Abstract
Recent studies have demonstrated the potential of natural killer (NK) cells in immunotherapy to treat multiple types of cancer. NK cells are innate lymphoid cells that play essential roles in tumor surveillance and control that efficiently kill the tumor and do not require the major histocompatibility complex. The discovery of the NK’s potential as a promising therapeutic target for cancer is a relief to oncologists as they face the challenge of increased chemo-resistant cancers. NK cells show great potential against solid and hematologic tumors and have progressively shown promise as a therapeutic target for cancer immunotherapy. The effector role of these cells is reliant on the balance of inhibitory and activating signals. Understanding the role of various immune checkpoint molecules in the exhaustion and impairment of NK cells when their inhibitory receptors are excessively expressed is particularly important in cancer immunotherapy studies and clinical implementation. Emerging immune checkpoint receptors and molecules have been found to mediate NK cell dysfunction in the tumor microenvironment; this has brought up the need to explore further additional NK cell-related immune checkpoints that may be exploited to enhance the immune response to refractory cancers. Accordingly, this review will focus on the recent findings concerning the roles of immune checkpoint molecules and receptors in the regulation of NK cell function, as well as their potential application in tumor immunotherapy.
Subject terms: Cancer, Immunology
Introduction
Natural killer (NK) cells are cytotoxic lymphocytes in the family of innate lymphoid cells that play essential roles in the first line of defense against cancer and viral infections.1–3 Human NK cells make up to 15 percent of the circulating lymphocytes and are associated with the killing and destruction of microbially infected and malignantly autologous and allogenic cells.4
NK cells have been determined to demonstrate antitumor cell cytotoxicity in the absence of prior sensitization and the subsequent production of cytokines alongside chemokines that have an effect on regulating certain immune responses.4 Some types of cancer, such as invasive breast carcinoma, bladder urothelial carcinoma, renal clear cell carcinoma, colon adenocarcinoma, lung squamous cell carcinoma, lower-grade glioma, pancreatic adenocarcinoma, stomach adenocarcinoma, cutaneous melanoma, uterine corpus endometrial carcinoma, and thyroid carcinoma, are associated with a deficiency of the number of NK cells leading to poor clinical outcomes.5 Many cancer types have demonstrated an imbalance of immune-regulated signals in NK cells. Inhibitory and activating receptors are expressed on the surface of NK cells and contribute to the execution of various NK cell functions.4 NK cells express the non-HLA-class I-specific inhibitory receptors (such as programmed cell death protein 1 [PD-1],6,7 T cell immunoreceptor with Ig and immunoreceptor tyrosine-based inhibition motif [ITIM]8 domains [TIGIT],9,10 a cluster of differentiation 112 receptor [CD112R], CD96, interleukin-1 receptor 8 [IL-1R8], and T cell immunoglobulin and mucin-domain-containing molecule 3 [TIM-3]), as well as activating receptors (such as NK group 2 [NKG2] family of receptor D [NKG2D] and coreceptor [CD226]).4,11–13 NK cells also express the HLA-class I-specific inhibitory receptors (such as killer cell immunoglobin-like receptor (KIR), NKG2A, and lymphocyte activation gene-3 [LAG-3]).14 Although more researches have shown that the blockade of checkpoint inhibitory receptors may be crucial in effectively reversing T cell exhaustion and the restoration of the antitumor capacity of T cells,15 there has been growing interest in the immune effects these treatments may have on NK cells as well.
Immune checkpoint inhibitors (ICIs) represent some of the most efficient immunotherapeutic approaches currently used to treat cancer.16 ICIs are antibodies that serve the role of binding to inhibitory molecules on the tumor-infiltrating surfaces of lymphocytes and thereby allowing the reactivation of antitumor immune responses.17 One of the promising discoveries to enhance antitumor activity and survival is the dual targeting of chimeric antigen-based receptor-engineered NK cells of glioblastoma, which has been found to overcome the heterogeneity of specific expression of target antigens and enhances the antitumor activity and survival.17,18 Moreover, immune checkpoint receptors (IC) contribute either positively or negatively in the regulation of activating the host immune response, preventing unwanted reactions that may affect healthy tissues.19
The term IC is mainly used about the inhibitory ICs critical in controlling NK and cytotoxic CD8+ T cells because of its high cytotoxic potential.19 Therefore, specific ICs are described concerning their regulation of either NK cell or T cell activity.19 NK cell activation is mainly regulated by receptors from the natural cytotoxicity receptor, NKG2, and KIR families.19–22 For example, activating and inhibitory KIR receptors serve to control the development and function of NK cell immunity while adjusting to the tumor microenvironment (TME).23,24 The interactions between KIRs and the corresponding HLA class I ligands contribute to the mediation of NK cell self-tolerance or cytotoxicity against the transformed cells.24,25 The control of unwanted damage of healthy cells by the NK cells is regulated by HLA-class I-specific receptors, which contain a fail-safe mechanism. Recent studies show that NKG2A forms a crucial checkpoint for controlling T cell and NK cell activation in cancerous conditions.24,26 The checkpoint receptors mediate the delivery of multiple signals, the balance of which determines if NK cells kill their target cells, such as stressed cells and tumor cells, or remain inactive.27–29
Dysregulation of checkpoint receptor signaling contributes to NK cell dysfunction.30 Furthermore, the downregulation of activating NK cell receptors and the upregulation of inhibitory receptors have been reported in multiple tumors. For example, NK cells expressed decreased levels of CD226 in various types of cancers, including myeloma, breast cancer, and myeloid leukemia.14,15,31 Notably, the downregulation of activating NK cell receptors, sometimes by helix-loop-helix protein ID2, serves as an active suppression and can be restored in the TME, such as by the repression of GSK3.14,32,33 As highlighted earlier, ICs can further be described as being membrane molecules that are mainly located, although not exclusively, on T lymphocytes and NK cells that act after recognizing appropriate ligands on the antigen-presenting cells (APC) or the target cells. Therefore, ICs can play either a negative or positive role in the processes of lymphocyte activation. The detrimental regulation of NK cell function also involves the upregulation of inhibitory receptors. For example, PD-1 upregulation was recently found in NK cells from multiple myeloma, Kaposi sarcoma, and head and neck cancer patients.16,34–36 TIGIT expression on NK cells was further upregulated in tumor regions compared with peritumoral regions in colorectal tumors.10,24,37–39 CD96, CD112R, TIM-3, NKG2A, LAG-3, and IL-1R8 are other significant receptors that have been proved to be upregulated on NK cells in cancers.26,40,41
Aside from this, the expression of various ligands on cancer cells has been reported to target common signaling molecules. The targeted interaction modifies the function of NK cells by regulating its numerous inhibitory and activating receptors. For instance, transformed cells may lose or decrease their HLA-I expression during cancer progression due to the activation of inhibitory NK cell receptors bound to their surfaces.26 Increased activation of inhibitory receptors and decreased stimulation of activating receptors reduce the antitumor response in the NK cells, and facilitate the induction of tumor immune escape. Several solid tumor types create an immunosuppressive microenvironment that prevents T cell or NK cell-mediated lysis through inhibitory ligands, such as the PD-L1 expressed on the surfaces of the cancer cells.6,42–45 The modulation of these checkpoint molecules and the development of inhibitory receptor blockade provide a vital strategy to improve NK cell function, such as by Diacylglycerol Kinase zeta.46–49
NK cells have essential roles in tumor surveillance and control. Some studies have identified the presence of dysfunctional NK cells in some cancer patients.4,14,16,50 Due to the inability to preserve their primary role, the dysfunctional NK cells allow certain cancers to escape the immune surveillance.17,51,52 Thus, restoring the function of NK cells in cancer patients may be a promising approach for immunotherapy.53,54 In the following sections, a detailed analysis of NK cell immune checkpoint receptors and their ligands will be discussed along with their prospective influence in cancer immunotherapy.55–57
NK cell phenotype and function
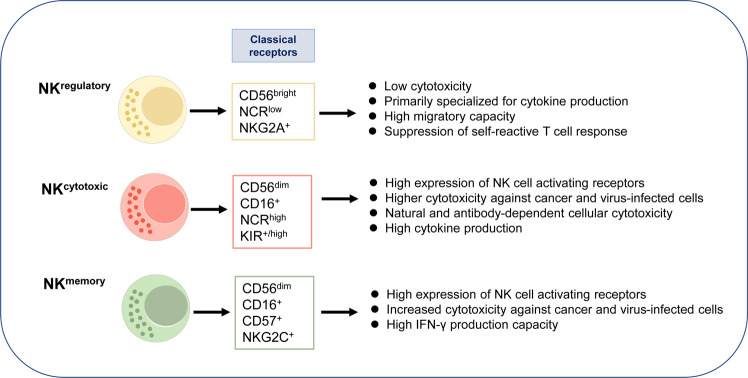
NK cells display a range of phenotypic and functional characteristics (Fig. 1). The activated phenotype of NK cells is directly related to the progression of a disease. For example, there are phenotypic and functional differences that NK cells exhibit in patients with the immune reconstitution inflammatory syndrome compared with healthy individuals.58 The phenotype and cytotoxic function of NK cells are closely associated. Based on the experiment conducted to explore the extent to which NK cells in patients with cancers can be activated to kill cancer cells when stimulated by cytokines, NK cells have natural cytotoxicity that destroys cancer cells in cancer patients.59
Fig. 1.
Phenotypical and functional properties of NK cells. According to classical receptors (for example, adhesion molecules-CD56 and CD57, activating receptors-CD16, NCR, KIR and NKG2C, inhibitory receptors-NKG2A), NK cells are subdivided into regulatory and cytotoxic phenotypes and memory NK cells. Therefore, these subsets of NK cells appear to be functionally different
On the other hand, cytokine-mediated cytotoxicity is regulated by a balance between the inhibitory and activating receptors. NK cells’ natural cytotoxicity requires co-engagement of several activating receptors such as KIR2DSs.60 Stimulated NK cells, such as tumor-primed cells, are phenotypically different from resting NK cells and characteristic with upregulation of NK cells’ expression of the inhibitory receptors and/or downregulation of the activating receptors.59
Checkpoint receptors and ligands in NK cell dysfunction
As previously mentioned, NK cell function is regulated and maintained by the balance of inhibitory and activating receptors responsible for the preservation of the cell’s steady internal environment (cell homeostasis). In cancer microenvironments, the function of antitumor NK cells is affected by the decreasing expression of activating receptors and their ligands, as well as the increased expression of inhibitory immune checkpoint molecules; this facilitates the tumor immune escape (Figs. 2 and 3) and allows for the development of various cancer types.61–64 NK cell activation is primarily controlled by the dynamic balance between inhibitory and activating receptor signaling.65 As an example, NK cells express cell surface inhibitory receptors antagonizing to the activation pathways through protein tyrosine phosphatases.66 The nature of the typical inhibitory receptor, KIR, contributes to the recognition of self-antigens and subsequently provides negative signals that lead to the eventual suppression of NK cell activation through inhibition of the stimulatory signaling pathway. The ligand of the KIR is the major histocompatibility complex (MHC) class I molecule and is present in healthy cells. The inhibitory cell surface receptors are innately characterized by ITIM present in the cytoplasmic domains of various inhibitory receptors.66
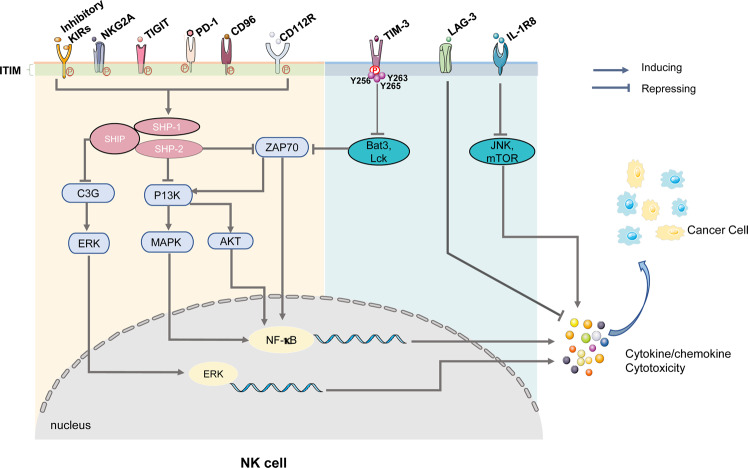
Fig. 2.
NK cell inhibitory receptor signaling is required for immune escape. Augmentation of inhibitory checkpoint NK cell receptor activation reduces the antitumor response in NK cells and facilitates the induction of tumor immune escape. In the tumor microenvironment, most of these receptors’ stimulation results in the phosphorylation of immunoreceptor tyrosine tail (ITT)-like motifs on their intracytoplasmic tails. After that, these immunoreceptor tyrosine-based inhibition motifs (ITIM) recruit phosphatases (such as Src homology domain-containing tyrosine phosphatase [SHP] and SH2 domain-containing inositol-5-phosphatase [SHIP]) that inhibit downstream signaling and ultimately suppress NK cell cytotoxic activity. Further research is necessary to understand better the signal mechanisms of these checkpoint receptors (including CD96, CD112R, and LAG-3). Nonetheless, studies have shown that these inhibitory signals attenuate NK cell cytotoxicity and antitumor cytokine/chemokine release, allowing tumor immune escape
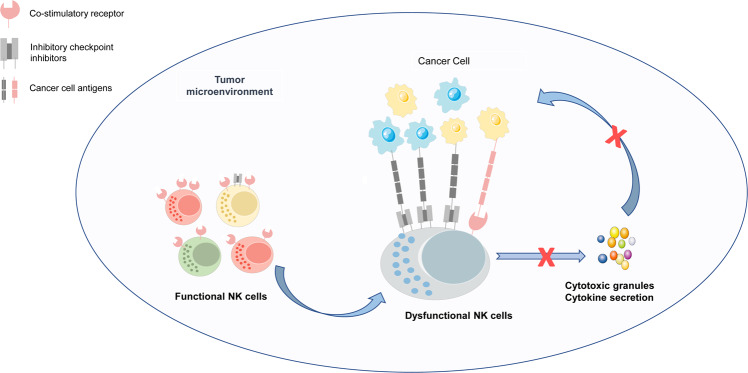
Fig. 3.
Schematic representation of a model of NK cell immune checkpoint receptors in tumor cell recognition. NK cell function is regulated and maintained by the balance of inhibitory and activating receptor signals. In the tumor microenvironment, NK cells transform to become dysfunctional cells with enhanced expression of inhibitory immune checkpoints and decreased expression of activating immune checkpoints. This imbalance of signaling transmission induces NK cell dysfunction and the extinction of antitumor immune response. Blockade of these inhibitory checkpoint molecules with the use of immune receptor inhibitors (ICI) (e.g., anti-KIR (lirilumab), anti-PD-1 (sintilimab and pembrolizumab), anti-NKG2A (monalizumab)), as well as activation of co-stimulatory receptor signaling by inducing co-stimulatory receptor expression (e.g., NKG2D ligand α3 domain-specific antibodies, cytokines (IL-2, IL-15, and IL-21), demethylating agents, histamine, anti-TGF-β monoclonal antibodies, TGF-β receptor inhibitors, TGF-β antisense oligonucleotides), can restore the antitumor activity of NK cells
Killer cell immunoglobulin-like receptor (KIR) family
As highlighted earlier, NK cells across development express inhibitory KIR receptors that are specific for MHC class I antigens in a variegated and stochastic manner.21,67 KIRs are divided into haplotypes A and B, where A is more frequent and mainly constituted of inhibitory receptors, while B contains both types of receptors with a predominance of the activating type.67 The repertoire of NK cell KIR is highly dependent on both HLA and KIR polymorphisms.21 The inhibitory KIRs inhibit NK cell activity through ITIM-based recruitment of protein tyrosine phosphatases (SHP-1 and SHP-2) responsible for dephosphorylation of the surrounding tyrosine kinases and the adapter proteins, which include DAP-10.67 As a diversified and polymorphic group of NK cell receptors that comprise inhibitory and activating KIRs, each member of the KIR family recognizes a particular HLA class I allotype from -A, -B, -C as the ligand.26 As an example, the inhibitory KIR2DL1, KIR2DL2, and KIR2DL3 affiliate with HLA-C as their ligand, whereas HLA-A and HLA-B serve as the ligands attached to other KIRs that include the inhibitory KIR3DL2 and KIR3DL3.68,69 Furthermore, the KIRs, ligands for KIRs, and MHC-I molecules exhibit high natural polymorphism.26
PD-1
In the context of cancer immunology, co-inhibitory signaling molecules are well described for T cells. One of the most notable is PD-1, also known as CD279. This receptor also occurs in NK cells and has been shown to reduce NK cell response.70 PD-1 is highly expressed on CD56dim NK cells in the peripheral blood of approximately one-quarter of healthy humans.71 In ovarian carcinoma, the CD56dim NK cells with high PD-1 expression have been found to have the phenotypic characteristics of fully mature NK cells with the NKG2A-KIR+CD57+ phenotype and are increased in patients.72 A recent study reported PD-1 expression by NK cells in the malignant pleural effusions of patients with primary mesothelioma or metastatic tumors, including lung cancer, intestinal adenocarcinoma, uterine cancer, breast cancer, and bladder carcinoma.73,74 Another recent study warned of the possibility of PD-1 leading promoting hyperprogression of cancer if amplified by PD-1 blockade,36 which can be the mechanism of how the receptor downregulates NK cell response.75,76
PD-1 binds to the ligands PD-L1 and PD-L2 to mediate inactivation of immune cells, including NK cells.44,77,78 Studies showed that PD-1/PD-L1 interaction in NK cells was required in MHC+ and MHC– tumors. In mouse lymphoma tumor models, PD-1 inhibits NK cell-dependent immune surveillance and facilitates the escape of tumor cells from the NK cell response.79–81 More so, PD-1 contains an extracellular variable immunoglobulin (IgV) domain, a transmembrane region, and an intracellular tail with two phosphorylation sites; one phosphorylation site is in an immunoreceptor tyrosine-based inhibitory motif, and the other is in an immunoreceptor tyrosine-based switch motif.72,82,83 To be more specific, Y248 (a PD-1 tyrosine-based switch motif) interacts with SHP-2, which is a requirement of the inhibition of proximal signaling molecules (including ZAP70, PI3K/AKT, C3G, and ERK) that consequently leads to the suppression of immune cell activation.79 In contrast, the activity of NK cells can be enhanced by histidine-rich glycoprotein, which modulates PD-1 expression via anti-C-type lectin-like receptor 1B.82,84 These findings provide potential mechanisms involved in the upregulation of PD-1 in the peripheral blood NK cells of patients with Kaposi sarcoma, NK cells from ovarian cancer ascites, and in the peripheral and tumor-infiltrating NK cells of patients with digestive cancer.76,85–89
TIGIT and CD96
Two additional inhibitory receptors, CD96 and T cell immunoreceptor with Ig and ITIM domains (TIGIT), bind to the DNAM-1 ligand and serve to oppose DNAM-1 function.90 TIGIT, also known as WUCAM and Vstm3, is an immune checkpoint molecule that inhibits the activation of T cells and NK cells.7,91–95 It contains an IgV domain, a transmembrane domain, and an immunoreceptor tyrosine-based inhibitory motif (ITIM).92 TIGIT has the capacity of disrupting DNAM-1 significantly through cis interactions to form heterodimers. Subsequent blockade of TIGIT with monoclonal antibodies augment the antitumor and antiviral activity of NK cells and T cells based on studies on mouse models.96,97 The expression of TIGIT plays a vital role in suppressing activation and maturation of NK cells.92,98–100 Therefore, TIGIT has a role in tumor immunosurveillance, similar to the role of the PD-1/PD-L1 axis during tumor immunosuppression.44
Studies have shown that the interaction of TIGIT with the poliovirus receptor (PVR) and poliovirus receptor-like 2 (PVRL2), also named CD112, Nectin-2, and PRR2, directly inhibits NK cell cytotoxicity.92,101,102 In addition, TIGIT has immunosuppressive effects, in that it competes with DNAM-1 for nectin-like ligands. An excellent example of the nectin-like ligand is CD155, the primary ligand for TIGIT. CD155 is expressed in many types of cancer cells.103 As highlighted, the intracellular domain of TIGIT consists of an immunoreceptor tyrosine tail (ITT) and ITIM.10 ITT–like motifs play a crucial role in inhibiting signals. The engagement of TIGIT with CD155 encourages its phosphorylation through the Src-family kinases Fyn and Lck; this results in the recruitment of SHIP-1, which in turn downregulates the PI3K, MAPK and NF-κB signaling pathways in modulating immune cell function.92,104,105 TIGIT can be readily detected on resting human NK cells but not on mouse NK cells. The engagement of TIGIT with CD155 prevents human NK cytotoxicity and cytokine production; this is made possible by counterbalancing DNAM-1 mediated activation, which can be reversed by antibody-mediated TIGIT blockade.106,107 The blockade of TIGIT makes NK cells resistant to inhibition by myeloid-derived suppressor cells.96,108 In like manner, a recent study showed that downregulated TIGIT expression inhibited the proliferation of colorectal cancer cells.37,109,110
CD96, also known as TACTILE (T cell activation, increased late expression), is a member of the immunoglobulin gene superfamily and an immune inhibitory receptor expressed on resting NK cells.111–115 The protein, CD96, facilitates adhesion of NK cells and T cells during immune responses.114 CD96 is similar to TIGIT, based on its competition with DNAM-1 for nectin and nectin-like ligands, and inhibits the activity of NK cells.116,117 The binding of CD96 to CD155 inhibits IFN-γ production by NK cells.111,118 Furthermore, studies of metastatic lung tumors in the mouse model demonstrated that the antibody-mediated blockade of CD96 promoted IFN-γ production by NK cells and improved the control of this cancer.111,119,120 The effect of antibody-mediated blockade of CD96 on NK cell function and its impact on human cancer patients remains unknown; thus, further study is needed to understand its potential as a targeted molecule for immunotherapy.
TIM-3
TIM-3 is in the TIM family of receptor proteins, all of which have similar structures. The extracellular regions of TIMs consist of a single membrane-bound distal IgV domain and a glycosylated mucin domain of variable length that is close to the membrane.12,121–123 The intracellular domain consists of a C-terminal cytoplasmic tail with five conserved tyrosine residues that interact with multiple components of the T cell receptor complex.124,125 TIM-3 is expressed on nearly all mature CD56dimCD16+ NK cells and is upregulated following stimulation with IL-12 and IL-18, and by IL-15 mediated NK cell maturation;126–128 this suggests that TIM-3 acts as a marker for NK cell maturation or activation, and TIM-3 cross-linking can inhibit NK cell-mediated cytotoxicity.129,130 Importantly, upregulation of TIM-3 has been found in gastric cancer, lung cancer, renal cancer, head and neck cancer, melanoma, schwannoma, follicular B-cell non-Hodgkin lymphoma, cervical cancer, prostate cancer, colorectal cancer, urothelial bladder carcinoma, esophageal cancer, and in the peripheral blood of patients with ovarian cancer.35,93,131–143 The co-expression of PD-1 and TIM-3 correlates with acute myelogenous leukemia progression, suggesting that TIM-3 has potential as a prognostic biomarker for various cancers.
Some of the cognate ligands for TIM-3 include galectin-9, phosphatidylserine, high mobility group protein 1, and carcinoembryonic antigen-related cell adhesion molecule 1.126,144–148 TIM-3’s cytoplasmic tail lacks a classical signaling motif and is replaced by five conserved tyrosine residues where some of them serve for signaling, and they include Y256, Y263, and Y265.148,149 The tyrosine residues signal through regulated interaction with HLA-B associated transcript-3 (Bat3). Bat3 binds to TIM-3 while in a steady-state, and sequentially recruits Lck, which is catalytically activated and promotes T cell signaling.150,151 Y256 and Y263 become phosphorylated, leading to the dissociation of Bat3; this, in turn, promotes T cell inhibition.150,151 TIM-3 is conservatively increased on both resting and activated T cells. In contrast, TIM-3 is only marginally increased on NK cells to oppose NK cell activation.152 Therefore, further research should better determine the role of TIM-3 in tumor surveillance to better understand its potential application in NK cell-mediated cancer treatment.
TIM-3 targeting effects do not affect non-tumor bearing tissues, although it does not seem to have any specificity.143 This remains one of the cancer research mysteries that although specificity remains a crucial factor in regulating immune responses in tissue.122,153,154 The upregulation of TIM-3 in several types of cancer raises concerns about the safety of all other tissues. However, research has shown that the safety of NK cells may be improved by irradiation.126,155 TIM-3 ligands expressed in tumor tissues, such as the Ceacam-1 and galectin-9. The effect of the TIM-3 blockade on tumor tissue is preferential.131,156 Therefore, targeting TIM-3 in a person with neck cancer will only affect other tissues if those tissues have some known ligands of the TIM-3 blockade.157 TIM-3 blockade is regulated by functional specification, a concept through which pathways such as the TIM-3 pathway sometimes regulate immune responses’ distinct features; thus, some other tissues in a patient with the neck cancer can remain unaffected by the targeting.134,156,157
CD112R
CD112R, also known as poliovirus receptor-related immunoglobulin domain protein (PVRIG), is a newly identified inhibitory checkpoint receptor in the PVR-like family. CD112R is constitutively expressed on mouse NK cells, natural killer T cells, and CD8+ T cells, and also expressed on human T cells and NK cells.101,158–160 Based on research findings, the expression of CD112R on CD16+ NK cells from human PBMCs was nearly equal to that on CD16− NK cells.160 CD112R was also highly expressed on NK cells from ovarian, endometrial, kidney, prostate, lung, and breast cancers.11 The receptor interacts with PVRL2 but not PVR (CD155) and competes with CD226 for PVRL2 binding to inhibit T cell and NK cell function.11,158,160 Human tumors with high PVRL2 expression and lower PVR expression are more likely to respond to CD112R blockade ex vivo.11
In the CD112R-deficient mouse TME of colon carcinoma and melanoma, increased CD8+ T cell effector function inhibited tumor growth compared with wild-type mice. Furthermore, it has been established that anti-CD112R antagonists combined with anti-PD-L1 could also reduce tumor growth or metastasis.155,158,161,162 In an ex vivo study on human tumor-infiltrating lymphocytes, CD112R blockade enhanced T-cell function, and in combination with TIGIT or PD-1 blockade, further enhanced the effect.11,163 However, its function in human NK cells is still inadequately known. In a preclinical study, it was established that the blockade of CD112R alone or together with TIGIT-blockade enhances the trastuzumab-triggered antitumor response in human NK cells and improves the efficacy of trastuzumab therapy for breast cancer.104,160,164 This finding suggests that CD112R on NK cells may be a potential target for breast cancer treatment in the future, although its efficacy and safety remain to be further verified through animal experiments and clinical trials.
IL-1R8
Interleukin-1 receptor 8 (IL-1R8) is also called a single immunoglobin IL-1-related receptor and is a member of the IL-1 receptor family. It has functional and structural characteristics that negatively regulate ILR and Toll-like receptor.75,165,166 IL-1R8 has been identified as a checkpoint protein in NK cells that regulate antitumor activity in solid cancers. The peripheral blood of Il1r8−/− mice proved to have an increase in NK cell number and maturity. The attenuation of IL-18 destroyed NK cell maturation in Il1r8−/− cells.75,167 The deficiency of IL-1R8 can also result in enhanced IL-18-dependent activation of the JNK and mTOR pathways essential for the control of NK cell differentiation, activation, and metabolism. NK cells with low IL-1R8 levels tend to sustain more extended activation after stimulation. These NK cells also indicate high antitumor immunity levels (like IFN-γ); whereas, small pro-inflammatory cytokine (CXCL1, IL-6, CCL2, IL-1β, and TNF-α) levels attribute to promoting tumor growth.142,167,168 Importantly, a genetic IL-1R8 blockade can result in NK cell-mediated resistance to hematogenous liver metastasis, hepatic carcinogenesis, and lung metastasis.108,167 Interventions to overcome this resistance are already in their various testing stages; chimeric antigen receptors (CARs) engineered NK cells are one of them that has been found to be sufficiently effective.169–173 These findings show that IL-1R8 is an NK cell checkpoint protein that may be inhibited to promote antitumor activity.
LAG-3
Structurally, LAG-3 is similar to CD4, yet it manifests a higher binding affinity to MHC class II molecules than CD4. It is expressed on activated T cells and NK cells.174 Another of its potential ligand is LSECtin, a member of the DCSIGN family.175,176 There is a high expression of LAG-3 in patients with Hodgkin lymphoma, acute myelocytic leukemia, and chronic lymphocytic leukemia.177–179 Its cytoplasmic tail is made up of three unique and conserved regions in mice and humans. It includes a serene phosphorylation site, a glutamic-acid proline (EP) repeats, and a KIEELE motif. Of the three, the KIEELE motif is the one that serves as the inhibitory function of LAG-3 in CD4+ cells.180 The effector function of T cells is inhibited by the engagement of LAG-3 and enhanced by the blockade of LAG-3.180,181 It is interesting to learn that LAG-3 is involved in the exhaustion of T cells as a result of pathophysiological basics; hence its combination with PD-1 synergizes to reinstate T cell function.182,183 The role of LAG-3, however, in regulating NK cell function is not yet clear and therefore calls for more research and investigation. NK cells from LAG-3 deficient mice show defects in the killing of specific cancer cells.184 It is worth noting that blocking the LAG-3 pathway with the use of LAG-3 antibodies or even soluble LAG-3 does not have any effect on the cytotoxicity of human NK cells.176,185 Even so, targeting LAG-3 may be useful in immunotherapy due to its influence on T cell and NK cell effector function.
NKG2A
NK group 2 member A (NKG2A) is a NK cell receptor of the NKG2 family, a type II membrane receptor that forms a heterodimer with CD94.16,28 It dimerizes with CD94 to form an inhibitory receptor that is related to C-type lectins and recognizes HLA-E (also known as MHC class I antigen E).28 These inhibitory receptors interact with MHC I ligands on target cells, leading to complete inhibition of NK cell granule polarization and the prevention of cytotoxic granule release.186 NKG2A contains two immunoreceptor tyrosine-based inhibition motifs (ITIMs) in its cytoplasmic tail. These ITIMs are phosphorylated following ligation of the ITIM-bearing receptor and facilitate this leads to the recruitment of tyrosine phosphatases, such as SH2 domain-containing phosphatase (SHP)-1 and SHP-2.187 The recruitment of SHP-1 by the ITIM-bearing receptors seems to inhibit the initiation of signaling in that it blocks most downstream signals in NK cells.187 The selective dephosphorylation of Vav1, the only protein detectably associated with the catalytic site of SHP-1, by SHP-1 leads to the inhibition of NK cell cytotoxicity, whereas the phosphorylation of an adapter protein CRK leads to the inhibition of NK cell function after ligation of NKG2A to HLA-E.188–190 Previous studies have shown that NK cells express NKG2A in cancers of the breast, cervix, liver, and lung.191–194 Importantly, tumor immunity increases when the inhibitory NKG2A receptor is blocked. The strong capability of NKG2A to suppress NK cells means that the blockade of NKG2A will effectively lead to the restoration of NK cells. For example, the blockade of NKG2A in an experiment using an anti-NKG2A antibody against NKG2A ligand in mice led to the effective alleviation of the functional impairment of NK cells.98,195 The prospective use of immune checkpoint inhibitors, as demonstrated by NKG2A, propels the development of cancer immunotherapy.42,196–198
Activating immune checkpoint receptors
Some additional co-stimulatory pathways are involved in regulating NK cell responses, which impede the development of cancer and contribute to cancer immunotherapy. For example, CD226, also known as PTA-1 or DNAM-1, is an activating immune receptor that is expressed on NK cells, which has cytolytic activity and functions in lymphokine secretion.116 It contains two Ig-like domains on its extracellular portion and has a cytoplasmic tail containing three tyrosine residues. CD226 is in the immunoglobulin superfamily and modulates NK cell functions by interacting with PVR, Nectin-2, CD96, and TIGIT.84,199–203 In murine models, downregulation of CD96 enhanced antitumor functions of CD226, thereby reducing lung metastases and tumor growth. These findings indicate that CD226 has potential as an activating checkpoint receptor for immunotherapy.
NKG2D is an activating immune receptor expressed on NK cells that can trigger cytotoxicity and is in the CD94/NKG2 family of C-type lectin-like receptors.200,204 NKG2D binds to its ligands that occur on the surface of tumor cells, such as stem-like tumor cells, and alters these cells to be more susceptible to immune destruction.205,206 Alternatively, tumor cells can evade immune surveillance by shedding soluble NKG2D ligands. Recent studies showed that NKG2D targeting might be a practical immunotherapeutic approach for the treatment of cancer.203,207,208 Inducing activating receptors and their ligands can be targeted by some potential strategies such as NKG2D ligand α3 domain-specific antibodies, cytokines (IL-2, IL-15, and IL-21), histamine, anti-TGF-β monoclonal antibodies, TGF-β receptor I kinase inhibitors, TGF-β antisense oligonucleotides demethylating agents (5-azacytidine and 5-aza-2’-deoxycytidine) (Fig. 3).209–216 In particular, targeting the NKG2D/soluble MIC, which is highly expressed in many malignant carcinoma cells (like melanoma and leukemia), maybe a potential approach for the treatment of these cancers.
NK cell as a promising therapeutic target for cancer
NK cells belong to the innate immune system and are active in the early fight against cancer through their ability to kill abnormal cells.101,217 They do not require previous stimulation and can induce an effective antitumor response that is mainly mediated by specialized cell-surface receptors, indicating malignancy.218 There is evidence from preclinical experiments and clinical trials for the development of NK cell-targeted immunotherapies (Tables 1 and 2) as a solution to chemo-resistant cancers, such as non-Hodgkin lymphoma.80,219 Monoclonal antibodies (mAbs) have been shown to elicit or augment prevailing antitumor immune responses. Their activity is referred to as ‘checkpoint blockade mAbs’ and is based on the principle of disturbing suppressive signals made by inhibitory receptors of lymphocytes. Recent methodologies have also been developed using the mAb-mediated blockade of definite NK cell immune checkpoints.48,52,74,157,220
Table 1.
Preclinical experiments in promising cancer target of immune checkpoints
| Immune checkpoint receptors | Treatment | Ligands | Signaling motif | Receptor adapter | Key results |
|---|---|---|---|---|---|
| Stimulating family | |||||
| NKG2D | NKG2D ligand α3 domain-specific antibodies, anti-NKG2A protein expression blockers (PEBLs) | MICA, MICB, and the ULBP family | YINM | DAP-10, DAP-12 | The α3 domain-specific MICA/B antibody stimulated NK cells to produce IFN-γ and TNF-α209 |
| KIR2DS/KIR3DS | Transfection of KIR2DS and DAP-12 | classical HLA class I | ITAM | DAP-12 | DAP-12 expression enhanced surface expression on NK cells and stability of KIR2DS225 |
| Inhibitory family | |||||
| KIRs | Transfection of KIR-Fc GL183, EB6, DF200 and Pan2D (NKVSF1) | classical HLA class I | ITIM | KIR and HLA class I ligand interactions modulate NK cell immunity and antitumor activity25,22,228 | |
| TIGIT | 13G6, SHIP-1 silencing, Y225A mutation | CD155, CD112, CD113 | ITIM/ITT | Blockade of TIGIT signaling prevents NK cell exhaustion and enhances potent antitumor immunity92,97,105 | |
| CD96 | 3.3 mAb, 6A6 and 8B10 | CD155 | ITIM/YXXM | Anti-CD96 mAbs promote NK cell anti-metastatic activity in CD115-dependent and CD115-independent manner120 | |
| LAG-3 | mAbs blocking FGL1/LAG-3 binding | MHC class II molecules, Fibrinogen-like Protein 1 | KIEELE | mAbs blocking FGL1/LAG-3 binding stimulates tumor immunity181 | |
| NKG2A | anti-NKG2A PEBLs | HLA-E | ITIM | Anti-NKG2A PEBL transduction inhibited NKG2A expression without affecting NK cell proliferation, and generated more potent cytotoxicity than an anti-NKG2A antibody. The NKG2Anull NK cells increased NK cell killing of HLA-E–expressing tumors in vivo43 | |
| TIM-3 | mAbs blocking TIM3-Fc binding to Gal-9, phosphatidylserine, Ceacam-1 | Gal-9, phosphatidylserine, HMGB1, Ceacam-1 | Tyrosine | Anti-Tim-3 antibodies inhibited Tim-3 signaling to improve the proliferation and cytotoxic activity of CD8+ TILs and reduce tumor-promoting cytokines production146,147 |
Table 2.
Clinical trials targeting NK cell-inhibitory checkpoints in cancer treatment
| Target | Agent | Clinical trials number | Phase | Status | Combination immunotherapy | Combination immunotherapy target | Tumor |
|---|---|---|---|---|---|---|---|
| TIGIT | MTIG7192A | NCT02794571 | I | Recruiting | Atezolizumab | PD-L1 | Advanced/metastatic tumors |
| NCT03563716 | II | Active, not recruiting | Atezolizumab | PD-L1 | NSCLC | ||
| BMS-986207 | NCT02913313 | I/II | Active, not recruiting | Nivolumab | PD-1 | Broad solid tumor | |
| MK-7684-001 | NCT02964013 | I | Recruiting | Pembrolizumab | PD-1 | Advanced solid tumors | |
| AB154 | NCT03628677 | I | Recruiting | Zimberelimab | PD-1 | Advanced solid tumor | |
| ASP8374 | NCT03260322 | I | Recruiting | Pembrolizumab | PD-1 | Advanced solid tumors | |
| TrasGEX™ | NCT01409343 | I | Completed | Solid tumors | |||
| LAG-3 | Relatlimab | NCT03642067 | II | Recruiting | Nivolumab | PD-1 | MSS colorectal adenocarcinomas, colorectal adenocarcinoma |
| NCT03743766 | II | Recruiting | Nivolumab | PD-1 | Metastatic melanoma | ||
| NCT03607890 | II | Recruiting | Nivolumab | PD-1 | Refractory MSI-H solid tumors prior of PD-(L)1 therapy, MSI-H tumors | ||
| NCT03724968 | II | Active, not recruiting | Nivolumab, Ipilimumab | PD-1, CTLA-4 | Metastatic melanoma stratified by MHC-II expression | ||
| NCT03044613 | I | Recruiting | Nivolumab | PD-1 | Gastric cancer, esophageal cancer, GEJ cancer | ||
| NCT03459222 | I/II | Recruiting | Nivolumab, Ipilimumab, BMS-986205 | PD-1,CTLA-4, IDO1 | Advanced cancer | ||
| NCT01968109 | I/II | Recruiting | Nivolumab, BMS-986213 | PD-1 | NSCLC, gastric cancer, HCC, RCC, bladder cancer, SCCHN, melanoma | ||
| NCT02966548 | I | Recruiting | Nivolumab | PD-1 | Advanced solid tumors | ||
| NCT02996110 | II | Recruiting | Ipilimumab, Nivolumab, BMS-986205 | CTLA-4,PD-1,IDO1 | Advanced RCC | ||
| NCT02935634 | II | Recruiting | Ipilimumab, Nivolumab, BMS-986205 | CTLA-4,PD-1,IDO1 | Advanced gastric cancer | ||
| NCT02750514 | II | Active, not recruiting | Ipilimumab, Nivolumab, BMS-986205, Dasatinib | CTLA-4,PD-1,IDO1,LAG3 | Advanced NSCLC | ||
| NCT03335540 | I | Recruiting | Nivolumab, Cabiralizumab, Ipilimumab, IDO1 Inhibitor | PD-1,CSF-1R, CTLA-4,IDO1, KIR2DL1/2/3 | Advanced cancer | ||
| NCT03704077 | II | Withdrawn | Nivolumab | PD-1 | Gastric cancer, GEJ adenocarcinoma | ||
| NCT03623854 | II | Recruiting | Nivolumab | PD-1 | Advanced chordoma | ||
| NCT03470922 | II/III | Recruiting | Nivolumab | PD-1 | Advanced melanoma | ||
| IMP321 | NCT02676869 | I | Completed | Pembrolizumab | PD-1 | Stage IV or III melanoma | |
| NCT02614833 | II | Active, not recruiting | Adenocarcinoma breast stage IV | ||||
| NCT03252938 | I | Active, not recruiting | Avelumab | PD-L1 | Solid tumors, peritoneal carcinomatosis | ||
| NCT03625323 | II | Recruiting | Pembrolizumab | PD-1 | NSCLC, SCCHN | ||
| NCT00349934 | I | Completed | Metastatic breast cancer | ||||
| NCT00351949 | I | Completed | Stage IV RCC | ||||
| XmAb®22841 | NCT03849469 | I | Recruiting | Pembrolizumab (Keytruda®) | Melanoma, Cervical carcinoma, Pancreatic carcinoma, TNBC, HCC, Urothelial carcinoma, SCCHN, Nasopharyngeal carcinoma, RCC, NSCLC, SCLC, Gastric or GEJ adenocarcinoma, Advanced or metastatic solid tumors, Prostate carcinoma, MSI-H, Mismatch repair deficiency, Epithelial ovarian cancer, Fallopian tube cancer, Primary peritoneal carcinoma, Intrahepatic cholangiocarcinoma | ||
| TSR-033 | NCT03250832 | I | Recruiting | Dostarlimab | PD-1 | Advanced solid tumors | |
| Sym022 | NCT03489369 | I | Completed | Metastatic cancer, Solid tumor, lymphoma | |||
| REGN3767 | NCT03005782 | I | Recruiting | Cemiplimab (REGN2810) | PD-1 | Advanced malignancies, including lymphoma | |
| MK4280 | NCT03598608 | I/II | Recruiting | Pembrolizumab | Hodgkin disease, non-Hodgkin lymphoma, B-cell lymphoma | ||
| NCT02720068 | I | Recruiting | Pembrolizumab | PD-1 | Advanced solid tumors | ||
| MGD013 | NCT03219268 | I | Recruiting | Advanced solid tumors, Hematologic neoplasms, Gastric Cancer, Ovarian cancer, GEJ cancer, HER2-positive breast cancer, HER2-positive gastric cancer, DLBCL | |||
| LAG525 | NCT03365791 | II | Active, not recruiting | PDR001 | PD-1 | SCLC, Gastric adenocarcinoma, Esophageal adenocarcinoma, Castration-resistant prostate adenocarcinoma, Soft tissue sarcoma, Ovarian adenocarcinoma, Advanced well-differentiated neuroendocrine tumors, DLBCL | |
| NCT02460224 | I/II | Active, not recruiting | PDR001 | PD-1 | Advanced solid tumors | ||
| NCT03499899 | II | Active, not recruiting | PDR001 | PD-1 | Advanced TNBC | ||
| NCT03742349 | I | Recruiting | Spartalizumab (PDR001), NIR178, MCS110, | PD-1, Adenosine A2A receptor, M-CSF | TNBC | ||
| INCAGN0 2385 | NCT03538028 | I | Recruiting | Cervical cancer, MSI-H endometrial cancer, Gastric cancer (including GEJ), Esophageal cancer, HCC, Melanoma (uveal melanoma excluded), Merkel cell carcinoma, Mesothelioma, MSI-H colorectal cancer, NSCLC, Ovarian cancer, SCCHN, SCLC, RCC, TNBC, Urothelial carcinoma, DLBCL | |||
| FS118 | NCT03440437 | I | Active, not recruiting | Advanced cancer, Metastatic cancer | |||
| BI 754111 | NCT03780725 | I | Recruiting | BI 754091 | PD-1 | NSCLC, Head and neck neoplasms | |
| NCT03156114 | I | Recruiting | BI 754091 | PD-1 | Advanced solid tumors, NSCLC | ||
| NCT03433898 | Early Phase I | Recruiting | BI 754091 | PD-1 | Advanced solid tumors | ||
| NCT03697304 | II | Suspended | BI 754091 | PD-1 | Advanced and/or Metastatic solid tumors | ||
| NKG2A | Monalizumab | NCT02921685 | I | Recruiting | Hematologic malignancies | ||
| NCT02671435 | I/II | Active, not recruiting | Durvalumab | PD-L1 | Advanced solid tumors | ||
| NCT02643550 | I/II | Recruiting | Anti-PD(L)1 | PD(L)1 | Recurrent or metastatic SCCHN | ||
| NCT02459301 | I | Completed | Gynecologic cancer | ||||
| TIM-3 | TSR022 | NCT02817633 | I | Recruiting | Nivolumab, TSR-042, TSR-033 | PD-1, LAG-3 | Advanced or metastatic solid tumors |
| NCT03680508 | II | Recruiting | TSR-042 | PD-1 | Locally advanced or metastatic liver cancer | ||
| Sym023 | NCT03489343 | I | Active, not recruiting | Metastatic cancer, solid tumor, lymphoma | |||
| NCT03311412 | I | Recruiting | Sym021, Sym022 | PD-1, LAG-3 | Metastatic cancer, solid tumor, lymphoma | ||
| SHR-1702 | NCT03871855 | I | Not yet recruiting | Camrelizumab | PD-1 | Advanced solid tumor | |
| RO7121661 | NCT03708328 | I | Recruiting | Solid tumors, Metastatic melanoma, NSCLC, SCLC, Esophageal squamous cell carcinoma | |||
| MBG453 | NCT03066648 | I | Recruiting | PDR001 | PD-1 | AML, high risk MDS | |
| NCT02608268 | I/II | Recruiting | PDR001 | PD-1 | Advanced solid tumor | ||
| LY3321367 | NCT03099109 | I | Active, not recruiting | LY3300054 | PD-L1 | Advanced relapsed/refractory solid tumor | |
| INCAGN2390 | NCT03652077 | I | Recruiting | Cervical cancer, Gastric cancer, GEJ cancer, Esophageal cancer, HCC, Melanoma, Uveal melanoma, Merkel cell carcinoma, Mesothelioma, MSI, NSCLC, Ovarian cancer, SCCHN, SCLC, RCC, TNBC, Urothelial carcinoma, Mismatch repair deficiency | |||
| BMS-986258 | NCT03446040 | I/II | Recruiting | Nivolumab | PD-1 | Advanced cancer | |
| BGB-A425 | NCT03744468 | I/II | Recruiting | Tislelizumab | PD-1 | Locally advanced or metastatic solid tumors | |
| NKG2D | CYAD-101(NKR-2) | NCT03692429 | I | Recruiting | Colorectal cancer | ||
| NCT03466320 | I/II | Recruiting | AML, Adult MDS | ||||
| NCT03370198 | I | Active, not recruiting | Colon cancer liver metastasis | ||||
| NCT03310008 | I | Active, not recruiting | Colon cancer liver metastasis | ||||
| NCT03018405 | I/II | Recruiting | AML, MDS, Multiple myeloma |
MSS microsatellite stable, NSCLC non-small cell lung cancer, MSI-H microsatellite instability high, GEJ gastroesophageal junction, SCLC small cell lung cancer, SCCHN squamous cell carcinoma of the head and neck, DLBCL diffuse large B-cell lymphoma, HCC hepatocellular carcinoma, RCC renal cell carcinoma, TNBC triple-negative breast cancer, AML acute myeloid leukemia, MDS myelodysplastic syndromes
The restoration of NK cell activity against HLA-I+ tumor cells is essential in the therapeutic targeting for novel immunotherapies that depend on therapeutic monoclonal antibodies, such as anti-pan-KIR2D.67,221,222 In the absence of infection, inhibitory HLA-KIR signals dominate and protect tumor cells from NK cell-mediated lysis, while intracytoplasmic binding of KIR2DL or KIR3DL with ITIMs prevent NK cell lysis activity.68,223 Conversely, KIR2DS and KIR3DS are noncovalently associated with an ITAM–bearing adapter molecule DAP-12, which results in phosphorylation of tyrosine residue in the ITAM and recruiting ZAP-70 or Syk, leading to cellular activation and increases the capacity of NK cells to recognize tumor cells; this is an essential target for cancer treatment.26,224,225 KIR has been established to play a critical role in mediating self-tolerance along with the facilitation of cytotoxicity against infected cells and transformed cells. According to research, KIR-HLA relationships can be linked to the incidence and course of both solid tumors and hematologic malignancies.67,226 The inhibitory KIR-HLA relationships have been established as being overrepresented in patients with acute and chronic leukemia, breast cancer, Hodgkin lymphoma, and melanoma.226 The assertion can be understood based on matched autologous inhibitory KIR-HLA interactions preventing the lysis of cancerous target cells.227 Autologous NK cell therapy, which is a novel expansion method and will help patients at the advanced stage of digestive cancer, is in phase I trial.227 As part of targeting cancer therapies, the expression of activating KIRs in different circumstances can be linked to better outcomes in some malignancies.26 KIR-ligand modulation is vital for cancer therapy as exemplified by the initial therapeutic manipulation of KIR-ligand relationships in the case of T cell-depleted, haploidentical hematopoietic stem cell transplantation for hematologic malignancies. The inhibitory KIR-ligand relationship has been established as important in the realization of NK cell alloreactive potential with special attention on the balance of activating and inhibitory signals that direct cytotoxicity.26,228
Preclinical studies and clinical trials of PD-1 blockade therapy have led to impressive results in mediating tumor eradication. As discussed, NK cells secrete pro-inflammatory cytokines that promote the expression of PD-L1 in tumor cells and enhance the inhibiting role of PD-1. At the same time, PD-1 antibodies can bind to NK cell surface PD-1, prevent NK cell depletion, and enhance NK cell antitumor response.229,230 Pidilizumab (previously CT-011) is an anti-PD-1 antibody that recently entered clinical trials. Pidilizumab can enhance human NK cell activity against autologous primary multiple myeloma cells.4,231–233 In addition, highly dense activated human primary NK cells can kill colorectal carcinoma cells grown in 3D cultures independent of PD-L1 expression, suggesting that the use of allogeneic activated NK cells could be an effective treatment of this cancer.38,77,93,234–236 Immune evasion via PD-1/PD-L1 in NK cells and monocytes/macrophages is prominent in Hodgkin’s lymphoma.38,77,93,234–237 Also, one of the immune checkpoint molecules that have already been employed in specific preclinical tumor models is the establishment of efficacy in blocking TIGIT to modulate NK cell function and has resulted in improved clinical responses in patients with cancer.7,94,95
There are ongoing phase I/II trials of the application of NK cells combined with PD-1 antibodies (sintilimab and pembrolizumab) in the treatment of patients with advanced non-small-cell lung cancer (NCT03958097) and advanced biliary tract cancer (NCT03937895). Thus, there are many ongoing studies of immunotherapies for PD-1 blockade by targeting NK cells in cancer treatment.
Lirilumab targeting pan-KIR2D and monalizumab targeting NKG2A have been demonstrated to disrupt the interactions of inhibitory KIR on NK cells with classical HLA class I-peptide complex on tumor cells or NKG2A with nonclassical HLA-I molecular HLA-E, which could unleash the antitumor NK cell cytotoxic activity mimicking “missing-self” response.16,69,238 Both lirilumab and monalizumab are safe with limited side effects upon prolonged treatment in phase I clinical trials.237 Markedly, all dosages of lirilumab induced high levels of free KIR2Ds (>95%).237 These agents are currently undergoing phase I/II clinical trials across a range of hematologic and solid tumors in monotherapy or combination with other immune checkpoint blockades, including PD-1 inhibition (NCT02671435 and NCT02643550).239
The role of NK cells in cancer therapy also promotes the activating receptor CD226, which is a mediator of the cells’ responses against tumors. Although, the mechanism by which the CD226 receptors exert control over the function of NK cell is a challenge, the engagement of the receptor with CD155 in the transcription factor FOXO1 phosphorylation, which results in the inactivation of the receptor’s negative regulatory control over the effector function of NK cell.226,240
As discussed, the activated NKG2D receptor is a critical NKG2 family receptor on NK cells and can bind to NKG2D ligands expressed on tumor cells. This binding allows NK cells to activate and destroy tumor cells, as well as produce cytokines. The NKG2D/NKG2D ligand axis has been recognized to play an essential role in antitumor activity. Infusions of activated NK cells can scavenge circulating soluble MICA in cancer patients, leading to reinstate NKG2D-mediated immune surveillance.235 Although our understanding of the control of NKG2D ligand expression remains limited, research in recent years revealed various cellular mechanisms by which cancer cells evaded detection by reducing the “stress-induced ligands” expression. Recently, NKG2D ligand (MICA and MICB) α3 domain-specific antibodies were developed to restore NK cell-mediated tumor immunity by increasing the density of stimulatory MICA and MICB ligands on the surface of tumor cells along with reducing the shedding of both MICA and MICB.236 Therefore, targeting co-stimulatory receptor signaling may serve as an alternative strategy to boost NK cell function and prove beneficial for cancer treatment (Fig. 3).
The difference between the current treatment strategies and the promising immune checkpoint molecules such as TIGIT in NK cells is that they can be modulated to enhance clinical responses. The diverse receptors expressed on the tumor-infiltrating surfaces of NK cells would increase the binding of tumors once activated and destroy them.121 The establishment of the receptors, especially the IL-1R2 and IL-1R8, which are effective immune regulators, will improve immune surveillance and control.165 Single immunoglobulin IL-1R-related receptor, SIGIRR, also known as TIR8 or IL-1R8, PD-1, LAG-3, CTLA4, and TIM-3 has distinct functional and structural characteristics that adapt it better to regulate the expression of NK cells in a TME.13,241 Other receptors that can complement the role of NK cell subsets to increase their prediction of malignancy are TIM-3 and IL-15.154,242
Given different cancer types and tumor staging, it is hard to say which is better for restoring NK cell-mediated tumor immunity between targeting the NKG2D receptor and targeting KIR or NKG2A. Although one recent in vitro study found blocking the inhibitory signal (blocking of HLA–KIR) is not so efficient as the activating signal increases (Ara-C treatment that induced NKG2D ligands) potentiate NK cell responses against acute myelocytic leukemia (AML) cells, more in vitro and in vivo studies are needed to demonstrate it. However, this study provides evidence that a combination of both treatments has better efficiency.243
Recently, adoptive cell therapy strategies, including cancer-targeting CARs-engineered NK cell infusion have been explored in preclinical and clinical studies.169–173 Chang et al. demonstrated a chimeric receptor with the NK cell-activating molecule NKG2D, plus two vital signaling molecules, DAP-10 and CD3ζ, enhanced the antitumor effect of peripheral blood NK cells on osteosarcoma.169 More recently, human induced pluripotent stem cells (iPSCs) have been proven to be more efficient in genetically modifying and expressing CAR constructs specifically designed to enhance NK cell antitumor activity.244 To date, CAR engineered NK cells, which are from autogenic or allogeneic peripheral blood mononuclear cell, umbilical cord blood, human embryonic stem cell, human iPSC, and NK cell lines,244–254 are at the beginning but greatly promising in tumor immunotherapy.
The increasing resistance of several cancers to chemotherapy demands a clinical strategy to increase immune checkpoint molecules’ effectiveness. Findings from a study indicated that the elimination of regulatory T cells (Treg) using IL-2–diphtheria fusion would have a therapeutic benefit if haploidentical NK cells are infused in AML patients. The study shows that Treg cells have a negative role in population expansion of NK cells following transfer. In this first clinical trial, an administration of recombinant IL-15 clearing lung cancer lesions in metastatic cancer patients astatic further supports the effectiveness of immune checkpoint molecules in NK cells as target cancer immunotherapy.255 Combined therapy is emerging more effective strategy for treating cancer. Although targeting NK cells is a leading intervention cancer immunotherapy through their direct lysis of tumor cells, the intervention’s effectiveness is reduced by immune evasion.256–258 Combination therapy provides an alternative strategy for increasing the effectiveness of NK cells’ antitumor ability.55 One of the target combinations is NK cells with nanomaterials and oncolytic viruses. TIGIT combined with CD112 and CD155; blocking CD96 is considered another promising therapeutic role.259 Research findings have proved that a combination of TIGIT blockade and anti-PD-1/PD-L1 antibodies forms a synergy that may be used in preclinical trials.156 Other findings from a recent study indicated that the checkpoint receptor TIGIT blockage prevents the exhaustion of NK cell and triggers the elicitation of T-cell immunity that are potent tumor-specific in a NK cell-dependent manner.19,260 A combination of engineering NK cells with specific immune checkpoint signaling domains also provides a promising therapeutic potential for the treatment of recurrent and refractory cancer.
Study and progress of adenosine in the NK cell cancer immunotherapy
To complement the general role of control and surveillance of malignant cells that the immune system play, the development of adoptive cellular therapy and checkpoint blockade has brought about a revolution in the landscape of cancer therapy and promises the potential in which oncologists can utilize a patient’s immune system to treat cancer.185,261–263 Purinergic signaling axis has gained prominence as one of the mechanisms of tumor-mediated immunosuppressions, in which the purine nucleoside adenosine produced in the TME has the ability of potently suppressing NK cell function.264–266 During this event, the production of extracellular adenosine is triggered by the mediation of the cell surface ectoenzymes CD38, CD39, and CD73,267–269 and the development of therapeutic agents has focused on targeting these enzymes as responses.270 The therapeutic agents also target receptors, including the downstream adenosine receptors (AR) such as A1R, A2BR, A2AR, and A3R, to promote antitumor immune responses.16,264,271
In NK cells, the signaling of adenosine (ADO) signals via A2AR suppresses their cytokine production and cytotoxicity function.272,273 ADO signaling plays a critical role in immune regulation, as further underscored by the NK cells total dysfunction in individuals presenting with a variant of severe combined immunodeficiency caused when adenosine deaminase (ADA) undergoes a mutation that catalyzes ADO conversion to inosine.262,274,275 Also, the expression of CD38 on tumors, T cells, and NK cells promote the generation of ADO followed by proliferation and suppression of T cell function. In addition to suppressing T cell responses, cAMP has an overall inhibitory effect on NK cells.264,276–278
NK cells express high A2AR levels, specific A2AR agonists, or ADO suppressed NK cells due to their cytokine production and cytotoxic function.142,279–281 Intracellular cAMP concentration enhancement mediated by A2AR is believed to be the predominant mechanism explaining how ADO suppresses NK cell activity (Fig. 4).264,282,283 Like T cells, NK cells express the A3R, the function of which has been found to regulate A3R positively and NK cells, resulting in metastatic and primary tumor growth both in human and mouse models of melanoma and colon cancer.284
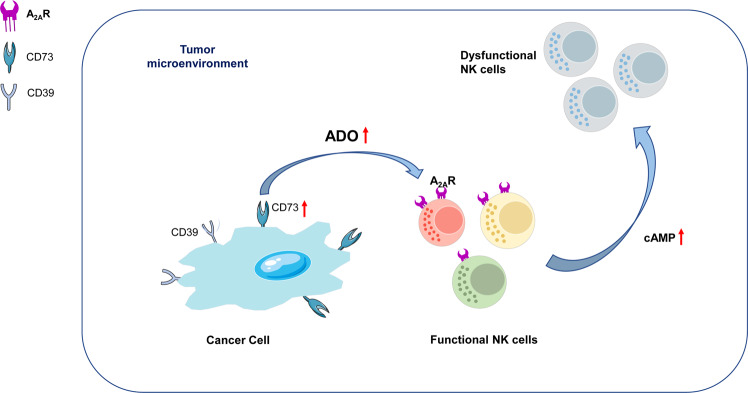
Fig. 4.
Adenosine-axis in the tumor microenvironment mediates immune suppression of NK cells. In the tumor microenvironment, stress such as hypoxia increases extracellular ATP levels and CD73 expression on cancer cells, leading to adenosine (ADO) upregulation and the activation of downstream signaling through A2AR on NK cells. ADO/A2AR signaling results in dysfunction of NK cell metabolic and effector functions
There are several trials on approaches involving a combination of CD38 with anti-PD-1 that have been found to stimulate enhanced antitumor responses by T cell-mediated by enhanced Granzyme B and IFN-γ expression by CD8+ T cells.79,264,285 Findings from another study showed that A2AR cells had enhanced the penetration in hypoxic tumors.286 Although the focus of most of the combination approaches indicates the possibility of ADO targeting aimed at enhancing T cell responses, ADO axis targeting, including NK cells, can enhance other immune subsets comprised in the TME.287 The accumulation of ADO predominantly in the TME when the extracellular ATP is catabolized to ADO by CD38, CD39, and CD73 is expressed in immune and tumor cells, and it has been reported that ADO mediates the suppression of antitumor immunity when the ADO receptors.185,268
Extracellular ADO production in the TME
TME forms site for high ADO concentrations caused by inflammation, tissue disruption, and contribution of stromal and immune cells.288,289 Hypoxia is another predominant driver of increased ADO concentration through stimulation of large ATP formation.290 It also contributes to the expression of HIF-1α, a well-defined transcription factor, which stimulates the expression of ectoenzymes CD73 (5’-NT) and CD39 (NTPDase1) on stromal cells, tumor cells, as well as tumor-infiltrating immunosuppressive cell subsets, for example, myeloid-derived suppressor cells and Treg cells.242,291 Part of the evidence to support the role of CD39 and CD73 in tumorigenesis and inflammation is that mice deficient in CD73 or CD39 are susceptible to autoimmunity/inflammation and be tumor resistant as a result of ADO-mediated immunosuppression alleviation.292–294
Targeting the adenosine pathway in TME to improve immunotherapies
Cancer immunotherapies are currently considered the fourth pillar in the treatment of cancer.295 Several research studies on the role of ADO negatively regulating NK cell responses through A2AR, and naturally targeting the ADO pathway are part of cancer immunotherapy interventions that may further boost the efficacy of approved immunotherapies in the clinic.35,267,296,297 Although ADO has been found to thwart the antitumor immune responses that radiotherapy elicits, when ADO receptors are antagonized in cancers, the approach allows the interruption of the ADO-dependent immune evasion that results in antitumor immune responses.298 The immunosuppressive role of NK cells, combination strategies rationally targeting the pathway with adoptive cell therapies (ACT), and checkpoint inhibitors can synergistically promote the function of antitumor immune cell function, a process that utilizes ATP generated in the TME (Fig. 4) (Table 3).271,299,300
Table 3.
Clinical evaluation targeting the adenosine pathway
| Molecular target | Clinical trial number | Study phase | Cancer type | Agents | Combination |
|---|---|---|---|---|---|
| A2AR | NCT03207867 | II | Solid tumors and non-hodgkin lymphoma | NIR178 | PDR001 (anti-PD-1) |
| A2AR | NCT02403193 | I/II | Non-small cell lung cancer (NSCLC) | PBF-509 | PDR001 (anti-PD-1) |
| A2AR | NCT02655822 | I | Advanced cancers | Ciforadenant | Atezolizumab (anti-PD-L1) |
| A2AR | NCT04266665 | IV | Glioma | Dexmedetomidine | Craniotomy |
| A2AR | NCT04280328 | I | Relapsed or refractory multiple myeloma | CPI-444 | Daratumumab (anti-CD38) |
| A2AR and A2BR | NCT04262856 | II | Non-small cell lung cancer (ARC-7) | AB928 | Zimberelimab (anti-PD-1), AB154 (anti-TIGIT) |
| A2AR and A2BR | NCT03629756 | I | Advanced malignancies | AB928 | AB122 (anti-PD-1) |
| A2AR and A2BR | NCT03719326 | I | Triple-negative breast cancer or gynecologic malignancies | AB928 | Multiple drug combinations |
| A2AR and A2BR | NCT03846310 | I | NSCLC | AB928 | Zimberelimab (anti-PD-1), Pembrolizumab (anti-PD-1), Carboplatin, Pemetrexed |
| A2AR and A2BR | NCT03720678 | I | Advanced metastatic gastroesophageal Cancer (GEC) or colorectal cancer (CRC) | AB928 | mFOLFOX |
| A2AR, A2BR, and CD73 | NCT04381832 | I/II | Metastatic castrate-resistant prostate cancer (mCRPC) | AB928, AB680 | Zimberelimab (anti-PD-1), Enzalutamide (androgen receptor inhibitor), Docetaxel |
| A2AR, CD73 | NCT02111330 | I | NSCLC | AZD4635, MEDI9447 | Osimertinib (EGFR Kinase inhibitor) |
| A2AR, CD73 | NCT04089553 | II | Prostate cancer | AZD4635, Oleclumab | Durvalumab (anti-PD-1) |
| A2AR, CD73 | NCT03549000 | I | Advanced cancers | NIR178, NZV930 | PDR001 (anti-PD-1) |
| A2BR | NCT03274479 | I | NSCLC | PBF-1129 |
Limitations of immune checkpoint molecules in NK cell for cancer immunotherapy
Age is the main contributor to NK cell altered expression of checkpoint molecules in NK cells.47,301 Activating receptors alteration has been associated with chronic exposure of the receptors to ligands on tumor cells.302 There is also a synergistic effect between age and cancer that diminishes NK cell-mediated tumor immunosurveillance, which may also be associated with NK cell immunoresistance.257,303 However, oncologists have made significant successes in developing different strategies to harness the power of NK cells aimed at targeting tumor cells, such as adoptive transfer therapy, which involves allogeneic or autologous expanded NK cells.2,304
Future directions to study the NK cells in cancer immunotherapy
Although numerous trials have been carried out on various innovations on cancer therapy, cancer treatment is still a significant challenge. Proposals by academia, such as identifying allogeneic NK cells as one of the most effective cancer treatment options, have not made much progress in identifying a cancer therapy applicable to a majority of cancers without issues such as NK cell receptor downregulation.305 In my opinion, leaving these studies the academia and biotech companies would not bring a solution any time soon. I propose that the World Health Organization developed governments and other non-governmental organization fund multi-disciplinary groups and organizations in the healthcare field to conduct researches aimed at both preventive and curative recommendations for cancer therapy, especially for stage I to III treatment strategies for non‐small cell lung cancer, in which radiotherapy and surgery are usually employed.306 Therefore, considering combinatorial approaches drawing together different treatment strategies that involve NK cell functions would be worthwhile. NK cell research companies should consider developing NK cell-specific treatments underlying scientific findings and principles of their best product pipelines, demonstrating highly innovative concepts and practices that herald future sustainable and cost-effective clinical applications.
Since the role of NK cell-based immunotherapy strategies has gained immense support as a proven intervention against cancer, I advise that everyone should not consider it as the bottom-line solution based on which all future research will focus. NK cells can only be effective in a few types of cancer, considering their complex networking, heterogeneity, as well as the inherent adaptability of a wide range of tumors that evade destruction by immune cells. In the meantime, future studies should focus resources on improving the efficacy of NK cell products that are currently available.94 The discoveries of all NK cell-based immunotherapies should proceed to clinical implementation.306
Conclusion
Human NK cells are essential and play a significant role in the development of therapeutic treatments for cancer. In the tumor microenvironment, NK cells transform to become dysfunctional cells with enhanced expression of inhibitory immune checkpoints, including the non-HLA-class I-specific inhibitory receptors (such as PD-1, TIGIT, CD112R, CD96, IL-1R8, and TIM-3) and HLA-class I-specific inhibitory receptors (such as KIR, NKG2A, and LAG-3), as well as decreased expression of activating receptors (such as NKG2D and CD226). Adenosine-A2AR signaling is emerging as a novel inhibitory immune checkpoint pathway in NK cells. Blockade of these inhibitory checkpoint molecules with the use of immune receptor inhibitors, as well as activation of co-stimulatory receptor signaling by inducing co-stimulatory receptor expression, can reinstate the antitumor activity of NK cells. Many primary researches support the efficacy of NK cell-targeted immunotherapies based on immune checkpoint molecules in NK cells at preclinical phase and are ongoing at different clinical phases. In addition, more research is needed on the safety, tolerability, and clinical efficacy of drugs targeting these checkpoints and their combination therapy. There is a need to identify highly effective strategies of overcoming immune resistance, the main challenge in cancer immunotherapy, such as through CAR engineered NK cells. Above all, an in-depth understanding of immune checkpoint molecules that target NK cells could help develop new therapeutic strategies against refractory cancers.
Supplementary information
Acknowledgements
This review was funded by the National Natural Science Foundation of China (No. 81400659, 81974377), Natural Science Fund of Liaoning Province (No. 2017225032, 20180551193, 2020-MS-181), Shenyang Science and Technology Project (No. 17-230-9-16), and the 345 Talent Project of Shengjing Hospital (No. 40B).
Author contributions
Conceptualization: C.D., R.S., and F.X.; writing—original draft preparation: Y.C. and X.W.; writing—review and editing: T.J., Y.T., C.W., R.S., and F.X.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Yuqing Cao, Xiaoyu Wang
Contributor Information
Rui Song, Email: rsong01@llu.edu.
Feng Xu, Email: xuf@sj-hospital.org.
Supplementary information
The online version of this article (10.1038/s41392-020-00348-8) contains supplementary material, which is available to authorized users.
References
- 1.Mandal A, Viswanathan C. Natural killer cells: In health and disease. Hematol. Oncol. Stem Cell Ther. 2015;8:47–55. doi: 10.1016/j.hemonc.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Seki, S. et al. The liver as a crucial organ in the first line of host defense: the roles of Kupffer cells, natural killer (NK) cells and NK1.1 Ag+ T cells in T helper 1 immune responses. Immunol. Rev.174, 35–46 (2000). [DOI] [PubMed]
- 3.Muraro, E. et al. Improved Natural Killer cell activity and retained anti-tumor CD8+ T cell responses contribute to the induction of a pathological complete response in HER2-positive breast cancer patients undergoing neoadjuvant chemotherapy. J. Transl. Med.13, 204 (2015). [DOI] [PMC free article] [PubMed]
- 4.Sivori S, et al. Human NK cells: surface receptors, inhibitory checkpoints, and translational applications. Cell. Mol. Immunol. 2019;16:430–441. doi: 10.1038/s41423-019-0206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu X, et al. Association of germline variants in natural killer cells with tumor immune microenvironment subtypes, tumor-infiltrating lymphocytes, immunotherapy response, clinical outcomes, and cancer risk. JAMA Netw. Open. 2019;2:e199292. doi: 10.1001/jamanetworkopen.2019.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Z, et al. Prognostic impact of adenosine receptor 2 (A2aR) and programmed cell death ligand 1 (PD-L1) expression in colorectal cancer. Biomed. Res. Int. 2019;2019:8014627. doi: 10.1155/2019/8014627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adachi K, Tamada K. Immune checkpoint blockade opens an avenue of cancer immunotherapy with a potent clinical efficacy. Cancer Sci. 2015;106:945–950. doi: 10.1111/cas.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bern MD, et al. Immunoreceptor tyrosine-based inhibitory motif-dependent functions of an MHC class I-specific NK cell receptor. Proc. Natl Acad. Sci. USA. 2017;114:E8440–E8447. doi: 10.1073/pnas.1713064114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solomon BL, Garrido-Laguna I. TIGIT: a novel immunotherapy target moving from bench to bedside. Cancer Immunol. Immunother. 2018;67:1659–1667. doi: 10.1007/s00262-018-2246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li K, Tian H. Development of small-molecule immune checkpoint inhibitors of PD-1/PD-L1 as a new therapeutic strategy for tumour immunotherapy. J. Drug Target. 2019;27:244–256. doi: 10.1080/1061186X.2018.1440400. [DOI] [PubMed] [Google Scholar]
- 11.Whelan, S. et al. PVRIG and PVRL2 are induced in cancer and inhibit CD8+ T-cell function. Cancer Immunol. Res.7, 257–268 (2019). [DOI] [PMC free article] [PubMed]
- 12.Gurjao C, et al. Intrinsic resistance to immune checkpoint blockade in a mismatch repair-deficient colorectal cancer. Cancer Immunol. Res. 2019;7:1230–1236. doi: 10.1158/2326-6066.CIR-18-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tu L, et al. Assessment of the expression of the immune checkpoint molecules PD-1, CTLA4, TIM-3 and LAG-3 across different cancers in relation to treatment response, tumor-infiltrating immune cells and survival. Int. J. Cancer. 2020;147:423–439. doi: 10.1002/ijc.32785. [DOI] [PubMed] [Google Scholar]
- 14.Souza-Fonseca-Guimaraes F, Cursons J, Huntington ND. The emergence of natural killer cells as a major target in cancer immunotherapy. Trends Immunol. 2019;40:142–158. doi: 10.1016/j.it.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Zou W. Mechanistic insights into cancer immunity and immunotherapy. Cell. Mol. Immunol. 2018;15:419–420. doi: 10.1038/s41423-018-0011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.André, P. et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK Cells. Cell175, 1731–1743 (2018). [DOI] [PMC free article] [PubMed]
- 17.Carotta S. Targeting NK cells for anticancer immunotherapy: clinical and preclinical approaches. Front. Immunol. 2016;7:152. doi: 10.3389/fimmu.2016.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genßler S, et al. Dual targeting of glioblastoma with chimeric antigen receptor-engineered natural killer cells overcomes heterogeneity of target antigen expression and enhances antitumor activity and survival. Oncoimmunology. 2016;5:e1119354. doi: 10.1080/2162402X.2015.1119354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blake SJ, Dougall WC, Miles JJ, Teng MW, Smyth MJ. Molecular pathways: targeting CD96 and TIGIT for cancer immunotherapy. Clin. Cancer Res. 2016;22:5183–5188. doi: 10.1158/1078-0432.CCR-16-0933. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, et al. Human fused NKG2D-IL-15 protein controls xenografted human gastric cancer through the recruitment and activation of NK cells. Cell. Mol. Immunol. 2017;14:293–307. doi: 10.1038/cmi.2015.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nowak J, et al. Role of donor activating KIR-HLA ligand-mediated NK cell education status in control of malignancy in hematopoietic cell transplant recipients. Biol. Blood Marrow Transplant. 2015;21:829–839. doi: 10.1016/j.bbmt.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 22.van der Ploeg K, et al. Modulation of human leukocyte antigen-C by human cytomegalovirus stimulates KIR2DS1 recognition by natural killer cells. Front. Immunol. 2017;8:298. doi: 10.3389/fimmu.2017.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balsamo M, et al. Hypoxia downregulates the expression of activating receptors involved in NK-cell-mediated target cell killing without affecting ADCC. Eur. J. Immunol. 2013;43:2756–2764. doi: 10.1002/eji.201343448. [DOI] [PubMed] [Google Scholar]
- 24.Sun H, Sun C. The rise of NK cell checkpoints as promising therapeutic targets in cancer immunotherapy. Front. Immunol. 2019;10:2354. doi: 10.3389/fimmu.2019.02354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romagne F, et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood. 2009;114:2667–2677. doi: 10.1182/blood-2009-02-206532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan M, Arooj S, Wang H. NK cell-based immune checkpoint inhibition. Front. Immunol. 2020;11:167. doi: 10.3389/fimmu.2020.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019;18:175–196. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandoval-Borrego D, et al. Overexpression of CD158 and NKG2A inhibitory receptors and underexpression of NKG2D and NKp46 activating receptors on NK cells in acute myeloid leukemia. Arch. Med. Res. 2016;47:55–64. doi: 10.1016/j.arcmed.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Mingari MC, Pietra G, Moretta L. Immune checkpoint inhibitors: anti-NKG2A antibodies on board. Trends Immunol. 2019;40:83–85. doi: 10.1016/j.it.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Market, M., Baxter, K. E., Angka, L., Kennedy, M. A. & Auer, R. C. The potential for cancer immunotherapy in targeting surgery-induced natural killer cell dysfunction. Cancers11, 2 (2018). [DOI] [PMC free article] [PubMed]
- 31.Guillerey C, et al. Immunosurveillance and therapy of multiple myeloma are CD226 dependent. J. Clin. Invest. 2015;125:2077–2089. doi: 10.1172/JCI77181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parameswaran R, et al. Repression of GSK3 restores NK cell cytotoxicity in AML patients. Nat Commun. 2016;7:11154. doi: 10.1038/ncomms11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delconte RB, et al. The helix-loop-helix protein ID2 governs NK cell fate by tuning their sensitivity to interleukin-15. Immunity. 2016;44:103–115. doi: 10.1016/j.immuni.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Blake SJ, et al. Suppression of metastases using a new lymphocyte checkpoint target for cancer immunotherapy. Cancer Discov. 2016;6:446–459. doi: 10.1158/2159-8290.CD-15-0944. [DOI] [PubMed] [Google Scholar]
- 35.Ma, S. R. et al. Blockade of adenosine A2A receptor enhances CD8+ T cells response and decreases regulatory T cells in head and neck squamous cell carcinoma. Mol. Cancer16, 99 (2017). [DOI] [PMC free article] [PubMed]
- 36.Kamada, T. et al. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc. Natl Acad. Sci. USA116, 9999–10008 (2019). [DOI] [PMC free article] [PubMed]
- 37.Qin S, et al. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol. Cancer. 2019;18:155. doi: 10.1186/s12943-019-1091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lanuza PM, et al. Activated human primary NK cells efficiently kill colorectal cancer cells in 3D spheroid cultures irrespectively of the level of PD-L1 expression. Oncoimmunology. 2018;7:e1395123. doi: 10.1080/2162402X.2017.1395123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frazao A, et al. NKG2D/NKG2-ligand pathway offers new opportunities in cancer treatment. Front. Immunol. 2019;10:661. doi: 10.3389/fimmu.2019.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bauman Y, et al. Downregulation of the stress-induced ligand ULBP1 following SV40 infection confers viral evasion from NK cell cytotoxicity. Oncotarget. 2016;7:15369–15381. doi: 10.18632/oncotarget.8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruggeri L, et al. Effects of anti-NKG2A antibody administration on leukemia and normal hematopoietic cells. Haematologica. 2016;101:626–633. doi: 10.3324/haematol.2015.135301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp. Mol. Med. 2018;50:1–11. doi: 10.1038/s12276-018-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamiya T, Seow SV, Wong D, Robinson M, Campana D. Blocking expression of inhibitory receptor NKG2A overcomes tumor resistance to NK cells. J. Clin. Invest. 2019;129:2094–2106. doi: 10.1172/JCI123955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang Y, Chen M, Nie H, Yuan Y. PD-1 and PD-L1 in cancer immunotherapy: clinical implications and future considerations. Hum. Vaccin. Immunother. 2019;15:1111–1122. doi: 10.1080/21645515.2019.1571892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He, W. et al. CD155T/TIGIT signaling regulates CD8+ T-cell metabolism and promotes tumor progression in human gastric cancer. Cancer Res.77, 6375–6388 (2017). [DOI] [PubMed]
- 46.Sanchez-Correa B, et al. Modulation of NK cells with checkpoint inhibitors in the context of cancer immunotherapy. Cancer Immunol. Immunother. 2019;68:861–870. doi: 10.1007/s00262-019-02336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou G, et al. Reduction of immunosuppressive tumor microenvironment in cholangiocarcinoma by ex vivo targeting immune checkpoint molecules. J. Hepatol. 2019;71:753–762. doi: 10.1016/j.jhep.2019.05.026. [DOI] [PubMed] [Google Scholar]
- 48.Feng M, et al. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat. Rev. Cancer. 2019;19:568–586. doi: 10.1038/s41568-019-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang E, Singh BK, Paustian AM, Kambayashi T. Diacylglycerol Kinase ζ is a target to enhance NK cell function. J. Immunol. 2016;197:934–941. doi: 10.4049/jimmunol.1600581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lanuza PM, et al. Recalling the biological significance of immune checkpoints on NK cells: a chance to overcome LAG3, PD1, and CTLA4 inhibitory pathways by adoptive NK cell transfer? Front. Immunol. 2019;10:3010. doi: 10.3389/fimmu.2019.03010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meza Guzman, L. G., Keating, N. & Nicholson, S. E. Natural killer cells: tumor surveillance and signaling. Cancers12, 952 (2020). [DOI] [PMC free article] [PubMed]
- 52.Teratake Y, et al. Development of a protein-based system for transient epigenetic repression of immune checkpoint molecule and enhancement of antitumour activity of natural killer cells. Br. J. Cancer. 2020;122:823–834. doi: 10.1038/s41416-019-0708-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller JS, Lanier LL. Natural killer cells in cancer immunotherapy. Ann. Rev. Cancer Biol. 2019;3:77–103. doi: 10.1146/annurev-cancerbio-030518-055653. [DOI] [Google Scholar]
- 54.Fang F, Xiao W, Tian Z. Challenges of NK cell-based immunotherapy in the new era. Front. Med. 2018;12:440–450. doi: 10.1007/s11684-018-0653-9. [DOI] [PubMed] [Google Scholar]
- 55.Battella S, Cox MC, Santoni A, Palmieri G. Natural killer (NK) cells and anti-tumor therapeutic mAb: unexplored interactions. J. Leukoc. Biol. 2016;99:87–96. doi: 10.1189/jlb.5VMR0415-141R. [DOI] [PubMed] [Google Scholar]
- 56.Xu-Monette ZY, et al. Immune profiling and quantitative analysis decipher the clinical role of immune-checkpoint expression in the tumor immune microenvironment of DLBCL. Cancer Immunol. Res. 2019;7:644–657. doi: 10.1158/2326-6066.CIR-18-0439. [DOI] [PubMed] [Google Scholar]
- 57.Cantoni C, et al. NK cells, tumor cell transition, and tumor progression in solid malignancies: new hints for NK-based immunotherapy? J. Immunol. Res. 2016;2016:4684268. doi: 10.1155/2016/4684268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nabatanzi R, Cose S, Joloba M, Jones SR, Nakanjako D. Effects of HIV infection and ART on phenotype and function of circulating monocytes, natural killer, and innate lymphoid cells. AIDS Res. Ther. 2018;15:7. doi: 10.1186/s12981-018-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hood SP, et al. Phenotype and function of activated natural killer cells from patients with prostate cancer: patient-dependent responses to priming and IL-2 activation. Front. Immunol. 2018;9:3169. doi: 10.3389/fimmu.2018.03169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bjorkstrom, N. K. et al. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood116, 3853–3864 (2010). [DOI] [PubMed]
- 61.Chiossone L, Vienne M, Kerdiles YM, Vivier E. Natural killer cell immunotherapies against cancer: checkpoint inhibitors and more. Semin. Immunol. 2017;31:55–63. doi: 10.1016/j.smim.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 62.Munari E, et al. PD-L1 expression heterogeneity in non-small cell lung cancer: defining criteria for harmonization between biopsy specimens and whole sections. J. Thorac. Oncol. 2018;13:1113–1120. doi: 10.1016/j.jtho.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 63.Chretien AS, et al. Cancer-induced alterations of NK-mediated target recognition: current and investigational pharmacological strategies aiming at restoring NK-mediated anti-tumor activity. Front. Immunol. 2014;5:122. doi: 10.3389/fimmu.2014.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang R, et al. Loss of Fas expression and high expression of HLA-E promoting the immune escape of early colorectal cancer cells. Oncol. Lett. 2017;13:3379–3386. doi: 10.3892/ol.2017.5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun H, et al. Human CD96 correlates to natural killer cell exhaustion and predicts the prognosis of human hepatocellular Carcinoma. Hepatology. 2019;70:168–183. doi: 10.1002/hep.30347. [DOI] [PubMed] [Google Scholar]
- 66.Yoon SR, Kim TD, Choi I. Understanding of molecular mechanisms in natural killer cell therapy. Exp. Mol. Med. 2015;47:e141. doi: 10.1038/emm.2014.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Konjević, G., Vuletić, A. & Mirjačić Martinović, K. Natural killer cell receptors: alterations and therapeutic targeting in malignancies. Immunol. Res.64, 25–35 (2016). [DOI] [PubMed]
- 68.Thiruchelvam-Kyle, L. et al. The activating human NK cell receptor KIR2DS2 recognizes a β2-microglobulin-independent ligand on cancer cells. J. Immunol.198, 2556–2567 (2017). [DOI] [PubMed]
- 69.Chaganty BK, et al. Trastuzumab upregulates expression of HLA-ABC and T cell costimulatory molecules through engagement of natural killer cells and stimulation of IFNγ secretion. Oncoimmunology. 2016;5:e1100790. doi: 10.1080/2162402X.2015.1100790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Torphy, R. J., Schulick, R. D. & Zhu, Y. Newly emerging immune checkpoints: promises for future cancer therapy. Int. J. Mol. Sci. 18, 2642 (2017). [DOI] [PMC free article] [PubMed]
- 71.Xu L, et al. Increased Tim-3 expression in peripheral NK cells predicts a poorer prognosis and Tim-3 blockade improves NK cell-mediated cytotoxicity in human lung adenocarcinoma. Int. Immunopharmacol. 2015;29:635–641. doi: 10.1016/j.intimp.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 72.Pesce S, et al. Identification of a subset of human natural killer cells expressing high levels of programmed death 1: a phenotypic and functional characterization. J. Allergy Clin. Immunol. 2017;139:335–346.e333. doi: 10.1016/j.jaci.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 73.Tumino N, et al. Presence of innate lymphoid cells in pleural effusions of primary and metastatic tumors: functional analysis and expression of PD-1 receptor. Int. J. Cancer. 2019;145:1660–1668. doi: 10.1002/ijc.32262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Muntasell A, et al. Targeting NK-cell checkpoints for cancer immunotherapy. Curr. Opin. Immunol. 2017;45:73–81. doi: 10.1016/j.coi.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 75.Wang Q, Gao J, Wu X. Pseudoprogression and hyperprogression after checkpoint blockade. Int. Immunopharmacol. 2018;58:125–135. doi: 10.1016/j.intimp.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 76.Paul S, Lal G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front. Immunol. 2017;8:1124. doi: 10.3389/fimmu.2017.01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vari F, et al. Immune evasion via PD-1/PD-L1 on NK cells and monocyte/macrophages is more prominent in Hodgkin lymphoma than DLBCL. Blood. 2018;131:1809–1819. doi: 10.1182/blood-2017-07-796342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jung HI, et al. Overexpression of PD-L1 and PD-L2 is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Res. Treat. 2017;49:246–254. doi: 10.4143/crt.2016.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peled M, et al. Affinity purification mass spectrometry analysis of PD-1 uncovers SAP as a new checkpoint inhibitor. Proc. Natl Acad. Sci. USA. 2018;115:E468–e477. doi: 10.1073/pnas.1710437115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Herndler-Brandstetter D, et al. Humanized mouse model supports development, function, and tissue residency of human natural killer cells. Proc. Natl Acad. Sci. USA. 2017;114:E9626–e9634. doi: 10.1073/pnas.1705301114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hsu J, et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J. Clin. Invest. 2018;128:4654–4668. doi: 10.1172/JCI99317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nishimura Y, et al. Histidine-rich glycoprotein augments natural killer cell function by modulating PD-1 expression via CLEC-1B. Pharmacol. Res. Perspect. 2019;7:e00481. doi: 10.1002/prp2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jeffery HC, et al. Changes in natural killer cells and exhausted memory regulatory T Cells with corticosteroid therapy in acute autoimmune hepatitis. Hepatol. Commun. 2018;2:421–436. doi: 10.1002/hep4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Delconte RB, et al. CIS is a potent checkpoint in NK cell-mediated tumor immunity. Nat. Immunol. 2016;17:816–824. doi: 10.1038/ni.3470. [DOI] [PubMed] [Google Scholar]
- 85.Beldi-Ferchiou A, et al. PD-1 mediates functional exhaustion of activated NK cells in patients with Kaposi sarcoma. Oncotarget. 2016;7:72961–72977. doi: 10.18632/oncotarget.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tabellini G, et al. Primitive neuroectodermal tumor in an ovarian cystic teratoma: natural killer and neuroblastoma cell analysis. Case Rep. Oncol. 2014;7:70–78. doi: 10.1159/000357802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu Y, et al. Increased expression of programmed cell death protein 1 on NK cells inhibits NK-cell-mediated anti-tumor function and indicates poor prognosis in digestive cancers. Oncogene. 2017;36:6143–6153. doi: 10.1038/onc.2017.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xie S, Chen J, Zhang M, Wu Z. Allogenic natural killer cell immunotherapy of sizeable ovarian cancer: a case report. Mol. Clin. Oncol. 2017;6:903–906. doi: 10.3892/mco.2017.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shitara K, Nishikawa H. Regulatory T cells: a potential target in cancer immunotherapy. Ann. NY Acad. Sci. 2018;1417:104–115. doi: 10.1111/nyas.13625. [DOI] [PubMed] [Google Scholar]
- 90.Stein N, Tsukerman P, Mandelboim O. The paired receptors TIGIT and DNAM-1 as targets for therapeutic antibodies. Hum. Antibodies. 2017;25:111–119. doi: 10.3233/HAB-160307. [DOI] [PubMed] [Google Scholar]
- 91.Mahaweni NM, Ehlers FAI, Bos GMJ, Wieten L. Tuning natural killer cell anti-multiple myeloma reactivity by targeting inhibitory signaling via KIR and NKG2A. Front. Immunol. 2018;9:2848. doi: 10.3389/fimmu.2018.02848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li M, et al. T-cell immunoglobulin and ITIM domain (TIGIT) receptor/poliovirus receptor (PVR) ligand engagement suppresses interferon-γ production of natural killer cells via β-arrestin 2-mediated negative signaling. J. Biol. Chem. 2014;289:17647–17657. doi: 10.1074/jbc.M114.572420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hong X, Wang X, Wang T, Zhang X. Correlation of T cell immunoglobulin and ITIM domain (TIGIT) and programmed death 1 (PD-1) with clinicopathological characteristics of renal cell carcinoma may indicate potential targets for treatment. Med. Sci. Monit. 2018;24:6861–6872. doi: 10.12659/MSM.910388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cho YH, et al. Natural killer cells as a potential biomarker for predicting immunotherapy efficacy in patients with non-small cell lung cancer. Target. Oncol. 2020;15:241–247. doi: 10.1007/s11523-020-00712-2. [DOI] [PubMed] [Google Scholar]
- 95.Wagner J, et al. A two-phase expansion protocol combining interleukin (IL)-15 and IL-21 improves natural killer cell proliferation and cytotoxicity against rhabdomyosarcoma. Front. Immunol. 2017;8:676. doi: 10.3389/fimmu.2017.00676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat. Rev. Cancer. 2016;16:7–19. doi: 10.1038/nrc.2015.5. [DOI] [PubMed] [Google Scholar]
- 97.Zhang Q, et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 2018;19:723–732. doi: 10.1038/s41590-018-0132-0. [DOI] [PubMed] [Google Scholar]
- 98.Zhang B, et al. Immunoreceptor TIGIT inhibits the cytotoxicity of human cytokine-induced killer cells by interacting with CD155. Cancer Immunol. Immunother. 2016;65:305–314. doi: 10.1007/s00262-016-1799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kurtulus S, et al. TIGIT predominantly regulates the immune response via regulatory T cells. J. Clin. Invest. 2015;125:4053–4062. doi: 10.1172/JCI81187. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 100.Inozume T, et al. Melanoma cells control antimelanoma CTL responses via interaction between TIGIT and CD155 in the effector phase. J. Invest. Dermatol. 2016;136:255–263. doi: 10.1038/JID.2015.404. [DOI] [PubMed] [Google Scholar]
- 101.Kim N, et al. Natural killer cells as a promising therapeutic target for cancer immunotherapy. Arch. Pharm. Res. 2019;42:591–606. doi: 10.1007/s12272-019-01143-y. [DOI] [PubMed] [Google Scholar]
- 102.Jong AY, et al. Large-scale isolation and cytotoxicity of extracellular vesicles derived from activated human natural killer cells. J. Extracell. Vesicles. 2017;6:1294368. doi: 10.1080/20013078.2017.1294368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun Y, et al. Combined evaluation of the expression status of CD155 and TIGIT plays an important role in the prognosis of LUAD (lung adenocarcinoma) Int. Immunopharmacol. 2020;80:106198. doi: 10.1016/j.intimp.2020.106198. [DOI] [PubMed] [Google Scholar]
- 104.Kim N, Kim HS. Targeting checkpoint receptors and molecules for therapeutic modulation of natural killer cells. Front. Immunol. 2018;9:2041. doi: 10.3389/fimmu.2018.02041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu S, et al. Recruitment of Grb2 and SHIP1 by the ITT-like motif of TIGIT suppresses granule polarization and cytotoxicity of NK cells. Cell Death Differ. 2013;20:456–464. doi: 10.1038/cdd.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Manieri NA, Chiang EY, Grogan JL. TIGIT: a key inhibitor of the cancer immunity cycle. Trends Immunol. 2017;38:20–28. doi: 10.1016/j.it.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 107.Boyerinas B, et al. Antibody-dependent cellular cytotoxicity activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol. Res. 2015;3:1148–1157. doi: 10.1158/2326-6066.CIR-15-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sarhan D, et al. Adaptive NK cells resist regulatory T-cell suppression driven by IL37. Cancer Immunol. Res. 2018;6:766–IL775. doi: 10.1158/2326-6066.CIR-17-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ma B, et al. Use of aspirin in the prevention of colorectal cancer through TIGIT-CD155 pathway. J. Cell. Mol. Med. 2019;23:4514–4522. doi: 10.1111/jcmm.14332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Meckawy GR, et al. Natural killer NKG2A and NKG2D in patients with colorectal cancer. J. Gastrointest. Oncol. 2019;10:218–225. doi: 10.21037/jgo.2018.12.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chan CJ, et al. The receptors CD96 and CD226 oppose each other in the regulation of natural killer cell functions. Nat. Immunol. 2014;15:431–438. doi: 10.1038/ni.2850. [DOI] [PubMed] [Google Scholar]
- 112.Bernhardt G. TACTILE becomes tangible: CD96 discloses its inhibitory peculiarities. Nat. Immunol. 2014;15:406–408. doi: 10.1038/ni.2855. [DOI] [PubMed] [Google Scholar]
- 113.Carlsten M, Childs RW. Genetic manipulation of NK cells for cancer immunotherapy: techniques and clinical implications. Front. Immunol. 2015;6:266. doi: 10.3389/fimmu.2015.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rezvani K, Rouce R, Liu E, Shpall E. Engineering natural killer cells for cancer immunotherapy. Mol. Ther. 2017;25:1769–1781. doi: 10.1016/j.ymthe.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hu W, Wang G, Huang D, Sui M, Xu Y. Cancer immunotherapy based on natural killer cells: current progress and new opportunities. Front. Immunol. 2019;10:1205. doi: 10.3389/fimmu.2019.01205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Deuss FA, Gully BS, Rossjohn J, Berry R. Recognition of nectin-2 by the natural killer cell receptor T cell immunoglobulin and ITIM domain (TIGIT) J. Biol. Chem. 2017;292:11413–11422. doi: 10.1074/jbc.M117.786483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Holmes VM, et al. Interaction between nectin-1 and the human natural killer cell receptor CD96. PLoS One. 2019;14:e0212443. doi: 10.1371/journal.pone.0212443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Deuss FA, Watson GM, Fu Z, Rossjohn J, Berry R. Structural basis for CD96 immune receptor recognition of nectin-like protein-5, CD155. Structure. 2019;27:219–228.e213. doi: 10.1016/j.str.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 119.Hodgins JJ, Khan ST, Park MM, Auer RC, Ardolino M. Killers 2.0: NK cell therapies at the forefront of cancer control. J. Clin. Invest. 2019;129:3499–3510. doi: 10.1172/JCI129338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Roman Aguilera A, et al. CD96 targeted antibodies need not block CD96-CD155 interactions to promote NK cell anti-metastatic activity. Oncoimmunology. 2018;7:e1424677. doi: 10.1080/2162402X.2018.1424677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vitale M, Cantoni C, Pietra G, Mingari MC, Moretta L. Effect of tumor cells and tumor microenvironment on NK-cell function. Eur. J. Immunol. 2014;44:1582–1592. doi: 10.1002/eji.201344272. [DOI] [PubMed] [Google Scholar]
- 122.Wang Z, et al. The clinical significance of abnormal Tim-3 expression on NK cells from patients with gastric cancer. Immunol. Invest. 2015;44:578–589. doi: 10.3109/08820139.2015.1052145. [DOI] [PubMed] [Google Scholar]
- 123.Komita H, et al. Expression of immune checkpoint molecules of T cell immunoglobulin and mucin protein 3/galectin-9 for NK cell suppression in human gastrointestinal stromal tumors. Oncol. Rep. 2015;34:2099–2105. doi: 10.3892/or.2015.4149. [DOI] [PubMed] [Google Scholar]
- 124.Cekic C, Day YJ, Sag D, Linden J. Myeloid expression of adenosine A2A receptor suppresses T and NK cell responses in the solid tumor microenvironment. Cancer Res. 2014;74:7250–7259. doi: 10.1158/0008-5472.CAN-13-3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Meggyes M, et al. Peripheral blood TIM-3 positive NK and CD8+ T cells throughout pregnancy: TIM-3/galectin-9 interaction and its possible role during pregnancy. PLoS ONE. 2014;9:e92371. doi: 10.1371/journal.pone.0092371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kikushige Y, et al. A TIM-3/Gal-9 autocrine stimulatory loop drives self-renewal of human myeloid leukemia stem cells and leukemic progression. Cell Stem Cell. 2015;17:341–352. doi: 10.1016/j.stem.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 127.Vega-Carrascal I, et al. Dysregulation of TIM-3-galectin-9 pathway in the cystic fibrosis airways. J. Immunol. 2011;186:2897–2909. doi: 10.4049/jimmunol.1003187. [DOI] [PubMed] [Google Scholar]
- 128.Van Audenaerde JRM, et al. Interleukin-15 stimulates natural killer cell-mediated killing of both human pancreatic cancer and stellate cells. Oncotarget. 2017;8:56968–56979. doi: 10.18632/oncotarget.18185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.So EC, et al. NK cell expression of Tim-3: first impressions matter. Immunobiology. 2019;224:362–370. doi: 10.1016/j.imbio.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 130.Hadadi L, et al. Dysregulated expression of Tim-3 and NKp30 receptors on NK cells of patients with chronic lymphocytic leukemia. Oncol Res Treat. 2019;42:202–208. doi: 10.1159/000497208. [DOI] [PubMed] [Google Scholar]
- 131.Lu, X. et al. Tumor antigen-specific CD8+ T cells are negatively regulated by PD-1 and Tim-3 in human gastric cancer. Cell. Immunol.313, 43–51 (2017). [DOI] [PubMed]
- 132.Gorman JV, Colgan JD. Regulation of T cell responses by the receptor molecule Tim-3. Immunol. Res. 2014;59:56–65. doi: 10.1007/s12026-014-8524-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Komohara Y, et al. The coordinated actions of TIM-3 on cancer and myeloid cells in the regulation of tumorigenicity and clinical prognosis in clear cell renal cell carcinomas. Cancer Immunol. Res. 2015;3:999–1007. doi: 10.1158/2326-6066.CIR-14-0156. [DOI] [PubMed] [Google Scholar]
- 134.Shayan G, et al. Adaptive resistance to anti-PD1 therapy by Tim-3 upregulation is mediated by the PI3K-Akt pathway in head and neck cancer. Oncoimmunology. 2017;6:e1261779. doi: 10.1080/2162402X.2016.1261779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Freud AG, Yu J, Caligiuri MA. Human natural killer cell development in secondary lymphoid tissues. Semin. Immunol. 2014;26:132–137. doi: 10.1016/j.smim.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li Z, Liu X, Guo R, Wang P. TIM-3 plays a more important role than PD-1 in the functional impairments of cytotoxic T cells of malignant Schwannomas. Tumour Biol. 2017;39:1010428317698352. doi: 10.1177/1010428317698352. [DOI] [PubMed] [Google Scholar]
- 137.Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2018;175:313–326. doi: 10.1016/j.cell.2018.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.He Y, et al. TIM-3, a promising target for cancer immunotherapy. Onco Targets Ther. 2018;11:7005–7009. doi: 10.2147/OTT.S170385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ndhlovu LC, et al. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood. 2012;119:3734–3743. doi: 10.1182/blood-2011-11-392951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang Y, et al. Preoperative Tim‑3 expression on peripheral NK cells is correlated with pathologic TNM staging in colorectal cancer. Mol. Med. Rep. 2017;15:3810–3818. doi: 10.3892/mmr.2017.6482. [DOI] [PubMed] [Google Scholar]
- 141.Yang M, et al. T-cell immunoglobulin mucin-3 expression in bladder urothelial carcinoma: clinicopathologic correlations and association with survival. J. Surg. Oncol. 2015;112:430–435. doi: 10.1002/jso.24012. [DOI] [PubMed] [Google Scholar]
- 142.Zhang, Y. & Schmidt-Wolf, I. G. H. Ten-year update of the international registry on cytokine-induced killer cells in cancer immunotherapy. J. Cell. Physiol. 235, 9291–9303 (2020). [DOI] [PubMed]
- 143.Ju Y, et al. T cell immunoglobulin- and mucin-domain-containing molecule-3 (Tim-3) mediates natural killer cell suppression in chronic hepatitis B. J. Hepatol. 2010;52:322–329. doi: 10.1016/j.jhep.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 144.Huang YH, et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 2015;517:386–390. doi: 10.1038/nature13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Melero I, Rouzaut A, Motz GT, Coukos G. T-cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious cancer immunotherapy. Cancer Discov. 2014;4:522–526. doi: 10.1158/2159-8290.CD-13-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang X, et al. Increased Tim-3 expression on TILs during treatment with the Anchored GM-CSF vaccine and anti-PD-1 antibodies is inversely correlated with response in prostate cancer. J. Cancer. 2020;11:648–656. doi: 10.7150/jca.29705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sabatos-Peyton CA, et al. Blockade of Tim-3 binding to phosphatidylserine and CEACAM1 is a shared feature of anti-Tim-3 antibodies that have functional efficacy. Oncoimmunology. 2018;7:e1385690. doi: 10.1080/2162402X.2017.1385690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.van de Weyer PS, et al. A highly conserved tyrosine of Tim-3 is phosphorylated upon stimulation by its ligand galectin-9. Biochem. Biophys. Res. Commun. 2006;351:571–576. doi: 10.1016/j.bbrc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 149.Lee J, et al. Phosphotyrosine-dependent coupling of Tim-3 to T-cell receptor signaling pathways. Mol. Cell. Biol. 2011;31:3963–3974. doi: 10.1128/MCB.05297-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rangachari M, et al. Bat3 promotes T cell responses and autoimmunity by repressing Tim-3-mediated cell death and exhaustion. Nat. Med. 2012;18:1394–1400. doi: 10.1038/nm.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ji J, et al. Long non-coding RNA Lnc-Tim3 exacerbates CD8 T cell exhaustion via binding to Tim-3 and inducing nuclear translocation of Bat3 in HCC. Cell Death Dis. 2018;9:478. doi: 10.1038/s41419-018-0528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.da Silva IP, et al. Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer Immunol. Res. 2014;2:410–422. doi: 10.1158/2326-6066.CIR-13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Du, W. et al. TIM-3 as a target for cancer immunotherapy and mechanisms of action. Int. J. Mol. Sci. 18, 645 (2017). [DOI] [PMC free article] [PubMed]
- 154.Tallerico R, et al. IL-15, TIM-3 and NK cells subsets predict responsiveness to anti-CTLA-4 treatment in melanoma patients. Oncoimmunology. 2017;6:e1261242. doi: 10.1080/2162402X.2016.1261242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schönfeld K, et al. Selective inhibition of tumor growth by clonal NK cells expressing an ErbB2/HER2-specific chimeric antigen receptor. Mol. Ther. 2015;23:330–338. doi: 10.1038/mt.2014.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44:989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.van de Donk NW, et al. Clinical efficacy and management of monoclonal antibodies targeting CD38 and SLAMF7 in multiple myeloma. Blood. 2016;127:681–695. doi: 10.1182/blood-2015-10-646810. [DOI] [PubMed] [Google Scholar]
- 158.Murter, B. et al. Mouse PVRIG has CD8+ T cell-specific coinhibitory functions and dampens antitumor immunity. Cancer Immunol. Res.7, 244–256 (2019). [DOI] [PMC free article] [PubMed]
- 159.Zhu Y, et al. Identification of CD112R as a novel checkpoint for human T cells. J. Exp. Med. 2016;213:167–176. doi: 10.1084/jem.20150785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xu F, et al. Blockade of CD112R and TIGIT signaling sensitizes human natural killer cell functions. Cancer Immunol. Immunother. 2017;66:1367–1375. doi: 10.1007/s00262-017-2031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Krasnova Y, Putz EM, Smyth MJ, Souza-Fonseca-Guimaraes F. Bench to bedside: NK cells and control of metastasis. Clin. Immunol. 2017;177:50–59. doi: 10.1016/j.clim.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 162.Julia EP, Amante A, Pampena MB, Mordoh J, Levy EM. Avelumab, an IgG1 anti-PD-L1 immune checkpoint inhibitor, triggers NK cell-mediated cytotoxicity and cytokine production against triple negative breast cancer cells. Front. Immunol. 2018;9:2140. doi: 10.3389/fimmu.2018.02140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Capone M, et al. Frequency of circulating CD8+CD73+T cells is associated with survival in nivolumab-treated melanoma patients. J. Transl. Med. 2020;18:121. doi: 10.1186/s12967-020-02285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lupo, K. B. & Matosevic, S. Natural killer cells as allogeneic effectors in adoptive cancer immunotherapy. Cancers11, 769 (2019). [DOI] [PMC free article] [PubMed]
- 165.Molgora M, Supino D, Mantovani A, Garlanda C. Tuning inflammation and immunity by the negative regulators IL-1R2 and IL-1R8. Immunol. Rev. 2018;281:233–247. doi: 10.1111/imr.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Guillerey C, et al. Toll-like receptor 3 regulates NK cell responses to cytokines and controls experimental metastasis. Oncoimmunology. 2015;4:e1027468. doi: 10.1080/2162402X.2015.1027468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Molgora M, et al. IL-1R8 is a checkpoint in NK cells regulating anti-tumour and anti-viral activity. Nature. 2017;551:110–114. doi: 10.1038/nature24293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Seo H, et al. IL-21-mediated reversal of NK cell exhaustion facilitates anti-tumour immunity in MHC class I-deficient tumours. Nat. Commun. 2017;8:15776. doi: 10.1038/ncomms15776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chang YH, et al. A chimeric receptor with NKG2D specificity enhances natural killer cell activation and killing of tumor cells. Cancer Res. 2013;73:1777–1786. doi: 10.1158/0008-5472.CAN-12-3558. [DOI] [PubMed] [Google Scholar]
- 170.Romanski A, et al. CD19-CAR engineered NK-92 cells are sufficient to overcome NK cell resistance in B-cell malignancies. J. Cell. Mol. Med. 2016;20:1287–1294. doi: 10.1111/jcmm.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Oelsner S, et al. Continuously expanding CAR NK-92 cells display selective cytotoxicity against B-cell leukemia and lymphoma. Cytotherapy. 2017;19:235–249. doi: 10.1016/j.jcyt.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 172.Ao, X. et al. Anti-αFR CAR-engineered NK-92 cells display potent cytotoxicity against αFR-positive ovarian cancer. J. Immunother.42, 284–296 (2019). [DOI] [PMC free article] [PubMed]
- 173.Montagner, I. M. et al. Anti-PSMA CAR-engineered NK-92 cells: an off-the-shelf cell therapy for prostate cancer. Cells9, 1382 (2020). [DOI] [PMC free article] [PubMed]
- 174.Ruffo E, Wu RC, Bruno TC, Workman CJ, Vignali DAA. Lymphocyte-activation gene 3 (LAG3): the next immune checkpoint receptor. Semin. Immunol. 2019;42:101305. doi: 10.1016/j.smim.2019.101305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Xu F, et al. LSECtin expressed on melanoma cells promotes tumor progression by inhibiting antitumor T-cell responses. Cancer Res. 2014;74:3418–3428. doi: 10.1158/0008-5472.CAN-13-2690. [DOI] [PubMed] [Google Scholar]
- 176.Andrews LP, Marciscano AE, Drake CG, Vignali DA. LAG3 (CD223) as a cancer immunotherapy target. Immunol. Rev. 2017;276:80–96. doi: 10.1111/imr.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Williams P, et al. The distribution of T-cell subsets and the expression of immune checkpoint receptors and ligands in patients with newly diagnosed and relapsed acute myeloid leukemia. Cancer. 2019;125:1470–1481. doi: 10.1002/cncr.31896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gandhi, M. K. et al. Expression of LAG-3 by tumor-infiltrating lymphocytes is coincident with the suppression of latent membrane antigen-specific CD8+ T-cell function in Hodgkin lymphoma patients. Blood108, 2280–2289 (2006). [DOI] [PubMed]
- 179.Shapiro M, et al. Lymphocyte activation gene 3: a novel therapeutic target in chronic lymphocytic leukemia. Haematologica. 2017;102:874–882. doi: 10.3324/haematol.2016.148965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Workman CJ, Dugger KJ, Vignali DA. Cutting edge: molecular analysis of the negative regulatory function of lymphocyte activation gene-3. J. Immunol. 2002;169:5392–5395. doi: 10.4049/jimmunol.169.10.5392. [DOI] [PubMed] [Google Scholar]
- 181.Wang J, et al. Fibrinogen-like protein 1 is a major immune inhibitory ligand of LAG-3. Cell. 2019;176:334–347 e312. doi: 10.1016/j.cell.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Burton BR, et al. Sequential transcriptional changes dictate safe and effective antigen-specific immunotherapy. Nat. Commun. 2014;5:4741. doi: 10.1038/ncomms5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Catakovic K, Klieser E, Neureiter D, Geisberger R. T cell exhaustion: from pathophysiological basics to tumor immunotherapy. Cell Commun. Signal. 2017;15:1. doi: 10.1186/s12964-016-0160-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Marçais A, et al. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat. Immunol. 2014;15:749–757. doi: 10.1038/ni.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Marhelava, K., Pilch, Z., Bajor, M., Graczyk-Jarzynka, A. & Zagozdzon, R. Targeting negative and positive immune checkpoints with monoclonal antibodies in therapy of cancer. Cancers11, 1756 (2019). [DOI] [PMC free article] [PubMed]
- 186.Das M, Zhu C, Kuchroo VK. Tim-3 and its role in regulating anti-tumor immunity. Immunol. Rev. 2017;276:97–111. doi: 10.1111/imr.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Meyer CE, et al. Expression of the inhibitory receptor NKG2A correlates with increased liver and splenic NK cell response to activating receptor engagement. Immun. Inflamm. Dis. 2017;5:177–189. doi: 10.1002/iid3.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ben-Shmuel, A., Biber, G., Sabag, B. & Barda-Saad, M. Modulation of the intracellular inhibitory checkpoint SHP-1 enhances the antitumor activity of engineered NK cells. Cell. Mol. Immunol. 10.1038/s41423-020-0443-6 (2020). [DOI] [PMC free article] [PubMed]
- 189.Kumar S. Natural killer cell cytotoxicity and its regulation by inhibitory receptors. Immunology. 2018;154:383–393. doi: 10.1111/imm.12921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lowry LE, Zehring WA. Potentiation of natural killer cells for cancer immunotherapy: a review of literature. Front. Immunol. 2017;8:1061. doi: 10.3389/fimmu.2017.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mukherjee, N. et al. Intratumoral CD56bright natural killer cells are associated with improved survival in bladder cancer. Oncotarget9, 36492–36502 (2018). [DOI] [PMC free article] [PubMed]
- 192.Chang WC, et al. Regulatory T cells suppress natural killer cell immunity in patients with human cervical carcinoma. Int. J. Gynecol. Cancer. 2016;26:156–162. doi: 10.1097/IGC.0000000000000578. [DOI] [PubMed] [Google Scholar]
- 193.Sun C, et al. High NKG2A expression contributes to NK cell exhaustion and predicts a poor prognosis of patients with liver cancer. Oncoimmunology. 2017;6:e1264562. doi: 10.1080/2162402X.2016.1264562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Böttcher JP, et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell. 2018;172:1022–1037.e1014. doi: 10.1016/j.cell.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nabekura T, et al. Cutting edge: NKG2D signaling enhances NK cell responses but alone is insufficient to drive expansion during mouse cytomegalovirus infection. J. Immunol. 2017;199:1567–1571. doi: 10.4049/jimmunol.1700799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Miyazato K, Hayakawa Y. Pharmacological targeting of natural killer cells for cancer immunotherapy. Cancer Sci. 2020;111:1869–1875. doi: 10.1111/cas.14418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Carlsten M, et al. Checkpoint inhibition of KIR2D with the monoclonal antibody IPH2101 induces contraction and hyporesponsiveness of NK cells in patients with myeloma. Clin. Cancer Res. 2016;22:5211–5222. doi: 10.1158/1078-0432.CCR-16-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 2018;62:29–39. doi: 10.1016/j.intimp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 199.Sanchez-Correa, B. et al. DNAM-1 and the TIGIT/PVRIG/TACTILE axis: novel immune checkpoints for natural killer cell-based cancer immunotherapy. Cancers11, 877 (2019). [DOI] [PMC free article] [PubMed]
- 200.Bauer S, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 201.Yang C, Li Y, Yang Y, Chen Z. Overview of strategies to improve therapy against tumors using natural killer cell. J Immunol. Res. 2020;2020:8459496. doi: 10.1155/2020/8459496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.van Hall T, et al. Monalizumab: inhibiting the novel immune checkpoint NKG2A. J. Immunother. Cancer. 2019;7:263. doi: 10.1186/s40425-019-0761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hosomi S, Grootjans J, Huang YH, Kaser A, Blumberg RS. New insights into the regulation of natural-killer group 2 member D (NKG2D) and NKG2D-ligands: endoplasmic reticulum stress and CEA-related cell adhesion molecule 1. Front. Immunol. 2018;9:1324. doi: 10.3389/fimmu.2018.01324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.McWilliams EM, et al. Therapeutic CD94/NKG2A blockade improves natural killer cell dysfunction in chronic lymphocytic leukemia. Oncoimmunology. 2016;5:e1226720. doi: 10.1080/2162402X.2016.1226720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Grossenbacher SK, Canter RJ, Murphy WJ. Natural killer cell immunotherapy to target stem-like tumor cells. J Immunother. Cancer. 2016;4:19. doi: 10.1186/s40425-016-0124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ames E, et al. NK cells preferentially target tumor cells with a cancer stem cell phenotype. J. Immunol. 2015;195:4010–4019. doi: 10.4049/jimmunol.1500447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Deng W, et al. Antitumor immunity. A shed NKG2D ligand that promotes natural killer cell activation and tumor rejection. Science. 2015;348:136–139. doi: 10.1126/science.1258867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Luo Q, et al. Tumor-derived soluble MICA obstructs the NKG2D pathway to restrain NK cytotoxicity. Aging Dis. 2020;11:118–128. doi: 10.14336/AD.2019.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Du C, et al. MICA immune complex formed with alpha 3 domain-specific antibody activates human NK cells in a Fc-dependent manner. J. Immunother. Cancer. 2019;7:207. doi: 10.1186/s40425-019-0687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wei F, et al. BPIFB1 (LPLUNC1) inhibits radioresistance in nasopharyngeal carcinoma by inhibiting VTN expression. Cell Death Dis. 2018;9:432. doi: 10.1038/s41419-018-0409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Pérez-Martínez A, et al. A phase I/II trial of interleukin-15-stimulated natural killer cell infusion after haplo-identical stem cell transplantation for pediatric refractory solid tumors. Cytotherapy. 2015;17:1594–1603. doi: 10.1016/j.jcyt.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 212.Kloess S, et al. Triplebody mediates increased anti-leukemic reactivity of IL-2 activated donor natural killer (NK) cells and impairs viability of their CD33-expressing NK subset. Front. Immunol. 2017;8:1100. doi: 10.3389/fimmu.2017.01100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Romero, A. I., Thoren, F. B., Brune, M. & Hellstrand, K. NKp46 and NKG2D receptor expression in NK cells with CD56dim and CD56bright phenotype: regulation by histamine and reactive oxygen species. Br. J. Haematol.132, 91–98 (2006). [DOI] [PubMed]
- 214.Lee JC, Lee KM, Ahn YO, Suh B, Heo DS. A possible mechanism of impaired NK cytotoxicity in cancer patients: down-regulation of DAP10 by TGF-beta1. Tumori J. 2011;97:350–357. doi: 10.1177/030089161109700316. [DOI] [PubMed] [Google Scholar]
- 215.Baragano Raneros A, et al. Methylation of NKG2D ligands contributes to immune system evasion in acute myeloid leukemia. Genes Immun. 2015;16:71–82. doi: 10.1038/gene.2014.58. [DOI] [PubMed] [Google Scholar]
- 216.Shah NN, et al. Acute GVHD in patients receiving IL-15/4-1BBL activated NK cells following T-cell-depleted stem cell transplantation. Blood. 2015;125:784–792. doi: 10.1182/blood-2014-07-592881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Li Y, Di Santo JP. Probing human NK cell biology using human immune system (HIS) mice. Curr. Top. Microbiol. Immunol. 2016;395:191–208. doi: 10.1007/82_2015_488. [DOI] [PubMed] [Google Scholar]
- 218.Kwon HJ, Kim N, Kim HS. Molecular checkpoints controlling natural killer cell activation and their modulation for cancer immunotherapy. Exp. Mol. Med. 2017;49:e311. doi: 10.1038/emm.2017.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bachanova V, et al. Haploidentical natural killer cells induce remissions in non-Hodgkin lymphoma patients with low levels of immune-suppressor cells. Cancer Immunol. Immunother. 2018;67:483–494. doi: 10.1007/s00262-017-2100-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.He Y, et al. The combination of anti-KIR monoclonal antibodies with anti-PD-1/PD-L1 monoclonal antibodies could be a critical breakthrough in overcoming tumor immune escape in NSCLC. Drug Des. Devel. Ther. 2018;12:981–986. doi: 10.2147/DDDT.S163304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kandilarova SM, et al. The influence of HLA and KIR genes on malignant melanoma development and progression. Arch. Immunol. Ther. Exp. 2016;64:73–81. doi: 10.1007/s00005-016-0437-3. [DOI] [PubMed] [Google Scholar]
- 222.Rautela J, et al. Molecular insight into targeting the NK cell immune response to cancer. Immunol. Cell Biol. 2018;96:477–484. doi: 10.1111/imcb.12045. [DOI] [PubMed] [Google Scholar]
- 223.Levi-Schaffer F, Mandelboim O. Inhibitory and coactivating receptors recognising the same ligand: immune homeostasis exploited by pathogens and tumours. Trends Immunol. 2018;39:112–122. doi: 10.1016/j.it.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zamora AE, Grossenbacher SK, Aguilar EG, Murphy WJ. Models to study NK cell biology and possible clinical application. Curr. Protoc. Immunol. 2015;110:14 37 11–14 37 14. doi: 10.1002/0471142735.im1437s110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Mulrooney TJ, Posch PE, Hurley CK. DAP12 impacts trafficking and surface stability of killer immunoglobulin-like receptors on natural killer cells. J. Leukoc. Biol. 2013;94:301–313. doi: 10.1189/jlb.0213093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Chambers AM, Matosevic S. Immunometabolic dysfunction of natural killer cells mediated by the hypoxia-CD73 axis in solid tumors. Front. Mol. Biosci. 2019;6:60. doi: 10.3389/fmolb.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sakamoto N, et al. Phase I clinical trial of autologous NK cell therapy using novel expansion method in patients with advanced digestive cancer. J. Transl. Med. 2015;13:277. doi: 10.1186/s12967-015-0632-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hilton HG, et al. The production of KIR-Fc fusion proteins and their use in a multiplex HLA class I binding assay. J. Immunol. Methods. 2015;425:79–87. doi: 10.1016/j.jim.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Garcia-Diaz A, et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 2017;19:1189–1201. doi: 10.1016/j.celrep.2017.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Quatrini, L. et al. Glucocorticoids and the cytokines IL-12, IL-15, and IL-18 present in the tumor microenvironment induce PD-1 expression on human natural killer cells. J. Allergy Clin. Immunol. 10.1016/j.jaci.2020.04.044 (2020). [DOI] [PubMed]
- 231.Benson DM, Jr., et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116:2286–2294. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Thomas LM. Current perspectives on natural killer cell education and tolerance: emerging roles for inhibitory receptors. Immunotargets Ther. 2015;4:45–53. doi: 10.2147/ITT.S61498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Shimasaki N, Jain A, Campana D. NK cells for cancer immunotherapy. Nat. Rev. Drug Discov. 2020;19:200–218. doi: 10.1038/s41573-019-0052-1. [DOI] [PubMed] [Google Scholar]
- 234.Benson DM, Jr., et al. A phase I Trial of the anti-KIR antibody IPH2101 and lenalidomide in patients with relapsed/refractory multiple myeloma. Clin. Cancer Res. 2015;21:4055–4061. doi: 10.1158/1078-0432.CCR-15-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Acebes-Huerta A, et al. Drug-induced hyperploidy stimulates an antitumor NK cell response mediated by NKG2D and DNAM-1 receptors. Oncoimmunology. 2016;5:e1074378. doi: 10.1080/2162402X.2015.1074378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ferrari de Andrade L, et al. Antibody-mediated inhibition of MICA and MICB shedding promotes NK cell-driven tumor immunity. Science. 2018;359:1537–1542. doi: 10.1126/science.aao0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Vey N, et al. A phase 1 study of lirilumab (antibody against killer immunoglobulin-like receptor antibody KIR2D; IPH2102) in patients with solid tumors and hematologic malignancies. Oncotarget. 2018;9:17675–17688. doi: 10.18632/oncotarget.24832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Lee N, et al. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc. Natl Acad. Sci. USA. 1998;95:5199–5204. doi: 10.1073/pnas.95.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lupo KB, Matosevic S. CD155 immunoregulation as a target for natural killer cell immunotherapy in glioblastoma. J. Hematol. Oncol. 2020;13:76. doi: 10.1186/s13045-020-00913-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Solinas C, Silva De,, Bron P, Willard-Gallo D, Sangiolo KD. Significance of TIM3 expression in cancer: from biology to the clinic. Semin. Oncol. 2019;46:372–379. doi: 10.1053/j.seminoncol.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 242.Della Chiesa, M. et al. Features of memory-like and PD-1+ human NK cell subsets. Front. Immunol.7, 351 (2016). [DOI] [PMC free article] [PubMed]
- 243.Holubova, M. et al. Improving the clinical application of natural killer cells by modulating signals signal from target cells. Int. J. Mol. Sci. 20, 3472 (2019). [DOI] [PMC free article] [PubMed]
- 244.Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell. 2018;23:181–192 e185. doi: 10.1016/j.stem.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Szmania S, et al. Ex vivo-expanded natural killer cells demonstrate robust proliferation in vivo in high-risk relapsed multiple myeloma patients. J. Immunother. 2015;38:24–36. doi: 10.1097/CJI.0000000000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Yoon, S. R. et al. Generation of donor natural killer cells from CD34+ progenitor cells and subsequent infusion after HLA-mismatched allogeneic hematopoietic cell transplantation: a feasibility study. Bone Marrow Transplant.45, 1038–1046 (2010). [DOI] [PubMed]
- 247.Geller MA, et al. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13:98–107. doi: 10.3109/14653249.2010.515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Curti A, et al. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood. 2011;118:3273–3279. doi: 10.1182/blood-2011-01-329508. [DOI] [PubMed] [Google Scholar]
- 249.Klingemann H, et al. Autologous stem cell transplant recipients tolerate haploidentical related-donor natural killer cell-enriched infusions. Transfusion. 2013;53:412–418. doi: 10.1111/j.1537-2995.2012.03764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Shaffer BC, et al. Phase II study of haploidentical natural killer cell infusion for treatment of relapsed or persistent myeloid malignancies following allogeneic hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2016;22:705–709. doi: 10.1016/j.bbmt.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lee DA, et al. Haploidentical natural killer cells infused before allogeneic stem cell transplantation for myeloid malignancies: a phase I trial. Biol. Blood Marrow Transplant. 2016;22:1290–1298. doi: 10.1016/j.bbmt.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Shah N, et al. Phase I study of cord blood-derived natural killer cells combined with autologous stem cell transplantation in multiple myeloma. Br. J. Haematol. 2017;177:457–466. doi: 10.1111/bjh.14570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ciurea SO, et al. Phase 1 clinical trial using mbIL21 ex vivo-expanded donor-derived NK cells after haploidentical transplantation. Blood. 2017;130:1857–1868. doi: 10.1182/blood-2017-05-785659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Habib S, Tariq SM, Tariq M. Chimeric antigen receptor-natural killer cells: the future of cancer immunotherapy. Ochsner J. 2019;19:186–187. doi: 10.31486/toj.19.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Conlon KC, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J. Clin. Oncol. 2015;33:74–82. doi: 10.1200/JCO.2014.57.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Josefsson SE, et al. TIGIT and PD-1 mark intratumoral T cells with reduced effector function in B-cell non-Hodgkin lymphoma. Cancer Immunol. Res. 2019;7:355–362. doi: 10.1158/2326-6066.CIR-18-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Becker PS, et al. Selection and expansion of natural killer cells for NK cell-based immunotherapy. Cancer Immunol. Immunother. 2016;65:477–484. doi: 10.1007/s00262-016-1792-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Burugu S, Dancsok AR, Nielsen TO. Emerging targets in cancer immunotherapy. Semin. Cancer Biol. 2018;52:39–52. doi: 10.1016/j.semcancer.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 259.Dougall WC, Kurtulus S, Smyth MJ, Anderson AC. TIGIT and CD96: new checkpoint receptor targets for cancer immunotherapy. Immunol. Rev. 2017;276:112–120. doi: 10.1111/imr.12518. [DOI] [PubMed] [Google Scholar]
- 260.Szturz P, Vermorken JB. Immunotherapy in head and neck cancer: aiming at EXTREME precision. BMC Med. 2017;15:110. doi: 10.1186/s12916-017-0879-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Fang L, et al. Targeting late-stage non-small cell lung cancer with a combination of DNT cellular therapy and PD-1 checkpoint blockade. J. Exp. Clin. Cancer Res. 2019;38:123. doi: 10.1186/s13046-019-1126-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Gao Y, et al. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat. Immunol. 2017;18:1004–1015. doi: 10.1038/ni.3800. [DOI] [PubMed] [Google Scholar]
- 263.Suck G, et al. NK-92: an ‘off-the-shelf therapeutic’ for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol. Immunother. 2016;65:485–492. doi: 10.1007/s00262-015-1761-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Sek, K. et al. Targeting adenosine receptor signaling in cancer immunotherapy. Int. J. Mol. Sci. 19, 3837 (2018). [DOI] [PMC free article] [PubMed]
- 265.Whiteside TL. Targeting adenosine in cancer immunotherapy: a review of recent progress. Expert Rev. Anticancer Ther. 2017;17:527–535. doi: 10.1080/14737140.2017.1316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Wang, J., Lupo, K. B., Chambers, A. M. & Matosevic, S. Purinergic targeting enhances immunotherapy of CD73+ solid tumors with piggyBac-engineered chimeric antigen receptor natural killer cells. J Immunother Cancer6, 136 (2018). [DOI] [PMC free article] [PubMed]
- 267.Chambers AM, et al. Adenosinergic signaling alters natural killer cell functional responses. Front. Immunol. 2018;9:2533. doi: 10.3389/fimmu.2018.02533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Torres, Á. et al. Extracellular adenosine promotes cell migration/invasion of glioblastoma stem-like cells through A3 adenosine receptor activation under hypoxia. Cancer Lett.446, 112–122 (2019). [DOI] [PubMed]
- 269.Arab S, Hadjati J. Adenosine blockage in tumor microenvironment and improvement of cancer immunotherapy. Immune Netw. 2019;19:e23. doi: 10.4110/in.2019.19.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Frazao, A. et al. CD16+NKG2Ahigh natural killer cells infiltrate breast cancer-draining lymph nodes. Cancer Immunol. Res.7, 208–218 (2019). [DOI] [PubMed]
- 271.Jin D, et al. CD73 on tumor cells impairs antitumor T-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res. 2010;70:2245–2255. doi: 10.1158/0008-5472.CAN-09-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Nowak M, et al. The A2aR adenosine receptor controls cytokine production in iNKT cells. Eur. J. Immunol. 2010;40:682–687. doi: 10.1002/eji.200939897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Festag, J. et al. Preventing ATP degradation by ASO-mediated knockdown of CD39 and CD73 results in A2aR independent rescue of T-cell proliferation. Mol. Ther.-Nucl. Acid.21, 656–669 (2020). [DOI] [PMC free article] [PubMed]
- 274.Horenstein AL, Bracci C, Morandi F, Malavasi F. CD38 in adenosinergic pathways and metabolic re-programming in human multiple myeloma cells: in-tandem insights from basic science to therapy. Front. Immunol. 2019;10:760. doi: 10.3389/fimmu.2019.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Young A, et al. A2AR adenosine signaling suppresses natural killer cell maturation in the tumor microenvironment. Cancer Res. 2018;78:1003–1016. doi: 10.1158/0008-5472.CAN-17-2826. [DOI] [PubMed] [Google Scholar]
- 276.Lee, M. Y. & Allen, C. T. Mechanisms of resistance to T cell-based immunotherapy in head and neck cancer. Head Neck42, 2722–2733 (2020). [DOI] [PubMed]
- 277.Muller L, et al. Human tumor-derived exosomes (TEX) regulate Treg functions via cell surface signaling rather than uptake mechanisms. Oncoimmunology. 2017;6:e1261243. doi: 10.1080/2162402X.2016.1261243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Su W, et al. The cAMP-adenosine feedback loop maintains the suppressive function of regulatory T cells. J. Immunol. 2019;203:1436–1446. doi: 10.4049/jimmunol.1801306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Seitz L, et al. Safety, tolerability, and pharmacology of AB928, a novel dual adenosine receptor antagonist, in a randomized, phase 1 study in healthy volunteers. Invest. New Drugs. 2019;37:711–721. doi: 10.1007/s10637-018-0706-6. [DOI] [PubMed] [Google Scholar]
- 280.Wang WT, et al. Elevated absolute NK cell counts in peripheral blood predict good prognosis in chronic lymphocytic leukemia. J. Cancer Res. Clin. Oncol. 2018;144:449–457. doi: 10.1007/s00432-017-2568-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Pico de Coaña Y, Choudhury A, Kiessling R. Checkpoint blockade for cancer therapy: revitalizing a suppressed immune system. Trends Mol. Med. 2015;21:482–491. doi: 10.1016/j.molmed.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 282.Willingham SB, et al. A2AR antagonism with CPI-444 induces antitumor responses and augments efficacy to anti-PD-(L)1 and Anti-CTLA-4 in preclinical models. Cancer Immunol. Res. 2018;6:1136–1149. doi: 10.1158/2326-6066.CIR-18-0056. [DOI] [PubMed] [Google Scholar]
- 283.Welihinda, A. A., Kaur, M., Raveendran, K. S. & Amento, E. P. Enhancement of inosine-mediated A2AR signaling through positive allosteric modulation. Cell. Signal.42, 227–235 (2018). [DOI] [PMC free article] [PubMed]
- 284.Zhao X, Li L, Starr TK, Subramanian S. Tumor location impacts immune response in mouse models of colon cancer. Oncotarget. 2017;8:54775–54787. doi: 10.18632/oncotarget.18423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Masjedi A, et al. Downregulation of A2AR by siRNA loaded PEG-chitosan-lactate nanoparticles restores the T cell mediated anti-tumor responses through blockage of PKA/CREB signaling pathway. Int. J. Biol. Macromol. 2019;133:436–445. doi: 10.1016/j.ijbiomac.2019.03.223. [DOI] [PubMed] [Google Scholar]
- 286.Fallah-Mehrjardi K, et al. Pharmacological targeting of immune checkpoint A2aR improves function of anti-CD19 CAR T cells in vitro. Immunol. Lett. 2020;223:44–52. doi: 10.1016/j.imlet.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 287.Yang, R. et al. Conversion of ATP to adenosine by CD39 and CD73 in multiple myeloma can be successfully targeted together with adenosine receptor A2A blockade. J. Immunother. Cancer8, e000610 (2020). [DOI] [PMC free article] [PubMed]
- 288.Hatfield SM, et al. Immunological mechanisms of the antitumor effects of supplemental oxygenation. Sci. Transl. Med. 2015;7:277ra230. doi: 10.1126/scitranslmed.aaa1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Yu M, et al. CD73 on cancer-associated fibroblasts enhanced by the A2B-mediated feedforward circuit enforces an immune checkpoint. Nat. Commun. 2020;11:515. doi: 10.1038/s41467-019-14060-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Losenkova, K. et al. Compartmentalization of adenosine metabolism in cancer cells and its modulation during acute hypoxia. J. Cell Sci. 133, jcs241463 (2020). [DOI] [PMC free article] [PubMed]
- 291.Wu M, Mei F, Liu W, Jiang J. Comprehensive characterization of tumor infiltrating natural killer cells and clinical significance in hepatocellular carcinoma based on gene expression profiles. Biomed. Pharmacother. 2020;121:109637. doi: 10.1016/j.biopha.2019.109637. [DOI] [PubMed] [Google Scholar]
- 292.Azambuja JH, et al. CD73 downregulation decreases in vitro and in vivo glioblastoma growth. Mol. Neurobiol. 2019;56:3260–3279. doi: 10.1007/s12035-018-1240-4. [DOI] [PubMed] [Google Scholar]
- 293.Allard D, Chrobak P, Allard B, Messaoudi N, Stagg J. Targeting the CD73-adenosine axis in immuno-oncology. Immunol. Lett. 2019;205:31–39. doi: 10.1016/j.imlet.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 294.Zhang B. CD73: a novel target for cancer immunotherapy. Cancer Res. 2010;70:6407–6411. doi: 10.1158/0008-5472.CAN-10-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Fujisawa T, Isayama H. Immunotherapy for pancreatic. Cancer. Juntendo Med. J. 2020;66:238–244. doi: 10.14789/jmj.2020.66.JMJ20-LN02. [DOI] [Google Scholar]
- 296.Francis DM, Thomas SN. Progress and opportunities for enhancing the delivery and efficacy of checkpoint inhibitors for cancer immunotherapy. Adv. Drug Deliv. Rev. 2017;114:33–42. doi: 10.1016/j.addr.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Leone RD, Lo YC, Powell JD. A2aR antagonists: next generation checkpoint blockade for cancer immunotherapy. Comput. Struct. Biotechnol. J. 2015;13:265–272. doi: 10.1016/j.csbj.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Vaupel P, Multhoff G. Adenosine can thwart antitumor immune responses elicited by radiotherapy: therapeutic strategies alleviating protumor ADO activities. Strahlenther. Onkol. 2016;192:279–287. doi: 10.1007/s00066-016-0948-1. [DOI] [PubMed] [Google Scholar]
- 299.Vigano S, et al. Targeting adenosine in cancer immunotherapy to enhance T-cell function. Front. Immunol. 2019;10:925. doi: 10.3389/fimmu.2019.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Zhu, Z. et al. Checkpoint inhibitors for stage I to III non‐small cell lung cancer treated with surgery or radiotherapy with curative intent: a generic protocol. Cochrane Database Syst. Rev. 6, CD013364 (2019).
- 301.Nicolai, C. J. et al. NK cells mediate clearance of CD8+ T cell-resistant tumors in response to STING agonists. Sci. Immunol.5, eaaz2738 (2020). [DOI] [PMC free article] [PubMed]
- 302.Parihar R, et al. NK cells expressing a chimeric activating receptor eliminate MDSCs and rescue impaired CAR-T cell activity against solid tumors. Cancer Immunol. Res. 2019;7:363–375. doi: 10.1158/2326-6066.CIR-18-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Tang M, et al. Tumoral NKG2D alters cell cycle of acute myeloid leukemic cells and reduces NK cell-mediated immune surveillance. Immunol. Res. 2016;64:754–764. doi: 10.1007/s12026-015-8769-3. [DOI] [PubMed] [Google Scholar]
- 304.Lim O, et al. GMP-compliant, large-scale expanded allogeneic natural killer cells have potent cytolytic activity against cancer cells in vitro and in vivo. PLoS ONE. 2013;8:e53611. doi: 10.1371/journal.pone.0053611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Han B, et al. Altered NKp30, NKp46, NKG2D, and DNAM-1 expression on circulating NK cells is associated with tumor progression in human gastric cancer. J. Immunol. Res. 2018;2018:6248590. doi: 10.1155/2018/6248590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Zhu, J. et al. Immunotherapy (excluding checkpoint inhibitors) for stage I to III non-small cell lung cancer treated with surgery or radiotherapy with curative intent. Cochrane Database Syst. Rev.12, CD011300 (2017). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.