Abstract
Background and Objectives
The relationship between the hospital percutaneous coronary intervention (PCI) volumes and the in-hospital clinical outcomes of patients with acute myocardial infarction (AMI) remains the subject of debate. This study aimed to determine whether the in-hospital clinical outcomes of patients with AMI in Korea are significantly associated with hospital PCI volumes.
Methods
We selected and analyzed 17,121 cases of AMI, that is, 8,839 cases of non-ST-segment elevation myocardial infarction and 8,282 cases of ST-segment elevation myocardial infarction, enrolled in the 2014 Korean percutaneous coronary intervention (K-PCI) registry. Patients were divided into 2 groups according to hospital annual PCI volume, that is, to a high-volume group (≥400/year) or a low-volume group (<400/year). Major adverse cardiovascular and cerebrovascular events (MACCEs) were defined as composites of death, cardiac death, non-fatal myocardial infarction (MI), stent thrombosis, stroke, and need for urgent PCI during index admission after PCI.
Results
Rates of MACCE and non-fatal MI were higher in the low-volume group than in the high-volume group (MACCE: 10.9% vs. 8.6%, p=0.001; non-fatal MI: 4.8% vs. 2.6%, p=0.001, respectively). Multivariate regression analysis showed PCI volume did not independently predict MACCE.
Conclusions
Hospital PCI volume was not found to be an independent predictor of in-hospital clinical outcomes in patients with AMI included in the 2014 K-PCI registry.
Keywords: Myocardial infarction, Percutaneous coronary intervention, Low-volume hospitals, Treatment outcome
INTRODUCTION
Coronary angiography and percutaneous coronary intervention (PCI) are performed worldwide and have saved many lives. PCI is also recommended as a first-line therapeutic strategy for acute myocardial infarction (AMI) by the American Heart Association (AHA), the American College of Cardiology (ACC), and the European Society of Cardiology (ESC).1),2),3) Primary PCI and early invasive PCI are widely implemented in Korea in accord with international guidelines, and the number of PCI-capable hospitals is increasing, however the numbers of procedures performed in each hospital range widely. Several clinical studies have demonstrated an inverse relationship between hospital PCI volume and in-hospital mortality for patients with AMI.4),5),6),7) In contrast, a recent study concluded that low- and high-volume PCI hospitals have similar in-hospital clinical outcomes for AMI patients that undergo primary PCI.8) Furthermore, a recent meta-analysis showed operator volume was not related to mortality, but that major adverse cardiac event (MACE) rates were lower for high volume PCI practitioners than low volume practitioners.9),10)
Current international guidelines recommend that an institution should have an annual institutional PCI volume of at least 400 cases.11),12) However, in Korea, about half of the hospitals that perform PCI treat fewer than 400 cases per year, and the relationship between hospital PCI volume and in-hospital clinical outcomes of patients with AMI has not been fully elucidated. Therefore, it is important to determine whether hospital PCI volumes are related to in-hospital clinical outcomes. Accordingly, the purpose of this study was to determine whether hospital PCI volume significantly affects the in-hospital clinical outcomes in a Korean cohort of patients with AMI.
METHODS
Study population and data collection
The 2014 Korean percutaneous coronary intervention (K-PCI) registry is a retrospective multicenter registry of coronary artery disease (CAD) patients that have undergone PCI, and includes clinical diagnostic information, procedural data, and details of adverse events during index admission after PCI procedures were conducted at 92 hospitals. The 2014 K-PCI registry governing committee centrally managed web-based registered data. All variables used correspond with written definitions in the case report form (CRF) dictionary.
We selected and analyzed 17,121 cases of AMI, which included 8,839 non-ST segment elevation myocardial infarction (NSTEMI) and 8,282 ST-segment elevation myocardial infarction (STEMI) cases treated from January 2014 to December 2014. AMI was diagnosed by the criteria described in the 3rd Universal Definition of Myocardial Infarction.13) Previous studies have reported that hospitals performing ≥400 PCI cases per year have lower major adverse event rates after PCI than hospitals performing <400 cases. Furthermore, international guidelines recommend a minimum annual institutional PCI volume of 400 cases per year to minimize the incidence of MACEs after PCI.2),11),12),14),15) Based on these findings, we divided patients treated at the participating hospitals into 2 groups based on annual hospital PCI volume, that is, a high-volume group (n=12,830; ≥400 PCI cases per annum) and a low-volume group (n=4,291; <400 cases per annum). The 49 high-volume hospitals and 43 low-volume hospitals participated in the present study. The study endpoint was major adverse cardiovascular and cerebrovascular events (MACCEs; defined as a composite of death, cardiac death, non-fatal myocardial infarction (non-fatal MI), stent thrombosis, stroke, and urgent PCI) during index admission after PCI (Supplementary Table 1).
All 92 hospital institutional review boards approved this study, and all waived the requirement for informed consent due to the retrospective nature of the study and lack of clinical follow-up.
Statistical analysis
Discrete demographic, clinical, procedural, and outcome variable data are presented as numbers (%) and were compared using the χ2 test. Continuous variables are presented as mean±standard deviation and were compared using the Student's t-test. Independent predictors of adverse events were identified by logistic regression analysis, which included clinical variables found to be associated with clinical outcomes at the p<0.10 level by univariate analysis. All p values were 2-tailed, and statistical significance was accepted for p values <0.05. The analysis was conducted using R version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Clinical, angiographic, and procedural characteristics
Baseline characteristics are summarized in Table 1.
Table 1. Baseline patient and clinical characteristics.
| Variables | High volume (≥400/year) (n=12,830) | Low volume (<400/year) (n=4,291) | p value | |
|---|---|---|---|---|
| Age (years) | 64.6±12.9 | 64.1±13.1 | 0.016 | |
| Sex | 0.496 | |||
| Male | 9,506 (74.1) | 3,156 (73.5) | ||
| Female | 3,324 (25.9) | 1,135 (26.5) | ||
| Diabetes mellitus | 4,241 (33.1) | 1,320 (30.8) | 0.006 | |
| Smoking | 0.306 | |||
| Never | 7,631 (59.6) | 2,499 (59.0) | ||
| Smoker | 4,611 (36.0) | 1,565 (37.0) | ||
| Ex-smoker | 570 (4.4) | 170 (4.0) | ||
| Hypertension | 7,149 (55.8) | 2,302 (53.7) | 0.019 | |
| Dyslipidemia | 4,474 (34.9) | 977 (22.9) | 0.001 | |
| FHx CAD | 714 (5.6) | 206 (5.0) | 0.148 | |
| Prior MI | 1,186 (9.3) | 375 (8.8) | 0.353 | |
| Prior PCI | 2,410 (18.8) | 647 (15.1) | 0.001 | |
| Prior CABG | 117 (0.9) | 21 (0.5) | 0.010 | |
| Renal failure | 0.134 | |||
| No | 11,977 (93.5) | 4,043 (94.4) | ||
| CKD | 530 (4.1) | 143 (3.3) | ||
| ESRD | 287 (2.2) | 94 (2.2) | ||
| KT | 14 (0.1) | 4 (0.1) | ||
| Cerebrovascular disease | 1,062 (8.3) | 278 (6.5) | 0.001 | |
| Peripheral artery disease | 239 (1.9) | 48 (1.1) | 0.001 | |
Values are presented as mean±standard deviation or number (%).
CABG = coronary artery bypass graft; CKD = chronic kidney disease; ESRD = end stage renal disease; FHx CAD = family history of coronary artery disease; MI = myocardial infarction; PCI = percutaneous coronary intervention; KT = kidney transplantation.
The high-volume group had a higher proportion of elderly patients than the low-volume group. No significant difference was observed between the gender distributions of the groups. The high-volume group had higher historic rates of diabetes mellitus, hypertension, dyslipidemia, PCI, coronary artery bypass graft (CABG) surgery, cerebrovascular accidents, and peripheral artery disease.
Clinical presentations and angiographic and procedural characteristics of the 2 groups are summarized in Table 2. The low-volume group had higher rates of cardiogenic shock and cardiac arrest before PCI. Intravascular ultrasound (IVUS) and fractional flow reserve (FFR) were performed more frequently during PCI in the high-volume group.
Table 2. Clinical, angiographic, and procedural characteristics.
| Variables | High volume (≥400/year) (n=12,830) | Low volume (<400/year) (n=4,291) | p value | |
|---|---|---|---|---|
| Diagnosis | 0.577 | |||
| NSTEMI | 6,640 (51.8) | 2,199 (51.2) | ||
| STEMI | 6,190 (48.2) | 2,092 (48.8) | ||
| Cardiogenic shock | 865 (6.7) | 389 (9.1) | 0.001 | |
| Cardiac arrest | 656 (5.1) | 269 (6.3) | 0.004 | |
| Left main lesion | 551 (4.3) | 155 (3.6) | 0.057 | |
| Proximal LAD lesion | 4,040 (31.5) | 1,316 (30.7) | 0.325 | |
| IVUS | 3,147 (24.5) | 863 (20.1) | 0.001 | |
| FFR | 220 (1.7) | 29 (0.7) | 0.001 | |
Values are presented as number (%).
FFR = fractional flow reserve; IVUS = intravascular ultrasound; LAD = left anterior descending; NSTEMI = non-ST-segment elevation myocardial infarction; STEMI = ST-segment elevation myocardial infarction.
In-hospital clinical outcomes after PCI
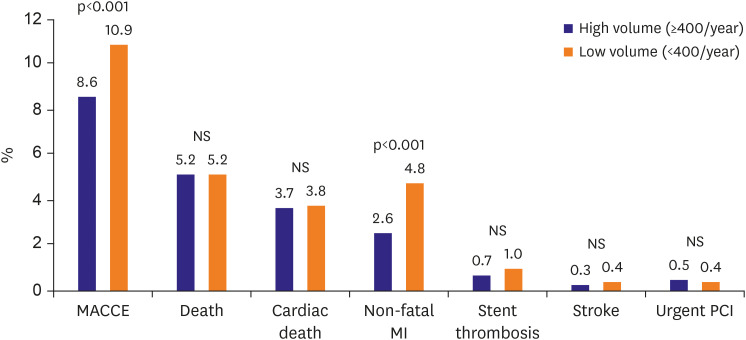
MACCE (467 [10.9%] vs. 1,109 [8.6%], p=0.001) and non-fatal MI (208 [4.8%] vs. 330 [2.6%], p=0.001) were more common in the low-volume group. However, no significant intergroup difference was observed between the rates of death from all causes, cardiac death, stent thrombosis, stroke, or urgent PCI (Figure 1).
Figure 1. In-hospital outcomes by PCI volume.
MACCE = major adverse cardiovascular and cerebrovascular events; MI = myocardial infarction; NS = not significant; PCI = percutaneous coronary intervention.
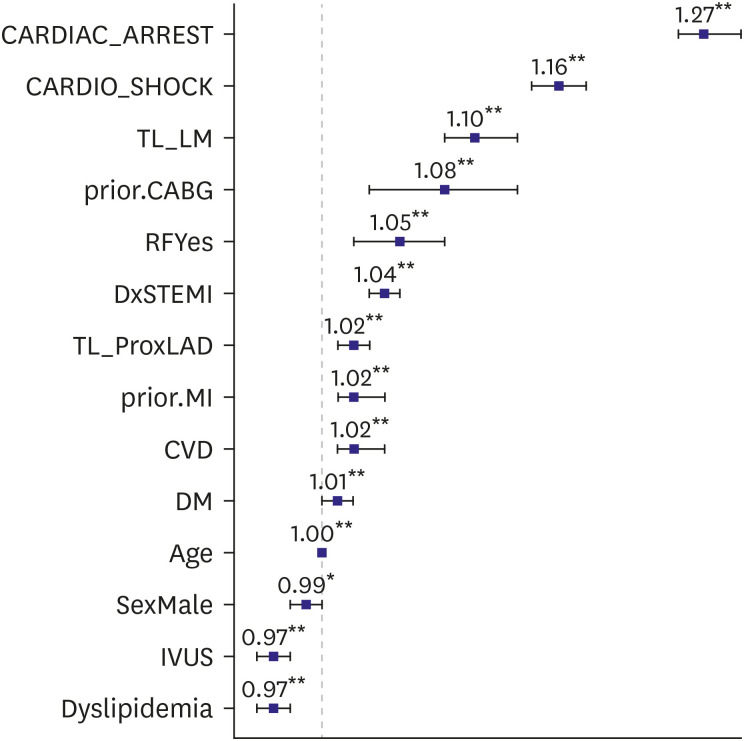
Multivariate analysis showed that age, diabetes mellitus, prior MI, prior CABG, renal failure, cerebrovascular disease, STEMI presentation, cardiogenic shock, cardiac arrest, and involvement of left main or proximal left anterior descending (LAD) independently predicted MACCE (Figure 2, Table 3). In addition, male sex, dyslipidemia, and IVUS usage were also identified as independent predictors of a reduced MACCE rate (Figure 2, Table 3). However, hospital PCI volume was not found to predict MACCE independently.
Figure 2. Odds ratios of major adverse cardiovascular and cerebrovascular events.
CARDIAC_ARREST = cardiac arrest; CARDIO_SHOCK = cardiogenic shock; CVD = cerebrovascular disease; DM = diabetes mellitus; DxSTEMI = diagnosis of ST-segment elevation myocardial infarction; IVUS = intravascular ultrasound; prior.CABG = prior history of coronary artery bypass graft; prior.MI = prior history of myocardial infarction; RFYse = renal failure; SexMale = male; TL_LM = target lesion_left main coronary artery; TL_ProxLAD = target lesion proximal left anterior descending artery.
**p<0.001.
Table 3. Independent predictors of major adverse cardiovascular and cerebrovascular events.
| Variable | Univariate analysis | p value | Multivariate analysis | p value | ||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |||
| PCI volume | 1.000 | 1.000–1.000 | 0.6957 | |||
| Age (years) | 1.026 | 1.021–1.030 | 0.0001 | 1.002 | 1.001–1.002 | 0.0001 |
| Male | 0.736 | 0.657–0.825 | 0.0001 | 0.988 | 0.978–0.998 | 0.0186 |
| Diabetes mellitus | 1.316 | 1.180–1.468 | 0.0001 | 1.012 | 1.003–1.021 | 0.0089 |
| Smoking | 0.751 | 0.682–0.828 | 0.0001 | |||
| Hypertension | 1.178 | 1.059–1.311 | 0.0026 | |||
| Dyslipidemia | 0.645 | 0.570–0.729 | 0.0001 | 0.973 | 0.964–0.982 | 0.0001 |
| FHx CAD | 0.784 | 0.609–1.010 | 0.0597 | |||
| Prior MI | 1.301 | 1.100–1.538 | 0.0021 | 1.023 | 1.008–1.038 | 0.0025 |
| Prior PCI | 1.060 | 0.926–1.213 | 0.3994 | |||
| Prior CABG | 2.308 | 1.489–3.577 | 0.0002 | 1.078 | 1.029–1.080 | 0.0016 |
| Renal failure | 1.776 | 1.337–2.360 | 0.0001 | 1.051 | 1.022–1.080 | 0.0005 |
| Cerebrovascular disease | 1.559 | 1.315–1.848 | 0.0001 | 1.025 | 1.009–1.041 | 0.0022 |
| Peripheral arteria disease | 1.286 | 0.886–1.865 | 0.1856 | |||
| STEMI | 1.766 | 1.586–1.967 | 0.0001 | 1.037 | 1.028–1.046 | 0.0001 |
| Cardiogenic shock | 7.276 | 6.370–8.311 | 0.0001 | 1.159 | 1.137–1.182 | 0.0098 |
| Cardiac arrest | 9.575 | 8.271–11.085 | 0.0001 | 1.275 | 1.247–1.303 | 0.0001 |
| Left main lesion | 3.131 | 2.597–3.774 | 0.0001 | 1.104 | 1.081–1.128 | 0.0001 |
| Proximal LAD lesion | 1.395 | 1.251–1.555 | 0.0001 | 1.021 | 1.012–1.030 | 0.0001 |
| IVUS | 0.581 | 0.504–0.670 | 0.0001 | 0.967 | 0.957–0.976 | 0.0001 |
| FFR | 0.457 | 0.249–0.837 | 0.0113 | |||
CABG = coronary artery bypass graft; CI = confidence interval; FFR = fractional flow reserve; FHx CAD = family history of coronary artery disease; IVUS = intravascular ultrasound; LAD = left anterior descending; MI = myocardial infarction; OR = odds ratio; PCI = percutaneous coronary intervention; STEMI = ST-segment elevation myocardial infarction.
DISCUSSION
This study is the first large registry study to analyze relationships between hospital PCI volume and in-hospital clinical outcomes of patients with AMI that underwent PCI in Korea. The main results of the study were that the low-volume group had a higher frequency of overall MACCE and non-fatal MI than the high-volume group. However, hospital PCI volume did not affect mortality rates and was not found to predict MACCE independently.
The 2011 American College of Cardiology Foundation/American Heart Association/Society of Cardiovascular Angiography and Intervention (ACCF/AHA/SCAI) guideline and the British Cardiovascular Intervention Society recommend a minimum annual hospital PCI volume of 400, but many rural hospitals in the USA do not meet this minimum requirement.2),12),16),17) Similarly, more than 50% of Korea hospitals in the provinces do not perform ≥400 PCI procedures annually, but nevertheless, frequently provide important services such as early invasive treatment for NSTEMI and primary PCI for STEMI.18)
The Paris Metropolitan PCI registry study (2001–2002) showed a clear inverse relationship exists between hospital PCI volume and in-hospital mortality after emergency procedures had been performed on patients presenting with AMI, cardiogenic shock, or out of hospital cardiac arrest (6.75% mortality at high-volume hospitals vs. 8.54% at low-volume hospitals, p=0.028). As was performed in the present study, the Paris Metropolitan PCI registry study divided the cohort into 2 groups based on hospital volumes of < or ≥400 PCI cases per annum.6) The difference between the results of the 2 studies might be explained as follows. First, the 2 studies were conducted 13 years apart, and in our study, the transradial approach was used in >50%, and drug-eluting stents (DES) were implanted in >90% of our patients. Second, PCI devices advanced considerably during this period, for example, coronary stents were introduced and reduced the mortality disparity between low-volume and high-volume PCI hospitals.7) Furthermore, new technologies like IVUS and FFR have been introduced, and PCI operators now have many opportunities to learn about PCI techniques at domestic and international live demonstration meetings and conferences.19),20),21)
Most studies have compared low-volume and high-volume PCI hospitals with respect to primary PCI for STEMI. A Japanese study concluded no differences in mortality between AMI patients that had undergone primary PCI at low- vs. high-volume primary PCI hospitals (9.9% vs. 10.5%, p=0.688). However, other studies conducted in the US, France, and a meta-analysis concluded mortality rates are higher at low-volume PCI hospitals.4),5),6),7),15),22)
The hospital PCI volume-outcome relationship has been discussed for a long time, which is not surprising because the findings of studies on the topic are discordant. These differences may have been caused by cohort heterogeneities in terms of CAD severity, concurrent diseases, methods of categorization, and the definition of PCI volume used.
A retrospective analysis of the database of the National Health Insurance Review & Assessment Service and Korean National Statistical Office failed to detect a difference in the risk-adjusted 30-day mortality rates after PCI between the low (<200 cases/year), medium (200–399 cases/year), and high (≥400 cases/year) volume hospitals.23)
The 2013 ACCF/AHA/SCAI clinical competence statement eased minimum requirements for PCI to >200 procedures per annum for institutes and ≥50 procedures per annum for operators, and the 2014 PCI guidelines of the ESC/European Association for Cardio-Thoracic Surgery recommend a minimum of 75 procedures per year for operators.24),25) A recent Japanese PCI (J-PCI) registry report showed the probability of mortality plateaued at approximately 100 procedures per year.21) Korean Society of Interventional Cardiology (KSIC) certified hospitals are required to carry out at least 100 PCI cases each year, and thus, the study subjects were divided and compared using a cut-off of 100 PCI cases per year. Therefore, the occurrence of MACCE, death, and non-fatal MI was statistically higher in hospitals with less than 100 PCI cases per year (Supplementary Figure 1). The results of the present study based on 2014 K-PCI registry data support the currently required minimum threshold of 100 PCI cases per year for the accreditation of institutes by the KSIC.
Currently, the KSIC recommends ≥150 PCI procedures every 2 years to meet interventional cardiologist certification requirements and ≥100 PCIs/year for an institute to be certified by the KSIC. However, in our opinion, the KSIC recommendation of ≥75 PCI procedures/year for each operator is over-demanding. Inohara et al.21) reported that lower institutional PCI volume was inversely related to in-hospital outcomes. However, the predicted probability of mortality decreased with increasing institutional PCI volume and flattened out at approximately >100 procedures per year. The association between annual operator volume and outcomes was less clear in the J-PCI registry. Furthermore, the median numbers of cases per annum per Japanese and US operator have been reported to be 28 and 33, respectively.9) However, we do not think the results of the J-PCI registry are much worse than those of the Korean K-PCI registry, and therefore, it would appear hospital PCI volume is more important than operator PCI volume.
In a recently published study, PCI hospitals were divided into 3 groups according to operator volume; the 75th percentile (>30 cases/year), between 75th and 25th percentiles (10–30 cases/year), and below the 25th percentile (<10 cases/year). The researchers reported that in-hospital clinical outcomes were not significantly dependent on operator volume, which was not found to predict adverse outcomes before discharge.10) We suggest the number of PCI cases required by KSIC for certified interventional cardiologists be reduced based on the findings of the present study and those of other studies.9),24),26)
The number of hospitals that perform fewer PCI cases is expected to gradually increase in Korea, as is the case in Japan. Even in low volume hospitals, at least 3 interventional cardiologists are required for primary PCI to treat STEMI patients, and thus, the number of operators who cannot perform 75 PCI cases/year will increase, and operators affiliated to low-volume centers are likely to give up KSIC certification. The 2013 ACCF/AHA/SCAI clinical competence statement has eased the minimum requirements for PCI performance (>200 PCIs per year per institute and ≥50 PCIs per year for operators) to reflect the recent decline in the number of PCIs performed.24),27),28)
In the present study, about 75% of all AMI patients were treated at a high-volume PCI hospital, and the remainder were treated at a low-volume PCI hospital. Low-volume PCI hospitals in small and medium-sized cities continue to make major contributions to the treatment of AMI, and thus, continuous quality control of low-volume PCI hospitals is critical.
We found significant variations between high vs. low-volume PCI hospitals in terms of baseline clinical and procedural characteristics and in-hospital outcomes. Furthermore, rates of atherosclerosis risk factors such as diabetes mellitus, hypertension and dyslipidemia, and histories of cardiovascular and cerebrovascular disease were higher in high-volume PCI hospitals. This concurs with that found in a study registered in the National Cardiovascular Data Registry conducted from July 1, 2009, to March 31, 2015, which showed high-risk CAD patients are more likely to visit high-volume PCI hospitals.29) Although no difference was observed between STEMI or NSTEMI rates among cases treated at high or low-volume hospitals, initial presentations of cardiogenic shock and cardiac arrest were found to be more common for low-volume PCI hospitals. These results differ from those of the Paris Metropolitan PCI registry study, in which rates of cardiogenic shock were similar for high and low-volume PCI hospitals, and the cardiac arrest rate was lower for low-volume PCI hospitals.
We compared patients in the 2 study groups that presented with cardiogenic shock or cardiac arrest. Baseline characteristics revealed no difference in clinical characteristics except for a higher prevalence of diabetes mellitus and dyslipidemia in the high-volume group. Like that observed for all study subjects, a greater proportion of patients with cardiogenic shock were treated in low-volume hospitals. However, there was no difference in the proportion of cardiac arrest, left main lesion, proximal LAD lesion, and using IVUS and FFR in the patients with cardiogenic shock and arrest between 2 groups. In addition, MACCE rates were similar in the 2 groups.
Several recent clinical trials and one meta-analysis have reported that IVUS guidance PCI significantly decreased MACE, death, and stent thrombosis rates in patients with complex lesions or acute coronary syndrome.19),30) We found IVUS was used more frequently in the high-volume group, and examined whether its use independently predicted MACCE. Multivariate analysis revealed that implementation of IVUS significantly reduced MACCE. Therefore, we cautiously suggest that increasing IVUS use during PCI in low-volume hospitals would reduce MACCE rates.
Technical issues are also important in terms of reducing MACCE rates after PCI, and according to a recent study based on 2014 K-PCI registry data, low volume PCI operators tend to experience higher rates of repeated PCI,10) which indicates technical issues are importantly associated with the rate of non-fatal MI after PCI.
Emergency PCI for AMI is being implemented in low volume PCI hospitals in rural areas and small-sized private hospitals in Korea. KSIC accreditation of high-quality hospitals and implementation of the interventional cardiologist accreditation system have led to significant improvements in the skills of doctors and catheterization room staff in low-volume PCI hospitals. Also, recently developed coronary PCI devices such as DES and PCI with IVUS guidance have effectively reduced MACCE rates, and thus, nowadays, the effects of hospital PCI volumes on outcomes may be substantially attenuated, which we propose is a reason why hospital PCI volume was not found to independently predict MACCE in the present study. We suggest that hospital and operator accreditation requirements could be eased in Korea to less than minimum 2013 ACCF/AHA/SCAI requirements (>200 PCIs per year per institute and ≥50 PCIs per year for operators). However, to do so, a well-planned prospective study is needed to confirm the relationship between hospital PCI volume and in-hospital outcomes after the treatment of AMI in Korea.
The present study has a number of limitations that warrant consideration. First, only the 92 hospitals that participated in the 2014 K-PCI registry study were included. Therefore, we need to be careful in incorporating our study findings into all of the PCI hospitals and patients in Korea. Nevertheless, the hospitals that participated were recognized by KSIC and most university hospitals participated. Second, the study was based on registry data, which raises the possibility of input bias as individual institutions submitted data. However, the K-PCI data management and inspection program determined whether collected data were accurate and complete, and CRF variables were refined based on the results obtained. The 2014 K-PCI committee also performed data management quality verification during data collection. Third, we could not take into account the experience of operators at low- or high-volume PCI centers, and did not analyze in-hospital outcomes according to the number of procedures performed annually by operators. Forth, we used in-hospital outcomes as the study endpoint, and thus, a long-term follow-up study is needed to confirm the relationship between hospital PCI volumes and long-term clinical outcomes. Lastly, in-hospital clinical outcomes could not be compared concerning the presence or absence of cardiac intensive care teams and facilities because the K-PCI registry did not collect such data from each study participated hospitals.
In conclusion, this is the first Korean study to evaluate the relationship between hospital PCI volume and in-hospital clinical outcomes after PCI for AMI patients using 2014 K-PCI registry data. The study shows MACCE and non-fatal MI rates were higher at low-volume PCI hospitals, but that hospital PCI volume did not independently predict in-hospital outcomes.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Kim BK, Nah DY, Choi KU, Bae JH, Rhee MY.
- Data curation: Kim BK, Nah DY, Jang JS, Moon KW.
- Formal analysis: Kim BK, Nah DY, Jang JS, Moon KW.
- Funding acquisition: Nah DY.
- Investigation: Kim BK, Nah DY.
- Methodology: Kim BK, Nah DY, Moon KW.
- Project administration: Kim BK, Nah DY.
- Resources: Kim BK, Nah DY.
- Software: Kim BK, Nah DY.
- Supervision: Nah DY, Choi KU, Bae JH, Rhee MY, Jang JS, Moon KW, Lee JH, Kim HY, Kang SH, Song WH, Lee SU, Shim BJ, Chung H, Hyon MS.
- Validation: Kim BK, Nah DY, Jang JS, Hyon MS.
- Visualization: Kim BK, Nah DY.
- Writing - original draft: Kim BK, Nah DY.
- Writing - review & editing: Kim BK, Nah DY, Choi KU, Bae JH, Rhee MY, Jang JS, Moon KW, Lee JH, Kim HY, Kang SH, Song WH, Lee SU, Shim BJ, Chung H, Hyon MS.
SUPPLEMENTARY MATERIALS
Major adverse cardiovascular and cerebrovascular events (in-hospital events variables)
In-hospital outcomes by PCI volume about a cut-off of 100 procedures per year.
References
- 1.Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. J Am Coll Cardiol. 2016;67:1235–1250. doi: 10.1016/j.jacc.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:2574–2609. doi: 10.1161/CIR.0b013e31823a5596. [DOI] [PubMed] [Google Scholar]
- 3.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. doi: 10.1093/eurheartj/ehy855. [DOI] [PubMed] [Google Scholar]
- 4.Canto JG, Every NR, Magid DJ, et al. The volume of primary angioplasty procedures and survival after acute myocardial infarction. National Registry of Myocardial Infarction 2 Investigators. N Engl J Med. 2000;342:1573–1580. doi: 10.1056/NEJM200005253422106. [DOI] [PubMed] [Google Scholar]
- 5.Vakili BA, Kaplan R, Brown DL. Volume-outcome relation for physicians and hospitals performing angioplasty for acute myocardial infarction in New York state. Circulation. 2001;104:2171–2176. doi: 10.1161/hc3901.096668. [DOI] [PubMed] [Google Scholar]
- 6.Spaulding C, Morice MC, Lancelin B, et al. Is the volume-outcome relation still an issue in the era of PCI with systematic stenting? Results of the greater Paris area PCI registry. Eur Heart J. 2006;27:1054–1060. doi: 10.1093/eurheartj/ehi843. [DOI] [PubMed] [Google Scholar]
- 7.Shiraishi J, Kohno Y, Sawada T, et al. Effects of hospital volume of primary percutaneous coronary interventions on angiographic results and in-hospital outcomes for acute myocardial infarction. Circ J. 2008;72:1041–1046. doi: 10.1253/circj.72.1041. [DOI] [PubMed] [Google Scholar]
- 8.Tsuchihashi M, Tsutsui H, Tada H, et al. Volume-outcome relation for hospitals performing angioplasty for acute myocardial infarction: results from the nationwide Japanese registry. Circ J. 2004;68:887–891. doi: 10.1253/circj.68.887. [DOI] [PubMed] [Google Scholar]
- 9.Badheka AO, Patel NJ, Grover P, et al. Impact of annual operator and institutional volume on percutaneous coronary intervention outcomes: a 5-year United States experience (2005-2009) Circulation. 2014;130:1392–1406. doi: 10.1161/CIRCULATIONAHA.114.009281. [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Eom SY, Kim U, et al. Effect of operator volume on in-hospital outcomes following primary percutaneous coronary intervention for ST-elevation myocardial infarction: based on the 2014 cohort of Korean percutaneous coronary intervention (K-PCI) registry. Korean Circ J. 2020;50:133–144. doi: 10.4070/kcj.2019.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirshfeld JW, Jr, Ellis SG, Faxon DP. Recommendations for the assessment and maintenance of proficiency in coronary interventional procedures: statement of the American College of Cardiology. J Am Coll Cardiol. 1998;31:722–743. doi: 10.1016/s0735-1097(98)00009-6. [DOI] [PubMed] [Google Scholar]
- 12.Milstein A, Galvin RS, Delbanco SF, Salber P, Buck CR., Jr Improving the safety of health care: the leapfrog initiative. Eff Clin Pract. 2000;3:313–316. [PubMed] [Google Scholar]
- 13.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J. 2012;33:2551–2567. doi: 10.1093/eurheartj/ehs184. [DOI] [PubMed] [Google Scholar]
- 14.Dawkins KD, Gershlick T, de Belder M, et al. Percutaneous coronary intervention: recommendations for good practice and training. Heart. 2005;91(Suppl 6):vi1–vi27. doi: 10.1136/hrt.2005.061457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Post PN, Kuijpers M, Ebels T, Zijlstra F. The relation between volume and outcome of coronary interventions: a systematic review and meta-analysis. Eur Heart J. 2010;31:1985–1992. doi: 10.1093/eurheartj/ehq151. [DOI] [PubMed] [Google Scholar]
- 16.Banning AP, Baumbach A, Blackman D, et al. Percutaneous coronary intervention in the UK: recommendations for good practice 2015. Heart. 2015;101(Suppl 3):1–13. doi: 10.1136/heartjnl-2015-307821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dehmer GJ, Weaver D, Roe MT, et al. A contemporary view of diagnostic cardiac catheterization and percutaneous coronary intervention in the United States: a report from the CathPCI Registry of the National Cardiovascular Data Registry, 2010 through June 2011. J Am Coll Cardiol. 2012;60:2017–2031. doi: 10.1016/j.jacc.2012.08.966. [DOI] [PubMed] [Google Scholar]
- 18.Jang JS, Han KR, Moon KW, et al. The current status of percutaneous coronary intervention in Korea: based on year 2014 cohort of Korean percutaneous coronary intervention (K-PCI) registry. Korean Circ J. 2017;47:328–340. doi: 10.4070/kcj.2017.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong SJ, Mintz GS, Ahn CM, et al. Effect of intravascular ultrasound-guided drug-eluting stent implantation: 5-year follow-up of the IVUS-XPL randomized trial. JACC Cardiovasc Interv. 2020;13:62–71. doi: 10.1016/j.jcin.2019.09.033. [DOI] [PubMed] [Google Scholar]
- 20.Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 21.Inohara T, Kohsaka S, Yamaji K, et al. Impact of institutional and operator volume on short-term outcomes of percutaneous coronary intervention: a report from the Japanese nationwide registry. JACC Cardiovasc Interv. 2017;10:918–927. doi: 10.1016/j.jcin.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Langabeer JR, 2nd, Kim J, Helton J. Exploring the relationship between volume and outcomes in hospital cardiovascular care. Qual Manag Health Care. 2017;26:160–164. doi: 10.1097/QMH.0000000000000142. [DOI] [PubMed] [Google Scholar]
- 23.Kim YH, Her AY. Relationship between hospital volume and risk-adjusted mortality rate following percutaneous coronary intervention in Korea, 2003 to 2004. Anadolu Kardiyol Derg. 2013;13:237–242. doi: 10.5152/akd.2013.070. [DOI] [PubMed] [Google Scholar]
- 24.Harold JG, Bass TA, Bashore TM, et al. ACCF/AHA/SCAI 2013 update of the clinical competence statement on coronary artery interventional procedures: a report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training (Writing Committee to Revise the 2007 Clinical Competence Statement on Cardiac Interventional Procedures) J Am Coll Cardiol. 2013;62:357–396. doi: 10.1016/j.jacc.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS guidelines on myocardial revascularization. EuroIntervention. 2015;10:1024–1094. doi: 10.4244/EIJY14M09_01. [DOI] [PubMed] [Google Scholar]
- 26.Kumbhani DJ, Cannon CP, Fonarow GC, et al. Association of hospital primary angioplasty volume in ST-segment elevation myocardial infarction with quality and outcomes. JAMA. 2009;302:2207–2213. doi: 10.1001/jama.2009.1715. [DOI] [PubMed] [Google Scholar]
- 27.Desai NR, Bradley SM, Parzynski CS, et al. Appropriate use criteria for coronary revascularization and trends in utilization, patient selection, and appropriateness of percutaneous coronary intervention. JAMA. 2015;314:2045–2053. doi: 10.1001/jama.2015.13764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maroney J, Khan S, Powell W, Klein LW. Current operator volumes of invasive coronary procedures in Medicare patients: implications for future manpower needs in the catheterization laboratory. Catheter Cardiovasc Interv. 2013;81:34–39. doi: 10.1002/ccd.24366. [DOI] [PubMed] [Google Scholar]
- 29.Fanaroff AC, Zakroysky P, Dai D, et al. Outcomes of PCI in relation to procedural characteristics and operator volumes in the United States. J Am Coll Cardiol. 2017;69:2913–2924. doi: 10.1016/j.jacc.2017.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darmoch F, Alraies MC, Al-Khadra Y, Moussa Pacha H, Pinto DS, Osborn EA. Intravascular ultrasound imaging-guided versus coronary angiography-guided percutaneous coronary intervention: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9:e013678. doi: 10.1161/JAHA.119.013678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Major adverse cardiovascular and cerebrovascular events (in-hospital events variables)
In-hospital outcomes by PCI volume about a cut-off of 100 procedures per year.