Abstract
Background & Aims
Chronic amino acid (AA) deficiency, as in kwashiorkor, reduces the size of the pancreas through an effect on mammalian target of rapamycin complex 1 (mTORC1). Because of the physiological importance of AAs and their role as a substrate, a stimulant of mTORC1, and protein synthesis, we studied the effect of acute protein and AA deficiency on the response to feeding.
Methods
ICR/CD-1 mice were fasted overnight and refed for 2 hours with 4 different isocaloric diets: control (20% Prot); Protein-free (0% Prot); control (AA-based diet), and a leucine-free (No Leu). Protein synthesis, polysomal profiling, and the activation of several protein translation factors were analyzed in pancreas samples.
Results
All diets stimulated the Protein Kinase-B (Akt)/mTORC1 pathway, increasing the phosphorylation of the kinase Akt, the ribosomal protein S6 (S6) and the formation of the eukaryotic initiation factor 4F (eIF4F) complex. Total protein synthesis and polysome formation were inhibited in the 0% Prot and No Leu groups to a similar extent, compared with the 20% Prot group. The 0% Prot diet partially reduced the Akt/mTORC1 pathway and the activity of the guanine nucleotide exchange factor eIF2B, without affecting eIF2α phosphorylation. The No Leu diet increased the phosphorylation of eIF2α and general control nonderepressible 2, and also inhibited eIF2B activity, without affecting mTORC1. Essential and nonessential AA levels in plasma and pancreas indicated a complex regulation of their cellular transport mechanisms and their specific effect on the synthesis of digestive enzymes.
Conclusions
These studies show that dietary AAs are important regulators of postprandial digestive enzyme synthesis, and their deficiency could induce pancreatic insufficiency and malnutrition.
Keywords: Protein Deficiency, mTORC1, Pancreatic Digestive Enzymes Synthesis, Malnutrition
Abbreviations used in this paper: AA, amino acid; Akt, Protein Kinase-B; BCAA, branched-chain amino acid; CCK, cholecystokinin; EAA, essential amino acid; eIF, eukaryotic initiation factor; ER, endoplasmic reticulum; 4E-BP1, eukaryotic initiation factor 4E binding protein 1; GCN2, general control nonderepressible 2; GDP, guanosine diphosphate; GSK3, glycogen synthase kinase-3; Leu, leucine; mRNA, messenger RNA; mTORC1, mammalian target of rapamycin complex 1; NEAA, nonessential amino acid; PCA, perchloric acid; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis; S6K, S6 kinase
Graphical abstract
Summary.
The lack of protein or just the amino acid leucine in the diet inhibits the pancreatic synthetic machinery to a similar extent, but through different regulation of protein translation mechanisms. This dietary deficiency could lead to pancreatic insufficiency and malnutrition.
Protein and amino acids (AAs) are an essential component of both human and animal nutrition and are involved in the maintenance of general health and well-being.1, 2, 3 Amino acids are the building blocks of protein, cell structures, and tissues, and also can act as regulators of protein metabolism and many physiological processes.4,5 The long-term deficiency of the essential amino acids (EAAs)6 in the diet causes depletion of plasma AAs, and can cause several metabolic problems such as protein-energy malnutrition,7 marasmus, or the kwashiorkor syndrome (a form of malnutrition caused by a lack of protein in the diet).8 It also can aggravate other health-related issues, such as hemoglobin production, infectious diseases such as human immunodeficiency virus/acquired immune deficiency syndrome,9 or tuberculosis,10 sarcopenia,11 or Pompe disease.12 Many studies have shown the therapeutic effect of AAs when administered to patients, especially to infants, for growth as well as to maintain metabolism.13,14 They also are used in parenteral nutrition in cases of maldigestion, malabsorption, short-bowel syndrome, eosinophilic gastrointestinal disorders, and gastrointestinal tract impairment.1,15,16 Amino acid supplementation specifically can benefit patients with chronic pancreatitis and pancreatic cancer because they have low circulating AA levels and suffer from malnutrition.17 In the past 15 years, AA levels in plasma and in different organs have been used in the diagnosis of animal and human disease (including pancreatitis, cancer, and diabetes), and monitor/predict their progression.18, 19, 20 Some studies also analyzed the role of the different AA transporters in normal conditions and during disease.19,21, 22, 23
The branched-chain amino acids (BCAAs), leucine, isoleucine, and valine, in particular, are considered biological regulators,24 and are available as supplements for patients with liver25,26 or kidney27 disease, and athletes with McArdle disease,28 among others. They play an important role in energy homeostasis,29 and are involved in the stimulation of cell proliferation in certain types of cancer.30 Moreover, the deficiency of BCAA in the diet improves metabolic health in mice31 and human beings.32
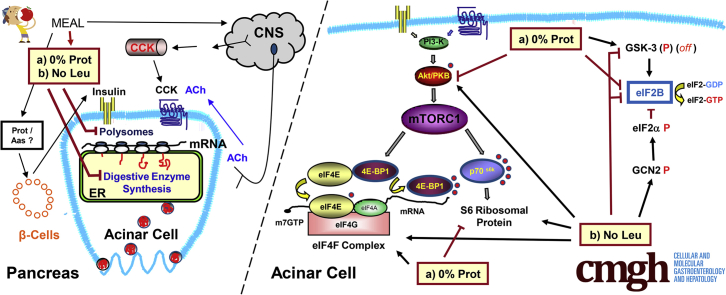
Clinical and histopathologic studies have documented the occurrence of pancreatic injury in response to a severe reduction of protein in the diet that lead to nutrient malabsorption and a state of malnutrition,7,33, 34, 35 which can be especially critical in children and young adults.21,36 The exocrine pancreas synthesizes and secretes between 6 and 20 g of digestive enzymes per day and requires optimal nutrition for enzyme synthesis.7,37 Consequently, the pancreas is extremely vulnerable to protein deficiency states. In fact, the pancreas of patients with the kwashiorkor syndrome is one of the most severely affected tissues, with reductions in size and secretory capacity.7,8,34 A study from our laboratory, mimicking this dietary protein deficiency, showed that mice fed a protein-deficient diet have pancreatic atrophy, and this process involves the mammalian target of rapamycin complex 1 (mTORC1) pathway.38 It has also been shown that protein and AAs can stimulate pancreas growth. A high-protein (40%) diet can induce pancreas growth in mice, independent of cholecystokinin (CCK).39 Other studies have shown that AAs can increase pancreatic trypsin levels and stimulate pancreas growth,40,41 and the AA leucine, specifically, modulates growth responses of pancreatic progenitors, involving mTORC1.42,43
Although these long-term effects occur through changes in the synthetic rates and messenger RNA (mRNA) levels during several days or weeks, little is known about their intracellular mechanisms in a short-term, more physiological setting. Short-term effects of protein and AAs seem mainly to target the regulation of the protein synthetic machinery of pancreatic acinar cells through mTORC1 activation. BCAAs, especially leucine, can stimulate mTORC1 and the pancreatic protein synthesis machinery of rats, independently of CCK and insulin.44 Other studies also have shown the role of leucine in the exocrine pancreas of rats,45 pigs,46 and ruminants.47
Protein synthesis is an acutely regulated process, involving translation of mRNA attached to ribosomes (polysome complexes) into protein.48, 49, 50 The initiation step involves the attachment of the mRNA to the ribosome and translation continues with the elongation of the polypeptide chain. Because ribosomal attachment can restart before peptide synthesis is completed this results in the formation of polysomes.50,51 Polysomal fractionation analysis shows that mRNAs are being translated actively into proteins.52,53 When the protein synthesis mechanisms are truncated, they can induce an accumulation of unfolded (or misfolded) proteins in the endoplasmic reticulum (ER), causing ER stress, and triggering the unfolded protein response.54,55
BCAAs have been shown to stimulate mTORC1 through an unknown rapamycin-insensitive pathway.56,57 Growth factors and AAs action converge on mTORC1, stimulating the mRNA binding step of translation initiation.58,59 mTORC1 regulates the phosphorylation of the translational repressor factor 4E (eIF4E) binding protein 1 (4E-BP1), the 70-kilodalton ribosomal protein S6 kinase (S6K),50,60 ribosomal proteins, and elongation factors.49
Our earlier studies showed that a short-term meal stimulated pancreatic protein synthesis at the translational level, with the activation of Protein Kinase-B (Akt) and mTORC1 downstream pathways, as well as the formation of the eIF4F complex,61 and that the protein elongation process is regulated by CCK in pancreatic acini.49 Because of the importance of protein and AA availability to the pancreas, we also studied the effects of BCAAs in the regulation of digestive enzyme synthesis, and leucine (Leu) was the one that had a more significant effect.44 We therefore now studied the impact that short-term dietary protein and a specific AA (Leu) deficiency could have on pancreas physiology, and the synthesis of pancreatic digestive enzymes. Protein was removed from one of the experimental diets, and in another group only the BCAA leuci234ne was removed from an isocaloric AA-based diet. The study involved overnight fasting before refeeding for 2 hours. Our findings show that both the lack of protein or only leucine in the diet inhibit total pancreatic protein synthesis and mRNA attachment to the polysomal fraction by different mechanisms, with different effects on mTORC1.
Results
Effects of Dietary Protein or Leucine Deficiency on Pancreatic Protein Synthesis
To show that dietary protein is important in the stimulation of pancreatic protein synthesis, mice were fasted for 16 hours and refed either a standard diet with 20% protein (20% Prot) or a protein-free diet (0% Prot) for 2 hours. Total pancreatic protein synthesis was analyzed by the flooding-dose technique. In addition, because we have shown that BCAAs, in particular leucine, stimulate pancreatic protein synthetic machinery,44 we analyzed whether the removal of only this specific amino acid from the diet would affect the regulation of pancreatic protein synthesis. The 2-hour refeeding time point was chosen because this is the time when maximal stimulation of pancreatic protein synthesis can be achieved by feeding fasted mice.61 The results showed that refeeding mice with the 20% protein control diet (20% Prot) increased total protein synthesis to 156.3% ± 14%, compared with the fasted group (Figure 1); the L-AA defined diet (AA) increased it to 138.7% ± 11%; the protein-free (0% Prot) and leucine-free (No Leu) diets induced a strong inhibition of total protein synthesis, to 57.6% and 41.3%, respectively, compared with the fasted group (Figure 1). Therefore, dietary protein and the BCAA leucine are required for the stimulation of total pancreatic protein synthesis after a meal.
Figure 1.
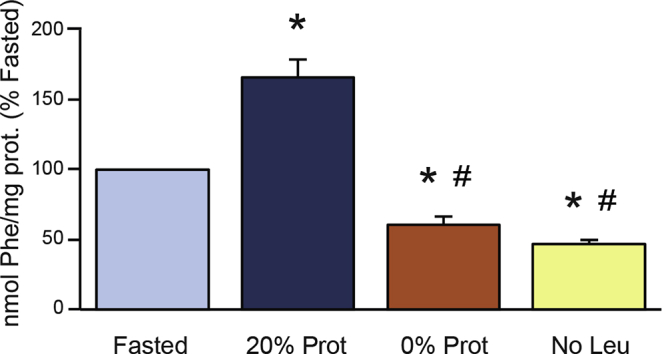
Effect of the different feeding treatments on total pancreatic protein synthesis, analyzed using the flooding-dose technique, and expressed as nanomoles of Phenylalanine incorporated into milligrams of pancreatic protein. Mice were fasted for 16 hours, and refed with 3 experimental diets: control diet (20% Protein), protein-deficient diet (0% Prot), and leucine-deficient diet (No Leu). ∗P < .05 vs control fasted group. #P < .05 vs control refed group.
Effects of Dietary Protein or Leucine Deficiency on Pancreatic Polysomal Profiles
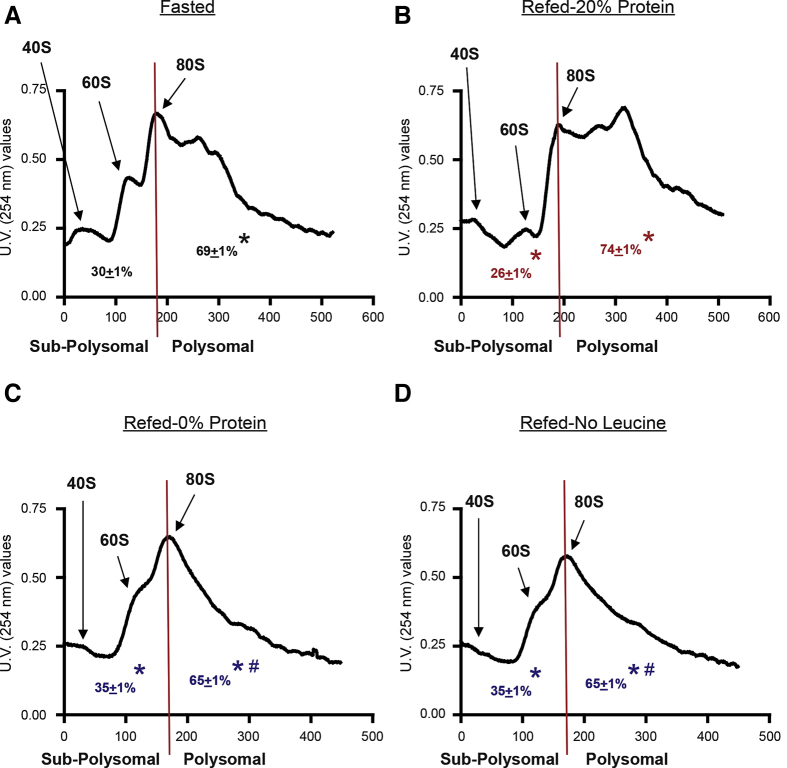
To study whether the effect of protein and leucine deficiency on the diet affected polysomal aggregation in the pancreas, polysomal profiling analysis was performed on pancreatic homogenates. The control refed group (20% Prot) showed a significant increase in the polysomal fraction (ie, more actively translated mRNAs into protein) than the fasted group (Figure 2A and B). This was reflected in the analysis of the area under the curve of the polysomal fractions (74.1% refed, compared with 69.1% in the fasted group) (Figure 2A and B). The group refed with the AA diet had a similar profile and area under the curve as the control diet with 20% protein (data not shown). The groups refed with a protein-free (0% Prot) or leucine-free (No Leu) diet, showed a very similar polysomal profile with a large reduction of the polysomal fraction, when compared with the polysomal fraction of the refed or fasted control groups (Figure 2C and D). These results were confirmed with the calculations of the area under the curve of their polysomal fraction (65.1% for both groups) (Figure 2). In addition, the analysis of the profile of the subpolysomal fraction clearly indicated the following: refeeding with a 20% protein diet reduced the amount of the free ribosomal subunits 40S and 60S (Figure 2) consistent with the increase in the 80S ribosomal unit, and the polysomal fraction; and the lack of protein or leucine in the diet increased the subpolysomal 40S and 60S peaks (area under the curve for both groups), compared with the refed control and fasted groups (Figure 2C and D). These data confirmed that total pancreatic protein synthesis was inhibited in the 0% Prot and No Leu groups, with a strong reduction of the mRNAs attached to the ribosomes for their translation into protein.
Figure 2.
Effect of the 4 different experimental treatments on pancreatic polysomal profiles. Each profile shows the peaks for the 40S and 60S ribosomal subunits, as well as for the whole ribosome (80S) and the different polysomes, in the polysomal fraction for representative fractionations. The graphs also show the calculations of the area under the curve for the subpolysomal and the polysomal fractions. The experimental groups were as follows: (A) Fasted; (B) refed (20% Prot); (C) refed protein-deficient diet (0% Prot); and (D) refed leucine-deficient diet (No Leu). N = 5–8/group. ∗P < .05 vs control group. #P < .05 vs control refed group.
Protein and Amino Acid Deficiency in the Diet Partially Inhibit the Akt/mTOR C1 Pathway
The mTOR C1 pathway is involved in the activation of pancreatic protein synthesis after feeding61 and after leucine administration.44 To assess the activation of the Akt/mTOR C1 pathway by dietary protein and leucine in this study, the phosphorylation (indicative of their activation) of Akt and the ribosomal protein S6 (downstream of mTORC1), as well as the formation of the eIF4F complex (also downstream of mTORC1). were analyzed. The No Protein feeding partially reduced the phosphorylation of Akt (Figure 3A) and S6 (Figure 3B). By contrast, the No Leu diet was not different from the 20% Prot in its effect on Akt and S6. Thus, there is a difference in the effect of protein and leucine deficiency. The phosphorylation of another effector downstream of mTORC1, 4E-BP1, was analyzed, and all the different diets stimulated its phosphorylation, mimicking the results for S6 phosphorylation.
Figure 3.
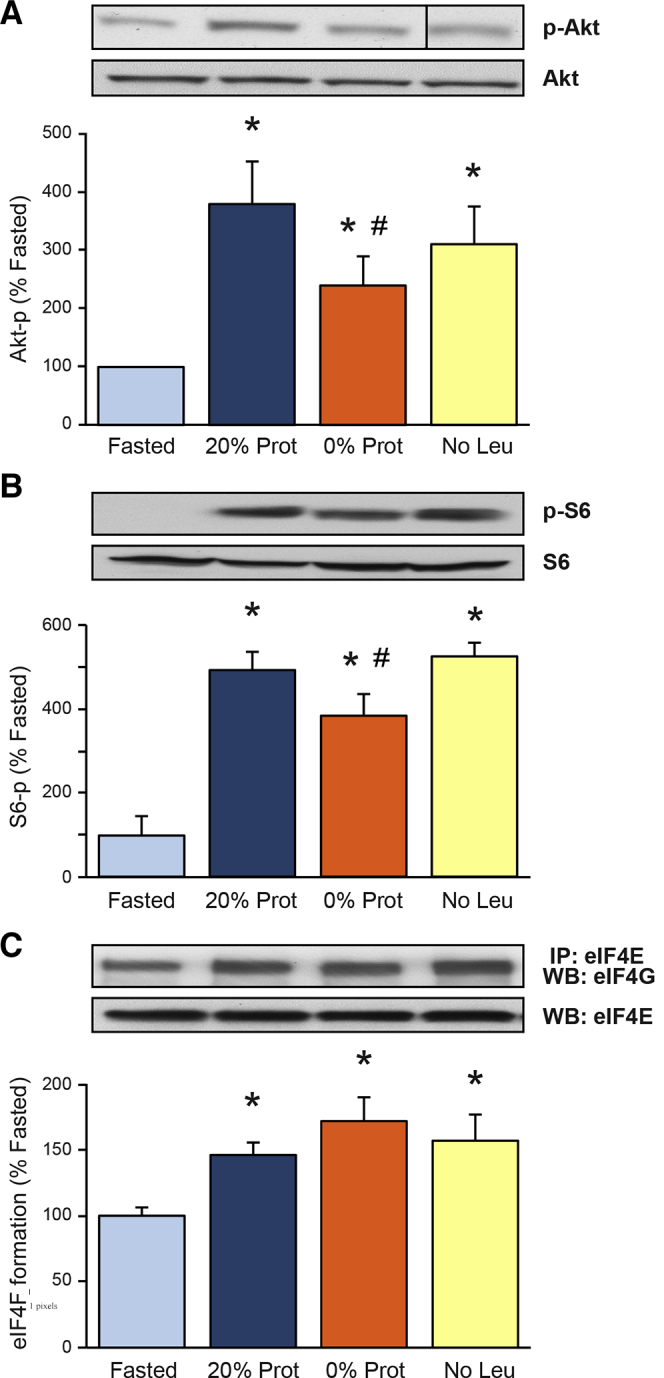
Effect of the different diets on (A) Protein Kinase-B and (B) ribosomal protein S6 phosphorylation, as well as on (C) eukaryotic initiation factor 4F (eIF4F) complex formation.Insets show representative immunoblots for phosphorylated Protein Kinase-B and S6, and loading controls in panels A and B. Results are expressed as a percentage of phosphorylation in the fasted group. (C) The eIF4F formation is expressed as a percentage of the co-immunoprecipitation of the initiation factor eIF4E with eIF4G compared with control values in the Fasted group. Inset shows representative immunoblot for eIF4G and eIF4E. ∗P < .05 vs control group. #P < .05 vs control refed group. (A) The representative image from the Western Blot signal for phosphorylated-Protein Kinase-B was taken at the same time, in the same gel and film, as the other ones, but it was not continuous to the 0% Prot, as shown in panel. It has been arranged to match the order of the groups in the graph. IP, immunoprecipitation.
The formation of the eIF4F complex is an important regulatory step in the protein synthesis mechanisms that catalyzes the recruitment and assembly of the capped mRNA with the 43S ribosomal complex. The formation of the eIF4F complex was analyzed by immunoprecipitation of eIF4G bound to eIF4E and was increased to more than 145% after feeding all the different diets compared with the fasted group (Figure 3C). eIF4E phosphorylation also was increased in response to all different diets, in correlation with the increase on eIF4F complex formation in the same groups. Therefore, the eIF4F complex formation can be activated independently of the dietary protein and AA content in a postprandial situation after a meal.
Amino Acid (Leucine) Deficiency in the Diet Increases eIF2α and General Control Nonderepressible 2 Phosphorylation
Pancreatic eIF2α phosphorylation is increased in stress situations such as acute pancreatitis in vivo and supraphysiological stimulation of isolated pancreatic acini in vitro.62,63 In this study, eIF2α phosphorylation was increased significantly only in the leucine-free diet (No Leu) group to 250% of the fasted group (Figure 4A). In concordance with this result, the described AA imbalance detector, the general control nonderepressible 2 (GCN2) kinase, also only was phosphorylated in the No Leu group to 160% of the fasted group (Figure 4B). We therefore conclude that the dietary AA imbalance caused by removing only leucine from the diet activates the ER stress mediator GCN2 and increases the phosphorylation of eIF2α, which, in turn, could be responsible for the inhibition of the total pancreatic protein synthesis and polysomal formation seen in the No Leu group (Figures 1 and 2).
Figure 4.
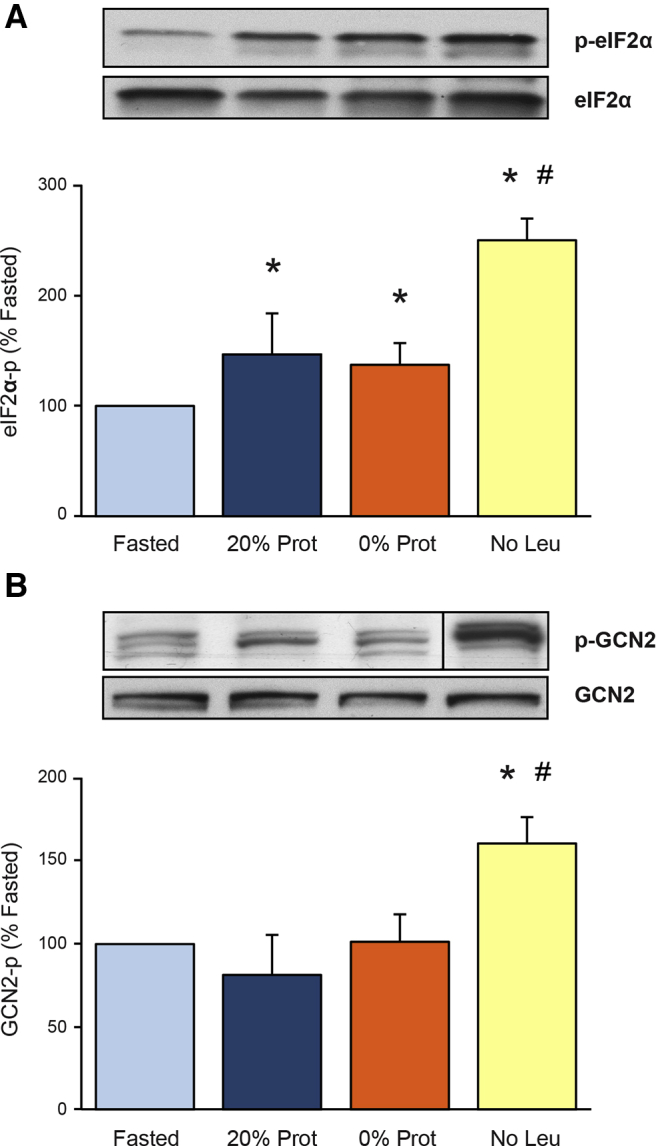
Effect of the different diets on the phosphorylation (activation) of (A) eukaryotic initiation factor 2α (eIF2α) and the (B) general control nonderepressible kinase 2 in mouse pancreas. Phosphorylation results are expressed as a percentage of the Fasted group. Insets show representative immunoblot for phosphorylated-eukaryotic initiation factor 2 and general control nonderepressible kinase 2, and their total amount in the cells. ∗P < .05 vs control fasted group. #P < .05 vs control refed group. (B) The representative image from the Western blot signal for phosphorylated-general control nonderepressible kinase 2 was taken at the same time, in the same gel and film, as the other ones, but it was not continuous to the 0% Prot, as shown in the figure. It has been arranged to match the order of the groups in the graph.
Protein and Leucine Deficiency in the Diet Inhibit eIF2B Activity
The guanine nucleotide exchange factor eIF2B is an important regulatory factor of protein synthesis, and its activity is inhibited by phosphorylated eIF2α.62 eIF2B activity was not enhanced (92% of the fasted group) by feeding the control diet (20% Prot), but was reduced significantly in both the 0% Prot (to 76.5%) and the No Leu (to 58.7% of the fasted group) groups (Figure 5A). Therefore, the lack of protein or the AA leucine in the diet significantly inhibited pancreatic eIF2B activity after 2-hour refeeding (Figure 5A). This result partially can explain the reduction in total protein synthesis and polysomal formation in the 0% Prot and No Leu groups.
Figure 5.
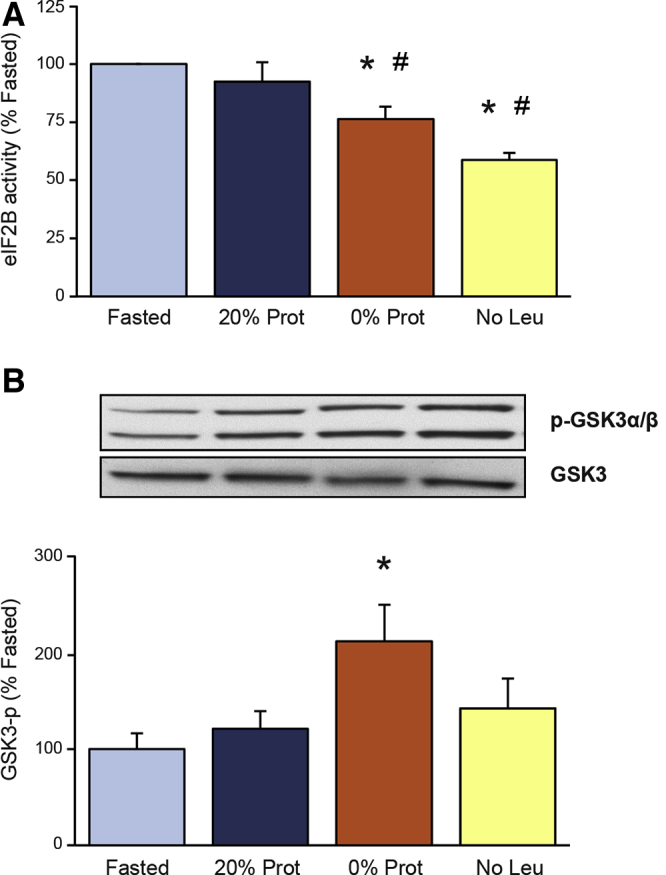
Effects of the different diets on (A) eukaryotic initiation factor 2B (eIF2B) activity and (B) glycogen synthase kinase-3 phosphorylation. Measurements of eIF2B activity are described in the Materials and Methods section. Both eIF2B activity and glycogen synthase kinase-3 phosphorylation (p-GSK3α/β) levels are expressed as a percentage of the fasted group. ∗P < .05 vs control fasted group. #P < .05 vs control refed group.
eIF2α phosphorylation was not increased in the 0% Prot group compared with the refed control group (20% Prot), and therefore could not account for the inhibition of eIF2B seen in this group. To study which other mechanisms could be involved with the inhibition of eIF2B activity, the phosphorylation status of glycogen synthase kinase-3 (GSK3) (eIF2B activator) also was analyzed. GSK3 usually is dephosphorylated when active and phosphorylation of its Ser 21/9 residues inhibits its activity. In this study, GSK3 phosphorylation was increased significantly in the 0% Prot group to 177.3% ± 34% of the fasted group (Figure 5B). This enhanced phosphorylation is associated with GSK3 inhibition and therefore could not be responsible for the inhibition of eIF2B activity in the same group. Therefore, eIF2B activity in the 0% Prot group has to be inhibited through a different mechanism.
EAA and Nonessential AA Concentrations in Plasma and Pancreas After Refeeding
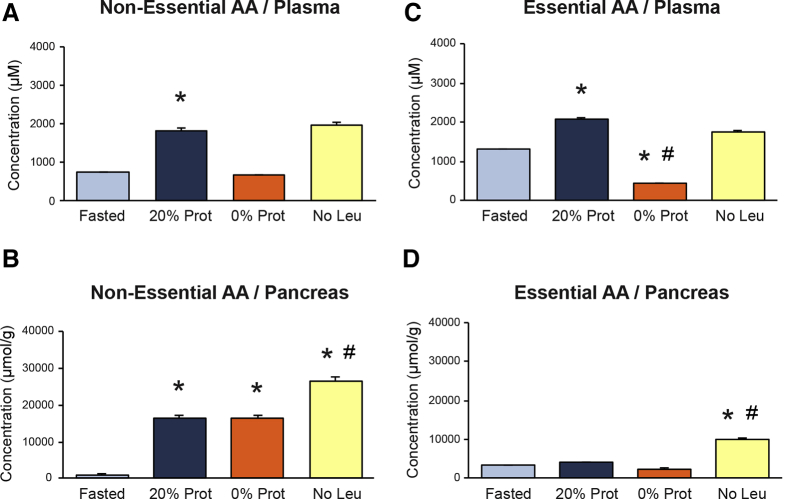
AA concentrations in plasma and the pancreas were analyzed to document any metabolic change resulting from the administration of the different diets. We measured the amount of 18 AAs in all the experimental groups, and analyzed whether there was a difference between EAAs and nonessential AAs (NEAAs) in the pancreas and plasma. The fasted group showed low levels of both types of AAs in plasma and pancreas, which doubled in the refed control group with 20% of protein, except for the EAAs in the pancreas, which showed no change, compared with the fasted group (Figure 6). The group with the 0% Prot diet showed a marked reduction (approximately half of the fasted group) of circulating EAAs and NEAAs in plasma (Figure 6A and B), but did not show this decrease in the pancreas (Figure 6C and D). The AA levels in the pancreas of the 0% Prot group were the same as the 20% Prot group, indicating that the pancreas captures, and/or retains, a similar amount of AAs in the postprandial situation, independently of the amount being fed. This also could indicate that these AAs must come from an internal body pool (not from food), and that they accumulate inside the acinar cell because they are not used for protein synthesis and might even keep being recruited to the pancreas by another postprandial stimulatory effect. The plasma AA levels in the No Leu group followed a pattern similar to the 20% Prot group (Figure 6A and B). In the pancreas of the same group (No Leu), the amount of both EAAs and NEAAs was higher than in any other group, indicating that the pancreas was capturing and retaining a larger amount of AAs without decreasing their plasma levels. These AAs also might come from other body sources and contribute to an AA imbalance situation inside the acinar cells (Figure 6C and D).
Figure 6.
Effect of the different diets on the concentration of essential amino acids (AAs) and nonessential AAs in (A and B) plasma and in (C and D) pancreas, respectively. Plasma AA concentrations are expressed in micromolar units. Pancreas AA concentrations are expressed as micromoles of AA per gram of tissue. ∗P < .05 vs control fasted group. #P < .05 vs control refed group
The amount of NEAAs inside the pancreas (compared with the total amount of AAs), was higher (>65%) than the amount of EAAs; reaching a maximum (87%) in the 0% Prot group. The amount of EAAs inside the pancreas was lower than 35% in all groups, and was very low (approximately 13%) in the 0% Prot group, most likely because of the lack of EAAs coming from this diet. These results indicate that most of the AAs in the pancreas were NEAAs and had to come from internal sources. NEAAs most likely are needed as building blocks for protein synthesis and not for activating the process.
The levels of all individual AAs in the plasma and in the pancreas are listed on Tables 1 and 2, respectively. Refeeding a balanced diet (20% Prot) increased the plasma concentration of most of the AAs compared with the fasted group, except for Cys, Gly, Iso, Leu, and Trp. Removing protein from the diet (0% Prot) caused a decrease in the plasma concentration of most AAs, except for Cys. After removing only leucine (No Leu) from the diet, many of them increased (Ala, Asp, Glu, Gly, Iso, Phe, Ser, Thr, Trp, Tyr, and Val), did not change significantly (Cys, His, Pro, Ser, Trp, and Tyr), although some were reduced significantly (Asn, Leu, Lys, and Met), compared with the 20% Prot group (Table 1).
Table 1.
Plasma Concentrations of Different Amino Acids After 2 Hours of Refeeding Different Diets
| Amino acid | Different diets |
|||
|---|---|---|---|---|
| Fasted | Refed (20% Prot) | Refed (0% Prot) | Refed (No-Leu) | |
| Essential | ||||
| Histidine | 61.0 ± 3.0 | 91.7 ± 7.3a | 50.9 ± 1.5b | 83.9 ± 4.0a,c |
| Lysine | 287.6 ± 24.1 | 513.3 ± 54.1a | 148.8 ± 4.2a,b | 354.2 ± 27.9b,c |
| Methionine | 40.3 ± 9.8 | 126.2 ± 12.0a | 19.6 ± 3.3a,b | 99.2 ± 5.0b,c |
| Phenylalanine | 86.3 ± 5.4 | 109.7 ± 10.5a | 38.7 ± 1.5a,b | 96.6 ± 4.4c |
| Threonine | 113.1 ± 18.1 | 266.1 ± 27.6a | 45.4 ± 7.6a,b | 275.8 ± 20.3a,c |
| Tryptophan | 41.1 ± 2.4 | 54.3 ± 3.5a | 33.3 ± 1.6b | 52.8 ± 3.3a,c |
| BCAA | ||||
| Leucine | 226.3 ± 16.7 | 254.9 ± 19.1 | 36.1 ± 1.5a,b | 24.5 ± 2.2a,b |
| Isoleucine | 138.7 ± 11.7 | 143.4 ± 10.6 | 15.0 ± 1.4a,b | 190.4 ± 15.8a,b,c |
| Valine | 320.2 ± 25.6 | 507.4 ± 34.1a | 45.3 ± 1.3a,b | 561.8 ± 22.6a,c |
| Nonessential | ||||
| Alanine | 255.5 ± 46.4 | 791.6 ± 79.6a | 308.4 ± 15.0a,b | 1006.4 ± 47.6a,b,c |
| Asparagine | 30.1 ± 5.7 | 63.9 ± 4.8a | 12.3 ± 3.7b | 20.8 ± 4.6b |
| Aspartic acid | 3.3 ± 1.5 | 4.5 ± 1.2 | 2.3 ± 1.0a,b | 10.4 ± 2.3a,b,c |
| Cysteine | 44.4 ± 1.8 | 44.9 ± 1.7 | 43.4 ± 1.8 | 44.6 ± 1.7 |
| Glutamic acid | 27.7 ± 1.8 | 53.2 ± 20.4 | 148.8 ± 4.2a,b | 67.3 ± 14.5c |
| Glycine | 196.6 ± 17.1 | 171.4 ± 18.8 | 138.0 ± 9.3a,b | 191.3 ± 13.7c |
| Proline | 90.3 ± 26.5 | 439.9 ± 54.3a | 58.9 ± 1.8b | 398.6 ± 28.2a,c |
| Serine | 71.7 ± 6.4 | 123.5 ± 20.6a | 45.5 ± 5.0a,b | 121.1 ± 9.6a,c |
| Tyrosine | 52.0 ± 7.2 | 136.0 ± 22.6a | 22.0 ± 2.3b | 123.2 ± 10.2a,c |
NOTE. Results are the average and SEM of 6 samples per group. Measurements are shown in micromolars.
BCAA, branched-chain amino acid; No-Leu, leucine-free diet; 20% Prot, standard diet with 20% protein; 0% Prot, protein-free diet.
P < .05 vs the control fasted group.
P < .05 vs the control refed group.
P < .05 vs the 0% Prot group.
Table 2.
Pancreas Content of Different Amino Acids After 2-Hour of Refeeding Different Diets
| Amino acid | Different diets |
|||
|---|---|---|---|---|
| Fasted | Refed (20% Prot) | Refed (0% Prot) | Refed (No-Leu) | |
| Essential | ||||
| Histidine | 643.8 ± 79.0 | 657.8 ± 97.9 | 665.8 ± 98.2 | 809.9 ± 119.3 |
| Lysine | 640.4 ± 25.1 | 705.9 ± 39.1 | 586.5 ± 55.2 | 1020.6 ± 96.7a,b,c |
| Methionine | 64.0 ± 11.4 | 284.7 ± 29.2a | 96.3 ± 14.2b | 530.8 ± 42.2a,b,c |
| Phenylalanine | 183.6 ± 29.5 | 251.8 ± 18.7 | 203.8 ± 18.8 | 667.9 ± 76.7a,b,c |
| Threonine | 399.8 ± 128.9 | 844.6 ± 66.9a | 417.6 ± 71.4b | 2033.1 ± 166.6a,b,c |
| Tryptophan | 207.8 ± 35.1 | 189.4 ± 41.6 | 223.0 ± 43.9 | 255.7 ± 119.3 |
| BCAA | ||||
| Leucine | 441.6 ± 68.2 | 338.5 ± 29.4 | 98.2 ± 15.2a,b | 53.0 ± 11.1a,b |
| Isoleucine | 225.3 ± 44.7 | 126.5 ± 12.5 | 34.7 ± 3.5 | 1147.9 ± 175.1a,b,c |
| Valine | 541.0 ± 117.2 | 781.1 ± 101.3 | 125.0 ± 11.9a,b | 3531.8 ± 216.6a,b,c |
| Nonessential | ||||
| Alanine | 1096.4 ± 178.7 | 4512.7 ± 253.0a | 3967.8 ± 323.0a | 8948.4 ± 579.7a,b,c |
| Asparagine | 225.6 ± 19.1 | 218.1 ± 13.0 | 299.2 ± 30.1 | 360.3 ± 27.5a,b |
| Aspartic acid | 417.7 ± 82.0 | 766.9 ± 73.5a | 451.8 ± 51.0b | 911.3 ± 99.3a,c |
| Cysteine | 48.4 ± 0.2 | 48.7 ± 0.3 | 47.3 ± 0.0 | 48.5 ± 0.1 |
| Glutamic acid | 2446.3 ± 168 | 4050.5 ± 255.3a | 2383.6 ± 228.5b | 3971.6 ± 406.2a,c |
| Glycine | 3820.6 ± 221.3 | 3331.7 ± 463.6 | 7462.1 ± 949.4a,b | 7263.8 ± 803.6a,b |
| Proline | 417.0 ± 57.6 | 2724.7 ± 308.4a | 1023.0 ± 115.9b | 3174.7 ± 377.3a,c |
| Serine | 359.8 ± 66.6 | 412.4 ± 52.1 | 535.7 ± 53.6 | 744.7 ± 87.7a,b,c |
| Tyrosine | 127.9 ± 41.0 | 464.8 ± 64.6a | 242.3 ± 28.2b | 1101.1 ± 101.5a,b,c |
NOTE. Results are the average and SEM of 6 samples per group. Measurements are shown in micromoles per gram.
BCAA, branched-chain amino acid; No-Leu, leucine-free diet; 20% Prot, standard diet with 20% protein; 0% Prot, protein-free diet.
P < .05 vs the control fasted group.
P < .05 vs the control refed group.
P < .05 vs the 0% Prot group.
In the pancreas, the levels of all AAs in the fasted group were higher than in the plasma, except for Cys, Glu, and Met. Refeeding a balanced diet (20% Prot) increased the pancreas content of most of them, except for Asn, Cys, His, Iso, Leu, Lys, Ser, and Trp. In the 0% Prot group there was a decrease in the pancreas content of most AAs, except for Asn, His, Phe, and Trp. Gly and Ser levels, on the contrary, increased significantly. In the No Leu group, there was no significant change in Cys and Glu levels, but the majority of them increased to levels higher than the refed 20% Prot group. As expected, leucine levels were very low in this group (Table 2).
Together, these results indicate that the ingestion of the 20% Prot diet increased the concentration of most circulating AAs in plasma and pancreas, presumably from the diet, thus making them available as a substrate for tissue or organ protein synthesis. The protein-free diet (0% Prot) significantly reduced their plasma levels, but increased the concentration of the NEAAs in the pancreas. The AA analysis in the No Leu group clearly shows an AA imbalance in plasma and pancreas that very likely could cause the stimulation of the ER stress mediators eIF2α and GCN2 (Figure 4), and the inhibition of pancreatic protein synthesis seen in this group (Figures 1 and 2). More studies need to be performed to determine the transport of the different AAs to pancreatic acinar cells, and their role on pancreatic digestive enzyme synthesis and pancreas physiology.
The Effect of Dietary Protein on Plasma Glucose and Insulin Levels
To assess whether metabolic changes were induced by feeding the different diets, plasma glucose and insulin were analyzed. Plasma glucose levels increased to normal postprandial levels (between 150 and 250 mg/dL in all groups) (Figure 7A). Plasma insulin levels were increased significantly after refeeding control or protein-free diets compared with basal (Figure 7B), but in the No Leu group it was reduced significantly compared with the control 20% Prot group (from 163 to 30 μU/mL). These reduced insulin levels could contribute to the reduction in protein synthesis in the No Leu group, but it does not appear to act through reduction of mTORC1 (Figure 3).
Figure 7.
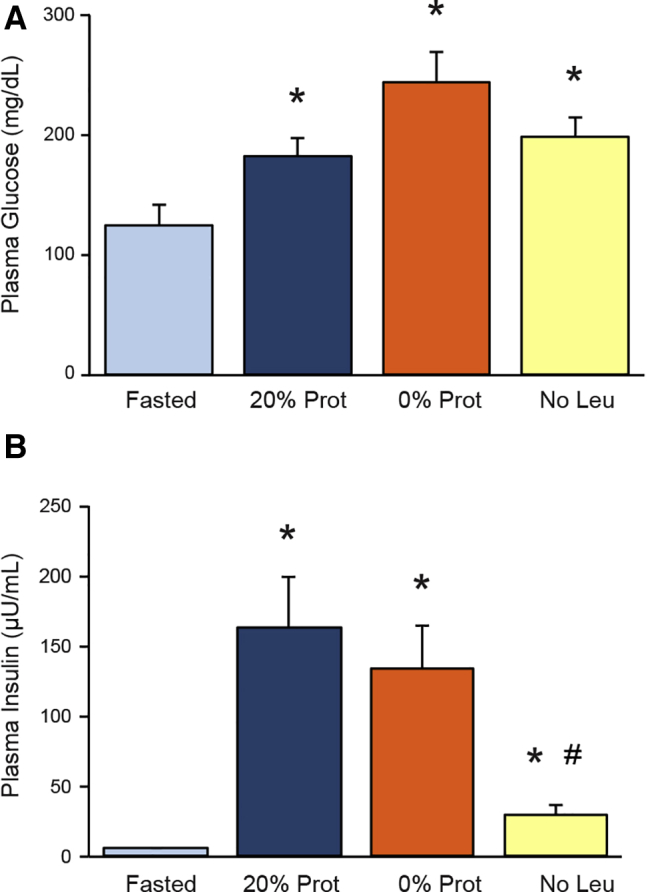
Effects of the different diets on (A) glucose and (B) insulin levels in plasma. Glucose levels are expressed in milligrams per deciliter, and insulin concentration in microunits per milliliter. ∗P < .05 vs control fasted group. #P < .05 vs control refed group.
Discussion
Dietary protein and AAs, especially the EAAs,6 are indispensable components of human and animal nutrition and health, as part of a protein-containing diet, and as supplements, because they are involved in many physiological processes. AAs, taken as supplements, improve general well-being, athletic performance, stimulate muscle protein synthesis,64, 65, 66 and play an important role in medical nutrition.1 In excess, however, they can lead to metabolic problems involving the brain, liver, muscle, kidneys, and/or the endocrine and exocrine pancreas.67, 68, 69 Their continued deficiency in the diet causes severe malnutrition, affecting growth in young individuals, as well as many other effects in human and animal metabolism.2,70,71 In the case of the exocrine pancreas, its high protein turnover makes this organ very vulnerable to protein-calorie malnutrition.37 The kwashiorkor syndrome affects the pancreas by reducing its size and overall pancreatic secretion.7,34,37,38 This reduction of pancreas size, over a period of several days, has been linked to an mTORC1-dependent mechanism in mice.38 mTORC1 also is involved in the short-term stimulation of pancreatic protein synthesis in response to eating a balanced meal,61 CCK stimulation,72 and by acute administration of BCAAs, more specifically, leucine, to mice and rats,44 dairy goats,73 and calves.74
Protein-free diets have a very relevant clinical impact32 because AAs (especially leucine) also act as nutrient signals and physiologic regulators on several tissues.29,75 Their mechanisms of action on protein synthesis in the pancreas are not completely understood. We therefore studied the effects of protein (AAs) and leucine in short-term pancreatic physiology by feeding mice a protein- or leucine-deficient diet, after an overnight fast, and studied the responses to these dietary deficiencies at the cellular, molecular, and physiological levels. This was performed by analyzing the effects on mTORC1 and pancreatic digestive enzyme synthesis after a meal because of its relevance for optimal digestion of nutrients in the gastrointestinal tract that can affect whole-body nutritional status and changes in overall well-being.
In many cell types, including pancreatic acinar cells,48,61,72 the stimulation of protein synthesis has been associated with the stimulation of mTORC1.76 mTORC1 coordinates several upstream signals including growth factors, intracellular amino acid availability, and energy status to regulate protein synthesis, autophagy, and cell growth,76 and is implicated in many human diseases including cancer, epilepsy, obesity, and diabetes.77 The ribosomal protein S6K and the translational repressor 4E-BP1 are mTORC1 downstream effectors involved in the regulation of protein synthesis.48,61,76 It has been shown that AAs (especially leucine, arginine, and methionine) can regulate mTORC1 activity, and thus protein synthesis in several cell types.78, 79, 80 Somewhat surprisingly, but perhaps because of the short time scale, in our study the experimental groups without protein (0% Prot) and leucine (No Leu) inhibited protein synthesis but did not cause a major inhibitory effect on mTORC1 activity (Figure 3). Only the 0% Prot group showed a partial reduction of Akt and ribosomal protein S6 phosphorylation, which partially could account for the inhibition of total protein synthesis seen in this group (Figure 1), but did not affect the formation of the eIF4F complex (Figure 3C). The mechanisms regulated downstream of S6K and the ribosomal protein S681 therefore could be responsible in part for the inhibition of total pancreatic protein synthesis (Figure 1) and polysomal formation (Figure 2) seen in the 0% Prot group. In the absence of leucine in the diet, mTORC1 activity was not affected, and thus it cannot be responsible for the inhibition of total protein synthesis and polysomal aggregation seen in the No Leu group (Figures 1 and 2).
Because leucine has been described as an AA that is able to affect several metabolic processes, and it is known that AA depletion reduces translation by a mechanism involving phosphorylation of eIF2α,82,83 we have compared the effects that dietary protein deficiency can cause to the exocrine pancreas protein synthetic machinery with the effects caused by removing only leucine from the diet. In our study, both groups caused a similar inhibition of total pancreatic protein synthesis (Figure 1), compared with the control group fed standard chow with 20% protein content.61 These results were correlated strongly with a reduction in the amount of ribosomes engaged on the mRNA translation process into protein (polysomes) (Figure 2), similarly to what occurs in hepatocytes84 and in other systems.85 So far, these results indicate that protein (AAs) and leucine deficiency in the diet inhibit overall pancreatic protein synthesis, hence, pancreatic digestive enzyme synthesis, and that this inhibition goes to the same extent in both groups, despite the differences in the AA composition of the diets. These results are in concordance with what has been described in another study, in which feeding a protein-deficient diet showed a reduction in the content of pancreatic digestive enzymes in blood.7 The lack of protein or certain AAs in the diet also can affect the synthesis of specific proteins in other tissues, as in the case of albumin in the liver,86 the myofibrillar protein in the muscle,87 or casein in mammary epithelial cells of dairy cows.88 Leucine, among other actions, can be a postprandial effector of the digestive system. For instance, leucine stimulates insulin secretion,89 and, at high concentrations, it has been linked to a reduction of food intake, and impaired cognitive outcome, among other general toxic effects.90, 91, 92 Clinically, persistent low levels of leucine also can cause decreased appetite, lethargy, poor growth, weight and hair loss, skin rashes, and desquamation.93 Leucine-free diets can be prescribed to patients with Maple syrup urine disease to counteract their high levels of leucine in plasma.92 When there is a dietary restriction of certain AAs, this AA imbalance situation (seen in Figure 6 and Table 2) triggers a stress response that activates the GCN2 kinase and phosphorylates eIF2α on Ser-51,94 and, in the brain, leads to a reduction of food intake.95 eIF2α phosphorylation has been described as a brake to protein synthesis.96 In our study, removing leucine from the diet caused the activation of GCN2, seen by an increase on its phosphorylation (Figure 4B), and increased eIF2α phosphorylation (Figure 4A), that most likely would account for the inhibition of total protein synthesis and the initiation of protein translation that we see in Figures 1 and 2.48,62,97 This phosphorylation reduces the levels of the active form of eIF2 (eIF2-guanosine triphosphate) that binds the initiator methionyl–transfer RNA (with the AUG codon) (Met–transfer RNAi) to the 40S ribosomal subunit and forms the ternary complex that starts translation, and inhibits the activity of the guanine nucleotide exchange factor eIF2B62,98,99; inhibition that we also see in our results (Figure 5A). eIF2B activity also can be regulated by GSK3.48,62,96,100 GSK3 phosphorylates the ε subunit of eIF2B (eIF2Bε) on Ser-540, inhibiting it.101 When GSK3 gets phosphorylated becomes inactive.96,98 This way, a stimulatory signal by insulin, for example, would phosphorylate GSK3, rendering it inactive, meaning that eIF2Bε would not get phosphorylated and be active.101 Based on these premises, our results indicate that the inhibition of eIF2B activity that we see in the No Leu group (Figure 5A) also could be explained by the reduction of GSK3 phosphorylation (Figure 5B), in addition to the phosphorylation of eIF2α.
As mentioned earlier, leucine stimulates insulin secretion, and because we have shown that insulin stimulates pancreatic protein synthesis,102 it also can be argued that the inhibition of pancreatic protein synthesis seen by removing leucine from the diet also could be the result of a reduction of postprandial insulin release (Figure 7B). Unpublished results from our laboratory have indicated that very low insulin levels during experimental diabetes inhibit pancreatic digestive enzyme synthesis and reduce Akt, S6, and eIF2α phosphorylation after a 2-hour refeeding compared with control refed mice with standard chow (20% Prot). Pancreatic acinar cell insulin receptor conditional knockout mice with normal plasma insulin levels but no insulin signaling to pancreatic acinar cells show a decrease on Akt and S6 phosphorylation, but an increase on eIF2α phosphorylation after a 2-hour normal chow refeeding (20% Prot).102 This discordance on the effects of no insulin signal to acinar cells on eIF2α warrants continued study. In summary, our own results on this topic indicate that insulin is involved in the stimulation of protein synthesis in the exocrine pancreas through the Akt/mTORC1 pathway, and the lack of insulin signaling during diabetes can lead to pancreatic insufficiency. The results from the current study indicate that insulin levels in the No Leu refeeding group are higher than the ones during diabetes, and they most likely contribute to stimulation (rather than to inhibition) of the synthetic machinery of the exocrine pancreas. In this case, a reduction on mTORC1 pathway stimulation also would be expected,103 but this was not observed (Figure 3). We therefore conclude that the inhibition of pancreatic protein synthesis seen in the No Leu group most likely is owing to the activation of GCN2 and phosphorylation of eIF2α, induced by accumulation of most of the amino acids in the pancreas, which creates an amino acid imbalance94 (Figure 6, and Table 2), and by the inhibition of GSK3 (Figure 5B) similar to other studies performed in the liver.104,105
Removing all the AAs from the diet (0% Prot Group) inhibited eIF2B activity in the pancreas (Figure 5A), but this reduction has to be induced by a mechanism other than eIF2α phosphorylation or GSK3 dephosphorylation. It could be argued that glucose or insulin levels could account for this inhibition, but these parameters do not change in the 0% Prot group (Figure 7), making this option less likely to happen. In this case, the inhibition of protein synthesis also could be explained by the lack of the building blocks for making more protein in plasma, specifically methionine (Tables 1 and 2), because it can be the main limiting one to start all translation processes, but it also can act through a mechanism independent of the Met–transfer RNA charging, the activation of a transcriptionally regulated mechanism via the activating transcription factor-4.106, 107, 108 It is worth noting that when all the AAs from the diet were removed, insulin levels were not affected (Figure 7). The amino acid imbalance created by the No Leu diet seems to be the main event that triggers the inhibition of digestive enzyme synthesis after feeding the No Leu diet, and not the lower insulin levels.
The analysis of the individual concentrations of AAs in plasma and pancreas shows their metabolic profiles and allows the assessment of their anabolic and catabolic states for each group. In addition, it suggests that changes in dietary composition induce selective adaptive responses in the transport of the different AAs to the pancreas, as has been described previously.109, 110, 111, 112, 113, 114, 115 Interestingly, glycine levels increase (approximately double) in the 0% Prot and No Leu groups (Table 2).
In the pancreas, EAA and NEAA levels are not reduced in the groups in which protein synthesis is inhibited, compared with the 20% Prot group (Figure 6C and D). In fact, in the No Leu group, it shows the highest amount of both types of AAs, compared with all the other groups (Figure 6C and D).
Glycine transport to the pancreas has been shown to be increased after refeeding because its levels seem to be limiting for AA incorporation into protein.116 In our study, the high Gly levels in the pancreas of these 2 groups (in which pancreatic protein synthesis is inhibited) are difficult to explain, but there could be associated mechanisms that activate its transport to the pancreas through its transporter (SLC6A5/GLYT1).117 Gly accumulation in the pancreas potentially could be stimulated by signals triggered by feeding, mobilizing it from other internal sources, and independent of the low amino acid content in the diet and plasma (Figure 6 and Tables 1 and 2), or simply could be the result of an accumulation of the AA because it is not being used for protein synthesis. Gly is a nonessential amino acid involved in the stimulation of insulin secretion,23,117 and its high levels (especially in the No Leu group) could indicate a need for a feedback mechanism that would increase postprandial insulin levels because of the lack of leucine in the diet (Figure 7B). However, again, it is very interesting to note that in the 0% Prot group, which is also without leucine, insulin levels are similar to the 20% Prot group ones.
Together, the results from the 0% Prot group indicate that the inhibition of protein synthesis is not likely owing to a reduction in the transport of EAAs and NEAAs to the pancreas (Figure 6C and D), despite the reduction in their plasma levels (Figure 6A and B). The inhibition of pancreatic protein synthesis also could be owing to a lack (or reduction) of CCK stimulation because it has been shown that protein and AAs stimulate CCK release from the inclusion-cells in the intestine,118 or to a reduction on cholinergic stimulation, which potentially would affect the mTORC1 pathway and the activation of eIF2B.
In the No Leu group, the levels of EAAs and NEAAs in plasma and pancreas are not reduced, they are actually higher in the pancreas and this cannot account for a reduction in their transport or availability for de novo synthesis. The AA imbalance, created by the lack of leucine, could trigger the stress response, which inhibits total protein synthesis through the activation of GCN2 and activating transcription factor-4, and GCN2, in turn, also could be responsible for the transcription of genes involved in amino acid transport and biosynthesis of NEAAs,119 partially explaining the increase of their overall amount in the pancreas. The source of these AAs most likely is coming from a stimulation of catabolic mechanisms known to be induced by low levels of leucine.93,120,121 Of relevance, in the No Leu group, catabolism seems to be activated because of the high levels of AA found in the pancreas.
In summary, our results show that a meal feeding without the ingestion of protein (AAs), or just lacking the AA leucine in the diet, acutely inhibits the pancreatic synthetic machinery through a partial inhibition of the mTORC1 pathway and the activation of the AA imbalance stress response through GCN2 and eIF2α, despite the availability of AAs in the pancreas owing to AA transport to acinar cells from internal sources, or intracellular accumulation owing to the inhibition of protein synthesis. This inhibition of protein synthesis would reduce pancreatic digestive enzyme synthesis, leading to pancreatic insufficiency, malnutrition, and other gastrointestinal effects that could impact overall body well-being and be very relevant in the clinical setting. Additional studies indeed are needed to determine the effects of the individual AAs on the regulation of pancreatic digestive enzyme synthesis and pancreatic insufficiency in combination with other physiological stimulants (CCK, insulin, muscarinic stimulation).
Materials and Methods
Materials
Phenylalanine (for the flooding-dose technique), TritonX-100, and SYBR Green were from Sigma Chemical Co (St. Louis, MO). Goat anti-rabbit and anti-mouse IgG conjugated to horseradish peroxidase and Enhanced Chemiluminiscence reagent were from Amersham Pharmacia Biotech (Piscataway, NJ); 10%, 15%, and 4%–20% Tris-HCl precast gels and broad range prestained sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) standard markers were from Bio-Rad (Hercules, CA); and nitrocellulose membranes were from Schleicher and Schuell (Keene, NH). Polyclonal rabbit antibodies to Akt, phospho-Akt (Ser-473), ribosomal protein S6 and phosphorylated S6 (Ser-240/244), GSK3 and phosphorylated GSK3 (Ser 21/9), eIF2 and phosphorylated eIF2 (Ser 51), and GCN2 and phosphorylated GCN2 (Thr 898) were from Cell Signaling (Beverly, MA). Mouse anti-eIF4E antibody was provided by Dr S. R. Kimball (Pennsylvania State University, Hershey, PA), and rabbit anti-eIF4G antibody was a gift from Dr R. E. Rhoads (Louisiana State University, Shreveport, LA). [3H]guanosine 5’-diphosphate (11.3 Ci/mmol) was from NEN Life Science Products, Inc (Boston, MA); the scintillation liquid Filtron-X was from National Diagnostics (Atlanta, GA); 25-mm nitrocellulose filter discs (HAWP) were from Millipore (Milford, MA). Protein A–linked Sepharose beads were obtained from Pierce Chemical (Rockford, IL). All experimental diets were from Dyets, Inc (Bethlehem, PA).
Animals and Experimental Design
Male ICR mice (Harlan Sprague-Dawley, Indianapolis, IN), 5–6 weeks old, were used, and the experiments were approved by the University of Michigan Committee on Use and Care of Animals. After arriving in the university facilities, mice were housed in specific pathogen-free areas, fed Purina 5001 chow (LabDiet, St. Louis, MO), and placed in a 12:12 hour light-dark cycle changing at 6:00 AM and 6:00 PM with free access to water. The day before the experiment, mice were separated (3 per cage) in different groups, as follows: (1) fed (fed ad libitum), (2) fasted (fasted for 16 h, starting at 5:00 PM), (3) refed control (20% Prot; AIN-93G), (4) refed 0% Prot (AIN-93G [Dyets, Inc, Bethlehem, PA] without protein; calorically replaced with carbohydrates), (5) refed AA (L-AA defined AIN-93G diet; the AA-based diet, control of the Leu-deficient diet), and (6) refed No Leu (L-AA defined AIN-93G without leucine). All the refed groups were fasted for 16 hours and refed the specific diets for 2 hours. The diets were isocaloric and contained 10% fat.
Evaluation of the Phosphorylation State of Akt, Ribosomal Protein S6, GSK3, eIF2, and GCN2
The phosphorylation state of Akt, ribosomal protein S6, GSK3, eIF2, and GCN2 was determined by the relative amount of the protein in their phosphorylated form, quantitated by protein immunoblot analysis using phospho-specific affinity-purified antibodies. Portions of the pancreas were homogenized in lysis buffer122 and aliquots of pancreatic homogenates or acinar lysates (from in vitro studies) were resolved in a 10% SDS-PAGE gel, transferred to nitrocellulose, followed by Western blot analysis using anti-phospho Akt (Ser 473), anti-phospho S6 (Ser 240/244), anti-phospho GSK3 (Ser 21/9), anti-phospho eIF2α (Ser 51), anti-phospho GCN2 (Thr 898) antibodies, and detected by Enhanced Chemiluminiscence. To ensure that total protein was not changed by the experimental treatments the same samples were run in parallel gels and Western blot with polyclonal antibodies to total Akt, S6, GSK3, eIF2α, and GCN2 at 1:500.
Measurement of Pancreatic Protein Synthesis
Pancreatic protein synthesis was determined using the flooding-dose technique as we have described previously for mice.61,63 Briefly, 0.4 μCi/g of L-[3H]Phe together with unlabeled L-Phe (1.5 μmol/g) were injected in the peritoneal cavity. Ten minutes after L-[3H]Phe administration, mice were euthanized and the pancreas rapidly was removed and frozen in liquid nitrogen. Frozen pancreas subsequently was homogenized in 10 vol of 0.6 N perchloric acid (PCA) and processed as described previously.61 L-Phe was measured by high-performance liquid chromatography and protein synthesis was expressed as nanomoles of L-Phe per milligram of protein.
Association of Eukaryotic Initiation Factors eIF4G and eIF4E and Formation of the eIF4F Complex
To analyze the formation of the eIF4F complex, we determined the association of eIF4E with eIF4G by quantitating the amount of eIF4G bound to immunoprecipitated eIF4E using specific anti-eIF4E antibody, as previously described.44,63 Briefly, pancreatic samples were homogenized in 2 mL of buffer, centrifuged at 10,000 × g for 10 minutes at 4°C, and the supernatant, containing microsomes and soluble protein, was used to precipitate eIF4E from 0.5 mg of protein. The immunoprecipitates were resolved on 4%–20% gradient gel SDS-PAGE followed by Western blot analysis using anti-eIF4G antibody (1:2000).44,63
Polysomal Fractionation
The polysomal fractionation technique85,123 used sucrose gradient separation of pancreas homogenates in a BIOCOMP gradient fractionator (Fredericton, Canada). Briefly, whole pancreases were homogenized in 10 volumes of buffer containing 40 mmol/L HEPES (pH 7.5), 100 mmol/L KCl, and 50 mmol/L MgCl. The homogenate then was centrifuged at 8800 × g for 15 minutes at 4°C. Nine volumes of pancreas homogenate were mixed with 1 volume of detergent mix (10% Triton X-100, 10% sodium deoxycholate) and loaded onto linear 10%–50% sucrose density gradients. The gradients were centrifuged at 39,000 rpm in a Beckman (Chaska, MN) SW 41 rotor for 120 minutes at 4°C and fractions were collected in the BIOCOMP gradient fractionator with UV absorption at 254 nm continuously recorded. On the basis of the absorption obtained the area corresponding to the 40, 60, and 80 S ribosomal units were designated subpolysomal. These fractions usually contain protein, RNA, free ribosomal subunits, and monosomes. The last fraction corresponding to disomes, trisomes, and polysomes of increasing number bound to (and therefore, actively translating) mRNA was designated polysomal.
Analysis of eIF2B Activity
Determination of eIF2B activity in pancreatic tissue was performed as described previously by measuring the rate of exchange of [3H]Guanosine Diphosphate (GDP) present in an exogenous eIF2-[3H]GDP complex for free nonradiolabeled GDP in pancreatic tissue samples.62,124 The guanine nucleotide exchange activity was measured as a decrease in eIF2-[3H]GDP complex bound to nitrocellulose filters and expressed as nanomoles of GDP exchanged per minute per milligram of acinar protein or as a percentage of the control group.62,63
Analysis of Plasma and Pancreas Individual AA Content by Gas Chromatography
Plasma samples were precipitated 1:1 with 1.2 N PCA, and frozen pancreas samples were homogenized with 10 volumes of 0.6 N PCA. Both precipitates were centrifuged at 3000 rpm for 10 minutes and the supernatants were used for AA analysis using an EZ:faast kit for free AA analysis via gas chromatography–mass spectrometry from Phenomenex (Torrance, CA).125 Briefly, before analysis, an internal standard (norvaline) was added to 100 μL of plasma or tissue extract to be analyzed. AAs in the sample were concentrated by cation exchange solid-phase extraction at an acidic pH, were washed with a 2-propanol/water solution, and then were eluted using an alkaline solution of sodium hydroxide in water mixed with 2-propanol. The eluate was derivatized by the addition of propyl chloroformate, followed by a 2-minute reaction period at room temperature. The AA derivatives were extracted into an organic layer containing chloroform and isooctane. This layer was collected and the solvent evaporated, and the residue was reconstituted in solvent for gas chromatography–mass spectrometry analysis. Separation and detection was performed using a Zebron ZB-AAA column from Phenomenex on a 6890 Gas Chromatography with a 5973 Mass Selective Detector. Individual AAs were quantitated based on calibration curves generated by injection of AA standard mixtures derivatized in parallel with the samples. This analysis was performed at the Metabolomics Core of the Biomedical Research Core Facilities (Office of Research, University of Michigan Medical School).
Measurement of Plasma Insulin
The plasma insulin concentration was determined by a standard procedure at the Michigan Diabetes Research and Training Center with double-antibody radioimmunoassay using a 125I-Human insulin tracer and a guinea pig anti-rat insulin first antibody from Linco Research (St. Charles, MO), a rat insulin standard from Novo Research Institute (Bagsvaerd, Denmark), and a sheep anti-guinea pig gamma globulin secondary antibody, with 3% of Polyethylene Glycol 6000 added, developed in the same center. The limit of sensitivity for the assay is 1 μU/mL.
Statistical Analysis
Data from mouse experiments are represented as means and SEM and were obtained from 3 to 4 different experiments, with 4–6 animals per group studied in each, unless otherwise indicated in the figure legend. Statistical analysis was performed by 1-way analysis of variance and differences with a P value less than .05 were considered significant.
All authors had access to the study data and reviewed and approved the final manuscript.
CRediT Authorship Contributions
Maria Dolors Sans Gili (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Investigation: Lead; Methodology: Lead; Validation: Lead; Writing – original draft: Lead);Stephen J Crozier (Investigation: Supporting; Writing – review & editing: Supporting);Nancy L Vogel (Investigation: Supporting; Resources: Supporting; Writing – review & editing: Supporting);Louis G D’Alecy (Methodology: Supporting; Resources: Supporting; Writing – review & editing: Supporting);John A Williams (Conceptualization: Supporting; Funding acquisition: Lead; Project administration: Lead; Supervision: Supporting; Validation: Supporting; Writing – review & editing: Supporting).
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Supported by National Institutes of Health grant DK-059578 (J.A.W.).
References
- 1.Karau A., Grayson I. Amino acids in human and animal nutrition. Adv Biochem Eng Biotechnol. 2014;143:189–228. doi: 10.1007/10_2014_269. [DOI] [PubMed] [Google Scholar]
- 2.Freeman H.J., Kim Y.S., Sleisenger M.H. Protein digestion and absorption in man. Normal mechanisms and protein-energy malnutrition. Am J Med. 1979;67:1030–1036. doi: 10.1016/0002-9343(79)90645-4. [DOI] [PubMed] [Google Scholar]
- 3.Silk D.B., Grimble G.K., Rees R.G. Protein digestion and amino acid and peptide absorption. Proc Nutr Soc. 1985;44:63–72. doi: 10.1079/pns19850011. [DOI] [PubMed] [Google Scholar]
- 4.Massey K.A., Blakeslee C.H., Pitkow H.S. A review of physiological and metabolic effects of essential amino acids. Amino Acids. 1998;14:271–300. doi: 10.1007/BF01318848. [DOI] [PubMed] [Google Scholar]
- 5.Santos C.S., Nascimento F.E.L. Isolated branched-chain amino acid intake and muscle protein synthesis in humans: a biochemical review. Einstein (Sao Paulo) 2019;17 doi: 10.31744/einstein_journal/2019RB4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou Y., Wu G. Nutritionally essential amino acids. Adv Nutr. 2018;9:849–851. doi: 10.1093/advances/nmy054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Hodhod M.A., Nassar M.F., Hetta O.A., Gomaa S.M. Pancreatic size in protein energy malnutrition: a predictor of nutritional recovery. Eur J Clin Nutr. 2005;59:467–473. doi: 10.1038/sj.ejcn.1602053. [DOI] [PubMed] [Google Scholar]
- 8.Williams C.D., Oxon B.M., Lond H. Kwashiorkor: a nutritional disease of children associated with a maize diet. 1935. Bull World Health Organ. 2003;81:912–913. [PMC free article] [PubMed] [Google Scholar]
- 9.Butorov E.V. Influence of L-lysine amino acid on the HIV-1 RNA replication in vitro. Antivir Chem Chemother. 2015;24:39–46. doi: 10.1177/2040206614566582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rieck B., Degiacomi G., Zimmermann M., Cascioferro A., Boldrin F., Lazar-Adler N.R., Bottrill A.R., le Chevalier F., Frigui W., Bellinzoni M., Lisa M.N., Alzari P.M., Nguyen L., Brosch R., Sauer U., Manganelli R., O’Hare H.M. PknG senses amino acid availability to control metabolism and virulence of Mycobacterium tuberculosis. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez-Arnau F.M., Fonfria-Vivas R., Cauli O. beneficial effects of leucine supplementation on criteria for sarcopenia: a systematic review. Nutrients. 2019;11:2504. doi: 10.3390/nu11102504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarnopolsky M.A., Nilsson M.I. Nutrition and exercise in Pompe disease. Ann Transl Med. 2019;7:282. doi: 10.21037/atm.2019.05.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Millward D.J. Identifying recommended dietary allowances for protein and amino acids: a critique of the 2007 WHO/FAO/UNU report. Br J Nutr. 2012;108(Suppl 2):S3–S21. doi: 10.1017/S0007114512002450. [DOI] [PubMed] [Google Scholar]
- 14.Millward D.J. Knowledge gained from studies of leucine consumption in animals and humans. J Nutr. 2012;142:2212S–2219S. doi: 10.3945/jn.111.157370. [DOI] [PubMed] [Google Scholar]
- 15.Dudrick S.J. History of parenteral nutrition. J Am Coll Nutr. 2009;28:243–251. doi: 10.1080/07315724.2009.10719778. [DOI] [PubMed] [Google Scholar]
- 16.O’Morain C., Segal A.W., Levi A.J. Elemental diet as primary treatment of acute Crohn’s disease: a controlled trial. Br Med J (Clin Res Ed) 1984;288:1859–1862. doi: 10.1136/bmj.288.6434.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schrader H., Menge B.A., Belyaev O., Uhl W., Schmidt W.E., Meier J.J. Amino acid malnutrition in patients with chronic pancreatitis and pancreatic carcinoma. Pancreas. 2009;38:416–421. doi: 10.1097/MPA.0b013e318194fc7a. [DOI] [PubMed] [Google Scholar]
- 18.Noguchi Y., Zhang Q.W., Sugimoto T., Furuhata Y., Sakai R., Mori M., Takahashi M., Kimura T. Network analysis of plasma and tissue amino acids and the generation of an amino index for potential diagnostic use. Am J Clin Nutr. 2006;83:513S–519S. doi: 10.1093/ajcn/83.2.513S. [DOI] [PubMed] [Google Scholar]
- 19.Adrych K., Smoczynski M., Stojek M., Sledzinski T., Slominska E., Goyke E., Smolenski R.T., Swierczynski J. Decreased serum essential and aromatic amino acids in patients with chronic pancreatitis. World J Gastroenterol. 2010;16:4422–4427. doi: 10.3748/wjg.v16.i35.4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bi X., Henry C.J. Plasma-free amino acid profiles are predictors of cancer and diabetes development. Nutr Diabetes. 2017;7:e249. doi: 10.1038/nutd.2016.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broer S., Broer A. Amino acid homeostasis and signalling in mammalian cells and organisms. Biochem J. 2017;474:1935–1963. doi: 10.1042/BCJ20160822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang-Sattler R., Yu Z., Herder C., Messias A.C., Floegel A., He Y., Heim K., Campillos M., Holzapfel C., Thorand B., Grallert H., Xu T., Bader E., Huth C., Mittelstrass K., Doring A., Meisinger C., Gieger C., Prehn C., Roemisch-Margl W., Carstensen M., Xie L., Yamanaka-Okumura H., Xing G., Ceglarek U., Thiery J., Giani G., Lickert H., Lin X., Li Y., Boeing H., Joost H.G., de Angelis M.H., Rathmann W., Suhre K., Prokisch H., Peters A., Meitinger T., Roden M., Wichmann H.E., Pischon T., Adamski J., Illig T. Novel biomarkers for pre-diabetes identified by metabolomics. Mol Syst Biol. 2012;8:615. doi: 10.1038/msb.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Floegel A., Stefan N., Yu Z., Muhlenbruch K., Drogan D., Joost H.G., Fritsche A., Haring H.U., Hrabe de Angelis M., Peters A., Roden M., Prehn C., Wang-Sattler R., Illig T., Schulze M.B., Adamski J., Boeing H., Pischon T. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes. 2013;62:639–648. doi: 10.2337/db12-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshizawa F. New therapeutic strategy for amino acid medicine: notable functions of branched chain amino acids as biological regulators. J Pharmacol Sci. 2012;118:149–155. doi: 10.1254/jphs.11r05fm. [DOI] [PubMed] [Google Scholar]
- 25.Holecek M. Branched-chain amino acid supplementation in treatment of liver cirrhosis: updated views on how to attenuate their harmful effects on cataplerosis and ammonia formation. Nutrition. 2017;41:80–85. doi: 10.1016/j.nut.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Gluud L.L., Dam G., Les I., Cordoba J., Marchesini G., Borre M., Aagaard N.K., Vilstrup H. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2015;9:CD001939. doi: 10.1002/14651858.CD001939.pub3. [DOI] [PubMed] [Google Scholar]
- 27.Deleaval P., Luaire B., Laffay P., Jambut-Cadon D., Stauss-Grabo M., Canaud B., Chazot C. Short-term effects of branched-chain amino acids-enriched dialysis fluid on branched-chain amino acids plasma level and mass balance: a randomized cross-over study. J Ren Nutr. 2020;30:61–68. doi: 10.1053/j.jrn.2019.03.079. [DOI] [PubMed] [Google Scholar]
- 28.Wagenmakers A.J., Coakley J.H., Edwards R.H. Metabolism of branched-chain amino acids and ammonia during exercise: clues from McArdle’s disease. Int J Sports Med. 1990;11(Suppl 2):S101–S113. doi: 10.1055/s-2007-1024861. [DOI] [PubMed] [Google Scholar]
- 29.Zhang S., Zeng X., Ren M., Mao X., Qiao S. Novel metabolic and physiological functions of branched chain amino acids: a review. J Anim Sci Biotechnol. 2017;8:10. doi: 10.1186/s40104-016-0139-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao F., Wang C., Yin H., Yu J., Chen S., Fang J., Guo F. Leucine deprivation inhibits proliferation and induces apoptosis of human breast cancer cells via fatty acid synthase. Oncotarget. 2016;7:63679–63689. doi: 10.18632/oncotarget.11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei S., Zhao J., Wang S., Huang M., Wang Y., Chen Y. Intermittent administration of a leucine-deprived diet is able to intervene in type 2 diabetes in db/db mice. Heliyon. 2018;4 doi: 10.1016/j.heliyon.2018.e00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fontana L., Cummings N.E., Arriola Apelo S.I., Neuman J.C., Kasza I., Schmidt B.A., Cava E., Spelta F., Tosti V., Syed F.A., Baar E.L., Veronese N., Cottrell S.E., Fenske R.J., Bertozzi B., Brar H.K., Pietka T., Bullock A.D., Figenshau R.S., Andriole G.L., Merrins M.J., Alexander C.M., Kimple M.E., Lamming D.W. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep. 2016;16:520–530. doi: 10.1016/j.celrep.2016.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu J.W., Badaloo A., Wilson L., Taylor-Bryan C., Chambers B., Reid M., Forrester T., Jahoor F. Dietary supplementation with aromatic amino acids increases protein synthesis in children with severe acute malnutrition. J Nutr. 2014;144:660–666. doi: 10.3945/jn.113.184523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pitchumoni C.S. Pancreas in primary malnutrition disorders. Am J Clin Nutr. 1973;26:374–379. doi: 10.1093/ajcn/26.3.374. [DOI] [PubMed] [Google Scholar]
- 35.Henley E.C., Taylor J.R., Obukosia S.D. The importance of dietary protein in human health: combating protein deficiency in sub-Saharan Africa through transgenic biofortified sorghum. Adv Food Nutr Res. 2010;60:21–52. doi: 10.1016/S1043-4526(10)60002-2. [DOI] [PubMed] [Google Scholar]
- 36.Moore S.E., Collinson A.C., N’Gom P.T., Prentice A.M. Maternal malnutrition and the risk of infection in later life. Nestle Nutr Workshop Ser Pediatr Program. 2005;55:153–164. doi: 10.1159/000082600. discussion 164–167. [DOI] [PubMed] [Google Scholar]
- 37.Descos L., Duclieu J., Minaire Y. Exocrine pancreatic insufficiency and primitive malnutrition. Digestion. 1977;15:90–95. doi: 10.1159/000197988. [DOI] [PubMed] [Google Scholar]
- 38.Crozier S.J., D’Alecy L.G., Ernst S.A., Ginsburg L.E., Williams J.A. Molecular mechanisms of pancreatic dysfunction induced by protein malnutrition. Gastroenterology. 2009;137:1093–1101. doi: 10.1053/j.gastro.2009.04.058. 1101 e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crozier S.J., Sans M.D., Wang J.Y., Lentz S.I., Ernst S.A., Williams J.A. CCK-independent mTORC1 activation during dietary protein-induced exocrine pancreas growth. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1154–G1163. doi: 10.1152/ajpgi.00445.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hara H., Hashimoto N., Akatsuka N., Kasai T. Induction of pancreatic trypsin by dietary amino acids in rats: four trypsinogen isozymes and cholecystokinin messenger RNA. J Nutr Biochem. 2000;11:52–59. doi: 10.1016/s0955-2863(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 41.Hara H., Narakino H., Kiriyama S., Kasai T. Induction of pancreatic growth and proteases by feeding a high amino acid diet does not depend on cholecystokinin in rats. J Nutr. 1995;125:1143–1149. doi: 10.1093/jn/125.5.1143. [DOI] [PubMed] [Google Scholar]
- 42.Elghazi L., Blandino-Rosano M., Alejandro E., Cras-Meneur C., Bernal-Mizrachi E. Role of nutrients and mTOR signaling in the regulation of pancreatic progenitors development. Mol Metab. 2017;6:560–573. doi: 10.1016/j.molmet.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sans M.D., Williams J.A. Exocrine Pancreas Knowledge Base; Pancreapedia: 2017. The mTOR signaling pathway and regulation of pancreatic function. [DOI] [Google Scholar]
- 44.Sans M.D., Tashiro M., Vogel N.L., Kimball S.R., D’Alecy L.G., Williams J.A. Leucine activates pancreatic translational machinery in rats and mice through mTOR independently of CCK and insulin. J Nutr. 2006;136:1792–1799. doi: 10.1093/jn/136.7.1792. [DOI] [PubMed] [Google Scholar]
- 45.Hashimoto N., Hara H. Dietary amino acids promote pancreatic protease synthesis at the translation stage in rats. J Nutr. 2003;133:3052–3057. doi: 10.1093/jn/133.10.3052. [DOI] [PubMed] [Google Scholar]
- 46.Morales A., Buenabad L., Castillo G., Vazquez L., Espinoza S., Htoo J.K., Cervantes M. Dietary levels of protein and free amino acids affect pancreatic proteases activities, amino acids transporters expression and serum amino acid concentrations in starter pigs. J Anim Physiol Anim Nutr (Berl) 2017;101:723–732. doi: 10.1111/jpn.12515. [DOI] [PubMed] [Google Scholar]
- 47.Cao Y.C., Yang X.J., Guo L., Zheng C., Wang D.D., Cai C.J., Liu S.M., Yao J.H. Effects of dietary leucine and phenylalanine on pancreas development, enzyme activity, and relative gene expression in milk-fed Holstein dairy calves. J Dairy Sci. 2018;101:4235–4244. doi: 10.3168/jds.2017-13987. [DOI] [PubMed] [Google Scholar]
- 48.Sans M.D., Williams J.A. Translational control of protein synthesis in pancreatic acinar cells. Int J Gastrointest Cancer. 2002;31:107–115. doi: 10.1385/IJGC:31:1-3:107. [DOI] [PubMed] [Google Scholar]
- 49.Sans M.D., Xie Q., Williams J.A. Regulation of translation elongation and phosphorylation of eEF2 in rat pancreatic acini. Biochem Biophys Res Commun. 2004;319:144–151. doi: 10.1016/j.bbrc.2004.04.164. [DOI] [PubMed] [Google Scholar]
- 50.Roux P.P., Topisirovic I. Signaling pathways involved in the regulation of mRNA translation. Mol Cell Biol. 2018;38 doi: 10.1128/MCB.00070-18. e00070-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shirokikh N.E., Preiss T. Translation initiation by cap-dependent ribosome recruitment: recent insights and open questions. Wiley Interdiscip Rev RNA. 2018;9:e1473. doi: 10.1002/wrna.1473. [DOI] [PubMed] [Google Scholar]
- 52.Chasse H., Boulben S., Costache V., Cormier P., Morales J. Analysis of translation using polysome profiling. Nucleic Acids Res. 2017;45:e15. doi: 10.1093/nar/gkw907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kudla M., Karginov F.V. Measuring mRNA translation by polysome profiling. Methods Mol Biol. 2016;1421:127–135. doi: 10.1007/978-1-4939-3591-8_11. [DOI] [PubMed] [Google Scholar]
- 54.Sicari D., Delaunay-Moisan A., Combettes L., Chevet E., Igbaria A. A guide to assessing endoplasmic reticulum homeostasis and stress in mammalian systems. FEBS J. 2020;287:27–42. doi: 10.1111/febs.15107. [DOI] [PubMed] [Google Scholar]
- 55.Lukas J., Pospech J., Oppermann C., Hund C., Iwanov K., Pantoom S., Petters J., Frech M., Seemann S., Thiel F.G., Modenbach J.M., Bolsmann R., de Freitas Chama L., Kraatz F., El-Hage F., Gronbach M., Klein A., Muller R., Salloch S., Weiss F.U., Simon P., Wagh P., Klemenz A., Kruger E., Mayerle J., Delcea M., Kragl U., Beller M., Rolfs A., Lerch M.M., Sendler M. Role of endoplasmic reticulum stress and protein misfolding in disorders of the liver and pancreas. Adv Med Sci. 2019;64:315–323. doi: 10.1016/j.advms.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 56.Lynch C.J., Patson B.J., Anthony J., Vaval A., Jefferson L.S., Vary T.C. Leucine is a direct-acting nutrient signal that regulates protein synthesis in adipose tissue. Am J Physiol Endocrinol Metab. 2002;283:E503–E513. doi: 10.1152/ajpendo.00084.2002. [DOI] [PubMed] [Google Scholar]
- 57.Tang H., Hornstein E., Stolovich M., Levy G., Livingstone M., Templeton D., Avruch J., Meyuhas O. Amino acid-induced translation of TOP mRNAs is fully dependent on phosphatidylinositol 3-kinase-mediated signaling, is partially inhibited by rapamycin, and is independent of S6K1 and rpS6 phosphorylation. Mol Cell Biol. 2001;21:8671–8683. doi: 10.1128/MCB.21.24.8671-8683.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Proud C.G. mTOR-mediated regulation of translation factors by amino acids. Biochem Biophys Res Commun. 2004;313:429–436. doi: 10.1016/j.bbrc.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 59.Xu G., Kwon G., Cruz W.S., Marshall C.A., McDaniel M.L. Metabolic regulation by leucine of translation initiation through the mTOR-signaling pathway by pancreatic beta-cells. Diabetes. 2001;50:353–360. doi: 10.2337/diabetes.50.2.353. [DOI] [PubMed] [Google Scholar]
- 60.Reiter A.K., Anthony T.G., Anthony J.C., Jefferson L.S., Kimball S.R. The mTOR signaling pathway mediates control of ribosomal protein mRNA translation in rat liver. Int J Biochem Cell Biol. 2004;36:2169–2179. doi: 10.1016/j.biocel.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 61.Sans M.D., Lee S.H., D’Alecy L.G., Williams J.A. Feeding activates protein synthesis in mouse pancreas at the translational level without increase in mRNA. Am J Physiol Gastrointest Liver Physiol. 2004;287:G667–G675. doi: 10.1152/ajpgi.00505.2003. [DOI] [PubMed] [Google Scholar]
- 62.Sans M.D., Kimball S.R., Williams J.A. Effect of CCK and intracellular calcium to regulate eIF2B and protein synthesis in rat pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2002;282:G267–G276. doi: 10.1152/ajpgi.00274.2001. [DOI] [PubMed] [Google Scholar]
- 63.Sans M.D., DiMagno M.J., D’Alecy L.G., Williams J.A. Caerulein-induced acute pancreatitis inhibits protein synthesis through effects on eIF2B and eIF4F. Am J Physiol Gastrointest Liver Physiol. 2003;285:G517–G528. doi: 10.1152/ajpgi.00540.2002. [DOI] [PubMed] [Google Scholar]
- 64.Phillips S.M. A brief review of critical processes in exercise-induced muscular hypertrophy. Sports Med. 2014;44(Suppl 1):S71–S77. doi: 10.1007/s40279-014-0152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McGlory C., van Vliet S., Stokes T., Mittendorfer B., Phillips S.M. The impact of exercise and nutrition on the regulation of skeletal muscle mass. J Physiol. 2019;597:1251–1258. doi: 10.1113/JP275443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cholewa J.M., Dardevet D., Lima-Soares F., de Araujo Pessoa K., Oliveira P.H., Dos Santos Pinho J.R., Nicastro H., Xia Z., Cabido C.E., Zanchi N.E. Dietary proteins and amino acids in the control of the muscle mass during immobilization and aging: role of the MPS response. Amino Acids. 2017;49:811–820. doi: 10.1007/s00726-017-2390-9. [DOI] [PubMed] [Google Scholar]
- 67.Galsgaard K.D., Winther-Sorensen M., Orskov C., Kissow H., Poulsen S.S., Vilstrup H., Prehn C., Adamski J., Jepsen S.L., Hartmann B., Hunt J., Charron M.J., Pedersen J., Wewer Albrechtsen N.J., Holst J.J. Disruption of glucagon receptor signaling causes hyperaminoacidemia exposing a possible liver-alpha-cell axis. Am J Physiol Endocrinol Metab. 2018;314:E93–E103. doi: 10.1152/ajpendo.00198.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gar C., Rottenkolber M., Prehn C., Adamski J., Seissler J., Lechner A. Serum and plasma amino acids as markers of prediabetes, insulin resistance, and incident diabetes. Crit Rev Clin Lab Sci. 2018;55:21–32. doi: 10.1080/10408363.2017.1414143. [DOI] [PubMed] [Google Scholar]
- 69.Morabito M.V., Berman D.E., Schneider R.T., Zhang Y., Leibel R.L., Small S.A. Hyperleucinemia causes hippocampal retromer deficiency linking diabetes to Alzheimer’s disease. Neurobiol Dis. 2014;65:188–192. doi: 10.1016/j.nbd.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nie C., He T., Zhang W., Zhang G., Ma X. Branched chain amino acids: beyond nutrition metabolism. Int J Mol Sci. 2018;19:954. doi: 10.3390/ijms19040954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Siddik M.A.B., Shin A.C. Recent progress on branched-chain amino acids in obesity, diabetes, and beyond. Endocrinol Metab (Seoul) 2019;34:234–246. doi: 10.3803/EnM.2019.34.3.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bragado M.J., Groblewski G.E., Williams J.A. Regulation of protein synthesis by cholecystokinin in rat pancreatic acini involves PHAS-I and the p70 S6 kinase pathway. Gastroenterology. 1998;115:733–742. doi: 10.1016/s0016-5085(98)70153-2. [DOI] [PubMed] [Google Scholar]
- 73.Cao Y., Liu K., Liu S., Guo L., Yao J., Cai C. Leucine regulates the exocrine function in pancreatic tissue of dairy goats in vitro. Biomed Res Int. 2019;2019:7521715. doi: 10.1155/2019/7521715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo L., Yao J.H., Zheng C., Tian H.B., Liu Y.L., Liu S.M., Cai C.J., Xu X.R., Cao Y.C. Leucine regulates alpha-amylase and trypsin synthesis in dairy calf pancreatic tissue in vitro via the mammalian target of rapamycin signalling pathway. Animal. 2019;13:1899–1906. doi: 10.1017/S1751731118003683. [DOI] [PubMed] [Google Scholar]
- 75.Kimball S.R., Jefferson L.S. Amino acids as regulators of gene expression. Nutr Metab (Lond) 2004;1:3. doi: 10.1186/1743-7075-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Condon K.J., Sabatini D.M. Nutrient regulation of mTORC1 at a glance. J Cell Sci. 2019;132:jcs222570. doi: 10.1242/jcs.222570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saxton R.A., Sabatini D.M. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhuang Y., Wang X.X., He J., He S., Yin Y. Recent advances in understanding of amino acid signaling to mTORC1 activation. Front Biosci (Landmark Ed) 2019;24:971–982. doi: 10.2741/4762. [DOI] [PubMed] [Google Scholar]
- 79.Yao Y., Jones E., Inoki K. Lysosomal regulation of mTORC1 by amino acids in mammalian cells. Biomolecules. 2017;7:51. doi: 10.3390/biom7030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jewell J.L., Russell R.C., Guan K.L. Amino acid signalling upstream of mTOR. Nat Rev Mol Cell Biol. 2013;14:133–139. doi: 10.1038/nrm3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meyuhas O. Ribosomal protein S6 phosphorylation: four decades of research. Int Rev Cell Mol Biol. 2015;320:41–73. doi: 10.1016/bs.ircmb.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 82.Kobayashi H., Borsheim E., Anthony T.G., Traber D.L., Badalamenti J., Kimball S.R., Jefferson L.S., Wolfe R.R. Reduced amino acid availability inhibits muscle protein synthesis and decreases activity of initiation factor eIF2B. Am J Physiol Endocrinol Metab. 2003;284:E488–E498. doi: 10.1152/ajpendo.00094.2002. [DOI] [PubMed] [Google Scholar]
- 83.Kimball S.R., Horetsky R.L., Jefferson L.S. Implication of eIF2B rather than eIF4E in the regulation of global protein synthesis by amino acids in L6 myoblasts. J Biol Chem. 1998;273:30945–30953. doi: 10.1074/jbc.273.47.30945. [DOI] [PubMed] [Google Scholar]
- 84.Everson W.V., Flaim K.E., Susco D.M., Kimball S.R., Jefferson L.S. Effect of amino acid deprivation on initiation of protein synthesis in rat hepatocytes. Am J Physiol. 1989;256:C18–C27. doi: 10.1152/ajpcell.1989.256.1.C18. [DOI] [PubMed] [Google Scholar]
- 85.Pringle E.S., McCormick C., Cheng Z. Polysome profiling analysis of mRNA and associated proteins engaged in translation. Curr Protoc Mol Biol. 2019;125:e79. doi: 10.1002/cpmb.79. [DOI] [PubMed] [Google Scholar]
- 86.Flaim K.E., Liao W.S., Peavy D.E., Taylor J.M., Jefferson L.S. The role of amino acids in the regulation of protein synthesis in perfused rat liver. II. Effects of amino acid deficiency on peptide chain initiation, polysomal aggregation, and distribution of albumin mRNA. J Biol Chem. 1982;257:2939–2946. [PubMed] [Google Scholar]
- 87.Sugawara T., Ito Y., Nishizawa N., Nagasawa T. Supplementation with dietary leucine to a protein-deficient diet suppresses myofibrillar protein degradation in rats. J Nutr Sci Vitaminol (Tokyo) 2007;53:552–555. doi: 10.3177/jnsv.53.552. [DOI] [PubMed] [Google Scholar]
- 88.Zhang M.C., Zhao S.G., Wang S.S., Luo C.C., Gao H.N., Zheng N., Wang J.Q. d-Glucose and amino acid deficiency inhibits casein synthesis through JAK2/STAT5 and AMPK/mTOR signaling pathways in mammary epithelial cells of dairy cows. J Dairy Sci. 2018;101:1737–1746. doi: 10.3168/jds.2017-12926. [DOI] [PubMed] [Google Scholar]
- 89.Yang J., Dolinger M., Ritaccio G., Mazurkiewicz J., Conti D., Zhu X., Huang Y. Leucine stimulates insulin secretion via down-regulation of surface expression of adrenergic alpha2A receptor through the mTOR (mammalian target of rapamycin) pathway: implication in new-onset diabetes in renal transplantation. J Biol Chem. 2012;287:24795–24806. doi: 10.1074/jbc.M112.344259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Laeger T., Reed S.D., Henagan T.M., Fernandez D.H., Taghavi M., Addington A., Munzberg H., Martin R.J., Hutson S.M., Morrison C.D. Leucine acts in the brain to suppress food intake but does not function as a physiological signal of low dietary protein. Am J Physiol Regul Integr Comp Physiol. 2014;307:R310–R320. doi: 10.1152/ajpregu.00116.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hoffmann B., Helbling C., Schadewaldt P., Wendel U. Impact of longitudinal plasma leucine levels on the intellectual outcome in patients with classic MSUD. Pediatr Res. 2006;59:17–20. doi: 10.1203/01.pdr.0000190571.60385.34. [DOI] [PubMed] [Google Scholar]
- 92.Yudkoff M., Daikhin Y., Nissim I., Horyn O., Luhovyy B., Luhovyy B., Lazarow A., Nissim I. Brain amino acid requirements and toxicity: the example of leucine. J Nutr. 2005;135:1531S–1538S. doi: 10.1093/jn/135.6.1531S. [DOI] [PubMed] [Google Scholar]
- 93.Wappner R.S., Gibson K.M. Disorders of leucine metabolism. In: Blau N., Leonard J., Hoffmann G.F., Clarke J.T.R., editors. Vol 1. Springer-Verlag; Berlin: 2006. pp. 59–79. (Physician’s guide to the treatment and follow-up of metabolic diseases). [Google Scholar]
- 94.Zhang P., McGrath B.C., Reinert J., Olsen D.S., Lei L., Gill S., Wek S.A., Vattem K.M., Wek R.C., Kimball S.R., Jefferson L.S., Cavener D.R. The GCN2 eIF2alpha kinase is required for adaptation to amino acid deprivation in mice. Mol Cell Biol. 2002;22:6681–6688. doi: 10.1128/MCB.22.19.6681-6688.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maurin A.C., Benani A., Lorsignol A., Brenachot X., Parry L., Carraro V., Guissard C., Averous J., Jousse C., Bruhat A., Chaveroux C., B’Chir W., Muranishi Y., Ron D., Penicaud L., Fafournoux P. Hypothalamic eIF2alpha signaling regulates food intake. Cell Rep. 2014;6:438–444. doi: 10.1016/j.celrep.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pavitt G.D. Regulation of translation initiation factor eIF2B at the hub of the integrated stress response. Wiley Interdiscip Rev RNA. 2018;9:e1491. doi: 10.1002/wrna.1491. [DOI] [PubMed] [Google Scholar]
- 97.Wek R.C. Role of eIF2alpha kinases in translational control and adaptation to cellular stress. Cold Spring Harb Perspect Biol. 2018;10:a032870. doi: 10.1101/cshperspect.a032870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kimball S.R., Fabian J.R., Pavitt G.D., Hinnebusch A.G., Jefferson L.S. Regulation of guanine nucleotide exchange through phosphorylation of eukaryotic initiation factor eIF2alpha. Role of the alpha- and delta-subunits of eiF2b. J Biol Chem. 1998;273:12841–12845. doi: 10.1074/jbc.273.21.12841. [DOI] [PubMed] [Google Scholar]
- 99.Welsh G.I., Stokes C.M., Wang X., Sakaue H., Ogawa W., Kasuga M., Proud C.G. Activation of translation initiation factor eIF2B by insulin requires phosphatidyl inositol 3-kinase. FEBS Lett. 1997;410:418–422. doi: 10.1016/s0014-5793(97)00579-6. [DOI] [PubMed] [Google Scholar]
- 100.Proud C.G. Regulation of mRNA translation. Essays Biochem. 2001;37:97–108. doi: 10.1042/bse0370097. [DOI] [PubMed] [Google Scholar]
- 101.Wang X., Proud C.G. A novel mechanism for the control of translation initiation by amino acids, mediated by phosphorylation of eukaryotic initiation factor 2B. Mol Cell Biol. 2008;28:1429–1442. doi: 10.1128/MCB.01512-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sans M.D., Amin R.K., Vogel N.L., D’Alecy L.G., Kahn C.R., Williams J.A. Specific deletion of insulin receptors on pancreatic acinar cells defines the insulin-acinar axis: implications for pancreatic insufficiency in diabetes. Gastroenterology. 2011;140 A-233. [Google Scholar]
- 103.Yoon M.S. The role of mammalian target of rapamycin (mTOR) in insulin signaling. Nutrients. 2017;9:1176. doi: 10.3390/nu9111176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chotechuang N., Azzout-Marniche D., Bos C., Chaumontet C., Gausseres N., Steiler T., Gaudichon C., Tome D. mTOR, AMPK, and GCN2 coordinate the adaptation of hepatic energy metabolic pathways in response to protein intake in the rat. Am J Physiol Endocrinol Metab. 2009;297:E1313–E1323. doi: 10.1152/ajpendo.91000.2008. [DOI] [PubMed] [Google Scholar]
- 105.Anthony T.G., McDaniel B.J., Byerley R.L., McGrath B.C., Cavener D.R., McNurlan M.A., Wek R.C. Preservation of liver protein synthesis during dietary leucine deprivation occurs at the expense of skeletal muscle mass in mice deleted for eIF2 kinase GCN2. J Biol Chem. 2004;279:36553–36561. doi: 10.1074/jbc.M404559200. [DOI] [PubMed] [Google Scholar]
- 106.Liu G.M., Hanigan M.D., Lin X.Y., Zhao K., Jiang F.G., White R.R., Wang Y., Hu Z.Y., Wang Z.H. Methionine, leucine, isoleucine, or threonine effects on mammary cell signaling and pup growth in lactating mice. J Dairy Sci. 2017;100:4038–4050. doi: 10.3168/jds.2016-11973. [DOI] [PubMed] [Google Scholar]
- 107.Metayer-Coustard S., Mameri H., Seiliez I., Crochet S., Crepieux P., Mercier Y., Geraert P.A., Tesseraud S. Methionine deprivation regulates the S6K1 pathway and protein synthesis in avian QM7 myoblasts without activating the GCN2/eIF2 alpha cascade. J Nutr. 2010;140:1539–1545. doi: 10.3945/jn.110.122663. [DOI] [PubMed] [Google Scholar]
- 108.Pettit A.P., Jonsson W.O., Bargoud A.R., Mirek E.T., Peelor F.F., 3rd, Wang Y., Gettys T.W., Kimball S.R., Miller B.F., Hamilton K.L., Wek R.C., Anthony T.G. Dietary methionine restriction regulates liver protein synthesis and gene expression independently of eukaryotic initiation factor 2 phosphorylation in mice. J Nutr. 2017;147:1031–1040. doi: 10.3945/jn.116.246710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mann G.E., Peran S. Basolateral amino acid transport systems in the perfused exocrine pancreas: sodium-dependency and kinetic interactions between influx and efflux mechanisms. Biochim Biophys Acta. 1986;858:263–274. doi: 10.1016/0005-2736(86)90331-7. [DOI] [PubMed] [Google Scholar]
- 110.Mann G.E., Smith S.A., Norman P.S., Emery P.W. Fasting, refeeding and diabetes modulate free amino acid concentrations in the rat exocrine pancreas: role of transstimulation in amino acid efflux. Pancreas. 1988;3:67–76. doi: 10.1097/00006676-198802000-00012. [DOI] [PubMed] [Google Scholar]
- 111.Mann G.E., Munoz M., Peran S. Fasting and refeeding modulate neutral amino acid transport activity in the basolateral membrane of the rat exocrine pancreatic epithelium: fasting-induced insulin insensitivity. Biochim Biophys Acta. 1986;862:119–126. doi: 10.1016/0005-2736(86)90475-x. [DOI] [PubMed] [Google Scholar]
- 112.Fukushima D., Doi H., Fukushima K., Katsura K., Ogawa N., Sekiguchi S., Fujimori K., Sato A., Satomi S., Ishida K., Fukushima K. Glutamate exocrine dynamics augmented by plasma glutamine and the distribution of amino acid transporters of the rat pancreas. J Physiol Pharmacol. 2010;61:265–271. [PubMed] [Google Scholar]
- 113.Sweiry J.H., Munoz M., Mann G.E. Cis-inhibition and trans-stimulation of cationic amino acid transport in the perfused rat pancreas. Am J Physiol. 1991;261:C506–C514. doi: 10.1152/ajpcell.1991.261.3.C506. [DOI] [PubMed] [Google Scholar]
- 114.Munoz M., Emery P.W., Peran S., Mann G.E. Dietary regulation of amino acid transport activity in the exocrine pancreatic epithelium. Biochim Biophys Acta. 1988;945:273–280. doi: 10.1016/0005-2736(88)90489-0. [DOI] [PubMed] [Google Scholar]
- 115.Morisset J.A., Webster P.D. Effects of fasting and feeding on protein synthesis by the rat pancreas. J Clin Invest. 1972;51:1–8. doi: 10.1172/JCI106779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cheneval J.P., Johnstone R.M. Changes in amino acid transport in the rat pancreas in response to fasting and feeding. Biochim Biophys Acta. 1976;433:630–637. doi: 10.1016/0005-2736(76)90286-8. [DOI] [PubMed] [Google Scholar]
- 117.Yan-Do R., Duong E., Manning Fox J.E., Dai X., Suzuki K., Khan S., Bautista A., Ferdaoussi M., Lyon J., Wu X., Cheley S., MacDonald P.E., Braun M. A glycine-insulin autocrine feedback loop enhances insulin secretion from human beta-cells and is impaired in type 2 diabetes. Diabetes. 2016;65:2311–2321. doi: 10.2337/db15-1272. [DOI] [PubMed] [Google Scholar]
- 118.Wang Y., Chandra R., Samsa L.A., Gooch B., Fee B.E., Cook J.M., Vigna S.R., Grant A.O., Liddle R.A. Amino acids stimulate cholecystokinin release through the Ca2+-sensing receptor. Am J Physiol Gastrointest Liver Physiol. 2011;300:G528–G537. doi: 10.1152/ajpgi.00387.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Castilho B.A., Shanmugam R., Silva R.C., Ramesh R., Himme B.M., Sattlegger E. Keeping the eIF2 alpha kinase Gcn2 in check. Biochim Biophys Acta. 2014;1843:1948–1968. doi: 10.1016/j.bbamcr.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 120.DeArmond P.D., Dietzen D.J., Pyle-Eilola A.L. Amino acids disorders. In: Garg U., Smith L.D., editors. Biomarkers in inborn errors of metabolism: clinical aspects and laboratory determination. 1st ed. Elsevier, Inc; 2017. pp. 25–64. [Google Scholar]
- 121.Workeneh B., Mitch W.E. The influence of kidney disease on protein and amino acid metabolism. In: Kopple J.D., Massry S.G., Kalantar-Zadeh K., editors. 3rd ed. Vol 1. Elsevier Inc; 2013. pp. 1–16. (Nutritional management of renal disease). [Google Scholar]
- 122.Bragado M.J., Groblewski G.E., Williams J.A. p70s6k is activated by CCK in rat pancreatic acini. Am J Physiol. 1997;273:C101–C109. doi: 10.1152/ajpcell.1997.273.1.C101. [DOI] [PubMed] [Google Scholar]
- 123.Anthony T.G., Reiter A.K., Anthony J.C., Kimball S.R., Jefferson L.S. Deficiency of dietary EAA preferentially inhibits mRNA translation of ribosomal proteins in liver of meal-fed rats. Am J Physiol Endocrinol Metab. 2001;281:E430–E439. doi: 10.1152/ajpendo.2001.281.3.E430. [DOI] [PubMed] [Google Scholar]
- 124.Kimball S.R., Everson W.V., Flaim K.E., Jefferson L.S. Initiation of protein synthesis in a cell-free system prepared from rat hepatocytes. Am J Physiol. 1989;256:C28–C34. doi: 10.1152/ajpcell.1989.256.1.C28. [DOI] [PubMed] [Google Scholar]
- 125.Kugler F., Graneis S., Schreiter P.P., Stintzing F.C., Carle R. Determination of free amino compounds in betalainic fruits and vegetables by gas chromatography with flame ionization and mass spectrometric detection. J Agric Food Chem. 2006;54:4311–4318. doi: 10.1021/jf060245g. [DOI] [PubMed] [Google Scholar]