Abstract
Background and Aims
Floral food bodies (including edible trichomes) are a form of floral reward for pollinators. This type of nutritive reward has been recorded in several angiosperm families: Annonaceae, Araceae, Calycanthaceae, Eupomatiaceae, Himantandraceae, Nymphaeaceae, Orchidaceae, Pandanaceae and Winteraceae. Although these bodies are very diverse in their structure, their cells contain food material: starch grains, protein bodies or lipid droplets. In Pinguicula flowers, there are numerous multicellular clavate trichomes. Previous authors have proposed that these trichomes in the Pinguicula flower play the role of ‘futterhaare’ (‘feeding hairs’) and are eaten by pollinators. The main aim of this study was to investigate whether the floral non-glandular trichomes of Pinguicula contain food reserves and thus are a reward for pollinators. The trichomes from the Pinguicula groups, which differ in their taxonomy (species from the subgenera: Temnoceras, Pinguicula and Isoloba) as well as the types of their pollinators (butterflies/flies and bees/hummingbirds), were examined. Thus, it was determined whether there are any connections between the occurrence of food trichomes and phylogeny position or pollination biology. Additionally, we determined the phylogenetic history of edible trichomes and pollinator evolution in the Pinguicula species.
Methods
The species that were sampled were: Pinguicula moctezumae, P. esseriana, P. moranensis, P. emarginata, P. rectifolia, P. mesophytica, P. hemiepiphytica, P. agnata, P. albida, P. ibarrae, P. martinezii, P. filifolia, P. gigantea, P. lusitanica, P. alpina and P. vulgaris. Light microscopy, histochemistry, and scanning and transmission electron microscopy were used to address our aims with a phylogenetic perspective based on matK/trnK DNA sequences.
Key Results
No accumulation of protein bodies or lipid droplets was recorded in the floral non-glandular trichomes of any of the analysed species. Starch grains occurred in the cells of the trichomes of the bee-/fly-pollinated species: P. agnata, P. albida, P. ibarrae, P. martinezii, P. filifolia and P. gigantea, but not in P. alpina or P. vulgaris. Moreover, starch grains were not recorded in the cells of the trichomes of the Pinguicula species that have long spurs, which are pollinated by Lepidoptera (P. moctezumae, P. esseriana, P. moranensis, P. emarginata and P. rectifolia) or birds (P. mesophytica and P. hemiepihytica), or in species with a small and whitish corolla that self-pollinate (P. lusitanica). The results on the occurrence of edible trichomes and pollinator syndromes were mapped onto a phylogenetic reconstruction of the genus.
Conclusion
Floral non-glandular trichomes play the role of edible trichomes in some Pinguicula species (P. agnata, P. albida, P. ibarrae, P. martinezii, P. filifolia and P. gigantea), which are mainly classified as bee-pollinated species that had originated from Central and South America. It seems that in the Pinguicula that are pollinated by other pollinator groups (Lepidoptera and hummingbirds), the non-glandular trichomes in the flowers play a role other than that of a floral reward for their pollinators. Edible trichomes are symplesiomorphic for the Pinguicula species, and thus do not support a monophyletic group such as a synapomorphy. Nevertheless, edible trichomes are derived and are possibly a specialization for fly and bee pollinators by acting as a food reward for these visitors.
Keywords: Butterworts, carnivorous plants, floral micro-morphology, food hairs, Lentibulariaceae, trichome structure, Pinguicula, spur, trichomes
INTRODUCTION
Plants offer various floral rewards for pollinators that can be divided into two groups: non-nutritive rewards (e.g. nest materials, a place of shelter, heat sources, substances for production of sexual attractants or places for mating ) and nutritive rewards (e.g. brood site, floral sweet tissue, stigmatic secretion or fatty oils) (Simpson and Neff, 1981). The most common floral nutritive rewards are nectar and pollen (Faegri and van der Pijl, 1979; Nicolson et al., 2007). However, some species produce food bodies (including edible trichomes) that are eaten by their pollinators. The cells of these structures are rich with starch grains, protein bodies or oil droplets (Young, 1986; Thien et al., 2009; for orchids, see Pansarin and Maciel, 2017 and references therein). Food bodies have been recorded in several unrelated plant families: Annonaceae, Araceae, Calycanthaceae, Eupomatiaceae, Himantandraceae, Orchidaceae, Pandanaceae, Nymphaeaceae and Winteraceae (e.g. Faegri and van der Pijl, 1979; Rickson, 1979; Cox, 1982; Young, 1986; Davies et al., 2002; Thien et al., 2009; Endress, 2010; Pansarin and Maciel, 2017). Thus, this type of reward occurs in both evolutionarily old families via beetle pollination (Annonaceae, Calycanthaceae, Eupomatiaceae, Himantandraceae, Nymphaeaceae and Winteraceae; see Endress, 2010) as well as in the more evolutionarily derived family Orchidaceae, which now represents an evolutionary pick of diversity. Floral food bodies can be divided into two major groups: the first (which occurs, for example, in the older lineages of angiosperms, Endress, 2010) – the outgrowths (or tips) of the carpels, stamens, staminodes and tepals; and the second – the epidermal edible trichomes. These trichomes have been particularly well analysed in Orchidaceae and they were found to have evolved independently in this family about five times (genera: Cyanaeorchis, Dendrobium, Eria, Maxillaria and Polystachya; Pansarin and Maciel, 2017). In orchids, they are very diverse in their structure and morphology as well as in the storage of nutritive material in their cells (e.g. Davies et al., 2002; Davies and Turner, 2004; Pansarin and Maciel, 2017).
Pinguicula is a monophyletic genus within the Lentibulariaceae L. family (Jobson et al., 2003; Müller et al., 2004; Fleischmann and Roccia, 2018) and is among the Lamiales (Schäferhoff et al., 2010; Chase et al., 2016) and contains about 96 species. Pinguicula are well known for their carnivory (e.g. Alcalá and Domínguez, 2003, 2005; Darnowski et al., 2018; Heslop-Harrison, 1970; Heslop-Harrison and Heslop-Harrison, 1980; Vassilyev and Muravnik, 1988).
Pinguicula produce spurred zygomorphic flowers, which have nectar as a reward (Abrahamczyk et al., 2017; Fleischmann and Roccia, 2018; Lustofin et al., 2019). In Pinguicula flowers, there are numerous multicellular clavate trichomes at the base of the corolla – the throat; see Fig. 1A–I (Casper, 1966). Previous authors have proposed that these trichomes in the Pinguicula flower play the role of ‘futterhaare’ (‘feeding hairs’) and are eaten by their pollinators, or that some of them play the role of mimic pollen grains (see Fleischmann, 2016). Thus, the main aim of this study was to determine whether these trichomes of Pinguicula contain food reserves and thus may be a reward for potential pollinators. We selected species from the different clades, which are based on published phylogenetic proposals, within Pinguicula (members from three subgenera but focused on the Central American species) and also sampled species based on differences in their mating system. For this criterion, self- (i.e. a small flower with a whitish corolla) vs. outcross species (large, brightly coloured corollas, nectar guides and long spurs) were compared. Additionally, in our study, we considered the pollinator types (butterflies/fly and bees/hummingbirds). Fleischmann (2016) wrote that the clavate trichomes of Pinguicula are glandular, and therefore another task/aim was to determine whether these trichomes have the character of glands.
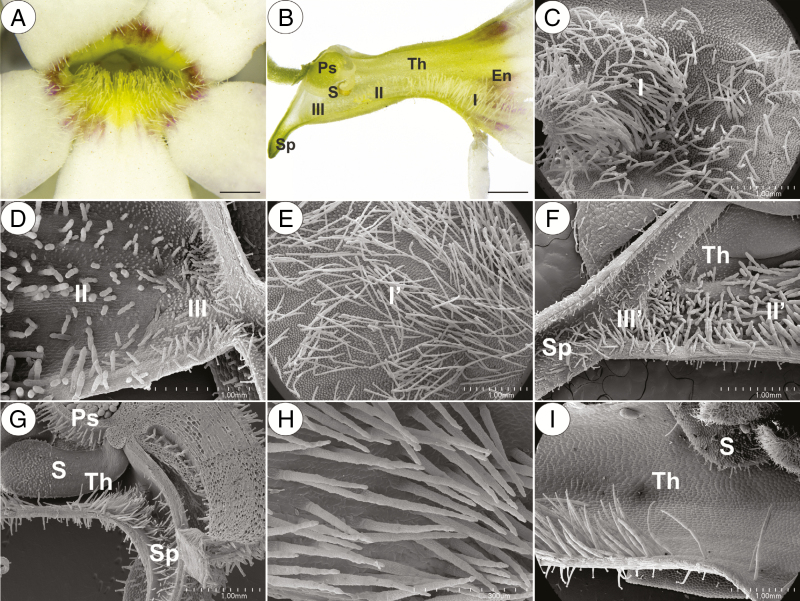
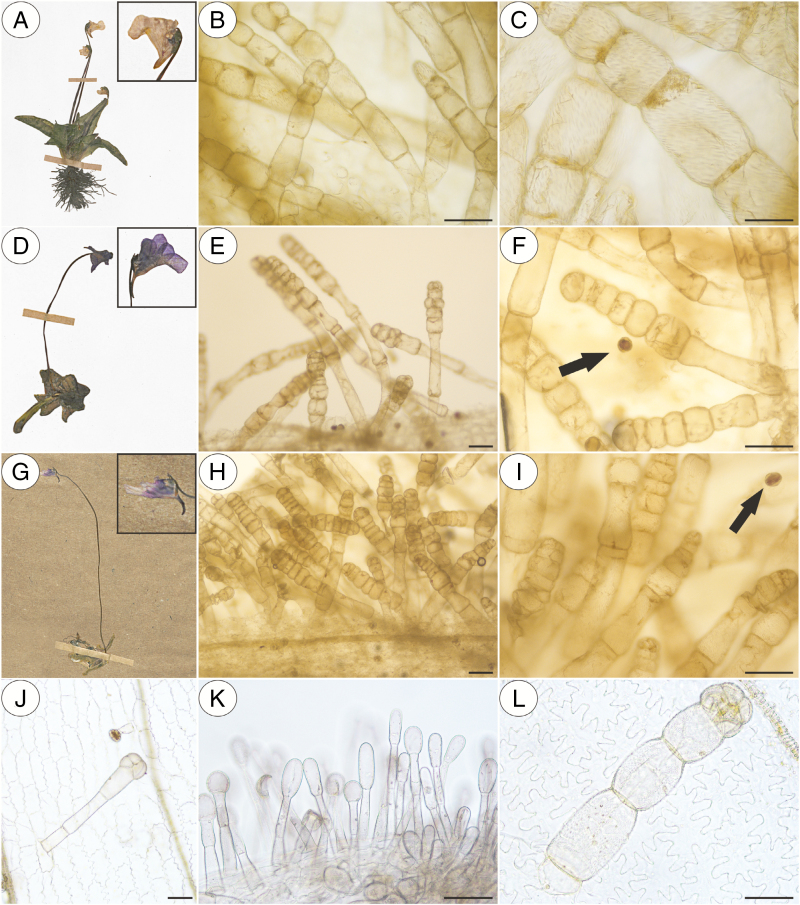
Fig. 1.
General morphology and micromorphology of the selected Pinguicula species that were examined. (A–D) General morphology and micromorphology of a P. agnata flower showing the entrance to the flower (En) with multicellular clavate slender trichomes (I), the throat (Th) with multicellular compact thick trichomes (II) in the front and two types of long and slender or short and compact non-glandular trichomes (III) that are located at the entrance to the spur (Sp); note the presence of a pistil (Ps) and a stamen (S) in the throat; scale bars = 2 mm, 2 mm, 1 mm and 1 mm, respectively. (E and F) Micromorphology of a P. gigantea flower; note the similar distribution and micromorphology of the non-glandular trichomes (Iʹ, IIʹ, IIIʹ) compared with P. agnata; scale bars = 1 mm and 1 mm, respectively. (G and H) Micromorphology of the P. rectifolia throat with generative organs and many celled uniseriate slender non-glandular trichomes indicated by an acute apical cell that is located in the throat and basal part of the spur; scale bars = 1 mm and 300 µm, respectively. (I) Micromorphology of the P. hemiepiphytica throat with long and slender multicellular non-glandular trichomes and a stamen; scale bar = 1 mm.
MATERIALS AND METHODS
Plant material
Seventeen taxa were sampled: Pinguicula moctezumae Zamudio & R.Z.Ortega, P. esseriana B.Kirchn., P. moranensis Kunth, P. emarginata Zamudio & Rzed., P. rectifolia Speta & F.Fuchs, P. mesophytica Zamudio, P. hemiepiphytica Zamudio & Rzed., P. agnata Casper, P. albida Wright ex Griseb., P. ibarrae Zamudio, P. martinezii Zamudio, P. filifolia C.Wright ex Griseb, P. gigantea Luhrs, P. lusitanica L., P. alpina L. and P. vulgaris L. [P. vulgaris subsp. vulgaris L. and P. vulgaris L. subsp. bicolor (Woł.) Á. Löve & D. Löve]. For our study, we primarily used living material (see Table 1). However, histochemical studies were used by some authors (e.g. Hernández and Katinas, 2019) in the case of herbarium material in order to show storage material or glandular structures. Therefore, we also used herbarium material of Pinguicula from the Herbarium of the Institute of Botany (KRA).
Table 1.
List of the Pinguicula species that were examined along with information regarding their infrageneric classification, the origin of the plant material and the type of pollinator for each species.
| Species | Infrageneric classification | Material origin | Type of pollinator |
|---|---|---|---|
| P. moctezumae Zamudio & R.Z.Ortega | Temnoceras | Botanical Garden of Jagiellonian University in Cracow (collected from: Mexico) | Lepidoptera (Abrahamczyk et al., 2017) |
| P. rectifolia Speta & F.Fuchs | Temnoceras | Botanical Garden of Jagiellonian University in Cracow (collected from: Mexico) | Lepidoptera (flower’s structure indicates that type of pollinator) |
| P. moranensis Kunth | Temnoceras | Botanical Garden of Jagiellonian University in Cracow (collected from: near Santiago Juxtlahuaca, Oaxaca, Mexico 1851 m) | Lepidoptera (Villegas and Alcalá, 2018) |
| P. emarginata Zamudio & Rzed. | Temnoceras | Botanical Garden of Jagiellonian University in Cracow (collected from: Mexico) | Lepidoptera (flower’s structure indicates that type of pollinator) |
| P. esseriana B.Kirchn. | Temnoceras | Botanical Garden of Jagiellonian University in Cracow (collected from: Mexico) | Lepidoptera (flower’s structure indicates that type of pollinator) |
| P. hemiepiphytica Zamudio & Rzed | Temnoceras | Botanical Garden of Jagiellonian University in Cracow (collected from: near Ixtlan de Juarez, Oaxaca, Mexico, 2209–2535 m.) | Most probably hummingbirds (Lampard et al., 2016) |
| P. mesophytica Zamudio | Temnoceras | Botanical Garden of Jagiellonian University in Cracow (collected from: Cerro Miramundo, El Salvador) | Ornithophily is presumed: a watercolour showing a species of hummingbird visiting a plants of Pinguicula mesophytica was shown in Roccia et al. (2016) |
| P. agnata Casper | Temnoceras | Botanical Garden of Jagiellonian University in Cracow (collected from: Mexico) | Diptera/Hymenoptera (flower’s structure indicates that type of pollinator) |
| P. gigantea Luhrs | Temnoceras | Botanical Garden of Jagiellonian University in Cracow (collected from: Mexico) | Diptera/Hymenoptera (Abrahamczyk et al., 2017) |
| P. ibarrae Zamudio | Temnoceras | Botanical Garden in Liberec | Diptera/Hymenoptera (flower’s structure indicates that type of pollinator) |
| P. martinezii Zamudio | Temnoceras | Diptera/Hymenoptera (flower’s structure indicates that type of pollinator) | |
| P. albida Wright ex Griseb. | Temnoceras | Hymenoptera (Dominguez et al., 2014) | |
| P. filifolia C.Wright ex Griseb. | Temnoceras | Hymenoptera (Dominguez et al., 2014) | |
| P. lusitanica L. | Isoloba | Botanical Garden of Jagiellonian University in Cracow (collected from: Europa) | Diptera/Hymenoptera(?), self-pollination (Heslop-Harrison, 2004) |
| P. alpina L. | Pinguicula | Herbarium of Jagiellonian University in Cracow (collected from: Alps, Innsbruck, Austria; KRA 0299930) | Diptera/Hymenoptera (Molau, 1993; Nordin, 2015) |
| P. vulgaris subsp.vulgaris L. | Pinguicula | Herbarium of Jagiellonian University in Cracow (collected from: Małe Pieniny, Rezerwat Zaskalskie, Poland; KRA 71415) | Diptera/Hymenoptera (Molau, 1993) |
| P. vulgaris L. subsp. bicolor (Woł.) Á. Löve & D. Löve | Pinguicula | Herbarium of Jagiellonian University in Cracow (collected from: Dąbrowa Górnicza, użytek ekologiczny ‘Młaki and Pogorią I’, Poland; KRA 0138573) |
Methods
The flowers were examined using light microscopy (LM), scanning electron microscopy (SEM) and transmission electron microscopy as described below. The material was fixed in a mixture of 2.5 or 5 % glutaraldehyde with 2.5 % formaldehyde in a 0.05 m cacodylate buffer (Sigma; pH 7.2) overnight or for several days, washed three times in a 0.1 m sodium cacodylate buffer and post-fixed in a 1 % osmium tetroxide solution at room temperature for 1.5 h. Next, the material was treated as was previously described (Płachno et al., 2017) and examined using a Hitachi H500 transmission electron microscope (Hitachi, Tokyo, Japan), which is housed at the University of Silesia in Katowice, at an accelerating voltage of 75 kV. The semi-thin sections (0.9–1.0 µm thick) that were prepared for LM were stained with aqueous methylene blue/azure II for 1–2 min (Humphrey and Pittman, 1974) and examined using Olympus BX60 and Nikon Eclipse E400 light microscopes to perform the general histology. The periodic acid–Schiff (PAS) reaction for LM (semi-thin sections) was also used to reveal the presence of insoluble polysaccharides (Wędzony, 1996), and Sudan Black B was used to detect the presence of lipids and cuticle material (Jensen, 1962).
Additionally, material that had been embedded in Technovit 7100 (Kulzer, Germany) was also examined. This material was fixed (as above), washed three times in a 0.1 m sodium cacodylate buffer, dehydrated in a graded ethanol series for 15 min at each concentration and kept overnight in absolute ethanol. Next, the samples were infiltrated for 1 h each in 3:1, 1:1 and 1:3 (v/v) mixtures of absolute ethanol and Technovit and then stored for 12 h in pure Technovit. The resin was polymerized by adding a hardener. The material was sectioned to 5 μm thickness using a rotary microtome (Microm, Adamas Instrumenten), stained with 0.1 % toluidine blue O and mounted in DPX (Sigma-Aldrich). The selected Technovit sections were stained with naphthol blue black (NBB) for total protein staining (Fisher, 1968; Mathe and Vieillescazes, 2002) or the PAS reaction was performed to visualize the starches (Wędzony, 1996).
In order to identify the main classes of the chemical compounds that are present in the trichomes, histochemical procedures with fresh or fixed flowers using Sudan III, Sudan Black B and Lugol’s solution were performed in order to detect the total lipids, starch grains and proteins (Johansen, 1940), respectively.
For SEM, the flowers were fixed (as above) and later dehydrated and critical point dried using CO2. They were then sputter-coated with gold and examined at an accelerating voltage of 20 kV using a Hitachi S-4700 scanning electron microscope, which is housed at the Institute of Geological Sciences, Jagiellonian University in Kraków, Poland.
Phylogenetic analyses
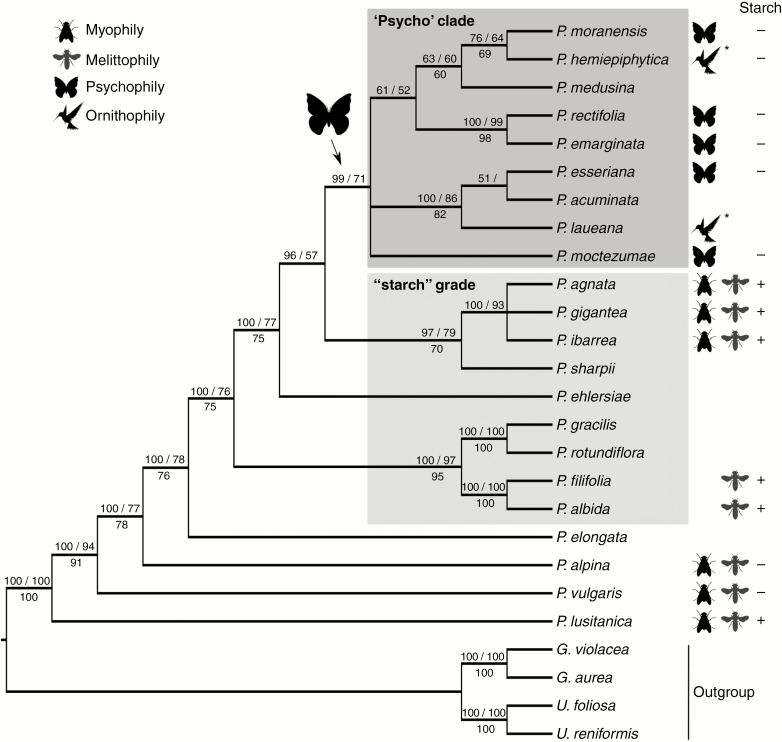
The available matK/trnK DNA sequences of the Pinguicula species [P. acuminata (DQ010652.1), P. agnata (AF531782.1), P. albida (LC348432.1), P. alpina (AF531783.1), P. ehlersiae Speta & F.Fuchs (NC_023463.1), P. elongata Benj. (FM200224.1), P. emarginata (AF531785.1), P. esseriana (DQ010656.1), P. filifolia (AF531786.1), P. gigantea (AF531789.1), P. gracilis Zamudio (AF531790.1), P. hemiepiphytica (LC348445.1), P. ibarrae (LC348446.1), P. laueana Speta & F.Fuchs (DQ010659.1), P. lusitanica (DQ010661.1), P. medusina Zamudio & Studnička (LC348454.1), P. moctezumae (AF531797.1), P. moranensis (AF531798.1), P. rectifolia (AF531801.1), P. rotundiflora Studnička (AF531802.1), P. sharpii Casper & K.Kondo (AF531803.1) and P. vulgaris (AF531807.1)] were obtained from GenBank (NCBI) to be the ingroup. For the outgroup, two Genlisea [G. aurea A.St.-Hil. (NC_037078.1) and G. violacea A.St.-Hil. (NC_037083.1)] and two Utricularia species [U. foliosa L. (KY025562.1) and U. reniformis A.St.-Hil. (NC_029719.2)] were used. The sequences were aligned using the online MAFFT v. 7.450 package (Katoh et al., 2019). All of the gaps were treated as missing. We used three approaches to create the phylogenetic reconstructions: Bayesian inference (BI), maximum likelihood (ML) and maximum parsimony (MP). BI was determined using Mr Bayes v. 3.2.7a (Ronquist et al., 2012) under the CIPRES Science Gateway v. 3.3 (Miller et al., 2010). For BI, 2 × 106 generations were calculated using two runs with four chains until the standard deviation reached a value <0.01. In each run, the trees were sampled every 100 generations at a sample frequency of 100. The first 25 % of the trees that were initially produced were discarded as burn-in. The BI was conducted using the GTR + G model and was calculated using MrModeltest v. 2.4 software (Nylander, 2004) following the Akaike information criterion (Akaike, 1973). ML was determined using the online IQ-TREE v. 1.6.12 (Nguyen et al., 2015) and the obtained branch supports with the ultrafast bootstrap (10 000 replicates) (Hoang et al., 2018). For the MP analyses, PAUP* v. 4.0a (build 166) program (Swofford, 2002) was used under the CIPRES Science Gateway v. 3.3 (Miller et al., 2010) to obtain the bootstrap values (2000 pseudoreplicates and a heuristic search with 1000 replicates with the random addition of sequences and the branch swapping algorithm TBR). The trees that were obtained were edited using FigTree v. 1.4.3 (Rambaut, 2016). To optimize the pollinators/syndromes on the tree, we used the BI tree, and the pollinators were plotted according to published studies (listed in Table 1). The pollinator silhouettes used in Fig. 4 were designed using Freepik (https://www.freepik.com).
Fig. 4.
Phylogeny of the Pinguicula species based on the Bayesian inference (BI), maximum likelihood (ML) and maximum parsimony (MP) analyses of the trnK/matK sequences. The numbers above the branches refer to the BI posterior probability and the ML bootstrap support, respectively, and below the MP, the bootstrap support. The animal silhouettes denote the pollinator for each species. ‘*’ indicates the homoplastic origin of the ornithophily for P. hemiepiphytica and P. laueana independently. ‘+’ or ‘–’ indicate the presence/absence of starch grains in the edible trichomes of the bee-/fly-pollinated species.
RESULTS
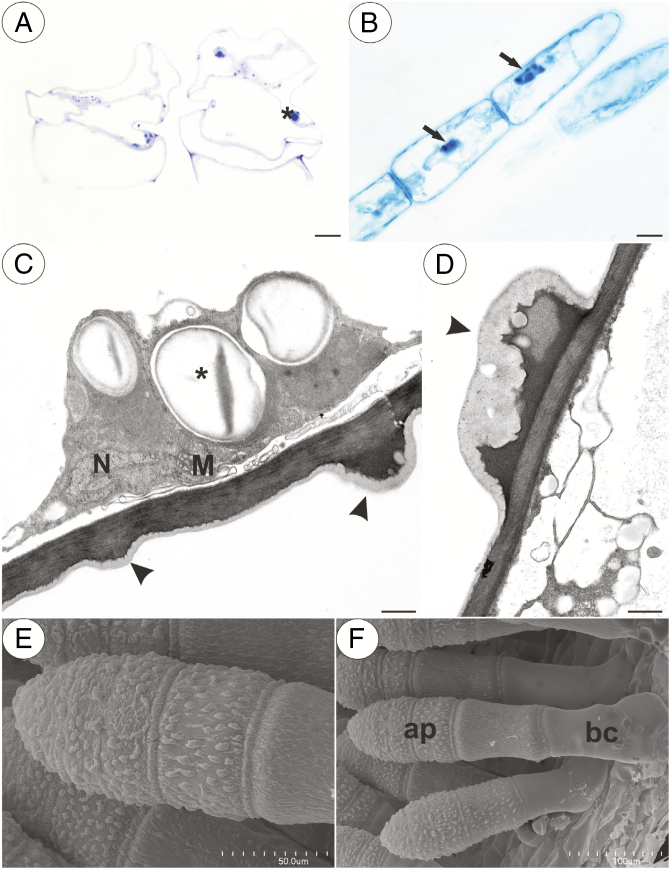
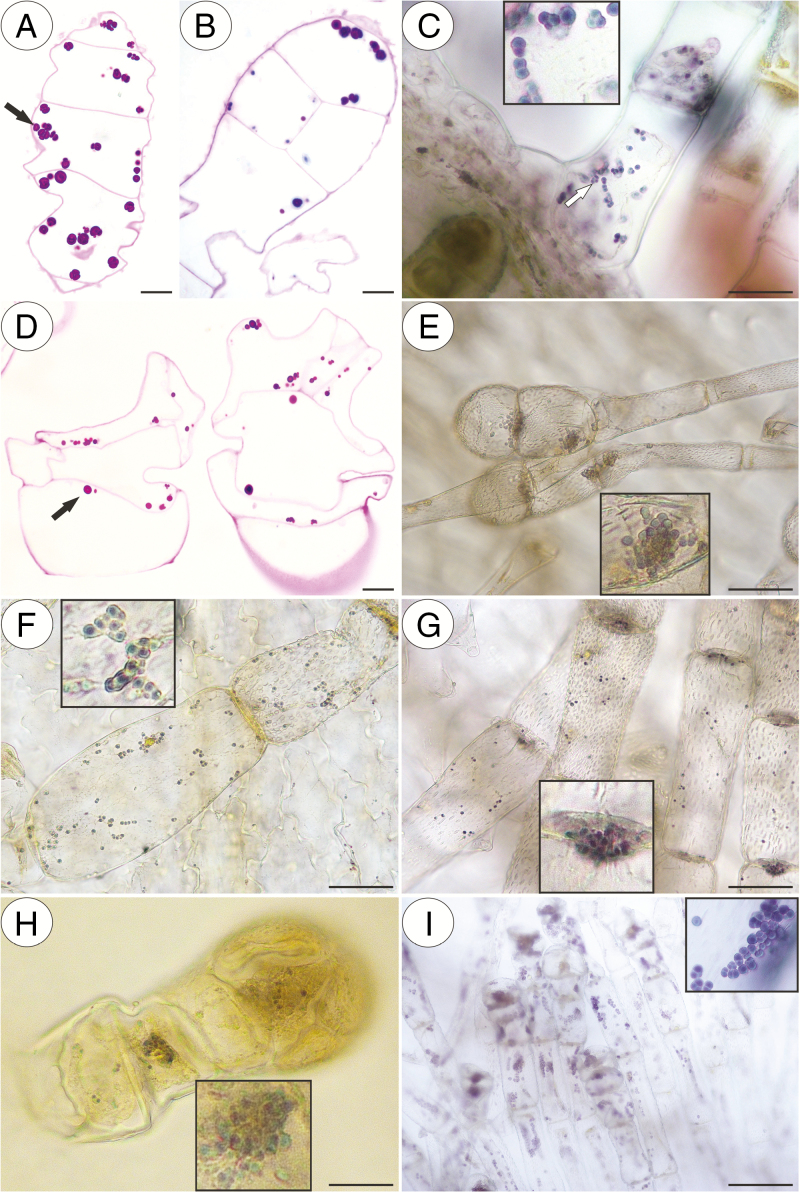
In our study, we observed various types of multicellular non-glandular trichomes, which differed in terms of their micromorphology (see Supplementary data Table S1). The trichome cells were highly vacuolated (Fig. 2A, B) and contained a peripheral cytoplasm with organelles such as a nucleus, mitochondria, plastids and an endoplasmic reticulum (Fig. 2C). Intranuclear paracrystalline bodies occurred in the nuclei (Fig. 2B). Staining with NBB revealed that these consisted of proteins (Fig. 2B). Some trichome cells had visible cuticular striations (Fig. 2D–F), while others had a smooth surface (Fig. 2F). The PAS reaction and Lugol’s staining revealed amyloplasts with starch grains in the cells of the trichomes of the species from the subgenus Temnoceras: P. agnata, P. albida, P. ibarrae, P. martinezii, P. filifolia and P. gigantea (Fig. 3A–I and see ‘starch’ grade in Fig. 4). Starch grains were observed in these species independent of the type of trichomes (Supplementary data Table S1). Lugol’s staining did not reveal any amyloplasts with starch grains in the cells of the trichomes of the species from the subgenus Pinguicula: P. alpina (Fig. 5A–C) and P. vulgaris (P. vulgaris subsp. vulgaris and P. vulgaris subsp. bicolor) (Fig. 5D–I) or the subgenus Isoloba: P. lusitanica (Fig. 5J–L). Moreover, this staining did not reveal any amyloplasts with starch grains in the trichome cells of species from the subgenus Temnoceras, which is pollinated by butterflies [P. moctezumae, P. esseriana, P. moranensis, P. emarginata and P. rectifolia; Fig. 4 (‘psycho’ clade) Fig. 6A–G] or birds (P. mesophytica and P. hemiepiphytica, Fig. 7A–D; Supplementary data Table S1). Staining with NBB did not reveal any protein bodies (in either the cytoplasm or the vacuoles) in the cells of the trichomes of any of the examined species (Fig. 8A–D; Supplementary data Table S1). Staining with Sudan III did not reveal any lipid droplets in the cells of the trichomes in any of the examined species (Fig. 9A–F); however, positive staining was recorded in the cuticular striations (Fig. 9A–F).
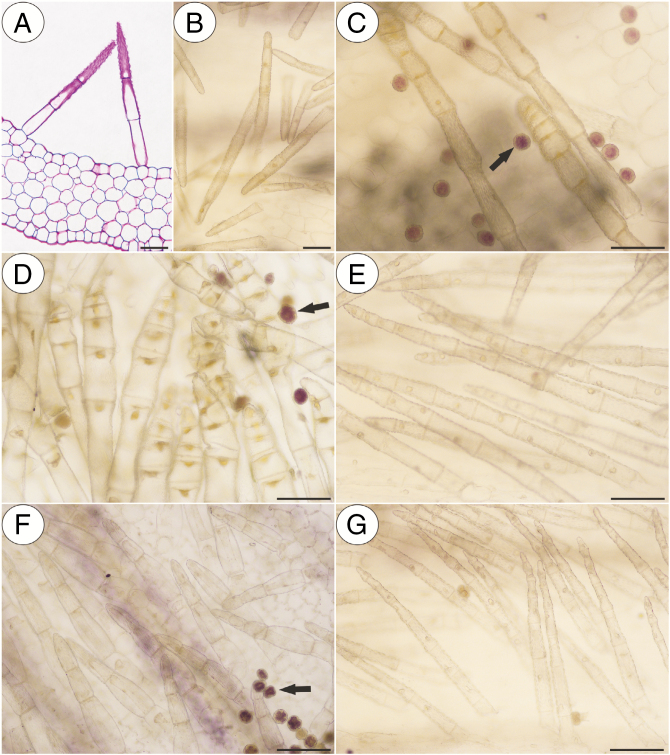
Fig. 2.
Structure of the non-glandular trichomes. (A) Section through the P. albida multicellular thick compact non-glandular trichomes that are located in the throat; note the numerous starch grains (asterisk); scale bar = 10 µm. (B) Naphthol blue black staining of a P. moctezumae multicellular non-glandular trichome showing the presence of a nucleus with a paracrystalline protein inclusion (arrow); note there are no protein bodies in the cytoplasm; scale bar = 10 µm. (C and D) Ultrastructure of a cell of a P. agnata non-glandular trichome; note the mitochondrion (M), nucleus (N) and prominent cuticular striations (arrowhead); scale bars = 0.7 µm and 0.5 µm, respectively. (E and F) Micromorphology of a P. agnata multicellular compact thick non-glandular trichome that is located in the front of the throat; note the cuticular striations on the surface of the apical cells (ap) and the smooth cuticle surface of the basal cell (bc); scale bars = 50 µm and 100 µm, respectively.
Fig. 3.
PAS reaction and Lugol’s staining of the Pinguicula species that were examined, which contain amyloplasts with starch grains (arrow, inserts) inside various types of non-glandular trichomes. (A–C) P. agnata; scale bars = 10 µm, 10 µm, 50 µm, respectively. (D and E) P. albida; scale bars = 10 µm and 50 µm, respectively. (F) P. ibarrae; scale bar = 50 µm. (G) P. martinezii; scale bar = 50 µm. (H) P. filifolia; scale bar = 50 µm. (I) P. gigantea; scale bar = 50 µm.
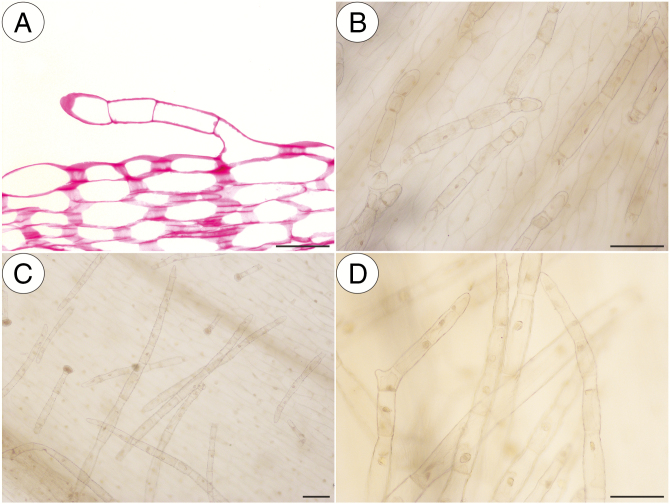
Fig. 5.
Histochemistry of the flower non-glandular trichomes from the species belonging to the Pinguicula and Isoloba subgenera; note the numerous starch grains inside the pollen grains (arrow). (A) Herbarium material of the P. alpina (KRA 0299930) that were examined. (B and C) Negative result of the Lugol’s staining of the P. alpina non-glandular trichomes; scale bars = 100 µm and 50 µm, respectively. (D) Herbarium material of the P. vulgaris subsp. vulgaris (KRA 71415) that were examined. (E and F) Negative result of the Lugol’s staining of the P. vulgaris subsp. vulgaris non-glandular trichomes; note the pollen grains (arrow) with a positive staining of the starch grains inside; scale bars = 100 µm and 100 µm, respectively. (G) Herbarium material of the P. vulgaris subsp. bicolor (KRA 0138573) that were examined. (H and I) Negative result of the Lugol’s staining of the P. vulgaris subsp. bicolor non-glandular trichomes; note the pollen grains (arrow) with a positive staining of the starch grains inside; scale bars = 100 µm and 100 µm, respectively. (J–L) Negative result of the Lugol’s staining of the P. lusitanica non-glandular trichomes; scale bars = 50 µm, 50 µm and 50 µm, respectively.
Fig. 6.
PAS reaction and Lugol’s staining of various non-glandular trichomes of the Pinguicula species that were examined that are pollinated by Lepidoptera; note the pollen grains (arrow) with a positive staining of the starch grains inside. (A) PAS reaction of the P. moctezumae non-glandular trichomes that are located in the basal part of the spur; scale bar = 50 µm. (B and C) Negative result of the Lugol’s staining of the P. moctezumae non-glandular trichomes; scale bars = 50 µm and 100 µm, respectively. (D) Negative result of the Lugol’s staining of the P. esseriana non-glandular trichomes; scale bar = 100 µm. (E) Negative result of the Lugol’s staining of the P. moranensis non-glandular trichomes; scale bar = 100 µm. (F) Negative result of the Lugol’s staining of the P. emarginata non-glandular trichomes; scale bar = 100 µm. (G) Negative result of the Lugol’s staining of the P. rectifolia non-glandular trichomes; scale bar = 100 µm.
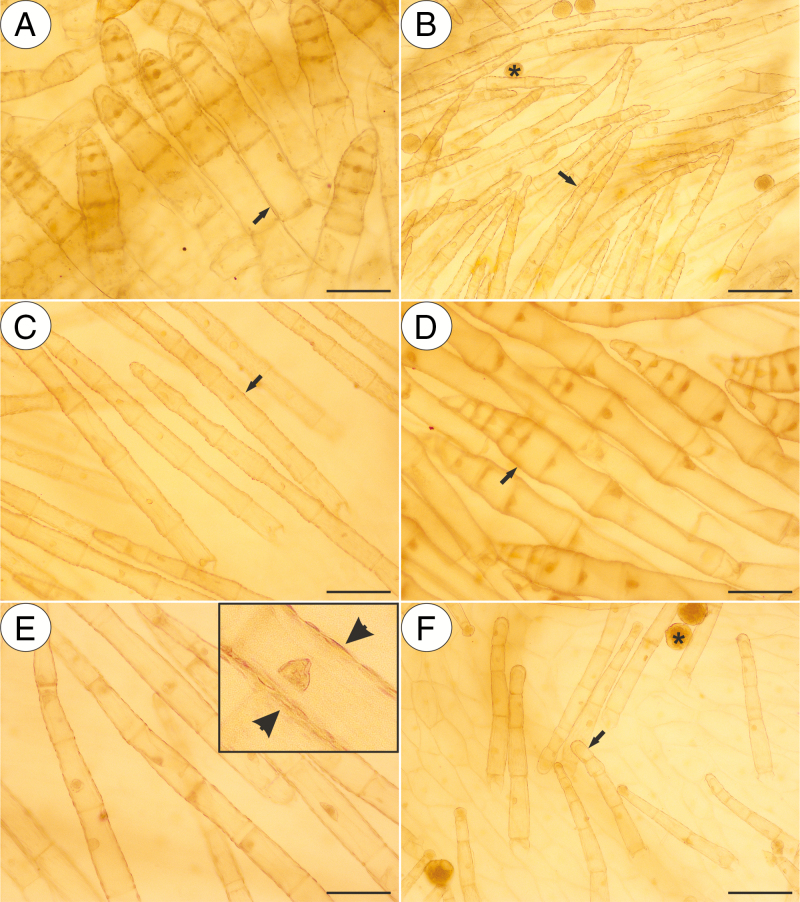
Fig. 7.
PAS reaction and Lugol’s staining of various non-glandular trichomes of the Pinguicula species that were examined that are most probably pollinated by hummingbirds. (A) PAS reaction of a P. mesophytica non-glandular trichome; scale bar = 50 µm. (B) Negative result of the Lugol’s staining of the P. mesophytica non-glandular trichomes; scale bar = 100 µm. (C and D) Negative result of the Lugol’s staining of the P. hemiepiphytica non-glandular trichomes; scale bars = 100 µm and 100 µm, respectively.
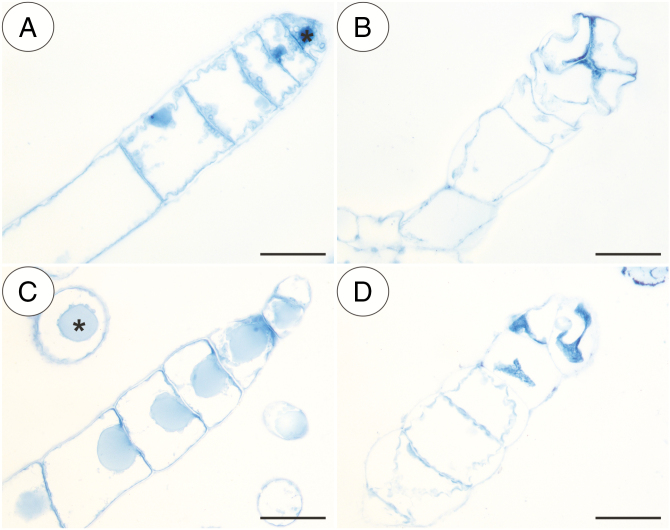
Fig. 8.
Naphthol blue black (NBB) staining of various non-glandular trichomes of the selected Pinguicula species that were examined; note the lack of protein bodies in the cytoplasm. Nucleus (asterisk). (A) P. agnata; scale bar = 50 µm. (B) P. albida; scale bar = 50 µm. (C) P. esseriana; scale bar = 50 µm. (D) P. vulgaris; scale bar = 50 µm.
Fig. 9.
Sudan III staining of various non-glandular trichomes of the selected Pinguicula species that were examined; note the positive staining of the cuticular striations of the non-glandular trichomes cells (arrow, insert and arrowhead) and lipids inside the pollen grains (asterisk). (A) P. agnata; scale bar = 100 µm. (B) P. rectifolia; scale bar = 100 µm. (C) P. moranensis; scale bar = 100 µm. (D) P. esseriana; scale bar = 100 µm. (E) P. hemiepiphytica; scale bar = 100 µm. (F) P. mesophytica; scale bar = 100 µm.
The phylogenetic hypothesis, which was based on the trnK/matK sequences (Fig. 4), supports the assumption that both psychophily and ornithophily are derived for the Pinguicula lineages, probably from the plesiomorphic condition of myophily and/or melittophily. The ornithophily was possibly derived from the psychophily (Fig. 4). Thus, the pollination by birds has emerged at least twice as homoplasies to the Pinguicula species independently.
DISCUSSION
We did not find the typical characters of glandular cells in the cells of the multicellular clavate trichomes. Therefore, we agree with Casper (1966, 2019) that these trichomes are non-glandular. We did show that the cells of the floral non-glandular trichomes of P. agnata, P. albida, P. ibarrae, P. martinezii, P. filifolia and P. gigantea were rich in amyloplasts that contained starch. Thus, these peculiar trichomes contain food reserves and probably function as edible trichomes. In orchids, edible trichomes (including pseudopollen-forming trichomes) are formed for a specific pollinator group, i.e. bees (Pansarin and Maciel, 2017). Thus, it is clear that in Pinguicula starch contained trichomes are recorded in species pollinated by bees, as showed in the ‘starch’ grade by the phylogenetical hypothesis (Fig. 4). Therefore, the lack of starch in the trichomes in the ‘psycho’ clade is a secondary loss, considering that P. alpina, P. lusitanica and P. vulgaris also did not present this character (Fig. 4). Pinguicula mesophytica is not represented in the tree but is a sister species to P. moranensis based on internal transcribed spacer (ITS) rDNA according to Shimai et al. (2007). Thus, pollination by birds is perhaps homoplastic in the Pinguicula species considering the known or supposed ornithophilic species (P. hemiepiphytica, P. laueana and P. mesophytica; Lampard et al., 2016; Roccia et al., 2016).
Interestingly, not all myophilic and melittophylic species had starch in these trichomes, which enabled us to infer that these traits are not a condition for those pollination syndromes. Moreover, we did not record food reserves in the trichomes of P. alpina and P. vulgaris, which are pollinated by bees and flies (Molau, 1993; Fleischmann, 2016). Fleischmann (2016) observed various dipterans dabbing at the yellow spots on the otherwise white corolla of P. alpina and on the white corolla marks on the violet corolla of P. vulgaris and P. leptoceras with their proboscis. He interpreted this behaviour as the insects trying to find nectar and pollen, and, therefore, in these species, the trichomes may guide insects to the spur. However, we do not agree with Fleischmann (2016) that they play the role of ‘feeding hairs’ in P. alpina and P. vulgaris because we did not find any reserve material in these trichomes. For this reason, these trichomes may play a tactile role and act as guides or they might mimic the edible trichomes of other species.
Most researchers accept that in Pinguicula the reward for pollinators is generally nectar because of the occurrence of a spur with glandular trichomes (Fleischmann and Roccia, 2018; Lustofin et al. 2019); however, actual observations of nectar secretion and nectar analysis are rare (Zamora, 1999; Abrahamczyk et al. 2017; Lustofin et al. 2019). Although edible trichomes may act as a reward in addition to nectar, a detailed study of nectar production and secretion in Pinguicula is required to be absolutely certain that all Pinguicula species produce nectar and in what quantities. In the related genera Utricularia (Hobbhahn et al., 2006; Clivati et al., 2014; Płachno et al., 2017, 2018, 2019a, b) and Genlisea (Aranguren et al. 2018), the reward for pollinators is nectar. However, in some species (U. antennifera, U. capilliflora, U. dunlopii, U. dunstaniae and U. lowriei), the spur is significantly reduced and the corolla forms filiform appendages (Taylor, 1989; Reut and Jobson, 2010). In U. dunlopii, the glandular trichomes (osmophores) are densely distributed on the modified floral appendages, and therefore their scent is most probably the attractant for visiting insects (Płachno et al., 2016). Although there are yellow non-glandular trichomes in the flower throats of U. multifida and U. tenella, they do not play the role of edible trichomes (Płachno et al., 2019a).
In orchids, the edible trichome cells (including the pseudopollen, which is formed by the disintegration of the trichomes) contain various types of food material (see Davies, 2009 and references therein). The main food material that is found in the edible trichome of orchids in the species from the Maxillaria genus is protein (Davies, 2009). Starch grains were recorded in the cells of the trichomes in the species from the genera Dendrobium (Davies and Turner, 2004), Cyanaeorchis (Pansarin and Maciel, 2017), Polystachya (Davies et al., 2002) and Maxillaria (Davies, 2009). Lipid droplets were recorded in the edible trichomes of Cyanaeorchis (Pansarin and Maciel, 2017). Thus, the edible trichomes of orchids are more diverse in the types of food material compared with Pinguicula.
From a phylogenetic perspective, edible trichomes are symplesiomorphic for the Pinguicula species and are found in the species of the ‘starch’ grade (Fig. 4), and therefore this does not support a monophyletic group such as a synapomorphy. However, the edible trichomes are derived and are possibly a specialization for fly and bee pollinators that act as a food reward for these visitors.
Field observations are needed to answer the question of whether insects consume ‘starch’ trichomes of Pinguicula flowers and thus whether these structures can be regarded as pollinators’ rewards. Checking if there is a correlation between the amount of nectar produced and the number of trichomes with starch also seems interesting.
Conclusion
Floral non-glandular trichomes play the role of edible trichomes in some Pinguicula species (P. agnata, P. albida, P. ibarrae, P. martinezii, P. filifolia and P. gigantea), which are primarily classified as bee-pollinated species that originated from Central and South America. It seems that in Pinguicula that are pollinated by other pollinator groups (Lepidoptera and hummingbirds), the non-glandular trichomes in the flowers play a role other than being a floral reward for their pollinators. However, even with a phylogenetic perspective, the gaps in knowledge are wide for several species, which does not permit a robust hypothesis. Thus, only when field studies have been undertaken can we be absolutely certain of the role of these trichomes.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of Table S1: micromorphology and histochemistry analyses of the food material content in various type of the Pinguicula flower non-glandular trichomes.
ACKNOWLEDGEMENTS
The authors would like to express their sincere and warm thanks to Dr Miroslav Studnička (director of Liberec Botanical Garden, Czech Republic) for providing some of the plant material for the study. We thank horticulturist Lucyna Kurleto for her conscientious care of the living collection of carnivorous plants that are located in the Botanical Garden of Jagiellonian University in Kraków and also thank the curator of KRA, Dr hab. Marcin Nobis for the opportunity to use the herbarium material. We cordially thank the reviewers and editors for comments, which have helped to improve the manuscript. There is no conflict of interest.
FUNDING
This research was financially supported by the Ministry of Science and Higher Education of Poland as part of the statutory activities of the Department of Plant Cytology and Embryology, Institute of Botany, Faculty of Biology, Jagiellonian University in Kraków (N18/DBS/000002). We cordially thank PIK INSTRUMENTS sp. z o.o. (https://micro-shop.pl/english/) for sponsoring our research.
LITERATURE CITED
- Abrahamczyk S, Kessler M, Hanley D, et al. 2017. Pollinator adaptation and the evolution of floral nectar sugar composition. Journal of Evolutionary Biology 30: 112–127. [DOI] [PubMed] [Google Scholar]
- Akaike H. 1973. Information theory and an extension of the maximum likelihood principle. In: Second International Symposium on Information Theory 267–281.
- Alcalá RE, Domínguez CA. 2003. Patterns of prey capture and prey availability among populations of the carnivorous plant Pinguicula moranensis (Lentibulariaceae) along an environmental gradient. American Journal of Botany 90: 1341–1348. [DOI] [PubMed] [Google Scholar]
- Alcalá RE, Domínguez CA. 2005. Differential selection for carnivory traits along an environmental gradient in Pinguicula moranensis. Ecology 86: 2652–2660. [Google Scholar]
- Aranguren Y, Płachno BJ, Stpiczyńska M, Miranda VFO. 2018. Reproductive biology and pollination of the carnivorous Genlisea violaceae (Lentibulariaceae). Plant Biology 20: 591–601. [DOI] [PubMed] [Google Scholar]
- Casper SJ. 1966. Monographie der Gattung Pinguicula L. Bibliotheca Botanica 127–128: 1–209. [Google Scholar]
- Casper SJ. 2019. The insectivorous genus Pinguicula (Lentibulariaceae) in the greater antilles. Berlin: Botanischer Garten und Botanishes Museum. [Google Scholar]
- Chase MW, Christenhusz MJM, Fay MF, et al. 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society 181: 1–20. [Google Scholar]
- Clivati D, Cordeiro GD, Płachno BJ, de Miranda VF. 2014. Reproductive biology and pollination of Utricularia reniformis A.St.-Hil. (Lentibulariaceae). Plant Biology (Stuttgart, Germany) 16: 677–682. [DOI] [PubMed] [Google Scholar]
- Cox PA. 1982. Vertebrate pollination and the maintenance of dioecism in Freycinetia. The American Naturalist 120: 65–80. [Google Scholar]
- Darnowski D, Bauer U, Moran J, et al. 2018. Prey selection and specialization by carnivorous plants. In: Ellison AM, Adamec L, eds. Carnivorous plants: physiology, ecology and evolution. Oxford: Oxford University Press, 285–293. [Google Scholar]
- Davies KL. 2009. Morphology. In: Kull T, Arditti J, Wong SM, eds. Orchid biology: reviews and perspectives, X. Dordrecht: Springer, 159–184. [Google Scholar]
- Davies KL, Turner MP. 2004. Pseudopollen in Dendrobium unicum Seidenf. (Orchidaceae): reward or deception? Annals of Botany 94: 129–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KL, Roberts DL, Turner MP. 2002. Pseudopollen and food-hair diversity in Polystachya Hook. (Orchidaceae). Annals of Botany 90: 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez Y, Silva SR, Valdés CMP, Miranda VFO. 2014. Inter- and intra-specific diversity of Cuban Pinguicula (Lentibulariaceae) based on morphometric analyses and its relation with geographical distribution. Plant Ecology & Diversity 7: 519–531. [Google Scholar]
- Endress PK. 2010. The evolution of floral biology in basal angiosperms. Philosophical Transactions of the Royal Society B: Biological Sciences 365: 411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faegri K, Van der Pijl L. 1979. The principles of pollination ecology. Oxford: Pergamon Press. [Google Scholar]
- Fisher DB. 1968. Protein staining of ribboned epon sections for light microscopy. Histochemie. Histochemistry. Histochimie 16: 92–96. [DOI] [PubMed] [Google Scholar]
- Fleischmann A. 2016. Pinguicula flowers with pollen imitations close at night – some observations on butterwort flower biology. Carnivorous Plant Newsletter 45: 84–92. [Google Scholar]
- Fleischmann A, Roccia A. 2018. Systematics and evolution of Lentibulariaceae: I. Pinguicula. In: Ellison AM, Adamec L, eds. Carnivorous plants: physiology, ecology and evolution. Oxford: Oxford University Press, 70–80. [Google Scholar]
- Hernández MP, Katinas L. 2019. Technique for the identification of osmophores in flowers of herbarium material (TIOFH). Protoplasma 256: 1753–1765. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison Y. 1970. Scanning electron microscopy of fresh leaves of Pinguicula. Science 167: 172–174. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison Y. 2004. Pinguicula L. Journal of Ecology 92: 1071–1118. [Google Scholar]
- Heslop-Harrison Y, Heslop-Harrison J. 1980. Chloride ion movement and enzyme secretion from the digestive glands of Pinguicula. Annals of Botany 45: 729–731. [Google Scholar]
- Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS. 2018. UFBoot2: improving the ultrafast bootstrap approximation. Molecular Biology and Evolution 35: 518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbhahn N, Küchmeister H, Porembski S. 2006. Pollination biology of mass flowering terrestrial Utricularia species (Lentibularaiceae) in the Indian Western Ghats. Plant Biology 8: 791–804. [DOI] [PubMed] [Google Scholar]
- Humphrey CD, Pittman FE. 1974. A simple methylene blue–azure II–basic fuchsin stain for epoxy-embedded tissue sections. Stain Technology 49: 9–14. [DOI] [PubMed] [Google Scholar]
- Jensen WA. 1962. Botanical histochemistry – principles and practice. San Francisco: W. H. Freeman and Company. [Google Scholar]
- Jobson RW, Playford J, Cameron KM, Albert VA. 2003. Molecular phylogenetics of Lentibulariaceae inferred from plastid rps16 intron and trnL-F DNA sequences: implications for character evolution and biogeography. Systematic Botany 28: 157–171. [Google Scholar]
- Johansen DA. 1940. Plant microtechnique. New York: McGraw-Hill Book Co. [Google Scholar]
- Katoh K, Rozewicki J, Yamada KD. 2019. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics 20: 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampard S, Gluch O, Robinson A, et al. 2016. Pinguicula of Latin America. Dorset, UK: Redfern Natural History. [Google Scholar]
- Lustofin K, Świątek P, Miranda VFO, Płachno BJ. 2019. Flower nectar trichome structure of carnivorous plants from the genus butterworts Pinguicula L. (Lentibulariaceae). Protoplasma 257: 245–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathe C, Vieillescazes C. 2002. Compréhension des mécanismes de coloration des liants protéiques picturaux à l’aide du Noir Amide 10B. L’Actualité Chimique 7: 11–14. [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Gateway Computing Environments Workshop, GCE 2010 (New Orleans) 1–8. [Google Scholar]
- Molau U. 1993. Reproductive ecology of the three Nordic Pinguicula species (Lentibulariaceae). Nordic Journal of Botany 13: 149–157. [Google Scholar]
- Müller K, Borsch T, Legendre L, Porembski S, Theisen I, Barthlott W. 2004. Evolution of carnivory in Lentibulariaceae and the Lamiales. Plant Biology (Stuttgart, Germany) 6: 477–490. [DOI] [PubMed] [Google Scholar]
- Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution 32: 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson SW, Nepi M, Pacini E, eds.2007. Nectaries and nectar. Dortrecht, The Netherlands: Springer. [Google Scholar]
- Nordin M. 2015. Pinguicula alpina (alpine butterwort) on the Swedish island of Gotland: pollination and reproduction. Thesis, Uppsala University(in Swedish).
- Nylander JAA. 2004. MrModeltest v2. Program distribued by the author. [Google Scholar]
- Pansarin ER, Maciel AA. 2017. Evolution of pollination systems involving edible trichomes in orchids. AoB Plants 9: plx033. [Google Scholar]
- Płachno BJ, Stpiczyńska M, Świątek P, Davies KL. 2016. Floral micromorphology of the Australian carnivorous bladderwort Utricularia dunlopii, a putative pseudocopulatory species. Protoplasma 253: 1463–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Płachno BJ, Stpiczyńska M, Davies KL, Świątek P, Miranda VFO. 2017. Floral ultrastructure of two Brazilian aquatic–epiphytic bladderworts: Utricularia cornigera Studnička and U. nelumbifolia Gardner (Lentibulariaceae). Protoplasma 254: 353–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Płachno BJ, Stpiczyńska M, Adamec L, Miranda VFO, Świątek P. 2018. Nectar trichome structure of aquatic bladderworts from the section Utricularia (Lentibulariaceae) with observation of flower visitors and pollinators. Protoplasma 255: 1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Płachno BJ, Stpiczyńska M, Świątek P, et al. 2019. a Floral micromorphology and nectar composition of the early evolutionary lineage Utricularia (subgenus Polypompholyx, Lentibulariaceae). Protoplasma 256: 1531–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Płachno BJ, Stpiczyńska M, Świątek P, et al. 2019. b Floral micromorphology of the bird-pollinated carnivorous plant species Utricularia menziesii R.Br. (Lentibulariaceae). Annals of Botany 123: 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaut A. 2016. FigTree. version 1.4.3. Institute of Evolutionary Biology, University of Edinburgh. [Google Scholar]
- Reut M, Jobson RW. 2010. A phylogenetic study of subgenus Polypompholyx: a parallel radiation of Utricularia (Lentibulariaceae) throughout Australasia. Australian Systematic Botany 23: 152–161. [Google Scholar]
- Rickson FR. 1979. Ultrastructural development of the beetle food tissue of Calycanthus flowers. American Journal of Botany 66: 80–86. [Google Scholar]
- Roccia A, Gluch O, Lampard S, et al. 2016. Pinguicula of the temperate North. Dorset, UK: Redfern Natural History. [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, et al. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäferhoff B, Fleischmann A, Fischer E, et al. 2010. Towards resolving Lamiales relationships: insights from rapidly evolving chloroplast sequences. BMC Evolutionary Biology 10: 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimai H, Kondo K. 2007. Phylogenetic analysis of Mexican and Central American Pinguicula (Lentibulariaceae) based on internal transcribed spacer (ITS) sequence. Chromosome Botany 2: 67–77. [Google Scholar]
- Simpson BB, Neff JL. 1981. Floral rewards: alternatives to pollen and nectar. Annals of the Missouri Botanical Garden 68: 301–322. [Google Scholar]
- Swofford DL. 2002. PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4.0. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Taylor P. 1989. The genus Utricularia – a taxonomic monograph. Kew Bulletin Additional Series 14: 1–724. [Google Scholar]
- Thien LB, Bernhardt P, Devall MS, et al. 2009. Pollination biology of basal angiosperms (ANITA grade). American Journal of Botany 96: 166–182. [DOI] [PubMed] [Google Scholar]
- Vassilyev AE, Muravnik LE. 1988. The ultrastructure of the digestive glands in Pinguicula vulgaris L. (Lentibulariaceae) relative to their function. I. The changes during maturation. Annals of Botany 62: 329–341. [Google Scholar]
- Villegas SG, Alcalá RE. 2018. Reproductive ecology of the carnivorous plant Pinguicula moranensis (Lentibulariaceae). Plant Biology (Stuttgart, Germany) 20: 205–212. [DOI] [PubMed] [Google Scholar]
- Wędzony M. 1996. Fluorescence microscopy for botanists. Kraków, Poland: Department of Plant Physiology Monographs 5; [in Polish]. [Google Scholar]
- Young HJ. 1986. Beetle pollination of Dieffenbachia longispatha (Araceae). American Journal of Botany 73: 931–944. [Google Scholar]
- Zamora R. 1999. Conditional outcomes of interactions: the pollinator–prey conflict of an insectivorous plant. Ecology 80: 786–795. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.