Abstract
The gut-brain axis is a bidirectional information interaction system between the central nervous system (CNS) and the gastrointestinal tract, in which gut microbiota plays a key role. The gut microbiota forms a complex network with the enteric nervous system, the autonomic nervous system, and the neuroendocrine and neuroimmunity of the CNS, which is called the microbiota-gut-brain axis. Due to the close anatomical and functional interaction of the gut-liver axis, the microbiota-gut-liver-brain axis has attracted increased attention in recent years. The microbiota-gut-liver-brain axis mediates the occurrence and development of many diseases, and it offers a direction for the research of disease treatment. In this review, we mainly discuss the role of the gut microbiota in the irritable bowel syndrome, inflammatory bowel disease, functional dyspepsia, non-alcoholic fatty liver disease, alcoholic liver disease, cirrhosis and hepatic encephalopathy via the gut-liver-brain axis, and the focus is to clarify the potential mechanisms and treatment of digestive diseases based on the further understanding of the microbiota-gut- liver-brain axis.
Keywords: Microbiota-gut-brain axis, Gut-liver axis, Gut microbiota, Digestive diseases, Herbaceous medications
Core Tip: Microbiota-gut-liver-brain axis regulates the occurrence and development of many diseases, and it offers a new direction for research on the treatment of diseases. In recent years, there have been more and more studies on the microbiota-gut-liver-brain axis, which not only increases the understanding of its pathogenesis, but also provides many novel treatment methods. We herein discuss the role of microbiota-gut-liver-brain axis in digestive diseases with a focus on clarifying the potential mechanisms and treatment.
INTRODUCTION
How does microbiome affect human health? According to related research, the ratio of microorganisms living in the human body to human cells is close to 1:1, and the vast majority live in the colon whereby the gut represents the largest reservoir of microorganisms[1]. Over the past decade, research on the trillions of microbiota living in the human body and their interaction with hosts has increased significantly. These previously neglected members has been recognized as providing the functions of host physiology, such as metabolism, immunity, cardiovascular function and neuronal development, and dysregulation of their structure or function can lead to the distortion of microbial-host homeostasis and may cause disease[2]. Therefore, it is important to understand the role of gut microbiota in the development of human diseases.
The gut microbiota forms a complex network along with the enteric nervous system (ENS), the autonomic nervous system (ANS), and the neuroendocrine and neuroimmunity of the central nervous system (CNS), which is called the microbiota-gut-brain axis. Signals of microbiota-gut-brain axis can occur through a variety of mechanisms. These mechanisms also affect physiological function at multiple levels. However, the signal pathways of the microbiota-gut-liver-brain axis need to be further studied, and the related therapies need to be further explored.
Recently, a growing number of studies have shown that gut microbiota is associated with many diseases, such as depression, autism, anxiety, obesity, schizophrenia, diabetes, Parkinson's disease and Alzheimer's disease. This means that the research of gut microbiota is crucial to the development of personalized health care strategies in the future, and people can directly adjust the gut microbiota to benefit the host. Given that gut microbiota play a key role in the gut-liver-brain axis, we mainly discuss the role of the gut microbiota in the irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), functional dyspepsia (FD), non-alcoholic fatty liver disease (NAFLD), alcoholic liver disease (ALD), cirrhosis and hepatic encephalopathy (HE) via the gut-liver-brain axis in this review, and focus on the potential mechanisms and treatment (Table 1).
Table 1.
Common treatments based on microbiota-gut-liver-brain axis
| Treatment | Mechanism | Example | Treated diseases | Ref. |
| Probiotics | Reduce depression and alter brain activity | Bifidobacterium longum subsp. Longum NCC3001 | Irritable bowel syndrome | [36] |
| Regulate immunity and diminish inflammation | VSL#3 | Ulcerative colitis | [61] | |
| Modulate gut microbiota, restore the intestinal barrier function and prevent mesenteric artery endothelial dysfunction, as well as enhance bile acids excretion, | LGG | Non-alcoholic fatty liver disease; hepatic encephalopathy; alcoholic liver disease | [115,141,168] | |
| Reduce ammonia levels by reducing gut microbiota imbalances | Lactobacillus and Bifidobacterium species | Hepatic encephalopathy | [143] | |
| FMT | Modulate gut microbiota, reduce endotoxin and inflammation factors, as well as reduce neuroinflammation | Irritable bowel syndrome; inflammatory bowel disease; non-alcoholic fatty liver disease; alcoholic liver disease; hepatic encephalopathy | [119,148] | |
| Antibiotic | Modulate gut microbiota and their end-products, as well as improve the cognitive function | Rifaximin | Hepatic encephalopathy; irritable bowel syndrome | [18,37] |
| Psychotherapy | Improve mental health | CBT | Irritable bowel syndrome; inflammatory bowel disease | [38,39] |
| Acupuncture and moxibustion | Regulate gut microbiota, repair mucosal tissue damage and improve gut mucosal immunity | Moxibustion | Irritable bowel syndrome; inflammatory bowel disease | [45,66] |
| Improve the balance of the HPA axis and anxiety behaviors | EA and MB | Functional dyspepsia | [68] | |
| Regulate gut-brain peptides and promote the gastric empty rate | Herbal cake-separated moxibustion | Alcoholic liver disease | [96] | |
| Herbaceous Medications | Improve gastrointestinal function, | DA-9701 (Motilitone) | Functional dyspepsia | [88] |
| Increase the production of ghrelin, cholecystokinin and vasoactive intestinal peptides | Xiangsha Liujunzi Decoction | Functional dyspepsia | [89] | |
| Increase the levels of motilin, gastrin and gastric emptying rate | XiaoErFuPi granules | Functional dyspepsia | [90] | |
| Regulate gut microbiota and promote the gastric empty rate | MA | Functional dyspepsia | [91] | |
| Treat both gastrointestinal and psychological symptoms | Rikkunshito | Functional dyspepsia | [92] | |
| Regulate gut mucosal barrier, lipid metabolism and liver function | Dachaihu decoction | Non-alcoholic fatty liver disease | [122] | |
| Polyphenol | Modulate gut microbiota, reduce inflammation factors and alleviate the pathological injuries, | Raw Bowl Tea polyphenol | Non-alcoholic fatty liver disease | [126] |
| Change the metabolism of bile acids | Green tea polyphenol | Non-alcoholic fatty liver disease | [127] |
LGG: Lactobacillus rhamnosus GG; CBT: Cognitive behavior therapy; EA: Electroacupuncture; MB: Moxibustion; HPA: Hypothalamus-pituitary-adrenal; MA: Magnoloside A.
MICROBIOTA
The gut microbiota is a highly dynamic system, whose density and composition are affected by a variety of exogenous and endogenous factors[3], however, the disorder of the gut microbiota is the basis for the occurrence of many diseases. Understanding the influencing factors of the microbiota can further regulate the microbiota and thus have a beneficial effect on the host. In addition, gut microbiota can produce bioactive peptides, including neurotransmitters, secondary bile acid conversion, short chain fatty acids (SCFAs), branched chain amino acids, and intestinal hormones[4]. These bioactive peptides are involved in the signals of the gut-brain axis, of which the representative is SCFAs, which enter the circulatory system and send signals to the brain via the gut-brain axis, and at the same time stimulate the hypothalamus-pituitary-adrenal (HPA) axis or it may directly affect the mucosal immune system, which can indirectly affect CNS transmission[5]. At the same time, more and more studies have shown that the gut microbiota plays an important role in regulating the body's basic functions such as metabolism, immunity, cardiovascular function and neuronal development[2,6]. Therefore, the gut microbiota may be a potential target for effective personalized medication for some diseases in the future. At present, whole-genome association research is underway, providing valuable microbial function potential for human studies, and the development of high throughput and low-cost sequencing methods, metabolomics, and proteomics has made an important contribution for the research.
MICROBIOTA-GUT-BRAIN AXIS
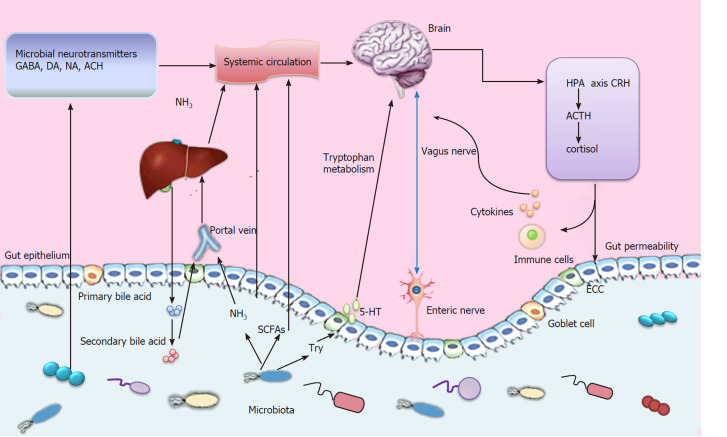
The gut-brain axis is a bidirectional information interaction system between the CNS and the gastrointestinal tract, involving neural, endocrine, and immune systems (Figure 1), which allows our brain and emotional state to influence gastrointestinal homeostasis and function from the top down, and regulates brain function and behavior from the bottom up[7]. This bidirectional interaction constitutes an up-down regulation and a down-up regulation system.
Figure 1.
Diagram showing the varied and complex bidirectional information interaction system for microbiota-gut-liver-brain axis, including neural, endocrine, and immune systems. GABA: Gamma-aminobutyric acid; DA: Dopamine; NA: Noradrenaline; Ach: Acetylcholine; 5-HT: Serotonin; HPA: Hypothalamus-pituitary-adrenal; CRH: Corticotropin-releasing hormone; ACTH: Adrenocorticotropic hormone; ECC: Enterochromaffin cell; SCFAs: Short chain fatty acids; Try: Tryptophan.
Up-down regulation. The regulation of the gastrointestinal tract by the nervous system is realized through the interaction of three levels: CNS, ANS and ENS. The ENS senses and responds to the dynamic ecosystem of the gastrointestinal tract by converting chemical signals from the environment into nerve impulses, which spread to the entire intestine and other organs of the body, including the CNS[8]. The ANS include sympathetic nerves and parasympathetic nerves, both of which can antagonize, cooperately or independently exert their autonomous effects. Also, the ANS controls the main functions of the gastrointestinal tract such as gastrointestinal motility, regulation of gastrointestinal blood flow, and secretion of digestive juice[9]. Moreover, studies have confirmed that the ANS affects intestinal epithelial stem cell proliferation[10]. The CNS affects the activity of the gastrointestinal tract by regulating the sympathetic and parasympathetic nerves and some structures of the CNS are involved in this process, such as amygdala, hypothalamus, nucleus tractus solitarius, etc[11]. In addition, the HPA axis plays an important role in mediating the effects of stress on the gastrointestinal tract[12].
Down-up regulation. Intestinal cells produce a variety of signal molecules, which can travel through the blood-brain barrier to the CNS after passing through the bloodstream. For example, a high-salt diet induces TH17 response in the intestine, resulting in an increase in circulating plasma interleukin-17 (IL-17), IL-17 in turn acts on brain endothelial cells, inhibiting the production of NO by endothelial cells, leading to reduced cerebral perfusion and cognitive dysfunction[13]. However, most neurotransmitters produced by microbiota, including serotonin, dopamine, and aminobutyric acid, usually cannot break through the blood-brain barrier that protects the brain. It can directly act on specific receptors of exogenous primary afferent neuron cell bodies, or it can cross the blood-brain barrier through neurotransmitter precursors and then is converted into active neurotransmitters. For example, gut microbiota can affect the metabolism of serotonin precursor tryptophan. This may affect serotoninergic signaling in the CNS, as tryptophan concentrations in plasma have been shown to correlate with serotonin levels in the brain[14].
The gut microbiota plays a key role in the gut-brain axis, which is proved by evidence in six different aspects: (1) Studies on sterile animals have shown that the brain is affected in the absence of a microbiome. For example, mice that grow in a sterile environment have a more exaggerated physiological response to stress, and Bifidobacterium infantis can reverse the excessive HPA stress response in sterile mice[15]; (2) Transplanting gut microbiota can change brain pathophysiology. For example, the intestinal microorganisms of patients with Parkinson′s disease are isolated and transplanted into the intestinal tract of a mouse model of Parkinson′s disease, which can aggravate the pathological changes in Parkinson′s disease[16]; (3) Extensive research shows that multiple probiotic strains eliminate stress, depression, and anxiety-like behaviors in preclinical models and human studies[4]; (4) Bacterial colonization of the gut is key to the development and maturation of important systems (including immune and endocrine systems) that influence programming and signaling in the CNS after birth[17]; (5) HE can be treated with antibiotics that target microorganisms, such as rifaximin, an oral antibiotic that modulates gut microbiota and their end-products[18]; and (6) the gut microbiota and their metabolites may affect specific brain structures, Labus et al[19] have demonstrated that the composition and function of gut microbiota in IBS are related to regional brain structure changes, and gut microbial composition is correlated with structural measures of brain regions including sensory- and salience-related regions. As a consequence, in the occurrence and development of the disease, we should pay attention to the role of the gut microbiota and the gut-brain axis, explore the mechanism of the disease, and develop promising strategies for future treatment.
MICROBIOTA-GUT-LIVER-BRAIN AXIS
In recent years, due to the high incidence of liver disease, the interaction between the gut and liver has gradually been recognized. The gut and liver communicate with each other through the portal vein, biliary tract, and systemic circulation. Intestinal products, such as host and/or microbial metabolites and microbial-associated molecular patterns (MAMPs), are transported to the liver through the portal vein and influence liver function. In parallel, the liver transports bile salts and antimicrobial molecules to the intestinal lumen through the biliary tract to maintain gut eubiosis by controlling unrestricted bacterial overgrowth[20]. Hence, gut dysbiosis can lead to metabolic disorders in the liver, which in turn leads to liver damage. For example, dysbiotic gut microbiota reduces the activation of nuclear bile acid receptor FXR and membrane G protein-coupled receptor TGR5, which leads to the decrease in the synthesis of secondary bile acids, thereby contributing to bile salt retention, small intestinal translocation and bacterial overgrowth, finally leading to liver disease[21]. The diseased liver cannot effectively inhibit the overgrowth of bacteria, remove the harmful microbial by-products, and accelerate the progress of the disease. Moreover, it is reported that liver damage is closely related to the severity of gut dysbiosis[22]. Therefore, in the occurrence and development of the disease, while paying attention to the important role of the gut-brain axis in the disease, the role of the liver in the gut-brain axis cannot be ignored, especially in liver diseases, such as HE, which is considered a typical microbiota-gut-liver-brain axis disease model.
MICROBIOTA-GUT-LIVER-BRAIN AXIS AND IBS
IBS is a common functional disease characterized by abdominal pain or discomfort accompanied by changes in bowel habits without organic lesions. It is the most common chronic visceral pain syndrome. The syndrome has no significant structural or biochemical abnormalities, and is defined by the criteria for symptoms. The Rome IV criteria are currently used for the diagnosis of IBS. IBS subtypes can be divided into IBS with predominant constipation, IBS with predominant diarrhea, IBS with mixed bowel habits, and IBS unclassified according to changes in predominant bowel habits[23]. Internal and external regulatory factors associated with the development of the disease include heredity, dietary intake, gastrointestinal infections, increased intestinal permeability, low inflammation, bile salt metabolism disorders, abnormal serotonin metabolism, CNS dysfunction, visceral allergies, gut microbiota imbalance. Patients with severe symptoms are more likely to be caused by a combination of factors[24,25].
There is increasing consensus that changes in the gut-brain axis plays an important role in the pathophysiology of IBS. Currently, the interaction of brain, gut and gut microbial metabolites is one of the important pathophysiological foundations of IBS. These metabolites are mainly involved in the subcortex but also in the cerebral cortex, which may alter the perception of pain in patients with IBS and may be mediated by microbial regulation of the intestinal serotoninergic system[26]. Labus et al[19] have demonstrated that the composition and function of gut microbiota in IBS are related to regional brain structure changes, and gut microbial composition is correlated with structural measures of brain regions including sensory- and salience-related regions, which suggests that the gut microbiota and their metabolites may affect specific brain structures, and gut microbiota may play a role in the development and formation of the microbiota-gut-brain axis. Based on this finding, it may play an important role in optimizing the treatment of IBS.
Treatment of IBS. (1) Diet control can improve symptoms. Among the many factors that have been proposed, intolerance to malabsorptive dietary carbohydrates has become a major cause of IBS, therefore dietary interventions is important[27]. Many studies have found that low-fermented oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAP) diets significantly improve symptoms[28,29], Zhou et al[30] fed rats with high doses of FODMAP feed, and observed elevated fecal Gram-negative bacteria and serum lipopolysaccharide levels, as well intestinal inflammation, barrier dysfunction, and visceral allergies response, and these symptoms can be prevented by antibiotics and reversed by a low FODMAP diet; (2) fecal microbiota transplantation (FMT), one of the effective methods for treating IBS, high-dose transplantation or repeated FMT can improve the response rate and intensity of FMT[31]. Use of FMT shortly after disturbance of the gut microbiota in IBS may improve efficacy[32], but the current evidence from randomized controlled trials does not suggest that FMT is beneficial for global IBS symptoms[33]. Therefore, the clinical efficacy of FMT in the treatment of IBS warrants more clinical research; (3) Probiotics can inhibit the excessive growth of pathogenic bacteria, competitively exclude pathogens and strengthen the intestinal barrier, enhance host immunity, increase the production of IgA, regulate the production of cytokines, produce or secrete SCFAs, and promote the absorption of ions and trace elements[34]. The use of multi-biotic probiotic supplements has the potential to improve symptoms of IBS. However, the specific symptoms improved by probiotics supplementation have not been consistent in different studies. Some studies have found general improvement in symptoms of IBS, while others have reported improvements in specific symptoms[35]. For example, Ray et al[36] demonstrated that Bifidobacterium longum subsp. Longum NCC3001 (BL) reduced the depression score and changed their brain activity in IBS patients, but BL had no effect on anxiety; (4) antibiotic therapy, short courses of non-absorbable antibiotics such as rifaximin have been shown to moderately improve the symptoms of IBS, especially bloating and flatulence[37], however, the exact duration of this improvement has not been determined, and further research is needed; (5) psychotherapy, especially face-to-face cognitive-behavioral therapy, can improve mental health and quality of life in patients with IBS[38]. Moreover, Dickson et al[39] followed adults with refractory IBS (12 mo of clinically significant symptoms despite first-line treatment), and found that compared with the conventional treatment group, the cognitive behavioral treatment group (including telephone and Web page method) has significant clinical and statistical improvements in IBS symptoms and effects on life and emotions, and there are no serious adverse reactions after treatment. Cognitive-behavioral research solidifies its status as the leading treatment for non-drug IBS, and emphasizes the importance of central processes under gut-brain interactions, which may pave the way for novel therapeutic strategies[40]; and (6) acupuncture and moxibustion can effectively regulate gastrointestinal motility, visceral hypersensitivity, gut-brain axis, neuroendocrine system and immune system[41]. In addition, acupuncture has a regulating effect on gut-brain peptides. For example, acupuncture at “Zusanli” and “Taichong” points can reduce the content of somatostatin, vasoactive intestinal peptide (VIP), and substance P (SP) in the ileum of IBS-D model rats[42]. Moreover, some studies have shown that oculo-acupuncture can not only up-regulate mRNA and protein expression of serotonin reuptake transporter in IBS model rats[43], but also significantly reduces the content of SP and VIP in serum and colon tissue of IBS rats, which improves the abnormal function of the gut-brain axis[44]. In addition, moxibustion treats IBS by regulating the gut microbiota. Wang et al[45] first tested the effect of moxibustion on the gut microbiota of IBS and found that IBS-related changes in the gut microbiota can be normalized by moxibustion, and the diversity of gut microbiota caused by moxibustion. As a consequence, acupuncture and moxibustion provide new ideas and targets for the clinical treatment of IBS.
MICROBIOTA-GUT-LIVER-BRAIN AXIS AND IBD
IBD is a chronic immune-mediated inflammatory disease of the intestinal mucosal tissues, mainly in two forms: Crohn's disease (CD) and ulcerative colitis (UC). CD is characterized by transmural inflammation of any part of the gastrointestinal tract, while UC affects the mucosal layers of the colon and rectum[46]. The incidence of IBD is increasing worldwide, with the highest incidence in Europe and North America, and the rising incidence in Asia[47]. Its etiology involves complex interactions between the environment, heredity, microbes, and immunity. Genome-wide association studies have confirmed an increase in IBD susceptibility sites to 163, most of which are related to CD and UC, and most of them are involved in regulating intestinal barrier function and host-microbe interactions[48]. And Chu et al[49] proposed that polymorphisms of susceptible genes promote disease through defects in the microbiome′s perception of protective signals, defining a potentially critical environmental etiology of IBD genes.
Recent advances in next-generation sequencing technology have confirmed adverse changes in the composition and function of the gut microbiota in IBD[50], such as reduced gut microbiota diversity, reduced SCFAs-producing bacteria, and increased hemolytic bacteria, sulfate-reducing bacteria, and pathogenic bacteria. This change can affect the integrity of the host′s immune system and barriers, leading to chronic diseases and abnormal immune responses. Furthermore, Imhann et al[51] found that healthy individuals with a high genetic risk load of IBD also had adverse changes in their gut microbiota. To our knowledge, the gut microbiota plays an important role in maintaining the intestinal environment balance, the development and activation of the host′s immune system. Therefore, adverse changes in the gut microbiota will lead to the development of disease. In addition, the gut microbiota also affects the host′s susceptibility to disease, and even affects the host′s metabolic function[50].
Empirical studies have found that under stress conditions such as lack of sleep and lack of physical activity, the brain (HPA axis) stimulates the production of pro-inflammatory cytokines, which can lead to increased intestinal permeability and changes in the gut microbiota. Moreover, personal habits (hygiene and smoking), long-term consumption of foods rich in fat and sugar, long-term use of drugs and genetic susceptibility can directly affect the composition of gut microbiota, and then affect the permeability of the intestine[46]. Therefore, these factors further lead to the development of IBD. More and more people believe that IBD is related to anxiety and depression-related symptoms, and behavioral disorders including anxiety and depression-like symptoms are also observed in animal models of IBD[52]. In a large primary care database study, Frolkis et al[53] found that depression is associated with an increased risk of IBD, and that treatment with antidepressant medications can decrease the incidence of IBD. In addition, Gracie et al[54] showed evidence of the bi-directional effect of IBD activity and mental disorders, that is, patients with normal anxiety scores at baseline and active disease were almost 6 times more likely to have abnormal anxiety scores during follow-up. Similarly, patients with quiescent disease activity at baseline, but abnormal anxiety scores, had 2-fold higher rates of flare of disease activity or need for glucocorticosteroids. These results highlight the bidirectional gut-brain axis interaction in patients with IBD.
During the course of IBD, symptoms such as abdominal pain, cramps, loose stools or bloody diarrhea, fatigue, anemia, or weight loss can occur. Fatigue is one of the most common and severe symptoms in IBD patients, which can lead to decreased quality of life and impaired productivity. An online survey from the European Crohn′s Disease Alliance and the Ulcerative Colitis Association found that 53% of patients with IBD and anemia and 40% of patients with IBD feel fatigued almost every day[55]. Recent research suggests that gut-brain axis may play a role in mediating fatigue. Key pathways include immunity (cytokines), metabolism (tryptophan), endocrine (cortisol), branch chains amino acids and SCFAs[56]. Therefore, IBD management should not only focus on inflammatory activities, but also the role of the gut-brain axis.
Treatment of IBD. (1) Control inflammatory response. Immune mediators are the main therapeutic targets for IBD. The homeostasis of effector cells and Treg cells in the intestine suggests the therapeutic potential of Treg cells in IBD patients, and studies have found that the gut microbiota is the key to inducing Treg cells and IL-10 production[57]. Therefore, regulating the gut microbiota can indirectly affect the homeostasis of Treg cells, thereby benefiting patients and providing a direction for future immunotherapy of IBD; (2) Intervention in gut microbiota: (a) Drug treatment: 5-Aminosalicylic acid (5-ASA) has a significant effect on bacterial gene expression[58], and Mesalazine reduces fecal and mucoadhesive bacteria concentrations[59]; (b) Probiotics: Many probiotics have been tested for their effectiveness in IBD. The mechanism is to regulate the microbiota and relieve the gut microbiota imbalance. A meta-analysis has found that probiotics can provide a similar effect to that of 5-ASA in maintaining UC remission[60], especially VSL#3 (a multi-organism combination), which may induce remission in patients with mild to moderate UC[61]. However, probiotic administration has no additional benefit for patients with relapsed CD under endoscopy, and its effect on CD needs to be further explored; and (c) FMT: Antibiotic pretreatment may increase the effectiveness of FMT or other microbial treatments[62]. But FMT is not as effective in IBD as in Clostridium infection[50]. In addition, there are still issues to be addressed in FMT. In addition to low effectiveness and safety uncertainty, other challenges include the lack of standardization of procedures, the risk of pathogen transfer, and the induction of unnecessary phenotypes (such as flares in UC patients)[63]. Therefore, more studies need to be done about the clinical application of FMT in order to reduce its adverse effects; (3) Psychotherapy and antidepressants. Integrating these treatments into the bio-psycho-social care model may improve the mental health and quality of life of some IBD patients, and change the natural history of the disease[64]; and (4) Acupuncture and moxibustion. Experimental and clinical studies have shown that acupuncture is a safe and effective treatment, and a meta-analysis has shown that acupuncture is more effective than oral sulphasalazine in the treatment of IBD[65]. Moxibustion plays a therapeutic role by repairing mucosal tissue damage, regulating gut microbiota and improving intestinal mucosal immunity[66,67] and short-term (7 d) rather than long-term (14 d) moxibustion may significantly affect the gut microbiota[66]. Studies by Wei et al[68] have shown that both electroacupuncture and moxibustion can improve anxiety behaviors in patients with colitis, and its effect comes in part from improving the balance of the HPA axis. In addition, studies have shown that changes in regional homogeneity in the subcortical regions of the electroacupuncture group and moxibustion group are associated with a decrease in the CD activity index. But the regulation mechanism is different, electroacupuncture regulates homeostatic afferent processing network, while moxibustion mainly regulates the default mode network of the brain[69].
MICROBIOTA-GUT-LIVER-BRAIN AXIS AND FD
FD is referred to as a series of symptoms in the gastroduodenal region of the upper gastrointestinal tract and is characterized by one or more of the followings: Postprandial fullness, early satiety, abdominal pain, epigastric burning, which are unexplained after a routine clinical evaluation. It includes two subgroups: postprandial distress syndrome, which is characterized by indigestion symptoms caused by meals, and epigastric pain syndrome, which does not occur only after meals, and these two subgroups can overlap[70]. About 21% of the world′s population has dyspepsia[71]. However, prevalence varies among different countries according to the differently defined criteria. About 10% of adults in the United States, Canada and the United Kingdom have FD based on Rome IV symptoms, and the highest prevalence is reported in the United States[72]. FD not only obviously affects the quality of life and work efficiency, but also poses a huge economic burden on the medical system[73].
The pathogenesis of FD is heterogeneous, and the relationship between possible pathophysiology factors is extremely complicated. It is impossible to have a unified pathological mechanism to explain the symptoms of all FD patients[74]. Various changes in the function and structure of the gastrointestinal tract in FD patients include changes in the stomach (impaired regulatory function, delayed gastric emptying and allergies), and changes in the duodenum (increased duodenal acid and/or lipid sensitivity and mild inflammation). These functional and structural abnormalities can interact[74], and impaired mucosal integrity, barrier dysfunction, low-level immune activation, and abnormal regulation of the gut-brain axis are also involved[70,74-76]. In a study of the Swedish population, anxiety increased FD risk by 7.6 times over the past 10 years[77]. There are also epidemiological studies showing that the prevalence of anxiety and depression is higher in patients with FD than in healthy people, which suggests that mental illness has an intrinsic role in the pathogenesis of FD, and pathophysiology research also shows that psychosocial factors and mental disorders may play a role in FD by regulating the processing of visceral signals in the brain[78]. In addition, the gut microbiota also plays an important role in the pathogenesis of FD. Zhong et al[79] used the 16S rRNA gene sequence to determine the relative abundance of bacterial genus in the duodenal mucosa. It was found that the relative abundance of Streptococcus was high in patients with FD, and the anaerobic genera Prevotella, Veillonella and Actinomyces was significantly reduced. And it was also found that duodenal mucosal bacterial load was related to FD symptoms. Furthermore, in a small number of patients with FD, Helicobacter pylori (Hp) infection is the cause of dyspepsia. Hp infection can cause chronic mucosal inflammation of the stomach and duodenum, which may lead to gastroduodenum movement and sensitivity abnormalities[80]. Therefore, in view of the fact that the abnormal regulation of the gut microbiota and the gut-brain axis play a key role in the pathogenesis of FD, the basis of microbiota-gut-brain axis dysfunction cannot be ignored in its treatment.
Treatment of FD. (1) Eradication of Hp. American College of Gastroenterology and the Canadian Association of Gastroenterology guidelines recommend that patients less than 60 years of age should be tested for non-invasive Hp and should be treated if the test is positive[81]. Eradication of Hp may improve symptoms in FD patients with epigastric pain and postprandial distress[80]. In addition, a meta-analysis shows that Hp eradication treatment leads to statistically significant improvement in long-term symptoms in patients with FD, although the effect is small[82]; (2) Acid inhibitory drug and prokinetics. Proton pump inhibitors (PPIs), histamine type 2 receptor antagonists and prokinetics have been recommended as first-line treatments for FD, patients with epigastric pain syndrome benefit from acid inhibitory drug, while patients with postprandial distress syndrome benefit from prokinetic drugs[83]. Recently, a meta-analysis has found that PPIs are more effective than prokinetic drugs[84], however, the use of PPIs is related to changes in the composition of the gut microbiota, which may lead to an increased risk of infection[85,86], therefore, both medical staff and researchers should consider the impact of PPIs on the gut microbiota when using PPIs; (3) Antipsychotics and antidepressants. Use of antidepressants showed a negative association with postprandial distress syndrome[72]. In addition, a systematic review and meta-analysis showed that psychotropic drugs may be effective for FD, but their effects seem to be limited to antipsychotics and tricyclic antidepressants[87]. Therefore, the exact efficacy of psychotropic drugs still needs to be confirmed by more research; (4) Herbaceous medications. Multi-component Chinese medicine for multiple targets may be a promising alternative therapy for FD. DA-9701 (Motilitone) is a botanical drug consisting of Corydalis tuber and morning glory seeds. It has been found to improve symptoms and gastrointestinal function in patients with FD, and to be safer than traditional medicine[88]. At the same time, studies have found that Xiangsha Liujunzi Decoction alleviates the symptoms of FD by increasing the production of ghrelin, cholecystokinin and VIP and increasing the levels of these neuropeptides in the circulation[89]. Moreover, Wei et al[90] found that XiaoErFuPi granules can increase the levels of motilin, gastrin and gastric emptying rate, and thus have a good effect on patients with FD. Xue et al[91] isolated and purified magnoloside A (MA) from Magnolia officinalis, and found that MA accelerated the delayed intestinal emptying of FD rats and increased the levels of gastrin, motilin, and calcitonin gene-related proteins, reducing the levels of serotonin, nitric oxide synthase, and VIP. On the other hand, MA can regulate the composition of the intestinal microbiota, leading to changes in SCFAs. In addition, a randomized, placebo-controlled, double-blind clinical trial found that Rikkunshito (a Japanese herbal medicine) may be beneficial for FD patients with both gastrointestinal and psychological symptoms[92]. These studies indicate that herbaceous plants and their extracts have great therapeutic potential in FD; (5) Acupuncture and moxibustion, there have been several studies showing that acupuncture therapy is superior to prokinetics in improving the symptoms and quality of life of FD patients[93,94]. Exact treatment mechanism is being explored. Fang et al[95] showed that the brain function of FD patients after treatment was close to that of the healthy control, The relief of gastrointestinal signs and symptoms by acupuncture is likely due to the normalization of gut-brain axis associated with FD. In addition, herbal cake-separated moxibustion can promote the gastric empty rate in FD rats, which may be associated with its effects in inhibiting stress induced decrease of hypothalamic 5-HT, DA and NE levels[96]. Therefore, acupuncture can be used as an effective supplement to routine treatment of patients with FD; and (6) Other treatments. Studies have found that mast cells increase during FD with or without inflammation, which may be caused by altering the gut-brain axis signal[97]. Mast cells play a key role in the regulation of the mucosal barrier. Signals from the intestinal nerve directly or through other lamina propria cells stimulate mast cells, releasing mediators through receptors, and in turn affect the epithelial barrier[98]. At the same time, the degree of paracellular permeability was positively correlated with the number of mast cells[76], therefore, the stabilization or blocking of mast cell surface receptors provides new insights into the treatment of FD. In addition, studies have found that child compound Endothelium corneum may enhance gastrointestinal motility by balancing homeostasis of the microbiota-gut-brain axis in FD rats[99], which also provides a new way for the treatment of FD.
MICROBIOTA-GUT-LIVE-BRAIN AXIS AND NAFLD
NAFLD is a common chronic liver disease, including a series of liver damage, from steatosis to steatohepatitis with or without fibrosis. Fibrosis may develop into cirrhosis and complications including hepatocellular carcinoma[100]. NAFLD can be divided into non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH) in histology[101]. The development of NAFLD is closely related to obesity, hyperlipidemia, hypertension, type-2 diabetes and metabolic syndrome. NAFLD is generally considered to be the liver manifestation of metabolic syndrome[102]. Currently, the understanding of the pathogenesis of NAFLD is still incomplete. It is believed to be the result of a combination of multiple damaging factors, including insulin resistance, oxidative stress, lipid metabolism alteration, inflammatory cytokines liberation, endoplasmic reticulum stress, gut dysbiosis or gut-liver axis activation, genetic and epigenetic factors[103,104]. In addition, studies have found that the neurobehavioral disorder of NAFLD is related to hyperammonemia, gut dysbiosis, metabolism and functional defects of brain regions[105]. Therefore, the microbiota-gut-live-brain axis plays an important regulatory role in the pathogenesis of NAFL/NASH, and the main participants are the gut microbiota, its bacterial products, and the intestinal barrier[106].
Colonization of the gut microbiota of patients with NASH can aggravate hepatic steatosis and inflammation in sterile mice fed a high-fat diet (HFD), confirming that the gut microbiota plays a vital role in the development of NAFLD[107]. And the study by Boursier et al[108] showed that the severity of NAFLD is related to gut dysbiosis and changes in the metabolic function of the gut microbiota. Changes in gut microbiota may stimulate liver fat deposition via the following mechanisms: Regulating intestinal permeability, increasing low-grade inflammation, regulating dietary choline metabolism, regulating bile acid metabolism, and producing endogenous ethanol[109]. And, gut microbiota has also been reported to play a role in the neuroendocrine regulation of lipid metabolism[110]. Furthermore, gut microbiota metabolites and ammonia may produce neurotoxic damage, which is related to the cognitive impairment of NASH[105], however, more research is necessary to fully understand the underlying mechanism of functional changes caused by cognitive impairment in NASH. Mouries et al[111] have recently found that the disruption of the intestinal epithelial barrier and gut vascular barrier (GVB) are early events in the onset of NASH, and HFD-mice in just one week will cause GVB damage and bacterial translocation. Hence, in addition to the important role of gut microbiota and its products in NAFLD, the intestinal barrier is also very important.
Treatment of NAFLD. In recent years, with in-depth research on the microbiota-gut-live-brain axis, the gut microbiota has become a therapeutic target for NAFLD: (1) Lifestyle interventions. First-line treatment for NAFLD includes diet and exercise. Studies have shown that the treatment of NAFLD diet and exercise is closely related to the intestinal microbiota. The Mediterranean diet (higher in monounsaturated fatty acids) has a significant impact on the composition and diversity of the gut microbiota. The polyphenols contained in it can cause the increase of bifidobacteria, and due to high dietary fiber intake, it can reduce Firmicutes and increase Bacteroides[112]. Moreover, Spinach consumption also has a similar effect, which can improve the liver dysfunction of NAFLD by regulating the gut microbiota[113]. In addition, it is interesting that exercise effectively offset the gut dysbiosis caused by the HFD, thereby preventing the imbalance of the gut-liver axis and improving the homeostasis of bile acid, which helps to control the development of NAFLD[114]; (2) Probiotics. Using probiotics to regulate the gut microbiota is a promising treatment for NAFLD. The probiotic Lactobacillus rhamnosus GG (LGG) increases beneficial bacteria in the distal small intestine, restores the intestinal barrier function, reduces liver inflammation and steatosis, and exerts protective effects on NAFL mice caused by high fructose diet[115]. However, the study by Naudin et al[116] found that in female mice on a high-fat, high-carbohydrate diet, dietary supplementation of Lactococcus lactis subspecies cremoris was more effective than dietary supplementation of LGG in reducing liver fat and inflammation development. In addition, a randomized clinical trial suggested that supplementation of VSL#3 for 4 mo can significantly improve NAFLD in children, and the mechanism may be the increase of glucagon-like peptide 1[117]. Another randomized clinical trial showed for the first time that a high potency multistrain probiotic can significantly improve liver histology, ALT and cytokines in adult patients with NAFLD[118]; (3) FMT. On the one hand, animal studies have shown that FMT can correct the gut dysbiosis in mice with steatohepatitis induced by HFD, increase the concentration of cecal butyrate and small intestinal tight junction protein ZO-1, as well as reduce endotoxin and inflammation factor generation[119], furthermore, recent studies by Porras et al[121] have shown that FMT in HFD-mice can cause metabolic phenotype transfer. For example, dHFD+ (responder to HFD donor) microbiota transplantation produces insulin resistance and moderate hepatic steatosis in control diet-fed recipients. On the other hand, clinical trials have shown that 6 wk after allogeneic FMT in NAFLD patients, the abnormal permeability of the small intestine is significantly reduced[121]. Although the results of existing studies are encouraging, large-scale FMT studies are still necessary to evaluate the effect of NAFLD; (4) Herbaceous medications. At present, a large number of studies are being carried out on herbal medicines to treat NAFLD. The Chinese herbal medicine Dachaihu decoction has been proven to have a good effect on NAFLD. Its mechanism may involve regulating the intestinal mucosal barrier, lipid metabolism and liver function to a certain extent[122]. And the Si-Ni-San freeze-dried powder prepared from four herbal medicines such as Bupleuri Radix, Paeoniae Alba Radix, Aurantii Immaturus Fructus, and Honey-fried Licorice Root in equal proportions not only reduce the total cholesterol, triglycerides and free fatty acids, but also change composition and function of gut microbiota[123]. Furthermore, Shenling Baizhu powder made from ten different traditional Chinese medicinal herbs not only increases the relative abundance of beneficial bacteria (Bifidobacterium and Anaerostipes), but also decreases levels of lipopolysaccharide, reduces serum endotoxin and inflammatory factors and improves liver function[124]. Feng et al[125] found that the traditional Chinese medicine Qushi Huayu Decoction (QHD) promotes the formation of regulatory T cell-induced microbiota in the gut, and at the same time enhances the liver′s antioxidant mechanism and decreases liver lipid synthesis; and (5) Polyphenols. Recently, animal studies showed that Raw Bowl Tea polyphenol not only reduced the level of Firmicutes in the feces of mice with NAFLD, and increased the minimum levels of Bacteroides and Akkermansia, but also reduced the production of inflammatory factors and alleviated the pathological injuries of liver and small intestinal tissues[126], moreover, green tea polyphenol (epigallocatechin-3-gallate) can affect the composition of the gut microbiota of mice fed a HFD and change the metabolism of bile acids[127]. These studies suggest that polyphenol has great therapeutic potential for patients with NAFLD. In addition, the flavonoid quercetin exerts its protective effect on HFD-induced NAFLD via its anti-inflammatory, antioxidant and prebiotic integrative response[128].
MICROBIOTA-GUT-LIVER-BRAIN AXIS AND ALD
ALD is the leading cause of chronic liver disease worldwide, ranging from simple steatosis, alcoholic hepatitis, fibrosis, cirrhosis, and hepatocellular carcinoma, the National Institute on Alcohol Abuse and Alcoholism showed that 48% of liver cirrhosis were alcohol related[129]. In the United States, hospitalizations for alcohol-related liver disease are increasing among young people, the more severe chronic liver failure, the more hospital resources they consume. The economic burden of this disease is increasing on young people[130]. Early research on the pathogenesis of the disease focused on oxidative stress[129]. Currently, there is increasing evidence that ALD is associated with changes in gut microbiota, which is the basis of the disorder of the gut-liver axis. The gut-liver axis is the interaction between the gut and its microbiota and the liver, which is established by the portal vein which enables transport of gut-derived products directly to the liver, as well as the liver feedback route of bile and antibody secretion to the intestine. Control of the microbial community is essential to maintain the homeostasis of the gut-liver axis, gut microbiota acts on the liver through various mechanisms such as increasing liver lipid metabolism, increasing alcohol production, increasing intestinal permeability, bacterial translocation, intestinal bacterial overgrowth, gut microbiota imbalance, and reduced bile secretion[131], and as part of the bidirectional communication, the liver shapes the gut microbial communities[132]. Microbial functions, especially those related to bile acid metabolism, can regulate alcohol-related damage even in cirrhosis and alcoholic hepatitis. Moreover, changes in the microbiota may also alter brain function, and specific changes in the gut-liver-brain axis are related to the interaction between the gut microbiota and alcohol addiction[133]. In addition to having a positive effect on the development of alcohol-dependent psychotic symptoms, the gut microbiota can also increase the risk of serious alcohol-related illnesses[134].
Studies have shown that direct toxicity to brain tissue, induction of neuroinflammation, and changes in gut microbiota by alcohol may be the mechanism of HE related to alcohol use[135]. In addition, alcohol intake can alter a variety of neurotransmitter in the brain. Studies by Tiwari et al[136] have shown that alcohol can reduce glutamatergic and GABA-ergic neurotransmitter to varying degrees, so cessation of alcohol is necessary for the treatment. However, Godlewski et al[137] showed that endocannabinoids act on the cannabinoid-1 receptor (CB1R) and ghrelin acts on its receptor (GHS-R1A) to promote alcohol intake through the gut-brain axis, therefore, inhibiting CB1R and GHS-R1A to reduce alcohol intake provides a new approach for the treatment of ALD. In a cross-sectional study, patients with alcoholic cirrhosis performed worse on cognitive tests than patients with non-alcoholic cirrhosis. MRI show that compared with patients with non-alcoholic cirrhosis, patients with alcoholic cirrhosis have greater effects of hyperammonemia and cerebral edema, and significantly higher cortical damage[138]. Furthermore, alcohol-induced disorders can also affect the gut-brain axis, further exacerbating abuse and emotional disorders. The brain is affected by alcohol-induced disorders in a wide range, from acute poisoning to changes in personality and behavior to dementia[133]. In conclusion, the occurrence and development of ALD are affected by the gut microbiota and the gut- brain axis. Some new treatments can be based on this.
Treatment of ALD. (1) The primary intervention focused on lifestyle changes-abstinence from alcohol. Long-term abstinence is the most effective strategy to prevent disease progression[139]. Complete cessation of alcohol is necessary to ensure meaningful reversals and sustained improvement of prognosis, even in the later stages of the disease[132], however, symptoms of abstinence should be monitored, prevented, and treated. Addolorato et al[140] found that baclofen can promote alcohol abstinence in patients with alcoholic cirrhosis and is well tolerated. Recently, Godlewski et al[137] have further shown that inhibition of CB1R by peripherally restricted drugs can reduce ethanol intake in mice, so this provides a safer treatment; (2) Intervention in gut microbiota. (a) Probiotics: A recent study has shown that supplementation with probiotics, LGG can reduce hepatic bile acids by increasing intestinal FXR/FGF15 signaling pathway-mediated suppression of bile acids de novo synthesis and enhances bile acids excretion, which prevents excessive bile acids-induced liver injury and fibrosis in mice[141]. In addition, alcohol exposure reduces the abundance of Akkermansia muciniphila in the intestines of mice and humans, however, Akkermansia muciniphila, a Gram-negative intestinal commensal, promotes barrier function by increasing mucus production, and studies have found that it can be recovered in experimental ALD by oral supplementation[142], therefore, patients with ALD may benefit from Akkermansia muciniphila. When ALD develops into alcoholic cirrhosis, attempts have been made to increase beneficial bacterial populations such as bifidobacteria and lactobacillu to reduce ammonia levels by reducing gut microbiota imbalances[143]; (b) FMT: Preliminary results from a randomized clinical trial comparing the efficacy of FMT and steroids for severe alcoholic hepatitis suggest that patients receiving FMT have greater survival benefits than patients receiving steroids[144], this result paves the way for FMT to become a potential treatment option for alcoholic hepatitis. Moreover, recent research by Bajaj et al[145] showed that FMT capsules after antibiotic pretreatment are well tolerated and safe for patients with cirrhosis and recurrent HE. However, the role and exact efficacy of FMT in ALD need further research and exploration; (c) Bacteriophages: Further research by Duan et al[146] showed that bacteriophages can specifically target cytolytic E. faecalis, and eliminate alcohol-induced liver disease in mice. But assessing bacteriophage safety and further patient testing are currently under study[147]; and (d) Synthetic human α-defensin 5 (HD5): Recent research by Zhong et al[148] found that chronic alcohol feeding resulted in microbial dysbiosis in mice and reduce antimicrobial peptides-α-defensins in Paneth cell. Knockout of functional α-defensins synergistically affected the bacterial composition and intestinal barrier of alcohol interference, and enhance the translocation of pathogen-associated molecular patterns and liver damage. Administration of HD5 effectively changes the cecal microbial composition, especially increases certain gut bacteria and reverses the harmful effects induced by alcohol. Therefore, HD5 may be a new and promising treatment for the treatment of alcoholic hepatitis; (3) Suppression of immunity: At present, many studies are further exploring the mechanism of immune suppressants on ALD and looking for potential therapeutic targets. Some studies have found that early application of glucocorticoids can improve the short-term survival of patients with severe alcoholic hepatitis[149]. In addition, Chu et al[150] confirmed that Candidalysin, a polypeptide toxin secreted by the symbiotic intestinal fungus Candida albicans, can increase the levels of Il1b, Cxcl1, and Cxcl2 mRNAs in the liver of mice after ethanol administration. These pro-inflammatory cytokines may further recruit immune cells and cause hepatocyte damage. This may directly lead to hepatocyte death induced by Candidalysin. They also found that Candidalysin is associated with the severity and mortality of liver disease in patients with alcoholic hepatitis. Therefore, Candidalysin may be an effective target for the treatment of alcohol-related liver disease. Inhibition of Candidalysin production can reduce the inflammatory response and thus benefit patients; (4) Acupuncture and moxibustion. Research has shown that electroacupuncture stimulation changed the levels of vasoactive substances to increase hepatic microcirculation perfusion and promote indocyanine green clearance, thereby improving hepatic microcirculation and reserve function, protecting liver function in animals with acute ALD[151]. Though the clinical application of acupuncture is widely studied, there are relatively few studies on acupuncture for ALD. Therefore, more research is needed to explore the exact mechanism and effectiveness of acupuncture for ALD; and (5) Liver transplantation: ALD is the main indication for liver transplantation worldwide. A retrospective analysis of 147 patients with early liver transplants (before 6 mo of abstinence) for severe alcoholic hepatitis found that most patients survived 1 year (94%) and 3 years (84%), similar to patients receiving liver transplantation for other indications[152]. But before and after liver transplantation, attention should not only be paid to alcohol recurrence, but also to prevention and treatment of modifiable risk factors such as obesity and smoking[149].
MICROBIOTA-GUT-LIVE-BRAIN AXIS AND CIRRHOSIS AND HE
Cirrhosis is the end-stage of various chronic liver diseases, and is clinically characterized by portal hypertension and decreased liver function. There are many causes of cirrhosis, alcohol abuse and viral hepatitis are the most common causes of liver cirrhosis. Decompensated cirrhosis can have any of the following complications, such as jaundice, bleeding from varicose veins, ascites or HE[153,154]. Bacterial translocation and its products such as endotoxin play a key role in the pathogenesis of HE, spontaneous bacterial peritonitis and other infections, more and more cirrhosis research has focused on gut microbiota. Bajaj et al[22] have found that the gut microbiota changes with disease progression. The cirrhosis dysbiosis ratio may be a useful quantitative indicator to describe the changes in the microbiome accompanying the progression of cirrhosis. Interestingly, a recent study has found that the diversity of circulating bacteria in patients with cirrhosis is consistent with the presence of dysbiosis in cirrhotics[155]. Moreover, specific taxa of gut microbes are associated with changes in neurons and astrocytes in brain dysfunction associated with cirrhosis[156]. Hence, changes in the microbiota-gut-liver-brain axis are closely related to the progression of cirrhosis. In addition, HE is considered a typical model of gut-liver-brain axis disease.
As it is known, HE manifests as a wide range of neurological or psychiatric abnormalities, from subclinical changes to coma[157]. It is believed to be related to harmful microbial by-products such as ammonia, indole, oxindole and endotoxin. The increase in the concentration of these toxic metabolites and the inability of the diseased liver to clear these products are considered to be important pathophysiological effects[143]. Furthermore, inflammation (systemic or local), leaky gut, bacterial translocation, and overgrowth of small intestinal bacteria are also critical to the pathogenesis of HE[158].
Treatment of cirrhosis and HE: So far, treatments for advanced cirrhosis and HE have focused on the regulation of gut microbiota. The efficacy of these intestinal-centric treatments further supports the importance of changes in the gut microbiota in disease progression: (1) Dietary approach. Diet plays a vital role in regulating the intestinal environment, and has received extensive attention in the treatment of advanced liver cirrhosis and HE. Since protein catabolism increases ammonia levels, early studies recommended limiting protein intake in patients with HE. However, this conclusion has been overturned. Existing studies have shown that normal protein intake is well tolerated by patients with HE[158]. Interestingly, a clinical trial study showed that a high-protein, high-calorie diet based on casein-vegetable can significantly reduce the blood ammonia level of patients with HE and improve mental status[159]. Oral supplementation of branched chain amino acids not only produces a nutritional effect on cirrhosis itself, but also produces an effect on reducing the risk of recurrence of HE[160]; (2) Non-absorbable disaccharides. Currently, non-absorbable disaccharides (lactulose and lactol) have been recommended as the first-line treatment for HE. Lactulose can not only treat and prevent overt hepatic encephalopathy (OHE), but also significantly improve the recovery rate of minimal hepatic encephalopathy (MHE)[161,162]. The mechanisms involved are laxative effect, reducing ammonia production, and exerting prebiotic effect to regulate gut microbiota[163]. In addition, animal studies have shown that lactulose can effectively improve cognitive function by enhancing the neuroplasticity of early HE model rats[164]; (3) Probiotics. With the in-depth research on the gut microbiota, the important role of probiotics has been observed in diseases. Existing studies have shown that probiotics can increase beneficial flora and reduce pathogenic bacteria; reduce endotoxemia and ammonia content, reduce physical and psychosocial sickness impact profile score and serious adverse events, significantly reverse MHE and reduce the development of OHE[165,166]. Moreover, the application of VSL#3 has received extensive attention from researchers. On the one hand, animal studies have shown that VSL#3, in addition to its significant anti-inflammatory effects, can also prevent mesenteric artery endothelial dysfunction, and induce the synthesis of bile acids in the liver of mice[167-169], on the other hand, clinical trials have shown that the intake of VSL#3 can significantly reduce the risk of hospitalization for HE, as well as Child-Turcotte-Pugh and model for end-stage liver disease scores[170]; (4) Antibiotics. Rifaximin is a non-absorbable antibiotic. It not only reduces the production of intestinal ammonia by increasing the expression of intestinal glutaminase, but also reduces the levels of serum soluble CD163 and mannose receptors and partially changes the gut microbiota to reduce endotoxemia[171,172]. And its effect on HE has been proved by clinical research[173]. Bajaj et al[18] found that rifaximin can also improve the cognitive function of MHE; and (5) FMT. The effect of FMT on advanced cirrhosis and HE is gradually being recognized. At present, safety and changes in the structure and function of the gut microbiota have been the main concern in FMT[174]. Clinical studies have shown that FMT can reduce the hospitalization rate of patients with cirrhosis and recurrent HE, improve cognition and gut dysbiosis[175], moreover, FMT can restore the decrease in microbial diversity and the changes in SCFAs and bile acid caused by antibiotic use[176].
CONCLUSION
With the deepening of understanding of microbiota and the gut-liver-brain axis, and further exploration of the microbiota-gut-liver-brain axis, the importance of gut-liver-brain interaction in the occurrence and development of many diseases has been discovered, which provides a new direction for further research on the treatment of diseases. In this review, we mainly discuss the role of the gut microbiota in IBS, IBD, FD, NAFLD, ALD, cirrhosis and HE via the gut-liver-brain axis. This article mainly focuses on clarifying the potential mechanisms and treatment. Although there are many studies based on this in preclinical models, clinical studies are required on its efficacy and safety, as well as patient tolerability. In addition, the specific signaling pathways of the microbiota-gut-liver-brain axis in the occurrence and development of gastrointestinal diseases are unknown, and further research is needed in order to find more personalized molecular targeted therapies.
Footnotes
Conflict-of-interest statement: The authors declare that they have no competing interests.
Manuscript source: Unsolicited manuscript
Peer-review started: July 8, 2020
First decision: August 8, 2020
Article in press: September 18, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gonzalez-Gallego J S-Editor: Huang P L-Editor: MedE-Ma JY P-Editor: Ma YJ
Ma YJ
Contributor Information
Jian-Hong Ding, Department of Gastroenterology, Affiliated Hospital to Zunyi Medical University, Zunyi 563003, Guizhou Province, China.
Zhe Jin, Department of Gastroenterology, Affiliated Hospital to Zunyi Medical University, Zunyi 563003, Guizhou Province, China.
Xiao-Xu Yang, Department of Gastroenterology, Affiliated Hospital to Zunyi Medical University, Zunyi 563003, Guizhou Province, China.
Jun Lou, Department of Gastroenterology, Affiliated Hospital to Zunyi Medical University, Zunyi 563003, Guizhou Province, China.
Wei-Xi Shan, Department of Gastroenterology, Affiliated Hospital to Zunyi Medical University, Zunyi 563003, Guizhou Province, China.
Yan-Xia Hu, Department of Gastroenterology, Affiliated Hospital to Zunyi Medical University, Zunyi 563003, Guizhou Province, China.
Qian Du, Department of Gastroenterology, Affiliated Hospital to Zunyi Medical University, Zunyi 563003, Guizhou Province, China.
Qiu-Shi Liao, Department of Gastroenterology, Affiliated Hospital to Zunyi Medical University, Zunyi 563003, Guizhou Province, China.
Rui Xie, Department of Gastroenterology, Affiliated Hospital to Zunyi Medical University, Zunyi 563003, Guizhou Province, China.
Jing-Yu Xu, Department of Gastroenterology, Affiliated Hospital to Zunyi Medical University, Zunyi 563003, Guizhou Province, China. xujingyu_gzzy@126.com.
References
- 1.Sender R, Fuchs S, Milo R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell. 2016;164:337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Zmora N, Suez J, Elinav E. You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16:35–56. doi: 10.1038/s41575-018-0061-2. [DOI] [PubMed] [Google Scholar]
- 3.Lynch SV, Pedersen O. The Human Intestinal Microbiome in Health and Disease. N Engl J Med. 2016;375:2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 4.Long-Smith C, O'Riordan KJ, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota-Gut-Brain Axis: New Therapeutic Opportunities. Annu Rev Pharmacol Toxicol. 2020;60:477–502. doi: 10.1146/annurev-pharmtox-010919-023628. [DOI] [PubMed] [Google Scholar]
- 5.Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PWJ. Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends Neurosci. 2016;39:763–781. doi: 10.1016/j.tins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, Leonard P, Li J, Burgdorf K, Grarup N, Jørgensen T, Brandslund I, Nielsen HB, Juncker AS, Bertalan M, Levenez F, Pons N, Rasmussen S, Sunagawa S, Tap J, Tims S, Zoetendal EG, Brunak S, Clément K, Doré J, Kleerebezem M, Kristiansen K, Renault P, Sicheritz-Ponten T, de Vos WM, Zucker JD, Raes J, Hansen T MetaHIT consortium, Bork P, Wang J, Ehrlich SD, Pedersen O. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 7.Butler MI, Cryan JF, Dinan TG. Man and the Microbiome: A New Theory of Everything? Annu Rev Clin Psychol. 2019;15:371–398. doi: 10.1146/annurev-clinpsy-050718-095432. [DOI] [PubMed] [Google Scholar]
- 8.Yoo BB, Mazmanian SK. The Enteric Network: Interactions between the Immune and Nervous Systems of the Gut. Immunity. 2017;46:910–926. doi: 10.1016/j.immuni.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wehrwein EA, Orer HS, Barman SM. Overview of the Anatomy, Physiology, and Pharmacology of the Autonomic Nervous System. Compr Physiol. 2016;6:1239–1278. doi: 10.1002/cphy.c150037. [DOI] [PubMed] [Google Scholar]
- 10.Davis EA, Zhou W, Dailey MJ. Evidence for a direct effect of the autonomic nervous system on intestinal epithelial stem cell proliferation. Physiol Rep. 2018;6:e13745. doi: 10.14814/phy2.13745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Browning KN, Travagli RA. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr Physiol. 2014;4:1339–1368. doi: 10.1002/cphy.c130055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 13.Faraco G, Brea D, Garcia-Bonilla L, Wang G, Racchumi G, Chang H, Buendia I, Santisteban MM, Segarra SG, Koizumi K, Sugiyama Y, Murphy M, Voss H, Anrather J, Iadecola C. Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated TH17 response. Nat Neurosci. 2018;21:240–249. doi: 10.1038/s41593-017-0059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson KV, Foster KR. Why does the microbiome affect behaviour? Nat Rev Microbiol. 2018;16:647–655. doi: 10.1038/s41579-018-0014-3. [DOI] [PubMed] [Google Scholar]
- 15.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet MF, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson's Disease. Cell. 2016;167:1469–1480.e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borre YE, Moloney RD, Clarke G, Dinan TG, Cryan JF. The impact of microbiota on brain and behavior: mechanisms & amp; therapeutic potential. Adv Exp Med Biol. 2014;817:373–403. doi: 10.1007/978-1-4939-0897-4_17. [DOI] [PubMed] [Google Scholar]
- 18.Bajaj JS, Heuman DM, Sanyal AJ, Hylemon PB, Sterling RK, Stravitz RT, Fuchs M, Ridlon JM, Daita K, Monteith P, Noble NA, White MB, Fisher A, Sikaroodi M, Rangwala H, Gillevet PM. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One. 2013;8:e60042. doi: 10.1371/journal.pone.0060042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labus JS, Hollister EB, Jacobs J, Kirbach K, Oezguen N, Gupta A, Acosta J, Luna RA, Aagaard K, Versalovic J, Savidge T, Hsiao E, Tillisch K, Mayer EA. Differences in gut microbial composition correlate with regional brain volumes in irritable bowel syndrome. Microbiome. 2017;5:49. doi: 10.1186/s40168-017-0260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, Knight R. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15:397–411. doi: 10.1038/s41575-018-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milosevic I, Vujovic A, Barac A, Djelic M, Korac M, Radovanovic Spurnic A, Gmizic I, Stevanovic O, Djordjevic V, Lekic N, Russo E, Amedei A. Gut-Liver Axis, Gut Microbiota, and Its Modulation in the Management of Liver Diseases: A Review of the Literature. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20020395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, Noble NA, Unser AB, Daita K, Fisher AR, Sikaroodi M, Gillevet PM. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60:940–947. doi: 10.1016/j.jhep.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. Bowel Disorders. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 24.Holtmann GJ, Ford AC, Talley NJ. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol Hepatol. 2016;1:133–146. doi: 10.1016/S2468-1253(16)30023-1. [DOI] [PubMed] [Google Scholar]
- 25.Hellström PM. Pathophysiology of the irritable bowel syndrome - Reflections of today. Best Pract Res Clin Gastroenterol. 2019;40-41:101620. doi: 10.1016/j.bpg.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Labus JS, Osadchiy V, Hsiao EY, Tap J, Derrien M, Gupta A, Tillisch K, Le Nevé B, Grinsvall C, Ljungberg M, Öhman L, Törnblom H, Simren M, Mayer EA. Evidence for an association of gut microbial Clostridia with brain functional connectivity and gastrointestinal sensorimotor function in patients with irritable bowel syndrome, based on tripartite network analysis. Microbiome. 2019;7:45. doi: 10.1186/s40168-019-0656-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbara G, Feinle-Bisset C, Ghoshal UC, Quigley EM, Santos J, Vanner S, Vergnolle N, Zoetendal EG. The Intestinal Microenvironment and Functional Gastrointestinal Disorders. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 28.Frieling T, Heise J, Krummen B, Hundorf C, Kalde S. Tolerability of FODMAP - reduced diet in irritable bowel syndrome - efficacy, adherence, and body weight course. Z Gastroenterol. 2019;57:740–744. doi: 10.1055/a-0859-7531. [DOI] [PubMed] [Google Scholar]
- 29.Dickson I. IBS: High FODMAP diet induces LPS-derived intestinal inflammation and visceral hypersensitivity. Nat Rev Gastroenterol Hepatol. 2018;15:68. doi: 10.1038/nrgastro.2017.187. [DOI] [PubMed] [Google Scholar]
- 30.Zhou SY, Gillilland M, 3rd, Wu X, Leelasinjaroen P, Zhang G, Zhou H, Ye B, Lu Y, Owyang C. FODMAP diet modulates visceral nociception by lipopolysaccharide-mediated intestinal inflammation and barrier dysfunction. J Clin Invest. 2018;128:267–280. doi: 10.1172/JCI92390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simrén M, Törnblom H, Palsson OS, Van Oudenhove L, Whitehead WE, Tack J. Cumulative Effects of Psychologic Distress, Visceral Hypersensitivity, and Abnormal Transit on Patient-reported Outcomes in Irritable Bowel Syndrome. Gastroenterology. 2019;157:391–402.e2. doi: 10.1053/j.gastro.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 32.Moayyedi P. Faecal microbiota transplantation for IBS: still a long way to go. Lancet Gastroenterol Hepatol. 2019;4:656–657. doi: 10.1016/S2468-1253(19)30226-2. [DOI] [PubMed] [Google Scholar]
- 33.Xu D, Chen VL, Steiner CA, Berinstein JA, Eswaran S, Waljee AK, Higgins PDR, Owyang C. Efficacy of Fecal Microbiota Transplantation in Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2019;114:1043–1050. doi: 10.14309/ajg.0000000000000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cremon C, Barbaro MR, Ventura M, Barbara G. Pre- and probiotic overview. Curr Opin Pharmacol. 2018;43:87–92. doi: 10.1016/j.coph.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Francavilla R, Piccolo M, Francavilla A, Polimeno L, Semeraro F, Cristofori F, Castellaneta S, Barone M, Indrio F, Gobbetti M, De Angelis M. Clinical and Microbiological Effect of a Multispecies Probiotic Supplementation in Celiac Patients With Persistent IBS-type Symptoms: A Randomized, Double-Blind, Placebo-controlled, Multicenter Trial. J Clin Gastroenterol. 2019;53:e117–e125. doi: 10.1097/MCG.0000000000001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ray K. IBS: Mindful of probiotics for psychiatric comorbidities in IBS. Nat Rev Gastroenterol Hepatol. 2017;14:386–387. doi: 10.1038/nrgastro.2017.70. [DOI] [PubMed] [Google Scholar]
- 37.Simrén M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, Vanner S, Verdu EF, Whorwell PJ, Zoetendal EG Rome Foundation Committee. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62:159–176. doi: 10.1136/gutjnl-2012-302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laird KT, Tanner-Smith EE, Russell AC, Hollon SD, Walker LS. Comparative efficacy of psychological therapies for improving mental health and daily functioning in irritable bowel syndrome: A systematic review and meta-analysis. Clin Psychol Rev. 2017;51:142–152. doi: 10.1016/j.cpr.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Dickson I. Remotely delivered cognitive behavioural therapy superior to treatment as usual for IBS. Nat Rev Gastroenterol Hepatol. 2019;16:326. doi: 10.1038/s41575-019-0153-7. [DOI] [PubMed] [Google Scholar]
- 40.Lackner JM, Jaccard J. Cognitive-behavioural therapy for IBS comes home: mapping a route for efficacy and efficiency in the digital age. Gut. 2019;68:1541–1542. doi: 10.1136/gutjnl-2019-318583. [DOI] [PubMed] [Google Scholar]
- 41.Ma XP, Hong J, An CP, Zhang D, Huang Y, Wu HG, Zhang CH, Meeuwsen S. Acupuncture-moxibustion in treating irritable bowel syndrome: how does it work? World J Gastroenterol. 2014;20:6044–6054. doi: 10.3748/wjg.v20.i20.6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu MR, Xiao RF, Peng ZP, Zuo HN, Zhu K, Wang SM. [Effect of acupuncture at "Zusanli" (ST 36 and "Taichong" (LR 3) on gastrointestinal hormone levels in rats with diarrhea type irritable bowel syndrome] Zhen Ci Yan Jiu. 2012;37:363–368. [PubMed] [Google Scholar]
- 43.Song SY, Wang YJ, Wang DS, Chai JY. [Effects of oculo-acupuncture therapy on colonic serotonin reuptake transporter expression in rats with irritable bowel syndrome] Zhen Ci Yan Jiu. 2011;36:101–104, 115. [PubMed] [Google Scholar]
- 44.Wang YJ, Wang DS, Guan HQ, Wang J, Chai JY, Zhao JR, Han XW. [Effects of eye-acupuncture therapy on serum and colonic SP and VIP contents in rats with irritable bowel syndrome] Zhen Ci Yan Jiu. 2010;35:8–11, 26. [PubMed] [Google Scholar]
- 45.Wang X, Qi Q, Wang Y, Wu H, Jin X, Yao H, Jin D, Liu Y, Wang C. Gut microbiota was modulated by moxibustion stimulation in rats with irritable bowel syndrome. Chin Med. 2018;13:63. doi: 10.1186/s13020-018-0220-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oligschlaeger Y, Yadati T, Houben T, Condello Oliván CM, Shiri-Sverdlov R. Inflammatory Bowel Disease: A Stressed "Gut/Feeling". Cells. 2019;8 doi: 10.3390/cells8070659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 48.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Büning C, Cohain A, Cichon S, D'Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chu H, Khosravi A, Kusumawardhani IP, Kwon AH, Vasconcelos AC, Cunha LD, Mayer AE, Shen Y, Wu WL, Kambal A, Targan SR, Xavier RJ, Ernst PB, Green DR, McGovern DP, Virgin HW, Mazmanian SK International IBD Genetics Consortium (IIBDGC), Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly MJ, Franke A, Parkes M, Vermeire S, Barrett JC, Cho JH. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science. 2016;352:1116–1120. doi: 10.1126/science.aad9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018;11:1–10. doi: 10.1007/s12328-017-0813-5. [DOI] [PubMed] [Google Scholar]
- 51.Imhann F, Vich Vila A, Bonder MJ, Fu J, Gevers D, Visschedijk MC, Spekhorst LM, Alberts R, Franke L, van Dullemen HM, Ter Steege RWF, Huttenhower C, Dijkstra G, Xavier RJ, Festen EAM, Wijmenga C, Zhernakova A, Weersma RK. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut. 2018;67:108–119. doi: 10.1136/gutjnl-2016-312135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abautret-Daly Á, Dempsey E, Parra-Blanco A, Medina C, Harkin A. Gut-brain actions underlying comorbid anxiety and depression associated with inflammatory bowel disease. Acta Neuropsychiatr. 2018;30:275–296. doi: 10.1017/neu.2017.3. [DOI] [PubMed] [Google Scholar]
- 53.Frolkis AD, Vallerand IA, Shaheen AA, Lowerison MW, Swain MG, Barnabe C, Patten SB, Kaplan GG. Depression increases the risk of inflammatory bowel disease, which may be mitigated by the use of antidepressants in the treatment of depression. Gut. 2019;68:1606–1612. doi: 10.1136/gutjnl-2018-317182. [DOI] [PubMed] [Google Scholar]
- 54.Gracie DJ, Guthrie EA, Hamlin PJ, Ford AC. Bi-directionality of Brain-Gut Interactions in Patients With Inflammatory Bowel Disease. Gastroenterology. 2018;154:1635–1646.e3. doi: 10.1053/j.gastro.2018.01.027. [DOI] [PubMed] [Google Scholar]
- 55.Danese S, Hoffman C, Vel S, Greco M, Szabo H, Wilson B, Avedano L. Anaemia from a patient perspective in inflammatory bowel disease: results from the European Federation of Crohn's and Ulcerative Colitis Association's online survey. Eur J Gastroenterol Hepatol. 2014;26:1385–1391. doi: 10.1097/MEG.0000000000000200. [DOI] [PubMed] [Google Scholar]
- 56.Borren NZ, van der Woude CJ, Ananthakrishnan AN. Fatigue in IBD: epidemiology, pathophysiology and management. Nat Rev Gastroenterol Hepatol. 2019;16:247–259. doi: 10.1038/s41575-018-0091-9. [DOI] [PubMed] [Google Scholar]
- 57.Zhou L, Sonnenberg GF. Essential immunologic orchestrators of intestinal homeostasis. Sci Immunol. 2018;3 doi: 10.1126/sciimmunol.aao1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaufman J, Griffiths TA, Surette MG, Ness S, Rioux KP. Effects of mesalamine (5-aminosalicylic acid) on bacterial gene expression. Inflamm Bowel Dis. 2009;15:985–996. doi: 10.1002/ibd.20876. [DOI] [PubMed] [Google Scholar]
- 59.Laghi L, Mastromarino P, Elisei W, Capobianco D, Zhu CL, Picchio M, Giorgetti G, Brandimarte G, Tursi A. Impact of treatments on fecal microbiota and fecal metabolome in symptomatic uncomplicated diverticular disease of the colon: a pilot study. J Biol Regul Homeost Agents. 2018;32:1421–1432. [PubMed] [Google Scholar]
- 60.Shen J, Zuo ZX, Mao AP. Effect of probiotics on inducing remission and maintaining therapy in ulcerative colitis, Crohn's disease, and pouchitis: meta-analysis of randomized controlled trials. Inflamm Bowel Dis. 2014;20:21–35. doi: 10.1097/01.MIB.0000437495.30052.be. [DOI] [PubMed] [Google Scholar]
- 61.Cheifetz AS, Gianotti R, Luber R, Gibson PR. Complementary and Alternative Medicines Used by Patients With Inflammatory Bowel Diseases. Gastroenterology. 2017;152:415–429.e15. doi: 10.1053/j.gastro.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 62.D'Haens GR, Jobin C. Fecal Microbial Transplantation for Diseases Beyond Recurrent Clostridium Difficile Infection. Gastroenterology. 2019;157:624–636. doi: 10.1053/j.gastro.2019.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Plichta DR, Graham DB, Subramanian S, Xavier RJ. Therapeutic Opportunities in Inflammatory Bowel Disease: Mechanistic Dissection of Host-Microbiome Relationships. Cell. 2019;178:1041–1056. doi: 10.1016/j.cell.2019.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gracie DJ, Hamlin PJ, Ford AC. The influence of the brain-gut axis in inflammatory bowel disease and possible implications for treatment. Lancet Gastroenterol Hepatol. 2019;4:632–642. doi: 10.1016/S2468-1253(19)30089-5. [DOI] [PubMed] [Google Scholar]
- 65.Ji J, Lu Y, Liu H, Feng H, Zhang F, Wu L, Cui Y, Wu H. Acupuncture and moxibustion for inflammatory bowel diseases: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2013;2013:158352. doi: 10.1155/2013/158352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qi Q, Liu YN, Jin XM, Zhang LS, Wang C, Bao CH, Liu HR, Wu HG, Wang XM. Moxibustion treatment modulates the gut microbiota and immune function in a dextran sulphate sodium-induced colitis rat model. World J Gastroenterol. 2018;24:3130–3144. doi: 10.3748/wjg.v24.i28.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang XM, Lu Y, Wu LY, Yu SG, Zhao BX, Hu HY, Wu HG, Bao CH, Liu HR, Wang JH, Yao Y, Hua XG, Guo HY, Shen LR. Moxibustion inhibits interleukin-12 and tumor necrosis factor alpha and modulates intestinal flora in rat with ulcerative colitis. World J Gastroenterol. 2012;18:6819–6828. doi: 10.3748/wjg.v18.i46.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wei D, Zhao N, Xie L, Huang B, Zhuang Z, Tang Y, Yu S, Wu Q. Electroacupuncture and Moxibustion Improved Anxiety Behavior in DSS-Induced Colitis Mice. Gastroenterol Res Pract. 2019;2019:2345890. doi: 10.1155/2019/2345890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bao C, Liu P, Liu H, Jin X, Calhoun VD, Wu L, Shi Y, Zhang J, Zeng X, Ma L, Qin W, Zhang J, Liu X, Tian J, Wu H. Different brain responses to electro-acupuncture and moxibustion treatment in patients with Crohn's disease. Sci Rep. 2016;6:36636. doi: 10.1038/srep36636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stanghellini V, Chan FK, Hasler WL, Malagelada JR, Suzuki H, Tack J, Talley NJ. Gastroduodenal Disorders. Gastroenterology. 2016;150:1380–1392. doi: 10.1053/j.gastro.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 71.Ford AC, Marwaha A, Sood R, Moayyedi P. Global prevalence of, and risk factors for, uninvestigated dyspepsia: a meta-analysis. Gut. 2015;64:1049–1057. doi: 10.1136/gutjnl-2014-307843. [DOI] [PubMed] [Google Scholar]
- 72.Aziz I, Palsson OS, Törnblom H, Sperber AD, Whitehead WE, Simrén M. Epidemiology, clinical characteristics, and associations for symptom-based Rome IV functional dyspepsia in adults in the USA, Canada, and the UK: a cross-sectional population-based study. Lancet Gastroenterol Hepatol. 2018;3:252–262. doi: 10.1016/S2468-1253(18)30003-7. [DOI] [PubMed] [Google Scholar]
- 73.Lacy BE, Weiser KT, Kennedy AT, Crowell MD, Talley NJ. Functional dyspepsia: the economic impact to patients. Aliment Pharmacol Ther. 2013;38:170–177. doi: 10.1111/apt.12355. [DOI] [PubMed] [Google Scholar]
- 74.Vanheel H, Farré R. Changes in gastrointestinal tract function and structure in functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013;10:142–149. doi: 10.1038/nrgastro.2012.255. [DOI] [PubMed] [Google Scholar]
- 75.Jung HK, Talley NJ. Role of the Duodenum in the Pathogenesis of Functional Dyspepsia: A Paradigm Shift. J Neurogastroenterol Motil. 2018;24:345–354. doi: 10.5056/jnm18060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ji R, Wang P, Kou GJ, Zuo XL, Wang X, Li YQ. Impaired gastric mucosal integrity identified by confocal endomicroscopy in Helicobacter pylori-negative functional dyspepsia. Neurogastroenterol Motil. 2020;32:e13719. doi: 10.1111/nmo.13719. [DOI] [PubMed] [Google Scholar]
- 77.Aro P, Talley NJ, Johansson SE, Agréus L, Ronkainen J. Anxiety Is Linked to New-Onset Dyspepsia in the Swedish Population: A 10-Year Follow-up Study. Gastroenterology. 2015;148:928–937. doi: 10.1053/j.gastro.2015.01.039. [DOI] [PubMed] [Google Scholar]
- 78.Van Oudenhove L, Aziz Q. The role of psychosocial factors and psychiatric disorders in functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013;10:158–167. doi: 10.1038/nrgastro.2013.10. [DOI] [PubMed] [Google Scholar]
- 79.Zhong L, Shanahan ER, Raj A, Koloski NA, Fletcher L, Morrison M, Walker MM, Talley NJ, Holtmann G. Dyspepsia and the microbiome: time to focus on the small intestine. Gut. 2017;66:1168–1169. doi: 10.1136/gutjnl-2016-312574. [DOI] [PubMed] [Google Scholar]
- 80.Suzuki H, Moayyedi P. Helicobacter pylori infection in functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013;10:168–174. doi: 10.1038/nrgastro.2013.9. [DOI] [PubMed] [Google Scholar]
- 81.Moayyedi P, Lacy BE, Andrews CN, Enns RA, Howden CW, Vakil N. ACG and CAG Clinical Guideline: Management of Dyspepsia. Am J Gastroenterol. 2017;112:988–1013. doi: 10.1038/ajg.2017.154. [DOI] [PubMed] [Google Scholar]
- 82.Kang SJ, Park B, Shin CM. Helicobacter pylori Eradication Therapy for Functional Dyspepsia: A Meta-Analysis by Region and H. pylori Prevalence. J Clin Med. 2019;8 doi: 10.3390/jcm8091324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miwa H, Kusano M, Arisawa T, Oshima T, Kato M, Joh T, Suzuki H, Tominaga K, Nakada K, Nagahara A, Futagami S, Manabe N, Inui A, Haruma K, Higuchi K, Yakabi K, Hongo M, Uemura N, Kinoshita Y, Sugano K, Shimosegawa T Japanese Society of Gastroenterology. Evidence-based clinical practice guidelines for functional dyspepsia. J Gastroenterol. 2015;50:125–139. doi: 10.1007/s00535-014-1022-3. [DOI] [PubMed] [Google Scholar]
- 84.Pinto-Sanchez MI, Yuan Y, Hassan A, Bercik P, Moayyedi P. Proton pump inhibitors for functional dyspepsia. Cochrane Database Syst Rev. 2017;11:CD011194. doi: 10.1002/14651858.CD011194.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jackson MA, Goodrich JK, Maxan ME, Freedberg DE, Abrams JA, Poole AC, Sutter JL, Welter D, Ley RE, Bell JT, Spector TD, Steves CJ. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65:749–756. doi: 10.1136/gutjnl-2015-310861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Imhann F, Bonder MJ, Vich Vila A, Fu J, Mujagic Z, Vork L, Tigchelaar EF, Jankipersadsing SA, Cenit MC, Harmsen HJ, Dijkstra G, Franke L, Xavier RJ, Jonkers D, Wijmenga C, Weersma RK, Zhernakova A. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:740–748. doi: 10.1136/gutjnl-2015-310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ford AC, Luthra P, Tack J, Boeckxstaens GE, Moayyedi P, Talley NJ. Efficacy of psychotropic drugs in functional dyspepsia: systematic review and meta-analysis. Gut. 2017;66:411–420. doi: 10.1136/gutjnl-2015-310721. [DOI] [PubMed] [Google Scholar]
- 88.Jin M, Son M. DA-9701 (Motilitone): A Multi-Targeting Botanical Drug for the Treatment of Functional Dyspepsia. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19124035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu J, Li F, Tang XD, Ma J, Ma X, Ge DY, Li GM, Wang Y. XiangshaLiujunzi decoction alleviates the symptoms of functional dyspepsia by regulating brain-gut axis and production of neuropeptides. BMC Complement Altern Med. 2015;15:387. doi: 10.1186/s12906-015-0913-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wei J, Man Q, Guo F, Xian M, Wang T, Tang C, Zhang Y, Li D, Tang D, Yang H, Huang L. Precise and systematic survey of the efficacy of multicomponent drugs against functional dyspepsia. Sci Rep. 2019;9:10713. doi: 10.1038/s41598-019-47300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xue Z, Wu C, Wei J, Xian M, Wang T, Yang B, Chen M. An orally administered magnoloside A ameliorates functional dyspepsia by modulating brain-gut peptides and gut microbiota. Life Sci. 2019;233:116749. doi: 10.1016/j.lfs.2019.116749. [DOI] [PubMed] [Google Scholar]
- 92.Tominaga K, Sakata Y, Kusunoki H, Odaka T, Sakurai K, Kawamura O, Nagahara A, Takeuchi T, Fujikawa Y, Oshima T, Kato M, Furuta T, Murakami K, Chiba T, Miwa H, Kinoshita Y, Higuchi K, Kusano M, Iwakiri R, Fujimoto K, Tack JF, Arakawa T. Rikkunshito simultaneously improves dyspepsia correlated with anxiety in patients with functional dyspepsia: A randomized clinical trial (the DREAM study) Neurogastroenterol Motil. 2018;30:e13319. doi: 10.1111/nmo.13319. [DOI] [PubMed] [Google Scholar]
- 93.Pang B, Jiang T, Du YH, Li J, Li B, Hu YC, Cai QH. Acupuncture for Functional Dyspepsia: What Strength Does It Have? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Evid Based Complement Alternat Med. 2016;2016:3862916. doi: 10.1155/2016/3862916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ho RST, Chung VCH, Wong CHL, Wu JCY, Wong SYS, Wu IXY. Acupuncture and related therapies used as add-on or alternative to prokinetics for functional dyspepsia: overview of systematic reviews and network meta-analysis. Sci Rep. 2017;7:10320. doi: 10.1038/s41598-017-09856-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fang J, Wang D, Zhao Q, Hong Y, Jin Y, Liu Z, Zhou K, Jing X, Yu X, Pan R, Chang A, Liu H, Zhu B. Brain-Gut Axis Modulation of Acupuncture in Functional Dyspepsia: A Preliminary Resting-State fcMRI Study. Evid Based Complement Alternat Med. 2015;2015:860463. doi: 10.1155/2015/860463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xiao LY, Liu WA, Wu QM, Wang LY, Zhou KM, Ye HM, Fu L. [Influence of Herbal-cake-separated Moxibustion on Contents of 5-HT, DA and NE in Hypothalamus in Rats with Functional Dyspepsia of Liver Stagnation and Spleen Deficiency Syndrome] Zhen Ci Yan Jiu. 2016;41:60–64. [PubMed] [Google Scholar]
- 97.Hall W, Buckley M, Crotty P, O'Morain CA. Gastric mucosal mast cells are increased in Helicobacter pylori-negative functional dyspepsia. Clin Gastroenterol Hepatol. 2003;1:363–369. doi: 10.1053/s1542-3565(03)00184-8. [DOI] [PubMed] [Google Scholar]
- 98.Keita ÅV, Söderholm JD. Mucosal permeability and mast cells as targets for functional gastrointestinal disorders. Curr Opin Pharmacol. 2018;43:66–71. doi: 10.1016/j.coph.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 99.He Y, Yang C, Wang P, Yang L, Wu H, Liu H, Qi M, Guo Z, Li J, Shi H, Wu X, Hu Z. Child compound Endothelium corneum attenuates gastrointestinal dysmotility through regulating the homeostasis of brain-gut-microbiota axis in functional dyspepsia rats. J Ethnopharmacol. 2019;240:111953. doi: 10.1016/j.jep.2019.111953. [DOI] [PubMed] [Google Scholar]
- 100.Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol. 2010;5:145–171. doi: 10.1146/annurev-pathol-121808-102132. [DOI] [PubMed] [Google Scholar]
- 101.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 102.Dietrich P, Hellerbrand C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract Res Clin Gastroenterol. 2014;28:637–653. doi: 10.1016/j.bpg.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 103.Porras D, Nistal E, Martínez-Flórez S, González-Gallego J, García-Mediavilla MV, Sánchez-Campos S. Intestinal Microbiota Modulation in Obesity-Related Non-alcoholic Fatty Liver Disease. Front Physiol. 2018;9:1813. doi: 10.3389/fphys.2018.01813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65:1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 105.Higarza SG, Arboleya S, Gueimonde M, Gómez-Lázaro E, Arias JL, Arias N. Neurobehavioral dysfunction in non-alcoholic steatohepatitis is associated with hyperammonemia, gut dysbiosis, and metabolic and functional brain regional deficits. PLoS One. 2019;14:e0223019. doi: 10.1371/journal.pone.0223019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Poeta M, Pierri L, Vajro P. Gut-Liver Axis Derangement in Non-Alcoholic Fatty Liver Disease. Children (Basel) 2017;4 doi: 10.3390/children4080066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chiu CC, Ching YH, Li YP, Liu JY, Huang YT, Huang YW, Yang SS, Huang WC, Chuang HL. Nonalcoholic Fatty Liver Disease Is Exacerbated in High-Fat Diet-Fed Gnotobiotic Mice by Colonization with the Gut Microbiota from Patients with Nonalcoholic Steatohepatitis. Nutrients. 2017;9 doi: 10.3390/nu9111220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, Guy CD, Seed PC, Rawls JF, David LA, Hunault G, Oberti F, Calès P, Diehl AM. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764–775. doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Arslan N. Obesity, fatty liver disease and intestinal microbiota. World J Gastroenterol. 2014;20:16452–16463. doi: 10.3748/wjg.v20.i44.16452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang SZ, Yu YJ, Adeli K. Role of Gut Microbiota in Neuroendocrine Regulation of Carbohydrate and Lipid Metabolism via the Microbiota-Gut-Brain-Liver Axis. Microorganisms. 2020;8 doi: 10.3390/microorganisms8040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mouries J, Brescia P, Silvestri A, Spadoni I, Sorribas M, Wiest R, Mileti E, Galbiati M, Invernizzi P, Adorini L, Penna G, Rescigno M. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J Hepatol. 2019;71:1216–1228. doi: 10.1016/j.jhep.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Anania C, Perla FM, Olivero F, Pacifico L, Chiesa C. Mediterranean diet and nonalcoholic fatty liver disease. World J Gastroenterol. 2018;24:2083–2094. doi: 10.3748/wjg.v24.i19.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Elvira-Torales LI, Periago MJ, González-Barrio R, Hidalgo N, Navarro-González I, Gómez-Gallego C, Masuero D, Soini E, Vrhovsek U, García-Alonso FJ. Spinach consumption ameliorates the gut microbiota and dislipaemia in rats with diet-induced non-alcoholic fatty liver disease (NAFLD) Food Funct. 2019;10:2148–2160. doi: 10.1039/c8fo01630e. [DOI] [PubMed] [Google Scholar]
- 114.Carbajo-Pescador S, Porras D, García-Mediavilla MV, Martínez-Flórez S, Juarez-Fernández M, Cuevas MJ, Mauriz JL, González-Gallego J, Nistal E, Sánchez-Campos S. Beneficial effects of exercise on gut microbiota functionality and barrier integrity, and gut-liver crosstalk in an in vivo model of early obesity and non-alcoholic fatty liver disease. Dis Model Mech. 2019;12 doi: 10.1242/dmm.039206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ritze Y, Bárdos G, Claus A, Ehrmann V, Bergheim I, Schwiertz A, Bischoff SC. Lactobacillus rhamnosus GG protects against non-alcoholic fatty liver disease in mice. PLoS One. 2014;9:e80169. doi: 10.1371/journal.pone.0080169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Naudin CR, Maner-Smith K, Owens JA, Wynn GM, Robinson BS, Matthews JD, Reedy AR, Luo L, Wolfarth AA, Darby TM, Ortlund EA, Jones RM. Lactococcus lactis Subspecies cremoris Elicits Protection Against Metabolic Changes Induced by a Western-Style Diet. Gastroenterology. 2020;159:639–651.e5. doi: 10.1053/j.gastro.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 117.Alisi A, Bedogni G, Baviera G, Giorgio V, Porro E, Paris C, Giammaria P, Reali L, Anania F, Nobili V. Randomised clinical trial: The beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2014;39:1276–1285. doi: 10.1111/apt.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Duseja A, Acharya SK, Mehta M, Chhabra S, Shalimar, Rana S, Das A, Dattagupta S, Dhiman RK, Chawla YK. High potency multistrain probiotic improves liver histology in non-alcoholic fatty liver disease (NAFLD): a randomised, double-blind, proof of concept study. BMJ Open Gastroenterol. 2019;6:e000315. doi: 10.1136/bmjgast-2019-000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhou D, Pan Q, Shen F, Cao HX, Ding WJ, Chen YW, Fan JG. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci Rep. 2017;7:1529. doi: 10.1038/s41598-017-01751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Porras D, Nistal E, Martínez-Flórez S, Olcoz JL, Jover R, Jorquera F, González-Gallego J, García-Mediavilla MV, Sánchez-Campos S. Functional Interactions between Gut Microbiota Transplantation, Quercetin, and High-Fat Diet Determine Non-Alcoholic Fatty Liver Disease Development in Germ-Free Mice. Mol Nutr Food Res. 2019;63:e1800930. doi: 10.1002/mnfr.201800930. [DOI] [PubMed] [Google Scholar]
- 121.Craven L, Rahman A, Nair Parvathy S, Beaton M, Silverman J, Qumosani K, Hramiak I, Hegele R, Joy T, Meddings J, Urquhart B, Harvie R, McKenzie C, Summers K, Reid G, Burton JP, Silverman M. Allogenic Fecal Microbiota Transplantation in Patients With Nonalcoholic Fatty Liver Disease Improves Abnormal Small Intestinal Permeability: A Randomized Control Trial. Am J Gastroenterol. 2020 doi: 10.14309/ajg.0000000000000661. [DOI] [PubMed] [Google Scholar]
- 122.Yang JM, Sun Y, Wang M, Zhang XL, Zhang SJ, Gao YS, Chen L, Wu MY, Zhou L, Zhou YM, Wang Y, Zheng FJ, Li YH. Regulatory effect of a Chinese herbal medicine formula on non-alcoholic fatty liver disease. World J Gastroenterol. 2019;25:5105–5119. doi: 10.3748/wjg.v25.i34.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhu F, Li YM, Feng TT, Wu Y, Zhang HX, Jin GY, Liu JP. Freeze-dried Si-Ni-San powder can ameliorate high fat diet-induced non-alcoholic fatty liver disease. World J Gastroenterol. 2019;25:3056–3068. doi: 10.3748/wjg.v25.i24.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang Y, Tang K, Deng Y, Chen R, Liang S, Xie H, He Y, Chen Y, Yang Q. Effects of shenling baizhu powder herbal formula on intestinal microbiota in high-fat diet-induced NAFLD rats. Biomed Pharmacother. 2018;102:1025–1036. doi: 10.1016/j.biopha.2018.03.158. [DOI] [PubMed] [Google Scholar]
- 125.Feng Q, Liu W, Baker SS, Li H, Chen C, Liu Q, Tang S, Guan L, Tsompana M, Kozielski R, Baker RD, Peng J, Liu P, Zhu R, Hu Y, Zhu L. Multi-targeting therapeutic mechanisms of the Chinese herbal medicine QHD in the treatment of non-alcoholic fatty liver disease. Oncotarget. 2017;8:27820–27838. doi: 10.18632/oncotarget.15482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu B, Zhang J, Sun P, Yi R, Han X, Zhao X. Raw Bowl Tea (Tuocha) Polyphenol Prevention of Nonalcoholic Fatty Liver Disease by Regulating Intestinal Function in Mice. Biomolecules. 2019;9 doi: 10.3390/biom9090435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ushiroda C, Naito Y, Takagi T, Uchiyama K, Mizushima K, Higashimura Y, Yasukawa Z, Okubo T, Inoue R, Honda A, Matsuzaki Y, Itoh Y. Green tea polyphenol (epigallocatechin-3-gallate) improves gut dysbiosis and serum bile acids dysregulation in high-fat diet-fed mice. J Clin Biochem Nutr. 2019;65:34–46. doi: 10.3164/jcbn.18-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Porras D, Nistal E, Martínez-Flórez S, Pisonero-Vaquero S, Olcoz JL, Jover R, González-Gallego J, García-Mediavilla MV, Sánchez-Campos S. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radic Biol Med. 2017;102:188–202. doi: 10.1016/j.freeradbiomed.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 129.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Singal AK, Arora S, Wong RJ, Satapathy SK, Shah VH, Kuo YF, Kamath PS. Increasing Burden of Acute-On-Chronic Liver Failure Among Alcohol-Associated Liver Disease in the Young Population in the United States. Am J Gastroenterol. 2020;115:88–95. doi: 10.14309/ajg.0000000000000411. [DOI] [PubMed] [Google Scholar]
- 131.Guohong-Liu, Qingxi-Zhao, Hongyun-Wei Characteristics of intestinal bacteria with fatty liver diseases and cirrhosis. Ann Hepatol. 2019;18:796–803. doi: 10.1016/j.aohep.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 132.Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. 2020;72:558–577. doi: 10.1016/j.jhep.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 133.Bajaj JS. Alcohol, liver disease and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16:235–246. doi: 10.1038/s41575-018-0099-1. [DOI] [PubMed] [Google Scholar]
- 134.Dubinkina VB, Tyakht AV, Odintsova VY, Yarygin KS, Kovarsky BA, Pavlenko AV, Ischenko DS, Popenko AS, Alexeev DG, Taraskina AY, Nasyrova RF, Krupitsky EM, Shalikiani NV, Bakulin IG, Shcherbakov PL, Skorodumova LO, Larin AK, Kostryukova ES, Abdulkhakov RA, Abdulkhakov SR, Malanin SY, Ismagilova RK, Grigoryeva TV, Ilina EN, Govorun VM. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome. 2017;5:141. doi: 10.1186/s40168-017-0359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Davis BC, Bajaj JS. Effects of Alcohol on the Brain in Cirrhosis: Beyond Hepatic Encephalopathy. Alcohol Clin Exp Res. 2018;42:660–667. doi: 10.1111/acer.13605. [DOI] [PubMed] [Google Scholar]
- 136.Tiwari V, Veeraiah P, Subramaniam V, Patel AB. Differential effects of ethanol on regional glutamatergic and GABAergic neurotransmitter pathways in mouse brain. J Neurochem. 2014;128:628–640. doi: 10.1111/jnc.12508. [DOI] [PubMed] [Google Scholar]
- 137.Godlewski G, Cinar R, Coffey NJ, Liu J, Jourdan T, Mukhopadhyay B, Chedester L, Liu Z, Osei-Hyiaman D, Iyer MR, Park JK, Smith RG, Iwakura H, Kunos G. Targeting Peripheral CB1 Receptors Reduces Ethanol Intake via a Gut-Brain Axis. Cell Metab. 2019;29:1320–1333.e8. doi: 10.1016/j.cmet.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ahluwalia V, Wade JB, Moeller FG, White MB, Unser AB, Gavis EA, Sterling RK, Stravitz RT, Sanyal AJ, Siddiqui MS, Puri P, Luketic V, Heuman DM, Fuchs M, Matherly S, Bajaj JS. The etiology of cirrhosis is a strong determinant of brain reserve: A multimodal magnetic resonance imaging study. Liver Transpl. 2015;21:1123–1132. doi: 10.1002/lt.24163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Singal AK, Bataller R, Ahn J, Kamath PS, Shah VH. ACG Clinical Guideline: Alcoholic Liver Disease. Am J Gastroenterol. 2018;113:175–194. doi: 10.1038/ajg.2017.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Addolorato G, Leggio L, Ferrulli A, Cardone S, Vonghia L, Mirijello A, Abenavoli L, D'Angelo C, Caputo F, Zambon A, Haber PS, Gasbarrini G. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370:1915–1922. doi: 10.1016/S0140-6736(07)61814-5. [DOI] [PubMed] [Google Scholar]
- 141.Liu Y, Chen K, Li F, Gu Z, Liu Q, He L, Shao T, Song Q, Zhu F, Zhang L, Jiang M, Zhou Y, Barve S, Zhang X, McClain CJ, Feng W. Probiotic Lactobacillus rhamnosus GG Prevents Liver Fibrosis Through Inhibiting Hepatic Bile Acid Synthesis and Enhancing Bile Acid Excretion in Mice. Hepatology. 2020;71:2050–2066. doi: 10.1002/hep.30975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Grander C, Adolph TE, Wieser V, Lowe P, Wrzosek L, Gyongyosi B, Ward DV, Grabherr F, Gerner RR, Pfister A, Enrich B, Ciocan D, Macheiner S, Mayr L, Drach M, Moser P, Moschen AR, Perlemuter G, Szabo G, Cassard AM, Tilg H. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut. 2018;67:891–901. doi: 10.1136/gutjnl-2016-313432. [DOI] [PubMed] [Google Scholar]
- 143.Mancini A, Campagna F, Amodio P, Tuohy KM. Gut : liver : brain axis: the microbial challenge in the hepatic encephalopathy. Food Funct. 2018;9:1373–1388. doi: 10.1039/c7fo01528c. [DOI] [PubMed] [Google Scholar]
- 144.Sarin SK, Pande A, Schnabl B. Microbiome as a therapeutic target in alcohol-related liver disease. J Hepatol. 2019;70:260–272. doi: 10.1016/j.jhep.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 145.Bajaj JS, Salzman NH, Acharya C, Sterling RK, White MB, Gavis EA, Fagan A, Hayward M, Holtz ML, Matherly S, Lee H, Osman M, Siddiqui MS, Fuchs M, Puri P, Sikaroodi M, Gillevet PM. Fecal Microbial Transplant Capsules Are Safe in Hepatic Encephalopathy: A Phase 1, Randomized, Placebo-Controlled Trial. Hepatology. 2019;70:1690–1703. doi: 10.1002/hep.30690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Duan Y, Llorente C, Lang S, Brandl K, Chu H, Jiang L, White RC, Clarke TH, Nguyen K, Torralba M, Shao Y, Liu J, Hernandez-Morales A, Lessor L, Rahman IR, Miyamoto Y, Ly M, Gao B, Sun W, Kiesel R, Hutmacher F, Lee S, Ventura-Cots M, Bosques-Padilla F, Verna EC, Abraldes JG, Brown RS, Jr, Vargas V, Altamirano J, Caballería J, Shawcross DL, Ho SB, Louvet A, Lucey MR, Mathurin P, Garcia-Tsao G, Bataller R, Tu XM, Eckmann L, van der Donk WA, Young R, Lawley TD, Stärkel P, Pride D, Fouts DE, Schnabl B. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature. 2019;575:505–511. doi: 10.1038/s41586-019-1742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Frederick E. Bacterial toxin linked to severe alcoholic liver disease. Science. 2019;366:784. doi: 10.1126/science.366.6467.784. [DOI] [PubMed] [Google Scholar]
- 148.Zhong W, Wei X, Hao L, Lin TD, Yue R, Sun X, Guo W, Dong H, Li T, Ahmadi AR, Sun Z, Zhang Q, Zhao J, Zhou Z. Paneth Cell Dysfunction Mediates Alcohol-related Steatohepatitis Through Promoting Bacterial Translocation in Mice: Role of Zinc Deficiency. Hepatology. 2020;71:1575–1591. doi: 10.1002/hep.30945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mathurin P, Bataller R. Trends in the management and burden of alcoholic liver disease. J Hepatol. 2015;62:S38–S46. doi: 10.1016/j.jhep.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chu H, Duan Y, Lang S, Jiang L, Wang Y, Llorente C, Liu J, Mogavero S, Bosques-Padilla F, Abraldes JG, Vargas V, Tu XM, Yang L, Hou X, Hube B, Stärkel P, Schnabl B. The Candida albicans exotoxin candidalysin promotes alcohol-associated liver disease. J Hepatol. 2020;72:391–400. doi: 10.1016/j.jhep.2019.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang D, Song XJ, Li SY, Wang SY, Chen BJ, Bai XD, Tang LM. Evaluation of liver function and electroacupuncture efficacy of animals with alcoholic liver injury by the novel imaging methods. Sci Rep. 2016;6:30119. doi: 10.1038/srep30119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lee BP, Mehta N, Platt L, Gurakar A, Rice JP, Lucey MR, Im GY, Therapondos G, Han H, Victor DW, Fix OK, Dinges L, Dronamraju D, Hsu C, Voigt MD, Rinella ME, Maddur H, Eswaran S, Hause J, Foley D, Ghobrial RM, Dodge JL, Li Z, Terrault NA. Outcomes of Early Liver Transplantation for Patients With Severe Alcoholic Hepatitis. Gastroenterology. 2018;155:422–430.e1. doi: 10.1053/j.gastro.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Garcia-Tsao G, Wiest R. Gut microflora in the pathogenesis of the complications of cirrhosis. Best Pract Res Clin Gastroenterol. 2004;18:353–372. doi: 10.1016/j.bpg.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 154.Starr SP, Raines D. Cirrhosis: diagnosis, management, and prevention. Am Fam Physician. 2011;84:1353–1359. [PubMed] [Google Scholar]
- 155.Kajihara M, Koido S, Kanai T, Ito Z, Matsumoto Y, Takakura K, Saruta M, Kato K, Odamaki T, Xiao JZ, Sato N, Ohkusa T. Characterisation of blood microbiota in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2019;31:1577–1583. doi: 10.1097/MEG.0000000000001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ahluwalia V, Betrapally NS, Hylemon PB, White MB, Gillevet PM, Unser AB, Fagan A, Daita K, Heuman DM, Zhou H, Sikaroodi M, Bajaj JS. Impaired Gut-Liver-Brain Axis in Patients with Cirrhosis. Sci Rep. 2016;6:26800. doi: 10.1038/srep26800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–735. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 158.Campion D, Giovo I, Ponzo P, Saracco GM, Balzola F, Alessandria C. Dietary approach and gut microbiota modulation for chronic hepatic encephalopathy in cirrhosis. World J Hepatol. 2019;11:489–512. doi: 10.4254/wjh.v11.i6.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gheorghe L, Iacob R, Vădan R, Iacob S, Gheorghe C. Improvement of hepatic encephalopathy using a modified high-calorie high-protein diet. Rom J Gastroenterol. 2005;14:231–238. [PubMed] [Google Scholar]
- 160.Amodio P, Canesso F, Montagnese S. Dietary management of hepatic encephalopathy revisited. Curr Opin Clin Nutr Metab Care. 2014;17:448–452. doi: 10.1097/MCO.0000000000000084. [DOI] [PubMed] [Google Scholar]
- 161.Sharma P, Sharma BC, Agrawal A, Sarin SK. Primary prophylaxis of overt hepatic encephalopathy in patients with cirrhosis: an open labeled randomized controlled trial of lactulose versus no lactulose. J Gastroenterol Hepatol. 2012;27:1329–1335. doi: 10.1111/j.1440-1746.2012.07186.x. [DOI] [PubMed] [Google Scholar]
- 162.Wang JY, Bajaj JS, Wang JB, Shang J, Zhou XM, Guo XL, Zhu X, Meng LN, Jiang HX, Mi YQ, Xu JM, Yang JH, Wang BS, Zhang NP. Lactulose improves cognition, quality of life, and gut microbiota in minimal hepatic encephalopathy: A multicenter, randomized controlled trial. J Dig Dis. 2019;20:547–556. doi: 10.1111/1751-2980.12816. [DOI] [PubMed] [Google Scholar]
- 163.Bothe MK, Maathuis AJH, Bellmann S, van der Vossen JMBM, Berressem D, Koehler A, Schwejda-Guettes S, Gaigg B, Kuchinka-Koch A, Stover JF. Dose-Dependent Prebiotic Effect of Lactulose in a Computer-Controlled In Vitro Model of the Human Large Intestine. Nutrients. 2017;9 doi: 10.3390/nu9070767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yang N, Liu H, Jiang Y, Zheng J, Li DM, Ji C, Liu YY, Zuo PP. Lactulose enhances neuroplasticity to improve cognitive function in early hepatic encephalopathy. Neural Regen Res. 2015;10:1457–1462. doi: 10.4103/1673-5374.165516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Viramontes Hörner D, Avery A, Stow R. The Effects of Probiotics and Symbiotics on Risk Factors for Hepatic Encephalopathy: A Systematic Review. J Clin Gastroenterol. 2017;51:312–323. doi: 10.1097/MCG.0000000000000789. [DOI] [PubMed] [Google Scholar]
- 166.Zhao LN, Yu T, Lan SY, Hou JT, Zhang ZZ, Wang SS, Liu FB. Probiotics can improve the clinical outcomes of hepatic encephalopathy: An update meta-analysis. Clin Res Hepatol Gastroenterol. 2015;39:674–682. doi: 10.1016/j.clinre.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 167.Hart AL, Lammers K, Brigidi P, Vitali B, Rizzello F, Gionchetti P, Campieri M, Kamm MA, Knight SC, Stagg AJ. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut. 2004;53:1602–1609. doi: 10.1136/gut.2003.037325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rashid SK, Idris-Khodja N, Auger C, Alhosin M, Boehm N, Oswald-Mammosser M, Schini-Kerth VB. Probiotics (VSL#3) prevent endothelial dysfunction in rats with portal hypertension: role of the angiotensin system. PLoS One. 2014;9:e97458. doi: 10.1371/journal.pone.0097458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Degirolamo C, Rainaldi S, Bovenga F, Murzilli S, Moschetta A. Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the Fxr-Fgf15 axis in mice. Cell Rep. 2014;7:12–18. doi: 10.1016/j.celrep.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 170.Dhiman RK, Rana B, Agrawal S, Garg A, Chopra M, Thumburu KK, Khattri A, Malhotra S, Duseja A, Chawla YK. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology. 2014;147:1327–37.e3. doi: 10.1053/j.gastro.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 171.Kang DJ, Kakiyama G, Betrapally NS, Herzog J, Nittono H, Hylemon PB, Zhou H, Carroll I, Yang J, Gillevet PM, Jiao C, Takei H, Pandak WM, Iida T, Heuman DM, Fan S, Fiehn O, Kurosawa T, Sikaroodi M, Sartor RB, Bajaj JS. Rifaximin Exerts Beneficial Effects Independent of its Ability to Alter Microbiota Composition. Clin Transl Gastroenterol. 2016;7:e187. doi: 10.1038/ctg.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kaji K, Saikawa S, Takaya H, Fujinaga Y, Furukawa M, Kitagawa K, Ozutsumi T, Kaya D, Tsuji Y, Sawada Y, Kawaratani H, Moriya K, Namisaki T, Akahane T, Mitoro A, Yoshiji H. Rifaximin Alleviates Endotoxemia with Decreased Serum Levels of Soluble CD163 and Mannose Receptor and Partial Modification of Gut Microbiota in Cirrhotic Patients. Antibiotics (Basel) 2020;9 doi: 10.3390/antibiotics9040145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kimer N, Krag A, Møller S, Bendtsen F, Gluud LL. Systematic review with meta-analysis: the effects of rifaximin in hepatic encephalopathy. Aliment Pharmacol Ther. 2014;40:123–132. doi: 10.1111/apt.12803. [DOI] [PubMed] [Google Scholar]
- 174.Bajaj JS, Khoruts A. Microbiota changes and intestinal microbiota transplantation in liver diseases and cirrhosis. J Hepatol. 2020;72:1003–1027. doi: 10.1016/j.jhep.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 175.Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu E, Cox IJ, Kheradman R, Heuman D, Wang J, Gurry T, Williams R, Sikaroodi M, Fuchs M, Alm E, John B, Thacker LR, Riva A, Smith M, Taylor-Robinson SD, Gillevet PM. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology. 2017;66:1727–1738. doi: 10.1002/hep.29306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bajaj JS, Kakiyama G, Savidge T, Takei H, Kassam ZA, Fagan A, Gavis EA, Pandak WM, Nittono H, Hylemon PB, Boonma P, Haag A, Heuman DM, Fuchs M, John B, Sikaroodi M, Gillevet PM. Antibiotic-Associated Disruption of Microbiota Composition and Function in Cirrhosis Is Restored by Fecal Transplant. Hepatology. 2018;68:1549–1558. doi: 10.1002/hep.30037. [DOI] [PubMed] [Google Scholar]