Abstract
Nitrate and nitrite supplement deficient endogenous nitric oxide (NO) formation. While these anions may generate NO, recent studies have shown that circulating nitrite levels do not necessarily correlate with the antihypertensive effect of oral nitrite administration and that formation of nitrosylated species (RXNO) in the stomach is critically involved in this effect. This study examined the possibility that RXNO formed in the stomach after oral nitrite administration promotes target protein nitrosylation in the vasculature, inhibits vasoconstriction and the hypertensive responses to angiotensin II. Our results show that oral nitrite treatment enhances circulating RXNO concentrations (measured by ozone-based chemiluminescence methods), increases aortic protein kinase C (PKC) nitrosylation (measured by resin-assisted capture SNO-RAC method), and reduces both angiotensin II-induced vasoconstriction (isolated aortic ring preparation) and hypertensive (in vivo invasive blood pressure measurements) effects implicating PKC nitrosylation as a key mechanism for the responses to oral nitrite. Treatment of rats with the nitrosylating compound S-nitrosoglutathione (GSNO) resulted in the same effects described for oral nitrite. Moreover, partial depletion of thiols with buthionine sulfoximine prevented PKC nitrosylation and the blood pressure effects of oral nitrite. Further confirming a role for PKC nitrosylation, preincubation of aortas with GSNO attenuated the responses to both angiotensin II and to a direct PKC activator, and this effect was attenuated by ascorbate (reverses GSNO-induced nitrosylation). GSNO-induced nitrosylation also inhibited the increases in Ca2+ mobilization in angiotensin II-stimulated HEK293T cells expressing angiotensin type 1 receptor. Together, these results are consistent with the idea that PKC nitrosylation in the vasculature may underlie oral nitrite treatment-induced reduction in the vascular and hypertensive responses to angiotensin II.
Keywords: Nitrite, S-nitrosylation, Angiotensin II, PKC
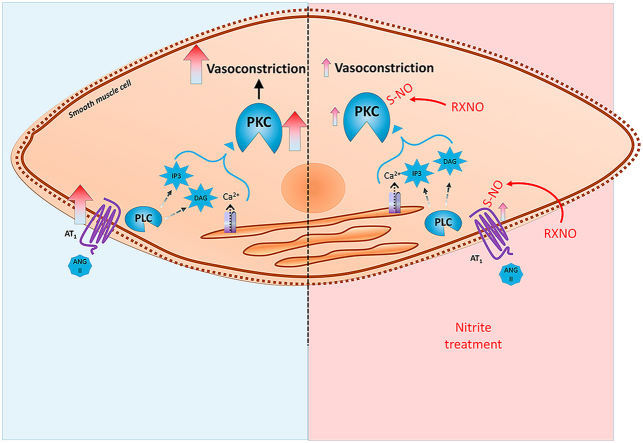
Graphical abstract
Highlights
-
•
Oral nitrite treatment exerts antihypertensive effects.
-
•
The mechanisms explaining such effects are not entirely known.
-
•
Oral nitrite treatment increases circulating concentrations of nitrosylating species.
-
•
Vascular PKC nitrosylation attenuates the vascular responses to angiotensin II.
1. Introduction
The cardiovascular effects of nitrate and nitrite have been extensively validated in the last decades and include antihypertensive responses, which were shown both in animal models and in humans [[1], [2], [3], [4]]. Most studies suggest that such antihypertensive effect is directly associated with increased systemic formation of nitric oxide (NO) [5,6]. In fact, nitrite may generate NO by enzymatic activity of xanthine oxide reductase [7,8] or by nitrite reductase activity of deoxyhemoglobin [5]. In addition, nitrite may form NO in the acid environment of the stomach [9] and elicit systemic effects [[10], [11], [12], [13]]. In last few years, our group has shown that increased plasma levels of nitrite do not necessarily correlate with the decreases in blood pressure after oral nitrite administration and that low gastric pH is critical to this response [3,13,14]. Our results suggest that oral nitrite administration increases the formation of nitrosylated species in the acidic environment of the stomach, which then reduce blood pressure [3,13,14].Other groups suggest that nitrite could form S-nitrosothiols directly in red blood cells under particular conditions [15]. Together, these previous studies suggest that S-nitrosothiols formation appears to be central to the vascular effects of nitrite. This mechanism may explain how a single dose of nitrite may decrease the blood pressure for up to 24 h, when circulating nitrite levels have long been normalized [16], and suggests that this effect may not depend exclusively on nitrite-derived NO formation and may in fact involve protein nitrosylation.
S-nitrosothiols are potent vasodilators [10,17] that may promote transnitrosylation reactions [18] resulting in nitrosylation of target proteins and important changes in protein function with persistent effects. An important example is nitrosylation of protein kinase C (PKC), which plays a critical role in the regulation of the vascular function. PKC nitrosylation results in inhibited enzyme activity, less protein phosphorylation and inhibition of vasoconstriction [17]. Given that PKC plays a central role in vascular signaling and that PKC nitrosylation could inhibit angiotensin type 1 receptors (AT1R) activation by angiotensin II or other receptors (adrenergic α1) mediating vasoconstriction, we hypothesized that treatment with oral nitrite could increase the circulating levels of S-nitrosylated species and promote vascular PKC nitrosylation, thus decreasing its activity. This mechanism could attenuate the vascular responses to angiotensin II and explain the blood pressure responses to oral nitrite treatment.
The present study addressed this possibility and provides further evidence supporting this mechanism by showing that oral nitrite treatment results in vascular responses that are very similar to those found with the use of S-nitrosoglutathione (GSNO), which also promotes vascular PKC nitrosylation and decreases the in vivo and ex vivo responses to angiotensin II.
2. Materials and methods
2.1. Animals
This study complied with guidelines of Ribeirao Preto Medical School, University of Sao Paulo, and the animals were handled according to the guiding principles published in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male Wistar rats (200–250 g) obtained from the colony at University of São Paulo were maintained on a 12-h light/dark cycle at room temperature (22–25 °C) with free access to standard rat chow and water.
2.2. Assessment of mean arterial pressure (MAP) in unanesthetized freely-moving rats
Invasive mean arterial blood pressure (MAP) was assessed in the present study. The animals were anesthetized with ketamine and xylazine (100 mg/kg and 10 mg/Kg i.p.) and the femoral artery and vein was cannulated (2 cm segment of a PE-10 tube connected to 14 cm of a PE-50 tubing; Clay Adams, Parsippany, NJ, USA). The catheters were tunneled subcutaneously and exteriorized through the back of the neck. After surgery, the nonsteroidal anti-inflammatory flunixine meglumine (2.5 mg/kg, s.c., Banamine®, Schering Plough, Brazil) was administered for post-operation analgesia. After 6 h of rest, the arterial cannula was connected to a pressure transducer and the MAP was recorded in freely moving rats using a data acquisition system (MP150CE; Biopac Systems Inc., CA, USA) connected to a computer (Acknowledge 3.2, for Windows). Before collecting data, we allowed at least 15 min of stabilization [11]. The catheter connected to femoral vein was used to infuse drugs.
2.3. Assessment of vascular reactivity
The rats were anesthetized with tribromoethanol (250 mg/kg i.p.) and their thoracic aortas were carefully isolated and cleaned of connective tissue and fat. Aortic rings, 4 mm in length, were cut and mounted for isometric tension recording as previously described [19]. The rings were placed in bath chambers (10 ml) for isolated organs containing modified Krebs salt solution with the following composition (mM): NaCl 130, CaCl2 1.6, MgSO4 1.2, KH2PO4 1.2, KCl 4.7, NaHCO3 14.9, glucose 5.5. The solution was kept at 37 °C, pH 7.4, and bubbled with 95% O2 and 5% CO2. The system was connected to an isometric force displacement transducer (Harvard Apparatus, Holliston, MA, USA) and the responses were recorded on a computer system using the LabChart version 4.0, PowerLab ADInstruments (2000) program. The aortic rings were subjected to a basal tension of 1.0 g during the 60 min equilibration period and endothelial integrity was examined by assessing the responses to acetylcholine (10−6 mol/l) under contractile tone induced by phenylephrine (10−7 mol/l). We used aortic rings with relaxing responses corresponding to at least 80% of the contraction induced by phenylephrine.
2.4. Effects of oral nitrite pretreatment on the acute responses to angiotensin II
A first series of experiments was designed to examine the effects of oral nitrite treatment on the blood pressure responses to intravenous infusions of angiotensin II. Therefore, the animals were randomly divided into two groups: normotensive rats that were treated for five days with sodium nitrite (0.2 mmol/kg by gavage) [3] and control rats (treated with water). On the fifth day, the rats were cannulated, as described above, and 6 h after the surgery, the arterial cannula was connected to a pressure transducer and MAP was recorded. After 15 min of stabilization, both groups of the animals received intravenous infusions of angiotensin II (0.03, 0.1, 0.3, 1.0, and 3.0 μg/kg) [20] and MAP was recorded.
Ten minutes after the last angiotensin II infusion, the animals were euthanized with an overdose of anesthetic and blood samples and aortic tissue were collected and stored at −70 °C. To preserve S-nitrosothiols, blood was centrifuged at 1000 g, and the plasma samples were stabilized with a solution containing N-ethylmaleimide (NEM) 10 mmol/l and diethylenetriaminepentaacetic acid (DTPA) 2 mmol/l [21]. Plasma samples were stored at −70 °C until used to measure their nitrite, nitrate, and nitrosylated species (RXNO) concentrations.
An independent group of rats treated with oral nitrite (or water) was used to assess the effects of oral nitrite treatment on the vascular reactivity to angiotensin II using that aortic reactivity assay described above. Aortic rings from rats treated with oral nitrite (or water) were incubated with angiotensin II (from 10−10 to 10−7 mol/l) and concentration-effect curves were compared between the two experimental groups.
2.5. Effects of oral S-nitrosogluthatione (GSNO) pretreatment on the acute responses to angiotensin II
A second series of experiments was designed to examine whether the oral treatment with S-nitrosogluthatione (GSNO) at equimolar dose relative to that of sodium nitrite could reproduce the blood pressure changes and the modifications in vascular reactivity induced by oral nitrite in the responses to angiotensin II.
Two groups of rats were used to test this possibility: normotensive rats that were treated for five days with GSNO (0.2 mmol/kg, by gavage) and control rats (treated with water). On the fifth day, the same procedures described above for animals treated with nitrite were repeated with these rats. They received the same angiontensin II infusions and MAP was recorded. Ten minutes after the last angiotensin II infusion, blood samples and aortic tissue were collected as described above and stored at −70 °C until used.
2.6. Assessment of how partial thiol depletion modifies the effects of oral nitrite pretreatment on the acute responses to angiotensin II
Given that nitrite-induced formation of S-nitrosothiols may mediate many of the effects associated with oral nitrite treatment, another series of experiments was designed to examine whether partial thiol depletion could attenuate the responses found with oral nitrite treatment. Therefore, we repeated the same blood pressure response experiments described in the first series of experiments using rats pretreated with oral nitrite (0.2 mmol/kg or vehicle for five days, by gavage) and the gluthathione synthase inhibitor buthionine sulfoximine (BSO) 1.4 mmol/kg every 12 h, i.p [22]. After five days of treatment, the same procedures described in the first series of experiments were carried out. Again, 10 min after the last angiotensin II infusion, blood samples and aortic tissue were collected as described above and stored at −70 °C until used.
2.7. Measurement of plasma nitrite and nitrosylated species concentrations
Plasma aliquots were analyzed in duplicate for their nitrite and nitrosylated species contents using an ozone-based reductive chemiluminescence assay as previously described [13,21]. Briefly, to measure nitrite concentrations in plasma, 50 μl of plasma samples were injected into a solution of acidified tri-iodide, purging with nitrogen in line with a gas-phase chemiluminescence NO analyzer (Sievers Model 280 NO analyzer; Boulder, CO, USA). To measure nitroso compounds (RXNO) concentrations, 500 μl of plasma samples were treated with acid sulfanilamide (5% sulfanilamide in HCl 1 mol/L) for 5 min before injection into the solution of acidified tri-iodide purged with nitrogen in line with the NO analyzer.
2.8. Assessment of total protein nitrosylation and PKC nitrosylation by resin-assisted capture (SNO-RAC) method
Total nitrosylated proteins and PKC nitrosylation were determined using the SNO-RAC method [3,23] 20–21 with modifications. Proteins were extracted from aortic tissue and HEK293T cells with a buffer (25 mM HEPES, 50 mM NaCl, 0.1 mM EDTA, 1% NP40 and 0.1% SDS pH 7.4) supplemented with protease inhibitor cocktail (Sigmafasttm Sigma) and centrifuged at 12,000 g at 4 °C for 10 min. The supernatant was added to 1.6 ml of blocking buffer (HEN Bufer: 100 mM HEPES, 1 mM EDTA and 0.1 mM neocuproine, pH 8.1 plus 2.5% SDS and 20 mM methylmethanethiosulfonate) for 20 min at 50 °C, mixing every 5 min. Then 6 ml of pre-chilled acetone were added to precipitate the proteins for 20 min at −20 °C. The samples were centrifuged at 2000 g for 10 min at 4 °C and the pellets were washed four times with acetone 70% and suspended in 0.5 ml of HEN buffer with 1% SDS. Then the samples were incubated overnight at 4 °C with 20 mM ascorbate and 40 μl of thiopropyl-sepharose 6B under rotation. All the steps were carried out in the absence of light. The resin was washed four times with 1 ml of HEN buffer plus 1% SDS and five times with HEN buffer diluted 1:10 with 1% SDS (HEN Buffer/10 SDS) followed by elution with HEN Buffer/10 SDS plus 2% of 2-mercaptoethanol for 1 h at room temperature. To quantify the proportion of nitrosylated proteins, both control input (i) and output (o) samples are run on a 5% SDS/PAGE gel combined with a 10%–5% SDS/PAGE gel. The run was stopped when the samples reached the 10% gel. Then the gels were stained with Coomassie Blue 0.05% and nitrosylated proteins were quantified (Amersham Image 600, GE Healthcare, Little Chalfont, Buckinghamshire, UK) using the ImageJ Program (NIH, USA). Given the low protein concentration in the output samples, we loaded two times higher protein concentration for output samples as compared to input samples. The percentage of protein S-nitrosylation was calculated as 100% x 2(i)/(o). To quantify PKC nitrosylation, a western blotting analysis of PKC was run with the control (input) and the output samples obtained from SNO-RAC.
2.9. Western blotting analysis of PKC
Western blotting analysis of vascular PKC expression was carried out to assess PKC expression and to quantify PKC S-nitrosylation in aortic samples processed by SNO-RAC technique as described above. Briefly, input samples corresponding to 10 μg of protein extracts and output samples corresponding to three times as much as the input volume were separated by SDS-PAGE using an 10% polyacrylamide gel. The proteins were transferred to nitrocellulose membranes (GE Healthcare, Madison, WI, USA). After blocking with 3% BSA, the membranes were incubated overnight at 4 °C with primary antibody directed against PKC (1:500; sc-937). Then the membranes were incubated with horseradish peroxidase (HRP)-secondary goat anti-rabbit antibody (1:2000; Millipore) and revealed with ECL chemiluminescence kit (GE Healthcare). PKC expression was normalized with respect to β-actin expression (1:1000; Millipore). Total nitrosylated PKC were calculated as 100% x 3(i)/(o).
2.10. Assessment of how GSNO-induced nitrosylation affects the vascular responses to PKC activation by angiotensin II or by a direct PKC activator
Given that both oral nitrite or GSNO increased vascular protein nitrosylation and PKC nitrosylation, a series of experiments using the vascular reactivity assay described above was carried out to examine whether incubation of aortic rings with GSNO could attenuate the vascular responses to angiotensin II and to the direct PKC activator Phorbol 12,13-dibutyrate (PDBu). Therefore aortic rings from normotensive rats were preincubated for 5 min with GSNO 1 mmol/l (or vehicle) and then washed out with modified Krebs salt solution to eliminate any residual GSNO. This procedure avoided residual effects of GSNO (NO release) in the bath chamber during the vascular reactivity assay. Then the responses to cumulative concentrations of angiotensin II (10−10 to 10−7 mol/l) or Phorbol 12,13-dibutyrate (PDBu, (10−9 to 10−5 mol/l) were measured to construct concentration–response curves. The curves were fitted using a nonlinear interactive fitting program (GraphPad Prism 5.0; GraphPad Software, Inc.).
In addition to the experiments carried out with aortic rings preincubated with GSNO (or vehicle) for 5 min, another similar series of experiments was carried out with aortic rings preincubated with GSNO (or vehicle) and ascorbate 5 mmol/l (or vehicle) for additional 15 min. This approach was used to destroy any GSNO-induced protein nitrosylation during the GSNO incubation.
Control experiments identical to those described above with GSNO were carried out using aortic rings preincubated for 5 min with sodium nitrite 1 mmol/l (or vehicle) instead of GSNO, and then washed out with modified Krebs salt solution to eliminate any residual nitrite. These experiments were carried out to examine whether incubation of aortic rings with nitrite could attenuate the vascular responses to angiotensin II or to the direct PKC activator PDBu.
2.11. Effects of GSNO on angiotensin II-mediated intracellular Ca2+ mobilization
Given that angiotensin II promotes vasoconstriction by activating cellular mechanisms involving increasing Ca2+ mobilization, we examined whether GSNO-induced nitrosylation could inhibit this mechanism in angiotensin II-stimulated HEK293T cells expressing the angiotensin type 1 receptor (AT1R). Intracellular calcium mobilization was determined in HEK293T cells (previously transfected with the AT1R), which were loaded with a Ca2+-sensitive dye (FLIPR® Calcium 5 Assay Kit – Molecular Devices, Sunnyvale, CA, USA) as previously decribed [24]. Briefly, 48 h after transfection with plasmids encoding AT1R or oxytocin receptor (control experiments), HEK293T cells seeded in 96-well clear-bottom black plates (Cellstar – Greiner Bio-One, Monroe, NC, USA) were washed with PBS and loaded with Ca2+ sensitive dye containing 2.5 mM probenecid and incubated for 1 h at 37 °C and 5%CO2. The plates were incubated for 15 min with GSNO 1 mM + cysteine 0.1 mM or vehicle (PBS pH 7.4). Then the plates were transferred to a FlexStation 3 microplate reader (Molecular Devices) and stimulated with a complete dose-response curve with angiotensin II or oxytocin and fluorescence was measured at excitation and emission wavelengths of 485 nm and 525 nm, respectively. Three or four independent experiments were performed with each drug.
2.12. Drugs and solutions
Thiopropyl-sepharose 6B was purchased from GE Healthcare (Little Chalfont, Buckinghamshire, UK) and all other drugs and reagents were purchased from Sigma Chemical Co. (St Louis, MO, USA). All solutions were prepared immediately before use.
2.13. Statistical analysis
The results are expressed as means ± S.E.M. The comparisons between groups were assessed by one-way or two-way analysis of variance followed by the Tukey test. A probability value P < 0.05 was considered signficant.
3. Results
3.1. Oral nitrite treatment enhances circulating S-nitrosylated species concentrations and attenuates the responses to angiotensin II by mechanisms associated with vascular PKC nitrosylation
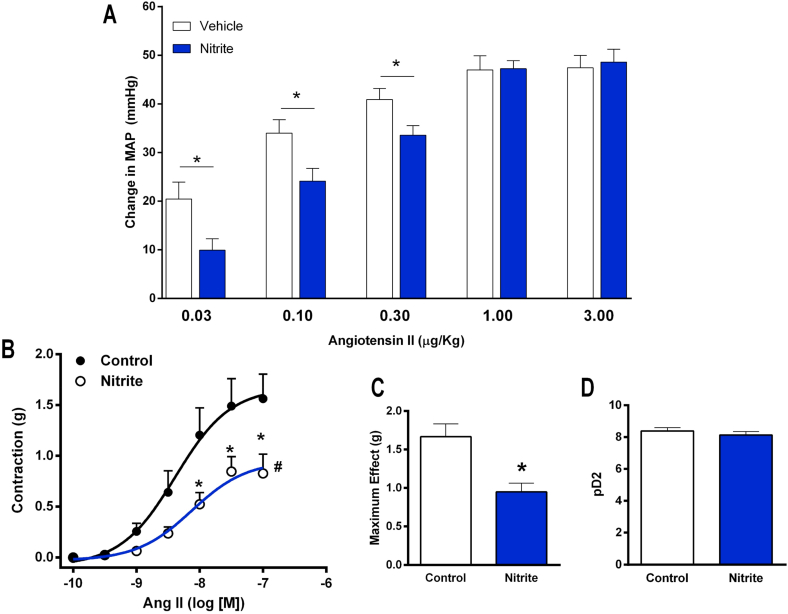
Rats were pretreated with oral nitrite for five days to examine whether this treatment affects the responses to angiotensin II. The blood pressure responses to angiotensin II infusions are shown in Fig. 1A, which clearly shows that pretreatment with oral nitrite attenuates the increases in MAP after the lower doses of angiotensin II (MAP increased by 9 ± 2 versus 20 ± 3 mmHg in controls after angiotensin II 0.03 μg/kg; by 24 ± 2 versus 34 ± 2 mmHg after angiotensin II 0.01 μg/kg in controls; and by 33 ± 2 versus 40 ± 2 mmHg after angiotensin II 0.3 μg/kg in controls; all P < 0.05; Fig. 1A). This in vivo evidence showing an effect of oral nitrite was confirmed by vascular reactivity experiments ex vivo. As shown in Fig. 1B, C and 1D, pretreatment with nitrite attenuated the maximum effect of angiotensin II by >44% (maximum effect = 1.6 ± 0.1 in controls versus 0.9 ± 0.1 g in aortic rings from nitrite-treated animals; P < 0.05).
Fig. 1.
Pretreatment with nitrite attenuates the blood pressure responses to angiotensin II in vivo and decreases angiontensin II-induced vascular contraction ex vivo. Panel A shows the increases in mean arterial blood pressure (MAP) after infusion of angiotensin II (0.03, 0.1, 0.3, 1.0 and 3.0 μg/kg, i.v.) in rats pretreated with oral nitrite (15 mg/kg) or vehicle for five days. Panel B shows the concentration-effect curves in response to angiotensin II (from 10−10 to 10−7 mol/l) using aortas from rats pretreated with oral nitrite (15 mg/kg) or vehicle for five days. Panels C and D show the maximum effect and the pD2 values (the negative logarithm of the concentration producing half maximum effect), respectively. Data are shown as mean ± S.E.M. (n = 8–10/group for Panel A, and n = 6/group for Panels B,C and D). *P < 0.05 versus Vehicle. #P < 0.05 versus Vehicle for the concentration-effect curve.
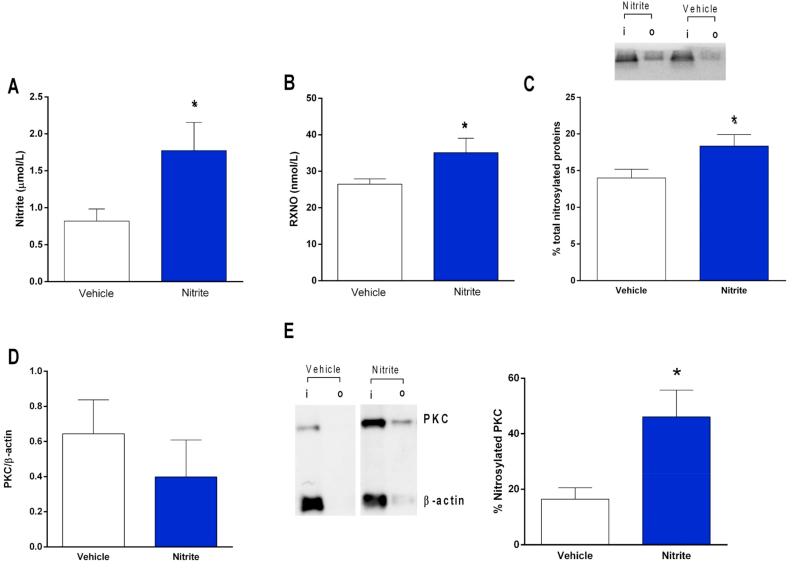
In order to show a mechanism involved in the effects of oral nitrite on MAP and vascular reactivity, we assessed circulating and vascular concentrations on nitrosylated species and measured vascular S-nitrosylation of PKC, which is a key mechanism attenuating vasoconstriction [17]. Fig. 2A and B show that oral nitrite treatment increased both plasma nitrite (0.7 ± 0.1 μmol/l in controls and 1.7 ± 0.2 μmol/l in nitrite-treated rats) and nitrosylated species (RXNO; 27 ± 1 nmol/l in controls and 35 ± 2 nmol/l in nitrite-treated rats), respectively (both P < 0.05). The increases in circulating RXNO concentrations were also associated with increased total protein nitrosylation in the aortic tissue (by >25%; Fig. 2C; P < 0.05). Whereas oral nitrite treatment had no effect on vascular PKC expression (Fig. 2D and E), aortic PKC nitrosylation increased from 16 ± 4% to 46 ± 9% in nitrite-treated animals (Fig. 2E); P < 0.05).
Fig. 2.
Pretreatment with nitrite increases the concentrations of nitrosylated species (RXNO) in plasma, and enhances total protein and PKC nitrosylation in the vessels. Panels A and B show the concentrations of nitrite and nitrosylated species (RXNO), respectively, in plasma from rats pretreated with oral nitrite (15 mg/kg) or vehicle for five days. Panel C shows a representative SDS/PAGE gel stained with Coomassie Blue to quantify total protein nitrosylation in aortic samples from nitrite or vehicle-treated animals using the SNO-RAC method (“I” corresponds to input: total protein; “o” corresponds to output: nitrosylated protein). The bar graphic shows the quantification of total nitrosylated proteins in the aortic samples. Panel D shows the quantification of PKC expression in the aortas from nitrite or vehicle-treated animals using Western blotting analysis. Panels E show a representative western blotting marking to quantification of PKC nitrosylation in aortic samples from nitrite or vehicle-treated animals. Data are shown as mean ± S.E.M. (n = 5–10/group). *P < 0.05 versus Vehicle. . (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2. GSNO treatment also enhances circulating S-nitrosylated species concentrations and attenuates the responses to angiotensin II by mechanisms associated with vascular PKC nitrosylation
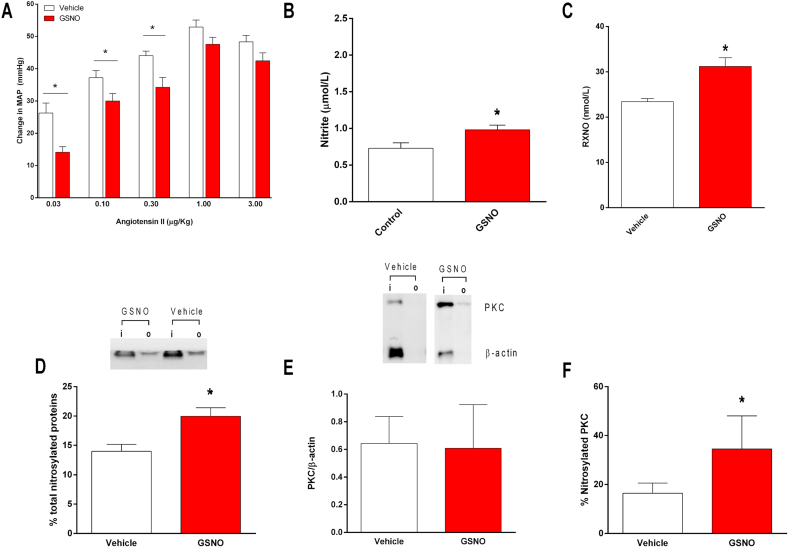
Given than many effects associated with oral nitrite treatment were associated with nitrosylation mechanisms, rats were pretreated with oral GSNO to examine whether this treatment could result in functional and biochemical changes similar to those found with oral nitrite treatment. Again, Fig. 3A shows that the blood pressure responses to angiotensin II infusions were attenuated by the pretreatment with GSNO when the lower doses of angiotensin II were used (MAP increased by 14 ± 1 versus 26 ± 3 mmHg in controls after angiotensin II 0.03 μg/kg; by 30 ± 2 versus 37 ± 2 mmHg after angiotensin II 0.01 μg/kg in controls; and by 34 ± 3 versus 44 ± 1 mmHg after angiotensin II 0.3 μg/kg in controls; all P < 0.05; Fig. 3A).
Fig. 3.
Pretreatment with S-nitrosogluthatione (GSNO) increases the concentrations of nitrosylated species (RXNO) in plasma, enhances total protein and PKC nitrosylation in the vessels and attenuates the blood pressure responses to angiotensin II in vivo. Panel A shows the increases in mean arterial blood pressure (MAP) after infusion of angiotensin II (0.03, 0.1, 0.3, 1.0 and 3.0 μg/kg, i.v.) in rats pretreated with oral GSNO (0.2 mmol/kg) or vehicle for five days. Panels B and C show the concentrations of nitrite and nitrosylated species (RXNO), respectively, in plasma from rats pretreated with oral GSNO or vehicle. Panel D shows a representative SDS/PAGE gel stained with Coomassie Blue to quantify total protein nitrosylation in aortic samples from GSNO or vehicle-treated animals using the SNO-RAC method (“I” corresponds to input: total protein; “o” corresponds to output: nitrosylated protein). The bar graphic shows the quantification of total nitrosylated proteins in the aortic samples. Panel E shows the quantification of PKC expression in the aortas using Western blotting analysis and show a representative marking to quantify PKC nitrosylation. Panel F show the quantification of PKC nitrosylation in aortic samples from GSNO or vehicle-treated animals. Data are shown as mean ± S.E.M. (n = 5–10/group). *P < 0.05 versus Vehicle. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Consistently, this in vivo effect of GSNO on the responses to angiotensin II were associated with increased plasma nitrite (0.7 ± 0.1 μmol/l in controls and 0.9 ± 0.1 μmol/l in GSNO-treated rats) and nitrosylated species (RXNO; 23 ± 1 nmol/l in controls and 36 ± 2 nmol/l in GSNO-treated rats), as shown in Fig. 3B and C, respectively (both P < 0.05). The increases in circulating RXNO concentrations were also associated with increased total protein nitrosylation in the aortic tissue (by >35%; Fig. 3D; P < 0.05). Whereas GSNO treatment had no effect on vascular PKC expression (Fig. 3E), aortic PKC nitrosylation increased from 16 ± 4% to 34 ± 10% in GSNO-treated animals (Fig. 3E and F; P < 0.05).
3.3. Partial thiol depletion prevents the effects of oral nitrite on blood pressure and vascular PKC nitrosylation
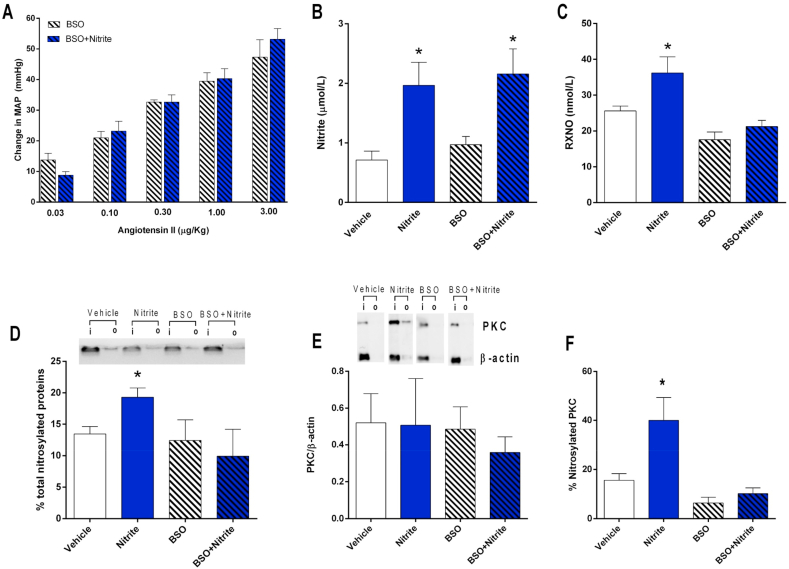
The effects of oral nitrite shown in the present study were very similar to those found with GSNO and apparently involve the formation of RXNO and nitrosylation mechanisms. To further validate this hypothesis, the effects of same oral nitrite treatment were studied in rats treated with BSO to cause partial thiol depletion, which could attenuate nitrite-induced increases in plasma RXNO concentrations and vascular protein nitrosylation. Interestingly, Fig. 4A shows that, in the context of partial thiol depletion with BSO, treatment with oral nitrite does not modify the blood pressure responses to angiotensin II (P > 0.05).
Fig. 4.
Partial thiol depletion prevents oral nitrite from attenuating the blood pressure responses to angiotensin II and blunts oral nitrite-induced increases in plasma concentrations of nitrosylated species, in total protein and PKC nitrosylation in the vessels. Panel A shows the increases in mean arterial blood pressure (MAP) after infusion of angiotensin II (0.03, 0.1, 0.3, 1.0 and 3.0 μg/kg, i.v.) in rats pretreated with the glutathione synthase inhibitor buthionine sulfoximine (BSO) 1.4 mmol/kg every 12 h, i.p., and with oral nitrite (15 mg/kg) or vehicle for five days. BSO pretreatment blunted the effects of oral nitrite, which were found in control animals treated with vehicle instead of BSO and are shown in Fig. 1A). Panels B and C show the concentrations of nitrite and nitrosylated species (RXNO), respectively, in plasma from rats pretreated with vehicle or nitrite, and with BSO or BSO and nitrite for five days. Panel D shows a representative SDS/PAGE gel stained with Coomassie Blue to quantify total protein nitrosylation in aortic samples from the four experimental groups using the SNO-RAC method (“I” corresponds to input: total protein; “o” corresponds to output: nitrosylated protein). The bar graphic shows the quantification of total nitrosylated proteins in the aortic samples. Panel E shows the quantification of PKC expression in the aortas from the four experimental groups using Western blotting analysis and show a representative marking to quantify PKC nitrosylation. Panel F show the quantification of PKC nitrosylation in aortic samples from the four experimental groups. Data are shown as mean ± S.E.M. (n = 5–10/group). *P < 0.05 versus Vehicle. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 4B and C show plasma nitrite and RXNO concentrations in the four groups of animals. Fig. 4B clearly shows similar increases in plasma nitrite concentrations after nitrite treatment in controls and in BSO-treated animals (P > 0.05). In contrast, nitrite treatment increased RXNO concentrations by approximately 40% in controls, whereas no significant increases in RXNO were found in animals treated with BSO. These results indicate that BSO prevented nitrite from increasing RXNO concentrations.
In agreement with these results, the quantification of vascular protein nitrosylation showed that nitrite treatment increased this biochemical parameter by approximately 35% in control animals (P < 0.05), whereas no significant increases in vascular protein nitrosylation were found in animals treated with BSO (P > 0.05; Fig. 4D). In parallel with total protein nitrosylation, the quantification of vascular PKC nitrosylation showed similar results. Treatment with oral nitrite or BSO did not affect the vascular expression of PKC (Fig. 4E, P > 0.05). However, nitrite treatment more than doubled PKC nitrosylation in control rats (P < 0.05), whereas no significant changes were found in BSO treated animals (P > 0.05, Fig. 4E and F). These results indicate that BSO prevented nitrite from increasing total protein and PKC nitrosylation in the aorta.
3.4. GSNO-induced nitrosylation attenuates the vascular responses to angiotensin II and to a direct PKC activator
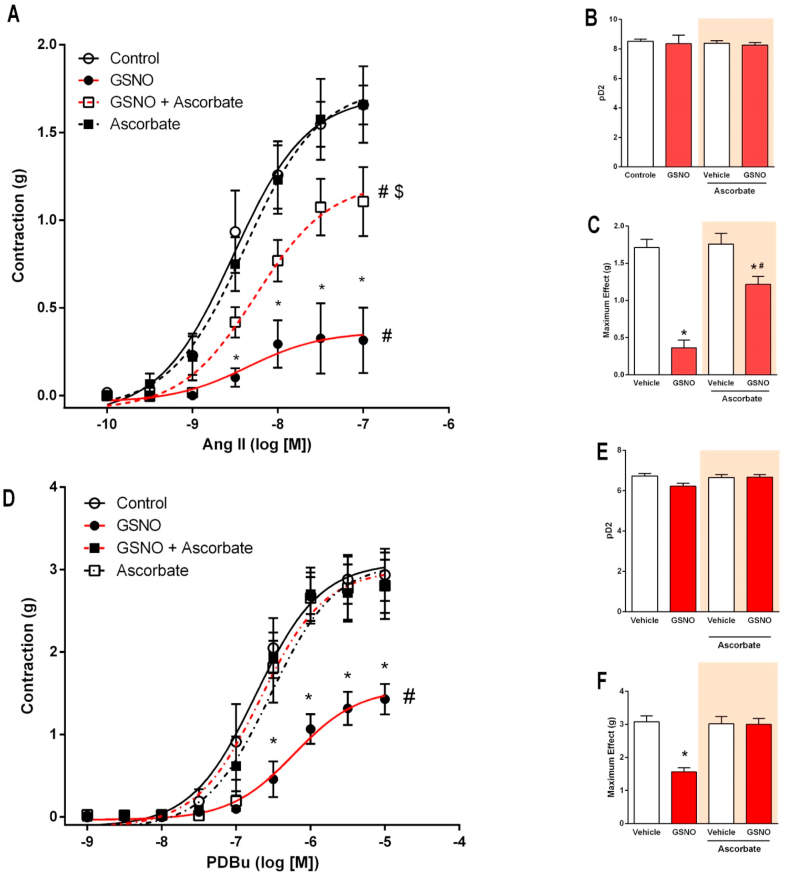
Treatment with oral nitrite or GSNO increased total protein and PKC nitrosylation in the vessels in association with decreased responsiveness to angiotensin II. Therefore, vascular reactivity experiments were designed to assess whether incubation of aortic rings with GSNO could attenuate the vascular responses to angiotensin II and to a direct PKC activator (PDBu), and whether this effect is reversible by ascorbate, which reverses GSNO-induced protein nitrosylation during the GSNO incubation. Fig. 5A, 5B, and 5C show that preincubation with GSNO attenuated the maximum contraction in response to angiotensin II by approximately 80% (P < 0.05). Whereas ascorbate alone had no effects, ascorbate prevented approximately 70% of GSNO-induced effects (P < 0.05). Similar results were found when the direct PKC activator PDBu was used (Fig. 5D, 5E, and 5F). Whereas GSNO attenuated the maximum contraction in response to angiotensin II by approximately 50% (P < 0.05), ascorbate totally prevented GSNO-induced effects (P < 0.05). Together, these results are consistent with the idea that PKC nitrosylation may underlie the reduced responses to angiotensin II.
Fig. 5.
Incubation with S-nitrosogluthatione (GSNO) impairs the vascular contraction induced by angiotensin II or by the PKC activator PDBu and this effect is prevented by ascorbate. Panel A shows the vasoconstriction induced by angiotensin II (10−10 to 10−7 mol/l) in aortic ring preparations. The aortic rings were preincubated for 5 min with GSNO 1 mmol/l (or vehicle) in the bath chambers and washed out extensively with modified Krebs salt solution. After eliminating residual GSNO from the bath chambers, the aortic rings were further incubated with ascorbate 5 mmol/l (or vehicle) for additional 15 min and then the concentration-response curves in response to angiotensin II were constructed. Panels B and C show the corresponding pD2 values (the negative logarithm of the concentration producing half maximum effect) and maximum effect, respectively. Panel D shows the vasoconstriction induced by the PKC activator PDBu (10−9 to 10−5 mol/l) in aortic ring preparations preincubated with GSNO (or vehicle) and ascorbate (or vehicle) as described above. Panels E and F show the corresponding pD2 values (the negative logarithm of the concentration producing half maximum effect) and maximum effect, respectively. Data are shown as mean ± S.E.M. (n = 6/group). *P < 0.05 versus respective Vehicle group. #P < 0.05 versus Control group. $ P < 0.05 versus GSNO group.
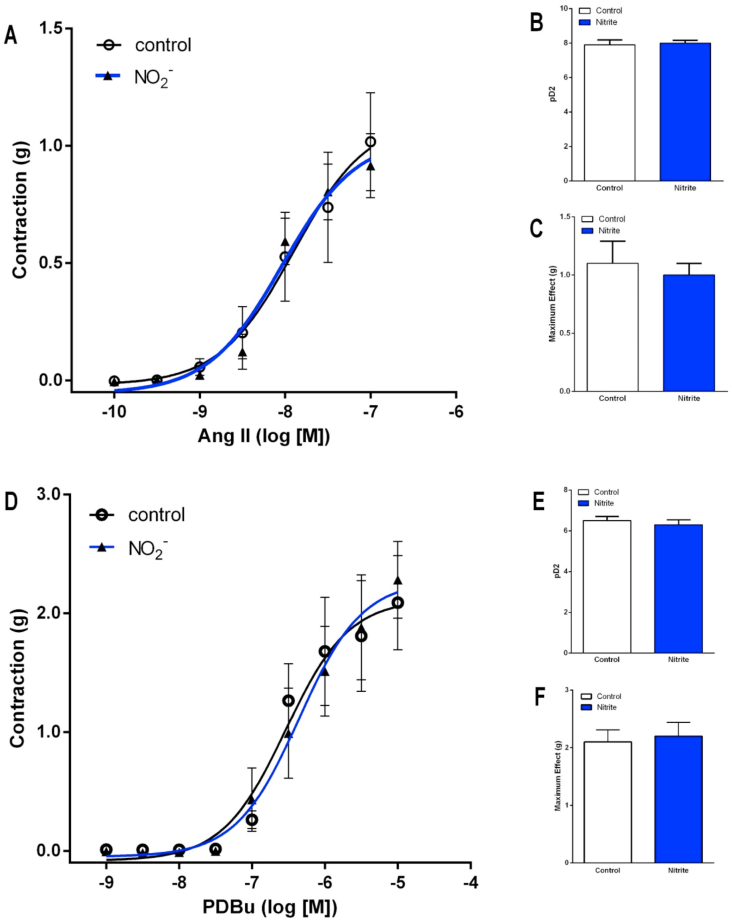
Control experiments carried out to examine the possibility that vascular incubation with sodium nitrite could attenuate the vascular responses to angiotensin II or to the direct PKC activator PDBu showed no effects of nitrite (Fig. 6; all P > 0.05).
Fig. 6.
Incubation with sodium nitrite does not affect the vascular contraction induced by angiotensin II or by the PKC activator PDBu. Panel A shows the vasoconstriction induced by angiotensin II (10−10 to 10−7 mol/l) in aortic ring preparations. The aortic rings were preincubated for 5 min with sodium nitrite 1 mmol/l (or vehicle) in the bath chambers and washed out extensively with modified Krebs salt solution. After eliminating residual sodium nitrite from the bath chambers, concentration-response curves in response to angiotensin II were constructed. Panels B and C show the corresponding pD2 values (the negative logarithm of the concentration producing half maximum effect) and maximum effect, respectively. Panel D shows the vasoconstriction induced by the PKC activator PDBu (10−9 to 10−5 mol/l) in aortic ring preparations preincubated with sodium nitrite (or vehicle) as described above. Panels E and F show the corresponding pD2 values (the negative logarithm of the concentration producing half maximum effect) and maximum effect, respectively. Data are shown as mean ± S.E.M. (n = 6/group).
3.5. GSNO-induced nitrosylation attenuates angiotensin II-mediated intracellular Ca2+ mobilization
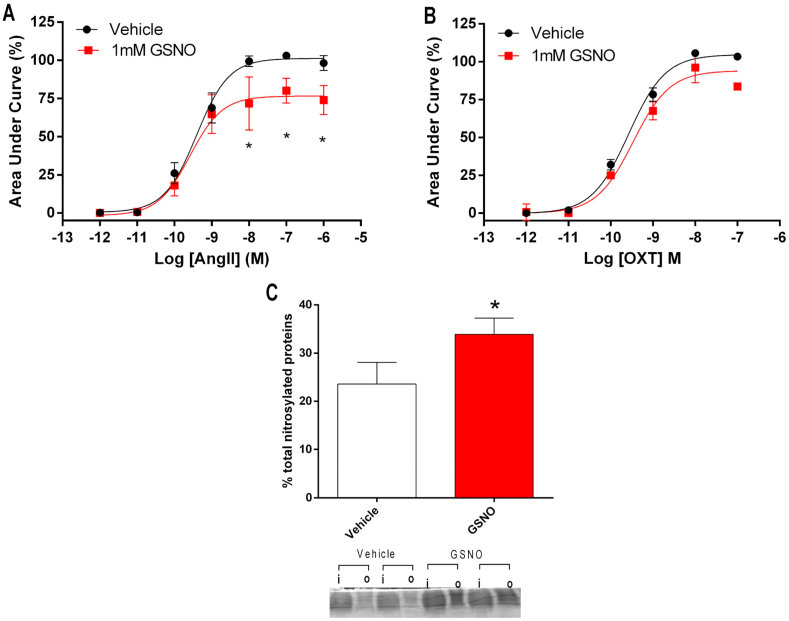
To further explore critical cellular mechanisms involved in AT1R signaling and vasoconstriction that could be modulated by nitrosylation, we examined whether GSNO-induced nitrosylation inhibits the increases in Ca2+ mobilization in angiotensin II-stimulated HEK293T cells expressing the AT1R. Interestingly, preincubation with GSNO 1 mM reduced calcium mobilization after stimulation with angiotensin II by approximately 25%, as shown in Fig. 7A (P < 0.05). This GSNO-induced effect was not found in control experiments examining the effects of oxytocin in HEK293T cells expressing the oxytocin receptor (Fig. 7B; P > 0.05), which shares with the AT1R the same intracellular machinery. These results suggest that GSNO impairs AT1R function by mechanisms probably involving nitrosylation. In fact, GSNO incubation increased total protein nitrosylation from 23 ± 4% in vehicle-treated cells to 33 ± 3% in GSNO-pretreated cells (P < 0.05; Fig. 7C).
Fig. 7.
Incubation of HEK293T cells expressing angiotensin II type 1 receptors (AT1R) with GSNO impairs angiotensin II (but not oxytocin)-stimulated intracellular calcium mobilization. Panel A shows intracellular Ca2+ mobilization in HEK293T cells expressing AT1R and loaded with a Ca2+-sensitive dye and stimulated with angiotensin II (10−12 to 10−6 mol/l) after treatment with GSNO (1 mM) or vehicle for 15 min. Panel B shows control experiments using the same cells expressing oxytocin receptor, which were stimulated with oxytocin (10−12 to 10−7 mol/l) after treatment with GSNO (1 mM) or vehicle for 15 min. Panel C shows a representative SDS/PAGE gel stained with Coomassie Blue to quantify total protein nitrosylation in HEK293T cells (treated with from GSNO or vehicle) using the SNO-RAC method (“I” corresponds to input: total protein; “o” corresponds to output: nitrosylated protein). The bar graphic shows the quantification of total nitrosylated proteins. Data are shown as mean ± S.E.M. (n = 3–4/group). *P < 0.05 versus Vehicle. . (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
This study shows for the first time that treatment with oral nitrite attenuates the vasoconstriction and the hypertensive responses to angiotensin II by mechanisms promoting S-nitrosylation of vascular proteins, in particular of PKC, which is critically involved in cellular signaling after activation of AT1R, and this effect may help to explain the blood pressure effects of oral nitrite administration.
Recent data from our group suggest that oral nitrite treatment increases circulating S-nitrosothiols concentrations and restores the protein nitrosylation levels in aorta from 2K1C hypertensive rats, which become hypertensive after primary activation of the renin-angiotensin axis, particularly during the initial weeks of hypertension [25]. This is important because the vascular redox environment of the vessels in 2K1C hypertension usually shows pro-oxidant modifications resulting of increased angiotensin II activity and decreased protein nitrosylation, as we have described before [10]. In the present study, the initial experiments demonstrated that oral nitrite treatment results in increased circulating levels of nitrosylated species and attenuates the vascular and blood pressure responses to angiotensin II in association with increased vascular PKC nitrosylation. This initial evidence implicating PKC nitrosylation as a mediator of the blood pressure effects of oral nitrite is consistent with the critical role played by PKC in controlling the vascular tone and a decrease in PKC activity when this enzyme is nitrosylated [17]. These results may help to explain the mechanisms contributing to the antihypertensive effects of both nitrate and nitrite anions [[1], [2], [3],26]. In fact, oral nitrite treatment increased PKC nitrosylation and prevented the vasoconstriction and the pressor effects of angiotensin II, and therefore, although it was not the focus of the present study, it is highly probable that similar attenuation of the effects caused by other mediators including vasoconstrictor catecholamines would be found. Interestingly, the effects reported here are consistent with previous findings showing that oral nitrite administration resulted in antihypertensive effects lasting >24 h after a single daily nitrite administration which increased protein nitrosylation in the aortas from hypertensive animals [3]. In fact, this response was also found with nitrate administration and significantly attenuated by the use of oral mouthwash, which impaired the enterosalivary cycle of nitrate and prevented nitrate from being reduced to nitrite, thus reducing the increases in circulating S-nitrosothiols and vascular protein nitrosylation [3,10,11]. Several recent studies by other groups have described vascular effects of nitrite that are not necessarily associated with increased NO production [[27], [28], [29]], thus strongly suggesting the involvement of other pathways such as catalase inhibition [28] or increased S-nitrosylation26 as possible mechanisms activated by nitrite.
To strengthen the hypothesis of PKC S-nitrosylation results in decreased response to angiotensin II, animals were treated with the nitrosylating agent GSNO [18,30] and similar experiments were carried out. GSNO treatment resulted in blood pressure responses very similar to those found with oral nitrite. Again, vascular protein nitrosylation, in particular PKC nitrosylation, was probably implicated in the functional effects described here. Further supporting this hypothesis, we found that partial thiol depletion with BSO prevented the increases in circulating RXNO concentrations, which may explain the blunting of the increases in total protein and PKC nitrosylation caused by oral nitrite treatment, thus resulting in attenuation of its blood pressure effects. These results are consistent with previous findings showing that treatment with BSO blunts the effects of oral nitrite treatment [10].
Next, to confirm that PKC nitrosylation may be a critical mechanism explaining the effects of oral nitrite treatment, vascular reactivity experiments were carried out to show how incubation with GSNO modifies the vascular responses to angiotensin II and to PDBu (PKC activator). Interestingly, GSNO-induced nitrosylation attenuated the vascular responses to angiotensin II and to PBDu, and this effect was significantly prevented by ascorbate, which reverses GSNO-induced protein nitrosylation. Given that there was no GSNO in the bath chambers during vascular reactivity experiments, these results are consistent with the idea that PKC nitrosylation, and not NO released from nitrosothiols [31], is involved in the attenuation of the vascular responses to angiotensin II or to PDBu. These results are consistent with the notion that PKC S-nitrosylation down-regulates vasoconstriction [17].
Despite Increased vascular PKC nitrosylation, other molecules could be nitrosylated in AT1R pathway and should result in attenuation of angiotensin II-mediated Ca2+ mobilization. To test this hypothesis, we studied the effects of GSNO on the increases in Ca2+ mobilization in angiotensin II-stimulated HEK293T cells expressing the AT1R. GSNO reduced Ca2+ mobilization significantly in cells with increased protein nitrosylation induced by GSNO incubation. The same was not true in control experiments using oxytocin to stimulate HEK293T cells expressing the oxytocin receptor, thus suggesting that nitrosylation affected AT1R, reducing the response to angiotensin II. Supporting this observation, AT1R nitrosylation was described and resulted in impaired binding of angiotensin II to AT1R [32].
This work focused on the possibility that oral nitrite could promote PKC nitrosylation as an important mechanism to attenuate vascular responsiveness to vasoconstrictors, as previously shown by others as a mechanism clearly decreasing PKC activity [17]. However, several other proteins involved in vasoconstriction and increased blood pressure could also be nitrosylated and also mediate the decrease in blood pressure after nitrite treatment. Indeed, AT1R [32], α1-adrenergic receptors [33], and NADPH oxidase [34] are among possible targets for oral nitrite treatment-induced protein nitrosylation. While our findings do not rule out the possible contribution of other nitrosylation targets in addition to PKC, they provide strong evidence that this mechanism directly linked to AT1R activation and intracellular calcium mobilization is involved in the blood pressure responses to oral nitrite administration. In addition, despite the fact that we have not addressed the contribution of cycle GMP (cGMP) to the effects of sodium nitrite in the present study, several previous reports have showed evidence supporting the participation of cGMP in various biological responses to nitrite under different conditions [35,36], and our data not exclude this possibility.
In conclusion, our results show that oral nitrite treatment increases the formation of nitrosylating species concentrations, thus promoting vascular protein nitrosylation including PKC as a target. As a result of these biochemical modifications, the responses to angiotensin II are attenuated. These findings may have several implications in the therapy of hypertension and other cardiovascular diseases resulting of abnormal activation of the renin-angiotensin system.
Conflicts of interest
All authors declare no conflicts of interest.
Declaration of competing interest
None.
Acknowledgements
We gratefully acknowledge Mrs. Sandra de Oliveira Conde Tella for excellent technical support. This work was supported by Fundação de Aparo a Pesquisa do Estado de São Paulo (FAPESP Grant numbers 2014-23946-0 and 2015-22228-9), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
References
- 1.Carlström M., Persson A.E.G., Larsson E., Hezel M., Scheffer P.G., Teerlink T., Weitzberg E., Lundberg J.O. Dietary nitrate attenuates oxidative stress, prevents cardiac and renal injuries, and reduces blood pressure in salt-induced hypertension. Cardiovasc. Res. 2011;89:574–585. doi: 10.1093/cvr/cvq366. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh S.M., Kapil V., Fuentes-Calvo I., Bubb K.J., Pearl V., Milsom A.B., Khambata R., Maleki-Toyserkani S., Yousuf M., Benjamin N., Webb A.J., Caulfield M.J., Hobbs A.J., Ahluwalia A. Enhanced vasodilator activity of nitrite in hypertension. Hypertension. 2013;61:1091–1102. doi: 10.1161/HYPERTENSIONAHA.111.00933. [DOI] [PubMed] [Google Scholar]
- 3.Pinheiro L.C., Ferreira G.C., Amaral J.H., Portella R.L., Tella S. de O.C., Passos M.A., Tanus-Santos J.E. Oral nitrite circumvents antiseptic mouthwash-induced disruption of enterosalivary circuit of nitrate and promotes nitrosation and blood pressure lowering effect. Free Radic. Biol. Med. 2016;101:226–235. doi: 10.1016/j.freeradbiomed.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Sonoda K., Ohtake K., Uchida H., Ito J., Uchida M., Natsume H., Tamada H., Kobayashi J. Dietary nitrite supplementation attenuates cardiac remodeling in l -NAME-induced hypertensive rats. Nitric Oxide. 2017;67:1–9. doi: 10.1016/j.niox.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Cosby K., Partovi K.S., Crawford J.H., Patel R.P., Reiter C.D., Martyr S., Yang B.K., a Waclawiw M., Zalos G., Xu X., Huang K.T., Shields H., Kim-shapiro D.B., Schechter A.N., Iii R.O.C., Gladwin M.T., Cannon R.O. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003;9 doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 6.Hendgen-Cotta U.B., Merx M.W., Shiva S., Schmitz J., Becher S., Klare J.P., Steinhoff H.-J., Goedecke A., Schrader J., Gladwin M.T., Kelm M., Rassaf T. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proc. Natl. Acad. Sci. 2008;105:10256–10261. doi: 10.1073/pnas.0801336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zweier J.L., Li H., Samouilov A., Liu X. Mechanisms of nitrite reduction to nitric oxide in the heart and vessel wall. Nitric Oxide. 2010;22:83–90. doi: 10.1016/j.niox.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damacena-Angelis C., Oliveira-Paula G.H., Pinheiro L.C., Crevelin E.J., Portella R.L., Moraes L.A.B., Tanus-Santos J.E. Nitrate decreases xanthine oxidoreductase-mediated nitrite reductase activity and attenuates vascular and blood pressure responses to nitrite. Redox. Biol. 2017;12:291–299. doi: 10.1016/j.redox.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lundberg J.O.M., Weitzberg E., Lundberg J.O.M., Alving K. Intragastric nitric oxide production in humans: measurements in expelled air. Gut. 1994;35:1543–1546. doi: 10.1136/gut.35.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinheiro L.C., Amaral J.H., Ferreira G.C., Portella R.L., Ceron C.S., Montenegro M.F., Toledo J.C., Tanus-Santos J.E. Gastric S-nitrosothiol formation drives the antihypertensive effects of oral sodium nitrite and nitrate in a rat model of renovascular hypertension. Free Radic. Biol. Med. 2015;87:252–262. doi: 10.1016/j.freeradbiomed.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 11.Pinheiro L.C., Montenegro M.F., Amaral J.H., Ferreira G.C., Oliveira A.M., Tanus-Santos J.E. Increase in gastric pH reduces hypotensive effect of oral sodium nitrite in rats. Free Radic. Biol. Med. 2012;53:701–709. doi: 10.1016/j.freeradbiomed.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Montenegro M.F., Sundqvist M.L., Larsen F.J., Zhuge Z., Carlström M., Weitzberg E., Lundberg J.O. Blood pressure–lowering effect of orally ingested nitrite is abolished by a proton pump inhibitor. Hypertension. 2017;69:23–31. doi: 10.1161/HYPERTENSIONAHA.116.08081. [DOI] [PubMed] [Google Scholar]
- 13.Pinheiro L.C., Ferreira G.C., Vilalva K.H., Toledo J.C., Tanus-Santos J.E. Contrasting effects of low versus high ascorbate doses on blood pressure responses to oral nitrite in L-NAME-induced hypertension. Nitric Oxide. 2018;74:65–73. doi: 10.1016/j.niox.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Sanches-Lopes J.M., Ferreira G.C., Pinheiro L.C., Kemp R., Tanus-Santos J.E. Consistent gastric pH-dependent effects of suppressors of gastric acid secretion on the antihypertensive responses to oral nitrite. Biochem. Pharmacol. 2020;177:113940. doi: 10.1016/j.bcp.2020.113940. [DOI] [PubMed] [Google Scholar]
- 15.Angelo M., Singel D.J., Stamler J.S. An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate. Proc. Natl. Acad. Sci. U. S. A. 2006;103:8366–8371. doi: 10.1073/pnas.0600942103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montenegro M.F., Pinheiro L.C., Amaral J.H., Marçal D.M.O., Palei A.C.T., Costa-Filho A.J., Tanus-Santos J.E. Antihypertensive and antioxidant effects of a single daily dose of sodium nitrite in a model of renovascular hypertension. Naunyn. Schmiedebergs. Arch. Pharmacol. 2012;385:509–517. doi: 10.1007/s00210-011-0712-0. [DOI] [PubMed] [Google Scholar]
- 17.Choi H., Tostes R.C., Webb R.C. S-nitrosylation inhibits protein kinase C–mediated contraction in mouse aorta. J. Cardiovasc. Pharmacol. 2011;57:65–71. doi: 10.1097/FJC.0b013e3181fef9cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broniowska K.A., Hogg N. The chemical biology of S-nitrosothiols. Antioxid. Redox Signal. 2012;17:969–980. doi: 10.1089/ars.2012.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinheiro L.C., Oliveira-Paula G.H., Portella R.L., Guimarães D.A., de Angelis C.D., Tanus-Santos J.E. Omeprazole impairs vascular redox biology and causes xanthine oxidoreductase-mediated endothelial dysfunction. Redox. Biol. 2016;9:134–143. doi: 10.1016/j.redox.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neto-Neves E.M., Montenegro M.F., Dias-Junior C.A., Spiller F., Kanashiro A., Tanus-Santos J.E. Chronic treatment with quercetin does not inhibit angiotensin-converting enzyme in vivo or in vitro. Basic Clin. Pharmacol. Toxicol. 2010;107:825–829. doi: 10.1111/j.1742-7843.2010.00583.x. [DOI] [PubMed] [Google Scholar]
- 21.Feelisch M., Rassaf T., Mnaimneh S., Singh N., Bryan N.S., Kelm M., Jourd’heuil D. Concomitant S-, N-, and heme-nitros (yl) ation in biological tissues and fluids: implications for the fate of NO in vivo. Faseb. J. 2002;16:1775–1785. doi: 10.1096/fj.02-0363com. [DOI] [PubMed] [Google Scholar]
- 22.Vaziri N.D., Wang X.Q., Oveisi F., Rad B., Vaziri N.D., Wang X.Q., Oveisi F., Rad B. Induction of oxidative stress by glutathione depletion causes severe hypertension in normal rats. Hypertension. 2000;36:142–146. doi: 10.1161/01.hyp.36.1.142. [DOI] [PubMed] [Google Scholar]
- 23.Figueiredo-Freitas C., Dulce R.A., Foster M.W., Liang J., Yamashita A.M.S., Lima-Rosa F.L., Thompson J.W., Moseley M.A., Hare J.M., Nogueira L., Sorenson M.M., Pinto J.R. S -nitrosylation of sarcomeric proteins depresses myofilament Ca 2+ sensitivity in intact cardiomyocytes. Antioxid. Redox Signal. 2015;23:1017–1034. doi: 10.1089/ars.2015.6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teixeira L.B., Parreiras-E-Silva L.T., Bruder-Nascimento T., Duarte D.A., Simões S.C., Costa R.M., Rodríguez D.Y., Ferreira P.A.B., Silva C.A.A., Abrao E.P., Oliveira E.B., Bouvier M., Tostes R.C., Costa-Neto C.M. Ang-(1-7) is an endogenous β-arrestin-biased agonist of the AT1 receptor with protective action in cardiac hypertrophy. Sci. Rep. 2017;7:1–10. doi: 10.1038/s41598-017-12074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martins-Oliveira A., Guimaraes D.A., Ceron C.S., Rizzi E., Oliveira D.M.M., Tirapelli C.R., Casarini D.E., Fernandes F.B., Pinheiro L.C., Tanus-Santos J.E. Direct renin inhibition is not enough to prevent reactive oxygen species generation and vascular dysfunction in renovascular hypertension. Eur. J. Pharmacol. 2018;821:97–104. doi: 10.1016/j.ejphar.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Tsuchiya K., Kanematsu Y., Yoshizumi M., Ohnishi H., Kirima K., Izawa Y., Shikishima M., Ishida T., Kondo S., Kagami S., Takiguchi Y., Tamaki T. Nitrite is an alternative source of NO in vivo. Am. J. Physiol. Circ. Physiol. 2005;288:H2163–H2170. doi: 10.1152/ajpheart.00525.2004. [DOI] [PubMed] [Google Scholar]
- 27.Rosenbaek J.B., Pedersen E.B., Bech J.N. The effect of sodium nitrite infusion on renal function, brachial and central blood pressure during enzyme inhibition by allopurinol, enalapril or acetazolamide in healthy subjects: a randomized, double-blinded, placebo-controlled, crossover study. BMC Nephrol. 2018;19:244. doi: 10.1186/s12882-018-1035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feelisch M., Akaike T., Griffiths K., Ida T., Prysyazhna O., Goodwin J.J., Gollop N.D., Fernandez B.O., Minnion M., Cortese-Krott M.M., Borgognone A., Hayes R.M., Eaton P., Frenneaux M.P., Madhani M. Long-lasting blood pressure lowering effects of nitrite are NO-independent and mediated by hydrogen peroxide, persulfides, and oxidation of protein kinase G1α redox signalling. Cardiovasc. Res. 2019;44:12. doi: 10.1093/cvr/cvz202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jankov R.P., Daniel K.L., Iny S., Kantores C., Ivanovska J., Ben Fadel N., Jain A. Sodium nitrite augments lung S -nitrosylation and reverses chronic hypoxic pulmonary hypertension in juvenile rats. Am. J. Physiol. Cell. Mol. Physiol. 2018;315:L742–L751. doi: 10.1152/ajplung.00184.2018. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y., Hogg N., S-Nitrosothiols Cellular formation and transport. Free Radic. Biol. Med. 2005;38:831–838. doi: 10.1016/j.freeradbiomed.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 31.Smith J.N., Dasgupta T.P. Kinetics and mechanism of the decomposition of S-nitrosoglutathione by l-ascorbic acid and copper ions in aqueous solution to produce nitric oxide. Nitric Oxide. 2000;4:57–66. doi: 10.1006/niox.2000.0272. [DOI] [PubMed] [Google Scholar]
- 32.Leclerc P.C., Lanctot P.M., Auger-Messier M., Escher E., Leduc R., Guillemette G. S-nitrosylation of cysteine 289 of the AT1 receptor decreases its binding affinity for angiotensin II. Br. J. Pharmacol. 2006;148:306–313. doi: 10.1038/sj.bjp.0706725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nozik-Grayck E., Whalen E.J., Stamler J.S., McMahon T.J., Chitano P., Piantadosi C.A. S -nitrosoglutathione inhibits α 1 -adrenergic receptor-mediated vasoconstriction and ligand binding in pulmonary artery. Am. J. Physiol. Cell. Mol. Physiol. 2006;290:L136–L143. doi: 10.1152/ajplung.00230.2005. [DOI] [PubMed] [Google Scholar]
- 34.Qian J., Chen F., Kovalenkov Y., Pandey D., Moseley M.A., Foster M.W., Black S.M., Venema R.C., Stepp D.W., Fulton D.J.R. Nitric oxide reduces NADPH oxidase 5 (Nox5) activity by reversible S-nitrosylation. Free Radic. Biol. Med. 2012;52:1806–1819. doi: 10.1016/j.freeradbiomed.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orts H., Nystr T., Huang Z., Zhang F., Larsen F.J., Weitzberg E., Lundberg J.O., Sj A., Nyström T., Ortsäter H., Sjöholm Å. Inorganic nitrite stimulates pancreatic islet blood flow and insulin secretion. Free Radic. Biol. Med. 2012;53:1017–1023. doi: 10.1016/j.freeradbiomed.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 36.Velmurugan S., Kapil V., Ghosh S.M., Davies S., McKnight A., Aboud Z., Khambata R.S., Webb A.J., Poole A., Ahluwalia A. Antiplatelet effects of dietary nitrate in healthy volunteers: involvement of cGMP and influence of sex. Free Radic. Biol. Med. 2013;65:1521–1532. doi: 10.1016/j.freeradbiomed.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]