Abstract
Despite a robust literature examining the association between sleep problems and cognitive abilities in childhood, little is known about this association in toddlerhood, a period of rapid cognitive development. The present study examined the association between various sleep problems, using actigraphy, and performance on a standardized test of cognitive abilities, longitudinally across three ages (30, 36, and 42 months) in a large sample of toddlers (N = 493). Results revealed a between-subject effect in which the children who had more delayed sleep schedules on average also showed poorer cognitive abilities on average but did not support a within-subjects effect. Results also showed that delayed sleep explains part of the association between family socioeconomic context and child cognitive abilities.
Nearly 25% of children experience some type of sleep problem (Owens, 2007), ranging from insufficient sleep to erratic sleep schedules to poor sleep consolidation. Sleep problems have been implicated in a variety of maladaptive outcomes in childhood, including obesity (Chen, Beydoun, & Wang, 2008), poorer immune functioning (Bryant, Trinder, & Curtis, 2004), and behavioral and emotional problems (Gregory & Sadeh, 2012). Findings regarding the potential consequences of sleep problems have increased calls for public health campaigns to educate families and caregivers about the importance of children’s sleep (Mindell et al., 2011).
Sleep problems have also been implicated in difficulties in the general domain of cognitive abilities. Research with both adults and children has established an association between sleep difficulties and various cognitive abilities, including memory, attention, intelligence, information processing, and verbal abilities (Astill, Van der Heijden, Van IJzendoorn, & Van Someren, 2012; Beebe, 2011; Ednick et al., 2009; Goel, Rao, Durmer, & Dinges, 2009; Lim & Dinges, 2010). Although there is emerging evidence of an association between sleep difficulties and cognitive abilities, important questions remain. One important question is which cognitive tasks are most susceptible to the effects of sleep problems (e.g., simple vs. complex cognitive tasks), and a second is at which ages the effects of sleep difficulties are shown. A third important question is what are the developmental processes through which these associations emerge and are maintained (Beebe, 2011). The goal of the present study was to clarify the developmental associations between sleep difficulties and cognitive abilities, particularly performance on a complex, standardized cognitive assessment, in an important and understudied age range, toddlerhood (Galland, Taylor, Elder, & Herbison, 2012). To our knowledge, this is the first longitudinal study to examine these processes in a sample of toddlers using objective measures of sleep and cognitive abilities.
Theories About How Sleep Difficulties Affect Cognitive Abilities
There is a strong theoretical basis for expecting sleep deficits to affect cognitive abilities (Ednick et al., 2009; Sadeh, 2007). Sleep loss likely affects cognitive functioning through several overlapping mechanisms. First, sleep is thought to be crucial for maintaining optimal neural functioning by facilitating neural plasticity through widespread synaptic downscaling (Tononi & Cirelli, 2014). Such downscaling, along with a multitude of other sleep-related neural phenomena, is thought to facilitate multiple cognitive processes, including learning and memory consolidation (Diekelmann & Born, 2010). Hence, sleep loss may affect cognitive abilities by disrupting these fundamental cognitive processes. In addition, the prefrontal cortex, the seat of higher order cognitive functioning, is thought to be particularly vulnerable to sleep loss, so the effects of sleep loss on executive functioning, crucial to cognitive functioning, are often pronounced (Jones & Harrison, 2001; Muzur, Pace-Schott, & Hobson, 2002). Sleep deficits may also increase daytime sleepiness, leading to a reduction in overall alertness that causes subsequent impairments in cognitive functions that require sustained mental effort (Short & Banks, 2014).
Although there has been substantially more research on the consequences of sleep loss in adults and older children, there is reason to hypothesize that similar consequences are present in early childhood as well. Indeed, one could expect the consequences of sleep loss on cognitive functioning in early childhood to be especially consequential, given the rapid rate of cognitive and neural development occurring during this developmental window (Beebe, 2011). The toddler and preschool years are characterized by substantial improvements in language abilities, theory of mind, symbolic representation, and executive functioning (Goswami, 2011), and cognitive development is supported by neural development in anterior regions of the brain, particularly the prefrontal cortex (Diamond, 2002). The rapid rate of neural and cognitive development occurring during the toddler and preschool years likely makes children of this age more susceptible to the effects of sleep problems, as varied forms of sleep loss can be considered a type of toxic stress (Beebe, 2011). Sleep loss impairs functioning in the prefrontal cortex (Jones & Harrison, 2001) but could also have a more broad-based effect in young children. Some researchers have begun to examine this possibility, but there is comparatively less research on early childhood. More research on sleep loss in early childhood cognitive development is needed because the toddler and preschool years are periods of substantial changes in normative sleep patterns, including decreases in daytime sleep (Acebo et al., 2005), decreases in night wakings (Gaylor, Burnham, Goodlin-Jones, & Anders, 2005), and decreases in the total amount of sleep (Galland et al., 2012).
Although there has been less research focusing on sleep problems and cognitive abilities in toddlers and preschoolers, the studies focused on this developmental window are highlighted in the following sections, along with the research on similar questions using samples of older children.
Experimental Sleep Restriction Studies With Children
Studies that incorporate experimental sleep manipulations provide information about the direct effects of sleep loss on cognitive functioning. Experimental sleep manipulations have the added benefit of controlling for individual differences by examining change within an individual before and after sleep disruptions. However, given the difficulties associated with implementing this methodology with children, relatively few studies have used experimental sleep restriction or extension with child samples, and those that have done so typically focus on school-age children. These studies provide evidence that, in middle childhood (ages 6–12), sleep restrictions of even 1 hr less per night can cause decrements in several domains of cognitive functioning, including attention (Gruber et al., 2011; Peters et al., 2009; Sadeh, Gruber, & Raviv, 2003; Vriend et al., 2013) and executive functions such as working memory (Vriend et al., 2013) and complex cognitive tasks (e.g., cognitive flexibility and problem solving; Randazzo, Muehlbach, Schweitzer, & Walsh, 1998). Sleep restriction in middle childhood affects academic skills, including math problem-solving fluency (Vriend et al., 2013) and general functioning in the classroom as reported by teachers (Fallone, Acebo, Seifer, & Carskadon, 2005). Of the existing child studies focusing on sleep restriction and subsequent cognitive performance, none has included a sample of toddlers or preschools (although see Berger, Miller, Seifer, Cares, & Lebourgeois, 2012, for a study focusing on sleep restriction and emotional regulation in toddlers).
Relatedly, researchers have examined the association between experimental extensions of sleep and cognitive outcomes. Sleep extension, of even small amounts, is associated with improved neurobehavioral functioning (Sadeh et al., 2003), improved performance on cognitive tasks requiring visuospatial processing (Dewald-Kaufmann, Oort, & Meijer, 2013), and improved academic performance (Gruber, Somerville, Bergmame, Fontil, & Paquin, 2016).
Naturalistic Studies of Sleep and Cognitive Abilities in Children
Although sleep restriction and extension studies provide important information about the causal effects of sleep loss and gain, they do not describe the effects of naturally occurring variations in sleep. Naturalistic correlational studies can show the association between individual differences in sleep and cognitive functioning in children as they occur in the child’s normal environment, even if they do not directly support causal inferences about the short-term effects of sleep loss.
Cross-Sectional Studies
Most of the correlational studies examining the association between sleep difficulties and cognitive functioning have used cross-sectional designs, examining this association at a single point in development. Astill et al. (2012) and Dewald, Meijer, Oort, Kerkhof, and Bögels (2010) meta-analyzed cross-sectional studies of the association between sleep duration (i.e., the amount of sleep per night or day) and sleep efficiency (i.e., the amount of time children are asleep during a given sleep period) and various cognitive outcomes, including sustained attention, executive functioning, multiple-domain cognitive functioning (i.e., complex tasks that require the integration of multiple higher-order cognitive abilities), explicit memory, implicit memory, intelligence, and school performance, across childhood (age 5) to adolescence. Despite extensive variability between studies, longer sleep durations were associated with better executive functioning, performance on the multiple-domain cognitive tasks (Astill et al., 2012), and school performance (Astill et al., 2012; Dewald et al., 2010). However, performance on tests of sustained attention, explicit memory, implicit memory, and formal intelligence were not associated with sleep duration (although it is likely that methodological issues were responsible for the low meta-analytic association between these variables; Astill et al., 2012). Astill et al. (2012) speculated that executive functions and multiple-domain cognitive function tasks were particularly susceptible to the effects of sleep loss because of their extensive reliance on prefrontal regions of the brain. School performance, which is an aggregate measure of academic performance across multiple domains over an extended period of time, may be more likely to show the cumulative effects of sleep loss. Researchers have speculated that many factors, including the direct effects of sleep on cognitive abilities as well as the indirect effects of sleep loss on motivation and emotion regulation may account for noticeable decrements in school performance in children with sleep difficulties (Buckhalt, 2011).
Longitudinal Studies
Cross-sectional studies can show concurrent associations and mean level differences across ages, but can, at times, be misleading when used to describe developmental changes (Kraemer, Yesavage, Taylor, & Kupfer, 2000). Longitudinal research, following the same children over time, is better for describing developmental change and clarifying directional relations among variables. Both experimental sleep restriction studies and the above-mentioned cross-sectional studies can only tap into the relatively short-term effects of sleep problems. Sleep problems at any given time could have an effect on near-term cognitive functioning, but it is also possible that long-term effects may exist in which sleep disruptions across time accumulate into more substantial deficits, which can be tested in longitudinal studies. As El-Sheikh and Buckhalt (2015) have argued, longitudinal, developmental studies of sleep are important.
Results from longitudinal studies with school-aged children that measure sleep and academic or cognitive abilities at two time points in development have indicated that earlier sleep difficulties are associated with decrements in later cognitive functioning, controlling for initial levels of cognitive functioning (Buckhalt, El-Sheikh, Keller, & Kelly, 2009), and school performance (Quach, Hiscock, Canterford, & Wake, 2009). Similarly, increased sleep consolidation in infancy, a marker of more mature child sleep, was associated with improved higher order cognitive functioning (e.g., executive functioning) in early childhood, even when controlling for prior cognitive functioning (Bernier, Beauchamp, Bouvette-Turcot, Carlson, & Carrier, 2013). Improving upon these studies with two time points, some longitudinal studies with three or more time points have examined the association between subjectively reported sleep (i.e., parent or child reports of sleep) and various cognitive outcomes. Studies that have examined the association between trajectories of change in sleep duration and problems during childhood and cognitive outcomes show that persistently short sleepers (< 10 hr a night) were more likely to perform worse on tests of verbal and nonverbal abilities at 5 and 6 years of age (Touchette et al., 2007) and have diminished receptive vocabulary at age 10, even when controlling for prior levels of receptive vocabulary (Seegers et al., 2016). In addition, children who decreased in parent-reported sleep problems across childhood showed better executive functioning at age 17 than peers whose sleep problems remained stable or increased (Friedman, Corley, Hewitt, & Wright, 2009). Likewise, children with consistent parent-reported sleep problems showed poorer complex cognitive functioning capacities at age 13 than children whose sleep problems were decreasing (Gregory, Caspi, Moffitt, & Poulton, 2009). However, these longitudinal studies that compare the cognitive abilities among different groups of children, as determined by the stability of their sleep durations or problems, can make only limited claims about causality, despite their longitudinal designs.
Studies that examine both sleep and cognitive abilities across at least three time points in development allow for more complex models for examining the nature of the association between these two constructs. However, to our knowledge, only one study, Bub, Buckhalt, and El-Sheikh (2011), has used this type of longitudinal design. Bub et al. (2011) examined sleep using child reports of sleep or wake problems and cognitive abilities using tests from the Woodcock–Johnson Tests of Cognitive Ability–III (Woodcock, McGrew, & Mather, 2001), at yearly intervals from third to fifth grades. Using individual growth curves, they found that both lower initial levels of child-reported sleepiness and increases in child-reported sleepiness were associated with diminished growth of verbal abilities (Bub et al., 2011). While this longitudinal design is a methodological improvement, further refinements are needed. We are especially interested in objective measures of sleep durations and awakenings (for example), because subjective reports of sleep, parent or child, may be less accurate than objective measures of sleep (e.g., actigraphy; Dayyat, Spruyt, Molfese, & Gozal, 2011). However, to our knowledge, no studies with three or more longitudinal assessments of both sleep and cognitive abilities have used objective measures of sleep.
The Role of SES in Association Between Sleep Problems and Cognitive Abilities
Across childhood, there is evidence of an association between family socioeconomic status (SES) and sleep problems in children, such that children from low SES backgrounds sleep significantly less overall, have later bedtimes, and show more variability in their sleep schedules (El-Sheikh et al., 2013; Gellis, 2011; Kelly, Kelly, & Sacker, 2013). Several explanations for these associations have been proposed. Children from low SES backgrounds are less likely to have high-quality sleep environments that are dark, comfortable, noncrowded, and noise-free spaces. Across childhood, nonideal sleep environments could affect the quantity, quality, and variability of the child’s sleep (Bagley, Kelly, Buckhalt, & El-Sheikh, 2015; Brown & Low, 2008; Chung et al., 2014). Relatedly, poor sleep hygiene (e.g., inconsistent sleep or wake schedules, poor-quality bedtime routines, consumption of caffeinated beverages prior to bedtime) is more prevalent in low SES children and in children with sleep problems (Jones & Ball, 2014). Differences in sleep hygiene may underlie the association between SES and sleep problems (Patrick, Millet, & Mindell, 2016). School-age children from low SES backgrounds also show increased presleep worries (potentially due to their increased exposure to environmental stressors), which mediate the association between SES and sleep or wake problems as measured via parent report (Bagley et al., 2015). Finally, across childhood, health problems such as asthma, obesity, and poorer immune functioning are more prevalent in low SES children, and they are associated with increased sleep problems (Chen et al., 2008). Although much of the research focusing on the association between sleep and SES has focused on older children, two studies suggest that similar associations may be present during toddlerhood (Barazzetta & Ghislandi, 2016; Van Tassel, 1985). Indeed, it is plausible that toddlers might be particularly susceptible to the proposed mechanisms through which SES affects sleep, because young children’s sleep routines are primarily shaped by caregivers and family members rather than the children themselves. Hence, parental stress might be more salient to young children who are put to bed by their caregivers than to older children who are primarily responsible for their own bedtime routine.
The association of sleep and SES, coupled with the well-established literature on the association between SES and cognitive development (Bradley & Corwyn, 2002), suggests the possibility that sleep problems are one mechanism through which SES affects cognitive development (Buckhalt, 2011). Sleep may mediate the association between SES and cognitive abilities; however, we are aware of only one study that has examined this potential mediation model. Brown and Low (2008) reported that in preschool-aged children, parent-reported sleep problems mediated the association between parent impressions of chaos in the home environment and responses to academic challenge, such that one process through which chaotic living conditions might affect children’s persistence in an academic task is through sleep problems. Brown and Low (2008) note several limitations to their study including the use of a small sample size, the use of cross-sectional mediation models, and the use of a single informant (parents) for all three study variables, suggesting the results may be due, in part, to shared method variance. Based on the promise of this study, however, more research focusing on the role of SES in the association between sleep problems and cognitive abilities, with an improved design, is warranted.
Current Study
The purpose of the present study is to describe how sleep difficulties and cognitive abilities change over time, both independently and jointly, during toddlerhood, an important period for cognitive development (Goswami, 2011). As noted earlier, many have hypothesized that naturally occurring sleep difficulties cause poorer cognitive abilities. We sought to examine the long-term effects of sleep problems on cognitive abilities in a sample of children in which both sleep and cognitive abilities were measured objectively at three time points across 1 year (at the ages of 30, 36, and 42 months). We considered two plausible, though not mutually exclusive, ways in which changes in sleep and cognitive abilities may be related. First, it may be the case that children who experience more sleep difficulties on average have poorer cognitive abilities, which would be a systematic, between-person effect. Between-person effects quantify differences between individuals in a sample. Additionally, it is also possible that changes in sleep difficulties, from each individual’s average level, lead to subsequent changes in cognitive functioning, which would be a within-person effect. Within-person effects quantify changes in an individual across time. Both such effects (between and within) have been theoretically proposed in the longitudinal association between sleep difficulties and cognitive abilities in older children and adults. However, to our knowledge, no study has explicitly and distinctly examined such hypothesized longitudinal relations in toddlerhood.
The present study has two main goals: (a) to determine if indeed sleep difficulties temporally precede changes in cognitive ability, by examining the directionality of the association between objectively measured sleep difficulties and cognitive abilities, and (b) disentangle between- and within-person effects of sleep on cognitive ability. If sleep does not show both between- and within-person effects, it is unlikely that this process is a causal one, and alternative, noncausal associations between these variables should be considered, including that the association is based on a spurious or unmeasured effect.
Based on the literature we reviewed, we expected that there to be both a between- and within-person effect of sleep difficulties on verbal and nonverbal cognitive abilities. An additional goal of the present study was to comprehensively and systematically examine the associations of multiple, objectively reported sleep parameters with cognitive outcomes. In addition to associations with subjectively reported sleep measures, including sleep or wake problems, daytime sleepiness, and sleep duration, associations have been noted for various types of objectively reported sleep difficulties, including short sleep durations (Buckhalt et al., 2009; Gruber et al., 2010; Paavonen et al., 2010; Vaughn, Elmore-Staton, Shin, & El-Sheikh, 2015), variability in the sleep schedule (Buckhalt, El-Sheikh, & Keller, 2007; Buckhalt et al., 2009), and poor sleep efficiency (Buckhalt et al., 2007, 2009). There also is evidence that different types of sleep problems may be differentially associated with cognitive outcomes. For example, Gruber et al. (2014) found that sleep efficiency, but not sleep duration, was associated with poorer academic performance in middle childhood, whereas Bernier et al. (2013) found that low levels of sleep consolidation (the extent to which sleep occurred at night vs. during the day) in infancy were associated with later higher order cognitive functioning in preschoolers. Such findings indicate a need to continue to refine our understanding of how different types of sleep problems are differentially related to cognitive outcomes. In addition, some important aspects of individual differences in sleep, including the lateness of the child’s bedtime, have yet to be examined in association with cognitive abilities. Given these contradictions and gaps in the literature, a systematic investigation of the association between cognitive abilities and sleep problems is warranted. In the present study, we examined a broad range of actigraphy variables (including those of duration, quality, variability, and timing of sleep) to further explore the associations between various objective measures of sleep. We hypothesized, based on previous findings, that many, if not all, of the indexes of sleep examined in the present study would show the hypothesized association with cognitive abilities (both verbal and nonverbal abilities).
Finally, given intriguing findings in the literature that sleep difficulties mediate the association between SES and aspects of academic performance in older children, an additional goal of the present study was to examine if sleep difficulties would mediate the association between SES and cognitive abilities in a sample of toddlers. Previous studies have established associations between sleep problems and SES as well as SES and cognitive development in toddlers, so it is possible that the prerequisites for statistical mediation may be met. However, for the present study, this was a follow-up, exploratory aim that we planned to pursue only if the association between sleep difficulties and cognitive abilities emerged.
Method
Participants
Participants in this study included toddlers recruited at 30 months of age, who were assessed at three time points: 30, 36, and 42 months of age. Data from three slightly different iterations of the same study are included in the current analysis. The first study (the Toddler Sleep Study; 2003–2005) was a pilot version of the second study (the Toddler Development Study 1; 2007–2012). The second study served as a basis for the third, ongoing study, which has been expanded to include multiple sites (the Toddler Development Study 2, 2012–present). Although each study had a slightly different protocol, the variables used in the present article were measured in identical ways across iterations of the study. Hence, all three study data sets were analyzed together. The number of participants at each age across each iteration of the study is included in Table S1. At 30 months, 493 children in total (female = 228) were recruited into the study. However, the number of children included in the final sample at each age is reduced for several reasons. In the first iteration of the study (the Toddler Sleep Study), planned missingness was used such that not all participants were assessed at all waves, and some only participated at 36 (n = 8) and 42 (n = 13) months of age. Next, a portion of the combined sample were part of an ongoing longitudinal study, so not all children who had participated at 30 months of age were old enough to participate in the second (n = 57) or third (n = 114) assessments. Data missing for this reason were considered to be missing at random (MAR), because the date at which the participant was recruited into the study explains why a participant was missing observations. Finally, some toddlers who participated in the first assessment did not participate in subsequent assessments (n = 60 at 36 months; n = 66 at 42 months). Missingness for each variable included in analyses is detailed in Table S2. We chose not to use the case deletion approach because the complete-data subsample may not be representative of the full population, the statistical power of subsequent analyses would be greatly reduced, and case-wise deletion would introduce undue bias when the data are MAR (Schafer & Graham, 2002). Instead, to account for this missing data from various sources, we used full information maximum likelihood during model estimation, a technique that provides accurate parameter estimates when data are considered to be MAR (Schafer & Graham, 2002).
Participants were recruited from two mid-sized, Midwestern cities and surrounding rural communities. The final sample was predominately Caucasian (76%, 4% Latino, 2% Black, 1% Mixed Race, 3% Other, and 13% unknown, not reported, or missing), reflecting the general racial breakdown of the two Midwestern cities (both cities were approximately 6%–8% Black and Hispanic according to recent U.S. Census Data) and came from two-parent households (84%, 7% single parent, 4% Other, 5% not reported). Primary caregivers in the samples were primarily college educated (76% college degree, 13% some college, 5% high school diploma or less, 6% not reported). The family’s SES was calculated using the Hollingshead Four-Factor Index (Hollingshead, 1975), which takes into account the parents’ educational attainment and occupational prestige (based on U.S. Census codes), with both parents’ education and occupation scores equally informing estimates when both parents are employed but with only one parent’s score informing estimates in families with a single parent or a couple with a nonemployed spouse. Parent occupation and education level were the only variables related to SES that were collected in the current sample, so the Hollingshead Index (which accounts for both education and occupation) was used. SES estimates ranged from 12.5 to 66, with M = 47.59 (SD = 13.26), suggesting that the sample was predominantly middle class, although not uniformly so. Based on parent reports on two standardized measures of child sleep disorders, the Child Sleep Habits Questionnaire (Owens, Spirito, & McGuinn, 2000) and the Kosair sleep questionnaire (Montomery-Downs et al., 2004), no child in the final sample had a score on either instrument elevated enough to indicate the presence of a childhood sleep disorder such as sleep disordered breathing or one of the various parasomnias.
Measures
Objective Measure of Sleep: Actigraphy
At each time point, sleep was measured using MicroMini Motionlogger actigraphs from Ambulatory Monitoring Inc. (Ardsley, NY), watch-like devices worn by the children continuously for 1–2 weeks (at 30 months: M = 10.92 nights, SD = 4.28; at 36 months: M = 10.16 nights, SD = 4.53; at 42 months: M = 12.35 nights, SD = 7.77). Actigraphs contain an accelerometer that measures minute-by-minute motor activity, which allows for the estimation of sleep and wake patterns. Actigraphs were typically worn on the child’s nondominant wrist, but some toddlers, who refused to wear the actigraph on their wrist, were allowed to wear the device on their ankle (9% of the sample were reported to have worn their actigraph on their ankle on at least one assessment). There were no systematic differences in how the child wore the actigraph based on family SES, child sex, or parent-reported sleep problems. However, age was associated with how the child wore the actigraph, such that older children were less likely to wear the actigraph on their ankle (r = −.14, p = .001). The number of nights of actigraphy data collected varied because family preference was taken into consideration when scheduling the lab visits (actigraphs were returned to the research assistants during this visit), as well as toddler noncompliance with wearing the device. There were no systematic differences in the number of nights the child wore the actigraph based on family SES, child sex, or parent-reported sleep problems. However, there was a significant association between the number of nights the child wore the actigraph and age (r = .13, p = .001), such that older children tended to have more nights of actigraphy data. This likely occurred because children were more compliant with wearing the actigraphs as they got older. Sleep diaries were completed each night by the child’s primary caregiver, and were used to mark the child’s bedtime, wake time, nap start and end time, and any times when the child was not wearing the actigraph.
Actigraphy data were processed using the Sadeh algorithm (a processing algorithm validated for use with children; Sadeh, Sharkey, & Carskadon, 1994). A large set of raw actigraphy variables that (a) were used in prior actigraphy research, (b) were consistent with major areas of sleep behavior (e.g., activity, amount, timing), and (c) were not merely a linear combination of already selected variables, were exported from the AW2 software package (Motionlogger Analysis Software Package Action W-2 software, version 2.6.92; Ambulatory Monitoring Inc). For the Toddler Development Study 1 portion of the current study’s sample (N = 109), these variables were reduced using principal components analysis to four composite sleep factors: sleep activity, sleep duration, sleep variability, and sleep timing. The composites were formed by summing unweighted standardized indexes for each relevant variable. See Table 1 for full-sample means and standard deviations of the variables included in each composite index. The sleep activity composite indexes activity and wake episodes that occur during the sleep period. The sleep duration composite indexes the general length of the child’s night-time sleep period. The sleep variability composite indexes night-to-night variability in the timing and duration of the child’s sleep. The sleep timing composite indexes the relative lateness of a child’s sleep schedule. These composites represent broad dimensions of actigraphy that are often examined in the child sleep literature (Meltzer, Montgomery-Downs, Insana, & Walsh, 2012; Meltzer, Walsh, Traylor, & Westin, 2012).
Table 1.
Actigraphy Variables Included in Each Composite Index, Along With Means and Standard Deviations of These Variables at Each Age
| Composite index | Variable names | 30 months | 36 months | 42 months |
|---|---|---|---|---|
| Sleep activity | Avg. time (min) awake after sleep onset | 89.19 (48.58) | 75.45 (41.96) | 70.29 (41.67) |
| SD of avg. min to min activity levels | 34.74 (10.48) | 31.84 (8.9) | 31.63 (8.61) | |
| Avg. number of awakenings (lasting 5 min or more) | 4.72 (2.31) | 4.19 (2.13) | 4.06 (2.15) | |
| Avg. duration (min) of longest wake episode (after sleep onset) | 31.18 (20.71) | 25.77 (14.94) | 23.89 (13.49) | |
| Avg. percent of active epochs (after sleep onset) | 51.47 (12.32) | 47.73 (12.07) | 46.13 (12.19) | |
| Sleep variability | SD of time of sleep onset | 0.71 (0.46) | 0.72 (0.42) | 0.72 (0.41) |
| SD of duration of time in bed | 49.62 (23.54) | 47.91 (20.78) | 46.68 (21.75) | |
| SD of duration of sleep period | 57.79 (29.2) | 55.27 (25.32) | 52.56 (25.44) | |
| SD of time of midsleep | 0.52 (0.29) | 0.52 (0.27) | 0.52 (0.25) | |
| SD of bedtime | 0.58 (0.38) | 0.58 (0.32) | 0.59 (0.34) | |
| SD of min asleep in bed | 60.16 (29.64) | 56.78 (21.63) | 54.46 (25.03) | |
| Sleep timing | Avg. time of midsleep (HH:MM in 24-hr time) | 02:19 (00:45) | 02:25 (00:49) | 02:22 (00:46) |
| Avg. time of sleep onset (HH:MM in 24-hr time) | 21:30 (00:54) | 21:35 (00:57) | 21:33 (00:53) | |
| Avg. bedtime (HH:MM in 24-hr time) | 20:52 (00:48) | 20:57 (00:52) | 20:57 (00:51) | |
| Sleep duration | Avg. sleep period (min) | 581.96 (42.88) | 584.37 (47.24) | 583.18 (42.07) |
| Avg. duration of time in bed (min) | 624.48 (41.63) | 626.11 (43.70) | 621.76 (38.83) | |
| Avg. min asleep in bed | 490.81 (68.51) | 506.01 (63.31) | 510.54 (59.76) |
Note. Avg. = average; min = minutes; SD = standard deviation.
In the combined sample used in the current study, the four composite indexes show adequate reliability (sleep activity: 30 months α= .72, 36 months α= .72, and 42 months α= .71; sleep duration: 30 months α = .80, 36 months α = .86, and 42 months α = .83; sleep variability: 30 months α = .69, 36 months α = .66, and 42 months α = .68; and sleep timing: 30 months α = .95, 36 months α = .95, and 42 months α = .96) and are relatively stable across the three ages assessed in the present study. We chose to use composites when examining our actigraphy data for several reasons. First, a survey of the child sleep literature observed wide differences across studies in the selection and the use of actigraph variables (Meltzer, Montgomery-Downs, et al., 2012; Meltzer, Walsh, et al., 2012). We sought to find a systematic way to examine multiple dimensions of sleep without rerunning our analyses for each of the numerous variables exported from AW2. In addition, it is well known that aggregate measures provide important measurement advantages over single-variable analysis (Rushton, Brainerd, & Pressley, 1983). Finally, using these composites allowed us to parsimoniously examine many of the important features of sleep without having to run, and correct for, as many tests as we would in a single-variable analysis. These composites were used in all models, along with a more traditional, single index of total sleep minutes (including sleep at night and during the day). This single index of total sleep minutes was included so that sleep during the day, in the form of naps, was also considered.
Cognitive Abilities
The Differential Abilities Scale Early Years Battery was used to assess preacademic cognitive abilities at each time point (Elliott, 1990). The Differential Abilities Scale (DAS)–Early Years Battery is a standardized assessment useful for children ages 2”–3” that includes four cognitive tests, including Verbal Comprehension, Naming Vocabulary, Picture Similarities, and Block Design. These tests load onto two higher-order factors, a verbal ability factor (Verbal Comprehension and Naming Vocabulary) and a nonverbal ability factor (Block Design and Picture Similarity). All tests load onto an overall general cognitive ability (GCA) factor. To model change in cognitive ability over time, raw scores, rather than age-standardized scores, were used. Higher raw scores on each subscale indicate better performance. The Cronbach’s alpha values were .85, .70, and .84 for the verbal, nonverbal, and GCA subscales, respectively.
SES
As described earlier, SES of the child’s family was estimated using the Hollingshead Four-Factor Index (Hollingshead, 1975). Although this index of SES is unlikely to be a true proxy for such indexes as income and accumulated wealth, it is an index of a nuclear family’s SES, and it is somewhat more comprehensive than any single index (Braveman et al., 2005).
Procedures
During a home visit at each age, a trained experimenter visited the family’s home and administered the DAS to the child (this assessment took 20–30 min to complete). The DAS was administered to the child in a quiet location, and caregivers were asked to remain silent and to not give any help or feedback to the child throughout the administration of the test. During this visit, the experimenter also gave the child an actigraph to wear over the next week or two and provided instructions to the caregiver about actigraph use. The child’s primary caregiver also completed several questionnaires, including reporting on demographic information and completing daily sleep diaries. All procedures were approved by the relevant institutional review boards at the two research sites.
Analysis Plan
To examine how sleep difficulties and cognitive abilities change and are related to one another over time, an autoregressive latent trajectory model with structured residuals (ALT-SR; Curran, Howard, Bainter, Lane, & McGinley, 2014) was used. The ALT-SR model is a variation on the ALT model (Bollen & Curran, 2004). The ALT model combines popular developmental modeling techniques, a bivariate latent growth curve model, in which the underlying latent growth trajectories for two constructs are estimated across all available time points for each individual, and a bivariate autoregressive model, in which the longitudinal association between two variables is estimated above and beyond the longitudinal prediction of the variable by itself. The ALT model combines the most beneficial aspects of each model in order to (a) estimate latent slope and intercept values for each variable series for each individual and (b) consider both lagged autoregressive and cross-lagged effects in models of the associations between variables.
A modification of ALT model, the ALT-SR (Curran et al., 2014), was used to disaggregate the between- and within-person effects of the predictor on the outcome. Unlike traditional ALT models, in which cross-lagged estimates amalgamate between-person and within-person effects, the ALT-SR model allows one to estimate traditional cross-lagged paths while effectively disentangling between- and within-person effects (Berry & Willoughby, 2017). This is accomplished through the creation of structured residuals, latent variables that absorb within-person residual variance at each time point and are examined as unique entities within the model. The structured residuals, as time-specific deviations from the underlying variable trajectory, are used to estimate autoregressive associations and cross-lagged associations between variables. These residuals can be examined to show the within-person effects of the theorized predictor variable on the outcome. The autoregression of these residuals does not affect the mean of the latent intercept and slope for each variable series, and these latter fixed effects can be used to estimate the between-person effect of the theorized predictor variable on the outcome variable. All models were estimated using Mplus 3.01 (Muthen & Muthen, 1998–2011) with maximum-likelihood estimation and robust standard errors, using the relevant syntax provided in Berry and Willoughby (2017).
Results
Descriptive Statistics
Descriptive statistics for each variable included in the analysis are presented in Table 2.
Table 2.
Descriptive Statistics and Cross-Sectional Correlations of Study Variables at (A) 30 Months, (B) 36 Months, and (C) 42 Months
| Sleep duration | Sleep timing | Sleep variability | Sleep activity | Total sleep minutes | GCA | Verbal ability | Nonverbal ability | SES (30 months) | |
|---|---|---|---|---|---|---|---|---|---|
| (A) | |||||||||
| Sleep duration | 1 | ||||||||
| Sleep timing | −.37*** | 1 | |||||||
| Sleep variability | −.12* | .33*** | 1 | ||||||
| Sleep activity | −.37*** | .07 | .26*** | 1 | |||||
| Total sleep minutes | .64*** | −.19*** | −.15** | −.77*** | 1 | ||||
| GCA | .00 | −.10* | −.13** | −.02 | .03 | 1 | |||
| Verbal ability | .06 | −.16** | −.12* | −.02 | .05 | .77*** | 1 | ||
| Nonverbal ability | −.03 | −.07 | −.13* | .00 | .02 | .80*** | .46*** | 1 | |
| SES (30 month) | .16** | −.22*** | −.19*** | −.08^ | .15** | .24*** | .25*** | .13** | 1 |
| N | 420 | 419 | 419 | 419 | 416 | 430 | 438 | 437 | 442 |
| M | −0.04 | −0.05 | 0.02 | 0.18 | 572.17 | 99.08 | 77.63 | 60.99 | 47.59 |
| SD | 0.87 | 0.91 | 0.84 | 0.90 | 82.33 | 13.31 | 18.05 | 16.20 | 13.26 |
| (B) | |||||||||
| Sleep duration | 1 | ||||||||
| Sleep timing | −0.32*** | 1 | |||||||
| Sleep variability | −.12* | .26*** | 1 | ||||||
| Sleep activity | −.24*** | .08 | .24*** | 1 | |||||
| Total sleep minutes | .42*** | −.23*** | −.32*** | −.70*** | 1 | ||||
| GCA | .09 | −.13* | −.01 | −.01 | .05 | 1 | |||
| Verbal ability | .07 | −.19** | −.01 | −.07 | .16** | .68*** | 1 | ||
| Nonverbal ability | .05 | −.10^ | .01 | −.01 | .04 | .81*** | .41*** | 1 | |
| SES (30 month) | −.07 | −.22*** | −.11^ | .01 | .05 | .17** | .21*** | .06 | 1 |
| N | 324 | 322 | 324 | 322 | 325 | 334 | 335 | 336 | 442 |
| M | 0.07 | 0.04 | −0.01 | −0.11 | 575.22 | 103.43 | 93.44 | 77.82 | 47.59 |
| SD | 0.90 | 0.98 | 0.76 | 0.8 | 90.06 | 15.87 | 17.65 | 19.98 | 13.26 |
| (C) | |||||||||
| Sleep duration | 1 | ||||||||
| Sleep timing | −.27*** | 1 | |||||||
| Sleep variability | −.10^ | .33*** | 1 | ||||||
| Sleep activity | −.30*** | .01 | .15* | 1 | |||||
| Total sleep minutes | .55*** | −.11^ | −.1 | −.70*** | 1 | ||||
| GCA | .04 | −.11^ | −.02 | −.13^ | .06 | 1 | |||
| Verbal ability | .10 | −.12^ | .03 | −.11^ | .14* | .69*** | 1 | ||
| Nonverbal ability | .03 | −.09 | .03 | −.08 | .00 | .76*** | .45*** | 1 | |
| SES (30 month) | .03 | −.19** | −.15* | −.11^ | .13* | .16** | .19** | .09 | 1 |
| N | 276 | 276 | 276 | 276 | 275 | 275 | 276 | 276 | 442 |
| M | 0.05 | 0.00 | −0.06 | −0.20 | 576.49 | 108.72 | 105.15 | 100.04 | 47.59 |
| SD | 0.80 | 0.95 | 0.73 | 0.79 | 64.76 | 14.20 | 16.30 | 19.47 | 13.26 |
Note. GCA = general cognitive ability; SES = socioeconomic status.
p ≤ .10.
p ≤ .05.
p ≤ .01.
p ≤ .001.
Cross-Sectional Associations
The cross-sectional associations between actigraphic sleep and cognitive abilities are presented in Table 2. We expected, based on previous cross-sectional studies and meta-analytic evidence, that there would be modest, within-time correlations between the complex cognitive abilities assessed by the DAS and sleep. A number of significant cross-sectional associations emerged between multiple sleep composites and cognitive abilities composites, but few were consistent across ages. Of the five actigraphy indexes used (four sleep composites and the index of total sleep minutes), only the sleep timing composite was associated with GCA scores across multiple ages. Sleep timing was significantly negatively associated with GCA scores at 30 months (r = −.10, p = .05), 36 months (r = −.12, p = .03), with a trend the same direction at 42 months (r = −.13, p = .09), such that the later the child’s sleep schedule (higher values on the sleep timing composite), the worse they performed on the DAS. When we regressed GCA scores on all of the actigraphy indexes at each age, sleep timing emerged as the only significant predictor of cognitive ability at ages 36 and 42 months. Based on these results, we chose to examine only sleep timing as a predictor in further models.
ALT-SR Model
Model Building
Based on the recommendations of Curran et al. (2014), the model-building procedure included three steps. First, latent curve models (LCM) were fitted for sleep and cognitive variables, independently, to estimate mean intercepts and growth parameters. Next, structured residuals were added to the best fitting LCM for each variable. Finally, the best fitting LCM (with structured residuals) for each construct was combined into a single model. From this model, cross-construct associations were estimated.
LCM With Structured Residuals
The sleep timing data were adequately represented by a random-intercepts LCM: χ2(4) = 16.62, p = .002, comparative fit index (CFI) = .97, root mean square error of approximation (RMSEA) = .08. The mean value of the intercept for sleep timing was not significant, suggesting it was not different from zero (this was expected as the variable is a composite of standardized variables). The variance component of the intercept was significant, suggesting that there was substantial between-child variability in intercept values for this index. Including a linear slope in this LCM did not improve model fit, suggesting that linear changes in sleep timing were not significant across this time period. Consequently, the intercept-only model was retained. Adding structured residuals to the intercept-only LCM model resulted in minimally improved model fit: χ2(3) = 7.22, p = .065, CFI = .99, RMSEA = .05; however, for theoretical reasons, this model was retained. Model results are presented in Table 3.
Table 3.
Tests of Mean and Variances for Sleep Timing and GCA LCM (With Structured Residuals)
| LCM with SR (sleep timing) |
LCM with SR (GCA) |
ALT-SR |
||||
|---|---|---|---|---|---|---|
| B | SE | B | SE | B | SE | |
| Fixed effects | ||||||
| Timing36 on Timing30 | 0.35* | 0.11 | ||||
| Timing42 on Timing36 | 0.32* | 0.10 | ||||
| GCA36 on GCA30 | 0.24* | 0.09 | ||||
| GCA42 on GCA36 | 0.28* | 0.11 | ||||
| Timing36 on GCA30 | −0.01 | 0.08 | ||||
| Timing42 on GCA36 | −0.01 | 0.09 | ||||
| GCA36 on Timing30 | −0.09 | 0.09 | ||||
| GCA42 on Timing36 | −0.10 | 0.10 | ||||
| M | ||||||
| Timingint | 0.003 | 0.06 | 0.01 | 0.04 | ||
| GCAint | 98.50** | 0.64 | 98.48** | 0.65 | ||
| GCAslope | 5.01** | 0.37 | 4.99** | 0.40 | ||
| (Co)Variances | ||||||
| Timingint | 0.65** | 0.52 | 0.55** | 0.07 | ||
| GCAint | 153.16** | 19.90 | 107.49** | 15.65 | ||
| GCAslope | 33.74** | 9.04 | 0.00 | 0.00 | ||
| Timingint with GCAint | −0.19* | 0.96 | ||||
| Fit statistics | ||||||
| χ2 | 16.62** | 0.20 | 24.95** | |||
| df | 4 | 1 | 11 | |||
| RMSEA | .08 | .00 | .51 | |||
| CFI | .97 | 1.00 | .98 | |||
Note. LCM = latent curve model; SR = structured residuals; ALT-SR = autoregressive latent trajectory model with structured residuals; Timing = sleep timing; GCA = general cognitive ability; RMSEA = root mean square error of approximation; CFI = comparative fit index.
p ≤ .05.
p ≤ .01.
The cognitive abilities data were modeled by a LCM with random intercepts and a linear slope with acceptable fit: χ2(1) = 0.20, p = .65, CFI = 1.00, RMSEA = .00. The means and variances of the intercept and linear slope were significant, indicating that the GCA cognitive abilities score improved significantly and linearly across toddlerhood, and that there was significant variability across individuals in both starting level and growth rate. The LCM for GCA scores was then expanded to include structured residuals. This model fit the data adequately: χ2(3) = 0.20, p = .65, CFI = .99, RMSEA = .05. Although the addition of the structured residuals did not substantially improve the fit of the model, again, the current study’s theoretical interest in differentiating the between- and within-person effects led us to include structured residuals. Model results are presented in Table 3.
ALT-SR Model Fitting and Interpretation
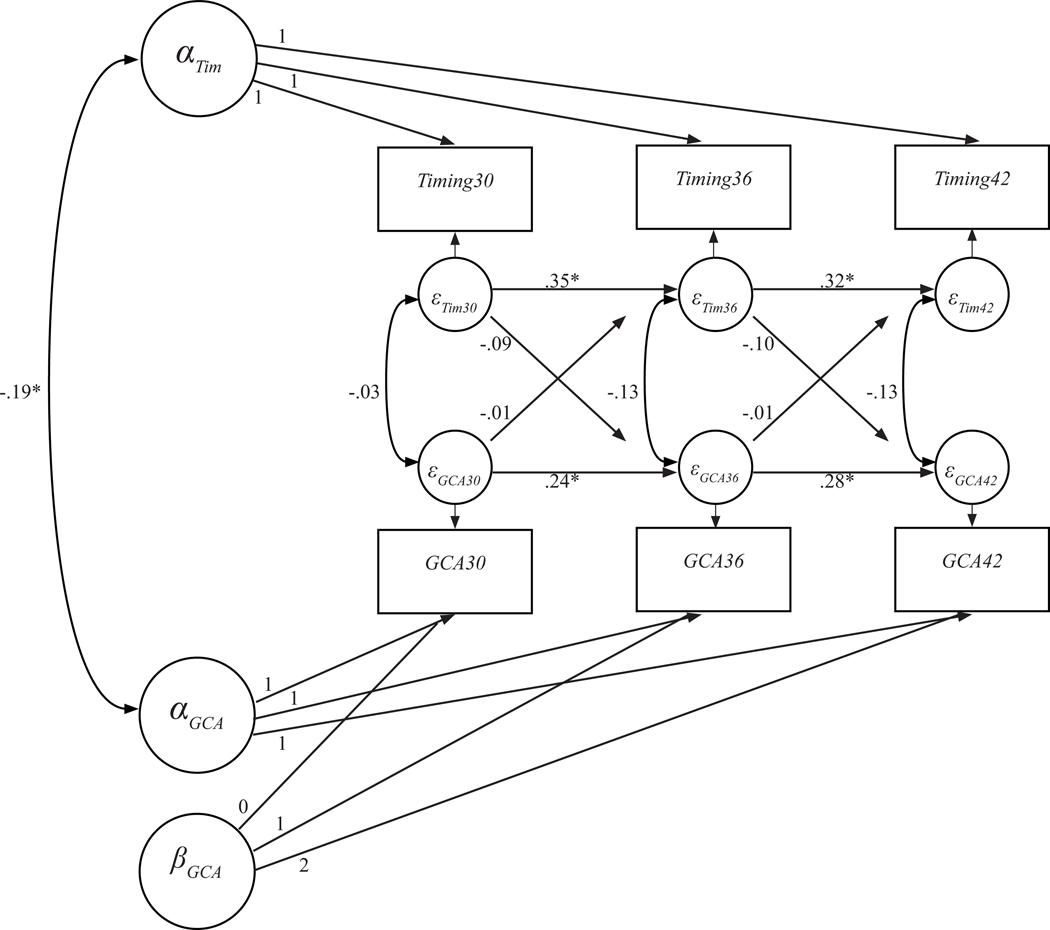
Once LCMs with structured residuals were fitted for each construct, these models were combined into a single model from which cross-construct associations and changes were examined. In this combined model, intercept values for both sleep timing and GCA were centered at the first time point (30 months) and allowed to vary randomly across children. Slope values in the model of GCA scores were linear and held constant across children (only an intercept value was included in the model of sleep timing, as described earlier). Latent intercept factors and the latent slope factor were allowed to covary. Time-specific, within-person residuals, which were modeled as structured residuals, were used to estimate covariance patterns and cross-lagged paths between the two constructs, as well as autoregressive paths within the construct. This combined model fit the data adequately: χ2(11) = 24.95, p = .009, CFI = .98, RMSEA = .05. Model results are presented in Table 3.
The final combined model, with standardized coefficients, is presented in Figure 1. This model, which disaggregates between- and within-person effects, suggests a pattern of between-person associations for sleep timing and cognitive abilities. This is evidenced by a significant covariance between the intercept factors for sleep timing and GCA (B = −.19, p = .047), which suggests that, during this 1-year window of development, children who had later sleep timing on average also had poorer cognitive abilities. However, this between-person effect size was relatively small. Additionally, as can be seen in the autoregressive paths of the structured residuals in Figure 1, there was continuity on both sleep timing and GCA scores across all three ages (B = .24 to .35, all p < .05), even after the latent intercepts and slopes were considered. Of note, effect sizes of the autoregressive effects were relatively small across both GCA and sleep timing, suggesting that a relatively small portion of the variance in each variable was accounted for by prior measurement occasion. Once the systematic, between-person association was accounted for, there was no evidence of any significant time-specific covariance or cross-lagged effects between either construct in either direction (B = −01 to −13, all p > .05). These results indicate that, once the between- and within-person effects were disaggregated, the association between sleep timing and cognitive abilities was a between-person association, not a within-person association. So, children with later bedtimes also had on average poorer cognitive abilities, but within-child fluctuations in the average level of bedtime lateness did not account for any within-child changes in cognitive abilities.
Figure 1.
Full ALT-SR model examining the association between sleep timing and cognitive abilities across three time points (30, 36, and 42 months), disentangling between- and within-person effects.
Note. ALT-SR = autoregressive latent trajectory model with structured residuals; Timing = sleep timing; GCA = general cognitive ability. *p ≤ .05.
Exploring the Between-Person Effect
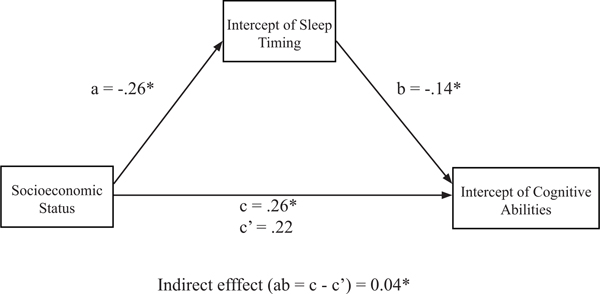
With a systematic, between-person association for late bedtimes and cognitive abilities evident, we sought to examine how SES might contribute to this association. Given the pattern of associations noted in the current study (i.e., the significant associations between SES and sleep timing, sleep timing and cognitive abilities, and SES and cognitive abilities), the preliminary conditions of statistical, cross-sectional mediation were satisfied. We examined whether the between-person variance associated with latent intercept values of the sleep timing variable mediated the association between-child SES and the between-person variance associated with latent intercept values of the cognitive abilities variable using regression analysis. Both of these latent intercept values were generated from the latent growth curve portion of the ALT-SR model described in the previous section. The standardized regression coefficients presented in Figure 2 indicate that lower SES was associated with later sleep timing (B = −.26, p < .001), and later sleep timing was associated with poorer cognitive abilities (B = −.14, p = .02).
Figure 2.
Standardized effects of socioeconomic status and sleep timing on cognitive abilities. Mediation includes intercept values for sleep timing and cognitive abilities variables as generated from the autoregressive latent trajectory model with structured residuals model.
*p ≤ .05.
There was also a direct effect between SES and cognitive abilities, such that lower SES was associated with poorer cognitive abilities (B = .22, p < .001). Based on the approach recommended by Hayes (2009, 2013) to test for statistical mediation, the indirect path between SES and cognitive abilities through the proposed mediator, sleep timing, was examined. The indirect path (ab) and 95% confidence intervals were estimated using a bootstrapping approach (with 5,000 iterations) and subsequent bias correction. The indirect effect from SES to cognitive abilities through sleep timing was significant (ab = .04, p = .05, 95% CI [.006, .068]), suggesting that the intercept values of sleep timing statistically mediated the association between SES and intercept values of cognitive abilities.
Discussion
The present study showed a longitudinal association between sleep difficulties and cognitive abilities in toddlerhood, and that this association was a between-person effect, not a within-person effect. Children who experience more sleep difficulties in the form of later bedtimes also have on average poorer cognitive abilities. However, within-child fluctuations in average levels of bedtime lateness did not account for any within-child changes in cognitive abilities. This suggests that, for the ways in which sleep and cognitive abilities were measured in the present study, there is no evidence that within-individual changes in late bedtimes across 6-month intervals account for changes in cognitive abilities, and our findings do not support a model in which changes in sleep affect subsequent cognitive abilities. Rather, the results support the interpretation that the same children who experience higher levels of sleep difficulties also show poorer cognitive abilities (although the size of the effect was small, consistent with associations typically noted between physiological and behavioral variables). A within-person association between sleep and cognitive abilities may exist, but the present study, with its large sample of toddlers measured across a year, at 6-month intervals, did not reveal such an association.
The fact that we did not find the within-person association we expected does not preclude the possibility that sleep difficulties in early childhood contribute substantially to poorer cognitive capacities. To examine this question, we tested whether sleep timing could explain some of the association between SES and cognitive abilities, and found that sleep timing significantly mediated the association between SES and cognitive abilities. Thus, sleep timing could explain some of the effect of SES on cognitive abilities. SES is a multifaceted variable, reflecting the influence of many processes. The findings of the present study help to identify one of the processes through which SES influences cognitive capacities in children and thus identify a potential target for efforts aimed at reducing adverse effects of lower SES on cognitive abilities. Promoting the importance of an early and developmentally appropriate sleep schedule in young children could help to reduce adverse effects on cognitive development associated with SES. However, future work will be necessary to evaluate the applicability of these findings. As there are known problems in assessing mediation cross-sectionally (Maxwell, Cole, & Mitchell, 2011), the results of this mediation model should be interpreted with some caution. The present study did not identify a longitudinal, within-person association between sleep timing and cognitive ability across this age, so a full longitudinal mediation model was unlikely to show an effect given that we also found no longitudinal association between sleep and cognitive abilities.
Although five major types of sleep difficulties were examined (duration, variability in the sleep schedule, activity during sleep, late timing of sleep, and total sleep minutes across 24 hr), only sleep timing, a composite index of the lateness of the child’s sleep schedule, consistently showed the expected associations with cognitive abilities across this developmental window, despite that a third of the correlations between the five sleep variables and the three indexes of cognitive abilities (verbal, nonverbal, and GCA) were significant and in the expected direction. Based on previous findings in the literature, namely that multiple domains of sleep difficulties are associated with cognitive abilities, we expected other dimensions of sleep, including duration, night-tonight variability, and activity (restlessness), to show more consistent effects. Although the effects were in the expected direction, they were not consistently significant across ages. In addition, the effect sizes of significant associations that we found between sleep difficulties and cognitive abilities were small in size (ranging from −.19 to −.10), suggesting that the associations observed in the present study were smaller than those that have been noted in previous studies. It is difficult to fully understand why previous findings did not replicate in our sample. We can consider several explanations of these null findings: First, it is possible that the young age of the sample contributed to the lack of significant findings. Both normative sleep patterns and cognitive abilities are changing rapidly during this time period, making it possible that assessments at any individual time point during this age range may not provide a stable picture of either construct. In addition, we made an effort to find consistency in the associations across the repeated measurement points, which is not possible in cross-sectional studies. Although some associations between sleep and cognitive abilities emerged at individual ages (Table 2), only associations that replicated across ages were considered further. This kept us from discussing other variables that we might have considered had we looked at each wave of data as if it were a separate, cross-sectional study. Finally, this is one of the first studies to examine the longitudinal association between objectively measured sleep and cognitive performance on a set of standardized tasks, so we did not capitalize on the kinds of shared method variance that may have inflated correlations in previous studies relying on subjective, parent-report measures. In short, our lack of full replication of previous findings could be due to our longitudinal rather than cross-sectional design, following younger children, and using more objective than subjective measures. In addition, when we regressed GCA scores on all of the sleep measures, sleep timing emerged as the only significant predictor of GCA scores at two of the three time points, lending support to the notion that sleep timing indexes an important influence on cognitive abilities even when controlling for other aspects of toddler sleep.
Little research has focused on sleep timing in young children. One exception, an epidemiological study, found that parent-reported late bedtimes at ages 3, 5, and 7 years were associated, both cross-sectionally and longitudinally, with lower cognitive scores on various standardized cognitive assessments at age 7 (Kelly et al., 2013). In research with adolescents, late sleep timing, also referred to as delayed sleep phase preference, is thought to be influenced by the adolescent’s circadian rhythm (so-called eveningness), their experienced sleep pressure, and the interference of other commitments and responsibilities. Such late bedtime preferences in adolescents have been associated with higher levels of emotional distress, poorer academic outcomes, and increased depressive symptoms (Asarnow, McGlinchey, & Harvey, 2014; Díaz-Morales & Escribano, 2013; Merikanto, Lahti, Puusniekka, & Partonen, 2013).
However, in toddlers, late bedtimes are less likely to represent a preference or choice of the child and are more likely determined by necessity or the preferences or choices of the child’s parents. We speculate that several potential factors may underlie a parent’s decision to put their child to bed at later times. First, it is possible that parental disorganization and a chaotic home environment in the evening may lead to later bedtimes. Similarly, it is also possible that the parents own lack of knowledge about appropriate bedtimes for toddlers and how to read toddlers’ signals of need and readiness for sleep leads them to put their children to bed late in the evening. Of course, the children’s own predisposition for noncompliance with the bedtime routine or emotional dysregulation may also cause their sleep to be delayed as the parents try to address this noncompliance or distress. Finally, it is also possible that parents’ own preference for later bedtimes (“eveningness”) may influence their decisions about when to put their children to bed. Although the factors underlying toddlers’ late sleep schedules may differ substantially from those of adolescents and adults, it is possible that some of the same mechanisms underlying the association between late bedtimes and cognitive abilities in adolescents and adults are also at work in children. For example, it is possible that toddlers’ late sleep timing leads to circadian rhythm disruptions that result in decreases in neural plasticity, which could lead to difficulties consolidating information (Tononi & Cirelli, 2014). Such difficulties in information consolidation could gradually accumulate over time, leading to meaningful cognitive deficits. Toddlerhood is regarded as a sensitive period for cognitive development (Bjorklund & Causey, 2017), so any information acquisition deficits could have an especially large impact. Although the present study does not provide evidence of a causal association between sleep difficulties and cognitive abilities, it is possible that such mechanisms may underlie the current findings of a between-persons association between these constructs. Additional processes, including the possibility that a genetic predisposition for a delayed sleep phase, could also confer risk for cognitive difficulties and contribute to the between-persons effect. Future research should seek to further clarify how late sleep schedules impact cognitive capacity in early childhood.
Strengths
The present study makes several important contributions. First, this is the first study, to our knowledge, to focus sharply on the association between sleep difficulties and cognitive abilities in toddlers. Second, although there is a large literature on the association between naturally occurring sleep difficulties and cognitive abilities in childhood (including meta-analytic evidence), there is a shortage of studies that use objective measurement coupled with an adequate longitudinal design to examine longitudinal developmental associations. The present study fills this gap in the literature by using objective measures of sleep and cognitive abilities, along with a three-wave longitudinal design. In addition, the longitudinal design allowed us to disentangle between- and within-person effects, while controlling for autoregressive pathways, using the ALT-SR model. Once the between- and within-person effects were examined separately, it was apparent that the association between sleep difficulties and cognitive abilities was a between-person effect, not a within-person effect, a finding that traditional modeling techniques would not have detected.
In addition, the present study provides a unique contribution to the field by examining how SES is associated with cognitive ability. Many studies have found that SES is associated with maladaptive outcomes, but they do not necessarily provide meaningful mechanistic information about the processes underlying these outcomes, because SES is likely to mark processes that or may not be present in every low SES family, such as chaotic households, higher exposure to stress, low levels of cognitive stimulation, maladaptive parenting practices, and, pertinent to the present study, inadequate sleep. The findings of present study add to the literature on potential mechanisms through which SES affects child development (Bradley & Corwyn, 2002; Linver, Brooks-Gunn, & Kohen, 2002) by suggesting that late bedtimes mediate the association between SES and cognitive abilities. This adds to the literature indicating that sleep problems may mediate the effect of SES on various health outcomes (Van Cauter & Spiegel, 1999).
Limitations
There are several limitations of the present study. First, given the protocol of the study, at each measurement point, cognitive abilities were assessed at the beginning of the 1- to 2-week window over which actigraphy data were collected. Because of this, it may be more difficult to examine the proximal effect of sleep on cognitive performance on the DAS. However, the focus of this study was not on the effect of a single night of sleep on cognitive performance but on a more naturalistic snapshot of the child’s sleep habits at each measurement point. For this reason, actigraphy indexes were averaged across a 1- to 2-week period, which presumably allows us to get a more realistic snapshot of the child’s typical sleep habits during that period of development. It is the influence of these typical sleep habits on cognitive development that we examined in the present study. In addition, there was no standardized time at which the DAS was administered across participants. Based on family preference, children could have been assessed at any time of the day, introducing the possibility that variations in alertness or sleep inertia could affect the child’s test performance. Sleep-deprived children have been shown to be more likely to have impaired neurobehavioral functioning when tested in the early morning as compared with afternoon (Sadeh, Gruber, & Raviv, 2002). Future studies should systematically evaluate the time of day when cognitive abilities are measured.
Next, the sample was predominantly middle class and White. If indeed sleep deficits are more likely to affect children from low SES backgrounds (as suggested by Buckhalt et al., 2009), our sample’s small percentage of lower SES families may have reduced our ability to detect these effects. Additionally, our index of SES was based only on parent education and occupation. This provides only a rough, summary measure of the stressors, resources, and attitudes that are embedded in SES. Future studies should consider including more detailed measures, such as income-to-needs ratio. Next, although the short, 6-month window between assessments has enabled us to do a fine-grained analysis of development across toddlerhood, it is possible that this window is too small to capture unfolding within-person effects of sleep problems on cognitive development. Studies focusing on the effect of sleep problems on cognitive development across a longer time span (e.g., several years) have sometimes shown associations with subsequent functioning (e.g., executive functioning in Bernier et al., 2013). In addition, it is possible that these effects are not present in toddlerhood. Nevertheless, given the rapid rate of cognitive development in toddlerhood, this seems a prime age during which an environmental variable, like sleep problems, might affect cognitive development.
Finally, the use of actigraphy data collection with very young children is known to result in measurement error due to the tendency for young children to be more active when they sleep (Meltzer, Montgomery-Downs, et al., 2012; Meltzer, Walsh, et al., 2012), thereby inflating estimates of sleep activity. This inflation could have skewed our sleep activity and sleep duration composite indexes, potentially accounting for some of the null findings with these composites. Although there are many benefits of collecting actigraphy data from young children (including its ease and its ability to capture natural sleep patterns outside of a lab setting), estimates of sleep using electroencephalography, although much more expensive than actigraphy, could reduce the activity-based measurement errors in sleep estimation.
Conclusions
The findings of the present study indicate the presence of a between-person, rather than a within-person, association between sleep difficulties and cognitive abilities. Without evidence of a within-person association, the present study does not directly support the interpretation that sleep difficulties play a causal role in cognitive deficits. Instead, toddlers who on average experience greater sleep difficulties in the form of later bedtimes also have, on average, poorer cognitive abilities. This association was only consistently present across measurement waves for a sleep variable indexing delayed sleep phase in the toddlers (late sleep timing) and not for any of the other sleep indexes examined (duration, activity, variability). Finally, the lateness of a child’s sleep schedule was found to mediate the association between-child SES and cognitive abilities, suggesting that delayed sleep phases may help explain how SES comes to be associated with child cognitive capacities. Sleep timing might be an important target not only for future developmental research but also for interventions aimed at improving child sleep and subsequent developmental outcomes.
Supplementary Material
Table S1. Number of Children Included at Each Age in Each Iteration of the Current Study
Table S2. Percent Missing at Each Age for Each Variable Included in Analysis
Acknowledgments
This research was supported by grants from the National Institutes of Mental Health (grant number MH099437) and the National Institute for Child Health and Human Development (grant number HD073202). Caroline Hoyniak is supported by a Graduate Research Fellowship from the National Science Foundation, grant number 1342962. We gratefully acknowledge the contribution of the research assistants, families, and children.
Footnotes
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher’s website:
Contributor Information
Caroline P. Hoyniak, Indiana University
John E. Bates, Indiana University
Angela D. Staples, Eastern Michigan University
Kathleen M. Rudasill, University of Nebraska—Lincoln
Dennis L. Molfese, University of Nebraska—Lincoln
Victoria J. Molfese, University of Nebraska—Lincoln
References
- Acebo C, Sadeh A, Seifer R, Tzischinsky O, Hafer A, & Carskadon MA (2005). Sleep/wake patterns derived from activity monitoring and maternal report for healthy 1-to 5-year-old children. Sleep, 28, 1568–1577. 10.1093/sleep/28.12.1568 [DOI] [PubMed] [Google Scholar]
- Asarnow LD, McGlinchey E, & Harvey AG (2014). The effects of bedtime and sleep duration on academic and emotional outcomes in a nationally representative sample of adolescents. Journal of Adolescent Health, 54, 350–356. 10.1016/j.jadohealth.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astill RG, Van der Heijden KB, Van IJzendoorn MH, & Van Someren EJ (2012). Sleep, cognition, and behavioral problems in school-age children: A century of research meta-analyzed. Psychological Bulletin, 138, 1109–1138. 10.1037/a0028204 [DOI] [PubMed] [Google Scholar]
- Bagley EJ, Kelly RJ, Buckhalt JA, & El-Sheikh M. (2015). What keeps low-SES children from sleeping well: The role of presleep worries and sleep environment. Sleep Medicine, 16, 496–502. 10.1016/j.sleep.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barazzetta M, & Ghislandi S. (2016). Family income and material deprivation: Do they matter for sleep quality and quantity in early life? Evidence from a longitudinal study. Sleep, 40: zsw066. 10.1093/sleep/zsw066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe DW (2011). Cognitive, behavioral, and functional consequences of inadequate sleep in children and adolescents. Pediatric Clinics of North America, 58, 649–665. 10.1016/j.pcl.2011.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger RH, Miller AL, Seifer R, Cares SR, & Lebourgeois MK (2012). Acute sleep restriction effects on emotion responses in 30- to 36-month-old children. Journal of Sleep Research, 21, 235–246. 10.1111/j.1365-2869.2011.00962.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier A, Beauchamp MH, Bouvette-Turcot AA, Carlson SM, & Carrier J. (2013). Sleep and cognition in preschool years: Specific links to executive functioning. Child Development, 84, 1542–1553. 10.1111/cdev.12063 [DOI] [PubMed] [Google Scholar]
- Berry D, & Willoughby MT (2017). On the practical interpretability of cross-lagged panel models: Rethinking a developmental workhorse. Child Development, 88, 1186–1206. 10.1111/cdev.12660 [DOI] [PubMed] [Google Scholar]
- Bjorklund DF, & Causey KB (2017). Children’s thinking: Cognitive development and individual differences. Thousand Oaks, CA: Sage. [Google Scholar]
- Bollen KA, & Curran PJ (2004). Autoregressive latent trajectory (ALT) models a synthesis of two traditions. Sociological Methods & Research, 32, 336–383. 10.1177/0049124103260222 [DOI] [Google Scholar]
- Bradley RH, & Corwyn RF (2002). Socioeconomic status and child development. Annual Review of Psychology, 53, 371–399. 10.1146/annurev.psych.53.100901.135233 [DOI] [PubMed] [Google Scholar]
- Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, & Posner S. (2005). Socioeconomic status in health research: One size does not fit all. Journal of the American Medical Association, 294, 2879–2888. 10.1001/jama.294.22.2879 [DOI] [PubMed] [Google Scholar]
- Brown ED, & Low CM (2008). Chaotic living conditions and sleep problems associated with children’s responses to academic challenge. Journal of Family Psychology, 22, 920–923. 10.1037/a0013652 [DOI] [PubMed] [Google Scholar]
- Bryant PA, Trinder J, & Curtis N. (2004). Sick and tired: Does sleep have a vital role in the immune system? Nature Reviews Immunology, 4, 457–467. 10.1038/nri1369 [DOI] [PubMed] [Google Scholar]
- Bub KL, Buckhalt JA, & El-Sheikh M. (2011). Children’s sleep and cognitive performance: A crossdomain analysis of change over time. Developmental Psychology, 47, 1504–1514. 10.1037/a0025535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckhalt JA (2011). Insufficient sleep and the socioeconomic status achievement gap. Child Development Perspectives, 5, 59–65. 10.1111/j.1750-8606.2010.00151.x [DOI] [Google Scholar]
- Buckhalt JA, El-Sheikh M, & Keller P. (2007). Children’s sleep and cognitive functioning: Race and socioeconomic status as moderators of effects. Child Development, 78, 213–231. 10.1111/j.1467-8624.2007.00993.x [DOI] [PubMed] [Google Scholar]
- Buckhalt JA, El-Sheikh M, Keller PS, & Kelly RJ (2009). Concurrent and longitudinal relations between children’s sleep and cognitive functioning: The moderating role of parent education. Child Development, 80, 875–892. 10.1111/j.1467-8624.2009.01303.x [DOI] [PubMed] [Google Scholar]
- Chen X, Beydoun MA, & Wang Y. (2008). Is sleep duration associated with childhood obesity? A systematic review and meta-analysis. Obesity, 16, 265–274. 10.1038/oby.2007.63 [DOI] [PubMed] [Google Scholar]
- Chung S, Wilson KE, Miller AL, Johnson D, Lumeng JC, & Chervin RD (2014). Home sleeping conditions and sleep quality in low-income preschool children. Sleep Medicine Research, 5, 29–32. 10.17241/smr.2014.5.1.29 [DOI] [Google Scholar]
- Curran PJ, Howard AL, Bainter SA, Lane ST, & McGinley JS (2014). The separation of between-person and within-person components of individual change over time: A latent curve model with structured residuals. Journal of Consulting and Clinical Psychology, 82, 879–894. 10.1037/a0035297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayyat EA, Spruyt K, Molfese DL, & Gozal D. (2011). Sleep estimates in children: Parental versus actigraphic assessments. Nature and Science of Sleep, 3, 115–123. 10.2147/NSS.S25676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald JF, Meijer AM, Oort FJ, Kerkhof GA, & Bogels SM (2010). The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: A meta-analytic review. Sleep Medicine Reviews, 14, 179–189. 10.1016/j.smrv.2009.10.004 [DOI] [PubMed] [Google Scholar]
- Dewald-Kaufmann JF, Oort F, & Meijer A. (2013). The effects of sleep extension on sleep and cognitive performance in adolescents with chronic sleep reduction: An experimental study. Sleep Medicine, 14, 510–517. 10.1016/j.sleep.2013.01.012 [DOI] [PubMed] [Google Scholar]
- Diamond A. (2002). Normal development of prefrontal cortex from birth to young adulthood: Cognitive functions, anatomy, and biochemistry In Stuss DT & Knight RT (Eds.), Principles of frontal lobe function (pp. 466–503). New York: Oxford University Press; 10.1093/acprof:oso/9780195134971.001.0001 [DOI] [Google Scholar]
- Díaz-Morales JF, & Escribano C. (2013). Predicting school achievement: The role of inductive reasoning, sleep length and morningness–eveningness. Personality and Individual Differences, 55, 106–111. 10.1016/j.paid.2013.02.011 [DOI] [Google Scholar]
- Diekelmann S, & Born J. (2010). The memory function of sleep. Nature Reviews Neuroscience, 11, 114–126. 10.1038/nrn2762 [DOI] [PubMed] [Google Scholar]
- Ednick M, Cohen AP, McPhail GL, Beebe D, Simaka-jornboon N, & Amin RS (2009). A review of the effects of sleep during the first year of life on cognitive, psychomotor, and temperament development. Sleep, 32, 1449–1458. 10.1093/sleep/32.11.1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott CD (1990). Differential Abilities Scale. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- El-Sheikh M, Bagley EJ, Keiley M, Elmore-Staton L, Chen E, & Buckhalt JA (2013). Economic adversity and children’s sleep problems: Multiple indicators and moderation of effects. Health Psychology, 32, 849–859. 10.1037/a0030413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, & Buckhalt JA (2015). II. Moving sleep and child development research forward: Priorities and recommendations from the SRCD-sponsored forum on sleep and child development. Monographs of the Society for Research in Child Development, 80(Serial No. 1), 15–32. 10.1111/mono.12142 [DOI] [PubMed] [Google Scholar]
- Fallone G, Acebo C, Seifer R, & Carskadon MA (2005). Experimental restriction of sleep opportunity in children: Effects on teacher ratings. Sleep, 28, 1561–1567. 10.1093/sleep/28.12.1561 [DOI] [PubMed] [Google Scholar]
- Friedman NP, Corley RP, Hewitt JK, & Wright KP Jr. (2009). Individual differences in childhood sleep problems predict later cognitive executive control. Sleep, 32, 323–333. 10.1093/sleep/32.3.323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galland BC, Taylor BJ, Elder DE, & Herbison P. (2012). Normal sleep patterns in infants and children: A systematic review of observational studies. Sleep Medicine Reviews, 16, 213–222. 10.1016/j.smrv.2011.06.001 [DOI] [PubMed] [Google Scholar]
- Gaylor EE, Burnham MM, Goodlin-Jones BL, & Anders TF (2005). A longitudinal follow-up study of young children’s sleep patterns using a developmental classification system. Behavioral Sleep Medicine, 3, 44–61. 10.1207/s15402010bsm0301_6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellis LA (2011). Children’s sleep in the context of socioeconomic status, race, and ethnicity In El-Sheikh M. (Ed.), Sleep and development: Familial and sociocultural considerations (pp. 219–244). Oxford, UK: Oxford University Press; 10.1093/acprof:oso/9780195395754.001.0001 [DOI] [Google Scholar]
- Goel N, Rao H, Durmer JS, & Dinges DF (2009). Neurocognitive consequences of sleep deprivation. Seminars in Neurology, 29, 320–339. 10.1055/s-0029-1237117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami U. (2011). The Wiley-Blackwell handbook of childhood cognitive development (Vol. 32, 2nd ed.). Oxford, UK: Blackwell. [Google Scholar]
- Gregory AM, Caspi A, Moffitt TE, & Poulton R. (2009). Sleep problems in childhood predict neuropsychological functioning in adolescence. Pediatrics, 123, 1171–1176. 10.1542/peds.2008-0825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory AM, & Sadeh A. (2012). Sleep, emotional and behavioral difficulties in children and adolescents. Sleep Medicine Reviews, 16, 129–136. 10.1016/j.smrv.2011.03.007 [DOI] [PubMed] [Google Scholar]
- Gruber R, Laviolette R, Deluca P, Monson E, Cornish K, & Carrier J. (2010). Short sleep duration is associated with poor performance on IQ measures in healthy school-age children. Sleep Medicine, 11, 289–294. 10.1016/j.sleep.2009.09.007 [DOI] [PubMed] [Google Scholar]
- Gruber R, Somerville G, Bergmame L, Fontil L, & Paquin S. (2016). School-based sleep education program improves sleep and academic performance of school-age children. Sleep Medicine, 21, 93–100. 10.1016/j.sleep.2016.01.012 [DOI] [PubMed] [Google Scholar]
- Gruber R, Somerville G, Enros P, Paquin S, Kestler M, & Gillies-Poitras E. (2014). Sleep efficiency (but not sleep duration) of healthy school-age children is associated with grades in math and languages. Sleep Medicine, 15, 1517–1525. 10.1016/j.sleep.2014.08.009 [DOI] [PubMed] [Google Scholar]
- Gruber R, Wiebe S, Montecalvo L, Brunetti B, Amsel R, & Carrier J. (2011). Impact of sleep restriction on neurobehavioral functioning of children with attention deficit hyperactivity disorder. Sleep, 34, 315–323. 10.1093/sleep/34.3.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2009). Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication Monographs, 76, 408–420. 10.1080/03637750903310360 [DOI] [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY: Guilford. [Google Scholar]
- Hollingshead AB (1975). Four factor index of social status. Unpublished work. [Google Scholar]
- Jones CH, & Ball H. (2014). Exploring socioeconomic differences in bedtime behaviours and sleep duration in English preschool children. Infant and Child Development, 23, 518–531. 10.1002/icd.1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K, & Harrison Y. (2001). Frontal lobe function, sleep loss and fragmented sleep. Sleep Medicine Reviews, 5, 463–475. 10.1053/smrv.2001.0203 [DOI] [PubMed] [Google Scholar]
- Kelly Y, Kelly J, & Sacker A. (2013). Time for bed: Associations with cognitive performance in 7-year-old children: A longitudinal population-based study. Journal of Epidemiology and Community Health, 67, 926–931. 10.1136/jech-2012-202024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Yesavage JA, Taylor JL, & Kupfer D. (2000). How can we learn about developmental processes from cross-sectional studies, or can we? American Journal of Psychiatry, 157, 163–171. 10.1176/appi.ajp.157.2.163 [DOI] [PubMed] [Google Scholar]
- Lim J, & Dinges DF (2010). A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychological Bulletin, 136, 375–389. 10.1037/a0018883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linver MR, Brooks-Gunn J, & Kohen DE (2002). Family processes as pathways from income to young children’s development. Developmental Psychology, 38, 719–734. 10.1037/0012-1649.38.5.719 [DOI] [PubMed] [Google Scholar]
- Maxwell SE, Cole DA, & Mitchell MA (2011). Bias in cross-sectional analyses of longitudinal mediation: Partial and complete mediation under an autoregressive model. Multivariate Behavioral Research, 46, 816–841. 10.1080/00273171.2011.606716 [DOI] [PubMed] [Google Scholar]
- Meltzer LJ, Montgomery-Downs HE, Insana SP, & Walsh CM (2012). Use of actigraphy for assessment in pediatric sleep research. Sleep Medicine Reviews, 16, 463–475. 10.1016/j.smrv.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer LJ, Walsh CM, Traylor J, & Westin A. (2012). Direct comparison of two new actigraphs and polysomnography in children and adolescents. Sleep, 35, 159–166. 10.5665/sleep.1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikanto I, Lahti T, Puusniekka R, & Partonen T. (2013). Late bedtimes weaken school performance and predispose adolescents to health hazards. Sleep Medicine, 14, 1105–1111. 10.1016/j.sleep.2013.06.009 [DOI] [PubMed] [Google Scholar]
- Mindell JA, Owens J, Alves R, Bruni O, Goh DY, Hiscock H, … Sadeh A (2011). Give children and adolescents the gift of a good night’s sleep: A call to action. Sleep Medicine, 12, 203–204. 10.1016/j.sleep.2011.01.003 [DOI] [PubMed] [Google Scholar]
- Muzur A, Pace-Schott EF, & Hobson JA (2002). The prefrontal cortex in sleep. Trends in Cognitive Sciences, 6, 475–481. 10.1016/S1364-6613(02)019927 [DOI] [PubMed] [Google Scholar]
- Montgomery-Downs HE, O’Brien LM, Holbrook CR, & Gozal D. (2004). Snoring and sleep-disordered breathing in young children: subjective and objective correlates. Sleep, 27, 87–94. doi: 10.1093/sleep/27.1.87 [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (1998–2011). Mplus user’s guide. Sixth Edition Los Angeles, CA: Author. [Google Scholar]
- Owens J. (2007). Classification and epidemiology of childhood sleep disorders. Sleep Medicine Clinics, 2, 353–361. 10.1016/j.jsmc.2007.05.009 [DOI] [Google Scholar]
- Owens JA, Spirito A, & McGuinn M. (2000). The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep, 23, 1043–1052. [PubMed] [Google Scholar]
- Paavonen EJ, R€aikkonen K, Pesonen A-K, Lahti J,€ Komsi N, Heinonen K, … Porkka-Heiskanen T (2010). Sleep quality and cognitive performance in 8-yearold children. Sleep Medicine, 11, 386–392. 10.1016/j.sleep.2009.09.009 [DOI] [PubMed] [Google Scholar]
- Patrick KE, Millet G, & Mindell JA (2016). Sleep differences by race in preschool children: The roles of parenting behaviors and socioeconomic status. Behavioral Sleep Medicine, 14, 467–479. 10.1080/15402002.2015.1017101 [DOI] [PubMed] [Google Scholar]
- Peters JD, Biggs SN, Bauer KM, Lushington K, Kennedy D, Martin J, & Dorrian J. (2009). The sensitivity of a PDA-based psychomotor vigilance task to sleep restriction in 10-year-old girls. Journal of Sleep Research, 18, 173–177. 10.1111/j.13652869.2008.00716.x [DOI] [PubMed] [Google Scholar]
- Quach J, Hiscock H, Canterford L, & Wake M. (2009). Outcomes of child sleep problems over the school-transition period: Australian population longitudinal study. Pediatrics, 123, 1287–1292. 10.1542/peds.2008-1860 [DOI] [PubMed] [Google Scholar]
- Randazzo AC, Muehlbach MJ, Schweitzer PK, & Walsh JK (1998). Cognitive function following acute sleep restriction in children ages 10–14. Sleep, 21, 861–868. 10.1093/sleep/21.8.861 [DOI] [PubMed] [Google Scholar]
- Rushton JP, Brainerd CJ, & Pressley M. (1983). Behavioral development and construct validity: The principle of aggregation. Psychological Bulletin, 94, 18–38. 10.1037/0033-2909.94.1.18 [DOI] [Google Scholar]
- Sadeh A. (2007). Consequences of sleep loss or sleep disruption in children. Sleep Medicine Clinics, 2, 513–520. 10.1016/j.jsmc.2007.05.012 [DOI] [Google Scholar]
- Sadeh A, Gruber R, & Raviv A. (2002). Sleep, neurobehavioral functioning, and behavior problems in school-age children. Child Development, 73, 405–417. 10.1111/1467-8624.00414 [DOI] [PubMed] [Google Scholar]
- Sadeh A, Gruber R, & Raviv A. (2003). The effects of sleep restriction and extension on school-age children: What a difference an hour makes. Child Development, 74, 444–455. 10.1111/1467-8624.7402008 [DOI] [PubMed] [Google Scholar]
- Sadeh A, Sharkey KM, & Carskadon MA (1994). Activity-based sleep-wake identification: An empirical test of methodological issues. Sleep, 17, 201–207. 10.1093/sleep/17.3.201 [DOI] [PubMed] [Google Scholar]
- Schafer JL, & Graham JW (2002). Missing data: Our view of the state of the art. Psychological Methods, 7, 147–177. 10.1037//1082-989X.7.2.147 [DOI] [PubMed] [Google Scholar]
- Seegers V, Touchette E, Dionne G, Petit D, Seguin JR, Montplaisir J, … Tremblay RE (2016). Short persistent sleep duration is associated with poor receptive vocabulary performance in middle childhood. Journal of Sleep Research, 25, 325–332. 10.1111/jsr.12375 [DOI] [PubMed] [Google Scholar]
- Short MA, & Banks S. (2014). The functional impact of sleep deprivation, sleep restriction, and sleep fragmentation In Bianchi MT (Ed.), Sleep deprivation and disease (pp. 13–26). New York, NY: Springer; 10.1007/978-1-4614-9087-6 [DOI] [Google Scholar]
- Tononi G, & Cirelli C. (2014). Sleep and the price of plasticity: From synaptic and cellular homeostasis to memory consolidation and integration. Neuron, 81, 12–34. 10.1016/j.neuron.2013.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touchette E, Petit D, S eguin JR, Boivin M, Tremblay RE, & Montplaisir JY (2007). Associations between sleep duration patterns and behavioral/cognitive functioning at school entry. Sleep, 30, 1213–1219. 10.1093/sleep/30.9.1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauter E, & Spiegel K. (1999). Sleep as a mediator of the relationship between socioeconomic status and health: A hypothesis. Annals of the New York Academy of Sciences, 896, 254–261. 10.1111/j.17496632.1999.tb08120.x [DOI] [PubMed] [Google Scholar]
- Van Tassel EB (1985). The relative influence of child and environmental characteristics on sleep disturbances in the first and second years of life. Journal of Developmental & Behavioral Pediatrics, 6, 81–86. 10.1097/00004703-198504000-00006 [DOI] [PubMed] [Google Scholar]
- Vaughn BE, Elmore-Staton L, Shin N, & El-Sheikh M. (2015). Sleep as a support for social competence, peer relations, and cognitive functioning in preschool children. Behavioral Sleep Medicine, 13, 92–106. 10.1080/15402002.2013.845778 [DOI] [PubMed] [Google Scholar]
- Vriend JL, Davidson FD, Corkum PV, Rusak B, Chambers CT, & McLaughlin EN (2013). Manipulating sleep duration alters emotional functioning and cognitive performance in children. Journal of Pediatric Psychology, 38, 1058–1069. 10.1093/jpepsy/jst033 [DOI] [PubMed] [Google Scholar]
- Woodcock RW, McGrew K, & Mather N. (2001). Woodcock Johnson Tests of Cognitive Ability III Rolling Variable Included in Analysis Meadows, IL: Riverside. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Number of Children Included at Each Age in Each Iteration of the Current Study
Table S2. Percent Missing at Each Age for Each Variable Included in Analysis