Abstract
In this study, we report the presence of a microbial community of bioremediation potential in terms of relative abundance and taxonomic biodiversity in sediment samples of river Ganga and Yamuna, India at nine different sites. Metagenomic libraries were constructed using TruSeq Nano DNA Library Prep Kit and sequenced on NextSeq 500 by Illumina Next Generation Sequencing (NGS) technology. Bioremediation bacteria belong to 45 genera with 92 species and fungi belong to 13 genera with 24 species have been classified using Kaiju taxonomical classification. The study revealed that Proteobacteria was the most dominant bacterial flora, followed by Actinobacteria, Firmicutes, and Deinococcus–Thermus. PCA analysis revealed that bioremediation bacteria viz. Streptomyces bikiniensis, Rhodococcus qingshengii, Bacillus aerophilus, Pseudomonas veronii, etc., were more dominant in highly polluted river stretch as compared to less polluted river stretch. Similarly, the relative abundance of bioremediation fungi viz. Phanerochaete chrysosporium and Rhizopus oryzae, etc., were significantly correlated with the polluted Kanpur stretch of river Ganga. Several protein domains, which play a pivotal role in bioremediation in the polluted environments, including urea ABC transporter, UrtA, UrtD, UrtE, zinc/cadmium/mercury/lead-transporting ATPase, etc., were identified using protein domain analysis. The protein domains involved in pesticide biodegradation viz. P450, short-chain dehydrogenases/reductases (SDR), etc., were also discovered in river sediment metagenomics data. This is the first report on the richness of bioremediation microbial communities in the Ganga and Yamuna riverine ecosystems, highlighting their importance in aquatic pollution management.
Keywords: metagenomics, Ganga, Yamuna, river sediment, bioremediation
Introduction
Aquatic environmental pollution is a global threat to natural biodiversity and human health (Nicolopoulou-Stamati et al., 2016). With the ever-increasing human population, the use of pesticides and other agro-input has been increased to achieve global food security (Ehrlich and Harte, 2015). Apart from regular use in the agricultural sector, pesticides are used in household purposes to control vector-borne diseases (VBD) like malaria, especially in developing countries (Van den Berg et al., 2012), including India. But the indiscriminate use of pesticides in agriculture and household ultimately results in a significant amount of their residues in an environment which eventually washes out to natural streams like rivers, wetlands, and finally to sea, causing a huge disruption in the natural aquatic biodiversity. It has been reported that pesticide residue found in wetlands, rivers, and sea in substantial amounts can cause the innumerable ill effect to aquatic organisms like endocrine disruption, growth reduction, etc. (Carvalho et al., 2009). Uses of organochlorine (OC) pesticides are banned in many countries for a long, but they are still found in the natural streams, sediments, aquatic flora, and fauna due to their high persistency. Several aquatic ecosystems including rivers, wetlands, and sea were being continuously monitored regularly for assessing the level of OC and their risk assessment on aquatic life (DiScenza et al., 2017; Fair et al., 2018; Unyimadu et al., 2018). Due to their high lipophilicity properties, they are being easily adsorbed on the river sediments and by the aquatic fauna. High concentrations of OC have been reported in the fish, Drapane africana (2237–6368 μg/kg) as compared to Mochokus niloticus (1006–3288 μg/kg) in the Niger River of Nigeria (Unyimadu et al., 2018). Gene networks involved in the reproduction and immune function of largemouth bass, are reported to disrupt in OC contaminated sites of the Lake Apopka (Martyniuk et al., 2016). Other than OC, the frequently used pesticides found in the natural stream belong to the group organophosphate, carbamates, pyrithroids, etc. Chlorpyrifos and other organophosphate pesticides are reported to impair immune function and structural integrity of the fish Cyprinus carpio L. through oxidative stress and apoptosis (Jiao et al., 2017). Lambda-cigalothrine, a pyrithroid insecticide has been reported to cause the alleviation of free amino acid content in the muscle, liver, and brain of fishes in the Alazani River (Dzhikiia et al., 2011).
Because of the toxic effect of pesticidal residues on the native flora and fauna, several physical and chemical methods are being used to eliminate these toxic xenobiotics from natural environments, like landfills, recycling, pyrolysis, etc., However, bioremediation using different microorganisms was found to be the most feasible technique, proved to be worked in many contaminated sites. It was reported that the presence of organophosphate hydrolase (OPH) enzyme in these microorganisms has the capacity of detoxifying them through cleavage of phosphate ester bonds (P-O, P-F, P-CN, and P-S) (Schofield and DiNovo, 2010). The yeast (Saccharomyces cerevisiae) capable of hydrolyzing the poorly hydrolyzed P-S class of organophosphate by integrating gene, encoding the wild-type OPH (enhanced variant enzyme S308L-OPH) into the ribosomal operon of the same (Makkar et al., 2013). Similarly, bacterial strain, Bacillus pumilis, was reported to be capable of bioremediation of methyl parathion, a P-S type OP pesticide, due to the presence of opdA gene (Ali et al., 2012). Several microorganisms belonging to the genera Bacillus, Brevibacillus, Ochrobactrum, Pseudomonas, Serratia, and Sphingobium are reported to be capable of degrading various pyrithroid pesticides through metabolic activity (Cycon and Piotrowska-Seget, 2016). Several genes with pyrithroid degrading ability were reported viz. estP in Klebsiella sp. ZD112 (Wu et al., 2006), pye3 from the metagenome of soil (Li et al., 2008), pytH in Sphingobium sp. JZ-1 (Wang et al., 2009), pytZ and pytY (from a genomic library of Ochrobactrum anthropi YZ-1) (Zhai et al., 2012). Not only bacteria, but several fungi were also reported with the ability of pesticide remediation through the catabolic or co-metabolic process. Fungi belonging to the genera Aspergillus, Candida, Cladosporium, Tricoderma, etc., were reported to degrade different pyrethroid pesticides like cyfluthrin, bifenthrin, deltamethrin, etc. (Cycon and Piotrowska-Seget, 2016).
The natural aquatic ecosystem holds and perishes a good amount of microbial population, which can degrade these toxic xenobiotic residues in situ. Therefore, to identify these potential microorganisms and their functional role, metagenomic studies have been conducted extensively in recent years. Metagenomic studies revealed a sediment microbiome in the Deepwater Horizon oil spill and the impact of oil deposition on microbial communities in surface sediments (Mason et al., 2014). Metagenome-assembled genomes (MAGs) revealed the microbial communities of two thermal pools in Kamchatka, Russia (Wilkins et al., 2019). Similarly, the key bacterial species were characterized in the Daphnia magna microbiota using shotgun metagenomics (Cooper and Cressler, 2020). Antibiotic Resistance Genes (AMRs) from the sediments of River Yamuna have been identified from the metagenomic study (Das et al., 2020). Using the metagenomics approach, potential microbial community involved in the biodegradation of phenanthrene, diesel, and hexadecane in the mangrove sediment were identified and the degrading bacteria belonged to genera Bacillus sp., Pseudomonas sp., Acinetobacter sp., and Staphylococcus sp. (Tiralerdpanich et al., 2018). The metagenomic approach was also utilized to demonstrate the role of IS1071, an insertion element flanks with xenobiotic degradation, in the formation and distribution of bacterial catabolic pathway gene cluster (Dunon et al., 2018). The biodegradation pathway of DDT, HCH, and atrazine in freshwater and marine sediments was analyzed through a metagenomics approach. The study identified 69 genera capable of degrading these persistent pesticides with major populations belonging to genus Plesiocystis sp., Anaerolinea sp., Jannaschia sp., and Mycobacterium sp., and found the presence of different genes viz. atzB, hdg, and hdt which encode for ethylaminohydrolase, dehalogenase, and hydratase, respectively (Fang et al., 2014). Carbamate pesticide degrading enzyme was also reported by functional metagenomic analysis of rumen samples of Holstein dairy cows (Ufarte et al., 2017). Similarly, the metagenomics approach was utilized to understand the underlying mechanism of biodegradation in situ and to predict the degradation potential of microbes in the soil of Queensland, Australia (Jeffries et al., 2018). However, there were not many studies on the occurrence of the native microbial population in the Ganga and Yamuna river ecosystem having bioremediation potential. With this background, the present study accentuates the identification and relative abundance of potential bioremediation microbes capable of degrading pollutants in these river sediments through the metagenomics approach. Furthermore, the study also aims to investigate the diversity and relative abundance of these bioremediation microbes in the different polluted and non-polluted sites of river Ganga and Yamuna.
Materials and Methods
Sample Collection and DNA Extraction
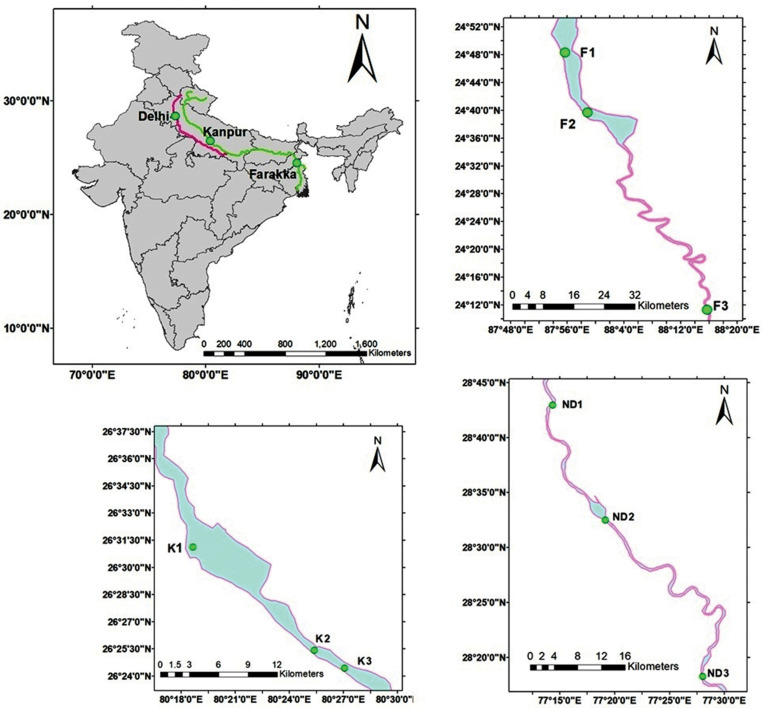
Sediment and water samples were collected from nine different sites of river Ganga viz. Ganga Barrage (K1, N 26°0.858″E 80°19.114″), Jajmau (K2, N 26°25.301″E 80°25.282″), Jana Village (K3, N 26°24.495″E 80°26.904″) near Kanpur, Uttar Pradesh, Farakka Barrage (F1, N 24°47.804″E 87°55.417″), Dhulian (F2, N 24°47.804″E 87°55.417″), Lalbagh (F3, N 29°11.087″E 88°16.079″) near Farakka, West Bengal and three different sites of river Yamuna at stretches viz. Wazaribad (ND1, N 28°42.39″E 77°13.57″), Okhla Barrage (ND2, N 28°2.51″E 77°18.30″), Faizupur Khaddar (ND3, N 28°18.43″E 77°27.52″) New Delhi, India during morning hours (8 to 11 AM) (Figure 1). All sediment samples were collected in plastic bags (sterile), sealed, and transported on ice (4°C) and stored at −80°C for metagenomics experiments. Metagenomic DNA from river sediment samples was extracted using a soil gDNA isolation Kit (Nucleospin Soil, Takara). After genomic DNA isolation, the quality was measured in Nanodrop 2000 and Qubit 3.0 Fluorometer and run in Agarose gel. Good quality sediment genomic DNA was used for next-generation library preparation.
FIGURE 1.
Map showing the sediment sampling sites. Sediments were collected from the river Ganga at six locations namely, K1 (Nawabganj, Kanpur), K2 (Jajmau, Kanpur), K3 (Jana village, Kanpur), F1 (Below Farakka bridge, West Bengal), F2 (Paharghati, West Bengal), and F3 (Lalbag, West Bengal) whereas, three locations from the river Yamuna viz. ND1 (Wazaribad, New Delhi), ND2 (Okhla Barrage, New Delhi), and ND3 (Faizupur Khadar, New Delhi).
Preparation of 2 × 150 NextSeq 500 Shotgun Libraries
Illumina Trueseq Nano DNA Library Prep Kit was used to prepare paired-end sequencing library. 200 ng of sediment gDNA was fragmented by CovarisM220 to produce a mean fragment allocation of 350 bp. CovarisM220 shearing method generates dsDNA fragments with 3/or 5/overhangs. All the fragments were then moved to end-repair. In the next step, products were PCR amplified with the index primer as provided in the Kit (TrueSeq DNA Nano Kit-Illumina). The libraries were analyzed in 4200 Tape Station System (Agilent Technologies) after PCR amplification using D1000 Screen tape.
Cluster Generation, Sequencing, Quality Control, and de novo Assembly
The Qubit fluorometric concentration for the libraries was measured and the mean peak size from Agilent Tape Station profile, the PE Illumina libraries were loaded into NextSeq 500 for cluster generation and sequencing. The CLC Genomics Workbench v8.5.1 was used to filter the high-quality reads of each sample and then processed into scaffolds.
Bioinformatics Analysis of Sediment Metagenome
The Trimmomatic v0.35 was used to process the sequenced raw data to obtain high-quality Clean read and to remove adapter sequences, ambiguous read (reads with unknown nucleotides “N” larger than 5%), and low-quality sequences (reads with more than 10% quality threshold {QV} < 20 phred Score). A minimum length of 100 nucleotides after trimming was applied. The high quality (QV > 20), paired-end reads were used for read assembly. Parameters considered for filtration such as, Adapter trimming; SLIDINGWINDOW: sliding window trimming of 20 bp, cutting once the average quality within the window falls below a threshold of 20; LEADING: cut bases off the start of a read, if bellow a threshold quality of 20; TRAILING: cut bases off the end of a read, if bellow a threshold quality of 20; MINLENGTH: Drop the read if it is below 100 bp length.
The filtered metagenomic reads were used for taxonomical assignment by the Kaiju web server1 for identification of bioremediation microbial species in the river sediment metagenome using NR protein database NCBI BLAST nr as reference database (Menzel et al., 2016; Cucio et al., 2018; Verce et al., 2019). Kaiju used Burrows-Wheeler transform algorithm for taxonomic classification on the protein-level. “Greedy” run mode was applied with a minimum match score of 70, and an allowance of five mismatches (Menzel et al., 2016). Further, other webserver/software viz. MG RAST and CLC Genomics Workbench v8.5.1 were used for validation of the identified bioremidation microbial communities. To study the phylogenetic relationship among the bioremediation bacterial species found in the river sediment metagenomes, multiple sequence analysis was carried out using MEGA 6 software (Tamura et al., 2013). The Neighbor-Joining method was used to infer evolutionary history (Saitou and Nei, 1987). The Maximum Composite Likelihood method (Tamura et al., 2004) was used to compute evolutionary distances. In this study, 92 identified bioremediation bacterial species, derived from the sediments of the rivers, Ganga and Yamuna were used. Heat map figures were generated using multiple experiment viewer (Mev), a web-based tool for visualizing the clustering of multivariate data (Howe et al., 2010). Following the identification of the bioremediation bacterial gene from metagenomic samples, the set of genes have been considered for ORF prediction. The resultant ORFs were then assessed for the identification of putative or conserved domains.
Analysis of Physico-Chemical Parameters
The DO (ppm), TDS (ppm), specific conductivity (μS/cm), salinity (%), pH, BOD (ppm), and COD (ppm) of collected river water samples from nine sampling sites were analyzed as per the standard methods of APHA (2012). The pH, specific conductivity (μS/cm), total nitrogen (%), available phosphate (mg/100 g), and organic C (%) of collected river sediment samples from nine sampling sites were also analyzed as per the standard methods of APHA (2012).
Statistical Analysis
The Principal Component Analysis (PCA) biplot and Scatter Plot Matrix along with correlation values between sampling sites and relative abundance of bioremediation microbes were developed in JMP Pro 10 after standardization of the estimated data. The Diversity index (β-diversity) analysis was carried out using Past software (version 4.03).
Results
Generation of Sediment Metagenome Data
Sediment samples were collected from the river Ganga at two locations namely, Kanpur (K1, K2, and K3) and Farakka (F1, F2, and F3), each with three sampling sites. While, from the river Yamuna, sediment samples were collected from the New Delhi stretch (ND1, ND2, and ND3) at three sampling sites. The measurements of isolated metagenomic DNA for quality and quantity have been presented in Table 1. The isolated river sediments metagenomic DNA from these nine sites were analyzed using high-throughput NGS technology and the total number of high quality reads (bp) of each sample collected from Kanpur, Farakka, and New Delhi stretches were 28718955(K1), 33703138(K2), 33887572(K3), 24929338(F1), 29128182(F2), 54496302(F3), 64876611(ND1), 64749798(ND2), and 62670420(ND3), respectively. All the high quality reads obtained from the sediments of different sites were considered for in silico analysis to discover the microbial diversity with bioremediation potential. Severe pollution by untreated sewage from the hundreds of tanneries, pharmaceutical industries, municipal waste, chemicals, pesticides, etc., were reported from New Delhi and Kanpur stretches. It was found that some critical pollution parameters (viz. BOD, COD, total nitrogen, and phosphate) were higher in these two sites as compared to the less polluted sites at Farraka. The details of the physicochemical parameters of water and sediments collected from these sites are presented in Table 2.
TABLE 1.
Average concentration of metagenomic DNA isolated from different river sediment samples.
| Sl. No. | Sample ID | Qubit nucleic acid concn. (ng/μ l) | Nano drop OD A260/280 |
| 1 | F1 | 6.60 | 1.80 |
| 2 | F2 | 6.20 | 1.83 |
| 3 | F3 | 46.00 | 1.87 |
| 4 | K1 | 45.80 | 1.80 |
| 5 | K2 | 80.20 | 1.85 |
| 6 | K3 | 185.60 | 1.80 |
| 7 | ND1 | 227.10 | 1.83 |
| 8 | ND2 | 186.50 | 1.82 |
| 9 | ND3 | 203.20 | 1.84 |
TABLE 2.
Physiochemical parameters of water and sediment at different sampling sites of river Ganga and Yamuna.
| Pollution Parameters |
River ganga |
River yamuna |
||||||||
|
Kanpur |
Farraka |
New Delhi |
||||||||
| Up side of ganga barrage (K1) | Jajmau (K2) | Jana village (K2) | Farakka barrage (F1) | Dhulian (F2) | Lalbagh (F3) | Wazaribad (ND1) | Okhla barrage (ND2) | Faizupur khaddar(ND3) | ||
| Water | DO (ppm) | 5.01 | 4.38 | 4.16 | 6.34 | 6.39 | 6.77 | 1.9 | 3.1 | 2.8 |
| TDS (ppm) | 170.4 | 316 | 227 | 95.5 | 94.3 | 96.5 | 342.5 | 466.3 | 356.15 | |
| Specific Conductivity (μS/cm) | 356 | 641 | 469 | 200.7 | 198.3 | 222.6 | 425.3 | 895 | 651.23 | |
| Salinity (%) | 0.17 | 0.31 | 0.23 | 0.09 | 0.09 | 0.09 | 0.23 | 0.14 | 0.15 | |
| pH | 8.10 | 7.84 | 7.9 | 8.20 | 7.78 | 7.82 | 7.98 | 7.65 | 7.237 | |
| BOD (ppm) | 1.0 | 6.0 | 6.2 | 0.2 | 0.8 | 0.5 | 5.1 | 7.9 | 4.23 | |
| COD (ppm) | 2.0 | 9.0 | 8.0 | 0.16 | 1.8 | 0.8 | 5.9 | 11.23 | 8.56 | |
| Sediment | pH | 7.2 | 7.8 | 8.1 | 7.01 | 7.5 | 7.6 | 7.5 | 7.9 | 7.8 |
| Specific Conductivity (μS/cm) | 0.09 | 0.54 | 0.89 | 0.06 | 0.12 | 0.11 | 0.15 | 0.65 | 0.35 | |
| Total Nitrogen (%) | 0.06 | 0.09 | 0.07 | 0.04 | 0.05 | 0.05 | 0.04 | 0.06 | 0.08 | |
| Available Phosphate (mg/100g) | 4.0 | 5.8 | 5.2 | 2.6 | 5.4 | 3.2 | 4.2 | 8.2 | 6.8 | |
| Organic C (%) | 0.10 | 0.32 | 0.19 | 0.35 | 0.43 | 0.21 | 0.09 | 0.3 | 0.47 | |
Taxonomical Classification of Sediment Metagenomes
Bioremediation bacteria belong to 45 genera with 92 species and fungi belong to 13 genera with 24 species have been classified using Kaiju taxonomical classification, MG RAST and CLC Genomics Workbench v8.5.1. A large number of bioremediation microbes with their relative abundance were identified from the sediment samples of river Ganga and Yamuna based on taxonomical classification (Supplementary Table S1). Twelve Bacillus sp., thirteen Pseudomonas sp., four Burkholderia sp., four Streptomyces sp., four Rhodococcus sp. three Sphingobium sp., Acinetobacter sp., Aeromonas sp., two Sphingomonas sp., Stenotrophomonas sp., Ochrobactrum sp., Geobacter sp., Enterobacter sp., Desulfovibrio sp. and more other bacterial species have been identified from the metagenomics data set. Among the phylums, it was found that Proteobacteria was most abundant, followed by Actinobacteria, Firmicutes, and Deinococcus-Thermus. Similarly, many bioremediation fungi viz. six Aspergillus sp., three Phanerochaete sp., two Bjerkandera sp., two Pleurotus sp., two Rhizopus sp., two Trametes sp., Clonostachys sp., Coprinus sp., Exophiala sp., Fusarium sp., Mucor sp., Penicillium sp. and Trichoderma sp. were also identified (Supplementary Table S2).
Phylogenetic Analysis of Bioremediation Bacteria
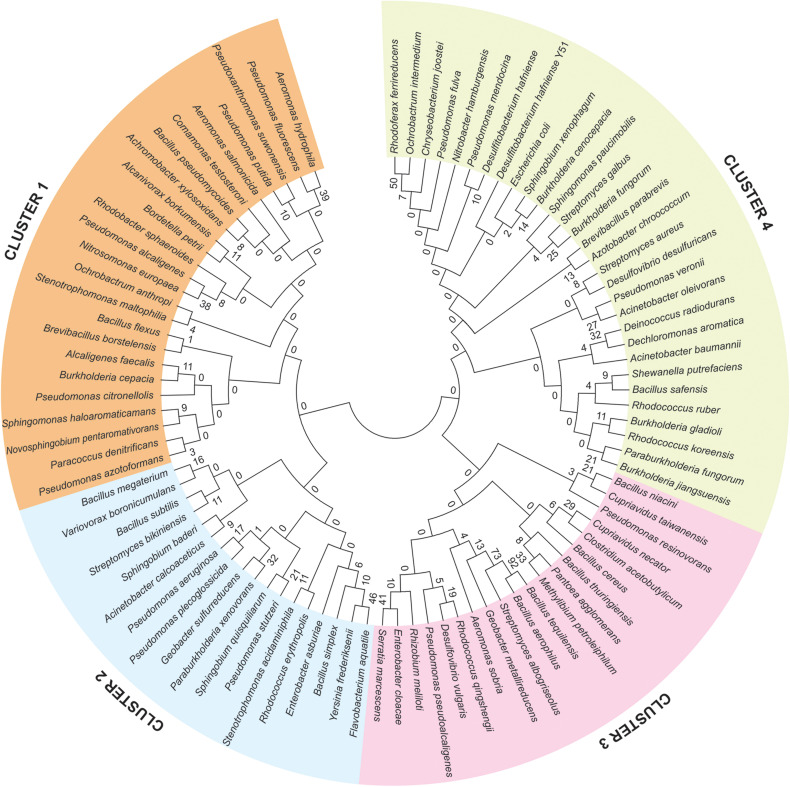
To understand the evolutionary relationship among the 92 identified genomes of bioremediation bacterial species, a Multiple Sequence Analysis (MSA) using MEGA 6 has been carried out. Based on the MSA, phylogenetic relationships were established. Out of four clusters, in cluster 1, Pseudomonas fluorescens and Aeromonas hydrophila lies in one clade with the highest bootstrap value. Similarly, Flavobacterium aquatile and Yersinia frederiksenii are closely related to each other in Cluster 2, while, Bacillus tequilensis and Bacillus aerophilus are phylogenetically closely related in Cluster 3. Rhodoferax ferrireducens and Ochrobactrum intermedium are very close to each other in Cluster 4 (Figure 2).
FIGURE 2.
Phylogenetic analysis of 92 identified genome of bioremediation bacterial species, derived from the sediments of the river Ganga and Yamuna. Phylogenetic cladogram clearly demarcated that, all the identified bacterial species shaped four different clusters represented in different colors and the bootstrap values are provided at the nodes of the phylogenetic tree.
Microbial Diversity Analysis
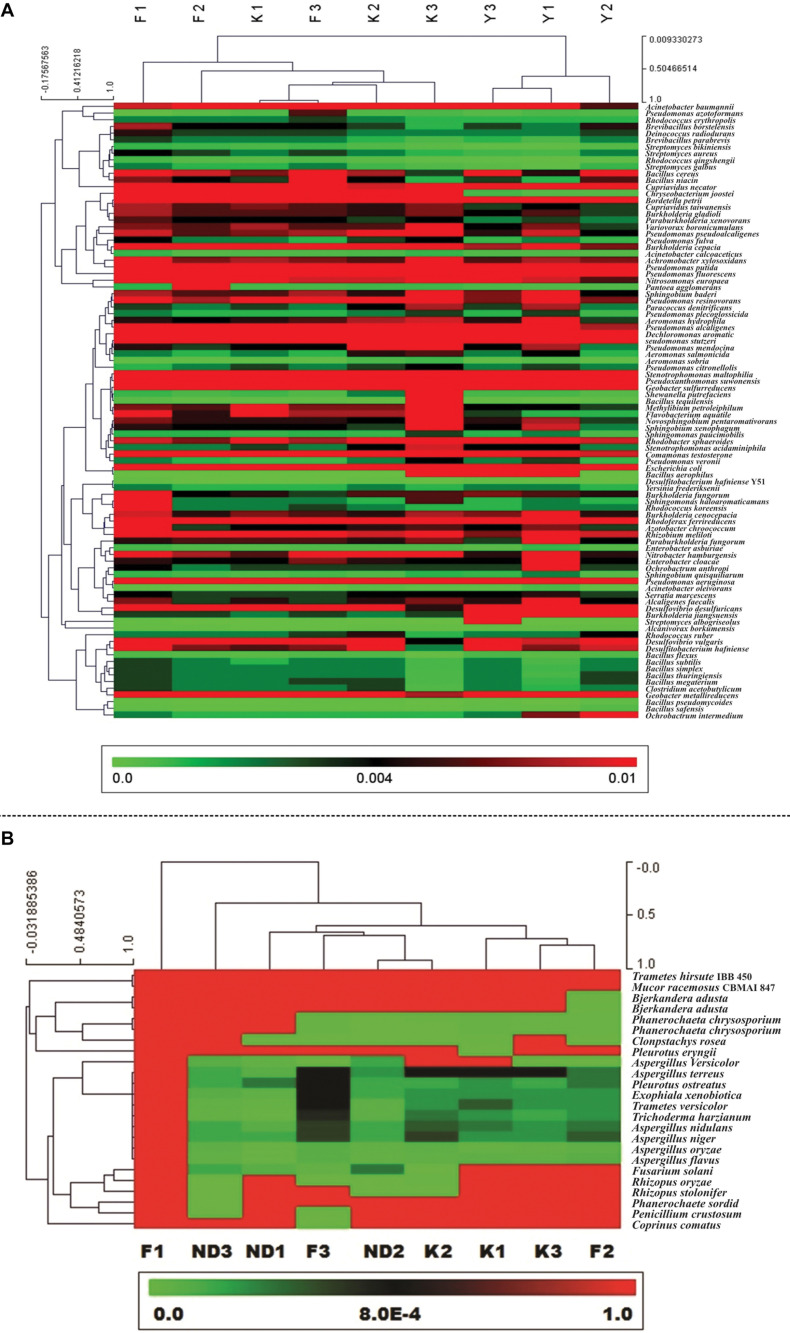
In the classified metagenomics data, a total of 92 bioremediation bacterial species from the 45 genera were considered for diversity analysis. Heat map analysis showed the clear distinction in the relative abundance of bioremediation bacteria between Kanpur and Farakka sediment samples which might be due to the higher level of pollution at Kanpur stretch of the river Ganga. The river Yamuna at New Delhi stretch was found to be highly polluted and contaminated by industrial and municipal swages. Heat map analysis portrayed a clear demarcation on the species relative abundance among nine different sites in the bacterial metagenomics data (Figure 3A) and 24 species of fungi (Figure 3B). Relative abundance analysis revealed that the various Streptomyces sp. were present in higher proportion in sediment samples of river Ganga (Farakka and Kanpur stretches) compare to river Yamuna. Streptomyces albogriseolus, Streptomyces aureus, and Streptomyces bikiniensis species were present in relatively high proportion at Farakka stretch of the river Ganga (Supplementary Table S1). From these metagenomics data, two Rhodococcus species (Rhodococcus erythropolis and Rhodococcus qingshengii) were found in higher abundance in Farakka stretch as compared to Yamuna stretch. Cupriavidus necator, Cupriavidus taiwanensis and Paraburkholderia xenovorans were also found higher abundance in Farakka stretch. Pseudomonas citronellolis was found in higher abundance in the Kanpur stretch as compared to Farakka and New Delhi stretches. Similarly, Flavobacterium aquatile, Methylibium petroleiphilum, Pantoea agglomerans, Bordetella petrii, Flavobacterium aquatile, Methylibium petroleiphilum were found higher in Kanpur stretch. Chryseobacterium joostei and Desulfovibrio desulfuricans were highly abundant in the New Delhi stretch as compared to Kanpur and Farakka stretches (Supplementary Table S1).
FIGURE 3.
Relative abundance Heat map of identified 92 bacterial species (A) and 24 fungal species (B) having bioremediation potential from nine different sediment metagenomes of river Ganga and Yamuna.
Among the bioremediation fungi, Aspergillus terreus, Trichoderma harzianum, and Trametes versicolor are found higher quantities in Kanpur stretch as compared to Farakka and New Delhi stretches. Aspergillus versicolor and Fusarium solani are observed in higher quantities in New Delhi stretch of the river Yamuna compared to the Kanpur and Farraka stretch of the river Ganga (Supplementary Table S2). The diversity index between nine sampling sites (β-diversity) with heat map was analyzed (Whittaker method) and presented in Supplementary Figures S1, S2. The β-diversity of bioremediation bacteria between F1 and ND3 was highest (0.027). Similarly, the β-diversity of bioremediation fungus was found to be the highest (0.379) between K1 and ND3.
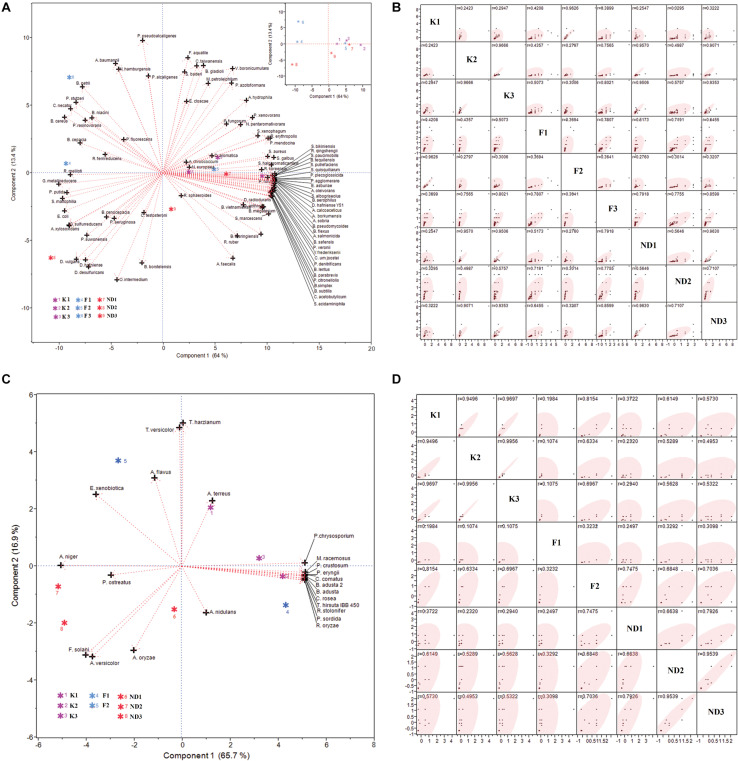
PCA Analysis
The relative abundance of 92 bacterial and 24 fungal species at nine different study sites was analyzed by PCA and correlation between sampling sites was observed in the Scatter Plot Matrix. The PCA biplot of bacterial relative abundance showed that the first two components together could explain 77.4% (PC1, 64% and PC2, 13.4%) variability in the relative abundance data and showed that, majority of the bioremediation bacteria are having high relative abundance at the polluted stretch of river Ganga i.e., Kanpur stretch and river Yamuna i.e., New Delhi stretch (Figure 4A). The Scatter Plot Matrix (Figure 4B) depicted that, some sites were correlated based on the relative abundance of bioremediation bacteria viz. K2 and K3 (r = 0.97); ND1 and ND3 (r = 0.96) and F2 and K1(r = 0.96). However, some sites were not found to be correlated with any other sites viz. F1, F3, and ND2.
FIGURE 4.
PCA biplot and Scatter Plot Matirx of relative abundance of bioremediation bacteria (A and B, respectively) and fungi (C and D, respectively) found at the test sites. PC1 and PC2 together could explain 77.4% and 82.6% of variability in relative abundance of bacteria and fungi, respectively.
Similarly, the PCA biplot of fungal relative abundance (PC1, 65.7%, and PC2, 16.9%) showed that the majority of the bioremediation fungi are having high relative abundance at the polluted stretch of river Ganga i.e., Kanpur stretch (Figure 4C). It was interesting to note that, the relative abundances of bioremediation fungi were found to zero at the site F1 and the sites F2 and F3 were not found correlated. The Scatter Plot Matrix (Figure 4D) also depicted that, Kanpur sites (K1, K2, and K3) were highly correlated (r > 0.94) so was ND2 and ND3 (r > 0.95).
Functional Metagenomics-Protein Domain Analysis
ORF’s were predicted from the identified genomes of bioremediation bacterial species, considered for conserved domain (CD) analysis. The CD-search analysis revealed that an immense number of domains related to bioremediation has been found in the sediment metagenome of these rivers. Protein domains related to bioremediation such as ABC transporter (cl25403, cd03214, TIGR03407, cd03297, TIGR02789, TIGR02770, TIGR03411, TIGR03410, cd03260, PRK10419, cd00625, and cl28564) is involved in the transport of a wide variety of different compounds, like molybdenum, nickel, arsenic and more complex organic molecules were found in the sediment metagenomes of river Ganga and Yamuna. Further, other transporter protein domain like, zinc/cadmium/mercury/lead-transporting ATPase (PRK11033), iron-dicitrate transporter (PRK11231), ferric transporter (PRK11432), cobalt transporter (PRK13647), nitrate transporter (TIGR01184), potassium transporter (COG2205), phosphonate/organophosphate ester transporter (PRK09984), ABC-type bacteriocin transporter (TIGR01193), P-type heavy metal-transporting ATPase (cd07546), which play a significant role in bioremediation have also been identified. In addition to that, several hydrolases like Ab-hydrolase, Metallo-dependent hydrolases, N-ethylammeline chlorohydrolase, which play a considerable role in bioremediation, were identified. The Cytochrome P450s domain which is haem-thiolate proteins and several Haloacid Dehalogenase like Hydrolases (HAD) (cd04302, cl21460, cd01427, pfam00702, TIGR01549, cd07512, cd16417, pfam13419) which play an active role in the oxidative degradation of various compounds, degradation of environmental toxins/mutagens and pesticide bioremediation has also been identified. Gene ontology (GO) analysis of Cytochrome P450s reveals that, P450s domain involved in the molecular function and biological process pathways for iron ion binding (GO:0003674), pigment biosynthetic process (GO:0008150), heme-binding (GO:0003674), and oxidoreductase activity (GO:0003674 and GO:0008150). Similarly, GO analysis of all the other identified domains has been carried out and given in Supplementary Figures S3–S6. All the domains related to bioremediation along with their accession number, super-family, and producing organism name has been tabulated in the Supplementary Table S3.
Discussion
The present metagenomics study identified the potential bioremediation microbes in the sediments of river Ganga and Yamuna and investigates the correlation between microbial relative abundance and diversity with polluted and non-polluted sites of these rivers. The lists of identified bioremediation microbes with target pollutants are given in Supplementary Tables S4, S5. In recent times, bioremediation of several toxic chemicals reported throughout the world. Sediments from rivers and lagoons of the Southern Gulf of Mexico were used for the biodegradation of hexadecane (Garcia-Cruz et al., 2018). The proteins of Brevibacillus brevis respond to Triphenyl phosphate and provide novel insights into the biodegradation process of bacteria under adverse environmental conditions (Wei et al., 2018). A previous study revealed that the bacterial isolates of a stream in Manaus-Amazon could bioremediate chromium-polluted ecosystems. These bacteria were classified in 10 genera viz. Micrococcus sp., Proteus sp., Acinetobacter sp., Acidovorax sp., Bacillus sp., Enterobacter sp., Comamonas sp., Alicycliphilus sp., Serratia sp., and Vagococcus sp. (Teles et al., 2018).
The present research was designed to identify bioremediation bacteria and fungi species of commercial importance and their relations with different riverine ecosystems using high-throughput sequencing and computational metagenomics. The sensitive taxonomical classification was used for the identification of microbial community biodiversity and their functions (Mendes et al., 2017). A large number of bioremediation bacteria and fungal species (Bacteria: 45 genera and 92 species; fungi: 13 genera and 24 species) (Supplementary Tables S1, S2) were found including pesticide degrading domains in the Ganga and Yamuna river sediments.
To investigate the evolutionary correlations among the identified bioremediation bacterial genome, a phylogenetic tree was constructed which showed the majority of the species are similar throughout the evolution. The phylogenetic tree of all the identified bioremediation species was configured in four different clusters. In Cluster 1, Pseudomonas fluorescens and Aeromonas hydrophila lies in one clade with the highest bootstrap value. The Bacillus tequilensis and Bacillus aerophilus are phylogenetically closely related in Cluster 3. The phylogenetic analysis based on the 16S rRNA gene of 181 type strains of Bacillus species and related taxa constituted nine clusters. The study revealed that Bacillus was not a monophyletic group. B. subtilis was in Group 1. Group 4 corresponds to thermophiles, Group 6 to halophilic or halotolerant bacilli, and Group 8 to alkaliphilic bacilli (Wang and Sun, 2009).
Relative abundance study showed that bioremediation bacteria of different genera were equally distributed among the three locations; however, few species dominate in one location over others, viz. Flavobacterium aquatile, Methylibium petroleiphilum, Pantoea agglomerans, Pseudomonas citronellolis were highly abundant in Kanpur location of river Ganga as compared to other locations. These location-specific changes of microbial diversity in the river sediments might be due to differential physicochemical properties and pollution level of the collected sediments. The present results also suggested that the relative abundance of bioremediation microbial communities was significantly correlated with polluted sites at river Ganga and Yamuna. The study revealed that polluted sites at New Delhi stretch of Yamuna and Kanpur stretch of Ganga were having a higher relative abundance of bioremediation bacteria and fungi as compared to less polluted Farakka sites.
Farakka stretch of the river Ganga was found to be less polluted (Samanta, 2013) whereas, the Kanpur stretch of Ganga and the New Delhi stretch of the river Yamuna were reported to be severely polluted due to the release of untreated metropolitan swages, factory effluents, etc. (Sehgal et al., 2012; Malik and Tauler, 2013). High concentrations of malathion (2.618 μg/l) and γ-HCH (0.259 μg/l) were detected in the surface water collected from the Ganga riverine ecosystem at Kanpur stretch. From the potable water samples in Delhi, Organochlorine pesticides, mainly isomers of hexachlorohexane, dichloro-diphenyl-trichloroethane, endrin, endosulfan, aldrin, dieldrin, and heptachlore were identified. It had been found α and β isomers of endosulfan residues in the Yamuna river (Agarwal et al., 2015). In the present investigation, the differential physicochemical parameters might be responsible for differences in the relative abundance of bioremediation microbes at different locations in the river Ganga and Yamuna. In the present study, a higher relative abundance of Bordetella petrii in Kanpur and Farakka was found as compared to the Yamuna. For that reason, it was postulated that pollution makes differences in the relative abundance of bioremediation bacteria species among different locations in the river Ganga and Yamuna. The β-diversity analysis also revealed that diversity indices of bioremediation bacteria and fungi of Farakka samples are different from the polluted sites of New Delhi.
Pesticide degrading bacteria identified in the present study are important as naturally occurring microbes can be manipulated to degrade highly toxic and carcinogenic compounds. Several microbes like Acephate degrading bacteria, Enterobacter asburiae, Bacillus cereus, Pantoea agglomerans was identified from sediments of the river Ganga and Yamuna, and these bacteria are reported earlier for pesticide decomposition by Ramya et al., 2016. Fipronil degrading Acinetobacter calcoaceticus and Acinetobacter oleivorans (Uniyal et al., 2016) were also found from the present sediment metagenomics at all locations. Pseudomonas putida and Rhodococcus sp. which degrade both the endosulfan isomers through oxidative and hydrolytic pathways were also found (Singh et al., 2017). Endosulfan degrading bacterial strains Pusillimonas sp. JW2 and Bordetella petrii NS were reported from polluted water and soil environments (Kong et al., 2018).
Bioremediation fungi Burkholderia jiangsuensis was reported to possess methyl parathion hydrolase (MPH) gene (bjmpd) (Liu et al., 2014). The present experiments found a significantly higher relative abundance of Burkholderia jiangsuensis in the Farakka stretch, whereas the same was not found at New Delhi stretch of river Yamuna. Cupriavidus necator was found to be involved in the degradation of aromatic compounds through 2, 3-dioxygenase pathway (Berezina et al., 2015). Cupriavidus necator was also found significantly higher quantities in Farakka locations in the present investigation. Highly competent chlorpyrifos degrading strain of Cupriavidus taiwanensis (Wang et al., 2015) was also found in higher proportion at Farakka locations.
Bioremediation fungi, Aspergillus terreus, found in the present study was reported to expresses Feruloyl esterases (FAEs) which is the key enzyme for biodegradation of lignocelluloses and hydrolysis of ester linkages between hemicellulose and lignin (Zhang et al., 2015). The study revealed that chlorfenvinphos could be degraded by Aspergillus fumigatus, Penicillium citrinum, Aspergillus terreus, and Trichoderma harzianum (Oliveira et al., 2015), and lots of these species were reported from the metagenomics samples. Phanerochaete chrysosporium, Aspergillus awamori, Aspergillus flavus, Trichoderma viride found to be involved in heavy metal bioremediation like Pb, Cd, Cr, and Ni (Joshi et al., 2011).
Aspergillus nidulans isolated from arsenic-contaminated soil was found to have the arsenic adsorption potential to be 84.35% (Maheswari and Murugesan, 2009). Aspergillus versicolor was reported to degrade antibiotic triclosan in simulated wastewater and semi-synthetic media (Tastan and Donmez, 2015). Exophiala xenobiotica was found to be dominant black yeast present in the car gasoline tanks, able to use toluene as a carbon source (Isola et al., 2013). Fusarium solani was reported to tolerate caffeine concentration of 8 g/L (Nayak et al., 2013). It is reported that Trametes versicolor (3000 UL–1) could produce laccase and Pleurotus ostreatus (2700 UL–1) showed the ability to degrade PAHs, phenanthrene (PHE) and pyrene (PYR) (Rosales et al., 2013). In our study, Aspergillus terreus, Trichoderma harzianum, and Trametes versicolor found higher quantities in Kanpur locations compared to Farakka and New Delhi stretches. Aspergillus versicolor and Fusarium solani were discovered in higher quantities in the New Delhi stretch of river Yamuna compared to the river Ganga.
PCA has been widely used to interpret significant bioremediation species from the experimental data set (Zitko, 1994; Vega et al., 1998; Shrestha and Kazama, 2007). The present analysis showed that the relative abundance of bacterial and fungal species of bioremediation potential is better correlated with the polluted sites of the river as compared to less polluted sites i.e., Farakka stretch. The study also revealed the location-wise clustering of data for Kanpur and New Delhi stretches of river Ganga and Yamuna due to a high correlation between pollution sites and relative abundance of microbes. Bioremediation fungal species showed a more prominent effect through a strong correlation with polluted sites viz. K2 and K3.
In the present metagenome, urea ABC transporter ATP binding protein Urtd gene in Alcaligenes faecalis, Bacillus cereus, Rhodococcus qingshengii, UrtE gene in Alcaligenes faecalis and Bacillus cereus bacteria were found in Farakka sample. In Kanpur stretch, UrtA gene was found in Ochrobactrum anthropi, UrtD, UrtE gene in Serratia marcescens. All members of this protein family ABC transporter ATP-binding subunits or gene clusters are expressed, producing the urea permease and connected with urea transport and metabolism. The role of these genes or domain in the bioremediation of urea can be supported by the previous finding (Valladares et al., 2002) where it was suggested that the involvement of a transporter of this superfamily in urea scavenging by some cyanobacteria in natural environments.
Another domain cd08419 which is a C-terminal substrate-binding of LysR-type transcriptional regulator (CbbR) of RubisCO operon, involved in carbon dioxide fixation was identified (Supplementary Table S3). The versatile function of various metal ion transport, amino acid transport, nickel, nitrate, phosphate, molybdenum, cobalt, Mn2+/Zn2+ transport, zinc/cadmium/mercury/lead-transporting ATPase, etc., found in the present metagenome were likely to play an active role in bioremediation process. In bacteria Pseudomonas putida, P450 was reported to be involved in Hexachlorobenzene and Pentachlorobenzene metabolism earlier (Scott et al., 2008). The Oxidoreductase gene was also reported in Pseudomonas sp. LBr, Agrobacterium strain T10 participates in Glyphosphate biodegradation (Scott et al., 2008). In the present study, enzymes from two different microbes, Burkholderia xenovorans and Flavobacterium aquatile were discovered which are actively involved in pesticide bioremediation.
The Metagenomic sequences have been submitted to the NCBI-SRA database under the Accession Nos: Kanpur Samples K1 (SRP190174), K2 (SRP190175), K3 (SRP189880), Farakka Samples, F1 (SRP191076), F2 (SRP191079), F3 (SRP191075), and New Delhi Samples, ND1 (SRP191073), ND2 (SRP191080), and ND3 (SRP191499) respectively.
Conclusion
This is the first report on the identification of bioremediation microbes in sediments of the river Ganga and Yamuna in India, using a metagenomic approach. The present study unraveled the in-depth insights on the relative abundance of important native bacteria and fungi convoluted in bioremediation in these rivers and their functional properties for possible biotechnological applications. The identified Nitrobacter humburgensis could reduce heavy metals like zinc, cadmium, mercury, and lead. Similarly, Rhodobacter sphaeroides could reduce arsenic, Shewanella putrefaciens for iron, Bacillus cereus for molybdenum and Alcaligenes faecalis for PAHs. The identified fungus like Aspergillus flaves could reduce lead, cadmium, chromium, and nickel, and Aspergillus nidulans for arsenic, etc., It was found that these bioremediation bacteria and fungi were more abundant in the sediments of a polluted stretch of the river. Several identified protein domains, which are actively involved in environmental bioremediation processes, could be explored for genome-scale engineering for industrial applications in the future.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/ Supplementary Material.
Author Contributions
BKB designed the metagenomics study and also collected samples for this research. HJC, BP and AKR performed bioinformatics data analysis. DJS and RR performed statistical analysis. BKB, BP, HJC, BD, AKR, DJS, PKP, ARR, AR, BKD, and TM wrote the manuscript. All authors participated in revision of the final manuscript for submission.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are thankful to Dr. J. K. Jena, Deputy Director General (Fisheries Science), Indian Council of Agricultural Research, New Delhi for his constant guidance and support. The authors also acknowledge the technical assistance of Mr. Asim Kumar Jana, Technical Officer for sample collection and laboratory analysis work.
Funding. This research work has been carried out under the CABin Project.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.556136/full#supplementary-material
References
- Agarwal A., Prajapati R., Singh O. P., Raza S. K., Thakur L. K. (2015). Pesticide residue in water- a challenging task in India. Environ. Monit. Assess. 187:54. 10.1007/s10661-015-4287-y [DOI] [PubMed] [Google Scholar]
- Ali M., Naqvi T. A., Kanwal M., Rasheed F., Hameed A., Ahmed S. (2012). Detection of the organophosphate degrading gene opdA in the newly isolated bacterial strain Bacillus pumilus W1. Ann. Microbiol. 62 233–239. 10.1007/s13213-011-0251-4 [DOI] [Google Scholar]
- APHA (2012). Standard Methods for The Examination of Water and Wastewater, 22nd Edn Washington, DC: American Public Health Association (APHA). [Google Scholar]
- Berezina N., Yada B., Lefebvre R. (2015). From organic pollutants to bioplastics: insights into the bioremediation of aromatic compounds by Cupriavidus necator. N. Biotechnol. 32 47–53. 10.1016/j.nbt.2014.09.003 [DOI] [PubMed] [Google Scholar]
- Carvalho F. P., Villeneuve J. P., Cattini C., Rendón J., Mota de Oliveira J. (2009). Pesticide and PCB residues in the aquatic ecosystems of Laguna de Terminos, a protected area of the coast of Campeche, Mexico. Chemosphere. 74 988–995. 10.1016/j.chemosphere.2008.09.092 [DOI] [PubMed] [Google Scholar]
- Cooper R. O., Cressler C. E. (2020). Characterization of key bacterial species in the Daphnia magna microbiota using shotgun metagenomics. Sci. Rep. 10:652. 10.1038/s41598-019-57367-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucio C., Overmars L., Engelen A. H., Muyzer G. (2018). Metagenomic analysis shows the presence of bacteria related to free-living forms of sulfur-oxidizing chemolithoautotrophic symbionts in the rhizosphere of the seagrass Zostera marina. Front. Mar. Sci. 5:171 10.3389/fmars.2018.00171 [DOI] [Google Scholar]
- Cycon M., Piotrowska-Seget Z. (2016). Pyrethroid-degrading microorganisms and their potential for the bioremediation of contaminated soils: a review. Front. Microb. 7:1463. 10.3389/fmicb.2016.01463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das B. K., Behera B. K., Chakraborty H. J., Paria P., Gangopadhyay A., Rout A. K., et al. (2020). Metagenomic study focusing on antibiotic resistance genes from the sediments of River Yamuna. Gene 758:144951. 10.1016/j.gene.2020.144951 [DOI] [PubMed] [Google Scholar]
- DiScenza D. J., Lynch J., Miller J., Verderame M., Levine M. (2017). Detection of organochlorine pesticides in contaminated marine environments via cyclodextrin-promoted fluorescence modulation. ACS Omega 2 8591–8599. 10.1021/acsomega.7b00991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunon V., Bers K., Lavigne R., Top E. M., Springael D. (2018). Targeted metagenomics demonstrates the ecological role of IS1071 in bacterial community adaptation to pesticide degradation. Environ. Microbiol. 20 4091–4111. 10.1111/1462-2920.14404 [DOI] [PubMed] [Google Scholar]
- Dzhikiia G. M., Mchedluri T. T., Nikolaishvili M. I., Zurabishvili Z. A., Iordanishvili G. S. (2011). The pyretroind pesticides lambda-cigalothrines influence on composition of free aminoacids in tissue of fish in Alazani River. Georgian Med. News 21 52–55. [PubMed] [Google Scholar]
- Ehrlich P. R., Harte J. (2015). Opinion: to feed the world in 2050 will require a global revolution. Proc. Natl. Acad. Sci. U.S.A. 112 14743–14744. 10.1073/pnas.1519841112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair P. A., White N. D., Wolf B., Arnott S. A., Kannan K., Karthikraj R., et al. (2018). Persistent organic pollutants in fish from Charleston Harbor and tributaries, South Carolina, United States: a risk assessment. Environ. Res. 167 598–613. 10.1016/j.envres.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H., Cai L., Yang Y., Ju F., Li X., Yu Y., et al. (2014). Metagenomic analysis reveals potential biodegradation pathways of persistent pesticides in freshwater and marine sediments. Sci. Total Environ. 470 983–992. 10.1016/j.scitotenv.2013.10.076 [DOI] [PubMed] [Google Scholar]
- Garcia-Cruz N. U., Sánchez-Avila J. I., Valdés-Lozano D., Gold-Bouchot G., Aguirre-Macedo L. (2018). Biodegradation of hexadecane using sediments from rivers and lagoons of the Southern Gulf of Mexico. Mar. Pollut. Bull. 128 202–207. 10.1016/j.marpolbul.2018.01.026 [DOI] [PubMed] [Google Scholar]
- Howe E., Holton K., Nair S., Schlauch D., Sinha R., Quackenbush J. (2010). “Mev: multiexperiment viewer,” in Biomedical Informatics for Cancer Research, eds Ochs M., Casagrande J., Davuluri R. (Boston, MA: Springer; ), 267–277. 10.1007/978-1-4419-5714-6_15 [DOI] [Google Scholar]
- Isola D., Selbmann L., de Hoog G. S., Fenice M., Onofri S., Prenafeta-Boldú F. X., et al. (2013). Isolation and screening of black fungi as degraders of volatile aromatic hydrocarbons. Mycopathologia 175 369–379. 10.1007/s11046-013-9635-2 [DOI] [PubMed] [Google Scholar]
- Jeffries T. C., Rayu S., Nielsen U. N., Lai K., Ijaz A., Nazaries L., et al. (2018). Metagenomic functional potential predicts degradation rates of a model organophosphorus xenobiotic in pesticide contaminated soils. Front. microbiol. 9:147. 10.3389/fmicb.2018.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao W., Han Q., Xu Y., Jiang H., Xing H., Teng X., et al. (2017). Impaired immune function and structural integrity in the gills of common carp (Cyprinus carpio L.) caused by chlorpyrifos exposure: through oxidative stress and apoptosis. Fish Shellfish Immunol. 67 604–611. 10.1016/j.fsi.2017.06.048 [DOI] [PubMed] [Google Scholar]
- Joshi P. K., Swarup A., Maheshwari S., Kumar R., Singh N. (2011). Bioremediation of heavy metals in liquid media through fungi isolated from contaminated sources. Indian J. Microbiol. 51 482–487. 10.1007/s12088-011-0110-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L., Zhang Y., Zhu L., Wang J., Wang J., Du Z., et al. (2018). Influence of isolated bacterial strains on the in situ biodegradation of endosulfan and the reduction of endosulfan- contaminated soil toxicity. Ecotoxicol. Environ. Saf. 160 75–83. 10.1016/j.ecoenv.2018.05.032 [DOI] [PubMed] [Google Scholar]
- Li G., Wang K., Liu Y. H. (2008). Molecular cloning and characterization of a novel pyrethroid-hydrolyzing esterase originating from the Metagenome. Microb. Cell. Fact. 7:38. 10.1186/1475-2859-7-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. Y., Li C. X., Luo X. J., Lai Q. L., Xu J. H. (2014). Burkholderia jiangsuensis sp. nov., a methyl parathion degrading bacterium, isolated from methyl parathion contaminated soil. Int. J. Syst. Evol. Microbiol. 64 3247–3253. 10.1099/ijs.0.064444-0 [DOI] [PubMed] [Google Scholar]
- Maheswari S., Murugesan A. G. (2009). Remediation of arsenic in soil by Aspergillus nidulans isolated from an arsenic-contaminated site. Environ. Technol. 30 921–926. 10.1080/09593330902971279 [DOI] [PubMed] [Google Scholar]
- Makkar R. S., DiNovo A. A., Westwater C., Schofield D. A. (2013). Enzyme-mediated bioremediation of organophosphates using stable yeast biocatalysts. J. Bioremed. Biodeg. 4:2 10.4172/2155-6199.1000182 [DOI] [Google Scholar]
- Malik A., Tauler R. (2013). Extension and application of multivariate curve resolution-alternating least squares to four-way quadrilinear data-obtained in the investigation of pollution patterns on Yamuna River, India-A case study. Anal. Chim. Acta 794 20–28. 10.1016/j.aca.2013.07.047 [DOI] [PubMed] [Google Scholar]
- Martyniuk C. J., Doperalski N. J., Prucha M. S., Zhang J. L., Kroll K. J., Conrow R., et al. (2016). High contaminant loads in Lake Apopka’s riparian wetland disrupt gene networks involved in reproduction and immune function in largemouth bass. Comp. Biochem. Physiol. D Genomics Proteomics 19 140–150. 10.1016/j.cbd.2016.06.003 [DOI] [PubMed] [Google Scholar]
- Mason O. U., Scott N. M., Gonzalez A., Robbins-Pianka A., Bælum J., Kimbrel J., et al. (2014). Metagenomics reveals sediment microbial community response to Deepwater Horizon oil spill. ISME J. 8 1464–1475. 10.1038/ismej.2013.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes L. W., Braga L. P. P., Navarrete A. A., Souza D. G., Silva G. G., Tsai S. M. (2017). Using metagenomics to connect microbial community biodiversity and functions. Curr. Issues Mol. Biol. 24 103–118. 10.21775/cimb.024.103 [DOI] [PubMed] [Google Scholar]
- Menzel P., Ng K. L., Krogh A. (2016). Fast and sensitive taxonomic classification for metagenomics with Kaiju. Nat. Commun. 7:11257. 10.1038/ncomms11257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak V., Pai P. V., Pai A., Pai S., Sushma Y. D., Vaman Rao C., et al. (2013). A comparative study of caffeine degradation by four different fungi. Bioremediatr. J. 17 79–85. 10.1080/10889868.2012.751960 [DOI] [Google Scholar]
- Nicolopoulou-Stamati P., Maipas S., Kotampasi C., Stamatis P., Hens L. (2016). Chemical pesticides and human health: the urgent need for a new concept in agriculture. Front. Public Health 4:148. 10.3389/fpubh.2016.00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira B. R., Penetra A., Cardoso V. V., Benoliel M. J., Barreto Crespo M. T., Samson R. A., et al. (2015). Biodegradation of pesticides using fungi species found in the aquatic environment. Environ. Sci. Pollut. Res. Int. 22 11781–11791. 10.1007/s11356-015-4472-0 [DOI] [PubMed] [Google Scholar]
- Ramya S. L., Venkatesan T., Murthy K. S., Jalali S. K., Varghese A. (2016). Degradation of acephate by Enterobacter asburiae, Bacillus cereus and Pantoea agglomerans isolated from diamondback moth Plutella xylostella (L), a pest of cruciferous crops. J. Environ. Biol. 37 611–618. [PubMed] [Google Scholar]
- Rosales E., Pazos M., Angeles Sanroman M. (2013). Feasibility of solid-state fermentation using spent fungi-substrate in the biodegradation of PAHs. Clean Soil Air Water 41 610–615. 10.1002/clen.201100305 [DOI] [Google Scholar]
- Saitou N., Nei M. (1987). The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4 406–425. [DOI] [PubMed] [Google Scholar]
- Samanta S. (2013). Metal and pesticide pollution scenario in Ganga River system. Aquat. Ecosyst. Health Manag. 16 454–464. 10.1080/14634988.2013.858587 [DOI] [Google Scholar]
- Schofield D. A., DiNovo A. A. (2010). Generation of a mutagenized organophosphorus hydrolase for the biodegradation of the organophosphate pesticides malathion and demeton-S. J. Appl. Microbiol. 109 548–557. 10.1111/j.1365-2672.2010.04672.x [DOI] [PubMed] [Google Scholar]
- Scott C., Pandey G., Hartley C. J., Jackson C. J., Cheesman M. J., Taylor M. C., et al. (2008). The enzymatic basis for pesticide bioremediation. Indian J. Microbiol. 48:65. 10.1007/s12088-008-0007-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal M., Garg A., Suresh R., Dagar P. (2012). Heavy metal contamination in the Delhi segment of Yamuna basin. Environ. Monit. Assess. 184 1181–1196. 10.1007/s10661-011-2031-9 [DOI] [PubMed] [Google Scholar]
- Shrestha S., Kazama F. (2007). Assessment of surface water quality using multivariate statistical techniques: a case study of the Fuji river basin, Japan. Environ. Model. Softw. 22 464–475. 10.1016/j.envsoft.2006.02.001 [DOI] [Google Scholar]
- Singh S. P., Guha S., Bose P. (2017). Impact of the composition of the bacterial population and additional carbon source on the pathway and kinetics of degradation of endosulfan isomers. Environ. Sci. Process. Impacts. 19 964–974. 10.1039/C7EM00154A [DOI] [PubMed] [Google Scholar]
- Tamura K., Nei M., Kumar S. (2004). Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. U.S.A. 101 11030–11035. 10.1073/pnas.0404206101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tastan B. E., Donmez G. (2015). Biodegradation of pesticide triclosan by A. Versicolor in simulated wastewater and semi-synthetic media. Pestic Biochem. Physiol. 118 33–37. 10.1016/j.pestbp.2014.11.002 [DOI] [PubMed] [Google Scholar]
- Teles Y. V., de Castro L. M., Junior ÉS., do Nascimento A. P., da Silva H. A., Costa R. S., et al. (2018). Potential of bacterial isolates from a stream in manaus-amazon to bioremediate chromium-contaminated environments. Water Air Soil Pollut. 229:266. 10.1007/s11270-018-3903-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiralerdpanich P., Sonthiphand P., Luepromchai E., Pinyakong O., Pokethitiyook P. (2018). Potential microbial consortium involved in the biodegradation of diesel, hexadecane and phenanthrene in mangrove sediment explored by metagenomics analysis. Mar Pollut Bull. 133 595–605. 10.1016/j.marpolbul.2018.06.015 [DOI] [PubMed] [Google Scholar]
- Ufarte L., Laville E., Duquesne S., Morgavi D., Robe P., Klopp C., et al. (2017). Discovery of carbamate degrading enzymes by functional metagenomics. PLoS One 12:e0189201. 10.1371/journal.pone.0189201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uniyal S., Paliwal R., Verma M., Sharma R. K., Rai J. P. (2016). Isolation and characterization of fipronil degrading Acinetobacter calcoaceticus and Acinetobacter oleivorans from rhizospheric zone of Zea mays. Bull. Environ. Contam. Toxicol. 96 833–838. 10.1007/s00128-016-1795-6 [DOI] [PubMed] [Google Scholar]
- Unyimadu J. P., Osibanjo O., Babayemi J. O. (2018). Levels of organochlorine pesticides in brackish water fish from Niger River, Nigeria. J. Environ. Res. Public Health 8 1–9. 10.1155/2018/2658306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valladares A., Montesinos M. L., Herrero A., Flores E. (2002). An ABC-type, high-affinity urea permease identified in cyanobacteria. Mol. Microbiol. 43 703–715. 10.1046/j.1365-2958.2002.02778.x [DOI] [PubMed] [Google Scholar]
- Van den Berg H., Zaim M., Yadav R. S., Soares A., Ameneshewa B., Mnzava A., et al. (2012). Global trends in the use of insecticides to control vector-borne diseases. Environ. Health Persp. 120 577–582. 10.1289/ehp.1104340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega M., Pardo R., Barrado E., Deban L. (1998). Assessment of seasonal and polluting effects on the quality of river water by exploratory data analysis. Water Res. 32 3581–3592. 10.1016/S0043-1354(98)00138-9 [DOI] [Google Scholar]
- Verce M., De Vuyst L., Weckx S. (2019). Shotgun metagenomics of a water kefir fermentation ecosystem reveals a novel oenococcus species. Front. Microbiol. 10:479. 10.3389/fmicb.2019.00479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B. Z., Guo P., Hang B.-J., Li L., He J., Li S.-P., et al. (2009). Cloning of a novel pyrethroid-hydrolyzing carboxylesterase gene from Sphingobium sp. strain JZ-1 and characterization of the gene product. J. Appl. Environ. Microbiol. 75 5496–5500. 10.1128/AEM.01298-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Xue Q., Zhou X., Tang X., Hua R. (2015). Isolation and characterization of a highly efficient chlorpyrifos degrading strain of Cupriavidus taiwanensis from sludge. J. Basic Microbiol. 55 229–235. 10.1002/jobm.201400571 [DOI] [PubMed] [Google Scholar]
- Wang W., Sun M. (2009). Phylogenetic relationships between Bacillus species and related genera inferred from 16S rDNA sequences. Braz. J. Microbiol. 40 505–521. 10.1590/S1517-83822009000300013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei K., Yin H., Peng H., Lu G., Dang Z. (2018). Bioremediation of triphenyl phosphate in river water microcosms: proteome alteration of Brevibacillus brevis and cytotoxicity assessments. Sci. Total Environ. 649 563–570. 10.1016/j.scitotenv.2018.08.342 [DOI] [PubMed] [Google Scholar]
- Wilkins L. G., Ettinger C. L., Jospin G., Eisen J. A. (2019). Metagenome-assembled genomes provide new insight into the microbial diversity of two thermal pools in Kamchatka, Russia. Sci. Rep. 9:3059. 10.1038/s41598-019-39576-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P. C., Liu Y. H., Wang Z. Y., Zhang X. Y., Li H., Liang W. Q., et al. (2006). Molecular cloning, purification, and biochemical characterization of a novel pyrethroid-hydrolyzing esterase from Klebsiella sp. strain ZD112. J. Agric. Food Chem. 54 836–842. 10.1021/jf052691u [DOI] [PubMed] [Google Scholar]
- Zhai Y., Li K., Song J., Shi Y., Yan Y. (2012). Molecular cloning, purification and biochemical characterization of a novel pyrethroid-hydrolyzing carboxylesterase gene from Ochrobactrum anthropi YZ-1. J. Hazard. Mater. 221 206–212. 10.1016/j.jhazmat.2012.04.031 [DOI] [PubMed] [Google Scholar]
- Zhang S. B., Wang L., Liu Y., Zhai H. C., Cai J. P. (2015). Expression of feruloyl esterase A from Aspergillus terreus and its application in biomass degradation. Protein Expr. Purif. 115 153–157. 10.1016/j.pep.2015.08.015 [DOI] [PubMed] [Google Scholar]
- Zitko V. (1994). Principal component analysis in the evaluation of environmental data. Mar. Pollut. Bull. 28 718–722. 10.1016/0025-326X(94)90329-8 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/ Supplementary Material.