Key Points
Question
What is the association between vision impairment and preventive care use among US residents 50 years and older?
Findings
In this cross-sectional study, those with self-reported vision impairment were less likely to report breast and colon cancer screening than those without vision impairment. However, there was no difference in the odds of reporting influenza and pneumococcal vaccines by vision impairment status.
Meaning
While this investigation cannot determine whether vision impairment affects preventive care, these results suggest that older US individuals with vision impairment may be less likely to use preventive care services and highlight an opportunity to improve health care use for this group.
Abstract
Importance
Preventive care is associated with decreased morbidity and mortality among older adults. Vision impairment may be a barrier to accessing care and health promotion information and therefore may contribute to decreased preventive care uptake.
Objective
To examine the association between self-reported vision impairment and uptake of preventive care services (ie, breast and colon cancer screenings and influenza and pneumococcal vaccinations).
Design, Setting, and Participants
Cross-sectional study using the 2015 and 2018 National Health Interview Survey (NHIS) and 2016 and 2018 Behavioral Risk Factor Surveillance System (BRFSS) data, national surveys of US residents conducted through in-person household interviews in NHIS, and state-based telephone interviews in BRFSS. Participants included respondents 50 years and older based on eligibility for each preventive care service examined.
Exposures
Vision impairment, defined as self-reported trouble seeing, in NHIS, and self-reported blindness/serious difficulty seeing in BRFSS.
Main Outcomes and Measures
Self-reported uptake of breast cancer screening (women aged 50-74 years), colon cancer screening (aged 50-74 years), influenza vaccination (50 years and older), and pneumococcal vaccination (65 years and older). Multivariable regression models adjusted for relevant confounders, including age, were used to examine the uptake of each preventive care service by vision impairment status.
Results
Among NHIS participants, older US individuals with vision impairment (prevalence between 14.3% and 16.3% in the different age groups; n = 12 120-29 654) were less likely to report breast cancer screening (odds ratio [OR], 0.82; 95% CI, 0.71-0.96) and colon cancer screening (OR, 0.89; 95% CI, 0.79-0.99) but not influenza (OR, 1.06; 95% CI, 0.97-1.15) and pneumococcal vaccination (OR, 1.03; 95% CI, 0.91-1.16), as compared with their counterparts without vision impairment. In BRFSS (n = 228 649-530 027), those with vision impairment (5.9%-6.8%) were less likely than those without vision impairment to report breast cancer screening (OR, 0.67; 95% CI, 0.59-0.75), colon cancer screening (OR, 0.70; 95% CI, 0.65-0.76), and pneumococcal vaccination (OR, 0.89; 95% CI, 0.81-0.99) but not influenza vaccination (OR, 0.95; 95% CI, 0.89-1.00).
Conclusions and Relevance
Older Americans with vision impairment may be less likely to use cancer-related preventive services as compared with their counterparts without vision impairments. These findings suggest that interventions to improve access to health information and health care services for individuals with vision impairment may be needed to improve cancer screening among this population.
This study examines the association between self-reported vision impairment and uptake of preventive care services (ie, breast and colon cancer screenings and influenza and pneumococcal vaccinations).
Introduction
Disease prevention and health promotion strategies are key to healthy aging.1 The 2011 National Prevention Strategy identified clinical and community preventive services as a measure to improve health and well-being through the prioritization of prevention. Preventive care services, such as screening tests, counseling, and immunizations, can reduce morbidity and mortality for older adults.2 These measures are also cost-effective and help decrease overall health care spending.2
Vision impairment prevalence is estimated to range from 5.3% among United States (US) adults aged 50 to 59 years to 50.0% among those 80 years and older.3 Vision impairment is associated with negative health outcomes, such as heart disease, stroke, and cancer,4,5,6,7 and negative health care use patterns, including increased readmissions and high health care costs.8 Moreover, individuals with vision impairment report greater barriers to access to care, including issues related to cost and transportation,9 and limited access to health promotion information.10
The presence of barriers to accessing health care that adults with vision impairment face may result in an overall decreased uptake of preventive care services, which could help explain the frequent comorbid health problems and increased health care spending reported in this group. Previous literature on vision impairment and preventive care has examined vision impairment within a broader context of disability,11,12,13 or compared those with vision impairment with people with other disabilities.14 Studies that have compared individuals with vision impairment with those without vision impairment in the general population have been limited to narrow geographic locations.15,16 To build on existing literature, we examined the association between self-reported vision impairment and self-reported preventive care uptake among US adults 50 years or older using 2 nationally representative surveys, the National Health Interview Survey (NHIS) and the Behavioral Risk Factor Surveillance System (BRFSS).
Methods
Study Populations
NHIS
The NHIS is an ongoing health survey of the US noninstitutionalized population conducted by the US Centers for Disease Control and Prevention (CDC) and US Census Bureau in all 50 states and the District of Columbia (DC) through in-person household interviews. Participants 50 years or older who completed the Sample Adult questionnaire in 2015 or 2018, when it contained detailed questions about cancer screening and immunizations, were eligible for inclusion in this study. The NHIS public use files were downloaded from IPUMS.17 The Research Ethics Review Board of National Center for Health Statistics approved the NHIS protocol.18
BRFSS
The BRFSS is an ongoing state-based telephone survey of adults conducted by the CDC in all US states, DC, and participating US territories. In 2016 and 2018, the core questionnaire included sections on cancer screening and immunization. Respondents from 2016 and 2018 50 years or older residing in any of the US states or DC were eligible for inclusion. The CDC determined that the BRFSS protocol is exempt from institutional review board approval.19 Verbal informed consent was obtained from participants in both studies, and no compensation or incentives were offered.
Preventive Care Outcomes
Outcomes included self-reported uptake of the following preventive care services:
Breast cancer screening: mammogram in the past 2 years;
Colon cancer screening: home blood stool test in the past year, sigmoidoscopy in the past 5 years, or colonoscopy in the past 10 years;
Influenza vaccination in the past year; and
Pneumococcal vaccination ever.
These outcomes were selected based on the Healthy People 2020 objective of increasing the proportion of older adults who are up to date on a set of clinical preventive services.20 Although this objective targets adults 65 years or older, breast and colon cancer screening and influenza vaccination are recommended for adults between the ages 50 to 64 years as well, and this group has been shown to benefit from these services.21 Moreover, the mortality benefit of breast and colon cancer screening may take around 10 years to accrue.22
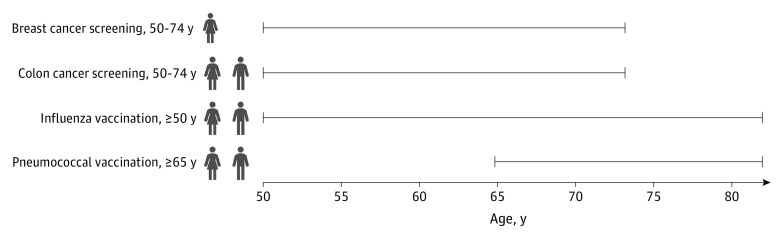
Based on the eligibility for each outcome, 4 groups were constructed from each study (ie, the NHIS and BRFSS) yielding 8 analytic samples in total (Figure 1). The age ranges for outcome eligibility were selected based on the US Preventive Task Force and CDC guidelines. The colon cancer screening population was restricted to those between the ages of 50 and 74 years rather than 50 and 75 years, in parallel with the breast cancer screening group.
Figure 1. Description of the Analytic Samples’ Eligibility Criteria.
Women between ages 50 and 74 years were included in the breast cancer screening sample; women and men between ages 50 and 74 years were included in the colon cancer screening sample; women and men 50 years or older were included in the influenza vaccination sample; and women and men 65 years or older were included in the pneumococcal vaccination sample. All 4 samples were created using National Health Interview Survey (NHIS) and Behavioral Risk Factor Surveillance System (BRFSS) data separately, resulting in a total of 8 samples.
For the cancer screening outcomes, NHIS and BRFSS participants were first asked if they ever had the given test (eg, “Have you ever had a mammogram?”). Following this, in NHIS, those who answered “yes” were asked: “When did you have your most recent mammogram?” Those who could not provide a definitive answer were then given an option to select a prespecified time since screening. In BRFSS, participants who had the screening test were asked: “How long has it been since you had your last mammogram?” Participants directly selected a prespecified time category. The final outcomes were computed based on the test and time specified previously (eg, those who had a mammography in the past 2 years were considered to have received breast cancer screening). Furthermore, NHIS participants who had a colonoscopy were asked about the reason they had the test; the colonoscopy was considered to have been completed for colon cancer screening only if it was “part of a routine examination.”
For the vaccination outcomes, NHIS and BRFSS participants were asked whether they received an influenza vaccination in the past year and whether they ever received a pneumococcal vaccination. Participants were considered to have received the influenza or pneumococcal vaccination if they answered “yes” to the respective question.
Vision Impairment
The main exposure was functional vision impairment, defined as self-reported difficulty seeing. In NHIS, participants were considered to be vision impaired if they responded “yes” to the question: “Do you have any trouble seeing, even when wearing glasses or contact lenses?” In BRFSS, participants who responded “yes” to the question “Are you blind or do you have serious difficulty seeing, even when wearing glasses?” were considered vision impaired.
Other Covariates
Sociodemographic characteristics included age, sex, and race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, and non-Hispanic other). In NHIS, the ratio of income to poverty (imputed values were provided) (<1.0, 1.00-1.99, and ≥2.0) was used as a proxy for socioeconomic status. In BRFSS, between 13.2% of respondents (colon cancer screening sample) and 19.5% of respondents (pneumococcal vaccination sample) had missing income information; therefore highest educational level (<high school, high school graduate, some college, and ≥college graduate) was used instead as a proxy for socioeconomic status.23 Access to care characteristics included having health insurance and a usual place of care (NHIS) or usual care clinician (BRFSS). A comorbidity count (0, 1-2, or ≥3 conditions) was computed based on self-reported chronic medical conditions, including coronary artery disease, other heart problems, chronic lung disease, cancer, arthritis, weak/failing kidneys, stroke, diabetes, and hypertension in NHIS. The same count was computed in BRFSS; however, hypertension and other heart problems were not available for this sample.
Statistical Analysis
Cross-sectional analyses examined the association between vision impairment and preventive care service uptake for each of the outcomes in the respective sample populations. Analyses accounted for the complex design of NHIS and BRFSS, using study weights accounting for aggregating years of data and analyzing subgroups. Population characteristics were described by vision impairment status using weighted percentages, and groupwise comparisons were performed using the χ2 tests of association for survey data. Multivariable logistic regression models examined the association between vision impairment and preventive care use. Models were adjusted for demographic characteristics (age, sex [except for breast cancer screening], and race/ethnicity), socioeconomic status (income-to-poverty ratio or educational attainment), access to care (usual place of care or care clinician), comorbidity count, and survey year. Because a large proportion of the population consisted of adults 65 years or older, and everyone in this age group is eligible for Medicare insurance, the main models were not adjusted for health insurance status; however, models that included health insurance status were run as a sensitivity analysis. There was no adjustment to the P values for multiple analyses because this is an exploratory study, and the aim was to control the probability of type I error individually for each comparison (comparisonwise error rate).24 The P values were 2-sided, and statistical level of significance was set at .05. Analyses were conducted using R: a language and environment for statistical computing (The R Foundation).25
Results
Population Characteristics
NHIS
Each of the 4 sample populations ranged in size from 12 120 to 29 654 participants (Table 1). Vision impairment was self-reported by 14.3% of the population in the colon cancer screening group, 15.0% in the influenza vaccination group, 15.8% in the breast cancer screening group, and 16.3% in the pneumococcal vaccination group. There was no difference in the age distribution between those with and without vision impairment in the cancer screening groups; however, in the vaccination groups, a higher proportion of people with vision impairment were 80 years or older. In all 4 populations, those with vision impairment were less likely than those without to be non-Hispanic White and more likely to be women (not applicable for the breast cancer screening group), have lower income, and have more comorbidities. Most people (93.0%-96.5%) had a usual place of care, and the percentages were similar among those with and without vision impairment.
Table 1. The 2015 and 2018 National Health Interview Survey Population Characteristics by Vision Impairment Status in Weighted Percentagesa.
| Characteristic | Cancer screening | Vaccination | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breast (n = 12 120) | Colon (n = 22 144) | Influenza (n = 29 654) | Pneumococcal (n = 14 771) | |||||||||
| Overall | No VI | VI | Overall | No VI | VI | Overall | No VI | VI | Overall | No VI | VI | |
| Weighted % | NA | 84.2 | 15.8 | NA | 85.7 | 14.3 | NA | 85.0 | 15.0 | NA | 83.7 | 16.3 |
| Age, y | ||||||||||||
| 50-54 | 23.1 | 23.4 | 21.3 | 23.5 | 23.7 | 22.1 | 19.3 | 19.7 | 17.2 | NAb | NAb | NAb |
| 55-59 | 22.6 | 22.6 | 22.8 | 22.6 | 22.4 | 23.8 | 18.6 | 18.7 | 18.5 | NAb | NAb | NAb |
| 60-64 | 21.7 | 21.7 | 21.5 | 21.8 | 21.9 | 21.4 | 18 | 18.2 | 16.4 | NAb | NAb | NAb |
| 65-69 | 18.3 | 18.1 | 19.5 | 18.1 | 18.1 | 18.5 | 14.8 | 14.9 | 14.2 | 33.8 | 34.6 | 29.9 |
| 70-74 | 14.3 | 14.2 | 14.9 | 13.9 | 13.8 | 14.3 | 11.4 | 11.4 | 11.0 | 25.9 | 26.4 | 23.2 |
| 75-79 | NAb | NAb | NAb | NAb | NAb | NAb | 7.6 | 7.5 | 8.3 | 17.4 | 17.4 | 17.5 |
| ≥80 | NAb | NAb | NAb | NAb | NAb | NAb | 10.2 | 9.5 | 14.2 | 22.9 | 21.6 | 29.3 |
| Female | 100 | 100 | 100 | 52.0 | 51.2 | 57.3 | 53.2 | 52.4 | 58.0 | 55.2 | 54.3 | 60.0 |
| Race/ethnicity | ||||||||||||
| Non-Hispanic White | 70.7 | 71.5 | 66.4 | 71.4 | 72.1 | 67.5 | 72.8 | 73.4 | 69.5 | 77.1 | 77.6 | 74.7 |
| Non-Hispanic Black | 11.1 | 10.5 | 14.2 | 10.6 | 10.0 | 13.7 | 10.2 | 9.7 | 13.2 | 8.7 | 8.2 | 11.0 |
| Hispanic | 11.3 | 10.9 | 13.8 | 11.3 | 11.0 | 13.1 | 10.5 | 10.3 | 11.5 | 8.3 | 8.2 | 8.7 |
| Non-Hispanic other | 6.8 | 7.1 | 5.7 | 6.7 | 6.9 | 5.7 | 6.5 | 6.6 | 5.8 | 5.9 | 6.0 | 5.7 |
| Ratio of income to poverty | ||||||||||||
| <1.0 | 10.5 | 8.8 | 19.1 | 9.3 | 8.1 | 17.0 | 9.4 | 8.2 | 15.9 | 8.6 | 7.5 | 14.2 |
| 1.00-1.99 | 15.9 | 14.9 | 21.5 | 15.3 | 14.3 | 21.4 | 17.2 | 16.1 | 23.3 | 20.7 | 19.7 | 25.8 |
| ≥2.0 | 73.6 | 76.3 | 59.4 | 75.4 | 77.7 | 61.6 | 73.4 | 75.7 | 60.8 | 70.7 | 72.8 | 60 |
| Health insurance | 94.7 | 95.0 | 92.8 | 93.8 | 94.1 | 92.2 | 94.9 | 95.1 | 93.9 | 99.5 | 99.5 | 99.5 |
| Usual place of care | 94.7 | 94.8 | 94.1 | 93.0 | 93.0 | 92.9 | 93.8 | 93.7 | 94.1 | 96.5 | 96.4 | 97.0 |
| Comorbidity countc | ||||||||||||
| 0 conditions | 26.7 | 29.1 | 13.8 | 27.2 | 29.1 | 15.4 | 24.1 | 26.0 | 13.2 | 12.5 | 13.7 | 6.8 |
| 1-2 conditions | 52.3 | 52.9 | 49.2 | 51.2 | 52.0 | 46.8 | 50.1 | 51.1 | 44.9 | 49.3 | 51.0 | 40.2 |
| ≥3 conditions | 21.0 | 18.0 | 37.0 | 21.6 | 18.9 | 37.7 | 25.8 | 22.9 | 41.9 | 38.2 | 35.3 | 53.0 |
Abbreviations: NA, not applicable; VI, vision impairment.
Vision impairment was defined as self-reported trouble seeing, even when wearing glasses/contact lenses.
Represents observations excluded from the analytic sample owing to age restrictions.
Comorbidity count: coronary artery disease (angina, myocardial infarction, or coronary heart disease), other heart problems, chronic lung disease (chronic bronchitis or emphysema), cancer, arthritis, weak or failing kidneys, stroke, diabetes, and hypertension.
BRFSS
The sample sizes for the BRFSS populations were larger than NHIS samples and ranged from 228 640 to 530 027 individuals (Table 2). The prevalence of vision impairment was lowest in the colon cancer screening group (5.9%) and highest in the pneumococcal vaccination group (6.8%), similar to NHIS. In addition, similar age, sex, race/ethnicity, and comorbidity trends to the NHIS samples were observed. Those with vision impairment were more likely than those without to have lower levels of education and not have a usual health care clinician.
Table 2. The 2016 and 2018 Behavioral Risk Factor Surveillance System Population Characteristics by Vision Impairment Status in Weighted Percentagesa.
| Characteristic | Cancer screening | Vaccination | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breast (n = 228 640) | Colon (n = 394 270) | Influenza (n = 530 027) | Pneumococcal (n = 279 618) | |||||||||
| Overall | No VI | VI | Overall | No VI | VI | Overall | No VI | VI | Overall | No VI | VI | |
| Weighted % | NA | 93.7 | 6.3 | NA | 94.1 | 5.9 | NA | 93.5 | 6.5 | NA | 93.2 | 6.8 |
| Age, y | ||||||||||||
| 50-54 | 23.0 | 23.0 | 23.6 | 23.6 | 23.6 | 22.9 | 19.3 | 19.4 | 17.5 | NAb | NAb | NAb |
| 55-59 | 21.4 | 21.4 | 21.9 | 21.7 | 21.6 | 22.8 | 17.7 | 17.8 | 17.4 | NAb | NAb | NAb |
| 60-64 | 22.8 | 22.8 | 23.1 | 22.7 | 22.7 | 23.7 | 18.6 | 18.7 | 17.9 | NAb | NAb | NAb |
| 65-69 | 17.8 | 17.9 | 17.6 | 17.9 | 17.9 | 17.0 | 14.7 | 14.8 | 13.0 | 33.1 | 33.5 | 27.8 |
| 70-74 | 14.9 | 15.0 | 13.8 | 14.1 | 14.1 | 13.6 | 11.7 | 11.8 | 10.5 | 26.3 | 26.6 | 22.3 |
| 75-79 | NAb | NAb | NAb | NAb | NAb | NAb | 8.5 | 8.5 | 9.4 | 19.3 | 19.2 | 20.1 |
| ≥80 | NAb | NAb | NAb | NAb | NAb | NAb | 9.5 | 9.2 | 14.2 | 21.3 | 20.7 | 29.8 |
| Female | 100.0 | 100.0 | 100.0 | 52.3 | 52.1 | 54.9 | 53.4 | 53.2 | 56.5 | 56.0 | 55.8 | 59.6 |
| Race/ethnicity | ||||||||||||
| Non-Hispanic White | 72.9 | 74.0 | 55.5 | 73.2 | 74.2 | 57.0 | 74.3 | 75.2 | 61.3 | 79.3 | 80.0 | 69.6 |
| Non-Hispanic Black | 11.2 | 10.8 | 17.1 | 10.7 | 10.3 | 17.0 | 10.4 | 10.0 | 15.3 | 8.8 | 8.5 | 12.0 |
| Hispanic | 10.1 | 9.4 | 21.1 | 10.2 | 9.6 | 19.1 | 9.7 | 9.2 | 17.0 | 7.0 | 6.7 | 12.4 |
| Non-Hispanic other | 5.8 | 5.8 | 6.2 | 5.9 | 5.9 | 6.9 | 5.7 | 5.6 | 6.4 | 4.9 | 4.8 | 6.0 |
| Education | ||||||||||||
| Less than HS | 11.7 | 10.4 | 30.4 | 12.2 | 11.0 | 30.8 | 13.5 | 12.2 | 31.2 | 14.2 | 13.2 | 28.6 |
| HS graduate | 27.2 | 27.1 | 29.4 | 27.6 | 27.4 | 30.4 | 28.5 | 28.3 | 31.2 | 29.2 | 29.1 | 30.6 |
| Some college | 33.2 | 33.5 | 29.3 | 31.5 | 31.7 | 27.4 | 30.8 | 31.1 | 25.7 | 30.7 | 31.0 | 26.6 |
| College graduate | 27.9 | 29.0 | 10.8 | 28.8 | 29.9 | 11.5 | 27.3 | 28.4 | 11.9 | 25.9 | 26.8 | 14.1 |
| Health insurance | 93.9 | 94.2 | 89.3 | 93.2 | 93.6 | 87.5 | 94.0 | 94.3 | 89.6 | 98.2 | 98.3 | 96.8 |
| Usual clinician | 91.7 | 91.8 | 89.6 | 89.1 | 89.3 | 85.9 | 90.2 | 90.3 | 87.9 | 94.4 | 94.6 | 92.8 |
| Comorbidity countc | ||||||||||||
| 0 conditions | 38.8 | 40.3 | 16.9 | 40.8 | 42.2 | 19.4 | 37.0 | 38.4 | 17.7 | 24.7 | 25.5 | 13.0 |
| 1-2 conditions | 51.6 | 51.4 | 55.1 | 49.6 | 49.3 | 53.4 | 51.7 | 51.5 | 54.2 | 59.2 | 59.5 | 54.9 |
| ≥3 conditions | 9.6 | 8.4 | 28.0 | 9.6 | 8.5 | 27.2 | 11.2 | 10.1 | 28.1 | 16.1 | 14.9 | 32.1 |
Abbreviations: HS, high school; NA, not applicable; VI, vision impairment.
Vision impairment was defined as self-reported blindness or serious difficulty seeing even when wearing glasses.
Represents observations excluded from the analytic sample owing to age restrictions.
Comorbidity count: coronary artery disease (coronary heart disease, angina, myocardial infarction), chronic lung disease, nonskin cancer, arthritis, kidney disease, stroke, and diabetes.
Unadjusted Analyses
NHIS
Overall, 72.3% of women between ages 50 and 74 years reported receiving breast cancer screening, and 55.4% of men and women between the ages 50 and 74 years reported colon cancer screening (Figure 2). The proportion of cancer screening was lower for those with vision impairment than those without (difference in proportions for breast cancer screening, −5.02%; 95% CI, −8.14 to −2.26%; P <.001; difference in proportions for colon cancer screening, −3.67%; 95% CI, −6.20 to −1.13%; P = .002). Report of influenza and pneumococcal vaccination use increased with age. Overall, the proportion of people receiving vaccinations was higher for those with vision impairment than those without (difference in proportions for influenza vaccination, 3.45%; 95% CI, 1.51-5.40; P <.001; difference in proportions for pneumococcal vaccination, 2.26%; 95% CI, 0.73-5.79; P = .02).
Figure 2. Preventive Care Services Uptake by Vision Impairment Status and Age Group in Weighted Percentages.
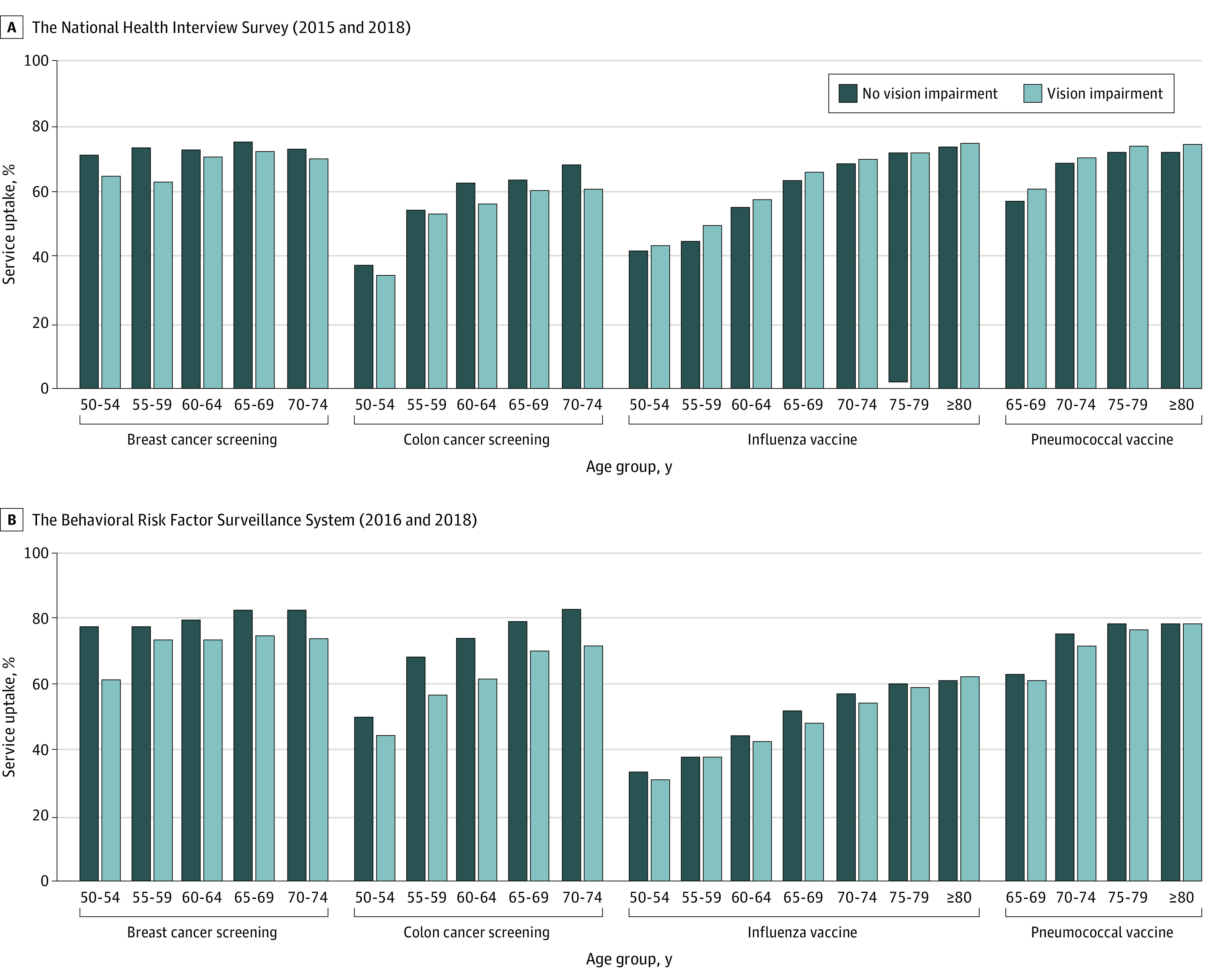
A, Source: 2015 and 2018 National Health Interview Survey (NHIS). Weighted percentages account for the NHIS complex survey design. Vision impairment was defined as self-reported trouble seeing, even when wearing glasses/contact lenses. B, Source: 2016 and 2018 Behavioral Risk Factor Surveillance System (BRFSS). Weighted percentages account for the BRFSS complex survey design. Vision impairment was defined as self-reported blindness or serious difficulty seeing, even when wearing glasses.
BRFSS
Similar trends were seen in the BRFSS populations for breast cancer screening (difference in proportions, −8.69%; 95% CI, −11.0% to −6.36%; P <.001) and colon cancer screening (difference in proportions, −9.85%; 95% CI, −11.61% to 8.10%, P value <.001). However, there was no difference in reported influenza (difference in proportions, −0.54%; 95% CI, −1.93% to 0.85%; P = .45) and pneumococcal vaccination (difference in proportions, −0.89%; 95% CI, −2.84% to 1.07%; P = .37) among those with and without vision impairment.
Adjusted Analyses
NHIS
In adjusted models, women with vision impairment had lower odds of reporting breast cancer screening compared with those without (odds ratio [OR], 0.82; 95% CI, 0.71-0.96) (Table 3). Likewise, men and women with vision impairment were less likely to report colon cancer screening (OR, 0.89; 95% CI, 0.79-0.99). However, there was no association between reporting influenza vaccination (OR, 1.06; 95% CI, 0.97-1.15) or pneumococcal vaccination (OR, 1.03; 95% CI, 0.91-1.16) and vision impairment.
Table 3. Odds Ratios and 95% Confidence Intervals of Preventive Care Services Uptake by Vision Impairment Statusa.
| Variable | Unadjusted models | Adjusted models | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| The National Health Interview Survey (2015 and 2018) | ||||
| Breast cancer screening (n = 12 120)b | ||||
| No vision impairment | 1 [Reference] | NA | 1 [Reference] | NA |
| Vision impairment | 0.78 (0.68-0.89) | <.001 | 0.82 (0.71-0.96) | .01 |
| Colon cancer screening (n = 22 144) | ||||
| No vision impairment | 1 [Reference] | NA | 1 [Reference] | NA |
| Vision impairment | 0.86 (0.78-0.95) | .005 | 0.89 (0.79-0.99) | .04 |
| Influenza vaccine (n = 29 654) | ||||
| No vision impairment | 1 [Reference] | NA | 1 [Reference] | NA |
| Vision impairment | 1.15 (1.06-1.25) | <.001 | 1.06 (0.97-1.15) | .21 |
| Pneumococcal vaccine (n = 14 771) | ||||
| No vision impairment | 1 [Reference] | NA | 1 [Reference] | NA |
| Vision impairment | 1.16 (1.03-1.31) | .013 | 1.03 (0.91-1.16) | .69 |
| The Behavioral Risk Factor Surveillance System (2016 and 2018)c | ||||
| Breast cancer screening (n = 228 640) | ||||
| No vision impairment | 1 [Reference] | NA | 1 [Reference] | NA |
| Vision impairment | 0.63 (0.56-0.70) | <.001 | 0.67 (0.59-0.75) | <.001 |
| Colon cancer screening (n = 394 270) | ||||
| No vision impairment | 1 [Reference] | NA | 1 [Reference] | NA |
| Vision impairment | 0.65 (0.60-0.70) | <.001 | 0.70 (0.65-0.76) | <.001 |
| Influenza vaccine (n = 530 027) | ||||
| No vision impairment | 1 [Reference] | NA | 1 [Reference] | NA |
| Vision impairment | 0.98 (0.93-1.03) | .45 | 0.95 (0.89-1.00) | .07 |
| Pneumococcal vaccine (n = 279 618) | ||||
| No vision impairment | 1 [Reference] | NA | 1 [Reference] | NA |
| Vision impairment | 0.96 (0.87-1.05) | .37 | 0.89 (0.81-0.99) | .03 |
Abbreviations: NA, not applicable; OR, odds ratio.
In the National Health Interview Survey, vision impairment was defined as self-reported trouble seeing, even when wearing glasses/contact lenses. In the Behavioral Risk Factor Surveillance System, vision impairment was defined as self-reported blindness or serious difficulty seeing even when wearing glasses.
Models adjusted for age (50-54 years, 55-59 years, 60-64 years, 65-69 years, 70-74 years, 75-79 years, and ≥80 years), sex (except for breast cancer screening), race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, and non-Hispanic other), income to poverty ratio (<1.00, 1.00-1.99, and ≥2.00), having a usual place of care (yes or no), comorbidity count (0, 1-2, and ≥3 conditions), and survey year (2015 or 2018).
Models adjusted for age (50-54 years, 55-59 years, 60-64 years, 65-69 years, 70-74 years, 75-79 years, and ≥80 years), sex (except for breast cancer screening), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and non-Hispanic other), education (less than high school, high school graduate, some college, or college graduate or higher), having a usual care clinician (yes or no), comorbidity count (0, 1-2, or 3+ conditions), and survey year (2016 or 2018).
BRFSS
In adjusted analyses, those with vision impairment were less likely than those without to report receiving breast cancer screening (OR, 0.67; 95%CI, 0.59-0.75) or colon cancer screening (OR, 0.70; 95% CI, 0.65-0.76). Adults with vision impairment had lower odds of reporting pneumococcal vaccination (OR, 0.89; 95% CI, 0.81-0.99) than those without visual impairments, but vision impairment was not associated with the odds of reporting influenza vaccination (OR, 0.95; 95% CI, 0.89-1.00).
Sensitivity Analyses
Adjusting for health insurance status did not change any of the associations (eTable in the Supplement).
Discussion
In this study including 2 nationally representative samples of US adults aged 50 years and older, individuals with vision impairment were less likely than those without vision impairment to report having received breast and colon cancer screening. However, there were little to no differences in self-report of influenza or pneumococcal vaccination between older US individuals with and without vision impairment.
While vision impairment was associated with lower odds of cancer screening in both study populations, the odds were substantially lower in the BRFSS than in the NHIS samples. This difference may be explained in part by how vision impairment was defined. While NHIS participants were asked about any difficulty seeing, BRFSS participants were asked specifically about severe difficulty or blindness; BRFSS may therefore be only capturing more severe vision impairment. Moreover, models with BRFSS data may have had more residual confounding; educational attainment was used as a proxy for socioeconomic status in BRFSS, while income, known to be more strongly and robustly associated with adverse health in old age, was used in NHIS.23
Our findings about cancer screening are consistent with previous studies. Using Medicare and Medicaid claims data from South Carolina,16 women with vision impairment were less likely than those without vision impairment to receive breast cancer screening (OR, 0.68; 95% CI, 0.58-0.80). These results are similar to our findings. However, this study population was limited to South Carolina residents eligible for Medicare or Medicaid, which includes people 65 years or older, those who have a disability, including blindness, or low income, and therefore our results may not be directly comparable. Using the same data source, men and women with blindness/low vision were less likely to be adherent with colon cancer screening relative to those without blindness/low vision or other disabilities (OR, 0.88; 95% CI, 0.80-0.96),15 which is consistent with our results.
We identified limited literature examining the association between vaccination uptake and vision impairment. In a study of women between the ages 51 and 64 years, those with any functional, activity, or sensory limitation, including vision impairment, were more likely to receive influenza screening in models adjusted for sociodemographic and medical characteristics.13 In our study, in both the NHIS and BRFSS samples, after adjusting for sociodemographic and health characteristics, uptake of influenza vaccination was not associated with vision impairment status. While vision impairment was not associated with report of pneumococcal vaccination in the NHIS (OR, 1.03; 95% CI, 0.91-1.16), it was associated with a lower pneumococcal vaccination in the BRFSS population in which vision impairment was defined more strictly (OR, 0.89; 95% CI, 0.81-0.99). Overall, these data suggest that vaccination uptake may not differ between older US individuals with and without vision impairment.
The differences noted in the association between vision impairment and cancer screening, as opposed to the results from our analyses of reported vaccination, is likely multifactorial. People with vision impairment have reported more barriers to health care access,9 and these may be magnified for cancer screening tests, which may be more costly, and require scheduling appointments and transportation to specific sites that provide them, as opposed to vaccinations that are routinely offered and easily accessible in various locations, including walk-in pharmacies. Cancer screening tests may also require participants to follow specific instructions (eg, colonoscopy preparation), which can be challenging for persons with vision impairment if the instructions are hard to read. Moreover, a survey conducted among women who did not complete breast cancer screening revealed that those with multiple disabilities, including vision impairment, were more likely to report lack of a physician recommendation as a reason.26 While the study was based on self-report, it is possible that physicians may be less likely to focus on preventive strategies when caring for persons with disabilities because they see more pressing health issues at hand to address.
Disease prevention is central to healthy aging. This is especially important among people who may already be at higher risk for chronic disease, disability, and cognitive impairment.27,28 Therefore, efforts to improve access to care among US adults with vision impairment should include preventive care, especially cancer screening. Making health promotion information more accessible to those with low vision may help adults with vision impairment make more informed decisions. Finally, using summary measures for preventive care surveillance (eg, combining uptake of vaccinations and screening), as in the Healthy People 2020 objective, may not permit evaluation of differences in the uptake of specific services. This differential uptake may be magnified for those with disabilities as the barriers to accessing certain services (eg, cancer screenings) may be greater than for other services (eg, vaccinations).
Limitations
This study had limitations that should be considered when interpreting the results. First, uptake of clinical services was based on self-report, which may be subject to recall bias. Likewise, we lacked objective measures about participants’ health, which can greatly affect physicians’ decisions to recommend cancer screening. However, the coherence of our findings across 2 different nationally representative samples of US adults increases the robustness of our results. Second, these data used self-reported visual functioning, which captures a different construct than objectively measured visual acuity but provides valuable insight on perceived vision impairment from a disability framework perspective29 and may affect preventive care use regardless of clinically assessed vision function. Third, we lacked information about the metropolitan status of respondents’ residence, which could differentially affect the uptake of health care services of those with and without vision impairment. Finally, the cross-sectional nature of the data does not allow us to determine temporality in the association between vision impairment and preventive care use.
While this work documented the association between vision impairment and cancer screening, future work would benefit from longitudinal analyses that include objective measures of vision, as well as medical record or health care claims data, and include assessments of health-related factors that may confound this association. Moreover, identifying mediators in the association between vision impairment and decreased cancer screening could reveal potential areas for intervention, such as vision correction, or point to vision rehabilitation or behavioral interventions to improve screening adherence. Finally, considering patients with vision loss in primary care settings by addressing structural barriers to access to care and increasing the use of nonvisual health promotion information could improve cancer screening among people with low vision.
Conclusions
In conclusion, across 2 different nationally representative surveys of older US individuals, we found that those with vision impairment were less likely than those without to have breast and colon cancer screening, while there was little difference in their influenza and pneumococcal vaccination uptake.
eTable. Multivariable-Adjusted Odds Ratios and 95% Confidence Intervals of Preventive Care Services Uptake by Vision Impairment Status
References
- 1.National Prevention Council Healthy Aging in Action. Washington, DC: US Department of Health and Human Services, Office of the Surgeon General; 2016. [PubMed] [Google Scholar]
- 2.National Prevention Council National Prevention Strategy. Washington, DC: US Department of Health and Human Services, Office of the Surgeon General; 2011. [Google Scholar]
- 3.Varma R, Vajaranant TS, Burkemper B, et al. Visual impairment and blindness in adults in the United States: demographic and geographic variations from 2015 to 2050. JAMA Ophthalmol. 2016;134(7):802-809. doi: 10.1001/jamaophthalmol.2016.1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Court H, McLean G, Guthrie B, Mercer SW, Smith DJ. Visual impairment is associated with physical and mental comorbidities in older adults: a cross-sectional study. BMC Med. 2014;12:181. doi: 10.1186/s12916-014-0181-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crews JE, Chou CF, Sekar S, Saaddine JB. The prevalence of chronic conditions and poor health among people with and without vision impairment, aged ≥65 years, 2010-2014. Am J Ophthalmol. 2017;182:18-30. doi: 10.1016/j.ajo.2017.06.038 [DOI] [PubMed] [Google Scholar]
- 6.Garin N, Olaya B, Lara E, et al. Visual impairment and multimorbidity in a representative sample of the Spanish population. BMC Public Health. 2014;14:815. doi: 10.1186/1471-2458-14-815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsueh CM, Wey JH, Yeh JS, Wu CH, Liou TH, Chang KH. Incidence and risk of major heart diseases in middle-aged adults with moderate to severe vision impairment: a population-based cohort study. Br J Ophthalmol. 2019;103(8):1054-1059. doi: 10.1136/bjophthalmol-2018-312471 [DOI] [PubMed] [Google Scholar]
- 8.Morse AR, Seiple W, Talwar N, Lee PP, Stein JD. Association of vision loss with hospital use and costs among older adults. JAMA Ophthalmol. 2019;137(6):634-640. doi: 10.1001/jamaophthalmol.2019.0446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spencer C, Frick K, Gower EW, Kempen JH, Wolff JL. Disparities in access to medical care for individuals with vision impairment. Ophthalmic Epidemiol. 2009;16(5):281-288. doi: 10.1080/09286580902999439 [DOI] [PubMed] [Google Scholar]
- 10.O’Day BL, Killeen M, Iezzoni LI. Improving health care experiences of persons who are blind or have low vision: suggestions from focus groups. Am J Med Qual. 2004;19(5):193-200. doi: 10.1177/106286060401900503 [DOI] [PubMed] [Google Scholar]
- 11.Miller NA, Kirk A, Alston B, Glos L. Effects of gender, disability, and age in the receipt of preventive services. Gerontologist. 2014;54(3):473-487. doi: 10.1093/geront/gnt012 [DOI] [PubMed] [Google Scholar]
- 12.Ramirez A, Farmer GC, Grant D, Papachristou T. Disability and preventive cancer screening: results from the 2001 California Health Interview Survey. Am J Public Health. 2005;95(11):2057-2064. doi: 10.2105/AJPH.2005.066118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei W, Findley PA, Sambamoorthi U. Disability and receipt of clinical preventive services among women. Womens Health Issues. 2006;16(6):286-296. doi: 10.1016/j.whi.2006.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horner-Johnson W, Dobbertin K, Lee JC, Andresen EM; Expert Panel on Disability and Health Disparities . Disparities in health care access and receipt of preventive services by disability type: analysis of the medical expenditure panel survey. Health Serv Res. 2014;49(6):1980-1999. doi: 10.1111/1475-6773.12195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deroche CB, McDermott SW, Mann JR, Hardin JW. Colorectal cancer screening adherence in selected disabilities over 10 years. Am J Prev Med. 2017;52(6):735-741. doi: 10.1016/j.amepre.2017.01.005 [DOI] [PubMed] [Google Scholar]
- 16.Xu X, Mann JR, McDermott SW, Deroche CB, Gustafson E, Hardin JW. Women with visual impairment and insured by Medicaid or Medicare are less likely to receive recommended screening for breast and cervical cancers. Ophthalmic Epidemiol. 2017;24(3):168-173. doi: 10.1080/09286586.2016.1213302 [DOI] [PubMed] [Google Scholar]
- 17.Blewett L, Rivera Drew J, King M, Williams KCW. IPUMS Health Surveys: National Health Interview Survey, Version 6.4. Minneapolis, MN: IPUMS, 2019. [Google Scholar]
- 18.Division of Health Interview Statistics National Center for Health Statistics 2018 National Health Interview Survey (NHIS) Public Use Data Release. Centers for Disease Control and Prevention US Department of Health and Human Services;2019. [Google Scholar]
- 19.Division of Population Health National Center for Chronic Disease Prevention Centers for Disease Control and Prevention BRFSS - OMB No. 0920-1061, Exp. Date 3/31/2018. Supporting Statement - Part A: Justification. 2016. Accessed September 22, 2020. https://www.reginfo.gov/public/
- 20.US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy people 2020. Accessed September 22, 2020. https://www.healthypeople.gov/2020/topics-objectives/topic/older-adults/objectives
- 21.Centers for Disease Control and Prevention , AARP AMA. Promoting Preventive Services for Adults 50-64: Community and Clinical Partnerships. Atlanta, GA: National Association of Chronic Disease Directors; 2009. [Google Scholar]
- 22.Lee SJ, Boscardin WJ, Stijacic-Cenzer I, Conell-Price J, O’Brien S, Walter LC. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441. doi: 10.1136/bmj.e8441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Darin-Mattsson A, Fors S, Kåreholt I. Different indicators of socioeconomic status and their relative importance as determinants of health in old age. Int J Equity Health. 2017;16(1):173. doi: 10.1186/s12939-017-0670-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bender R, Lange S. Adjusting for multiple testing--when and how? J Clin Epidemiol. 2001;54(4):343-349. doi: 10.1016/S0895-4356(00)00314-0 [DOI] [PubMed] [Google Scholar]
- 25.R: A Language and Environment for Statistical Computing [computer program]. Vienna, Austria: R Foundation for Statistical Computing; 2019.
- 26.Yankaskas BC, Dickens P, Bowling JM, et al. Barriers to adherence to screening mammography among women with disabilities. Am J Public Health. 2010;100(5):947-953. doi: 10.2105/AJPH.2008.150318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng DD, Swenor BK, Christ SL, West SK, Lam BL, Lee DJ. Longitudinal associations between visual impairment and cognitive functioning: the Salisbury eye evaluation study. JAMA Ophthalmol. 2018;136(9):989-995. doi: 10.1001/jamaophthalmol.2018.2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuller SD, Mudie LI, Siordia C, Swenor BK, Friedman DS. Nationwide prevalence of self-reported serious sensory impairments and their associations with self-reported cognitive and functional difficulties. Ophthalmology. 2018;125(4):476-485. doi: 10.1016/j.ophtha.2017.11.003 [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization Towards a common language for functioning, disability, and health: ICF. 2002. Accessed July 10, 2020. https://www.who.int/classifications/icf/icfbeginnersguide.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Multivariable-Adjusted Odds Ratios and 95% Confidence Intervals of Preventive Care Services Uptake by Vision Impairment Status