Tissue Eng. B. 2010;16(4):427–434. doi: 10.1089/ten.TEB.2009.0687. [DOI] [PubMed] [Google Scholar]
- 33.Krishnan L, Underwood CJ, Maas S, Ellis BJ, Kode TC, Hoying JB, Weiss JA. Effect of mechanical boundary conditions on orientation of angiogenic microvessels. Cardiovasc. Res. 2008;78(2):324–332. doi: 10.1093/cvr/cvn055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krishnan L, Willett NJ, Guldberg RE. Vascularization strategies for bone regeneration. Ann. Biomed. Eng. 2014;42(2):432–444. doi: 10.1007/s10439-014-0969-9. [DOI] [PubMed] [Google Scholar]
- 35.Laschke MW, Harder Y, Amon M, Martin I, Farhadi J, Ring A, Torio-Padron N, Schramm R, Rücker M, Junker D. Angiogenesis in tissue engineering: breathing life into constructed tissue substitutes. Tissue Eng. 2006;12(8):2093–2104. doi: 10.1089/ten.2006.12.2093. [DOI] [PubMed] [Google Scholar]
- 36.Laschke MW, Kleer S, Scheuer C, Schuler S, Garcia P, Eglin D, Alini M, Menger MD. Vascularisation of porous scaffolds is improved by incorporation of adipose tissue-derived microvascular fragments. Eur. Cell Mater. 2012;24:266–277. doi: 10.22203/ecm.v024a19. [DOI] [PubMed] [Google Scholar]
- 37.Laschke MW, Menger MD. Vascularization in tissue engineering: angiogenesis versus inosculation. Eur. Surg. Res. 2012;48(2):85–92. doi: 10.1159/000336876. [DOI] [PubMed] [Google Scholar]
- 38.Laschke MW, Menger MD. Adipose tissue-derived microvascular fragments: natural vascularization units for regenerative medicine. Trends Biotechnol. 2015;33(8):442–448. doi: 10.1016/j.tibtech.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Lee E, Ko J-Y, Kim J, Park J-W, Lee S, Im G-I. Osteogenesis and angiogenesis are simultaneously enhanced in BMP2-/VEGF-transfected adipose stem cells through activation of the YAP/TAZ signaling pathway. Biomater. Sci. 2019;7(11):4588–4602. doi: 10.1039/c9bm01037h. [DOI] [PubMed] [Google Scholar]
- 40.Lennon DP, Caplan AI. Isolation of rat marrow-derived mesenchymal stem cells. Exp. Hematol. 2006;34(11):1606–1607. doi: 10.1016/j.exphem.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 41.Liao Y, Wang J, Jaehnig EJ, Shi Z, Zhang B. WebGestalt 2019: gene set analysis toolkit with revamped UIs and APIs. Nucl. Acids Res. 2019;47(W1):W199–w205. doi: 10.1093/nar/gkz401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liberzon A, Subramanian A, Pinchback R, Thorvaldsdóttir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27(12):1739–1740. doi: 10.1093/bioinformatics/btr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maas, S. A., B. J. Ellis, G. A. Ateshian, and J. A. Weiss. FEBio: finite elements for biomechanics. J. Biomech. Eng. 134(1), 2012. [DOI] [PMC free article] [PubMed]
- 45.Maas SA, LaBelle SA, Ateshian GA, Weiss JA. A plugin framework for extending the simulation capabilities of FEBio. Biophys. J . 2018;115(9):1630–1637. doi: 10.1016/j.bpj.2018.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malda J, Klein TJ, Upton Z. The roles of hypoxia in the in vitro engineering of tissues. Tissue Eng. 2007;13(9):2153–2162. doi: 10.1089/ten.2006.0417. [DOI] [PubMed] [Google Scholar]
- 47.Matsubara H, Hogan DE, Morgan EF, Mortlock DP, Einhorn TA, Gerstenfeld LC. Vascular tissues are a primary source of BMP2 expression during bone formation induced by distraction osteogenesis. Bone. 2012;51(1):168–180. doi: 10.1016/j.bone.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McDaniel JS, Pilia M, Ward CL, Pollot BE, Rathbone CR. Characterization and multilineage potential of cells derived from isolated microvascular fragments. J. Surg. Res. 2014;192(1):214–222. doi: 10.1016/j.jss.2014.05.047. [DOI] [PubMed] [Google Scholar]
- 49.Müller P, Bulnheim U, Diener A, Lüthen F, Teller M, Klinkenberg ED, Neumann HG, Nebe B, Liebold A, Steinhoff G. Calcium phosphate surfaces promote osteogenic differentiation of mesenchymal stem cells. J. Cell Mol. Med. 2008;12(1):281–291. doi: 10.1111/j.1582-4934.2007.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagel T, Kelly DJ. Apparent behaviour of charged and neutral materials with ellipsoidal fibre distributions and cross-validation of finite element implementations. J. Mech. Behav. Biomed. Mater. 2012;9:122–129. doi: 10.1016/j.jmbbm.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 51.Nauman EA, Fong K, Keaveny T. Dependence of intertrabecular permeability on flow direction and anatomic site. Ann. Biomed. Eng. 1999;27(4):517–524. doi: 10.1114/1.195. [DOI] [PubMed] [Google Scholar]
- 52.Nunes SS, Krishnan L, Gerard CS, Dale JR, Maddie MA, Benton RL, Hoying JB. Angiogenic potential of microvessel fragments is independent of the tissue of origin and can be influenced by the cellular composition of the implants. Microcirculation. 2010;17(7):557–567. doi: 10.1111/j.1549-8719.2010.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park K-H, Kim H, Moon S, Na K. Bone morphogenic protein-2 (BMP-2) loaded nanoparticles mixed with human mesenchymal stem cell in fibrin hydrogel for bone tissue engineering. J. Biosci. Bioeng. 2009;108(6):530–537. doi: 10.1016/j.jbiosc.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 54.Pilia M, McDaniel JS, Guda T, Chen XK, Rhoads RP, Allen RE, Corona BT, Rathbone CR. Transplantation and perfusion of microvascular fragments in a rodent model of volumetric muscle loss injury. Eur. Cell Mater. 2014;28:11–23. doi: 10.22203/ecm.v028a02. [DOI] [PubMed] [Google Scholar]
- 55.Pilia M, McDaniel J, Guda T, Chen X, Rhoads R, Allen RE, Corona B, Rathbone C. Transplantation and perfusion of microvascular fragments in a rodent model of volumetric muscle loss injury. Eur. Cells Mater. 2014;28:11–24. doi: 10.22203/ecm.v028a02. [DOI] [PubMed] [Google Scholar]
- 56.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 57.Pollot, B. E., C. R. Rathbone, J. C. Wenke, and T. Guda. Natural polymeric hydrogel evaluation for skeletal muscle tissue engineering. J. Biomed. Mater. Res. B 2017. [DOI] [PubMed]
- 58.Rathbone C, Guda T, Singleton B, Oh D, Appleford M, Ong J, Wenke J. Effect of cell-seeded hydroxyapatite scaffolds on rabbit radius bone regeneration. J. Biomed. Mater. Res. A. 2014;102(5):1458–1466. doi: 10.1002/jbm.a.34834. [DOI] [PubMed] [Google Scholar]
- 59.Ruehle, M. A., E. A. Eastburn, S. A. LaBelle, L. Krishnan, J. A. Weiss, J. D. Boerckel, L. B. Wood, R. E. Guldberg, and N. J. Willett. Mechanical regulation of microvascular angiogenesis. bioRxiv 2020 [DOI] [PMC free article] [PubMed]
- 60.Sander EA, Nauman EA. Permeability of musculoskeletal tissues and scaffolding materials: experimental results and theoretical predictions. Crit. Rev. Biomed. Eng. 2003;31(1–2):1–26. doi: 10.1615/critrevbiomedeng.v31.i12.10. [DOI] [PubMed] [Google Scholar]
- 61.Santos MI, Reis RL. Vascularization in bone tissue engineering: physiology, current strategies, major hurdles and future challenges. Macromol. Biosci. 2010;10(1):12–27. doi: 10.1002/mabi.200900107. [DOI] [PubMed] [Google Scholar]
- 62.Santos MI, Tuzlakoglu K, Fuchs S, Gomes ME, Peters K, Unger RE, Piskin E, Reis RL, Kirkpatrick CJ. Endothelial cell colonization and angiogenic potential of combined nano- and micro-fibrous scaffolds for bone tissue engineering. Biomaterials. 2008;29(32):4306–4313. doi: 10.1016/j.biomaterials.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 63.Sato N, Sawasaki Y, Senoo A, Fuse Y, Hirano Y, Goto T. Development of capillary networks from rat microvascular fragments in vitro: the role of myofibroblastic cells. Microvasc. Res. 1987;33(2):194–210. doi: 10.1016/0026-2862(87)90017-3. [DOI] [PubMed] [Google Scholar]
- 64.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9(7):671. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schumann P, Von See C, Kampmann A, Lindhorst D, Tavassol F, Kokemüller H, Bormann KH, Gellrich NC, Rücker M. Comparably accelerated vascularization by preincorporation of aortic fragments and mesenchymal stem cells in implanted tissue engineering constructs. J. Biomed. Mater. Res. A. 2011;97(4):383–394. doi: 10.1002/jbm.a.33069. [DOI] [PubMed] [Google Scholar]
- 66.Später T, Frueh F, Menger M, Laschke M. Potentials and limitations of Integra® flowable wound matrix seeded with adipose tissue-derived microvascular fragments. Eur. Cells Mater. (ECM) 2017;33:268–278. doi: 10.22203/eCM.v033a20. [DOI] [PubMed] [Google Scholar]
- 67.Stegen S, Deprez S, Eelen G, Torrekens S, Van Looveren R, Goveia J, Ghesquière B, Carmeliet P, Carmeliet G. Adequate hypoxia inducible factor 1α signaling is indispensable for bone regeneration. Bone. 2016;87:176–186. doi: 10.1016/j.bone.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 68.Stevens MM. Biomaterials for bone tissue engineering. Mater. Today. 2008;11(5):18–25. [Google Scholar]
- 69.Stiers P-J, van Gastel N, Carmeliet G. Targeting the hypoxic response in bone tissue engineering: a balance between supply and consumption to improve bone regeneration. Mol. Cell. Endocrinol. 2016;432:96–105. doi: 10.1016/j.mce.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 70.Stone, I. R., and C. R. Rathbone. Microvascular fragment transplantation improves rat dorsal skin flap survival. Plast. Reconstruct. Surg. Global Open 4(12), 2016 [DOI] [PMC free article] [PubMed]
- 71.Suva LJ, Seedor GJ, Endo N, Quartuccio HA, Thompson DD, Bab I, Rodan GA. Pattern of gene expression following rat tibial marrow ablation. J. Bone Miner. Res. 1993;8(3):379–388. doi: 10.1002/jbmr.5650080315. [DOI] [PubMed] [Google Scholar]
- 72.Tang Y, Weiss SJ. Snail/Slug-YAP/TAZ complexes cooperatively regulate mesenchymal stem cell function and bone formation. Cell Cycle. 2017;16(5):399–405. doi: 10.1080/15384101.2017.1280643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Team RC. R: a language and environment for statistical computing. New York: RC Team; 2013. [Google Scholar]
- 74.Tsigkou O, Pomerantseva I, Spencer JA, Redondo PA, Hart AR, O’Doherty E, Lin Y, Friedrich CC, Daheron L, Lin CP. Engineered vascularized bone grafts. Proc. Natl. Acad. Sci. 2010;107(8):3311–3316. doi: 10.1073/pnas.0905445107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang X, Valls AF, Schermann G, Shen Y, Moya IM, Castro L, Urban S, Solecki GM, Winkler F, Riedemann L, Jain RK, Mazzone M, Schmidt T, Fischer T, Halder G, de Almodovar CR. YAP/TAZ orchestrate VEGF signaling during developmental angiogenesis. Dev. Cell. 2017;42(5):462–478.e7. doi: 10.1016/j.devcel.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 76.Weinreb M, Shinar D, Rodan GA. Different pattern of alkaline phosphatase, osteopontin, and osteocalcin expression in developing rat bone visualized by in situ hybridization. J. Bone Miner. Res. 1990;5(8):831–842. doi: 10.1002/jbmr.5650050806. [DOI] [PubMed] [Google Scholar]
- 77.Yang W, Guo D, Harris MA, Cui Y, Gluhak-Heinrich J, Wu J, Chen X-D, Skinner C, Nyman JS, Edwards JR. Bmp2 in osteoblasts of periosteum and trabecular bone links bone formation to vascularization and mesenchymal stem cells. J. Cell Sci. 2013;126(18):4085–4098. doi: 10.1242/jcs.118596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu H, VandeVord PJ, Mao L, Matthew HW, Wooley PH, Yang S-Y. Improved tissue-engineered bone regeneration by endothelial cell mediated vascularization. Biomaterials. 2009;30(4):508–517. doi: 10.1016/j.biomaterials.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 79.Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu A, Ibrahim JG, Love MI. Heavy-tailed prior distributions for sequence count data: removing the noise and preserving large differences. Bioinformatics. 2019;35(12):2084–2092. doi: 10.1093/bioinformatics/bty895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.