Abstract
Purpose
The coronavirus pandemic is affecting global health systems, endangering daily patient care. Hemato-oncological patients are particularly vulnerable to infection, requiring decisive recommendations on treatment and triage. The aim of this survey amongst experts on radiation therapy (RT) for lymphoma and leukemia is to delineate typical clinical scenarios and to provide counsel for high-quality care.
Methods
A multi-item questionnaire containing multiple-choice and free-text questions was developed in a peer-reviewed process and sent to members of the radiation oncology panels of the German Hodgkin Study Group and the German Lymphoma Alliance. Answers were assessed online and analyzed centrally.
Results
Omission of RT was only considered in a minority of cases if alternative treatment options were available. Hypofractionated regimens and reduced dosages may be used for indolent lymphoma and fractures due to multiple myeloma. Overall, there was a tendency to shorten RT rather than to postpone or omit it. Even in case of critical resource shortage, panelists agreed to start emergency RT for typical indications (intracranial pressure, spinal compression, superior vena cava syndrome) within 24 h. Possible criteria to consider for patient triage are the availability of (systemic) options, the underlying disease dynamic, and the treatment rationale (curative/palliative).
Conclusion
RT for hemato-oncological patients receives high-priority and should be maintained even in later stages of the pandemic. Hypofractionation and shortened treatment schedules are feasible options for well-defined constellations, but have to be discussed in the clinical context.
Electronic supplementary material
The online version of this article (10.1007/s00066-020-01705-w) contains supplementary material, which is available to authorized users.
Keywords: COVID, SARS-CoV-2, recommendation, lymphoma, hematology
Introduction
In December 2019, the first cases of an enigmatic pneumonia emerged in the city of Wuhan, Hubei Province, China. The causative virus was later identified as a member of the RNA-containing family of Coronaviridae, named severe acute respiratory syndrome coronavirus 2 (SARS-CoV‑2), with the resulting disease being called coronavirus disease 2019 (COVID-19) [1]. Clinical presentation shows great heterogeneity, from asymptomatic courses to typical pneumonia symptoms like fever, cough, and dyspnea, but also includes nausea, diarrhea, and kidney injury, which may require intensive care [2–4]. Particularly high fatality rates are seen amongst patients receiving mechanical ventilation due to respiratory failure [2–4]. At the time of submission of this article, 31.1 million persons worldwide had been infected, of whom 962,000 had succumbed to the disease [5].
Oncological patients may be particularly prone to infection, as Chinese authors claim an increase of infection rate, severe courses, and fatality rates for oncological patients, with the latter reaching up to 28.6% [6–9]. Focusing on hematological malignancies, reports from Europe and the United States displayed case-fatality rates of up to 40% [10–12].
Data from New York City reveal an increased rate of intubation amongst cancer patients with COVID-19, although augmented mortality was limited to patients <50 years of age [13]. Against this background, infection risks have to be weighed against the possible disadvantage of postponed or omitted therapy [14, 15].
Although the exact impact of COVID-19 on cancer patients is yet to be determined, many specialists in radiation oncology have already formulated emergency guidelines and recommendations for treatment of oncological patients during the pandemic [15–21]. Regarding hemato-oncological patients, the recent consensus paper by the International Lymphoma Radiation Oncology Group (ILROG) has outlined strategies for various entities. Still, there is an unmet need for treatment recommendations, which prompted us to form an expert panel consisting of the main radiation oncologists of the German Hodgkin Study Group (GHSG) and the German Lymphoma Alliance (GLA). The hereby presented paper analyzes typical scenarios and aims at providing guidance for clinical practice.
Methods
A questionnaire was developed by the radiation therapy (RT) expert panel in a peer-reviewed process and circulated within the group in May 2020 (Table 1 for clinical cases and supplementary Fig. 1 for full questionnaire). Recipients were either heads of departments of radiation oncology or consultants specialized in the field of hematological malignancies within the GHSG and GLA. Two radiation oncology departments did not answer the questionnaire. Answers were due on May 15, 2020. The survey is divided into two parts representing consecutive phases of the COVID-19 pandemic. The first scenario (phase 1) describes an early situation during the pandemic in which sufficient personal and treatment capacities are available, although meanwhile, the disease is spreading and thereby endangering patient care. Later, the second phase (phase 2) is characterized by a critical shortage in resources, which might require a triage concerning patient care. The survey was conducted as an online interrogation with a local adaption of the Lime survey (Lime Survey, Hamburg, Germany). Data were analyzed with Microsoft Excel 2016 (Microsoft Corporation, Redmond, USA).
Table 1.
Overview of the clinical scenarios for which different questions concerning priority, delay, and omission of therapy had to be answered
| Case 1: | Painful osteolytic lesion caused by multiple myeloma in non-weightbearing bones after stabilizing surgery |
| Case 2: | Osteolytic lesion of multiple myeloma in weightbearing bones (e.g., axial skeleton) without surgery |
| Case 3: | Limited-stage Hodgkin lymphoma, Ann–Arbor stage II without risk factors after completion of two cycles of ABVD |
| Case 4, 5: |
Diffuse large B‑cell lymphoma with initial abdominal bulky disease after completion of six cycles of R‑CHOP 4) With no information on PET status 5) PET-positive after treatment |
| Case 6: | Early-stage indolent lymphoma in noncritical location |
ABVD adriamycin, bleomycin, vinblastine, dacarbazine; R-CHOP rituximab, cyclophosphamide, hydroxydaunorubicin, oncovin, prednisone; PET positron emission tomography
Results
Overall, 10 participants answered the questionnaire. Most departments have taken protective measures for hemato-oncological RT patients (9; 90%), including the wearing of face masks by patients and staff and rigid basic hygiene (disinfection of treatment rooms, social distancing, reduction of waiting time) (Table 2). Additionally, screening with a decisive questionnaire is implemented in most departments in order to identify possible infectious patients. Tests for COVID-19 infection are widely applied, but to different populations (all patients, all in-patients, all symptomatic patients, all staff, all symptomatic staff).
Table 2.
Overview of answers provided for general questions (n = 10)
| Yes | No | Not answered/indecisive | |
|---|---|---|---|
| Hygiene measures taken | 9 | 1 | 0 |
| Phase 1 | |||
| Reduction of hemato-oncological patients | 0 | 10 | 0 |
| Critical indication for thoracic RT | 3 | 6 | 1 |
| CT during follow-up | 4 | 4 | 2 |
| Reduction of steroids | 3 | 6 | 1 |
| Phase 2 | |||
| Reduction of hemato-oncological patients | 3 | 4 | 3 |
| Chemotherapy-only for early-stage HL | 2 | 5 | 3 |
| Omission of TBI as conditioning regimen before allogenic SCT | 3 | 2 | 5 |
RT radiotherapy, CT computed tomography, HL Hodgkin lymphoma, TBI total body irradiation, SCT stem cell transplantation
Participants overwhelmingly declined to reduce hemato-oncological patient numbers in both phase 1 (10/10) and 2 (4/7). Three participants recommended a more critical indication for thoracic RT, whereas 6 denied this. For corticosteroids as an immunosuppressive agent, only a minority of participants considered a reduction (3/10). To facilitate differential diagnosis between viral pneumonia and radiation pneumonitis, 50% of participants advocated the use of a computed tomography (CT) scan (4 vs. 4).
Phase 1
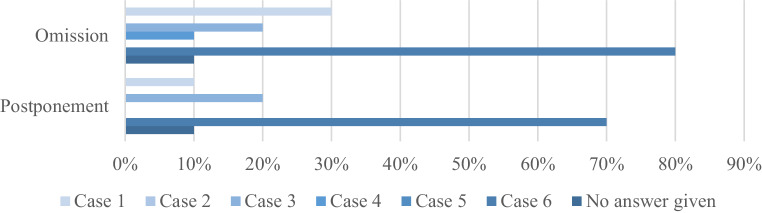
With multiple answers possible, there was a clear denial to delay RT in this phase of the pandemic, except for case 6 (7; 70%), case 3 (2; 20%), and case 1 (1; 10%; Fig. 1). The suggested time to postpone treatment was 4 weeks for case 1 and 3, 4–12 weeks of delay were proposed for case 6. Some answers argue for a wait-and-see strategy in this latter case, with a limitation of treatment to symptomatic/progressive disease. Omission of RT in case of resource shortage was considered for scenarios 6 (8; 80%), 1 (3; 30%), 3 (2; 20%), and 4 (1; 10%).
Fig. 1.
Overview of answers concerning postponement and omission of radiotherapy as provided by the participants for clinical cases in phase 1 of the pandemic (n = 10)
Shortening of treatment via hypofractionation or limitation of total dose was discussed for nearly all examples given with various dose prescriptions. There were suggestions to use hypofractionation in case 1 (5; 50%) with 1*8 Gy, 5*4 Gy, 8*3 Gy, and 10*3 Gy; case 2 (5; 50%) with 5*4 Gy and 8–12*3 Gy; case 4 (1; 10%) with 9–10*3 Gy; case 5 (1; 10%) with 12*3 Gy; and case 6 (2; 20%) with 1*4 Gy and 9*3 Gy. Dose-reduced concepts were proposed especially for scenario 6 (7; 70%), the majority of suggestions being 2*2 Gy.
In case of a COVID-19 infection before the onset of treatment, most participants agreed to postpone treatment for cases 6 (8; 80%), 1 (6; 60%), and 4 (5; 50%), with less than 50% approval for postponing treatment for the remaining three other cases. Similar cases were found to be eligible for interruption of treatment until COVID-19-negativity is established (case 1 and 6: 60%, respectively).
Phase 2
In contrast to phase 1, considerations for reduction of patients increased (3; 30% “yes” versus 4; 40% “no”; Table 2). Factors for clinical triage are largely disease dependent, for example the onset of symptoms, curative versus palliative treatment concept (7; 70% each), and the availability of alternative (systemic) treatments (6; 60%), whereas patient-related risk factors (smoking, hypertension, diabetes, age) were of less importance (4; 40%). The patient’s immune status, immunosuppressive potency of therapy, and the possibility to carry out treatment in an outpatient setting were deemed even less essential (3; 30% each).
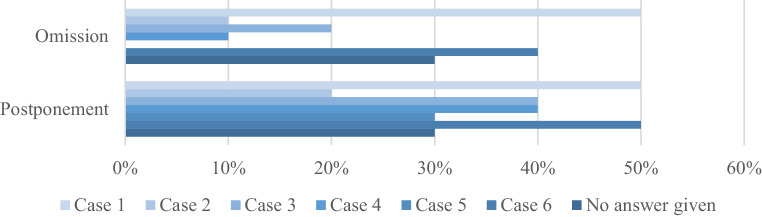
There was a broad consensus to commence emergency RT within 24 h for most given examples (7; 70% for superior vena cava syndrome, spinal cord compression, and amaurosis due to intraorbital disease; 6; 60% for intracranial pressure due to central nervous system lymphoma), except for the case of pain caused by vertebral involvement (2; 20%). No agreement was achieved concerning the omission of time-intense RT treatments like total body irradiation (TBI; 3; 30% in favor vs. 2; 20% against with 5; 50% being indecisive). Regarding the presented clinical situations, omission of RT due to limited capacities was considered for cases 1 (5; 50%) and 6 (4; 40%), with small percentages for the other scenarios (Fig. 2). Heterogeneous answers were received with regard to the question of which patients to postpone, focusing on cases 1 and 6 (5; 50% each) as well as cases 3 and 4 (4; 40% each). Additionally, the accepted delay until the beginning of RT differed significantly, being days up to 4 weeks for case 1, 4–6 weeks for case 2, 2–6 weeks for case 3, 3–8 weeks for case 4, 3–4 weeks for case 5, and 8–12 weeks for case 6.
Fig. 2.
Overview of answers concerning postponement and omission of radiotherapy as provided by the participants for clinical cases in phase 2 of the pandemic (n = 10)
Focusing on clinical case 3 (Hodgkin lymphoma), most participants disagreed on the idea of a chemotherapy-only regimen (5; 50% against vs. 2; 20% in favor). Ranking the different scenarios showed significant differences regarding urgency, with the highest priority for case 2 (4; 40%) and the lowest priority for case 6 (6; 60%). Median ranks, taking into account only complete answers, were: 4, 1, 2.5, 3, 2, and 6 for cases 1–6, respectively, which defines scenario 2 and 5 as the most prioritized cases.
Discussion
The hereby presented survey mirrors the complexity of hemato-oncological patient care in radiation oncology facing a pandemic. It emphasizes the importance for RT treatment for most clinical scenarios and illustrates feasible alternative concepts.
Cancer patients are defined as one group of persons at risk for a severe course of COVID-19 infection [22]. They rely on consequent basic hygiene and social distancing, which have been implemented in nearly all departments for patient and staff protection.
The immunosuppressive state of many oncological patients renders them susceptible to infection with increased rates of morbidity and mortality. Reports from China describe a doubled risk for COVID-19 infection and fatal course of disease for cancer patients [7, 8]. In accordance with this finding, a retrospective analysis of 28 cancer patients with COVID-19 identified anti-cancer treatment within the last 14 days as a risk factor for severe events (only one patient received RT in this group) [9]. Although RT has traditionally been regarded as an immunosuppressive treatment, recent studies in radiobiology rather suggest a complex immunomodulatory role, acting on both the cellular and humoral level of the immune system [23, 24].
Due to the increased risk profile of hemato-oncological patients, it may be attractive to increase screening efforts and to avoid supplementary toxicities. Nevertheless, there was no consent to decrease thoracic irradiations to prevent putative cardiopulmonary side effects. In accordance with this result, a decreased use of corticosteroids was not approved. A recent review from Guy’s Hospital failed to determine the use of steroids as a risk factor for COVID-19 patients and even pointed towards a positive role when used in early stages of infection [25]. The idea of CT scans as a means of differentiation between radiation pneumonitis and COVID pneumonia evoked mixed responses. Importantly, CT signs of COVID-19, like ground-glass opacities and consolidations, are unspecific and may also be caused by influenza or can be confused with radiation pneumonitis [26–28]. Consequently, a medical indication is needed, as thoracic CT scans neither replace laboratory tests nor should they be used as a screening method [26–28].
Recently, the ILROG published “emergency” guidelines for the treatment of leukemia and lymphoma patients in times of a pandemic, which define three key strategies: omission, delay, or shortening of RT [15].
Abandonment of RT may be possible for a palliative setting in which alternative options are at hand, as indicated in clinical scenario 1. Consequently, half of the participants agreed to omit or postpone treatment by up to 4 weeks for a comparable patient in a later phase of the pandemic. Additionally, 60% of answers suggest a delay or interruption of treatment in this case if the patient tests positive for SARS-CoV‑2. Importantly, most participants recommend shortening rather than omission of RT, especially in phase 1 of the pandemic. Regarding multiple myeloma, there were various concepts ranging from 1*8 Gy, over 5*4 Gy to 10–12*3 Gy. Although hypofractionation is feasible, the respective concepts have to be considered within the clinical context and adapted to the individual patient. Rades et al. demonstrated superior neurological recovery for myeloma patients with spinal cord compression by using RT doses of ≥30 Gy [29]. Additionally, solitary plasma cell lesions such as single osseous plasmocytoma or extramedullary plasmocytoma may benefit from dose escalation, with a dose recommendation of 40–50 Gy provided by the ILROG [30–32].
The questionnaire also aimed at judging the use of a chemotherapy-only regimen for early-stage Hodgkin lymphoma (case 3), which has been addressed by three multicenter trials. Noninferiority of unimodal treatment could not be proven, so combined-modality treatment of 2–3 cycles of ABVD followed by involved-site or involved-node RT remains standard of care for early-stage patients [33–35]. This corresponds to the low percentage of panelists who agreed to this chemotherapy-only strategy.
De-escalation of RT doses for lymphoma patients has been of interest in recent years, especially for indolent lymphoma [36, 37]. The FORT trial demonstrated feasibility and efficacy of 2*2 Gy in comparison to 24 Gy. Although the low-dose regimen showed inferior progression-free survival and local control, overall survival did not differ significantly [36]. Further studies elaborated on the feasibility of this concept for extranodal indolent lymphoma [38–41]. This rationale encouraged the suggestion of 2*2 Gy as an alternative treatment strategy for scenario 6. Other options may be the postponement of RT for 1 up to 3 months, which reached a high consent, or a wait-and-see strategy till the onset of symptoms.
This is contrasted by the aggressive biology of diffuse large B‑cell lymphoma described in cases 4 and 5 [42]. Delay in treatment delivery for these patients is only acceptable when facing a critical shortage of resources. Furthermore, hypofractionated concepts lack study validation, being based mainly on calculated equivalent biological efficacy [15].
Overall, treatment of leukemia and lymphoma patients is prioritized and shortage of treatment for these patients is neglected in most situations. This is in accordance with the agreement to start emergency radiation treatments for nearly all provided indications, even during the later phase of the pandemic. Moreover, TBI, as an established conditioning modality before allogenic stem cell transplantation, should not be discarded even in advanced pandemic stages [43].
However, adequate triage remains challenging both at the medical and the ethical level, being a multifactorial process. Panel members consented to integrate the distinction between curative and palliative regimens, onset of symptoms/biology of the underlying disease, and the availability of (systemic) alternatives in the decision process. It is the authors’ strong belief that access to high-quality RT has to be sustained for all patients, while maintaining treatment equality and personal dignity as delineated by the German federal ethic commission [44].
This survey bears several limitations, as only a limited number of radiation oncology experts in lymphoma treatment were consulted. Although the inclusion of different institutions aims at reflecting various concepts, this may not reveal the complete picture. Additionally, nearly all participants work at university hospitals, thus giving a one-sided view of treatment. Restriction of the survey to predefined scenarios facilitated the evaluation process, but may be prone to oversimplification of the complex clinical reality.
Nevertheless, this article provides concepts for typical indications for RT in hemato-oncological patients which are of interest for everyday clinical practice. The hereby provided recommendations are of value until the end of the current pandemic and may serve as a blueprint for future epidemics or crises. Altogether, treating physicians should focus on both maintaining public health by limiting virus spread within the population and guaranteeing state-of-the-art hemato-oncological cancer care.
Conclusion
COVID-19 will most likely remain a challenge for health systems at the national and international level in the months to come. RT for hemato-oncological patients receives high priority and should be maintained even in later stages of the pandemic. Hypofractionation and shortened treatment schedules are feasible options for well-defined constellations, but have to be discussed in the clinical context.
Caption Electronic Supplementary Material
Supp. Fig. 1 Full questionnaire as answered by the participants. Questions were assessed consecutively
Funding
Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
M. Oertel, K. Elsayad, R. Engenhart-Cabillic, G. Reinartz, C. Baues, H. Schmidberger, D. Vordermark, S. Marnitz, P. Lukas, C. Ruebe, A. Engert, G. Lenz, and H.T. Eich declare that they have no competing interests.
References
- 1.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-coV-2 admitted to ICus of the Lombardy region, Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W, Ni Z, Hu Y, et al. Clinical characteristics of Coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the new York city area. JAMA. 2020 doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johns Hopkins Univerity & Medicine Johns Hopkins Univerity&Medicine. https://coronavirus.jhu.edu/map.html. Accessed 21 Sept 2020
- 6.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 8.Yu J, Ouyang W, Chua MLK, Xie C. SARS-coV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020 doi: 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aries JA, Davies JK, Auer RL, et al. Clinical Outcome of Coronavirus Disease 2019 in Haemato-oncology Patients. Br J Haematol. 2020 doi: 10.1111/bjh.16852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malard F, Genthon A, Brissot E, et al. COVID-19 outcomes in patients with hematologic disease. Bone Marrow Transplant. 2020 doi: 10.1038/s41409-020-0931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of cancer patients with COVID-19 in a new York hospital system. Cancer Discov. 2020 doi: 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyashita H, Mikami T, Chopra N, et al. Do patients with cancer have a poorer prognosis of COVID-19? An experience in new York City. Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagar H, Formenti SC. Cancer and COVID-19—potentially deleterious effects of delaying radiotherapy. Nat Rev Clin Oncol. 2020 doi: 10.1038/s41571-020-0375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yahalom J, Dabaja BS, Ricardi U, et al. ILROG emergency guidelines for radiation therapy of hematological malignancies during the COVID-19 pandemic. Blood. 2020 doi: 10.1182/blood.2020006028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Combs SE, Belka C, Niyazi M, et al. First statement on preparation for the COVID-19 pandemic in large German Speaking University-based radiation oncology departments. Radiat Oncol. 2020;15:74. doi: 10.1186/s13014-020-01527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deutsche Gesellschaft für Radioonkologie (DEGRO) Hypofraktionierte Bestrahlungs-Regimes als Alternative zu konventioneller Bestrahlung in Zeiten reduzierter Strahlentherapie-Kapazitäten https://www.degro.org/wp-content/uploads/2020/03/Hypofraktionierte-Bestrahlungsregimes.pdf. Accessed: 05 July 2020
- 18.Deutsche Gesellschaft für Radioonkologie (DEGRO) ARO, Berufsverband Deutscher Strahlentherapeuten Stellungnahme der ARO, DEGRO und des Berufsverbandes zur Strahlentherapie während der COVID-19 Pandemie https://www.degro.org/wp-content/uploads/2020/03/Corona.pdf. Accessed: 05.July 2020
- 19.Guckenberger M, Belka C, Bezjak A, et al. Practice recommendations for lung cancer radiotherapy during the COVID-19 pandemic: An ESTRO-ASTRO consensus statement. Radiother Oncol. 2020 doi: 10.1016/j.radonc.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomson DJ, Palma D, Guckenberger M, et al. Practice recommendations for risk-adapted head and neck cancer radiotherapy during the COVID-19 pandemic: an ASTRO-ESTRO consensus statement. Int J Radiat Oncol Biol Phys. 2020 doi: 10.1016/j.ijrobp.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.German society of radiation oncology (DEGRO) (2020) 2. Stellungnahme der ARO, DEGRO und des Berufsverbandes zur Strahlentherapie während der COVID-19 Pandemie https://www.degro.org/wp-content/uploads/2020/03/Corona.pdf. Accessed: 05 July 2020
- 22.Robert-Koch-Institut (2020) Informationen und Hilfestellungen für Personen mit einem höheren Risiko für einen schweren COVID-19-Krankheitsverlauf. https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Risikogruppen.html?nn=13490888. Accessed 16 May 2020
- 23.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. JNCI J Natl Cancer Inst. 2013;105:256–265. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rückert M, Deloch L, Fietkau R, et al. Immune modulatory effects of radiotherapy as basis for well-reasoned radioimmunotherapies. Strahlenther Onkol. 2018;194:509–519. doi: 10.1007/s00066-018-1287-1. [DOI] [PubMed] [Google Scholar]
- 25.Russell B, Moss C, Rigg A, Van Hemelrijck M. COVID-19 and treatment with NSAIDs and corticosteroids: should we be limiting their use in the clinical setting? Ecancermedicalscience. 2020 doi: 10.3332/ecancer.2020.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi YW, Munden RF, Erasmus JJ, et al. Effects of radiation therapy on the lung: radiologic appearances and differential diagnosis. Radiographics. 2004;24:985–997. doi: 10.1148/rg.244035160. [DOI] [PubMed] [Google Scholar]
- 27.Deutsche Röntgengesellschaft (2020) COVID-19 – Information der AG Thoraxdiagnostik der Deutschen Röntgengesellschaft. https://www.drg.de/de-DE/5995/covid-19/. Accessed 16 May 2020 [DOI] [PubMed]
- 28.Zylka-Menhorn V. Radiologie und COVID-19: Ein Lungen-CT nicht ohne rechtfertigende Indikation. Dtsch Arztebl. 2020;117(17):A-872/B-732. [Google Scholar]
- 29.Rades D, Hoskin PJ, Stalpers LJA, et al. Short-course radiotherapy is not optimal for spinal cord compression due to myeloma. Int J Radiat Oncol Biol Phys. 2006;64:1452–1457. doi: 10.1016/j.ijrobp.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 30.Oertel M, Elsayad K, Kroeger KJ, et al. Impact of radiation dose on local control and survival in extramedullary head and neck plasmacytoma. Radiat Oncol. 2019;14:63. doi: 10.1186/s13014-019-1265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strojan P, Šoba E, Lamovec J, Munda A. Extramedullary plasmacytoma: clinical and histopathologic study. Int J Radiat Oncol. 2002;53:692–701. doi: 10.1016/S0360-3016(02)02780-3. [DOI] [PubMed] [Google Scholar]
- 32.Tsang RW, Campbell BA, Goda JS, et al. Radiation therapy for solitary plasmacytoma and multiple myeloma: guidelines from the international lymphoma radiation oncology group. Int J Radiat Oncol Biol Phys. 2018;101:794–808. doi: 10.1016/j.ijrobp.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Fuchs M, Goergen H, Kobe C, et al. Positron emission tomography-guided treatment in early-stage favorable hodgkin lymphoma: final results of the international, randomized phase III HD16 trial by the German Hodgkin study group. J Clin Oncol. 2019;37:2835–2845. doi: 10.1200/JCO.19.00964. [DOI] [PubMed] [Google Scholar]
- 34.Radford J, Illidge T, Counsell N, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J Med. 2015;372:1598–1607. doi: 10.1056/NEJMoa1408648. [DOI] [PubMed] [Google Scholar]
- 35.Raemaekers JMM, André MPE, Federico M, et al. Omitting radiotherapy in early positron emission tomography-negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: Clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol. 2014;32:1188–1194. doi: 10.1200/JCO.2013.51.9298. [DOI] [PubMed] [Google Scholar]
- 36.Hoskin PJ, Kirkwood AA, Popova B, et al. 4 Gy versus 24 Gy radiotherapy for patients with indolent lymphoma (FORT): a randomised phase 3 non-inferiority trial. Lancet Oncol. 2014;15:457–463. doi: 10.1016/S1470-2045(14)70036-1. [DOI] [PubMed] [Google Scholar]
- 37.Lowry L, Smith P, Qian W, et al. Reduced dose radiotherapy for local control in non-Hodgkin lymphoma: a randomised phase III trial. Radiother. Oncol. 2011;100:86–92. doi: 10.1016/j.radonc.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 38.Elsayad K, Susek KH, Eich HT. Total skin electron beam therapy as part of multimodal treatment strategies for primary cutaneous T-cell lymphoma. Oncol Res Treat. 2017;40:244–252. doi: 10.1159/000475634. [DOI] [PubMed] [Google Scholar]
- 39.König L, Hörner-Rieber J, Bernhardt D, et al. Response rates and recurrence patterns after low-dose radiotherapy with 4 Gy in patients with low-grade lymphomas. Strahlenther Onkol. 2018;194:454–461. doi: 10.1007/s00066-018-1277-3. [DOI] [PubMed] [Google Scholar]
- 40.König L, Stade R, Rieber J, et al. Radiotherapy of indolent orbital lymphomas: two radiation concepts. Strahlenther Onkol. 2016;192:414–421. doi: 10.1007/s00066-016-0962-3. [DOI] [PubMed] [Google Scholar]
- 41.Oertel M, Elsayad K, Weishaupt C, et al. De-escalated radiotherapy for indolent primary cutaneous B-cell lymphoma. Strahlenther Onkol. 2020;196:126–131. doi: 10.1007/s00066-019-01541-7. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization . WHO classification of tumours of haematopoietic and lymphoid tissues. 4. Lyon: International Agency for Research on Cancer; 2017. [Google Scholar]
- 43.Wong JYC, Filippi AR, Dabaja BS, et al. Total body irradiation: guidelines from the international Lymphoma radiation oncology group (ILROG) Int J Radiat Oncol Biol Phys. 2018;101:521–529. doi: 10.1016/j.ijrobp.2018.04.071. [DOI] [PubMed] [Google Scholar]
- 44.Deutscher Ethikrat (2020) Solidarity and responsibility during the Coronavirus crisis—ad hoc recommendation. https://www.ethikrat.org/publikationen/publikationsdetail/?tx_wwt3shop_detail%5Bproduct%5D=135&tx_wwt3shop_detail%5Baction%5D=index&tx_wwt3shop_detail%5Bcontroller%5D=Products&cHash=a37377aedcc6b8b131fce9a9146f9095. Accessed 16 May 2020
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp. Fig. 1 Full questionnaire as answered by the participants. Questions were assessed consecutively