Key Points
Question
Is there an interaction between the distribution of diabetic retinopathy lesions and the association of optical coherence tomography angiography scans and diabetic retinopathy severity?
Findings
This cross-sectional study found that, in eyes without, but not with, predominantly peripheral lesions, there is a strong association between increasing diabetic retinopathy severity and decreasing vessel density on optical coherence tomography angiography scans.
Meaning
This study suggests that eyes with predominantly peripheral lesions may have diabetic retinopathy severity that is associated primarily with peripheral, rather than posterior, nonperfusion.
Abstract
Importance
Studies have not yet determined whether the distribution of lesions in the retinal periphery alters the association between the severity of diabetic retinopathy (DR) and macular vessel density.
Objective
To evaluate the association of DR lesion distribution with optical coherence tomography angiography (OCTA) metrics and DR severity.
Design, Setting, and Participants
This cross-sectional observational study was conducted at a tertiary care center for diabetic eye disease among 225 patients with type 1 or 2 diabetes who had undergone imaging between February 15, 2016, and December 31, 2019.
Exposures
Optical coherence tomography angiography 3 × 3-mm macular scans and ultra-widefield color imaging.
Main Outcomes and Measures
Optical coherence tomography angiography vessel density in the superficial capillary plexus, intermediate capillary plexus, and deep capillary plexus and choriocapillaris flow density. The severity of DR and the predominantly peripheral lesions (PPL) were evaluated from ultra-widefield color imaging.
Results
The study evaluated 352 eyes (225 patients; 125 men [55.6%]; mean [SD] age, 52.1 [15.1] years), of which 183 eyes (52.0%) had mild nonproliferative diabetic retinopathy (NPDR), 71 eyes (20.2%) had moderate NPDR, and 98 eyes (27.8%) had severe NPDR or proliferative diabetic retinopathy (PDR). In eyes with no PPL (209 [59.4%]), the mean (SD) vessel density in the superficial capillary plexus (mild NPDR, 38.1% [4.7%]; moderate NPDR, 36.4% [4.6%]; severe NPDR or PDR, 34.1% [4.1%]; P < .001) and the deep capillary plexus (mild NPDR, 45.8% [3.0%]; moderate NPDR, 45.8% [2.2%]; severe NPDR or PDR, 44.5% [1.9%]; P = .002), as well as the mean (SD) choriocapillaris flow density (mild NPDR, 69.7% [6.2%]; moderate NPDR, 67.6% [5.6%]; severe NPDR or PDR, 67.1% [5.6%]; P = .01), decreased with increasing DR severity. These associations remained statistically significant even after correcting for age, signal strength index, spherical equivalent, duration of diabetes, type of diabetes, and correlation between eyes of the same patient. In eyes with PPL (143 [40.6%]), mean (SD) vessel density in the superficial capillary plexus (mild NPDR, 34.1% [4.1%]; moderate NPDR, 35.2% [4.1%]; severe NPDR or PDR, 36.0% [4.3%]; P = .42) and the deep capillary plexus (mild NPDR, 44.5% [1.7%]; moderate NPDR, 45.4% [1.4%]; severe NPDR or PDR, 44.9% [1.5%]; P = .81), as well as the mean (SD) choriocapillaris flow density (mild NPDR, 67.1% [5.6%]; moderate NPDR, 69.3% [4.6%]; severe NPDR or PDR, 68.3% [5.6%]; P = .49), did not appear to change with increasing DR severity.
Conclusions and Relevance
These results suggest that central retinal vessel density is associated with DR severity in eyes without, but not with, PPL. These findings suggest a potential need to stratify future optical coherence tomography angiography studies of eyes with DR by the presence or absence of PPL. If DR onset and worsening are associated with the location of retinal nonperfusion, assessment of global retinal nonperfusion using widefield angiography may improve the ability to evaluate DR severity and risk of DR worsening over time.
This cross-sectional study evaluates the association of the distribution of diabetic retinopathy lesions with optical coherence tomography angiography (OCTA) metrics and the severity of diabetic retinopathy.
Introduction
For decades, Early Treatment Diabetic Retinopathy Study (ETDRS) diabetic retinopathy (DR) severity grading has been the standard criterion for assessing diabetic eye disease.1,2,3 This classification system is reproducible and an increasing grade is closely associated with the risk of DR worsening. Recently, however, advances in retinal imaging have allowed us to visualize the retinal structure and function in greater detail and allowed assessment of new variables that might prove useful in disease management.4,5,6 One such approach uses ultra-widefield (UWF) imaging to identify predominantly peripheral lesions (PPL) in the retinal far periphery that may be associated with future risk of DR worsening independent of the baseline DR severity level.
The prevalence of eyes with PPL in patients with DR ranges between 37% and 41%.7,8,9 Compared with eyes without PPL (ie, a more central distribution of DR lesions), eyes with PPL are at higher risk of developing advanced retinopathy, with a 3.2-fold increased risk of a DR progression of 2 steps or more and a 4.7-fold increased risk for progression to proliferative DR (PDR) at 4 years.10,11 Eyes with PPL likely have greater amounts of peripheral nonperfusion, supporting the hypothesis that the mechanism underlying PPL development is associated with worsening of local nonperfusion.12 This hypothesis is also supported by past observations using standard fluorescein angiography of a pattern of DR lesion distribution where capillary nonperfusion is minimal in the posterior pole and most microaneurysms and nonperfusion are instead in the retinal periphery.13
Interactions between PPL and the association between central nonperfusion and DR severity have not been extensively explored, to our knowledge. Optical coherence tomography angiography (OCTA) is a noninvasive imaging technique that allows the visualization and quantification of distinct retinal vascular plexuses within the central macula.5 Many studies exploring OCTA in the eyes of patients with diabetes have demonstrated an association between worsening DR severity and decreased central macular vessel density (VD) or increased avascular retinal area.14,15 Although these associations have been consistent across multiple reports, the wide variability of VD measurements found across eyes with the same level of DR severity has limited their use as surrogate markers of DR severity within an individual eye. Furthermore, abnormalities in OCTA metrics are not always strongly associated with the extent of individual DR lesions, such as microaneurysms (MAs), intraretinal microvascular abnormalities (IRMAs), and venous beading, by which DR severity is evaluated.16
Given the association of PPL with DR progression and the association of OCTA metrics with DR severity, it is important to assess if the association between macular VD and DR severity is altered by the presence of PPL. Such data might help define when OCTA metrics are most representative of DR severity and clarify structural associations between PPL and disease progression. Thus, the aim of this study was to compare OCTA metrics across various DR severity levels in eyes with or without PPL.
Methods
Eligible patients for inclusion in this cross-sectional observational study were adults with type 1 or type 2 diabetes who had undergone OCTA, spectral-domain optical coherence tomography, and 200° Optos UWF color fundus photographic imaging at the Joslin Diabetes Center for clinical or investigational purposes between February 15, 2016, and December 31, 2019. This study was approved by the Joslin Diabetes Center Institutional Review Board and adhered to the tenets of the Declaration of Helsinki.17 Given that the data were collected retrospectively, informed consent was waived by the institutional review board.
Only eyes with mild nonproliferative DR (NPDR) or worse were included in the study. Although eyes with PDR were eligible for study inclusion, eyes with a history of panretinal photocoagulation were excluded. Exclusion criteria also included a spherical equivalent (SE) of less than −4 D or more than 2 D, nondiabetic macular pathologic characteristics (eg, retinal vein or artery occlusion or age-related macular degeneration), glaucoma, history of pars plana vitrectomy, macular edema (defined as central subfield thickness >320 μm in males and >305 μm in females on Heidelberg Spectralis optical coherence tomography [OCT]), vitreomacular traction, epiretinal membrane, or cystoid spaces in the central 3 × 3-mm scans. Eyes with any history of anti–vascular endothelial growth factor or corticosteroid intravitreal injections were also excluded given the unclear association of these treatments with OCTA VD measurements and their known association with DR severity.3,18
Standardized data collection forms were used to record patient and eye characteristics including SE, hemoglobin A1c measured within 3 months of imaging, and duration and type of diabetes. All spectral-domain optical coherence tomography (Heidelberg Engineering) images were evaluated for segmentation errors, and any errors were manually adjusted.
Widefield Imaging
Optos nonsimultaneous stereoscopic, on axis, nonsteered, 200° UWF color fundus photographs (Optos PLC) were acquired and graded by a certified Joslin Vision Network grader (M.A.) for ETDRS DR severity level.1,19 Using a previously validated protocol,9 the presence or absence of PPL was graded independently by 2 experienced retina specialists (M.A. and P.S.S.).
Each stereographically projected axial nonsteered UWF 200° image was digitally annotated using a proprietary software tool provided by the manufacturer (Optomapper tool; Optos PLC) using a described technique.20 Images with substantial artifacts or media opacities (eyes with PPL, 13 of 143 [9.1%]; eyes without PPL, 1 of 209 [0.5%]) were not selected for manual annotation. Microaneurysms and IRMAs were manually identified by 2 trained certified graders (M.A. and A.R.) as previously described.20 A subset of 45 eyes were annotated for MAs by both graders and the correlation coefficient for annotated MA lesions between both graders was 0.91. Lesion counts in the posterior pole (<10-mm–radius circle centered on the fovea), midperiphery (10- to 15-mm–radius circle centered on the fovea), and far periphery (>15-mm–radius circle centered on the fovea) were obtained.
OCTA Image Acquisition
Optical coherence tomography angiography imaging was performed using an RTVue XR Avanti spectral-domain optical coherence tomography device with AngioVue software (Optovue). The 3 × 3-mm macular region was imaged using 304 × 304 line scans. The AngioVue software uses the split-spectrum amplitude-decorrelation angiography algorithm.5 Angiovue software versions 2017.1.0.149 and 2017.1.0.151 were used, which included the 3-dimensional projection artifact removal–optical coherence tomography angiography (PAR-OCTA) algorithm that has been shown to affect VD measurements in DR.21
Images with signal strength index (SSI) less than 60 or quality index less than 7 were excluded. In addition, eyes with substantial motion artifacts, vessel doubling, displacement artifacts, and stretch artifacts were excluded (eyes with PPL excluded, 5 of 148 [3.4%]; eyes without PPL excluded, 9 of 218 [4.1%]).22 Automatic choriocapillaris flow density was acquired from the AngioVue software. The superficial capillary plexus (SCP), intermediate capillary plexus (ICP), and deep capillary plexus (DCP) were segmented as previously described.23,24,25,26 Details of the segmentation offsets are included in the eMethods in the Supplement. Parafoveal VD was manually processed from the OCTA images using a validated technique (eMethods and eFigure in the Supplement).
Statistical Analysis
The Shapiro-Wilk test was used to test for normality. Continuous variables were compared using either the t test or Mann-Whitney test, depending on whether the variables were normally distributed. Categorical variables were compared using the χ2 test. For differences in OCTA metrics across DR severity levels, generalized estimating equations correcting for the correlation between assessment of eyes from the same individual as well as common covariates known to affect OCTA metrics such as age, SE, and SSI were used.27,28,29 In addition, we corrected for diabetes type and duration, given the differences in the distribution of these characteristics between eyes with and eyes without PPL. Generalized estimating equations were also used to compare VD and choriocapillaris measurements between different DR severity levels. Pearson correlation was also performed by examining the association between MA or IRMA counts and VD measurements. Reported P values are 2-sided.
A sensitivity analysis was conducted by selecting a subgroup of eyes without PPL that matched the PPL group with regard to DR severity and type of diabetes. A random number generator was used to select 84 eyes with mild NPDR and 24 eyes with severe NPDR or PDR that matched the DR and diabetes type distribution present in the PPL group. We used SPSS statistical software, version 23 (IBM Corp) for statistical analysis.
Results
The study included 352 eyes of 225 patients (125 men [55.6%]; mean [SD] age, 52.1 [15.1] years); 143 eyes had PPL and 209 eyes did not have PPL. Patients with PPL were more likely to have type 1 diabetes (76 of 90 [84.4%] vs 77 of 135 [57.0%]) and a longer mean (SD) duration of diabetes (35.7 [15.1] vs 25.5 [13.7] years) than those without PPL (Table 1). There were no significant differences between the 2 groups with regard to sex, age, or hemoglobin A1c. A total of 183 eyes (52.0%) had mild NPDR, 71 eyes (20.2%) had moderate NPDR, and 98 eyes (27.8%) had severe NPDR or PDR. There was a greater number of eyes with severe NPDR or PDR in the no PPL group compared with the PPL group (74 [35.4%] vs 24 [16.8%]). There was no significant difference between eyes with and eyes without PPL in regard to the SE, central subfield thickness, SSI, or OCTA quality index.
Table 1. Comparison Between Patient, Eye, and Imaging Characteristics in Eyes With or Without PPL.
| Characteristic | Without PPL | With PPL |
|---|---|---|
| Patient characteristics | ||
| Eyes, No. | 209 | 143 |
| Female, No. (%) | 63/135 (46.7) | 37/90 (41.1) |
| Type 1 diabetes, No. (%) | 77/135 (57.0) | 76/90 (84.4) |
| Age, mean (SD), y | 50.5 (15.8) | 54.5 (13.7) |
| Duration of diabetes, mean (SD), y | 25.5 (13.7) | 35.7 (15.1) |
| Hemoglobin A1c, mean (SD), % | 8.3 (1.5) | 7.9 (1.2) |
| DR severity, No. (%) | ||
| Mild NPDR | 99 (47.4) | 84 (58.7) |
| Moderate NPDR | 36 (17.2) | 35 (24.5) |
| Severe NPDR or PDR | 74 (35.4) | 24 (16.8) |
| Eye and imaging characteristics | ||
| SE, mean (SD), D | −0.81 (1.68) | −0.88 (1.73) |
| CST, mean (SD), μm | 272.28 (29.86) | 277.17 (27.29) |
| SSI, mean (SD) | 72.16 (8.00) | 72.36 (7.36) |
| Quality index, mean (SD) | 7.89 (0.96) | 7.94 (0.84) |
Abbreviations: CST, central subfield thickness; DR, diabetic retinopathy; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; PPL, predominantly peripheral lesions; SE, spherical equivalent; SSI, signal strength index.
SI conversion factor: To convert hemoglobin A1c to proportion of total hemoglobin, multiply by 0.01.
In eyes with mild NPDR, mean (SD) SCP VD was greater in eyes without PPL than in those with PPL (38.1% [4.7%] vs 34.1% [4.1%]; unadjusted P = .03) (eTable 1 in the Supplement). In eyes with severe NPDR or PDR, mean (SD) SCP VD was lower in eyes without PPL than those with PPL (34.1% [4.1%] vs 36.0% [4.3%]; P = .05). No differences were identified for the ICP VD, DCP VD, or the choriocapillaris flow density between eyes with PPL and eyes without PPL for any DR severity level (eTable 1 in the Supplement).
Analyses stratified by presence or absence of PPL were performed by evaluating the association between VD metrics and DR severity. In eyes without PPL, the mean (SD) VD measurements were lower in eyes with more advanced DR compared with early DR in all vascular layers except the ICP (SCP: mild NPDR, 38.1% [4.7%]; moderate NPDR, 36.4% [4.6%]; severe NPDR or PDR, 34.1% [4.1%]; P < .001; ICP: mild NPDR, 45.2% [3.0%]; moderate NPDR, 45.3% [1.6%]; severe NPDR or PDR, 45.0% [1.7%]; P = .88; DCP: mild NPDR, 45.8% [3.0%]; moderate NPDR, 45.8% [2.2%]; severe NPDR or PDR, 44.5% [1.7%]; P = .002; mean [SD] choriocapillaris flow density: mild NPDR, 69.7% [6.2%]; moderate NPDR, 67.6% [5.6%]; severe NPDR or PDR, 67.1% [5.6%]; P = .01) (Table 2). These associations remained statistically significant even after correcting for age, SSI, SE, diabetes duration, type of diabetes, and correlation between eyes of the same patient. In contrast, in eyes with PPL, there were no differences noted in the mean (SD) VD measurements among any of the vascular layers with increasing DR severity (SCP: mild NPDR, 34.1% [4.1%]; moderate NPDR, 35.2% [4.1%]; severe NPDR or PDR, 36.0% [4.3%]; P = .42; ICP: mild NPDR, 45.0% [1.7%]; moderate NPDR, 45.5% [1.4%]; severe NPDR or PDR, 45.2% [2.0%]; P = .60; DCP: mild NPDR, 44.5% [1.7%]; moderate NPDR, 45.4% [1.4%]; severe NPDR or PDR, 44.9% [1.5%]; P = .81; mean [SD] choriocapillaris flow density: mild NPDR, 67.1% [5.6%]; moderate NPDR, 69.3% [4.6%]; severe NPDR or PDR, 68.3% [5.6%]; P = .49) (Table 2).
Table 2. Changes in Optical Coherence Tomography Angiography Parameters With Increasing Diabetic Retinopathy Severity in Eyes With or Without PPL.
| Parameter | Mean (SD) | P value | |||
|---|---|---|---|---|---|
| Mild NPDR | Moderate NPDR | Severe NPDR or PDR | Univariate analysis (ANOVA) | GEEsa | |
| Without PPL | |||||
| No. | 99 | 36 | 74 | NA | NA |
| Parafovea, % | |||||
| VD SCP | 38.1 (4.7) | 36.4 (4.6) | 34.1 (4.1) | <.001 | <.001 |
| VD ICP | 45.2 (3.0) | 45.3 (1.6) | 45.0 (1.7) | .88 | .80 |
| VD DCP | 45.8 (3.0) | 45.8 (2.2) | 44.5 (1.7) | .002 | <.001 |
| Choriocapillaris flow density, % | 69.7 (6.2) | 67.6 (5.6) | 67.1 (5.6) | .01 | .006 |
| With PPL | |||||
| No. | 84 | 35 | 24 | NA | NA |
| Parafovea, % | |||||
| VD SCP | 34.1 (4.1) | 35.2 (4.1) | 36.0 (4.3) | .42 | .47 |
| VD ICP | 45.0 (1.7) | 45.5 (1.4) | 45.2 (2.0) | .60 | .54 |
| VD DCP | 44.5 (1.7) | 45.4 (1.4) | 44.9 (1.5) | .81 | .67 |
| Choriocapillaris flow density, % | 67.1 (5.6) | 69.3 (4.6) | 68.3 (5.6) | .49 | .67 |
Abbreviations: ANOVA, analysis of variance; DCP, deep capillary plexus; GEEs, generalized estimating equations; ICP, intermediate capillary plexus; NA, not applicable; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; PPL, predominantly peripheral lesions; SCP, superficial capillary plexus; VD, vessel density.
Correcting for age, signal strength index, spherical equivalent, type of diabetes, diabetes duration, and correlation of both eyes in the same individual.
A sensitivity analysis was performed by selecting a subgroup of eyes without PPL that matched the PPL group with regards to DR severity and type of diabetes (eTable 3 in the Supplement). In this analysis, the percentage of eyes with severe NPDR or PDR in the group without PPL was 25.5% (41 of 161) vs 16.8% (24 of 143) in the PPL group. In this analysis, a decrease in SCP VD was still present across increasing DR severity levels even after correcting for age, SSI, SE, type of diabetes, diabetes duration, and correlation between eyes of the same patient. No significant association was found between ICP or DCP VD or choriocapillaris flow density and DR severity in this group of eyes.
On OCTA processing, the 3 × 3-mm image was initially cropped to exclude the central 1.5-mm–diameter circle to facilitate analysis of the parafoveal area without the confounding effect of the foveal avascular zone, which is widely variable. However, one problem in doing so is the potential exclusion of the macular vascular plexus immediately surrounding the foveal avascular zone. To address this problem, images were reprocessed with smaller-diameter (1 and 0.5 mm) central circles excluded. This sensitivity analysis yielded similar results, in that no association between SCP or DCP VD and DR severity was identified for eyes with PPL (eTable 2 in the Supplement).
In eyes without PPL that had images annotated for hemorrhages and MAs and IRMAs, both hemorrhages and MA and IRMA counts were weakly but negatively correlated with SCP VD in all the retinal zones (posterior pole: MA, r = −0.285; P < .001; IRMA, r = −0.243; P < .001; midperiphery: MA, r = −0.250; P = .001; IRMA, r = −0.206; P = .005; and far periphery: MA, r = −0.298; P < .001; IRMA, r = −0.243; P = .001) and the DCP VD in the far periphery (MA, −0.197; P = .007; IRMA −0.161; P = .03) (Table 3). In contrast, VD measurements in eyes with PPL were not correlated with either MA or IRMA counts regardless of the vascular zone.
Table 3. Pearson Correlation Between Optical Coherence Tomography Angiography and Manual Ultra-widefield Color Image Microaneurysm and Intraretinal Microvascular Abnormality Counts.
| Parameter | Microaneurysm counts | Intraretinal microvascular abnormality counts | ||||
|---|---|---|---|---|---|---|
| Posterior pole (10 mm) | Midperiphery (10-15 mm) | Far periphery (>15 mm) | Posterior pole (10 mm) | Midperiphery (10-15 mm) | Far periphery (>15 mm) | |
| Without PPL (n = 208) | ||||||
| Parafovea VD SCP | −0.285 | −0.250 | −0.298 | −0.243 | −0.206 | −0.243 |
| P value | <.001 | .001 | <.001 | <.001 | .005 | .001 |
| Parafovea VD ICP | 0.049 | 0.014 | 0.068 | 0.031 | 0.014 | 0.035 |
| P value | .51 | .85 | .36 | .68 | .85 | .64 |
| Parafovea VD DCP | −0.145 | −0.125 | −0.197 | −0.122 | −0.041 | −0.161 |
| P value | .05 | .09 | .007 | .10 | .58 | .03 |
| With PPL (n = 130) | ||||||
| Parafovea VD SCP | 0.032 | −0.056 | −0.012 | −0.063 | −0.032 | −0.023 |
| P value | .72 | .52 | .89 | .48 | .72 | .80 |
| Parafovea VD ICP | 0.036 | 0.031 | <0.001 | −0.036 | −0.016 | −0.058 |
| P value | .68 | .73 | >.99 | .68 | .86 | .51 |
| Parafovea VD DCP | 0.011 | 0.054 | 0.032 | 0.009 | 0.053 | 0.007 |
| P value | .90 | .54 | .72 | .92 | .55 | .94 |
Abbreviations: DCP, deep capillary plexus; ICP, intermediate capillary plexus; PPL, predominantly peripheral lesions; SCP, superficial capillary plexus; VD, vessel density.
Discussion
Previous studies that did not evaluate PPL have demonstrated an association between decreasing OCTA VD and worsening DR severity. Although our study found a statistically significant association in eyes without PPL, no association was found between central VD metrics and DR severity in eyes with PPL. Results remained consistent in a sensitivity analysis matching eyes without PPL with the PPL group in terms of DR severity and diabetes type and correcting for age, SSI, SE, type of diabetes, diabetes duration, and correlation between eyes of the same patient. Furthermore, an association between DR lesions and macular VD metrics was present only in eyes without PPL, with a stronger association noted with the SCP than the DCP. These findings are consistent with the theory that worsening DR severity in an individual eye may be associated primarily with either central or peripheral nonperfusion (Figure 1 and Figure 2).
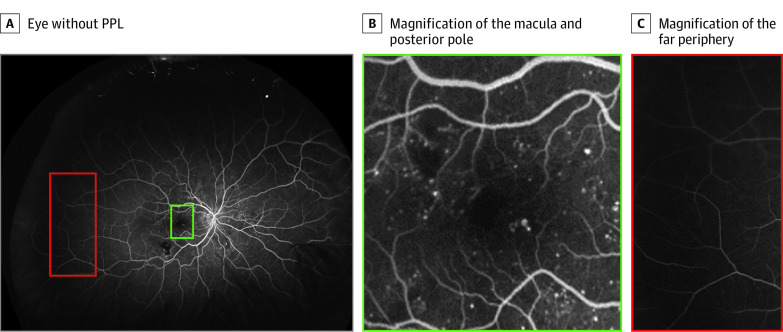
Figure 1. Distribution of Diabetic Retinopathy Lesions in an Example Eye Without Predominantly Peripheral Lesions (PPL).
A, Eye without PPL. B, Magnification of the macula and posterior pole. C, Magnification of the far periphery. Most of the lesions are localized to the macula and posterior pole (green) with minimal microaneurysms present in the far periphery (red).
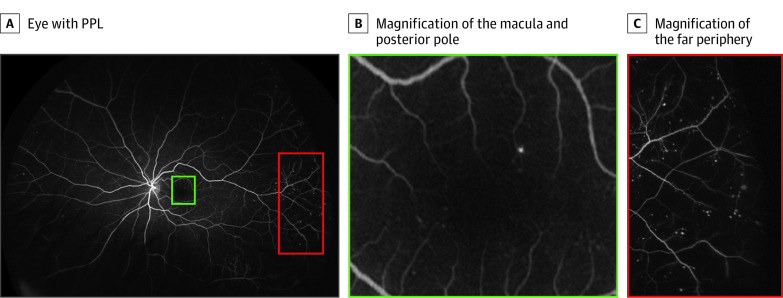
Figure 2. Distribution of Diabetic Retinopathy Lesions in an Eye With Predominantly Peripheral Lesions (PPL).
A, Eye with PPL. B, Magnification of the macula and posterior pole. C, Magnification of the far periphery. There are comparatively few microaneurysms in the macula and posterior pole (green) but a substantially greater number of diabetic lesions in the far periphery (red).
The finding that central nonperfusion may not be associated with DR severity in eyes with peripheral DR lesions has not been explored previously, to our knowledge. Prior studies evaluating changes in OCTA metrics generally have not graded the presence or absence of PPL.15,30,31 A study by Hafner et al32 evaluated peripheral lesions in eyes with early DR only and did not find significant differences in VD between eyes with and without peripheral lesions. That study used a different method of grading peripheral lesions relying on quadrants as opposed to distinct ETDRS fields and evaluated the presence or absence of peripheral lesions and not their predominant distribution.
We hypothesize that nonperfusion in the eye of an individual with diabetes likely exists across a spectrum that ranges from a predominance of peripheral nonperfusion to a predominance of posterior nonperfusion, and includes eyes with varying nonperfusion ratios in between. Earlier observations have reported instances in which the peripheral retina is affected, with little involvement in the central retina, which would be consistent with our findings.9,12,13 This disparity of OCTA parameter correlation with DR severity in eyes with PPL may affect interpretation of prior and future studies in this area. Because PPL are present in approximately 40% of all eyes with DR, this subpopulation may have had a substantial impact on the results of previous OCTA studies.8,9 Past studies have demonstrated differences in VD between eyes of increasing DR severity but have not found that OCTA metrics are sensitive or specific enough to assess DR severity in individual eyes.31 Stratification of eyes by PPL status might enable improved ability to detect differences in OCTA within eyes without PPL.
Our study is also the first, to our knowledge, to report associations between MA or IRMA counts and nonperfusion separately for eyes with or without PPL. An association between MA or IRMA counts with central OCTA metrics was evident only in eyes without PPL. This finding is consistent with the lack of significant differences in OCTA metrics across DR severity levels in eyes with PPL because DR severity grading is associated with lesion assessment. Previous work found a stronger association between MA counts and DCP VD compared with the current study, which found a stronger association with SCP VD.16 This finding may be explained by the use of UWF fluorescein angiography in the earlier study, which detects substantially more MAs compared with UWF color images and might be detecting MAs in the DCP that are masked in color images.20
Strengths and Limitations
This study has some strengths, including the detailed inclusion and exclusion criteria and use of only high-quality OCTA images (SSI>60; quality index >7). Another strength results from using PAR-OCTA software to reduce the effects of projection artifacts on the deeper vascular layers.21 The retina was segmented into 3 layers to negate the effect of the ICP on the overlying SCP and large retinal vessels were removed from the superficial vascular complex to ensure that only the microvascular changes were being analyzed.25
This study also has some limitations, including the relatively small number of eyes. Study eyes were limited in part owing to the exclusion of patients with diabetic macular edema and those with prior treatment with anti–vascular endothelial growth factor, corticosteroids, or panretinal photocoagulation because these conditions might confound the association between VD and DR severity. Thus, it is unclear if the current findings are applicable to eyes with diabetic macular edema and/or those receiving treatment. In addition, this study has a large percentage of eyes from individuals with type 1 vs type 2 diabetes. Thus, results may not fully represent associations in eyes of patients with type 2 diabetes. Future larger studies may evaluate eyes with each type of diabetes separately. Another potential limitation is associated with the offsets used to define the 3 vascular layers. Although validated techniques were used, no definitive consensus exists as to the correct location of these offsets by device. Use of different offsets may lead to different results depending on the extent of inclusion of the deeper layers in superficial vascular plexus measurements and vice versa.24,25 Finally, this study was a cross-sectional study from which we cannot assess changes in VD over time. A longitudinal study such as DRCR Retina Network Protocol AA, evaluating VD changes in eyes with or without PPL as they progress from mild to more advanced DR severity levels, might enable us to more fully understand how vascular loss develops over time in association with the presence of PPL.9 This would also allow a better understanding of how baseline OCTA metrics are associated with DR progression in eyes with or without PPL. Post hoc analysis of prior studies imaging the midperiphery and/or far-peripheral retina using either wide-field OCTA or UWF fluorescein angiography might also clarify longitudinal changes in the association between vascular density and DR severity in eyes with or without PPL.12,33
Conclusions
This study reports the finding that central retinal VD is not associated with DR severity in eyes with PPL, although it is evident in eyes without PPL. Because PPL are present in approximately 40% of all eyes with DR, these findings may have important implications for future studies evaluating OCTA metrics in people with DR. Optical coherence tomography angiography studies of eyes with DR should consider stratifying analyses based on the presence or absence of PPL. In addition, results from prior OCTA studies should be reevaluated with this concept in mind. If DR onset and worsening are associated with the location of retinal nonperfusion, global retinal nonperfusion assessment using widefield angiography may improve the ability of OCTA to assess DR severity and risk of DR worsening over time.
eMethods.
eReferences.
eFigure. Binarization Technique for the Superficial Vascular Complex (SVC)
eTable 1. Comparison of Optical Coherence Tomography Angiography (OCTA) Parameters Between Eyes With and Without Predominantly Peripheral Lesions (PPL) Stratified by Diabetic Retinopathy (DR) Severity
eTable 2. Parafoveal Vessel Density in Eyes With Predominantly Peripheral Lesions (PPL) After Cropping Central Circles of 1 mm and 0.5 mm
eTable 3. Sensitivity Analysis Evaluating a Subset of Eyes in the No Predominantly Peripheral Lesions (PPL) Group That Was Matched to the PPL Group With Regards to Diabetic Retinopathy Severity and Type of Diabetes Mellitus
References
- 1.Early Treatment Diabetic Retinopathy Study Research Group Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification: ETDRS report number 10. Ophthalmology. 1991;98(5)(suppl):786-806. doi: 10.1016/S0161-6420(13)38012-9 [DOI] [PubMed] [Google Scholar]
- 2.Early Treatment Diabetic Retinopathy Study Research Group Fundus photographic risk factors for progression of diabetic retinopathy: ETDRS report number 12. Ophthalmology. 1991;98(5)(suppl):823-833. doi: 10.1016/S0161-6420(13)38014-2 [DOI] [PubMed] [Google Scholar]
- 3.Bonnin S, Dupas B, Lavia C, et al. . Anti-vascular endothelial growth factor therapy can improve diabetic retinopathy score without change in retinal perfusion. Retina. 2019;39(3):426-434. doi: 10.1097/IAE.0000000000002422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.AbdelAl O, Ashraf M, Sampani K, Sun JK. “For mass eye and ear special issue” adaptive optics in the evaluation of diabetic retinopathy. Semin Ophthalmol. 2019;34(4):189-197. doi: 10.1080/08820538.2019.1620794 [DOI] [PubMed] [Google Scholar]
- 5.Jia Y, Tan O, Tokayer J, et al. . Split-spectrum amplitude–decorrelation angiography with optical coherence tomography. Opt Express. 2012;20(4):4710-4725. doi: 10.1364/OE.20.004710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun Z, Tang F, Wong R, et al. . OCT angiography metrics predict progression of diabetic retinopathy and development of diabetic macular edema: a prospective study. Ophthalmology. 2019;126(12):1675-1684. doi: 10.1016/j.ophtha.2019.06.016 [DOI] [PubMed] [Google Scholar]
- 7.Silva PS, Cavallerano JD, Sun JK, Soliman AZ, Aiello LM, Aiello LP. Peripheral lesions identified by mydriatic ultrawide field imaging: distribution and potential impact on diabetic retinopathy severity. Ophthalmology. 2013;120(12):2587-2595. doi: 10.1016/j.ophtha.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 8.Verma A, Alagorie AR, Ramasamy K, et al. ; Indian Retina Research Associates (IRRA) . Distribution of peripheral lesions identified by mydriatic ultra-wide field fundus imaging in diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2020;258(4):725-733. doi: 10.1007/s00417-020-04607-w [DOI] [PubMed] [Google Scholar]
- 9.Aiello LP, Odia I, Glassman AR, et al. ; Diabetic Retinopathy Clinical Research Network . Comparison of Early Treatment Diabetic Retinopathy Study standard 7-field imaging with ultrawide-field imaging for determining severity of diabetic retinopathy. JAMA Ophthalmol. 2019;137(1):65-73. doi: 10.1001/jamaophthalmol.2018.4982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva PS, Cavallerano JD, Haddad NM, et al. . Peripheral lesions identified on ultrawide field imaging predict increased risk of diabetic retinopathy progression over 4 years. Ophthalmology. 2015;122(5):949-956. doi: 10.1016/j.ophtha.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 11.Elmasry MA, Silva PS, Cavallerano J, et al. . Incorporating predominantly peripheral diabetic retinopathy (DR) lesion identification in a teleophthalmology program predicts DR progression over 4 years in eyes with early DR. Invest Ophthalmol Vis Sci. 2018;59(9):740. [Google Scholar]
- 12.Silva PS, Dela Cruz AJ, Ledesma MG, et al. . Diabetic retinopathy severity and peripheral lesions are associated with nonperfusion on ultrawide field angiography. Ophthalmology. 2015;122(12):2465-2472. doi: 10.1016/j.ophtha.2015.07.034 [DOI] [PubMed] [Google Scholar]
- 13.Niki T, Muraoka K, Shimizu K. Distribution of capillary nonperfusion in early-stage diabetic retinopathy. Ophthalmology. 1984;91(12):1431-1439. doi: 10.1016/S0161-6420(84)34126-4 [DOI] [PubMed] [Google Scholar]
- 14.Hwang TS, Hagag AM, Wang J, et al. . Automated quantification of nonperfusion areas in 3 vascular plexuses with optical coherence tomography angiography in eyes of patients with diabetes. JAMA Ophthalmol. 2018;136(8):929-936. doi: 10.1001/jamaophthalmol.2018.2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim AY, Chu Z, Shahidzadeh A, Wang RK, Puliafito CA, Kashani AH. Quantifying microvascular density and morphology in diabetic retinopathy using spectral-domain optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57(9):OCT362-OCT370. doi: 10.1167/iovs.15-18904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elmasry MA, Sampani K, Pitoc CM, et al. Differential association of macular superficial versus deep vascular density with microaneurysms and nonperfusion in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2019;60(9):5344. [Google Scholar]
- 17.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 18.Couturier A, Rey PA, Erginay A, et al. . Widefield OCT-angiography and fluorescein angiography assessments of nonperfusion in diabetic retinopathy and edema treated with anti-vascular endothelial growth factor. Ophthalmology. 2019;126(12):1685-1694. doi: 10.1016/j.ophtha.2019.06.022 [DOI] [PubMed] [Google Scholar]
- 19.Silva PS, Cavallerano JD, Sun JK, Noble J, Aiello LM, Aiello LP. Nonmydriatic ultrawide field retinal imaging compared with dilated standard 7-field 35-mm photography and retinal specialist examination for evaluation of diabetic retinopathy. Am J Ophthalmol. 2012;154(3):549-559. doi: 10.1016/j.ajo.2012.03.019 [DOI] [PubMed] [Google Scholar]
- 20.Ashraf M, Sampani K, AbdelAl O, et al. . Disparity of microaneurysm count between ultrawide field colour imaging and ultrawide field fluorescein angiography in eyes with diabetic retinopathy. Br J Ophthalmol. Published online February 28, 2020. doi: 10.1136/bjophthalmol-2019-315807 [DOI] [PubMed] [Google Scholar]
- 21.Ashraf M, Sampani K, Abu-Qamar O, et al. . Optical coherence tomography angiography projection artifact removal: impact on capillary density and interaction with diabetic retinopathy severity. Transl Vis Sci Technol. 2020;9(7):10. doi: 10.1167/tvst.9.7.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spaide RF, Fujimoto JG, Waheed NK. Image artifacts in optical coherence tomography angiography. Retina. 2015;35(11):2163-2180. doi: 10.1097/IAE.0000000000000765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell JP, Zhang M, Hwang TS, et al. . Detailed vascular anatomy of the human retina by projection-resolved optical coherence tomography angiography. Sci Rep. 2017;7:42201. doi: 10.1038/srep42201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garrity ST, Iafe NA, Phasukkijwatana N, Chen X, Sarraf D. Quantitative analysis of three distinct retinal capillary plexuses in healthy eyes using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2017;58(12):5548-5555. doi: 10.1167/iovs.17-22036 [DOI] [PubMed] [Google Scholar]
- 25.Ashraf M, Sampani K, Clermont A, et al. . Vascular density of deep, intermediate and superficial vascular plexuses are differentially affected by diabetic retinopathy severity. Invest Ophthalmol Vis Sci. 2020;61(10):53. doi: 10.1167/iovs.61.10.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park JJ, Soetikno BT, Fawzi AA. Characterization of the middle capillary plexus using optical coherence tomography angiography in healthy and diabetic eyes. Retina. 2016;36(11):2039-2050. doi: 10.1097/IAE.0000000000001077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu JJ, Camino A, Liu L, et al. . Signal strength reduction effects in OCT angiography. Ophthalmol Retina. 2019;3(10):835-842. doi: 10.1016/j.oret.2019.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leng Y, Tam EK, Falavarjani KG, Tsui I. Effect of age and myopia on retinal microvasculature. Ophthalmic Surg Lasers Imaging Retina. 2018;49(12):925-931. doi: 10.3928/23258160-20181203-03 [DOI] [PubMed] [Google Scholar]
- 29.Llanas S, Linderman RE, Chen FK, Carroll J. Assessing the use of incorrectly scaled optical coherence tomography angiography images in peer-reviewed studies: a systematic review. JAMA Ophthalmol. 2020;138(1):86-94. doi: 10.1001/jamaophthalmol.2019.4821 [DOI] [PubMed] [Google Scholar]
- 30.Agemy SA, Scripsema NK, Shah CM, et al. . Retinal vascular perfusion density mapping using optical coherence tomography angiography in normals and diabetic retinopathy patients. Retina. 2015;35(11):2353-2363. doi: 10.1097/IAE.0000000000000862 [DOI] [PubMed] [Google Scholar]
- 31.Ashraf M, Nesper PL, Jampol LM, Yu F, Fawzi AA. Statistical model of optical coherence tomography angiography parameters that correlate with severity of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2018;59(10):4292-4298. doi: 10.1167/iovs.18-24142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hafner J, Pollreisz A, Egner B, Pablik E, Schmidt-Erfurth U. Presence of peripheral lesions and correlation to macular perfusion, oxygenation and neurodegeneration in early type II diabetic retinal disease. Retina. 2020;40(10):1964-1971. doi: 10.1097/IAE.0000000000002704 [DOI] [PubMed] [Google Scholar]
- 33.Ehlers JP, Jiang AC, Boss JD, et al. . Quantitative ultra-widefield angiography and diabetic retinopathy severity: an assessment of panretinal leakage index, ischemic index and microaneurysm count. Ophthalmology. 2019;126(11):1527-1532. doi: 10.1016/j.ophtha.2019.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eReferences.
eFigure. Binarization Technique for the Superficial Vascular Complex (SVC)
eTable 1. Comparison of Optical Coherence Tomography Angiography (OCTA) Parameters Between Eyes With and Without Predominantly Peripheral Lesions (PPL) Stratified by Diabetic Retinopathy (DR) Severity
eTable 2. Parafoveal Vessel Density in Eyes With Predominantly Peripheral Lesions (PPL) After Cropping Central Circles of 1 mm and 0.5 mm
eTable 3. Sensitivity Analysis Evaluating a Subset of Eyes in the No Predominantly Peripheral Lesions (PPL) Group That Was Matched to the PPL Group With Regards to Diabetic Retinopathy Severity and Type of Diabetes Mellitus