Abstract
Background and Aims
Polyploidy is an important contributor to sympatric speciation and assortative mating is a key mechanism driving cytotype interactions in contact zones. While strong reproductive barriers can mediate the coexistence of different cytotypes in sympatry, positive frequency-dependent mating disadvantage ultimately drives the transition to single-ploidy populations. However, comprehensive estimates of reproductive isolation among cytotypes and across multiple barriers are rare. We quantify the strength of isolation across multiple reproductive stages in a tetraploid–octoploid contact zone to understand the potential for coexistence.
Methods
Assortative mating due to flowering asynchrony, pollinator behaviour, morphological overlap, self-fertilization and gametic competition between tetraploid and octoploid Gladiolus communis in a contact zone in the Western Iberian Peninsula were assessed in natural and experimental populations to quantify reproductive isolation (RI) between cytotypes.
Key Results
Tetraploids and octoploids have a high degree of overlap in flowering time and similar floral morphology, and are visited by generalist insects without cytotype foraging preferences, resulting in weak pre-pollination RI (from 0.00 to 0.21). In contrast, post-pollination isolation resulting from gametic selection was a strong barrier to inter-cytotype mating, with ploidy composition in stigmatic pollen loads determining the levels of RI (from 0.54 to 1.00). Between-cytotype cross-incompatibility was relatively high (RI from 0.54 to 0.63) as was isolation acquired through self-pollination (RI of 0.59 in tetraploids and 0.39 in octoploids).
Conclusions
Total RI was high for both tetraploids (from 0.90 to 1.00) and octoploids (from 0.78 to 0.98). Such high rates of assortative mating will enable cytotype coexistence in mixed-ploidy populations by weakening the impacts of minority cytotype exclusion. This study reveals the key role of gametic selection in cytotype siring success and highlights the importance of comprehensive estimates across multiple reproductive barriers to understand cytotype interactions at contact zones.
Keywords: Contact zone, cytotypes, gametic barriers, Gladiolus communis, hexaploid, octoploid, phenology, pollen load composition, pollinator preferences, polyploidy, tetraploid, unreduced gametes
INTRODUCTION
Whole-genome duplication, leading to polyploidy, is widespread in the evolutionary history of flowering plants, with all lineages having a polyploidization event at some point in their history (Soltis et al., 2007; Wood et al., 2009; Alix et al., 2017). The current incidence of polyploid species, defined by chromosome number relative to the generic base number, is also high, with estimates ranging from 20 to 40 % (Stebbins, 1938; Wood et al., 2009; Marques et al., 2017), and over 12 % of species are of mixed ploidy (Wood et al., 2009; Husband et al., 2013; Rice et al., 2015; Marques et al., 2017). Recent studies have also revealed surprisingly high cytogenetic diversity within some polyploid complexes (e.g. Baack, 2004; Kolář et al., 2009; Ståhlberg, 2009; Castro et al., 2012, 2018; Zozomová-Lihová et al., 2015). In most of these cases, cytotypes form contact zones where they occur in proximity and occasionally form mixed-ploidy populations (reviewed in Husband et al., 2013; Kolář et al., 2017). The spatial proximity creates the potential for ecological interactions between cytotypes and can result in the production of hybrids. Thus, contact zones are recognized as natural laboratories to study the patterns and processes involved in polyploid evolution (Lexer and van Loo, 2006). However, studies in these areas are reduced to a limited number of polyploid complexes (e.g. Baack, 2004, 2005; Lexer and van Loo, 2006; Castro et al., 2011; Zozomová-Lihová et al., 2015).
Theoretical models predict that mixed-ploidy populations are unstable and frequency-dependent selection will eliminate the minority cytotype as most of the crosses will occur with the dominant cytotype, reducing the fitness of the rare cytotype (minority cytotype exclusion theory; Levin, 1975; Rodriguez, 1996; Husband and Schemske, 2000). However, this theory has rarely been tested experimentally (Husband, 2000) and cytotype coexistence is more common than previously hypothesized (Husband et al., 2013; Kolář et al., 2017). Mixed-ploidy populations are possible if biological attributes, such as plant traits promoting assortative mating, large viability/fertility of polyploids and/or recurrent polyploid formation through unreduced gametes, can ameliorate fitness disadvantages of the rare cytotype (Rieseberg and Willis, 2007; Thompson and Merg, 2008; Paun et al., 2009; Jersáková et al., 2010).
Among the attributes mentioned above, barriers to inter-cytotype pollen flow and mating (acting separately or in concert) are key determinants of the levels of assortative mating in mixed-ploidy populations (Levin, 1975; Husband, 2000; Kolář et al., 2017). Inter-cytotype mating may be reduced by phenological, mechanical and behavioural mechanisms. Phenological isolation is determined by flowering time overlap and affects the probability of pollen exchange between cytotypes (phenological barrier, e.g. Van Dijk and Bijlsma, 1994; Petit et al., 1997; Nuismer and Cunningham, 2005; Jersáková et al., 2010; Martin and Husband, 2012). Morphological and/or physiological differences between cytotypes in flower characters may influence pollinator foraging behaviour (behavioural barrier, e.g. Segraves and Thompson, 1999; Kennedy et al., 2006). Differences in floral morphology might also affect pollen removal and deposition on the pollinator’s body (mechanical barrier, Grant, 1994), although this has rarely been studied in polyploid complexes (Segraves and Thompson, 1999; Jersáková et al., 2010; Borges et al., 2012). Even if pollen exchange between cytotypes does occur, gametic barriers caused by differential siring ability can still reduce inter-cytotype mating (e.g. pollen competition in mixed-ploidy loads, Baldwin and Husband, 2011; mentor effect, Mráz, 2003; or changes in reproductive strategies, Barringer, 2007; Kao, 2007). Finally, strong post-zygotic barriers, widely documented in diploid–polyploid crosses, can prevent hybrids from persisting (Ramsey and Schemske, 1998), although the strength of this barrier can vary among polyploid complexes and ploidy levels (e.g. Husband, 2004; Castro et al., 2011; Roccaforte et al., 2015; Sutherland and Galloway, 2017).
Despite the increased detection of mixed-ploidy species, the magnitude and influence of reproductive barriers on cytotype diversity and coexistence are poorly known. While research exists on individual factors that may weaken or overcome minority cytotype disadvantage, comprehensive studies exploring multiple factors promoting cytotype coexistence are rare (reviewed in Kolář et al., 2017). Only a few studies have quantified the contribution of multiple pre- and post-zygotic barriers to reproductive isolation between cytotypes (e.g. Van Dijk et al., 1992; Petit et al., 1997; Segraves and Thompson, 1999; Jersáková et al., 2010; Castro et al., 2011; Husband et al., 2016). Besides this, most studies are focused on diploid–tetraploid complexes (Kolář et al., 2017) and higher-ploidy systems are rarely addressed (e.g. 4x–8x Gymnadenia conopsea; Jersáková et al., 2010; 2x–6x Aster amellus, Castro et al., 2011). Higher-ploidy complexes differ from diploid–tetraploid ones as they may overcome the meiotic problems in anaphase II observed in odd-ploidy plants, and inter-cytotype offspring likely have higher reproductive fitness due to the production of even-ploidy progeny (e.g. 6x progeny in 4x–8x complexes). Studying multiple factors contributing to assortative mating and, consequently, to reproductive isolation in higher-ploidy systems will thus provide significant insights on cytotype interactions and maintenance of contact zones.
Gladiolus communis represents an ideal system to explore cytotype interactions at contact zones. This Mediterranean putative autopolyploid (Castro et al., 2018) is a tetraploid–octoploid complex (2n = 4x = 60 and 2n = 8x = 120 chromosomes), with occasional detection of hexaploids and hexaploid populations in the Iberian Peninsula (2n = 6x = 90; Fernandes et al., 1948; Fernandes and Queirós, 1971; Castro et al., 2018). Recent surveys reveal a complex cytotype distribution, with mixed-ploidy populations differing in cytotype composition being observed within and outside tetraploid–octoploid contact zones (Castro et al., 2018). However, the distribution of the dominant cytotypes (tetraploids and octoploids) cannot be explained by environmental differences (Castro et al., 2018) and nothing is yet known about the reproductive barriers governing the interactions between locally coexisting cytotypes.
Therefore, we quantify the contribution of multiple reproductive barriers to assortative mating (and reproductive isolation) in tetraploid–octoploid G. communis contact zones to better understand their role in cytotype coexistence. While strong reproductive barriers can mediate the coexistence of different cytotypes in sympatry, positive frequency-dependent mating disadvantage ultimately drives the transition to single-ploidy populations. We quantify reproductive isolation mediated by: (1) differences in flowering phenology; (2) differences in flower morphology that might affect pollen deposition on the pollinator’s body; (3) differences in pollinator behaviour and foraging preferences; (4) differences in self-fertilization rates; and (5) gametic selection against alternative cytotype pollen. Observations were made in natural populations and common-garden experiments; hand-pollinations were used to assess self-incompatibility differences and quantify the production of hexaploids under single- and mixed-ploidy pollen loads, and an experimental mixed-ploidy population was used to quantify the effect of all reproductive barriers.
MATERIALS AND METHODS
Study system
Gladiolus communis flowers from mid-April to mid-July, usually producing one spike inflorescence of pink hermaphroditic flowers on each plant. Flowers are zygomorphic, odourless and are visited by numerous Hymenoptera foraging for nectar and pollen, of which Bombus spp. (e.g. B. hortorum, B. pascuorum and B. terrestris), Anthophora sp., Colletes sp. and Anthidium florentinum are the most important pollinators (Castro, 2018). The stamens are unilateral, opening downwards, such that pollen is deposited on the upper part of the insect’s thorax during a visit. The pistil has a filiform three-lobed stigma that is exposed between the anthers and the upper petal (Hamilton, 1980; Alonso and Crespo, 2010).
Study populations and general experimental design
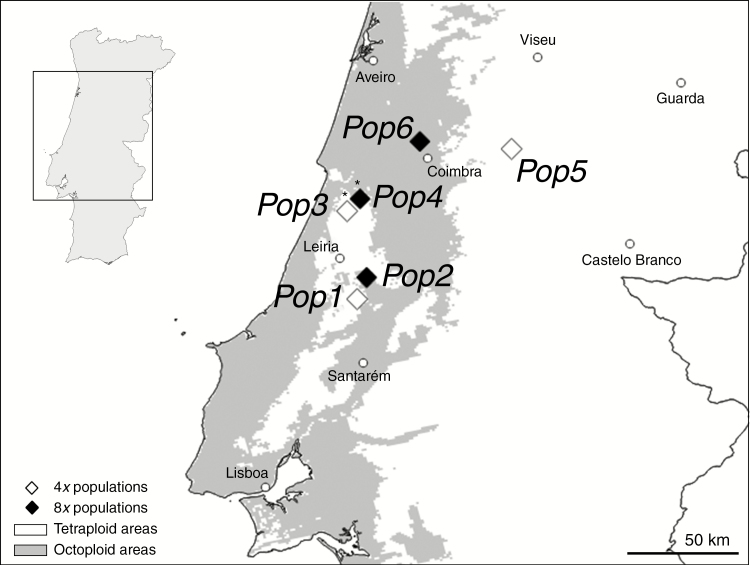
We used plants from the tetraploid–octoploid contact zone of central Portugal (Fig. 1), where cytotypes occur in proximity and occasionally in mixed-ploidy populations (Castro et al., 2018). We examined barriers to inter-cytotype mating using six single-ploidy populations (three tetraploid and three octoploid; Fig. 1). Plants from the same populations were also examined in a common-garden study at the Botanical Garden of the University of Coimbra. Bulbs were collected in the field in 2013 and placed in 2-L pots filled with commercial soil. DNA ploidy of these populations was originally assessed by Castro et al. (2018) and confirmed here using the same protocol (data not shown). In the common garden, the plants were used to assess flowering phenology under common conditions, perform hand-pollinations, and build an experimental mixed-ploidy population to quantify the effect of all reproductive barriers. In the field, we assessed flowering phenology, flower morphology and pollinator behaviour. Reproductive isolation indexes were calculated based on each barrier for tetraploids and octoploids separately, as well as the total.
Fig. 1.
Gladiolus communis populations (Pop1, Pop2, ...) studied in a tetraploid–octoploid contact zone. Populations marked with an asterisk were used to study flowering phenology in the field.
All analyses described in the following sections were performed in R software version 3.0.1 (R Core Development Team, 2016), using the packages ‘car’ for type-III analysis of variance (Fox et al., 2016), ‘lme4’ for generalized linear mixed models (GLMMs; Bates et al., 2014) and ‘multcomp’ for multiple comparisons after type-III analysis of variance (Hothorn et al., 2016). Year, population, individual and/or flower position were introduced as random factors, when applicable.
Flowering phenology
Flowering phenology was evaluated in natural populations (one tetraploid and one octoploid; Pop3 and Pop4 in Fig. 1) and in the common garden (six populations). In the field, 45 individuals per population were randomly selected and tagged before the flowering season. The number of open flowers in these individuals was monitored daily for up to 20 consecutive days, which covers the flowering period of the selected plants. In the common garden, the number of open flowers per plant (39 tetraploid and 21 octoploid plants, evenly distributed among the studied populations) was monitored daily for 50 d. Differences between cytotypes in total number of open flowers per day were tested using GLMMs with cytotype as fixed factor, total number of open flowers per day as response variable (Poisson distribution, log link function) and population as random factor.
A phenological reproductive isolation index (RIphenological) was calculated for each cytotype for natural populations and in the common garden separately, using the following formula (Husband and Sabara, 2004):
Flower morphology
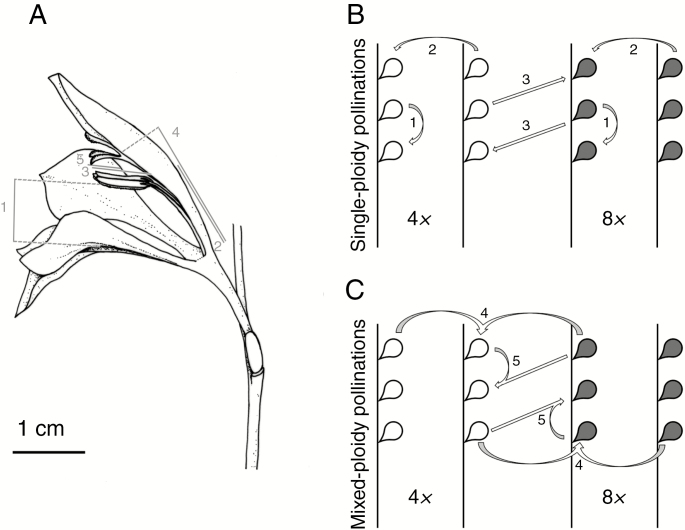
Ten individuals were haphazardly selected in each of the six natural populations and one flower per individual was characterized for distance between anther and lower tepal (which represents the size of pollinator entrance) and length of filament, anther, style and stigma (Fig. 2A). Differences between cytotypes were evaluated using GLMMs with cytotype as fixed factor, each flower trait as a response variable (Gaussian distribution, identity link function), and population and flower position as random factors.
Fig. 2.
Experimental designs. (A) Morphological parameters measured in Gladiolus communis flowers: 1, distance between anther and lower tepal (represents the size of pollinator entrance); 2, filament length; 3, anther length; 4, style length; 5, stigma length. (B, C) Hand-pollinations performed in tetraploid (white, 4x) and octoploid (grey, 8x) G. communis inflorescences involving single-ploidy (B) and mixed-ploidy (C) pollinations (1, self pollinations; 2, outcross within cytotypes; 3, outcross between cytotypes; 4, mixed outcross; 5, outcross between cytotypes and self-pollen). Arrows denote each pollination treatment, going from the donor plant(s) to the recipient one.
Morphological reproductive isolation (RImorphological) was calculated as:
where morphological overlap represents the physical overlap of male and female functions in pairwise comparisons between cytotypes and, thus, the likelihood that pollen can be transferred via pollinator from one cytotype to the other. Using the number of flowers with morphological overlap within cytotype enabled us to correct for herkogamy levels occurring in natural populations.
Pollinator foraging behaviour
Pollinator foraging behaviour was studied in artificial arrays within the six natural populations comprising tetraploid and octoploid individuals. Arrays were used due to the difficulty in finding suitable mixed-ploidy populations to study pollinator behaviour. Each array comprised ten inflorescences, five from the own population and five from the nearest population of the other cytotype, each with a similar number of open flowers and height, alternately arranged in a circle and separated by 20 cm. Three replicate arrays were established per population in areas without flowering Gladiolus plants and monitored during one entire day (0900 to 1600 h GMT) by several observers (21 h of observation per population, on average). For each foraging bout, we recorded the insect species and the visitation sequence to the plants in the array. For the five most abundant species, the movements between individuals were used to assess pollinator preferences (floral preference index) and behaviour (floral constancy index).
The floral preference index was calculated as the ratio between the number of visits to a given cytotype and the total number of visits recorded for a given pollinator. This index ranges from 0 to 1, where 0.5 indicates no preference, and 0 or 1 shows a preference for one of the cytotypes. The floral constancy index was calculated as the ratio between the number of movements within a cytotype and the total number of flights of the pollinator during the visit. A floral constancy index of 0 indicates an alternating foraging behaviour (all flights occur between cytotypes), a value of 0.5 indicates a random foraging behaviour, and a value of 1 indicates complete foraging constancy within a cytotype. Both indices were multiplied by a correction factor that accounts for the frequency and distances between plants of the same or different ploidy, following Husband et al. (2016), as the ratio between 0.5 (expected in random movements) and 0.58 (details provided in Supplementary Data Appendix 1). Only visits that comprised the interaction with three or more individuals were considered. For both indices and for each pollinator species, we tested for deviations from no preference (0.5) and from floral constancy (0.5) using χ2 tests.
A behavioural reproductive isolation index (RIbehavioural) due to pollinator behaviour was calculated incorporating the correction factor using the following formula:
This metric ranges from 0 (all matings are between-ploidy) to 1 (complete reproductive isolation), where 0.5 indicates random mating between the cytotypes.
Crossing ability under controlled conditions
Hand-pollinations were performed to assess the cross-compatibility of tetraploids and octoploids and the ability to produce hexaploids. Two pollination treatments, differing in the composition of pollen applied to the stigma, were performed: single-ploidy pollen loads and mixed-ploidy pollen loads (Fig. 2B, C). Three single-ploidy pollen load treatments were used: (1) self-pollination (anthers of the same inflorescence were used as pollen donor); (2) outcross within cytotypes (anthers of different individuals of the same cytotype were used as pollen donor); and (3) outcross between cytotypes (anthers of the other cytotype were used as pollen donor) (Fig. 2B). Two mixed-ploidy pollen load treatments were applied: (4) mixed-ploidy outcross (mix of tetraploid and octoploid anthers were used as pollen donors); and (5) outcross between cytotypes and self-pollen (anthers of the recipient individual and anthers of individuals of the other cytotype were used as donors) (Fig. 2C). These treatments enabled us to assess differences in self-incompatibility, evaluate the effect of single- and mixed-ploidy pollen loads on hexaploid production, and measure the impact of self-pollen deposition on reproductive isolation under mixed-ploidy pollen loads.
Pollination experiments were conducted during peak flowering (May 2014 and 2015) using 102 plants growing in a common garden. Before flowering, and until fruit collection, plants were protected with a nylon mesh to exclude pollinators. Except for the self-pollination treatment, all flowers used as pollen recipients were emasculated before their stigmas were receptive. In single-ploidy pollinations (other than self-pollination), anthers from three individuals (one anther per individual) were gently rubbed directly on the stigmatic papillae until saturated. In mixed-ploidy pollinations, three anthers of each cytotype were collected in a microtube and shaken; the pollen mix was then applied to the stigmatic papillae with a needle. Pollen for within-cytotype outcrosses was collected from plants of the same population as the recipient plant. Only the first four flowers of the inflorescence were pollinated to avoid resource allocation limitation within the inflorescence, and only one replicate per treatment was performed per plant. Fruits were collected when mature and seeds were counted. Fruit set (proportion of pollinated flowers that developed into fruit), seed:ovule ratio (S:O ratio, proportion of ovules that produced morphologically viable seeds) and reproductive success (fruit set × S:O ratio) were calculated. The S:O ratio was calculated using the mean number of ovules per flower (4x, 44.2 ± 0.5; 8x, 42.4 ± 0.5; mean ± s.e.; M. Castro, pers. comm.). Differences in fruit set (binomial distribution, logit link function), S:O ratio and reproductive success (Gaussian distribution and identity link function, after arcsine and square root transformation, respectively) were assessed using GLMMs, with cytotype and pollination treatment defined as fixed factors and population as random factor.
DNA ploidy of the offspring obtained from hand-pollinations was analysed using flow cytometry. Ten seeds per fruit were analysed using the protocol of Galbraith et al. (1983) with some adjustments (Castro et al., 2018). Briefly, two seeds per sample were simultaneously chopped with 0.5 cm2 of leaf tissue of a DNA standard, Pisum sativum (2C = 9.09 pg; Doležel et al., 1998), in woody plant buffer (Loureiro et al., 2007). The nuclear suspension was filtered and stained with propidium iodide for 5 min, and the samples were analysed with a CyFlow Space flow cytometer (Partec, Görlitz, Germany). At least 1300 nuclei in the sample and standard G1 peaks were analysed per sample, and only samples with coefficients of variation <5 % were accepted, otherwise a new sample was prepared and analysed. DNA ploidy was inferred for each seed following Castro et al. (2018). Differences in the proportion of hexaploids between treatments were assessed using GLMMs with ploidy of mother plant and pollination treatment as fixed factors, hexaploid proportion as response variable (binomial distribution, logit link function) and population as random factor.
Pollination treatments were used to calculate reproductive isolation caused by: (1) self-pollination, and (2) gametic isolation, reflecting differential gamete siring ability and zygote viability. Self-pollination is highly probable in G. communis as pollinators visit the flowers in sequence, from the base to the top of the inflorescence, and self-pollen is likely to be moved between flowers (M. Castro, field observations). The reproductive isolation index due to selfing (RIselfing) was calculated using the proportion of inter-cytotype offspring from treatment 5 (i.e. outcross between cytotypes and self-pollen) as follows:
The RIselfing value was corrected by multiplying by the proportion of within-inflorescence movements among the total number of movements recorded.
The gametic reproductive isolation index (RIgametic) was calculated as follows:
The reproductive success of single-ploidy cross-pollinations between cytotypes (treatment 3) and of mixed-ploidy pollinations (treatment 4) were used, separately, to calculate RIgametic under different pollen load compositions. In mixed-ploidy pollinations, only seeds that differed in DNA ploidy from the mother plant were used for RIgametic calculation, since offspring with the same ploidy of the mother were assumed to result from successful within-cytotype fertilization.
Effect of all reproductive barriers
The total RI index was calculated using the product of all separate RI indices (Ramsey et al., 2003). Additionally, we quantified the offspring produced by tetraploids and octoploids growing in sympatry under controlled conditions. To do this, we created an experimental mixed-ploidy population with 1:1 proportions of tetraploid and octoploid plants, comprising 250 pots, left to open pollination. In the end, 216 individuals flowered, including 122 tetraploids (56 %) and 94 octoploids (44 %). After flowering, 30 individuals per cytotype were randomly selected and fruits were collected (totalling 424 fruits). Fruit and seed production were quantified, and the DNA ploidy of the offspring was assessed as described above. The results were analysed statistically as described in the hand-pollination experiments.
RESULTS
Flowering phenology
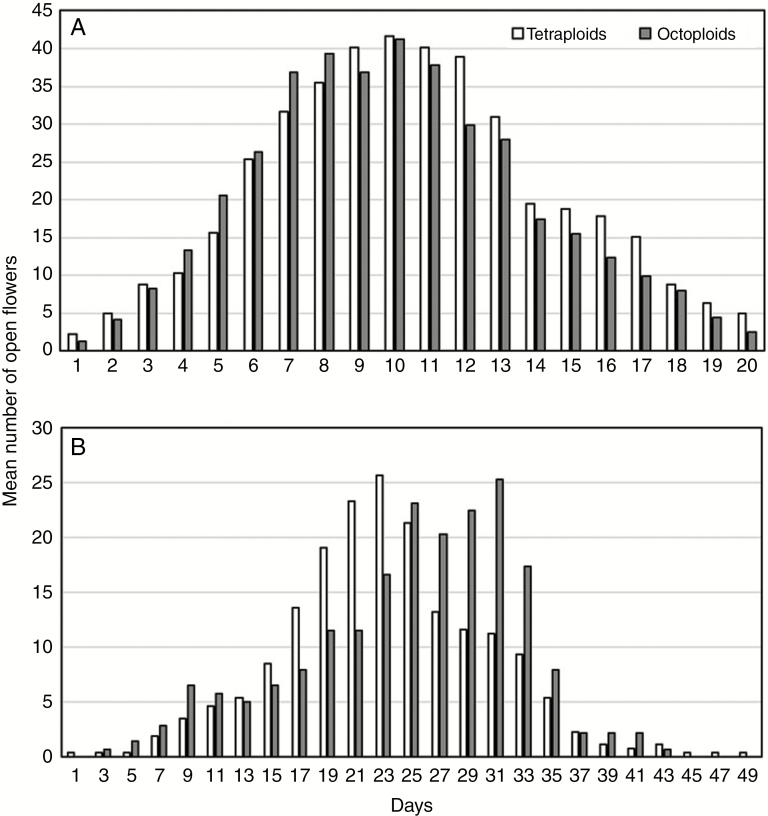
Flowering phenology of tetraploids and octoploids was almost completely overlapping (Fig. 3). Consequently, there were no significant differences in mean number of open flowers between cytotypes in both natural populations (F1,38 = 0.11, P = 0.747; Fig. 3A) and the common garden (F1,98 = 0.12, P = 0.735; Fig. 3B), although tetraploids tended to peak slightly earlier than octoploids in the common garden (Fig. 3B). These phenological patterns resulted in low reproductive isolation indices for both cytotypes under natural and common garden conditions (Table 1).
Fig. 3.
Flowering phenology of tetraploid and octoploid Gladiolus communis cytotypes in (A) natural populations and (B) a common garden. Values are given as mean number of open flowers per day, starting on the day of the first flower opening.
Table 1.
Reproductive isolation indices in Gladiolus communis polyploid complex. The isolation index for each barrier studied (RI individual barriers) is provided separately for tetraploids (RI 4x) and octoploids (RI 8x) and for the complex (RI Gc). Total reproductive isolation (total RI, in bold) is also provided for each scenario studied (single-ploidy inter-cytotype crosses and mixed-ploidy crosses)
| Reproductive barrier | RI individual barriers | |||
|---|---|---|---|---|
| RI 4x | RI 8x | RI Gc | ||
| Phenological | Natural populations | 0.05 | 0.00 | 0.05 |
| Common garden | 0.14 | 0.00 | 0.14 | |
| Morphological | 0.19 | 0.00 | 0.00 | |
| Pollinator behaviour | 0.13 | 0.21 | 0.29 | |
| Self-pollination | 0.59 | 0.39 | 0.49 | |
| Gametic | Single-ploidy inter-cytotype crosses | 0.63 | 0.54 | 0.58 |
| Mixed-ploidy crosses | 1.00 | 0.96 | 0.98 | |
| Total RI | Single-ploidy inter-cytotype crosses | 0.90 | 0.78 | 0.86 |
| Mixed-ploidy crosses | 1.00 | 0.98 | 0.99 | |
Flower morphology
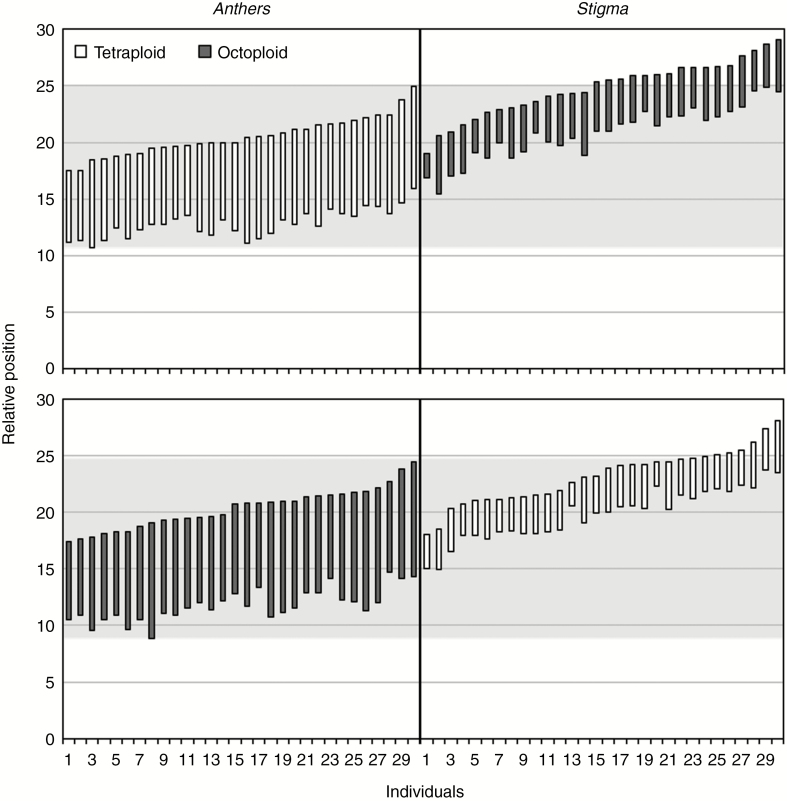
Tetraploid and octoploid flowers were morphologically similar, with no differences observed for the characters measured (Supplementary Data Table S1). Additionally, the relative positions of the sexual organs revealed a high overlap between tetraploid anthers and octoploid stigmas and between octoploid anthers and tetraploid stigmas (Fig. 4), leading to low morphological RI between cytotypes (RImorphological,4x = 0.19, RImorphological,8x = 0.00; Table 1).
Fig. 4.
Relative positions of sexual organs in tetraploid (4x) and octoploid (8x) Gladiolus communis flowers. White bars represent anther length (4x, top panel; 8x, bottom panel) and dark grey bars represent stigma length (8x, top panel; 4x, bottom panel) for individual flowers. Light grey areas represent the range of male organs, meaning that a stigma inside the box could be pollinated by the donor anthers.
Pollinator foraging behaviour
The main pollinator species had similar behavioural patterns when visiting G. communis (Table 2). The mean number of plants visited did not differ significantly among pollinator species (F4,193 = 1.50, P = 0.203) and ranged from 4.6 for B. terrestris to 8.5 for A. florentinum. Overall, no significant differences among pollinator species were found for preference and constancy indices (Table 2). Preference indices did not differ statistically from 0.5, indicating a lack of preference for a specific cytotype by each pollinator species (Table 2). Constancy indices revealed that A. florentinum visited cytotypes in an alternating foraging pattern (P = 0.016), while the remaining pollinators presented a random behaviour (P > 0.05; Table 2). The lack of preferences and the random/alternating behaviour by the pollinators resulted in low RI values due to pollinator behaviour (RIbehavioural = 0.29, RIbehavioural,4x = 0.13, RIbehavioural,8x = 0.21; Table 1).
Table 2.
Pollinator preferences and behaviour: preferences and constancy indices for the main pollinator species of Gladiolus communis. The mean number of plants visited per foraging flight (plants per visit), total number of flights (n) and total number of individuals visited (ni) are also given (all as mean ± s.e.). P-values for deviations from 0.5 are provided for each index and pollinator. Comparisons between pollinators are also presented
| Preference index | Constancy index | n (ni) | ||||
|---|---|---|---|---|---|---|
| Taxa | Plants per visit | Mean ± s.e. | P | Mean ± s.e. | P | |
| Anthidium florentinum | 8.4 ± 1.3 | 0.41 ± 0.0 | 0.384 | 0.14 ± 0.0 | 0.016 | 17 (142) |
| Anthophora sp. | 5.2 ± 0.3 | 0.40 ± 0.0 | 0.545 | 0.28 ± 0.0 | 0.370 | 39 (203) |
| Bombus pascuorum | 5.1 ± 0.2 | 0.39 ± 0.0 | 0.417 | 0.27 ± 0.0 | 0.334 | 106 (543) |
| Bombus terrestris | 4.6 ± 0.3 | 0.36 ± 0.0 | 0.371 | 0.26 ± 0.0 | 0.194 | 20 (91) |
| Colletes sp. | 6.5 ± 0.9 | 0.43 ± 0.1 | 0.731 | 0.28 ± 0.1 | 0.462 | 16 (104) |
| F 4,193; P-values | 1.50; 0.203 | 0.31; 0.869 | 0.75; 0.557 | |||
Crossing ability under controlled conditions
All pollination treatments produced fruits and seeds and significant differences were observed between cytotypes, pollination treatments and/or their interactions (Table 3 and Supplementary Data Table S2). Fruit set was similar between cytotypes and differed significantly among pollination treatments (Supplementary Data Table S2), with self-pollinations producing significantly lower fruit set than the remaining treatments, which presented similar values (Supplementary Data Fig. S1A). Reproductive success was similar to the S:O ratio (Supplementary Data Table S2 and Fig. S1B). Reproductive success differed significantly between cytotypes for two pollination treatments, selfing and inter-cytotype + selfing (Table 3), with octoploids having significantly higher reproductive success than tetraploids (P < 0.05; Fig. 5A). Within each cytotype, significant differences were observed among pollination treatments (Table 3). For tetraploids, treatments involving self-pollinations (treatments 1 and 5) resulted in significantly lower values and outcross within cytotype (treatment 2) significantly higher values than the remaining treatments (P < 0.05; Fig. 5B). For octoploids, self-pollination (treatment 1) had significantly lower values than the remaining treatments (P < 0.05; Fig. 5A).
Table 3.
Effects of ploidy and pollination treatment in reproductive success after hand-pollinations. Statistically significant differences at P < 0.05 are highlighted in bold
| Factor | Reproductive success | ||
|---|---|---|---|
| d.f. | F | P | |
| Effect of ploidy and pollination treatment | |||
| Ploidy | 1, 289 | 4.87 | 0.027 |
| Pollination treatment | 4, 289 | 222.76 | <0.001 |
| Pollination treatment × ploidy | 4, 289 | 10.80 | 0.029 |
| Differences between cytotypes within pollination treatment | |||
| Selfing | 1, 107 | 4.74 | 0.032 |
| Outcross (between cytotypes) | 1, 107 | 0.53 | 0.467 |
| Outcross (within cytotypes) | 1, 61 | 0.37 | 0.543 |
| Mixed outcross (4x + 8x) | 1, 43 | 0.95 | 0.330 |
| Outcross between cytotypes + selfing | 1, 48 | 18.92 | <0.001 |
| Differences among pollination treatments within cytotype | |||
| Tetraploids | 4, 256 | 212.50 | <0.001 |
| Octoploids | 4, 110 | 32.35 | <0.001 |
Fig. 5.
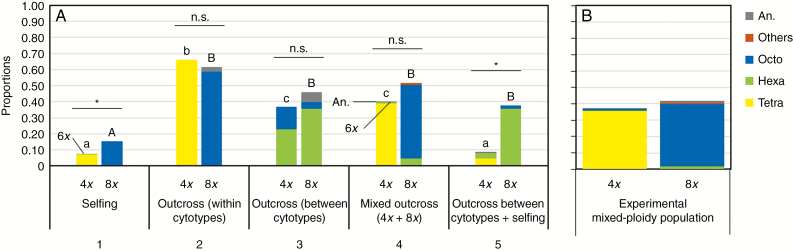
Reproductive success after different pollination treatments: (A) hand-pollinations; (B) experimental mixed-ploidy population. Statistical comparisons between ploidies within treatments: *P < 0.05; n.s., not significant at P > 0.05. Differences among treatments within cytotype are denoted by lower-case letters for tetraploids and upper-case letters for octoploids, with different letters representing significant differences at P < 0.05. Columns show the relative proportions of the ploidies observed in the offspring of each treatment (Tetra, tetraploids; Hexa, hexaploids; Octo, octoploids; Others, decaploids and dodecaploids; An., aneuploids), shown in different colours: number of analysed seeds per treatment for tetraploids and octoploids, respectively: (1) 131, 71; (2) 306, 235; (3) 21, 158; (4) 52, 113; (5) 118, 78; (experimental mixed-ploidy population) 313, 313.
The analyses of the DNA ploidy of the offspring (Fig. 5A) revealed that pollinations within the same cytotype (i.e. selfing and outcross within cytotypes) produced mostly offspring with the same ploidy as the parents. The production of a few hexaploid seeds after selfing of tetraploid individuals suggests the production of unreduced gametes.
For pollinations between cytotypes (treatments 3–5), the production of hexaploid seeds differed significantly between treatments for each cytotype (tetraploids, χ22,186 = 23.50, P < 0.001; octoploids, χ22,355 = 91.80, P < 0.001). Outcrossing between cytotypes (treatment 3) resulted in a high production of hexaploid seeds by both cytotypes, with no significant difference between them (χ21,187 = 0.08, P = 0.77), although octoploids produced more hexaploid seeds than the tetraploids (Fig. 5A). This pollination treatment revealed, once again, the production of unreduced gametes by tetraploid individuals, via both female and male gametes, detected by the production of octoploid seeds in both tetraploid and octoploid individuals.
The mixed-ploidy pollen load treatments (treatments 4 and 5) produced offspring with ploidy compositions that varied according to the ploidy of the maternal plant (Fig. 5A). In mixed-ploidy outcross pollinations (treatment 4), the offspring mostly had the ploidy of the mother, revealing a higher siring success of its own pollen. When the mixed-ploidy pollination involved the mother’s own pollen (treatment 5), octoploids produced mostly hexaploids, while tetraploids produced a few tetraploids through selfing and a few hexaploid seeds (resulting either from the fusion of self-unreduced gametes and/or from crosses between cytotypes). Octoploids produced significantly more hexaploid seeds than tetraploids in both treatments (treatment 4, χ21,186 = 4.20, P = 0.04; treatment 5, χ21,168 = 25.31, P < 0.001). Several aneuploid seeds were also observed, in particular when octoploids were involved (Fig. 5A).
The differences obtained in the inter-cytotype crosses (treatments 3–5) led to different reproductive isolation levels depending on the composition of the pollen loads (Table 1). Reproductive isolation caused by selfing differed between cytotypes: tetraploids presented an RIselfing higher than octoploids (RIselfing,4x = 0.59, RIselfing,8x = 0.39; Table 1). When the mother plant received a single-ploidy pollen load from the other cytotype, RIgametic of tetraploids continued to be slightly higher than that obtained for octoploids (RIgametic,4x = 0.63, RIgametic,8x = 0.54; Table 1). When the mother plant received a mixed-ploidy pollen load, RIgametic increased in both cytotypes (RIgametic,4x = 1.00, RIgametic,8x = 0.96), which resulted in almost complete reproductive isolation between cytotypes (RIgametic = 0.98; Table 1).
Effect of all reproductive barriers
Combining the effects of all reproductive stages resulted in total RI values ranging from 0.86 to 0.99 depending on the ploidy composition of pollen loads, with reproductive isolation indices caused by selfing and gametic selection having the highest contribution (Table 1).
The experimental mixed-ploidy population produced similar results (Fig. 5B) in comparison with mixed-ploidy outcross pollinations (treatment 4), resulting in high RI values for both cytotypes (RI4x = RI8x = 0.99). No statistical differences were observed in fruit set between cytotypes (z1,409 = −0.08, P = 0.938; Supplementary Data Fig. S1C), and, although octoploids had significantly higher S:O ratios than tetraploids (z1,409 = −2.26, P = 0.02; Supplementary Data Fig. S1D), there were no significant differences in final reproductive success (z1,409 = −1.34, P = 0.173; Fig. 5B). Most of the offspring produced had the ploidy of the mother plant (Fig. 5B). A few hexaploids were produced, but only by octoploid plants. Finally, we observed the production of octoploids by tetraploid mothers and decaploids by octoploid mothers, which suggests that unreduced gametes are occasionally produced. Some aneuploids were also produced by both cytotypes.
DISCUSSION
We quantified the contribution of several reproductive barriers to isolation between tetraploid and octoploid G. communis, which co-occur in several contact zones (Castro et al., 2018). Our results revealed weak pre-pollination and strong post-pollination barriers. No differences in flowering phenology and flower morphology were observed between cytotypes, and both were visited predominantly by generalist pollinators without cytotype foraging preferences. In contrast, post-pollination isolation, resulting from gametic selection, was a strong barrier to inter-cytotype mating, although inter-cytotype cross-ability was fairly high. Additionally, selfing could play an important role in mediating RI. Overall, the total RI likely weakens minority cytotype disadvantage, allowing cytotype coexistence.
Pre-pollination reproductive isolation
Our results show complete overlap in flowering between tetraploids and octoploids in the contact zone and when grown in a common garden. Thus, flowering phenology by itself cannot prevent inter-cytotype crossing. Previous studies have documented significant differentiation in flowering time between cytotypes, from total flowering divergence (Petit et al., 1997) to variable degrees of segregation (e.g. Felber, 1988; Bretagnolle and Thompson, 1996; Husband and Schemske, 2000; Ramsey, 2011; Laport et al., 2016). Complete overlap in flowering phenology is less common but has been observed in Aster amellus (Castro et al., 2011) and Gymnadenia conopsea (Jersáková et al., 2010). Similar flowering times can result from different processes; for example, recent or recurrent formation of octoploids from tetraploids, where tetraploid flowering alleles are recurrently introgressed into octoploids at contact zones (Laport et al., 2016), or environmental selection in similar habitats.
Floral traits can affect inter-cytotype mating by influencing pollinator preferences (e.g. Segraves and Thompson, 1999; Roccaforte et al., 2015) and by causing differential pollen deposition on the insect body (Grant, 1994). The main pollinators showed no preference for a specific cytotype and randomly visited inflorescences in mixed-ploidy arrays, revealing that pollinators do not discriminate between tetraploids and octoploids. This behaviour promotes pollen exchange between cytotypes and thus it does not prevent inter-cytotype crosses, resulting in low levels of RI. Research to date reports a diversity of pollinator foraging patterns, ranging from divergent cytotype pollinator communities to common flower visitors and asymmetrical visitation frequencies (e.g. Segraves and Thompson, 1999; Kennedy et al., 2006; reviewed in Segraves and Anneberg, 2016), and a lack of preferences by generalist pollinators or even by specific pollinator guilds (e.g. Jersáková et al., 2010; Castro et al., 2011; Borges et al., 2012). Together with a high overlap between the relative positions of sexual organs in tetraploid and octoploid flowers, the low differentiation of pollinators contributes to an absence of RI between G. communis cytotypes.
Altogether, when growing in sympatry, pre-pollination barriers (phenology, pollinator and morphology) in G. communis are very weak. In several polyploid complexes, pre-pollination barriers work in combination to generate RI (e.g. Van Dijk and Bijlsma, 1994; Segraves and Thompson, 1999; Nuismer and Cunningham, 2005; Roccaforte et al., 2015). However, when these barriers are weak or absent, as in G. communis, cytotypes are vulnerable to minority cytotype disadvantage.
Post-pollination reproductive isolation
Inter-cytotype crosses. Our hand-pollinations revealed RI levels dependent on the pollen load composition delivered to the stigmas. While single-ploidy inter-cytotype crosses revealed fairly high cross-ability between cytotypes and the production of hexaploid offspring, mixed-ploidy pollen loads revealed high levels of RI. G. communis occurs in mixed-ploidy populations with variable cytotype proportions (Castro et al., 2018). In these cases, mixed-ploidy pollen loads are expected (due to lack of pre-pollination barriers) and both tetraploid and octoploid mothers mostly produce offspring of their own ploidy. This result indicates pollen competition and gametic selection against alternative cytotype pollen, with higher siring success of pollen from the mother’s ploidy. Pollen competition is considered a key reproductive barrier for interspecific hybridization (Carney et al., 1996; Diaz and Macnair, 1999), being also observed in polyploid complexes. Different pollen tube growth rates have been observed, for example, in Cucumis melo (Tanaka and Mukai, 1955; Susiacue and Álvarez, 1997) and Chamaenerion angustifolium (Husband et al., 2002; Baldwin and Husband, 2011), while a mentor effect was observed in Centaurea crossing experiments (Koutecký et al., 2011), both affecting the fitness of inter-cytotype crosses. Overall, when growing in mixed-ploidy populations with similar cytotype proportions, post-pollination (and pre-zygotic) interactions are strong and lead to high RI between tetraploid and octoploid G. communis, ameliorating minority cytotype disadvantage.
Although siring success of inter-cytotype crosses was reduced by pollen–pistil interactions (as detected by Baldwin and Husband, 2011) and possibly also by post-zygotic processes (Müntzing, 1933; Van Dijk et al., 1992; Burton and Husband, 2000; reviewed in Lafon‐Placette and Köhler, 2016), single-ploidy inter-cytotype pollinations produced hexaploid offspring by both tetraploids and octoploids. Under these pollen loads, the levels of cross-ability recorded resulted in moderate RI between tetraploid and octoploid G. communis. Post-pollination barriers were shown to be weak in some diploid–tetraploid complexes, with triploids being observed in mixed-ploidy populations (e.g. Baack, 2004; Ståhlberg, 2009). More recently, post-zygotic isolation was also suggested to be weaker in higher-order ploidies than in diploids and tetraploids (Sutherland and Galloway, 2017) due to lower parental genomic imbalance (Sonnleitner et al., 2013). Our results suggest moderate post-pollination barriers for the rarer cytotype in the population (regardless of the cytotype), i.e. the rare cytotype will receive mostly pollen from the dominant cytotype, producing mostly hybrids. Under this scenario, pre-pollination reproductive barriers, although very weak, may act in concert with gametic barriers to ameliorate minority cytotype disadvantage.
Selfing. Pollinators can promote reproductive isolation by increasing self-pollination when moving among flowers of the same plant and reducing intermediate ploidy production (Husband and Sabara, 2004; Baack, 2005). In G. communis, pollinators frequently visit the bottom flower and then move upwards, visiting all open flowers sequentially. This behaviour promotes pollen transfer among the flowers of the same inflorescence (Jordan and Harder, 2006; Jordan et al., 2016) and, thus, most G. communis plants may experience self-pollination. Our hand-pollinations show that the presence of self-pollen significantly affected the fitness and offspring composition of G. communis cytotypes. In tetraploids, siring success after inter-cytotype outcrossing plus selfing was low, and the resulting seeds were composed of a few tetraploid and hexaploid seeds, whereas octoploids produced a significant number of hexaploid offspring (similar to that of inter-cytotype crosses). Thus, in octoploids, selfing does not reduce the production of hexaploids. Although octoploids are more self-compatible than tetraploids, 2x gametes (from tetraploids) are more successful in fertilizing ovules when competing against 4x gametes from octoploid self-pollen. Contrarily, in tetraploids, self-pollen deposition significantly reduced the development of hexaploid offspring when compared with inter-cytotype crosses, and consequently the cost associated with its production. The decrease in hexaploid offspring by tetraploids might have resulted from ovule blocking by self-pollen, producing a stronger post-pollination barrier than the one observed in octoploids. This resulted in higher RI through selfing in tetraploids than in octoploids. From our study, post-pollination interactions are determined by the origin and composition of the pollen delivered to stigmas and will govern the levels of reproductive isolation.
Cytotype co-existence in contact zones
When growing in sympatry under similar frequencies, post-pollination interactions were the strongest barrier leading to high reproductive isolation in both tetraploid and octoploid plants. These interactions were corroborated in an experimental mixed-ploidy population, in which resource availability and cytotype frequencies were controlled. Tetraploids and octoploids presented similar sexual reproductive success and produced offspring mainly of their own ploidy, supporting strong gametic selection. Strong RI may thus enable cytotype coexistence in mixed-ploidy populations. Still, it is worth noticing that some levels of gene flow might occur through the production of hexaploids and/or through unreduced gametes produced by tetraploids (however, genetic studies are necessary to confirm this). The coexistence of cytotypes has been observed in natural contact zones, such as in the Tripleurospermum inodorum diploid–tetraploid secondary contact zone (Čertner et al., 2017) or in the Knautia arvensis diploid–tetraploid primary contact zone (Hanzl et al., 2014), with mixed-ploidy populations being found and cytotypes being reproductively isolated. Still, although we attempted to quantify the contribution of multiple factors to assortative mating, the dynamics of the populations depend on several other factors. Factors such as patchy distribution generated by small-scale niche differentiation, differences in competitive abilities or vegetative reproduction that may confer an advantage to a given cytotype or promote inter-cytotype crosses due to plant clustering (e.g. Baack, 2004; Kao, 2007; Kolář et al., 2009; Collins et al., 2011), or differences in population dynamics parameters (e.g. Münzbergová, 2007), such as seed germination or seedling survival, may all have the potential to influence plant fitness and drive cytotype frequencies within the population.
Octoploid fitness advantages
Our study suggests that octoploids may experience fitness advantages over tetraploids. We detected the production of unreduced male and female gametes by tetraploid plants in natural populations, as supported by the occurrence of hexaploid individuals within tetraploid populations outside the tetraploid–octoploid contact areas (Castro et al., 2018). Unreduced gamete production causing recurrent octoploid formation may significantly contribute to the establishment of octoploid plants within tetraploid populations (Felber, 1991; Husband, 2004; Suda and Herben, 2013). Additionally, hexaploids produced after inter-cytotype crosses may serve as bridges (‘triploid bridge’ as defined by Ramsey and Schemske, 1998), contributing also to recurrent octoploid formation. The presence of flowering hexaploids in natural populations demonstrates that some hexaploid seeds are viable and that hexaploid plants can reach reproductive maturity (Castro et al., 2018). Finally, octoploids show higher reproductive success after selfing than tetraploids, which could represent a reproductive assurance under scenarios of low mating availability (Barringer, 2007; Borges et al., 2012; Siopa et al., 2018). Altogether, the levels of RI observed, combined with the contribution of unreduced gamete formation, hexaploid production and selfing, may provide suitable conditions for octoploids to emerge and increase their number within tetraploid populations.
The dynamics of contact zones of the polyploid G. communis complex is far from being completely understood and additional information on unreduced gamete formation, pollen tube growth rates, later-acting barriers and life-history traits, such as seed viability, dispersal capacity and asexual reproduction, need to be evaluated to understand the entire picture.
Conclusions
In sympatry, post-pollination gametic barriers are the most important reproductive barrier in the polyploid G. communis complex. However, the composition of the pollen delivered by pollinators will determine the magnitude of reproductive isolation and the production of hexaploids. Because the composition of the pollen load determines both cytotype fitness and offspring ploidy, and contact zones are characterized by different mixed-ploidy spatial arrangements, the interactions between cytotypes are expected to be complex in natural contact zones. Strong gametic selection against alternative cytotype pollen under mixed-ploidy pollen loads could maintain mixed tetraploid–octoploid populations. Additionally, the relative fitness of octoploids may increase with unreduced gamete formation, hexaploid production and higher selfing success than tetraploids. edna
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Appendix 1: floral preference and constancy index correction factor. Figure S1: fruit set and S:O ratio after hand-pollination treatments and in the experimental mixed-ploidy population. Table S1: morphological characterization and nectar production of tetraploid and octoploid Gladiolus communis flowers. Table S2: effect of ploidy and pollination treatment on fruit set and S:O ratio after hand-pollinations.
ACKNOWLEDGEMENTS
The authors are thankful to José Miguel Costa, Lucie Mota and Daniela Tavares for their help with pollinator monitoring, and to Francisco Núñez and José Miguel Costa for their assistance in insect identification. The authors are also thankful to the Handling Editor Dr Zuzana Münzbergová and three anonymous reviewers for their constructive and helpful comments, all of which enabled us to significantly improve the work.
FUNDING
This research was supported by POPH/FSE funds by the Portuguese Foundation for Science and Technology (FCT) with a doctoral grant to M.C. (SFRH/BD/89910/2012) and a starting grant and exploratory project to S.C. (IF/01267/2013), and by Project RENATURE financed by the Programa Operacional Regional do Centro 2014–2020 (Centro2020) – CENTRO-01-0145-FEDER-000007.
LITERATURE CITED
- Alix K, Gérard PR, Schwarzacher T, Heslop-Harrison JSP. 2017. Polyploidy and interspecific hybridization: partners for adaptation, speciation and evolution in plants. Annals of Botany 120: 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso MA, Crespo MB. 2010. Gladiolus L. In: Crespo MB, Herrero A, Quintana, eds. Flora Iberica Madrid: Real Jardín Botánico, 485–491. [Google Scholar]
- Baack EJ. 2004. Cytotype segregation on regional and microgeographic scales in snow buttercups (Ranunculus adoneus: Ranunculaceae). American Journal of Botany 91: 1783–1788. [DOI] [PubMed] [Google Scholar]
- Baack EJ. 2005. To succeed globally, disperse locally: effects of local pollen and seed dispersal on tetraploid establishment. Heredity 94: 538–546. [DOI] [PubMed] [Google Scholar]
- Baldwin S J, Husband BC. 2011. Genome duplication and the evolution of conspecific pollen precedence. Proceedings of the Royal Society of London B: Biological Sciences 278: 2010–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barringer BC. 2007. Polyploidy and self-fertilization in flowering plants. American Journal of Botany 94: 1527–1533. [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67: 1–48. [Google Scholar]
- Borges LA, Souza LGR, Guerra M, Machado IC, Lewis GP, Lopes AV. 2012. Reproductive isolation between diploid and tetraploid cytotypes of Libidibia ferrea (= Caesalpinia ferrea) (Leguminosae): ecological and taxonomic implications. Plant Systematics and Evolution 7: 1371–1381. [Google Scholar]
- Bretagnolle F, Thompson JD. 1996. An experimental study of ecological differences in winter growth between sympatric diploid and autotetraploid Dactylis glomerata. Journal of Ecology 84: 343–351. [Google Scholar]
- Burton TL, Husband BC. 2000. Fitness differences among diploids, tetraploids, and their triploid progeny in Chamerion angustifolium: mechanisms of inviability and implications for polyploid evolution. Evolution 4: 1182–1191. [DOI] [PubMed] [Google Scholar]
- Carney SE, Hodges SA, Arnold ML. 1996. Effects of differential pollen-tube growth on hybridization in the Louisiana irises. Evolution 50: 1871–1878. [DOI] [PubMed] [Google Scholar]
- Castro M. 2018. Evolutionary ecology of polyploids: understanding species coexistence at the contact zones. PhD Thesis, University of Coimbra, Portugal. [Google Scholar]
- Castro M, Castro S, Figueiredo A, Husband B, Loureiro J. 2018. Complex cytogeographical patterns reveal a dynamic tetraploid–octoploid contact zone. AoB Plants 10: ply012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro S, Münzbergová Z, Raabová J, Loureiro J. 2011. Breeding barriers at a diploid–hexaploid contact zone in Aster amellus. Evolutionary Ecology 25: 795–814. [Google Scholar]
- Castro S, Loureiro J, Procházka T, Münzbergová Z. 2012. Cytotype distribution at a diploid-hexaploid contact zone in Aster amellus (Asteraceae). Annals of Botany 110: 1047–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čertner M, Fenclová E, Kúr P, et al. 2017. Evolutionary dynamics of mixed-ploidy populations in an annual herb: dispersal, local persistence and recurrent origins of polyploids. Annals of Botany 120: 303–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AR, Naderi R, Mueller-Schaerer H. 2011. Competition between cytotypes changes across a longitudinal gradient in Centaurea stoebe (Asteraceae). American Journal of Botany 98: 1935–1942. [DOI] [PubMed] [Google Scholar]
- Diaz A, Macnair MR. 1999. Pollen tube competition as a mechanism of prezygotic reproductive isolation between Mimulus nasutus and its presumed progenitor M. guttatus. New Phytologist 144: 471–478. [DOI] [PubMed] [Google Scholar]
- Doležel J, Greilhuber J, Lucretti S, et al. 1998. Plant genome size estimation by flow cytometry: inter-laboratory comparison. Annals of Botany 82: 17–26. [Google Scholar]
- Felber F. 1988. Phenologie de la floraison de populations diploides et tetraploides d’Anthoxanthum alpinum et d’Anthoxanthum odoratum. Canadian Journal of Botany 66: 2258–2264. [Google Scholar]
- Felber F. 1991. Establishment of a tetraploid cytotype in a diploid population: effect of relative fitness of the cytotypes. Journal of Evolutionary Biology 4: 195–207. [Google Scholar]
- Fernandes A, Garcia J, Fernandes R. 1948. Herborizações nos domínios da fundação da Casa de Bragança. I - Vendas Novas. Memórias da Sociedade Broteriana 45: 143–176. [Google Scholar]
- Fernandes A, Queirós M. 1971. Sur la caryologie de quelques plantes réoltées pendant da III Reunion de Botanique Péninsulaire. Memórias da Sociedade Broteriana 21: 343–385. [Google Scholar]
- Fox J, Weisberg S, Adler D, et al. 2016. Package ‘car’. Companion to applied regression. R Package version 2–1. [Google Scholar]
- Galbraith DW, Harkins KR, Maddox JM, Ayres NM, Sharma DP, Firoozabady E. 1983. Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 220: 1049–1051. [DOI] [PubMed] [Google Scholar]
- Grant V. 1994. Modes and origins of mechanical and ethological isolation in angiosperms. Proceedings of the National Academy of Sciences of the USA 91: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AP. 1980. Gladiolus L. In: Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters S, eds. Flora Europaea, Vol. 5. Cambridge: Cambridge University Press, 101–102. [Google Scholar]
- Hanzl M, Kolář F, Nováková D, Suda J. 2014. Nonadaptive processes governing early stages of polyploid evolution: insights from a primary contact zone of relict serpentine Knautia arvensis (Caprifoliaceae). American Journal of Botany 101: 935–945. [DOI] [PubMed] [Google Scholar]
- Hothorn T, Bretz F, Westfall P, et al. 2016. Package ‘multcomp’. Simultaneous inference in general parametric models. R Package version 1.4–6. [DOI] [PubMed] [Google Scholar]
- Husband BC. 2000. Constraints on polyploid evolution: a test of the minority cytotype exclusion principle. Proceedings. Biological Sciences 267: 217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husband BC. 2004. The role of triploid hybrids in the evolutionary dynamics of mixed-ploidy populations. Biological Journal of the Linnean Society 82: 537–546. [Google Scholar]
- Husband BC, Sabara HA. 2004. Reproductive isolation between autotetraploids and their diploid progenitors in fireweed, Chamerion angustifolium (Onagraceae). New Phytologist 161: 703–713. [DOI] [PubMed] [Google Scholar]
- Husband BC, Schemske DW. 2000. Ecological mechanisms of reproductive isolation between diploid and tetraploid Chamerion angustifolium. Journal of Ecology 88: 689–701. [Google Scholar]
- Husband BC, Schemske DW, Burton TL, Goodwillie C. 2002. Pollen competition as a unilateral reproductive barrier between sympatric diploid and tetraploid Chamerion angustifolium. Proceedings. Biological Sciences 269: 2565–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husband BC, Baldwin SJ, Suda J. 2013. The incidence of polyploidy in natural plant populations: major patterns and evolutionary processes. In: Greilhuber J, Dolezel J, Wendel J, eds. Plant genome diversity, Vol. 2. Vienna: Springer, 255–276. [Google Scholar]
- Husband BC, Baldwin SJ, Sabara HA. 2016. Direct vs. indirect effects of whole-genome duplication on prezygotic isolation in Chamerion angustifolium: implications for rapid speciation. American Journal of Botany 103: 1259–1271. [DOI] [PubMed] [Google Scholar]
- Jersáková J, Castro S, Sonk N, et al. 2010. Absence of pollinator-mediated premating barriers in mixed-ploidy populations of Gymnadenia conopsea s.l. (Orchidaceae). Evolutionary Ecology 24: 1199–1218. [Google Scholar]
- Jordan CY, Harder LD. 2006. Manipulation of bee behavior by inflorescence architecture and its consequences for plant mating. American Naturalist 167: 496–509. [DOI] [PubMed] [Google Scholar]
- Jordan CY, Natta M, Harder LD. 2016. Flower orientation influences the consistency of bumblebee movement within inflorescences. Annals of Botany 118: 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao RH. 2007. Asexuality and the coexistence of cytotypes. New Phytologist 175: 764–772. [DOI] [PubMed] [Google Scholar]
- Kennedy BF, Sabara HA, Haydon D, Husband BC. 2006. Pollinator-mediated assortative mating in mixed ploidy populations of Chamerion angustifolium (Onagraceae). Oecologia 150: 398–408. [DOI] [PubMed] [Google Scholar]
- Kolář F, Čertner M, Suda J, Schönswetter P, Husband BC. 2017. Mixed-ploidy species: progress and opportunities in polyploid research. Trends in Plant Science 22: 1041–1055. [DOI] [PubMed] [Google Scholar]
- Kolář F, Štech M, Trávníček P, et al. 2009. Towards resolving the Knautia arvensis agg. (Dipsacaceae) puzzle: primary and secondary contact zones and ploidy segregation at landscape and microgeographic scales. Annals of Botany 103: 963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutecký P, Badurová T, Štech M, Košnar JAN, Karásek J. 2011. Hybridization between diploid Centaurea pseudophrygia and tetraploid C. jacea (Asteraceae): the role of mixed pollination, unreduced gametes, and mentor effects. Biological Journal of the Linnean Society 104: 93–106. [Google Scholar]
- Lafon-Placette C, Köhler C. 2016. Endosperm‐based postzygotic hybridization barriers: developmental mechanisms and evolutionary drivers. Molecular Ecology 25: 2620–2629. [DOI] [PubMed] [Google Scholar]
- Laport RG, Minckley RL, Ramsey J. 2016. Ecological distributions, phenological isolation, and genetic structure in sympatric and parapatric populations of the Larrea tridentata polyploid complex. American Journal of Botany 103: 1358–1374. [DOI] [PubMed] [Google Scholar]
- Levin DA. 1975. Minority cytotype exclusion in local plant populations. Taxon 24: 35–43. [Google Scholar]
- Lexer C, van Loo M. 2006. Contact zones: natural labs for studying evolutionary transitions. Current Biology 16: R407–R409. [DOI] [PubMed] [Google Scholar]
- Loureiro J, Rodriguez E, Dolezel J, Santos C. 2007. Two new nuclear isolation buffers for plant DNA flow cytometry: a test with 37 species. Annals of Botany 100: 875–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques I, Loureiro J, Draper D, Castro M, Castro S. 2017. How much do we know about the frequency of hybridization and polyploidy in the Mediterranean region? Plant Biology 20: 21–37. [DOI] [PubMed] [Google Scholar]
- Martin SL, Husband BC. 2012. Whole genome duplication affects evolvability of flowering time in an autotetraploid plant. PLoS ONE 7: e44784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mráz P. 2003. Mentor effects in the genus Hieracium s. str. (Compositae, Lactuceae). Folia Geobotanica 38: 345–350. [Google Scholar]
- Müntzing A. 1933. Hybrid incompatibility and the origin of polyploidy. Hereditas 18: 33–55. [Google Scholar]
- Münzbergová Z. 2007. Population dynamics of diploid and hexaploid populations of a perennial herb. Annals of Botany 100: 1259–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuismer SL, Cunningham BM. 2005. Selection for phenotypic divergence between diploid and autotetraploid Heuchera grossulariifolia. Evolution 59: 1928–1935. [PubMed] [Google Scholar]
- Paun O, Forest F, Fay MF, Chase MW. 2009. Hybrid speciation in angiosperms: parental divergence drives ploidy. New Phytologist 182: 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit C, Lesbros P, Ge X, Thompson JD. 1997. Variation in flowering phenology and selfing rate across a contact zone between diploid and tetraploid Arrhenatherum elatius (Poaceae). Heredity 79: 31–40. [Google Scholar]
- Ramsey J. 2011. Polyploidy and ecological adaptation in wild yarrow. Proceedings of the National Academy of Sciences of the USA 108: 7096–7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey J, Bradshaw HD Jr, Schemske DW. 2003. Components of reproductive isolation between the monkeyflowers Mimulus lewisii and M. cardinalis (Phrymaceae). Evolution 57: 1520–1534. [DOI] [PubMed] [Google Scholar]
- Ramsey J, Schemske DW. 1998. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annual Review of Ecology and Systematics 29: 467–501. [Google Scholar]
- R Core Development Team 2016. A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; http://www.R-project.org/. [Google Scholar]
- Rice A, Glick L, Abadi S, et al. 2015. The Chromosome Counts Database (CCDB) – a community resource of plant chromosome numbers. New Phytologist 206: 19–26. [DOI] [PubMed] [Google Scholar]
- Rieseberg LH, Willis JH. 2007. Plant speciation. Science 317: 910–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roccaforte K, Russo SE, Pilson D. 2015. Hybridization and reproductive isolation between diploid Erythronium mesochoreum and its tetraploid congener E. albidum (Liliaceae). Evolution 69: 1375–1389. [DOI] [PubMed] [Google Scholar]
- Rodriguez DJ. 1996. A model for the establishment of polyploidy in plants. American Naturalist 147: 33–46. [Google Scholar]
- Segraves KA, Anneberg TJ. 2016. Species interactions and plant polyploidy. American Journal of Botany 103: 1326–1335. [DOI] [PubMed] [Google Scholar]
- Segraves KA, Thompson JN. 1999. Plant polyploidy and pollination: floral traits and insect visits to diploid and tetraploid Heuchera grossulariifolia. Evolution 53: 1114–1127. [DOI] [PubMed] [Google Scholar]
- Siopa C, Dias MC, Castro M, Loureiro J, Castro S. 2018. Is selfing a reproductive assurance promoting polyploid establishment? Reduced fitness, leaky self‐incompatibility and lower inbreeding depression in neotetraploids. American Journal of Botany 107: 1–13. [DOI] [PubMed] [Google Scholar]
- Soltis DE, Soltis PS, Schemske DW, et al. 2007. Autopolyploidy in angiosperms: have we grossly underestimated the number of species? Taxon 56: 13–30. [Google Scholar]
- Sonnleitner M, Weis B, Flatscher R, et al. 2013. Parental ploidy strongly affects offspring fitness in heteroploid crosses among three cytotypes of autopolyploid Jacobaea carniolica (Asteraceae). PLoS ONE 8: e78959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ståhlberg D. 2009. Habitat differentiation, hybridization and gene flow patterns in mixed populations of diploid and autotetraploid Dactylorhiza maculata s.l. (Orchidaceae). Evolutionary Ecology 23: 295–328. [Google Scholar]
- Stebbins GL. 1938. Cytological characteristics associated with the different growth habits in the dicotyledons. American Journal of Botany 25: 189–198. [Google Scholar]
- Suda J, Herben T. 2013. Ploidy frequencies in plants with ploidy heterogeneity: fitting a general gametic model to empirical population data. Proceedings. Biological Sciences 280: 20122387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susiacue I, Álvarez JM. 1997. Fertility and pollen tube growth in polyploid melons (Cucumis melo L.). Euphytica 93: 369–373. [Google Scholar]
- Sutherland BL, Galloway LF. 2017. Postzygotic isolation varies by ploidy level within a polyploid complex. New Phytologist 213: 404–412. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Mukai T. 1955. Studies on artificially induced polyploid ‘Makma’ melon (Cucumis melo L. var. Makuwa Makino). III. Further study on pollen germination. Seiken Zihô 7: 86–93. [Google Scholar]
- Thompson JN, Merg KF. 2008. Evolution of polyploidy and the diversification of plant-pollinator interactions. Ecology 89: 2197–2206. [DOI] [PubMed] [Google Scholar]
- Van Dijk P, Bijlsma R. 1994. Simulations of flowering time displacement between two cytotypes that form inviable hybrids. Heredity 72: 522–522. [Google Scholar]
- Wood TE, Takebayashi N, Barker MS, Mayrose I, Greenspoon PB, Rieseberg LH. 2009. The frequency of polyploid speciation in vascular plants. Proceedings of the National Academy of Sciences of the USA 106: 13875–13879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zozomová-Lihová J, Malánová-Krásná I, Vít P, et al. 2015. Cytotype distribution patterns, ecological differentiation, and genetic structure in a diploid–tetraploid contact zone of Cardamine amara. American Journal of Botany 102: 1380–1395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.