Abstract
Tunicate larvae have a non-reproductive gonadotropin-releasing hormone (GnRH) system with multiple ligands and receptor heterodimerization enabling complex regulation. In Ciona intestinalis type A larvae, one of the gnrh genes, gnrh2, is conspicuously expressed in the motor ganglion and nerve cord, which are homologous structures to the hindbrain and spinal cord, respectively, of vertebrates. The gnrh2 gene is also expressed in the proto-placodal sensory neurons, which are the proposed homologue of vertebrate olfactory neurons. Tunicate larvae occupy a non-reproductive dispersal stage, yet the role of their GnRH system remains elusive. In this study, we investigated neuronal types of gnrh2-expressing cells in Ciona larvae and visualized the activity of these cells by fluorescence imaging using a calcium sensor protein. Some cholinergic neurons and dopaminergic cells express gnrh2, suggesting that GnRH plays a role in controlling swimming behavior. However, none of the gnrh2-expressing cells overlap with glycinergic or GABAergic neurons. A role in motor control is also suggested by a relationship between the activity of gnrh2-expressing cells and tail movements. Interestingly, gnrh2-positive ependymal cells in the nerve cord, known as a kind of glia cells, actively produced Ca2+ transients, suggesting that active intercellular signaling occurs in the glia cells of the nerve cord.
Subject terms: Evolutionary developmental biology, Peptide hormones, Glial biology, Motor control, Neural circuits
Introduction
Gonadotropin-releasing hormone (GnRH) is a key regulator of reproductive functions in vertebrates1,2. GnRH has also been suggested to play non-reproductive roles in the nervous system and during development3–9. Compared to its reproductive roles, however, the non-reproductive roles of GnRH are less well understood.
Tunicates are the sister group of vertebrates10,11. A conspicuous non-reproductive GnRH system has been reported in the larva of the sessile tunicate Ciona intestinalis type A (also called Ciona robusta)12,13. Six GnRH peptides and four receptors are encoded by the Ciona genome14–18. In the Ciona larva, the GnRH genes are strikingly expressed in the central nervous system (CNS) through the entire antero-posterior body axis12. Correspondingly, the GnRH receptor genes are specifically expressed in the tissues and organs located along the CNS, namely the notochord, the tail muscle, and the epidermal sensory neurons12. One of the Ciona gnrh genes, gnrh2, is conspicuously expressed in the motor ganglion and nerve cord of the larva, which are homologous structures to the hindbrain and spinal cord, respectively, of vertebrates. The gnrh2 gene is also expressed in the proto-placodal sensory neurons, which are the proposed homologue of vertebrate olfactory neurons19. Ciona GnRH has been suggested to play a pivotal role in the control of metamorphosis13. Considering the complex and well-developed nature of the larval GnRH system in Ciona, GnRH may play diverse and important roles in developmental and physiological processes in Ciona larvae. To date, however, the roles of the Ciona GnRH system remain elusive.
In this study, we investigated neuronal types of gnrh2-expressing cells in the Ciona larva and visualized the activity of these cells by fluorescence imaging using a calcium sensor protein. Some cholinergic motor neurons as well as unique cholinergic cells along the nerve cord were found to express gnrh2, suggesting that GnRH plays a role in the control of swimming behavior. By contrast, none of the gnrh2-expressing cells overlapped with glycinergic or GABAergic neurons. A role in motor control was also suggested by a relationship between the activity of some gnrh2-expressing cells and tail movements. Interestingly, gnrh2-positive ependymal cells in the nerve cord, known as a kind of glia cells, produced Ca2+ transients, suggesting that active intercellular signaling occurs in in the glia cells of the nerve cord.
Results
Gnrh2 is expressed in proto-placode-derived sensory neurons and caudal glial ependymal cells
The 4.3-kb upstream region of gnrh2 connected with a fluorescence reporter can recapitulate the expression patterns of gnrh2 12 (Fig. 1; Supplementary Fig. S1). This upstream region was used to transiently express mCherry and G-CaMP8 in cells expressing gnrh2 (Figs. 1, 2 and 3). Cell types were identified by double fluorescent staining of larvae with cell type-specific markers. The expression of electroporated transgenes displayed some mosaicism in Ciona larvae. Expression patterns of the reporter gene in each animal usually represent parts of the regions where the promoter can be activated, but the pattern obtained in dozens of larvae in at least three independent experiments per transgene was consistent. The mosaicism allowed us to visualize different populations of gnrh2-expressing cells. The number of larvae examined for each analysis are described in the figure legends, and additional data are presented in the Supplementary Information.
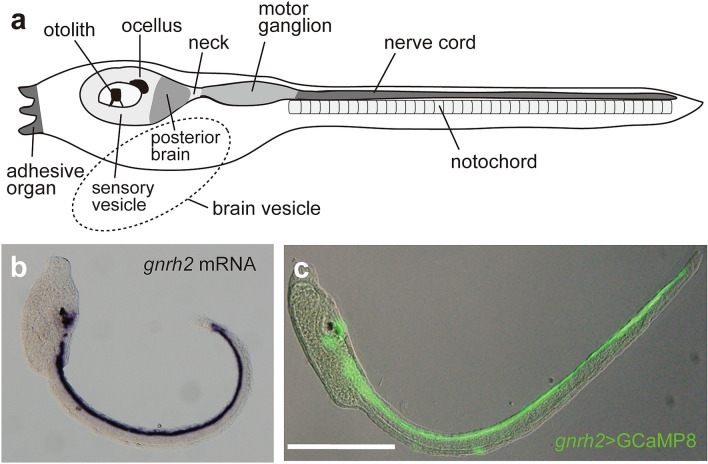
Figure 1.
Expression patterns of gnrh2 in the Ciona larva. (a) Schematic diagram showing the CNS of the larva. (b) Localization of gnrh2 mRNA visualized by in situ hybridization. (c) Immunofluorescent localization of G-CaMP8 expressed under the control of the cis-regulatory region of gnrh2 (gnrh2 > g-camp8) in a larva at 21 h post-fertilization (hpf). The expression patterns of g-camp8 were consistent with the endogenous gnrh2 expression in all larvae examined (n = 45). The results for eight other larvae are shown in Supplementary Figure S1. Scale bar, 200 µm.
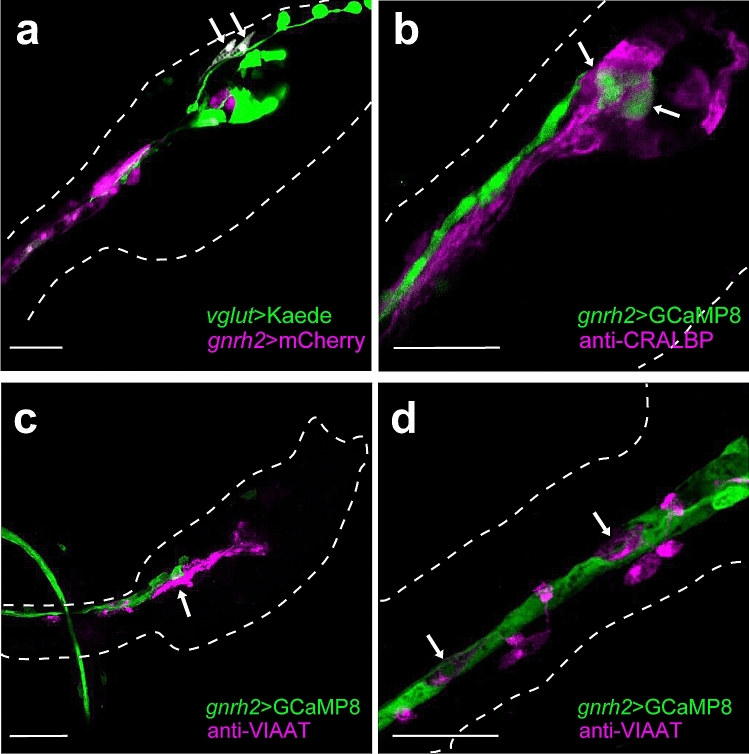
Figure 2.
Immunohistochemical identification of types of cells expressing gnrh2 in the Ciona larva at 21 hpf. (a) Glutamatergic neurons and gnrh2-expressing cells were labeled with Kaede (green) and mCherry (magenta), respectively. The proto-placode-derived sensory neurons (aATENs; arrows) were shown to express gnrh2 (6 of 6 larvae showed the expression; four additional examples are shown in Supplementary Figure S2). (b) CRALBP-positive cells (magenta) were not overlapped with gnrh2-expressing cells (green) (none of 16 larvae showed overlapped expression; four additional examples are shown in Supplementary Figure S3). Arrows indicate gnrh2-expressing cells in the brain vesicle. (c, d) GABAergic/glycinergic neurons were visualized by immunostaining with anti-VIAAT antibody (magenta). VIAAT-positive cells (magenta) were not overlapped with gnrh2-expressing cells (green) (none of 17 larvae showed overlapped expression; four additional examples are shown in Supplementary Figure S3). Arrows in (c) indicate GABAergic/glycinergic neurons in the motor ganglion. Arrows in (d) indicate VIAAT-positive ACINs. (a) Projection of 7 serial optical sections taken at 0.60 µm intervals. (b) Projection of 8 serial optical sections taken at 0.60 µm intervals. (c) Projection of 7 serial optical sections taken at 0.60 µm intervals. (d) Projection of 3 serial optical sections taken at 0.60 µm intervals. Scale bars, 30 µm.
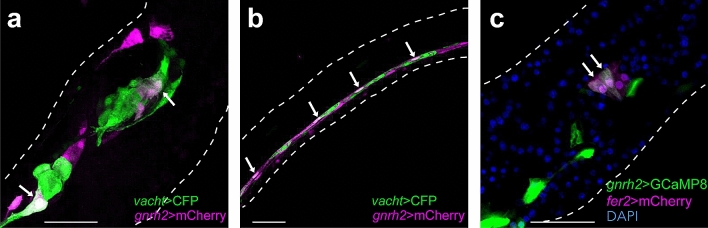
Figure 3.
Some cholinergic and dopaminergic neurons express gnrh2. (a, b) Cholinergic neurons and gnrh2-exressing cells were labeled with CFP (green) and mCherry (magenta), respectively. Arrows indicate cells that co-expressed both markers. In the motor ganglion, overlapped expression of two reporters was observed in 10 of 13 larvae (two additional examples are shown in Supplementary Figure S4). In the nerve cord, overlapped expression of two reporters was observed in 4 of 5 larvae (two additional examples are shown in Supplementary Figure S4). (c) Dopaminergic cells and gnrh2-expressing cells were labeled with mCherry (magenta) and G-CaMP8 (green), respectively. Some dopaminergic cells were also labeled with G-CaMP8 (arrows). Overlapped expression of mCherry and G-CaMP8 was observed in 10 of 16 larvae. Two additional examples are shown in Supplementary Figure S5. (a) Projection of 10 serial optical sections taken at 0.60 µm intervals. (b) Projection of 7 serial optical sections taken at 0.60 µm intervals. (c) Confocal image of a single optical section. Scale bars, 30 µm.
First, we examined whether gnrh2-expressing cells include glutamatergic neurons. In the Ciona larva, glutamate is a major neurotransmitter in the peripheral sensory neurons and photoreceptor cells20. Some interneurons in the posterior brain are also glutamatergic20. As previously reported19, proto-placode-derived gnrh2-expressing epidermal neurons (aATENs) are glutamatergic (Fig. 2a; Supplementary Fig. S2). In the CNS, glutamatergic neurons located at the ventral region of the posterior brain vesicle seem to express gnrh2 (Supplementary Fig. S2).
Next, we examined whether any of the GABAergic/glycinergic neurons express gnrh2 using vesicular inhibitory amino acid transporter (VIAAT) as a marker. None of the VIAAT-positive cells overlapped with the reporter expression under the control of the gnrh2 cis-regulatory region (Fig. 2c and d; Supplementary Fig. S3). In the anterior tail region, there are two pairs of VIAAT-positive neurons called anterior caudal inhibitory neurons (ACINs), which align with glial ependymal cells in the lateral wall of the anterior nerve cord21,22. Our result suggests that the ACINs do not express gnrh2, whereas the lateral ependymal cells express gnrh2 (Fig. 2d). The gnrh2 expression in the lateral wall ependymal cells of the nerve cord is consistent with the in situ hybridization data previously reported12.
Cellular retinaldehyde-binding protein (CRALBP) is specifically localized in the glial ependymal cells in the brain vesicle and the motor ganglion23,24. In our immunohistochemical analysis, CRALBP-positive cells were never overlapped with gnrh2-epxressing cells (Fig. 2b; Supplementary Fig. S3). Thus, in contrast to the conspicuous gnrh2 expression in the ependymal cells of the nerve cord, gnrh2 does not seem to be expressed in the ependymal cells in the brain vesicle and the motor ganglion.
Some cholinergic and dopaminergic neurons express gnrh2
Acetylcholine is a major neurotransmitter at the neuromuscular junctions of the Ciona larva21,25. Cholinergic neurons were visualized by a fluorescence protein expressed under the control of the cis-regulatory region of the vacht gene25. Gnrh2-expressing neurons were shown to be cholinergic both in the brain vesicle and the motor ganglion (Fig. 3a; Supplementary Fig. S4).
The caudal part of the CNS (nerve cord) mainly consists of non-neuronal ependymal cells26. The nerve cord also contains two types of neurons: ACINs and bilateral pairs of cholinergic caudal neurons21. Some of these cholinergic caudal neurons seem to express gnrh2 (Fig. 3b; Supplementary Fig. S4).
Another neurotransmitter that controls the swimming of Ciona tadpoles is dopamine27. Dopaminergic neurons are present in the brain vesicle27–29. Dopaminergic neurons were labeled with mCherry expressed under the control of the cis-regulatory region of the dopaminergic cell-specific gene fer227,29 (previously described as Ptf1a; see Gyoja & Satoh30 for the orthologous families of the bHLH transcription factors). Double fluorescence imaging of dopaminergic neurons and gnrh2-expressing cells suggested that some dopaminergic neurons express gnrh2 (Fig. 3c; Supplementary Fig. S5).
Active Ca2+ transients in aATENs and gnrh2-expressing cells of the posterior CNS
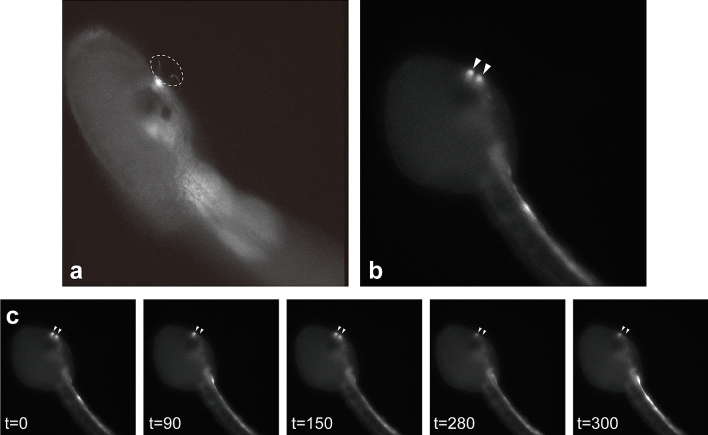
G-CaMP831 was used to monitor temporal changes in intracellular Ca2+ in gnrh2-expressing cells. We analyzed 51 larvae derived from 16 independent transfections (each transfection gave 1–5 larvae to the Ca2+ imaging analysis). Among the 51 larvae examined, 24, 40, and 41 larvae showed Ca2+ transients in aATENs, the motor ganglion, and the nerve cord, respectively. Active Ca2+ transients were observed in aATENs, the motor ganglion, and the caudal nerve cord (Figs. 4, 5, 6; Movies S1–S3). The larva contains two aATENs, and each has a sensory cilium19 (Fig. 4a,b). Both aATENs showed Ca2+ transients (Fig. 4c; Movie S1; Supplementary Fig. S6). In the larva shown in Fig. 4c, the activities of the two aATENs showed a moderate positive correlation (Supplementary Fig. S6).
Figure 4.
Calcium imaging of gnrh2-expressing chemosensory neurons. Fluorescence images of Ciona larvae expressing G-CaMP8 in the chemosensory aATENs. (a) The putative sensory cilum of each aATEN was labeled with G-CaMP8 fluorescence (dotted circle). (b, c) An example of a larva showing dynamic Ca2+ transients in a pair of aATENs (arrowheads) at 19 hpf. (c) Representative images of the larva recorded at the times indicated (in seconds). Serial images of the larva shown in (b, c) are shown in Movie S1. Quantitative analysis of the imaging data is shown in Supplementary Figure S6.
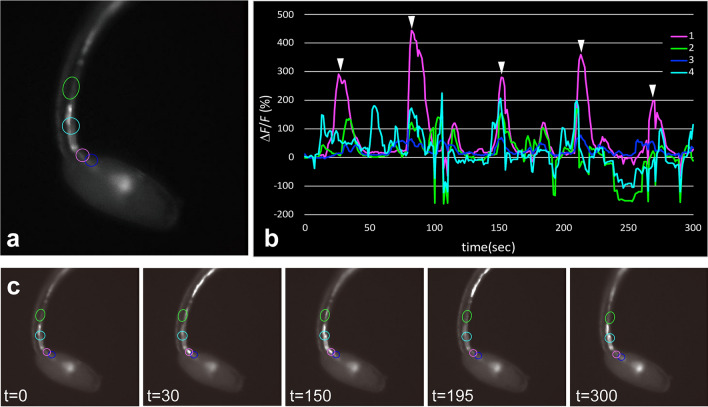
Some gnrh2-expressing cells at the posterior part of the motor ganglion exhibited active Ca2+ transients (Figs. 5 and 6). Periodic Ca2+ transients of a gnrh2-expressing neuron in the motor ganglion were observed at 20 h post-fertilization (hpf) (arrowheads in Fig. 6b). Similar periodic Ca2+ transients in the motor ganglion were observed in at least three additional larvae at 19–20 hpf. In the tail region, Ca2+ transients were observed through the entire length of the nerve cord (Figs. 5 and 6). Both cholinergic neurons and ependymal cells showed Ca2+ transients in the tail nerve cord. For example, the narrow cell indicated by an arrow in Fig. 5a is presumably a cholinergic neuron. Many cells showing Ca2+ transients were block-shaped, which is characteristic of caudal ependymal cells (Fig. 6c).
Figure 5.
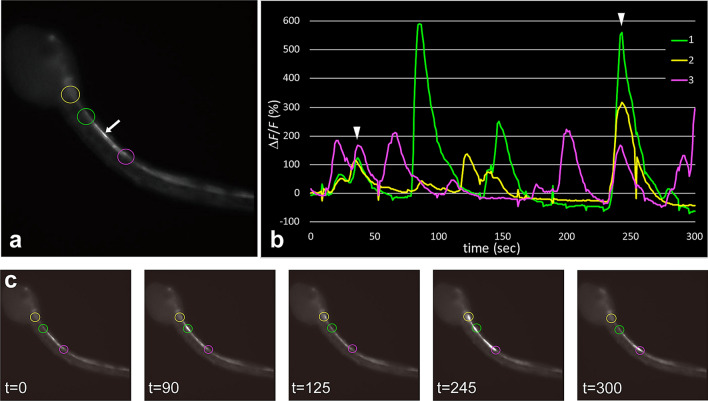
Interconnection between gnrh2-expressing cells in the larval CNS. (a) Fluorescence image of a larva at 19 hpf, showing G-CaMP8 fluorescence in the motor ganglion and the anterior nerve cord. (b) The graph shows the temporal patterns of fluorescence intensity at the three sites indicated by circles in (a). The colors of the lines correspond to the sites indicated by circles in the respective colors. The Ca2+ transients occurred independently of each other, but sometimes occurred at the same time (arrowheads). (c) Representative images of the larva recorded at the times indicated (in seconds). Serial images of the larva shown in (A) are shown in Movie S2.
Figure 6.
Periodic oscillation of Ca2+ transients in a gnrh2-expressing cell in the motor ganglion. (a) Fluorescence image of a larva at 20 hpf, showing G-CaMP8 fluorescence in the motor ganglion and the anterior nerve cord. (b) The graph shows the temporal patterns of fluorescence intensity at the four sites indicated by circles in (a). The colors of the lines correspond to the sites indicated by circles in the respective colors. Ca2+ spikes were periodically observed at regular intervals in the cell indicated by the magenta circle in (a) (arrowheads). (c) Representative images of the larva recorded at the times indicated (in seconds). Serial images of the larva shown in (a) are shown in Movie S3.
Ca2+ transients were observed at various times in cells located at different sites of the larva (Figs. 5 and 6). However, simultaneous activation of cells at different sites was occasionally observed (similar patterns were observed in at least 7 larvae), as indicated by arrowheads in Figs. 5a and 6b, suggesting the presence of a neural circuit connecting gnrh2-expressing cells at different sites.
Relationship between tail movements and Ca2+ transients in the motor ganglion and the anterior nerve cord
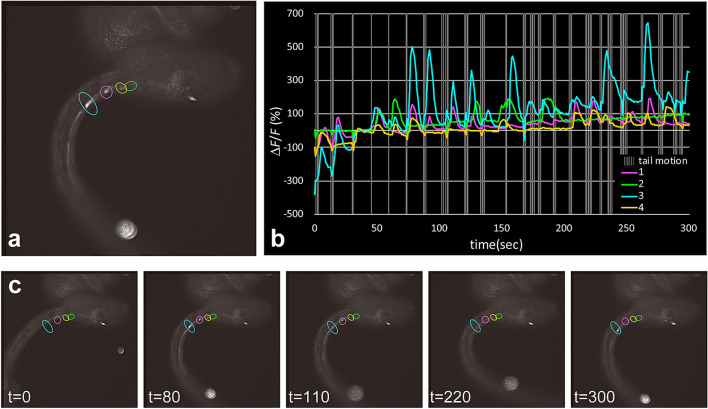
A neural circuit in the motor ganglion and the anterior nerve cord is thought to control muscle contraction in the tail21,32,33. Because active Ca2+ transients in gnrh2-expressing cells were observed in these regions, we examined the temporal relationship between tail movement and the activity of gnrh2-expressing cells. Due to extreme difficulties in obtaining Ca2+ imaging data from larvae with a quickly moving tail, we were only able to take serial fluorescence images from the single individual shown in Fig. 7. Ca2+ transients were frequently observed when the tail ceased its movement. Thus the tail movement often precedes the Ca2+ spike. This pattern can be interpreted as demonstrating that the cessation of tail movement leads to Ca2+ transients. This view is consistent with our observation that intracellular Ca2+ returned to a basal level before the tail began to move again (Supplementary Fig. S7). These findings suggest the possible involvement of gnrh2-expressing cells in the control of swimming behavior.
Figure 7.
Relationship between Ca2+ transients in gnrh2-expressing cells and tail movement. (a) Fluorescence image of a larva at 19 hpf, showing G-CaMP8 fluorescence in the anterior nerve cord. (b) The graph shows the temporal patterns of fluorescence intensity at the four sites encircled by colored lines. The colors of the lines in the graph correspond to the sites encircled by lines of the respective colors. Gray vertical lines indicate the period when the tail was moving. Ca2+ transients generally occurred when the tail movement stopped. (c) Representative images of the larva recorded at the times indicated (in seconds). Serial images of the larva shown in (a) are shown in Movie S4. A statistical evaluation of the relationship between Ca2+ transients and tail movement is shown in Supplementary Figure S7.
Discussion
In this study, we identified cell types of gnrh2-expressing cells and visualized their activity in the Ciona larva. Previously, the cells expressing GnRH-encoding genes had been only partially identified in Ciona. The caudal ependymal cells and the aATENs were reported to express gnrh212,19. We confirmed these findings and further identified CNS neurons expressing gnrh2.
In the brain vesicle, dopaminergic neurons, glutamatergic neurons located at the posterior ventral region, and a limited number of cholinergic neurons seem to express gnrh2. Pharmacological and behavioral analyses have suggested that dopaminergic cells modulate the light-off-induced swimming behavior of Ciona larvae27. The role of cholinergic neurons in the Ciona brain vesicle has not been elucidated. Our present observations are the first to show the heterogeneity of cholinergic neurons in the brain vesicle, and should provide clues for future investigations into the roles of these neurons.
Cholinergic neurons in the motor ganglion have been implicated in the regulation of tail muscle contraction21,32–34. Here we show that one subtype of cholinergic neurons in the motor ganglion expresses gnrh2 (Fig. 3a). These neurons extend axons posteriorly, but it is unclear whether they are motor neurons that directly innervate muscle cells or interneurons that connect to other CNS neurons in the caudal nerve cord. In the nerve cord, another class of cholinergic cells also expresses gnrh2 (Fig. 3b). One possible role of cholinergic/GnRH neurons in the motor ganglion and the nerve cord may be the control of swimming behavior. These neurons may also play a role in metamorphosis, because GnRH has been suggested to be involved in the regulation of metamorphosis13.
Calcium imaging has been applied to studies of Ciona development35–37. These previous studies focused on Ca2+ transients in embryos but not in larvae. The present study is thus the first to report the spatio-temporal patterns of Ca2+ transients in larvae of Ciona. Our observations included four novel findings: (i) active Ca2+ transients in the proto-placode-derived aATENs, (ii) periodic spikes in the motor ganglion, (iii) a relationship between Ca2+ transients and tail movements, and (iv) active Ca2+ transients in ependymal cells of the nerve cord.
The proto-placode-derived aATENs share morphological and molecular properties with vertebrate olfactory neurons and are thought to be chemosensory cells19. However, olfactory receptors have not been identified in Ciona, and the chemical cues that stimulate aATENs are not known. Calcium imaging with gnrh2 > G-CaMP8 could help us search for chemical cues that trigger the activation of aATENs in future studies.
Periodic Ca2+ transients observed in the motor ganglion are reminiscent of the spontaneous rhythmic activities observed in the developing nervous systems of vertebrates38–43. These periodic neuronal activities are thought to be important for the development of neural circuits in the CNS and the retina41,44,45. Similar rhythmic oscillation of Ca2+ transients was reported in the developing motor ganglion of the Ciona embryo37. By contrast, we observed rhythmic Ca2+ transients in larvae at 19–20 hpf. The swimming behavior of Ciona larvae reveals ontogenic changes; the larvae hatch at 18 hpf (18 °C) and their photo-responsiveness appears within 4 h after hatching46–48. Thus, the spontaneous rhythmic Ca2+ transients may play an important role in the neural circuit development of Ciona larvae.
We observed an association between the tail movements and Ca2+ transients in the motor ganglion and the nerve cord. This suggests that gnrh2-expressing cells are involved in the control of swimming locomotion. Ca2+ transients appeared when the tail stopped moving, and the Ca2+ signal was low when the tail was moving (Fig. 7b; Supplementary Fig. S7). In other words, the tail movement precedes the Ca2+ spike. This pattern suggests that these gnrh2-expressing cells are not motor neurons. In fact, the majority of the cells expressing gnrh2 in the nerve cord are ependymal cells, and we observed Ca2+ transients in ependymal cells. An intriguing possibility is that Ca2+ spikes are induced in ependymal cells by muscle contraction or motor axon excitation. If so, the ependymal cells may monitor the activity of muscle or motor neurons. It has been reported that various types of glia cells exhibit Ca2+ transients in response to neuronal activities and regulate neuronal functions in vertebrates49–52. The ependymal cells of Ciona larva may have similar regulatory roles, suggesting a deep evolutionary conservation of glia function between tunicates and vertebrates. Given the simplicity of its nervous system, the Ciona larva could serve as a unique model for the study of glia-neuron interaction.
In the present study, however, the observed relationship between the tail motion and the Ca2+ transients was largely associative, and the causal relationship and underlying molecular and cellular mechanisms remain unclear. These topics should be addressed by future optogenetic approaches, such as by controlling the activity of specific neurons, glia, and muscle cells by using light-activated ion channels53.
In conclusion, the present study revealed the presence of dynamic Ca2+ transients of gnrh2-expressing cells at various sites in the Ciona larva. Our findings suggest a connection between the activity of gnrh2-expressing cells and the tail movements of the larva. An important yet unsolved question is whether GnRH2 is involved in these processes. Future studies should address the developmental and physiological roles of gnrh2-expressing cells and GnRH peptides based on the findings of this study.
Methods
Ethical issues and approval
All animal treatments in this research were carried out in accordance with the Japanese Act on Welfare and Management of Animals (Act No. 105 of October 1, 1973; the latest revision is Act No. 51 of June 2, 2017, effective June 1, 2018). All experimental protocols were approved by the Institutional Animal Care and Use Committees of Konan University, Saitama University, Tohoku University, and the University of Tsukuba.
Biological materials
Mature adults of Ciona intestinalis type A were provided by the Maizuru Fisheries Research Station of Kyoto University and by the Misaki Marine Biological Station of the University of Tokyo through the National Bio-Resource Project of the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT), and were maintained in indoor tanks of artificial seawater (ASW) (Marine Art BR; Tomita Pharmaceutical, Tokushima, Japan) at 18 °C. The adults were also collected from the pond on the Fukae campus of Kobe University, Kobe, Japan and from the fishing harbor in Murotsu, Hyogo, Japan. Eggs and sperm were obtained surgically from the gonoducts, and the eggs were fertilized in vitro. After insemination, the embryos were raised in ASW containing 50 µg/ml streptomycin sulfate (S6501; Sigma-Aldrich, St. Louis, MO, USA) at 18 °C.
Preparation of reporter constructs and electroporation
Construction of the vglut > kaede was described previously27,54. The vacht > cfp plasmid was made by inserting the 3.8-kb upstream region of Ciona vacht25 into the SalI/BamHI site of pSP-CFP54. The 2.4-kb upstream region of Ciona fer2 (Gene ID KH.L116.39) was previously cloned into the pSP-CFP vector27. The reporter sequence was replaced with a DNA fragment coding for mCherry to generate fer2 > mcherry using NotI/EcoRI sites. The gnrh2 > kaede and gnrh2 > mcherry plasmids were made by inserting the 4.3-kb upstream region of Ciona gnrh212 into the XhoI/NotI sites of the pSP-Kaede vector and pSP-mCherry vector, respectively55. The gnrh2 upstream region was also used to generate the gnrh2 > g-camp8 construct. The Kaede coding sequence of pSP-Kaede was replaced with a DNA fragment coding for G-CaMP831 using NotI/EcoRI sites. The gnrh2 upstream region was amplified from the gnrh2 > kaede plasmid using a pair of nucleotide primers (5′-GAATCGGCCAACGCGGGATCCAGGAGCAGACGTCATAAGTA-3′ and 5′-TGACGCGGCCGCTGTTACGTTATCTCTCTAGAAG-3′), digested with BamHI and NotI, and then inserted into the BamHI/NotI sites upstream of the G-CaMP8 in the pSP vector. Plasmid DNA constructs were electroporated into fertilized Ciona eggs as described by Corbo et al.56.
Immunofluorescent staining
Immunofluorescent staining was carried out according to the method described by Nishitsuji et al.22. Photobleaching of fluorescent reporters, including CFP, mCherry, Kaede, and G-CaMP8, was not performed prior to immunofluorescent detection of these proteins. To avoid photoconversion of Kaede fluorescence from green to red, embryos and larvae transfected with the kaede transgene were kept away from short wavelength illumination during development, experiments, and observation, and no photoconversion was observed. All fluorescent images except those shown in Fig. 1 and Supplementary Fig. S1 were obtained by using a laser scanning confocal microscope (FV1200 IX83; Olympus, Tokyo) with a 40 × objective lens (numerical aperture (NA) 0.95; Olympus). The excitation/emission wavelengths for DAPI, Alexa Fluor 488, and Alexa Fluor 594 were 405 nm/461 nm, 473 nm/520 nm, and 559 nm/618 nm, respectively.
The fluorescent images shown in Fig. 1 and Supplementary Fig. S1 were obtained by using a fluorescent microscope (BX50; Olympus) with a 10 × objective lens (NA 0.40; Olympus) and a color fluorescence camera (DP74, Olympus). For these observations, the excitation and emission wavelengths were 470–490 nm and 515–550 nm, respectively.
To visualize the localization of cell type-specific proteins, a mouse antiserum against Ciona VIAAT21 or a rabbit antiserum against Ciona CRALBP23 was diluted 1:1000 in 10% goat serum in T-PBS (0.1% Triton X-100 in PBS) and used as the primary antibody. The secondary antibody was an Alexa Fluor 594-conjugated anti-mouse IgG (A11005; Thermo Fisher Scientific) or an Alexa Fluor 594-conjugated anti-rabbit IgG (A11012; Thermo Fisher Scientific).
The primary antibodies used to visualize the localization of fluorescent reporter proteins were rabbit anti-Kaede polyclonal (PM012; Medical & Biological Laboratories, Nagoya, Japan; for Kaede), rabbit anti-green fluorescent protein (GFP) polyclonal (A11122; Thermo Fisher Scientific; for G-CaMP8 and CFP), rat anti-red fluorescent protein (RFP) monoclonal (5F8; ChromoTek GmbH, Martinsried, Germany; for mCherry), and rat anti-GFP monoclonal (GF090R; Nacalai Tesque, Kyoto, Japan; for G-CaMP8 double-stained with anti-CRALBP) antibodies. All the primary antibodies were diluted 1000-fold as described above. The secondary antibodies were an Alexa Fluor 488-conjugated anti-rabbit IgG (A11008; Thermo Fisher Scientific) for G-CaMP8 and CFP, an Alexa Fluor 488-conjugated anti-rat IgG (A11006; Thermo Fisher Scientific) for G-CaMP8, and an Alexa Fluor 594-conjugated anti-rat IgG (A11007; Thermo Fisher Scientific) for mCherry.
In vivo Ca2+ imaging
Electroporated Ciona larvae expressing the G-CaMP8 transgene were placed in ASW on a 35-mm glass-based dish (coverslip diameter 12 mm, #3931-035; Iwaki, Japan). For imaging, a microscope (IX81; Olympus, Tokyo) equipped with an electron multiplying charge-coupled device (EMCCD) camera (EVOLVE512; Photometrics, Tucson, AZ) and a 20 × objective lens (NA 0.80; Olympus) was used. Fluorescence excitation was done using a Spectra 4 LED light source (Lumencor, Beaverton, OR, USA) at 475 nm center wavelength. Images were taken through a band-pass emission filter (510–550 nm) with a 50-ms exposure time per 1 s and 1 × 1 binning. For each larva, 300 images were taken in 5 min. Changes in intracellular calcium concentrations were measured as the changes in the green fluorescence of G-CaMP8. The fluorescence intensity was spatially averaged in each region of interest (ROI). The fluorescence change was defined as ΔF/F = (Ft – F0)/F0, where Ft is the fluorescence intensity at time t, and F0 is the baseline averaged for 4–5 s. The fluorescence change (ΔF/F) was calculated after subtracting the background fluorescence. A MetaMorph image analysis software system (Molecular Devices) was used to analyze the images. Image processing was also performed with ImageJ (U. S. National Institutes of Health, Bethesda, MD, USA; https://imagej.nih.gov/ij/).
Supplementary information
Acknowledgments
We thank the National BioResource Project of MEXT and all members of the Maizuru Fisheries Research Station and Yutaka Satou Lab of Kyoto University and the Misaki Marine Biological Station of the University of Tokyo for providing us with C. intestinalis adults. We also thank the Graduate School of Maritime Sciences, Kobe University for generously allowing us to collect C. intestinalis on the campus. This study was supported in part by Grants-in-Aid for Scientific Research from JSPS to T.G.K. (25650118, 25290067, 16H04724, 19H03213), A.K. (18H02484, 20H05074), J.N. (15H05723), and T.H. (16K07433, 19H03204). This study was also supported in part by research grants from the Takeda Science Foundation and the Hirao Taro Foundation of the Konan University Association for Academic Research.
Author contributions
T.G.K. and N.O. conceived the project and designed the experiments. N.O. and K.S. performed the experiments. A.K. and K.O. designed and built the Ca2+ imaging system. T.H., M.O., and J.N. provided essential materials. N.O. and T.G.K. analyzed and interpreted the data. K.S., T.H., J.N., and A.K. edited the manuscript. N.O. and T.G.K. wrote the manuscript. All authors reviewed the manuscript.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials and are available upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-75344-7.
References
- 1.Okubo K, Nagahama Y. Structural and functional evolution of gonadotropin-releasing hormone in vertebrates. Acta Physiol. 2008;193:3–15. doi: 10.1111/j.1748-1716.2008.01832.x. [DOI] [PubMed] [Google Scholar]
- 2.Oka Y. Three types of gonadotrophin-releasing hormone neurones and steroid-sensitive sexually dimorphic kisspeptin neurones in teleosts. J. Neuroendocrinol. 2009;21:334–338. doi: 10.1111/j.1365-2826.2009.01850.x. [DOI] [PubMed] [Google Scholar]
- 3.Dolan S, Evans NP, Richter TA, Nolan AM. Expression of gonadotropin-releasing hormone and gonadotropin-releasing hormone receptor in sheep spinal cord. Neurosci. Lett. 2003;346:120–122. doi: 10.1016/s0304-3940(03)00594-9. [DOI] [PubMed] [Google Scholar]
- 4.Albertson AJ, Talbott H, Wang Q, Jensen D, Skinner DC. The gonadotropin-releasing hormone type I receptor is expressed in the mouse cerebellum. Cerebellum. 2008;7:379–384. doi: 10.1007/s12311-008-0038-8. [DOI] [PubMed] [Google Scholar]
- 5.Sherwood NM, Wu S. Developmental role of GnRH and PACAP in a zebrafish model. Gen. Comp. Endocr. 2005;42:74–80. doi: 10.1016/j.ygcen.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Wu S, Page L, Sherwood NM. A role for GnRH in early brain regionalization and eye development in zebrafish. Mol. Cell. Endocrinol. 2006;257–258:47–64. doi: 10.1016/j.mce.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Abraham E, et al. Early development of forebrain gonadotrophin-releasing hormone (GnRH) neurons and the role of GnRH as an autocrine migration factor. J. Neuroendocrinol. 2008;20:394–405. doi: 10.1111/j.1365-2826.2008.01654.x. [DOI] [PubMed] [Google Scholar]
- 8.Kanaho YI, et al. Neurotrophic effect of gonadotropin-releasing hormone on neurite extension and neuronal migration of embryonic gonadotropin-releasing hormone neurons in chick olfactory nerve bundle culture. J. Neurosci. Res. 2009;87:2237–2244. doi: 10.1002/jnr.22051. [DOI] [PubMed] [Google Scholar]
- 9.Ramakrishnan S, Lee W, Navarre S, Kozlowski DJ, Wayne NL. Acquisition of spontaneous electrical activity during embryonic development of gonadotropin-releasing hormone-3 neurons located in the terminal nerve of transgenic zebrafish (Danio rerio) Gen. Comp. Endocrinol. 2010;168:401–407. doi: 10.1016/j.ygcen.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delsuc F, Brinkmann H, Chourrout D, Philippe H. Tunicates and not cephalochordates are the closest living relative of vertebrates. Nature. 2006;439:965–968. doi: 10.1038/nature04336. [DOI] [PubMed] [Google Scholar]
- 11.Putnam NH, et al. The amphioxus genome and the evolution of the chordate karyotype. Nature. 2008;453:1064–1071. doi: 10.1038/nature06967. [DOI] [PubMed] [Google Scholar]
- 12.Kusakabe TG, et al. A conserved non-reproductive GnRH system in chordates. PLoS ONE. 2012;7:e41955. doi: 10.1371/journal.pone.0041955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamiya C, et al. Nonreproductive role of gonadotropin-releasing hormone in the control of ascidian metamorphosis. Dev. Dyn. 2014;243:1524–1535. doi: 10.1002/dvdy.24176. [DOI] [PubMed] [Google Scholar]
- 14.Adams BA, et al. Six novel gonadotropin-releasing hormones are encoded as triplets on each of two genes in the protochordate, Ciona intestinalis. Endocrinology. 2003;144:1907–1919. doi: 10.1210/en.2002-0216. [DOI] [PubMed] [Google Scholar]
- 15.Kusakabe T, Mishima S, Shimada I, Kitajima Y, Tsuda M. Structure, expression, and cluster organization of genes encoding gonadotropin-releasing hormone receptors found in the neural complex of the ascidian Ciona intestinalis. Gene. 2003;322:77–84. doi: 10.1016/j.gene.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Tello JA, Rivier JE, Sherwood NM. Tunicate gonadotropin-releasing hormone (GnRH) peptides selectively activate Ciona intestinalis GnRH receptors and the green monkey type II GnRH receptor. Endocrinology. 2005;146:4061–4073. doi: 10.1210/en.2004-1558. [DOI] [PubMed] [Google Scholar]
- 17.Sakai T, Aoyama M, Kusakabe T, Tsuda M, Satake H. Functional diversity of signaling pathways through G protein-coupled receptor heterodimerization with a species-specific orphan receptor subtype. Mol. Biol. Evol. 2010;27:1097–1106. doi: 10.1093/molbev/msp319. [DOI] [PubMed] [Google Scholar]
- 18.Sakai T, et al. Evidence for differential regulation of GnRH signaling via heterodimerization among GnRH receptor paralogs in the protochordate, Ciona intestinalis. Endocrinology. 2012;153:1841–1849. doi: 10.1210/en.2011-1668. [DOI] [PubMed] [Google Scholar]
- 19.Abitua PB, et al. The pre-vertebrate origins of neurogenic placodes. Nature. 2015;524:462–465. doi: 10.1038/nature14657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horie T, Kusakabe T, Tsuda M. Glutamatergic networks in the Ciona intestinalis larva. J. Comp. Neurol. 2008;508:249–263. doi: 10.1002/cne.21678. [DOI] [PubMed] [Google Scholar]
- 21.Horie T, Nakagawa M, Sasakura Y, Kusakabe TG, Tsuda M. Simple motor system of the ascidian larva: neuronal complex comprising putative cholinergic and GABAergic/glycinergic neurons. Zool. Sci. 2010;27:181–190. doi: 10.2108/zsj.27.181. [DOI] [PubMed] [Google Scholar]
- 22.Nishitsuji K, et al. Cell lineage and cis-regulation for a unique GABAergic/glycinergic neuron type in the larval nerve cord of the ascidian Ciona intestinalis. Dev. Growth. Differ. 2012;54:177–186. doi: 10.1111/j.1440-169x.2011.01319.x. [DOI] [PubMed] [Google Scholar]
- 23.Tsuda M, et al. Origin of the vertebrate visual cycle. II. Visual cycle proteins are localized in whole brain including photoreceptor cells of a primitive chordate. Vis. Res. 2003;43:3045–3053. doi: 10.1016/j.visres.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Kusakabe TG, Takimoto N, Jin M, Tsuda M. Evolution and the origin of the visual retinoid cycle in vertebrates. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:2897–2910. doi: 10.1098/rstb.2009.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshida R, et al. Identification of neuron-specific promoters in Ciona intestinalis. Genesis. 2004;39:130–140. doi: 10.1002/gene.20032. [DOI] [PubMed] [Google Scholar]
- 26.Katz MJ. Comparative anatomy of the tunicate tadpole, Ciona intestinalis. Biol. Bull. 1983;164:1–27. [Google Scholar]
- 27.Razy-Krajka F, et al. Monoaminergic modulation of photoreception in ascidian: evidence for a proto-hypothalamo-retinal territory. BMC Biol. 2012;10:45. doi: 10.1186/1741-7007-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moret F, et al. The dopamine-synthesizing cells in the swimming larva of the tunicate Ciona intestinalis are located only in the hypothalamus-related domain of the sensory vesicle. Eur. J. Neurosci. 2005;21:3043–3055. doi: 10.1111/j.1460-9568.2005.04147.x. [DOI] [PubMed] [Google Scholar]
- 29.Horie T, et al. Regulatory cocktail for dopaminergic neurons in a protovertebrate identified by whole-embryo single-cell transcriptomics. Genes Dev. 2018;32:1297–1302. doi: 10.1101/gad.317669.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gyoja F, Satoh N. Evolutionary aspects of variability in bHLH orthologous families: Insights from the pearl oyster, Pinctada fucata. Zool. Sci. 2013;30:868–876. doi: 10.2108/zsj.30.868. [DOI] [PubMed] [Google Scholar]
- 31.Ohkura M, et al. Genetically encoded green fluorescent Ca2+ indicators with improved detectability for neuronal Ca2+ signals. PLoS ONE. 2012;7:e51286. doi: 10.1371/journal.pone.0051286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishino A, Okamura Y, Piscopo S, Brown ER. A glycine receptor is involved in the organization of swimming movements in an invertebrate chordate. BMC Neurosci. 2010;11:6. doi: 10.1186/1471-2202-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kusakabe TG. Identifying vertebrate brain prototypes in deuterostomes. In: Shigeno S, Murakami Y, Nomura T, editors. Brain evolution by design: from neural origin to cognitive architecture. Berlin: Springer; 2017. pp. 153–186. [Google Scholar]
- 34.Nishino A, Baba SA, Okamura Y. A mechanism for graded motor control encoded in the channel properties of the muscle ACh receptor. Proc. Natl. Acad. Sci. USA. 2011;108:2599–2604. doi: 10.1073/pnas.1013547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hackley C, Mulholland E, Kim GJ, Newman-Smith E, Smith WC. A transiently expressed connexin is essential for anterior neural plate development in Ciona intestinalis. Development. 2013;140:147–155. doi: 10.1242/dev.084681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdul-Wajid S, Morales-Diaz H, Khairallah SM, Smith WC. T-type calcium channel regulation of neural tube closure and EphrinA/EPHA expression. Cell Rep. 2015;13:829–839. doi: 10.1016/j.celrep.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akahoshi T, Hotta K, Oka K. Characterization of calcium transients during early embryogenesis in ascidians Ciona robusta (Ciona intestinalis type A) and Ciona savignyi. Dev. Biol. 2017;431:205–214. doi: 10.1016/j.ydbio.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 38.Gu X, Olson EC, Spitzer NC. Spontaneous neuronal calcium spikes and waves during early differentiation. J. Neurosci. 1994;14:6325–6335. doi: 10.1523/JNEUROSCI.14-11-06325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong RO, Chernjavsky A, Smith SJ, Shatz CJ. Early functional neural networks in the developing retina. Nature. 1995;374:716–718. doi: 10.1038/374716a0. [DOI] [PubMed] [Google Scholar]
- 40.Feller MB, Wellis DP, Stellwagen D, Werblin FS, Shatz CJ. Requirement for cholinergic synaptic transmission in the propagation of spontaneous retinal waves. Science. 1996;272:1182–1187. doi: 10.1126/science.272.5265.1182. [DOI] [PubMed] [Google Scholar]
- 41.Zhou ZJ. Direct participation of starburst amacrine cells in spontaneous rhythmic activities in the developing mammalian retina. J. Neurosci. 1998;18:4155–4165. doi: 10.1523/JNEUROSCI.18-11-04155.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kerr JN, Greenberg D, Helmchen F. Imaging input and output of neocortical networks in vivo. Proc. Natl. Acad. Sci. USA. 2005;102:14063–14068. doi: 10.1073/pnas.0506029102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang LW, Spitzer NC. Spontaneous calcium spike activity in embryonic spinal neurons is regulated by developmental expression of the Na+, K+-ATPase β3 subunit. J. Neurosci. 2009;29:7877–7885. doi: 10.1523/JNEUROSCI.4264-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feller MB. Spontaneous correlated activity in developing neural circuits. Neuron. 1999;22:653–656. doi: 10.1016/s0896-6273(00)80724-2. [DOI] [PubMed] [Google Scholar]
- 45.Spitzer NC, Lautermilch NJ, Smith RD, Gomez TM. Coding of neuronal differentiation by calcium transients. BioEssays. 2000;22:811–817. doi: 10.1002/1521-1878(200009)22:9<811::AID-BIES6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 46.Nakagawa M, Miyamoto T, Ohkuma M, Tsuda M. Action spectrum for the photophobic response of Ciona intestinalis (Ascidiacea, Urochordata) larvae implicates retinal protein. Photochem. Photobiol. 1999;70:359–362. [PubMed] [Google Scholar]
- 47.Tsuda M, Kawakami I, Shiraishi S. Sensitization and habituation of the swimming behavior in ascidian larvae to light. Zool. Sci. 2003;20:13–22. doi: 10.2108/zsj.20.13. [DOI] [PubMed] [Google Scholar]
- 48.Zega G, Thorndyke MC, Brown ER. Development of swimming behavior in the larva of the ascidian Ciona intestinalis. J. Exp. Biol. 2006;209:3405–3412. doi: 10.1242/jeb.02421. [DOI] [PubMed] [Google Scholar]
- 49.Reist NE, Smith SJ. Neurally evoked calcium transients in terminal Schwann cells at the neuromuscular junction. Proc. Natl. Acad. Sci. USA. 1992;89:7625–7629. doi: 10.1073/pnas.89.16.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Newman EA, Zahs KR. Calcium waves in retinal glial cells. Science. 1997;275:844–847. doi: 10.1126/science.275.5301.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robitaille R. Modulation of synaptic efficacy and synaptic depression by glial cells at the frog neuromuscular junction. Neuron. 1998;21:847–855. doi: 10.1016/s0896-6273(00)80600-5. [DOI] [PubMed] [Google Scholar]
- 52.Haydon PG. Glia: listening and talking to the synapse. Nat. Rev. Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- 53.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Ann. Rev. Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horie T, et al. Ependymal cells of chordate larvae are stem-like cells that form the adult nervous system. Nature. 2011;469:525–528. doi: 10.1038/nature09631. [DOI] [PubMed] [Google Scholar]
- 55.Hozumi A, et al. Efficient transposition of a single Minos transposon copy in the genome of the ascidian Ciona intestinalis with a transgenic line expressing transposase in eggs. Dev. Dynam. 2010;239:1076–1088. doi: 10.1002/dvdy.22254. [DOI] [PubMed] [Google Scholar]
- 56.Corbo JC, Levine M, Zeller RW. Characterization of a notochord-specific enhancer from the Brachyury promoter region of the ascidian, Ciona intestinalis. Development. 1997;124:589–602. doi: 10.1242/dev.124.3.589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials and are available upon request.