Invasive fungal infections pose a serious risk to public health on a global scale and cause almost 1.5 million deaths worldwide annually.1 Immunosuppressed patients are particularly at risk of developing fungal diseases such as candidemia, i.e., the presence of Candida species in the bloodstream. The mortality rates of hospital infections from C. albicans, a leading fungal pathogen, exceeds 40%. The introduction of echinocandin antifungal agents in clinics halted this trend, but growing incidences of the pathogen’s acquired drug resistance has threatened to undo this progress. In this issue of ACS Central Science, Fridman and co-workers shed light on the mechanism of action of this important class of antifungal agents by using fluorescently labeled caspofungin as a probe.2 In 2001, caspofungin acetate was the first within the class of echinocandin drugs to obtain United States Food and Drug Adminstration approval for intravenous fungal therapy.3 It interferes with fungal cell wall biosynthesis via noncompetitive inhibition of β(1,3)-d-glucan synthase, a membrane-bound protein complex, specifically at the Fks1p extracellular subdomain. Yet, it remains unclear how some Candida cells develop acquired resistance to echinocandin drugs. Given that they are becoming the go-to drugs for antifungal treatments, it is timely to compare and elucidate their mechanism as well as the dynamics of cell entry into both echinocandin-susceptible and -resistant strains.
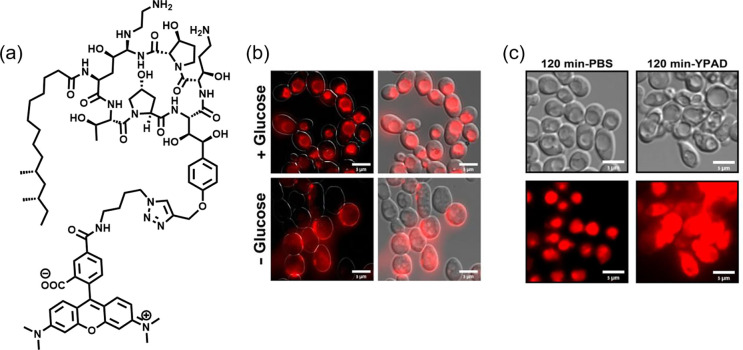
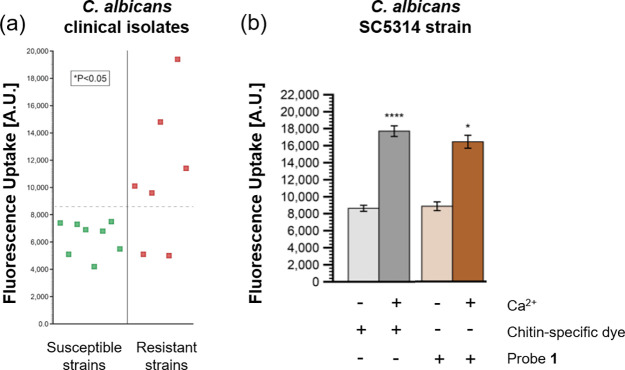
In this work, the authors presented an efficient way to assess echinocandin resistance in C. albicans and C. glabrata by examining uptake and subcellular localization of TMR-labeled caspofungin probe 1 (Figure 1a). Previously, the Fridman group also employed fluorescent-tagged azole drug analogues, another antifungal first-line therapeutic, in Candida to elucidate drug uptake and target site accumulation.4 In this paper, their findings showed that probe 1 was taken up into fungi cells within minutes via endocytosis and localized within the vacuole (Figure 1b). When incubated in nutrient-rich growth media, these treated cells experienced plasma membrane permeabilization, thus resulting in cell death during mitosis. In contrast, the absence of nutrients rendered the cells quiescent and viable even as probe 1 resided in the vacuole (Figure 1c). The report suggested that echinocandins were efficiently transported into Candida cells and only acted on metabolically active and dividing cells, but not on dormant ones. The authors also found a positive correlation between probe uptake and echinocandin resistance when probe 1 was screened against a panel of genetically mutated resistant strains (FKS genes) and clinically relevant strains (Figure 2a). This finding may offer an efficient way of evaluating resistance among Candida species and predicting the efficacy of the antifungal treatment.
Figure 1.
(a) TMR-labeled caspofungin probe 1. (b) Microscopy images of C. albicans cells treated with probe 1 (red) in the presence/absence of glucose, indicative of endocytosis. (c) DIC and fluorescent images of C. albicans remaining viable in PBS (left) but displaying fungal cell death characteristics when treated with probe 1 in YPAD media (right) after 120 min. Reproduced with permission from ref (2). Copyright 2020 American Chemical Society.
Figure 2.
(a) Probe 1 uptake associated with caspofungin resistance in susceptible and resistant C. albicans clinical isolates. (b) Susceptible C. albicans SC5314 strain treated with or without calcium, chitin-specific dye, and probe 1. Reproduced with permission from ref (2). Copyright 2020 American Chemical Society.
While this study established the relationship between increased fluorescent probe localization in vacuoles with echinocandin resistance, it utilized a caspofungin probe with a bulky fluorophore. This could have altered uptake characteristics and subsequent localization even though the authors took steps to ensure that the labeled probe would retain the same spectrum of antifungal activity. This potential alteration of uptake characteristics is evidenced by the fact that functionalization using NBD and BODIPY dyes (2 and CSF-BOD, respectively) increased MIC values, especially in susceptible strains. A viable alternative could be to exploit biorthogonal strategies such as click chemistry to perform post-entry labeling of functionalized caspofungin (e.g., 1a containing alkyne group) with a companion fluorogenic probe that contains an appropriate click handle (e.g., azido group). The modification of caspofungin with a less sterically demanding biorthogonal group can ensure closer modeling of uptake, transport, and localization profiles. A turn-on fluorophore will also reduce adventitious background noise and ensure a good fluorescence response. Additionally, the work can be extended to other fungi microbes’ models to verify that the capsofungin probe uptake is a sufficiently robust technique to measure susceptibility.
Another important contribution of this work is the development of a facile method to identify and classify echinocandin-resistant and susceptible strains. Current assays to determine resistance in pathogenic fungi rely on the chitin-specific dye Calcofluor White (CFW) to determine chitin levels as a marker for resistance.5 The fluorescence-based strategy employing probe 1 to quantify caspofungin accumulation may offer a more sensitive and rapid approach to evaluate resistance in the studied strains. As a proof of concept, susceptible C. albicans strains treated with calcium had an elevated resistance response through higher chitin output and resulting CFW levels, which is correlated with the increased fluorescence levels arising from accumulation of probe 1 (Figure 2b). In resistant strains with low chitin levels, such as C. glabrata, this distinction was even more pronounced since the CFW assay could not be adequately used.6 The fluorescence-based assay would fill this gap as a reliable indicator for resistance, especially in chitin-lacking strains. Taken together, a fluorescence-based approach by comparing accumulation of probe 1 in isolates provides a rapid method of identifying resistant strains when making therapeutic decisions, particularly in clinical settings where there is time pressure.
Overall, this strategy of utilizing a fluorescently labeled caspofungin probe has the potential to unlock a new paradigm for an expanded investigation of echinocandin resistance in pathogenic fungi. This fluorescent reporter approach paves the way for further studies of mode of actions in other antifungal drugs as well as their potential applications for clinical diagnosis of resistant strains and subsequent treatment regimens.
The authors declare no competing financial interest.
References
- Brown G. D.; Denning D. W.; Gow N. A. R.; Levitz S. M.; Netea M. G.; White T. C. Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 2012, 4 (165), 165rv13. 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- Jaber Q. Z.; Bibi M.; Ksiezopolska E.; Gabaldon T.; Berman J.; Fridman M., Elevated Vacuolar Uptake of Fluorescently Labeled Antifungal Drug Caspofungin Predicts Echinocandin Resistance in Pathogenic Yeast. ACS Cent. Sci. 2020. 10.1021/acscentsci.0c00813. [DOI] [PMC free article] [PubMed]
- Balkovec J. M.; Hughes D. L.; Masurekar P. S.; Sable C. A.; Schwartz R. E.; Singh S. B. Discovery and development of first in class antifungal caspofungin (CANCIDAS®)—A case study. Nat. Prod. Rep. 2014, 31 (1), 15–34. 10.1039/C3NP70070D. [DOI] [PubMed] [Google Scholar]
- Benhamou R. I.; Bibi M.; Steinbuch K. B.; Engel H.; Levin M.; Roichman Y.; Berman J.; Fridman M. Real-Time Imaging of the Azole Class of Antifungal Drugs in Live Candida Cells. ACS Chem. Biol. 2017, 12 (7), 1769–1777. 10.1021/acschembio.7b00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L. A.; Munro C. A.; de Bruijn I.; Lenardon M. D.; McKinnon A.; Gow N. A. R. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog. 2008, 4 (4), e1000040–e1000040. 10.1371/journal.ppat.1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot P. W. J.; Kraneveld E. A.; Yin Q. Y.; Dekker H. L.; Groß U.; Crielaard W.; de Koster C. G.; Bader O.; Klis F. M.; Weig M. The Cell Wall of the Human Pathogen Candida glabrata: Differential Incorporation of Novel Adhesin-Like Wall Proteins. Eukaryotic Cell 2008, 7 (11), 1951. 10.1128/EC.00284-08. [DOI] [PMC free article] [PubMed] [Google Scholar]