Abstract
Background and Aims
It remains unclear whether invasive species can maintain both high biomass and reproductive output across their invaded range. Along latitudinal gradients, allocation theory predicts that faster flowering onset at high latitudes results in maturation at smaller size and thus reduced reproductive output. For annual invasive plants, more favourable environmental conditions at low latitudes probably result in stronger competition of co-occurring species, potentially driving selection for higher investment in vegetative biomass, while harsher climatic conditions and associated reproductive uncertainty at higher latitudes could reduce selection for vegetative biomass and increased selection for high reproductive investment (stress-gradient hypothesis). Combined, these drivers could result in increased or constant reproductive allocation with increasing latitude.
Methods
We quantified life-history traits in the invasive annual plant Impatiens glandulifera along a latitudinal gradient in Europe. By growing two successive glasshouse generations, we assessed genetic differentiation in vegetative growth and reproductive output across six populations, and tested whether onset of flowering drives this divergence.
Key Results
Trait variation was mainly caused by genetic differentiation. As expected, flowering onset was progressively earlier in populations from higher latitudes. Plant height and vegetative biomass also decreased in populations from higher latitudes, as predicted by allocation theory, but their variation was independent of the variation in flowering onset. Reproductive output remained constant across latitudes, resulting in increased reproductive allocation towards higher latitudes, supporting the stress-gradient hypothesis. We also observed trait genetic differentiation among populations that was independent of latitude.
Conclusions
We show that an annual invasive plant evolved several life-history traits across its invaded range in ~150 years. The evolution of vegetative and reproductive traits seems unconstrained by evolution of flowering onset. This genetic decoupling between vegetative and reproductive traits possibly contributes to the invasion success of this species.
Keywords: Allocation theory, common garden, flowering onset, Impatiens glandulifera, latitudinal gradient, life-history theory, maternal effects, phenology, reproductive investment, seed size, stress-gradient hypothesis
INTRODUCTION
Invasive alien plant species and invaded habitats continue to increase across the globe (van Kleunen et al., 2015; Seebens et al., 2017). Much research has focused on explaining the success of invasive plant species, to help predict species invasiveness and manage the invasion process (Caño et al., 2008; Colautti et al., 2010). The ability of invasive species to successfully establish across a wide range of abiotic and biotic environmental conditions has been partly attributed to high phenotypic variation in several life-history traits (van Kleunen et al., 2010; Davidson et al., 2011; Liao et al., 2016). Population divergence in phenotypic traits can result from local adaptation, phenotypic plasticity or both (Sultan, 1995; Liao et al., 2016). The high rates of spread of many invaders and low levels of genetic diversity during the initial steps of the invasion process suggest that invasive species mostly rely on plasticity to maintain high fitness in new environments (Davidson et al., 2011; Molina-Montenegro and Naya, 2012; Murren and Dudash, 2012; Liao et al., 2016). High potential for plastic responses has indeed been observed as one of the characteristics shared by many invasive plant species (van Kleunen and Fischer, 2005). Nonetheless, certain invasive species are able to rapidly adapt genetically to new environments in the invaded range (Dlugosch and Parker, 2008; Colautti and Barrett, 2013; Oduor et al., 2016) and this high adaptive potential may be one of the key characteristics associated with invasiveness (Lee, 2002; Lavergne and Molofsky, 2007; Lee and Gelembiuk, 2008). With their relatively short generation times, annual invasive plants are expected to be even more responsive to local selective pressures, potentially resulting in relatively fast local adaptation (Sakai et al., 2001; Liao et al., 2016).
Compared to their native range, invasive plant species are often characterized by a relatively fast growth in their invaded range, resulting in higher vegetative biomass and reproductive output (Dlugosch and Parker, 2008; Mason et al., 2008; van Kleunen et al., 2010). It remains unclear, however, to what extent invasive species can maintain this high biomass and reproductive output across the environmental gradient of the entire invaded range (Hodgins et al., 2018), particularly when the range spans several latitudinal degrees. Because the length of the growing season usually decreases with latitude (at constant altitude), individuals from higher latitudes generally initiate flowering earlier during their ontogeny to ensure successful seed maturation by the end of the growing season (Olsson and Ågren, 2002; Stinchcombe et al., 2004). Life-history theory further predicts that selection for earlier flowering will result in maturation of smaller individuals due to physiological trade-offs (Colautti et al., 2010). This reduced size at maturation should, in turn, result in reduced reproductive output, with fewer and/or smaller seeds (Samson and Werk, 1986; Obeso, 2002; Weiner, 2004). Accordingly, changes in flowering onset of several invasive species towards higher latitudes have been found to negatively affect plant size and biomass at maturity and, in turn, decrease reproductive output (Weber and Schmid, 1998; Montague et al., 2008; Colautti and Barrett, 2013; Li et al., 2015). Therefore, changes in phenotypic traits along latitudinal gradients may result primarily from changes in the onset of flowering (Galloway and Burgess, 2012).
Alternatively, additional selective pressures, independent of those acting on flowering onset, could contribute to variation in reproductive output across latitudes. Assuming harsher climatic conditions at higher rather than lower latitudes, at least within temperate biomes, plants should experience both relatively low levels of competition (stress-gradient hypothesis) and higher levels of reproductive uncertainty at higher latitudes (Bertness and Callaway, 1994; Schemske et al., 2009; Bhattarai et al., 2017). Especially for annual species, this could result in reduced selection for competition-related traits (He et al., 2010) and increased selection for higher reproductive investment (Hickman, 1975) towards higher latitudes. Accordingly, genetically driven increased reproductive investment at high latitudes has been observed for several invasive plants (Hickman, 1975; Chun et al., 2011; Hodgins and Rieseberg, 2011; Liu et al., 2016). Shorter growing seasons are further expected to result in smaller seeds due to the shorter time available for fruit and seed maturation (Primack, 1987). Colonization trade-offs during range expansion are also expected to result in smaller average seed size and larger numbers of seeds towards the edge of the species range at higher latitudes (Hamilton et al., 2005). Populations at lower latitudes, on the other hand, may experience more intense competition from co-occurring species due to more favourable and benign environmental conditions, probably resulting in strong investment in competition-related traits such as large plant height and biomass (Hodgins et al., 2018) as well as in increased average seed size (Primack, 1987) to ensure successful establishment and survival until reproduction. Irrespective of these stress-gradient patterns, invasive species can also experience increased climatic stress at the edges of their distribution range. Overall, changes in vegetative biomass and reproductive allocation along latitudinal gradients will depend on the relative magnitude and direction of the different selection pressures along the gradients. Although several studies have simultaneously explored variation in vegetative and reproductive life-history traits for invasive plants along latitudinal gradients (Hickman, 1975; Chun et al., 2011; Hodgins and Rieseberg, 2011; Liu et al., 2016), no study has, to our knowledge, explored whether these traits changed independently from faster flowering onset at high latitudes.
Here we focus on the annual plant Impatiens glandulifera (Balsaminaceae) which is invasive in Europe, and originates from the western Himalaya. Although trait variation in I. glandulifera might be driven largely by genetic differentiation, based on its annual lifestyle (Liao et al., 2016), the observed reduced genetic diversity in its invasive range (Hagenblad et al., 2015) suggests that its ability for genetic differentiation could be limited. Although Pahl et al. (2013) suggested that phenotypic plasticity was the main driver of trait variation in above-ground biomass, specific leaf area, plant height and relative growth rate among populations from three different vegetation types in Germany, the common-garden experiment by Kollmann and Bañuelos (2004) suggested that genetic differentiation was the main cause of trait variation in plant height, above-ground biomass and flowering time across a latitudinal gradient. However, in the latter study the authors measured traits on plants grown from wild-collected seeds. Therefore, they were not able to separate the effects of genetic differentiation from those due to variation in the seed-maturation environment (i.e. non-genetic maternal effects).
In this study, we explore how several vegetative traits (plant height and above-ground biomass), reproductive traits (flowering onset, number of flowers, number of seeds per capsule and seed mass) and reproductive investment of I. glandulifera vary along a latitudinal gradient from northern France (49.9°N) to central Norway (63.5°N). This latitudinal gradient spans around the central half of the current invaded range of the species, thus excluding its range margins. By growing two successive generations in a standardized glasshouse environment, we were able to disentangle the effects of the environment experienced by the maternal plants from genetic differentiation on the among-population phenotypic differences along the latitudinal gradient. By comparing patterns of covariation among traits within and across populations, we assessed the following research questions:
Does selection for earlier flowering onset in the north result in reduced plant height, vegetative biomass and reproductive output?
Alternatively, is variation in these traits partly independent from selection on flowering onset, as predicted by the stress-gradient hypothesis? More specifically, do we see increased investment in plant height and vegetative biomass in the south due to increased competition and increased investment in reproduction in the north due to reproductive uncertainty?
Can we find additional sources of local genetic differentiation independent of the general clinal variation along the latitudinal gradient?
MATERIAL AND METHODS
Study species
Impatiens glandulifera Royle (Balsaminaceae; Himalayan balsam) is native to the western Himalaya, where it grows in road verges and around field borders between 2000 and 4000 m a.s.l. (Beerling and Perrins, 1993). The species was first introduced to Europe as an ornamental garden plant in the 1800s, and subsequently colonized riparian habitats along streams across its invaded range from northern Spain (41°N) to northern Norway (70°N) (Beerling, 1993; GBIF.org, 2019). The species is highly competitive and can affect several ecosystem functions, such as nutrient cycling and soil erosion control (Dassonville et al., 2008; Greenwood and Kuhn, 2014; Helsen et al., 2018) and is considered a problematic invasive species in several European countries.
This self-compatible, annual species bears insect-pollinated protandrous flowers and produces many seeds that are dispersed through ballistochory and hydrochory (Beerling and Perrins, 1993). It does not produce a persistent seed bank, making population persistence fully dependent on annual seedling establishment (Beerling and Perrins, 1993). Microsatellite analyses have shown that the species harbours less neutral genetic variation in its invaded range compared to its native range (Walker et al., 2009; Hagenblad et al., 2015; Nagy and Korpelainen, 2015), although genetic diversity seems to be slowly increasing with time across the invaded range (Helsen et al., 2019).
Sampling and glasshouse setup
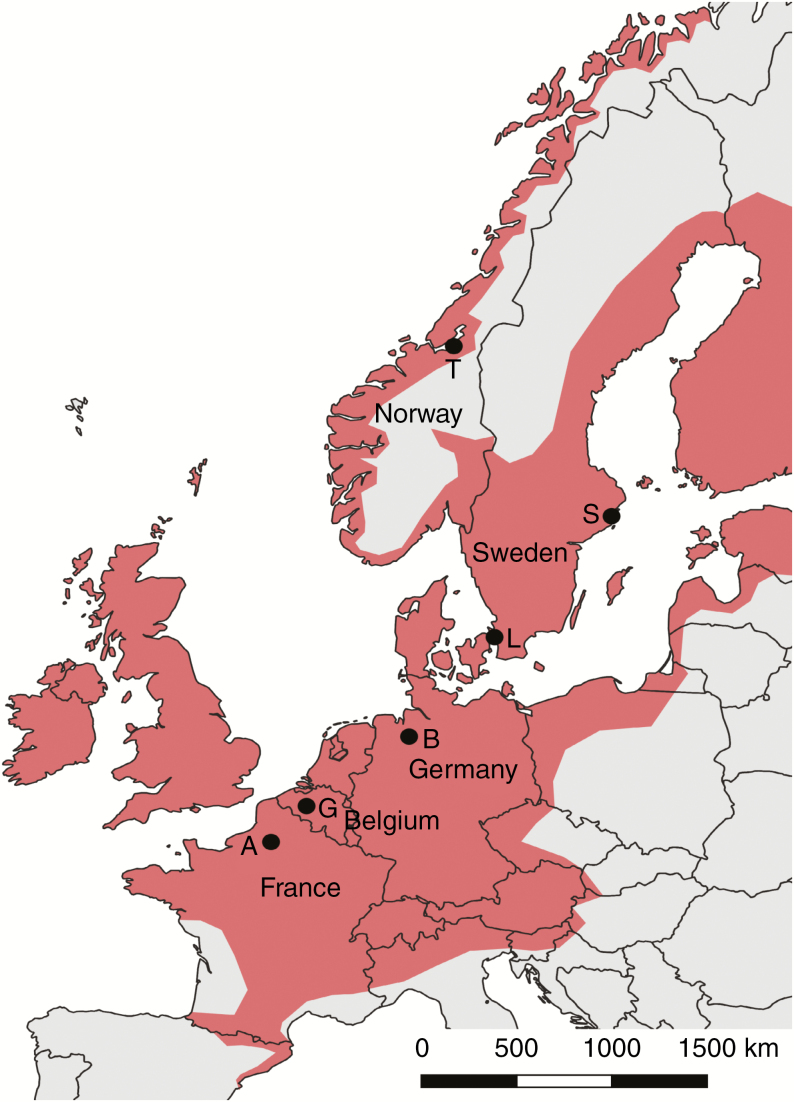
Seeds were sampled from one population for each of six study regions, distributed along a ~1600 km latitudinal transect in Western Europe (from Amiens, France, 49.9°N to Trondheim, Norway, 63.5°N) (Fig. 1, Table 1). In each population, we sampled all mature seeds of ten capsules from each of 30 random individuals during late summer of 2011. The collected seeds were stored in paper bags at room temperature until November 2011.
Fig. 1.
Sampling locations along the latitudinal gradient. Current distribution of Impatiens glandulifera in red, redrawn from GBIF.org (2019). A = Amiens, B = Bremen, G = Ghent, L = Lund, S = Stockholm, T = Trondheim.
Table 1.
Characteristics of the Impatiens glandulifera populations used for the glasshouse experiment sampled along a latitudinal gradient across Europe (cf. Fig. 1)
| Region | Latitude/longitude | Habitat | Population size |
|---|---|---|---|
| Amiens | 50.014°N/2.034°E | Lake shores/road sides/forest edges | 500–1000 |
| Ghent | 50.884°N/3.929°E | River banks/road sides | >1000 |
| Bremen | 53.192°N/8.662°E | Ditch bank | >1000 |
| Lund | 55.997°N/12.789°E | River bank | 200–500 |
| Stockholm | 59.163°N/18.169°E | River banks/forest edges | 200–500 |
| Trondheim | 63.477°N/10.969°E | Road sides | 500–1000 |
In November 2011, 30 seeds per maternal plant were stratified at 4 °C for 2 months. After germination, at the end of January 2012, five seedlings from each maternal plant were transferred to one plastic pot (12 cm diameter, 1.5 litre standard potting soil) in the glasshouse of the Norwegian University of Science and Technology (Trondheim). Upon reaching the two-leaf stage, seedlings were thinned to one individual per pot, giving a total of 30 individual plants (pots) per population, and a total of 180 individuals. Pots of the different populations were randomized in the glasshouse, spread across eight trays in two adjacent glasshouse compartments. Pots were randomly moved within compartments, once every week and across compartments every 2 weeks as long as it was possible to move them. For the first months, additional light was provided with a 12-h : 12-h light–dark cycle, using MASTER SON-T PIA Plus 400-W E E40 lamps. The average temperature was 10 °C during the day and 4 °C at night. After successful establishment (when plants were ~15 cm tall), the plants received an average temperature of 18 °C during the day and 8 °C at night. Plants were fertilized once a week and trays were continuously filled with standing water to maintain high soil moisture content. The applied nutrients (YaraLiva CacliNit) contained 15.5 % nitrogen and were supplied with a concentration of 1.2 mS/cm, which is within the range of the nutrient concentrations of the original soils.
When individuals had produced at least ten flowers, we cross-pollinated them by hand with other individuals from the same population. Pollen donors and receivers were randomly assigned within each population, and in each pollen receiver, ten flowers were pollinated. Only flowers that showed no sign of previous pollination were used as pollen receivers. Following hand pollination, the corolla, nectar glands and anthers of the pollen receiver flowers were removed to prevent additional cross- or self-pollination (Nienhuis and Stout, 2009). After pollination, paper tea bags were placed over the flowers, which prevented further pollination and collected the seeds after maturation. We used these seeds to produce the second glasshouse generation (F2) that was raised from January to June 2013 following the same protocol as for the first generation (F1), including hand-pollinations. The second generation also comprised 180 individuals (i.e. 30 individuals per population).
Trait measurements
We recorded the flowering onset as the time (in days) between germination and the opening of the first flower. At the end of the glasshouse trial (June 2012 and 2013), we measured plant height (cf. Pérez-Harguindeguy et al., 2013) and counted the total number of flowers produced by each individual. For flowers that had already fallen off we counted the scars of the inflorescences to quantify total flower number. Average seed mass and the average number of seeds per capsule were assessed from ten mature capsules collected per individual, obtained through hand pollination. Average seed mass (total mass divided by number of seeds) was quantified with a precision balance (0.1-mg accuracy), after air-drying all seeds for 8 weeks at room temperature. Reproductive output (total seed mass per individual) was estimated as the product of the number of flowers per individual with the number of seeds per capsule and the average seed mass. All vegetative above-ground biomass was then harvested and oven-dried at 60 °C for 72 h and subsequently weighted (0.01-g accuracy). Finally, we quantified reproductive investment as the ratio between the reproductive output and the sum of the above-ground biomass and reproductive output. All measurements were performed for both glasshouse generations (F1 and F2).
Statistical analyses
For all traits except reproductive investment (flowering onset, plant height, number of flowers, number of seeds per capsule, seed mass, above-ground biomass, reproductive output), we fitted full factorial linear mixed-effect models based on REML parameter estimations, with latitude (continuous variable), generation (factor with two levels: F1 vs. F2) and their interaction as predictor variables, and population as a random factor. Models were fitted with the lme4 package in R (Bates et al., 2015). Model selection was based on Akaike’s Information Criterion (ΔAIC > 2) on models fitted with maximum likelihood (ML), following the recommendations of Zuur et al. (2009) (Supplementary Data Appendix S1). For reproductive investment, we constructed a generalized linear mixed-effect model with beta distribution and logit link function, using the same fixed and random structure as for the other models, with the glmmTMB package in R (Brooks et al., 2017). Marginal pseudo-R2 values were calculated for each model with the MuMIn and sjstats packages in R (Barton, 2009; Nakagawa and Schielzeth, 2013; Lüdecke, 2019). In these models, the latitudinal effect observed at the F2 generation should mostly reflect genetic differentiation among populations. Generation differences in this latitudinal effect (i.e. significant interaction effect) could suggest either that population differences at the first generation partly resulted from environmental variation affecting the seeds produced in situ (i.e. maternal effects) or that differences in the glasshouse environment between generations combined with a genotype by environment interaction have generated two different latitudinal gradients in the phenotype expressed in the common environment. Latitude was entered in the model relative to the southernmost study region (Amiens thus had latitude = 0). Hence, model intercepts correspond to the expected mean value of each trait for the Amiens study region. Reproductive output was logarithmically transformed to meet model assumptions. Note that standard errors were calculated and presented for each fixed model term instead of P-values, to help assess (biological) significance of model parameters. All statistics were performed in R3.5.2 (R Core Team, 2018).
Additional environmental variation independent of the latitudinal gradient might occur across populations, potentially resulting in additional patterns of genetic differentiation. To test for this, we calculated the population mean residuals for each trait from the full-factorial linear models including latitude and generation but excluding population as a random factor. In the presence of latitude-independent local genetic differentiation, we expect the divergence of the population means from the predicted effect of latitude (residuals) to be correlated across generations. We thus tested whether the population mean residuals were correlated across generations with Spearman rank correlations.
Changes in the length of the growing season with latitude are expected to directly affect flowering onset (Stinchcombe et al., 2004). Because growth and reproduction are generally involved in some allocation trade-offs, these changes may, in turn, affect traits such as plant height, above-ground biomass and the number of flowers (Obeso, 2002; Weiner, 2004). Thus, for these traits, latitudinal changes may not reflect adaptation to specific conditions encountered along the gradient but may simply represent side effects of latitudinal changes in flowering onset. We tested this hypothesis by comparing patterns of covariance within and among populations using contextual models (Heisler and Damuth, 1987). Contextual models are multiple regressions including both group and individual predictors to estimate the contrast between within- and among-group relationships. In our case, these models allow us to estimate the relationship between the traits and the onset of flowering within and among populations. If latitude solely affects the traits via changes in the flowering onset, regressions between the traits and flowering onset should be similar at the within- and among-population levels. In other words, population differentiation in the various traits would be completely explained by the population differences in flowering onset. In contrast, different relationships at the two levels would indicate trait-specific genetic differentiation driven by the latitudinal gradient. For each contextual model, we fitted a mixed-effect model including both the population-mean-centred data (individual predictor) and the population means (group predictor) at each generation for the predictor variables and population identity as a random effect. We used the data from both generations because both predictor and response variables changed across generations. Because the number of flowers generally depends on plant size achieved at maturation, we first tested the effect of flowering onset on plant size (plant height and above-ground biomass) and subsequently analysed the effect of these two size variables on the number of flowers.
RESULTS
Effect of latitude and generation
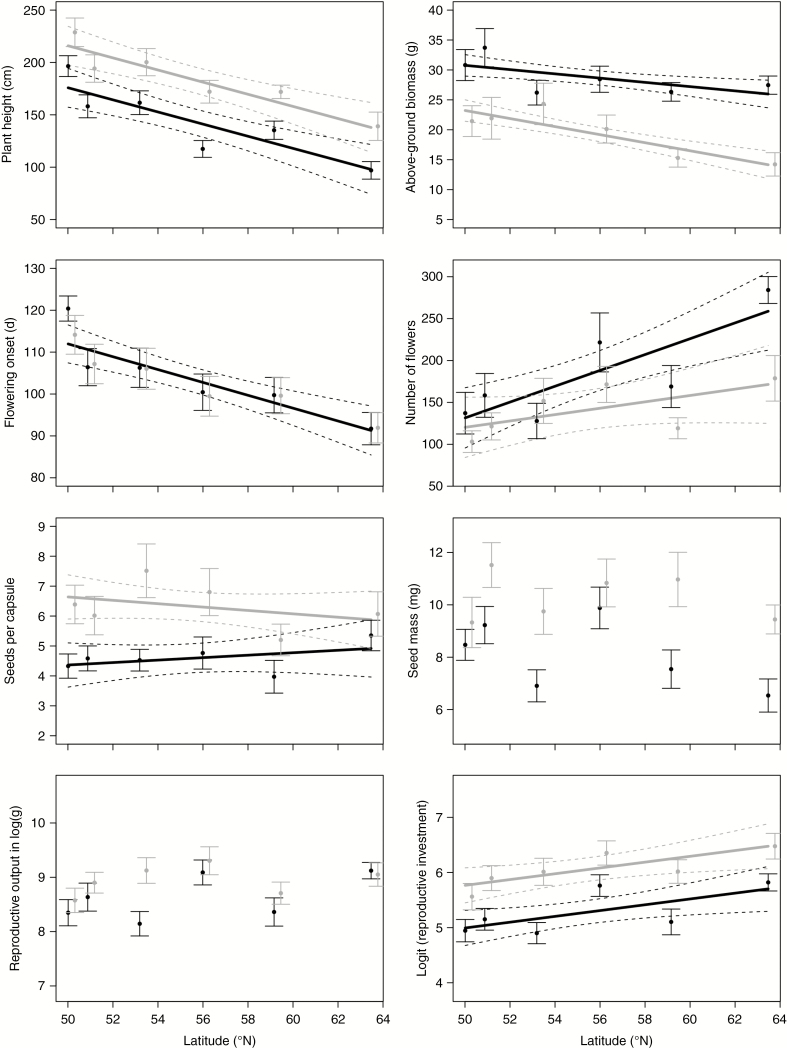
Plant height, above-ground biomass and flowering onset decreased with latitude, while the total number of flowers produced and reproductive investment increased with latitude in both generations (Table 2, Fig. 2; Supplementary Data Appendices S1 and S2). In contrast, no latitudinal cline was observed for seed mass or reproductive output (total seed mass per individual). The number of seeds per capsule showed a slight decrease with latitude, but only in the second generation (Table 2, Fig. 2; Appendix S2).
Table 2.
Parameter estimates (intercepts and slopes ± s.e.) of the reduced linear mixed-effect models testing the effect of generation, latitude and their interaction on the different traits in Impatiens glandulifera
| Trait | Generation | Latitude | |
|---|---|---|---|
| Intercept (s.e.) | Slope (s.e.) | R 2 | |
| Plant height (cm) | F 1: 175.82 (±9.45) F2: 215.90 (±9.45) | −5.79 (±1.29) | 0.504 |
| Above-ground biomass (g) | F 1: 30.76 (±0.91) F2: 23.22 (±0.91) | F 1: −0.356 (±0.127) F2: −0.673 (±0.127) | 0.358 |
| Flowering onset (d) | 111.96 (±2.31) | −1.53 (±0.32) | 0.252 |
| Number of flowers | F 1: 131.55 (±18.34) F2: 120.27 (±18.34) | F 1: 9.45 (±2.54) F2: 3.80 (±2.54) | 0.242 |
| Seeds per capsule | F 1: 4.36 (±0.38) F2: 6.63 (±0.38) | F 1: 0.04 (±0.052) F2: −0.06 (±0.052) | 0.204 |
| Seed mass (mg) | F 1: 8.09 (±0.43) F2: 10.31 (±0.43) | – | 0.171 |
| Reproductive output (g)* | F 1: 8.62 (±0.13) F2: 8.94 (±0.13) | – | 0.051 |
| Reproductive investment | F 1: 4.99 (±0.16) F2: 5.77 (±0.16) | 0.053 (±0.022) | 0.335 |
See Supplementary Data Appendix S1 for model selection and AIC values and Appendix S2 for the parameter estimates of the full models. Marginal pseudo-R2 is presented for each model. Note that slopes for latitude are provided for generation 1 (F1) and generation 2 (F2) separately for models with significant interaction terms.
*Log-transformed.
Fig. 2.
Plant traits in Impatiens glandulifera along a latitudinal gradient in first (black) and second (grey) generation in the glasshouse (average with 95 % confidence intervals). Regression slopes with 95 % confidence intervals are provided for traits with statistically significant latitude effects (Table 2).
In both generations, plant height decreased by no less than ~90 cm (45–50 % reduction), while flowering onset in the glasshouse advanced by ~25 d (~22 % change) for plants grown from seeds originating from the southernmost to the northernmost population. The strength of these two clines remained constant across generations (no statistically significant interaction, Table 2). This indicates that they essentially resulted from genetic differentiation among populations, because the slope of the cline would have decreased or disappeared if trait patterns were (partly) caused by environmental variation or maternal effects. Consistent between-generation differences in plant height occurred in all populations, with plants from the second generation being on average 40 cm taller than those of the first generation (Table 2). Above-ground biomass, on the other hand, was consistently lower in the second generation. The decrease in above-ground biomass towards higher latitude became slightly more pronounced in the second generation (model with interaction term) (Table 2, Fig. 2).
For the total number of flowers, the positive effect of latitude decreased from the first to the second generation (Table 2, Fig. 2). In the first generation, plants produced more than twice the number of flowers in the northernmost population compared to the southernmost population (from ~130 to 280 flowers), while in the second generation this increase in flower number was only 60 % (from 100 to 160 flowers). Reproductive output was higher for the second generation compared to the first, but was not affected by latitude (Table 2, Fig. 2). Reproductive investment, on the other hand, was almost double for the northernmost population, compared to the southernmost population, for both generations (Table 2, Fig. 2). As expected from the between-generation differences in above-ground biomass, consistent differences in reproductive investment occurred between the two generations in all populations, with reproductive investment being consistently higher at the second generation (Table 2, Fig. 2).
Local genetic differentiation
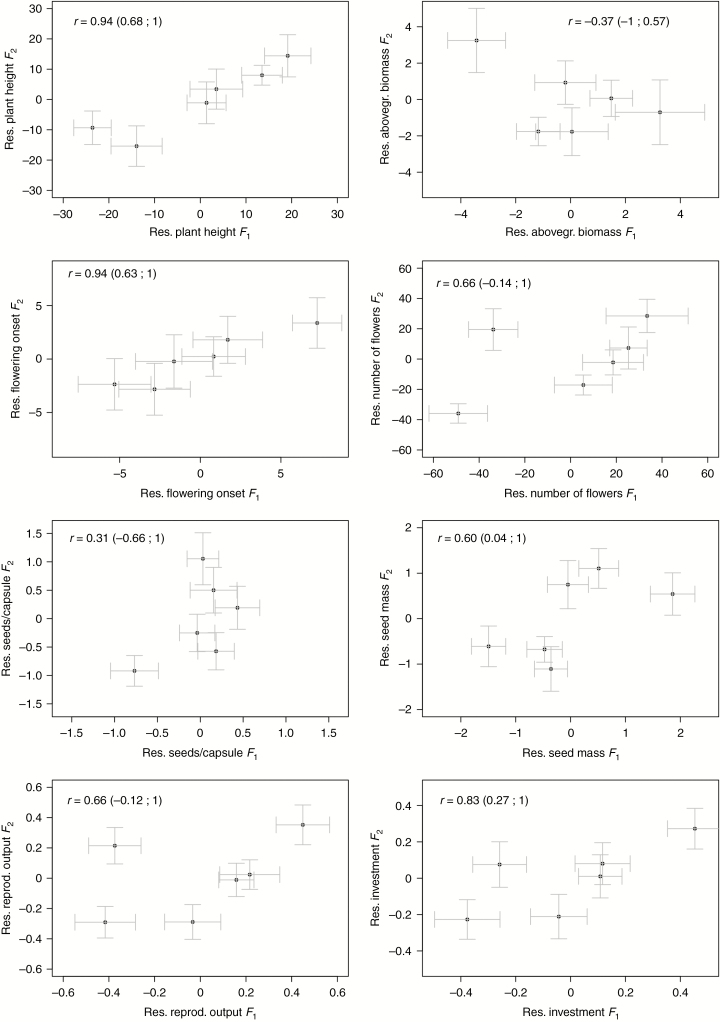
We found evidence for genetic differentiation independent of latitudinal effects for plant height, flowering onset, seed mass and reproductive investment. In these traits, deviations from the effect of latitude on the trait were correlated between the two generations (Fig. 3), indicating additional genetic differentiation among populations not due to latitudinal effects. For all other traits, although we observed positive correlations between generations, these were not statistically significant (Fig. 3).
Fig. 3.
Correlation between residuals obtained from the best model (results shown in Table 1) for the first (F1) and second (F2) generation for different traits in Impatiens glandulifera. Correlation coefficients (r) with 95 % confidence intervals are presented.
Independence of selection on the different traits
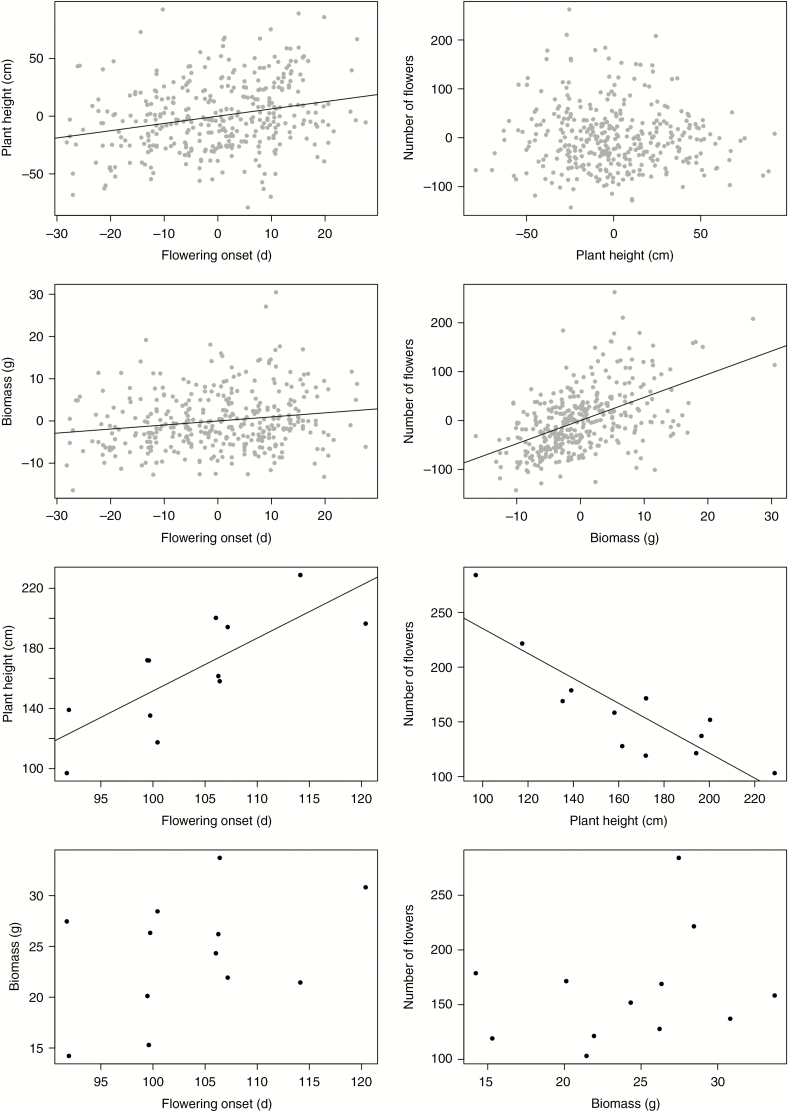
The effects of flowering onset on above-ground biomass and plant height differed between the within- and among-population levels (Fig. 4). While both size variables increased with later flowering onset within populations (parameter estimate ± s.e. for plant height β = 0.63 ± 0.13 cm d–1, above-ground biomass β = 0.09 ± 0.03 g d–1), the increase in plant height with flowering onset was about six times stronger at the among-population level (β = 3.52 ± 0.91 cm d–1), while above-ground biomass did not significantly covary with flowering onset among populations (β = 0.28 ± 0.20 g d–1). Because the range of flowering onset was similar at the two levels, these differences do not represent statistical artefacts generated by differences in the range of variation of the predictor variable. Similarly, the effect of above-ground biomass and plant height on the number of flowers differed between the two levels (Fig. 4). Within population, variation in plant height did not affect the number of flowers (β = −0.12 ± 0.11 flowers cm–1), while heavier plants produced more flowers (β = 4.74 ± 0.43 flowers g–1). In contrast, populations with taller plants produced fewer flowers per plant than populations with shorter plants (β = −1.14 ± 0.21 flowers cm–1), while among-population differences in plant biomass had no statistically significant effect on the number of flowers (β = 2.12 ± 2.61 flowers g–1).
Fig. 4.
Comparison of the effects of flowering onset on plant size (above-ground biomass and plant height) and the effect of plant size on the number of flowers between the within- and among-population levels of Impatiens glandulifera. Within-population relationships are presented in the upper four graphs where population-mean centred data are represented with grey dots, and among-population relationship are represented in the four lower graphs where each dot represents the mean for each population at each generation. Regression lines are only represented when statistically significant (see text for parameter estimates).
DISCUSSION
Effects of growing season (life-history theory)
Our results show that populations of the invasive Impatiens glandulifera across Europe are characterized by strong latitudinal clines for several traits. The persistence of this clinal variation for plant height, above-ground biomass and flowering onset, across two generations in a common garden, establishes that these previously observed trait clines (Kollmann and Bañuelos, 2004) are caused by genetic differentiation rather than maternal effects. For the reproductive traits, only the number of flowers and reproductive investment showed persistent clinal patterns related to latitude. Still, the decrease in the slope between the number of flowers and latitude at the second generation suggests that part of the variation in the F1 generation was due to phenotypic plasticity triggered by the environment in which seeds were produced.
As expected, flowering onset was progressively earlier towards higher latitudes, thus reflecting the expected adaptive response to variation in the length of the growing season along the latitudinal cline (Colautti et al., 2010; Hodgins et al., 2018). As predicted by allocation theory, this faster flowering was accompanied by reduced plant height and above-ground biomass. Similar latitudinal patterns in growth and phenology have been reported for invasive plants in Asia (Li et al., 2015), Europe (Weber and Schmid, 1998; Hodgins and Rieseberg, 2011) and North America (Montague et al., 2008; Colautti and Barrett, 2013).
Effects of competition and reproductive uncertainty (stress-gradient hypothesis)
Genetic differentiation in plant height and above-ground biomass along the latitudinal cline was partly independent of the genetic differentiation in the onset of flowering. Furthermore, reproductive output did not decrease with increasing latitude, contrary to allocation theory expectations (Primack, 1987; Hodgins et al., 2018). Instead, we observed that seed mass, seeds per capsules and reproductive output remained more or less constant, while the number of flowers (and thus seeds) increased with latitude. The observed independent evolution of the number of flowers from plant height and above-ground biomass supports the hypothesis that selection on reproductive traits is acting independently of, or in addition to, selection on flowering onset. These results also contradict the assumption of a positive correlation between biomass and fecundity (Kollmann and Bañuelos, 2004). Instead, these patterns suggest that I. glandulifera is able to invest in reproduction, independently of the variation in above-ground biomass, thus resulting in more reproductive allocation at higher latitudes. Similarly, variation in plant height/biomass and onset of flowering was also found to be uncorrelated in the invasive Senecio inaequidens along an altitudinal gradient (Monty and Mahy, 2009).
These results seemingly support the stress-gradient hypothesis, with populations at higher latitudes experiencing less severe competition, thus permitting plants to invest less energy in vegetative growth (biomass and plant height) (Bertness and Callaway, 1994; Schemske et al., 2009; Bhattarai et al., 2017). This is supported by measurements of competition-related functional traits in the I. glandulifera invaded communities. In Helsen et al. (2018), we observed a decrease in community-mean plant height, specific leaf area and Grime C-signature along the latitudinal gradient, indicating that competition is weakens with increasing latitude.
The high reproductive output, independent of latitude, suggests that selection for high seed set remains important along the latitudinal gradient, as can be expected for an annual species with no seedbank. This increased allocation to reproduction at higher latitudes has been observed for several other invasive plant species (Hickman, 1975; Chun et al., 2011; Hodgins and Rieseberg, 2011; Liu et al., 2016). Furthermore, although seed mass was not significantly correlated with latitude, the northernmost population was characterized by the lowest seed mass. This partly supports the expected latitudinal pattern for this trait. Indeed, at high latitudes, a larger number of seeds might represent an adaptation to increased dispersal ability at the range expansion edge and/or an adaptation to reduce the impact of reproductive failure in the short growing season (Primack, 1987; Hamilton et al., 2005).
Local genetic differentiation independent of the latitudinal gradient
Beside the latitudinal clines in genetic differentiation for most measured traits, we also observed a clear signal of genetic differentiation among populations, independent of the clinal variation for flowering onset, plant height, seed mass and reproductive investment. This suggests that different selective forces can act on several vegetative and reproductive traits simultaneously at different spatial scales. This also illustrates the importance of assessing potential genetic differentiation patterns that vary independently of latitudinal clines. The relatively low percentage of variation explained in these traits by latitude and generation (pseudo-R2 values between 17.1 and 50.4 %) indicates the potential of high variation present among populations at the local level. This additional genetic differentiation might be due to micro-climatic variation, variation in the strength of competition, or by variation in soil characteristics not accounted for by latitude. Future research focusing on specific potential drivers of selection is needed to enhance our understanding of the drivers of the observed genetic differentiation (e.g. Pahl et al., 2013).
Implications for invasion success and fitness
Theory suggests that trade-offs can, under certain conditions, constrain adaptive evolution and thus invasion success even in the presence of considerable genetic variation (Lande, 1979; Etterson and Shaw, 2001; Blows and Hoffmann, 2005; Eckert et al., 2008). The lack of covariation between flowering onset and size traits observed in I. glandulifera may therefore facilitate its rapid adaptation during invasion. Interestingly, genetic decoupling of traits can occur frequently in species with high sexual recombination (Barrick and Lenski, 2013). The protandrous breeding system and annual life history of I. glandulifera both indicate high outcrossing and recombination rates. Moreover, a recent meta-analysis has shown that self-incompatible invasive species exhibited exceptional levels of local adaptation (Oduor et al., 2016). This suggests that the genetic decoupling between important life history traits and their ability to evolve independently may be an important characteristic of invasive species. Whereas empirical studies assessing fitness-related trait trade-offs in invasive studies are extremely scarce, Colautti et al. (2010) tested for genetic covariation between the timing of flowering and size at maturity in Lythrum salicaria, invasive to North America. In contrast to our results, they found that genetic covariation between these traits probably constrained the invasion success of the species. It remains largely unexplored whether specific genetic architecture and evolutionary independence among life history traits may play a crucial role in invasion success.
It is worth noting that the patterns of trait variation observed in our study reflect life history strategies of these populations under near-optimal growing conditions (i.e. the glasshouse), which can generate plastic responses specific to these common conditions. Field trials and/or transplantation experiments are also needed to fully understand how these genetic differences in life-history strategies translate to growth, reproductive output and fitness along the gradient. The high invasion success of this species nonetheless suggests high fitness across its invaded range (Beerling and Perrins, 1993).
Our study shows that I. glandulifera was able to evolve several life history traits across its invaded range in only ~150 years (Beerling and Perrins, 1993), despite the relatively low neutral genetic diversity across the invaded range (Hagenblad et al., 2015; Helsen et al., 2019). This rapid evolution is probably generated by its short lifespan and the absence of a persistent seed bank, resulting in a short generation time (Liao et al., 2016), as well as some additive genetic variation in life history traits allowing populations to respond to selection. Moreover, in the absence of neutral genetic diversity, evolution can still occur where populations can retain high levels of adaptive genetic diversity (Whitlock, 2014; De Kort et al., 2015; Mittell et al., 2015). Comparably fast genetic differentiation has also been observed for other invasive plant species (Maron et al., 2004; Leger and Rice, 2007; Colautti and Barrett, 2013). These results show that I. glandulifera is able to successfully establish and adapt to a wide range of environmental conditions, probably explaining its large latitudinal invasive range (Beerling and Perrins, 1993). The increased reproductive investment in the northernmost population further indicates that the potential for range expansion is likely to remain high, because this probably facilitates (large-distance) seed dispersal and thus range expansion (Hamilton et al., 2005). If the current invasive range is environmentally constrained by temperature, this could imply a high potential for range expansion at the northern distribution edge, but potential contraction at the southern distribution edge, under progressing climate warming in the future.
CONCLUSIONS
By exploring trait variation across two consecutive glasshouse generations in Impatiens glandulifera, we showed that population differences along a latitudinal gradient were largely due to genetic differentiation, rather than by phenotypic plasticity. We also found clear support for genetic differentiation among populations that was independent of the main latitudinal gradient. We showed the ability of different life history traits to evolve independently of each other and independently of the changes in flowering onset. Indeed, we observed lower plant height and vegetative above-ground biomass, but higher reproductive investment at higher latitudes, which agrees with the stress-gradient hypothesis. We argue that the independent evolution of fitness traits may have contributed to the invasion success of I. glandulifera.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Appendix S1: AIC values for each performed linear mixed model testing the effect of generation, latitude and their interaction on the different traits in Impatiens glandulifera. Appendix S2: Parameter estimates and standard errors of the full linear mixed models testing the effect of generation, latitude and their interaction on the different traits in Impatiens glandulifera.
ACKNOWLEDGEMENTS
We are grateful to Martin Diekmann, Annika M. Felton, Annette Kolb, Isgard Lemke, Sigrid Lindmo, Sharmila Phuyal and Jan Plue for their help in the field and in processing samples in the laboratory and Guri Hanssen and Grete Rakvaag for taking care of plants in the glasshouse.
FUNDING
This work was supported by the research network FLEUR (http://www.fleur.ugent.be) funded by the Research Foundation – Flanders (FWO). K.H. held a postdoc fellowship of the FWO [1202817N]. C.P. is partly supported by the Research Council of Norway through its Centre of Excellence funding scheme (project no. 223257), and currently hosted by the Centre for Advanced Studies (CAS) in Oslo.
LITERATURE CITED
- Barrick JE, Lenski RE. 2013. Genome dynamics during experimental evolution. Nature 14: 827–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton K. 2009. Mu-MIn: Multi-model inference. R Package Version 0.12.2/r18 http://r-forge.r-project.org/projects/mumin/.
- Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67: 1–48. [Google Scholar]
- Beerling DJ. 1993. The impact of temperature on the northern distribution limits of the introduced species Fallopia japonica and Impatiens glandulifera in North-West Europe. Journal of Biogeography 20: 45–53. [Google Scholar]
- Beerling DJ, Perrins JM. 1993. Biological flora of the British Isles. Impatiens glandulifera Royle (Impatiens roylei Walp.). Journal of Ecology 81: 367–382. [Google Scholar]
- Bertness MD, Callaway R. 1994. Positive interactions in communities. Trends in Ecology and Evolution 9: 191–193. [DOI] [PubMed] [Google Scholar]
- Bhattarai GP, Meyerson LA, Cronin JT. 2017. Geographic variation in apparent competition between native and invasive Phragmites australis. Ecology 98: 349–358. [DOI] [PubMed] [Google Scholar]
- Blows MW, Hoffmann AA. 2005. A reassessment of genetic limits to evolutionary change. Ecology 86: 1371–1384. [Google Scholar]
- Brooks ME, Kristensen K, van Benthem KJ, et al. 2017. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal 9: 378–400. [Google Scholar]
- Caño L, Escarré J, Fleck I, Blanco-Moreno JM, Sans FX. 2008. Increased fitness and plasticity of an invasive species in its introduced range: a study using Senecio pterophorus. Journal of Ecology 96: 468–476. [Google Scholar]
- Chun YJ, Le Corre V, Bretagnolle F. 2011. Adaptive divergence for a fitness-related trait among invasive Ambrosia artemisiifolia populations in France. Molecular Ecology 20: 1378–1388. [DOI] [PubMed] [Google Scholar]
- Colautti RI, Barrett SCH. 2013. Rapid adaptation to climate facilitates range expansion of an invasive plant. Science 342: 364–367. [DOI] [PubMed] [Google Scholar]
- Colautti RI, Eckert CG, Barrett SCH. 2010. Evolutionary constraints on adaptive evolution during range expansion in an invasive plant. Proceedings of the Royal Society B: Biological Sciences 277: 1799–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassonville N, Vanderhoeven S, Vanparys V, Hayez M, Gruber W, Meerts P. 2008. Impacts of alien invasive plants on soil nutrients are correlated with initial site conditions in NW Europe. Oecologia 157: 131–140. [DOI] [PubMed] [Google Scholar]
- Davidson AM, Jennions M, Nicotra AB. 2011. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecology Letters 14: 419–431. [DOI] [PubMed] [Google Scholar]
- De Kort H, Vandepitte K, Mergeay J, Mijnsbrugge KV, Honnay O. 2015. The population genomic signature of environmental selection in the widespread insect-pollinated tree species Frangula alnus at different geographical scales. Heredity 115: 415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugosch KM, Parker IM. 2008. Invading populations of an ornamental shrub show rapid life history evolution despite genetic bottlenecks. Ecology Letters 11: 701–709. [DOI] [PubMed] [Google Scholar]
- Eckert CG, Samis KE, Lougheed SC. 2008. Genetic variation across species’ geographical ranges: the central – marginal hypothesis and beyond. Molecular Ecology 17: 1170–1188. [DOI] [PubMed] [Google Scholar]
- Etterson JR, Shaw RG. 2001. Constraint to adaptive evolution in response to global warming. Science 294: 151–154. [DOI] [PubMed] [Google Scholar]
- Galloway LF, Burgess KS. 2012. Artificial selection on flowering time: Influence on reproductive phenology across natural light environments. Journal of Ecology 100: 852–861. [Google Scholar]
- GBIF.org 2019. GBIF Occurrence Download. https://doi.org/10.15468/dl.onxiuf.
- Greenwood P, Kuhn NJ. 2014. Does the invasive plant, Impatiens glandulifera, promote soil erosion along the riparian zone? An investigation on a small watercourse in northwest Switzerland. Journal of Soils and Sediments 14: 637–650. [Google Scholar]
- Hagenblad J, Hülskötter J, Acharya KP, et al. 2015. Low genetic diversity despite multiple introductions of the invasive plant species Impatiens glandulifera in Europe. BMC Genetics 16: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton MA, Murray BR, Cadotte MW, et al. 2005. Life-history correlates of plant invasiveness at regional and continental scales. Ecology Letters 8: 1066–1074. [Google Scholar]
- He W-M, Thelen GC, Ridenour WM, Callaway RM. 2010. Is there a risk to living large? Large size correlates with reduced growth when stressed for knapweed populations. Biological Invasions 12: 3591–3598. [Google Scholar]
- Heisler IL, Damuth J. 1987. A method for analyzing selection in hierarchically structured populations. The American Naturalist 130: 582–602. [Google Scholar]
- Helsen K, Hagenblad J, Acharya KP, et al. 2019. No genetic erosion after five generations for Impatiens glandulifera populations across the invaded range in Europe. BMC Genetics 20: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helsen K, Smith SW, Brunet J, et al. 2018. Impact of an invasive alien plant on litter decomposition along a latitudinal gradient. Ecosphere 9: e02097. [Google Scholar]
- Hickman JC. 1975. Environmental unpredictability and plastic energy allocation strategies in the annual Polygonum cascadense (Polygonaceae). Journal of Ecology 63: 689–701. [Google Scholar]
- Hodgins KA, Bock DG, Rieseberg LH. 2018. Trait evolution in invasive species. Annual Plant Reviews 1: 1–37. [Google Scholar]
- Hodgins KA, Rieseberg L. 2011. Genetic differentiation in life-history traits of introduced and native common ragweed (Ambrosia artemisiifolia) populations. Journal of Evolutionary Biology 24: 2731–2749. [DOI] [PubMed] [Google Scholar]
- van Kleunen M, Dawson W, Essl F, et al. 2015. Global exchange and accumulation of non-native plants. Nature 525: 100–103. [DOI] [PubMed] [Google Scholar]
- van Kleunen M, Fischer M. 2005. Constraints on the evolution of adaptive phenotypic plasticity in plants. New Phytologist 166: 49–60. [DOI] [PubMed] [Google Scholar]
- van Kleunen M, Weber E, Fischer M. 2010. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecology Letters 13: 235–245. [DOI] [PubMed] [Google Scholar]
- Kollmann J, Bañuelos MJ. 2004. Latitudinal trends in growth and phenology of the invasive alien plant Impatiens glandulifera (Balsaminaceae). Diversity and Distributions 10: 377–385. [Google Scholar]
- Lande R. 1979. Quantitative genetic analysis of multivariate evolution, applied to brain: body size allometry. Evolution 33: 402–416. [DOI] [PubMed] [Google Scholar]
- Lavergne S, Molofsky J. 2007. Increased genetic variation and evolutionary potential drive the success of an invasive grass. Proceedings of the National Academy of Sciences USA 104: 3883–3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CE. 2002. Evolutionary genetics of invasive species. Trends in Ecology & Evolution 17: 386–391. [Google Scholar]
- Lee CE, Gelembiuk GW. 2008. Evolutionary origins of invasive populations. Evolutionary Applications 1: 427–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger EA, Rice KJ. 2007. Assessing the speed and predictability of local adaptation in invasive California poppies (Eschscholzia californica). Journal of Evolutionary Biology 20: 1090–1103. [DOI] [PubMed] [Google Scholar]
- Li X-M, She D-Y, Zhang D-Y, Liao W-J. 2015. Life history trait differentiation and local adaptation in invasive populations of Ambrosia artemisiifolia in China. Oecologia 177: 669–677. [DOI] [PubMed] [Google Scholar]
- Liao H, D’Antonio CM, Chen B, Huang Q, Peng S. 2016. How much do phenotypic plasticity and local genetic variation contribute to phenotypic divergences along environmental gradients in widespread invasive plants? A meta-analysis. Oikos 125: 905–917. [Google Scholar]
- Liu W, Maung-Douglass K, Strong DR, Pennings SC, Zhang Y. 2016. Geographical variation in vegetative growth and sexual reproduction of the invasive Spartina alterniflora in China. Journal of Ecology 104: 173–181. [Google Scholar]
- Lüdecke D. 2019. sjstats: Statistical functions for regression models (Version 0.17.5) https://cran.r-project.org/package=sjstats.
- Maron JL, Vilà M, Bommarco R, Elmendorf S, Beardsley P. 2004. Rapid evolution of an invasive plant. Ecological Monographs 74: 261–280. [Google Scholar]
- Mason RAB, Cooke J, Moles AT, Leishman MR. 2008. Reproductive output of invasive versus native plants. Global Ecology and Biogeography 17: 633–640. [Google Scholar]
- Mittell EA, Nakagawa S, Hadfield JD. 2015. Are molecular markers useful predictors of adaptive potential? Ecology Letters 18: 772–778. [DOI] [PubMed] [Google Scholar]
- Molina-Montenegro MA, Naya DE. 2012. Latitudinal patterns in phenotypic plasticity and fitness-related traits: assessing the climatic variability hypothesis (CVH) with an invasive plant species. PLoS One 7: e47620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague JL, Barrett SCH, Eckert CG. 2008. Re-establishment of clinal variation in flowering time among introduced populations of purple loosestrife (Lythrum salicaria, Lythraceae). Journal of Evolutionary Biology 21: 234–245. [DOI] [PubMed] [Google Scholar]
- Monty A, Mahy G. 2009. Clinal differentiation during invasion: Senecio inaequidens (Asteraceae) along altitudinal gradients in Europe. Oecologia 159: 305–315. [DOI] [PubMed] [Google Scholar]
- Murren CJ, Dudash MR. 2012. Variation in inbreeding depression and plasticity across native and non-native field environments. Annals of Botany 109: 621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A-M, Korpelainen H. 2015. Population genetics of Himalayan balsam (Impatiens glandulifera): comparison of native and introduced populations. Plant Ecology & Diversity 8: 317–321. [Google Scholar]
- Nakagawa S, Schielzeth H. 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods in Ecology and Evolution 4: 133–142. [Google Scholar]
- Nienhuis CM, Stout JC. 2009. Effectiveness of native bumblebees as pollinators of the alien invasive plant Impatiens glandulifera (Balsaminaceae) in Ireland. Journal of Pollination Ecology 1: 1–11. [Google Scholar]
- Obeso JR. 2002. The costs of reproduction in plants. New Phytologist 155: 321–348. [DOI] [PubMed] [Google Scholar]
- Oduor AMO, Leimu R, van Kleunen M. 2016. Invasive plant species are locally adapted just as frequently and at least as strongly as native plant species. Journal of Ecology 104: 957–968. [Google Scholar]
- Olsson K, Ågren J. 2002. Latitudinal population differentiation in phenology, life history and flower morphology in the perennial herb Lythrum salicaria. Journal of Evolutionary Biology 15: 983–996. [Google Scholar]
- Pahl AT, Kollmann J, Mayer A, Haider S. 2013. No evidence for local adaptation in an invasive alien plant: Field and greenhouse experiments tracing a colonization sequence. Annals of Botany 112: 1921–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Harguindeguy N, Diaz S, Garnier E, et al. 2013. New handbook for standardised measurement of plant functional traits worldwide. Australian Journal of Botany 61: 167–234. [Google Scholar]
- Primack RB. 1987. Relationships among flowers, fruits, and seeds. Annual Review of Ecology and Systematics 18: 409–430. [Google Scholar]
- R Core Team 2018. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; https://www.R-project.org/. [Google Scholar]
- Sakai AK, Allendorf FW, Holt JS, et al. 2001. The population biology of invasive species. Annual Review of Ecology and Systematics 32: 305–332. [Google Scholar]
- Samson DA, Werk KS. 1986. Size-dependent effects in the analysis of reproductive effort in plants. The American Naturalist 127: 667–680. [Google Scholar]
- Schemske DW, Mittelbach GG, Cornell HV, Sobel JM, Roy K. 2009. Is there a latitudinal gradient in the importance of biotic interactions? Annual Review of Ecology, Evolution, and Systematics 40: 245–269. [Google Scholar]
- Seebens H, Blackburn TM, Dyer EE, et al. 2017. No saturation in the accumulation of alien species worldwide. Nature Communications 8: 14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcombe JR, Weinig C, Ungerer M, et al. 2004. A latitudinal cline in flowering time in Arabidopsis thaliana modulated by the flowering time gene FRIGIDA. Proceedings of the National Academy of Sciences USA 101: 4712–4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan SE. 1995. Phenotypic plasticity and plant adaptation. Acta Botanica Neerlandica 44: 363–383. [Google Scholar]
- Walker NF, Hulme PE, Hoelzel AR. 2009. Population genetics of an invasive riparian species, Impatiens glandulifera. Plant Ecology 203: 243–252. [Google Scholar]
- Weber E, Schmid B. 1998. Latitudinal population differentiation in two species of Solidago (Asteraceae) introduced into Europe. American Journal of Botany 85: 1110–1121. [PubMed] [Google Scholar]
- Weiner J. 2004. Allocation, plasticity and allometry in plants. Perspectives in Plant Ecology, Evolution and Systematics 6: 207–215. [Google Scholar]
- Whitlock R. 2014. Relationships between adaptive and neutral genetic diversity and ecological structure and functioning: a meta-analysis. Journal of Ecology 102: 857–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. In: Gail M, Krickeberg K, Samet J, Tsiatis A, Wong W, eds. Statistics for biology and health. New York: Spring Science and Business Media. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.