Abstract
Carbon dioxide (CO2) hydrogenation to liquid fuels including gasoline, jet fuel, diesel, methanol, ethanol, and other higher alcohols via heterogeneous catalysis, using renewable energy, not only effectively alleviates environmental problems caused by massive CO2 emissions, but also reduces our excessive dependence on fossil fuels. In this Outlook, we review the latest development in the design of novel and very promising heterogeneous catalysts for direct CO2 hydrogenation to methanol, liquid hydrocarbons, and higher alcohols. Compared with methanol production, the synthesis of products with two or more carbons (C2+) faces greater challenges. Highly efficient synthesis of C2+ products from CO2 hydrogenation can be achieved by a reaction coupling strategy that first converts CO2 to carbon monoxide or methanol and then conducts a C–C coupling reaction over a bifunctional/multifunctional catalyst. Apart from the catalytic performance, unique catalyst design ideas, and structure–performance relationship, we also discuss current challenges in catalyst development and perspectives for industrial applications.
Short abstract
This Outlook highlights recent developments in the design of novel and very promising heterogeneous catalysts for direct CO2 hydrogenation to methanol, liquid hydrocarbons, and higher alcohols.
Introduction
The catalytic conversion of captured carbon dioxide (CO2) to liquid fuels can ameliorate global climate change and reduce our excessive dependence on fossil fuels. Since carbon in CO2 is in its highest oxidation state, its reduction and transformation need a large energy input. As a high-energy material, hydrogen is generally used for the large-scale catalytic transformation of CO2 to value-added products.1,2 At present, the hydrogen source mainly comes from syngas, petrochemical plants, methane steam reforming, chlorine alkali plants, and coke oven gas.3 Renewable energies such as solar, wind, geothermal, and hydro can be used along with CO2 and water to produce green liquid fuels. In 2017, Shih et al. proposed the “liquid sunshine” concept that employs solar energy to produce stable, easily transportable, energy dense liquid products.4 In Europe, power-to-liquid (PTL) fuels obtained from surplus renewable electricity via water electrolysis-derived H2 and CO2 have received much attention, and a number of PTL-related studies were recently reported.5−9
Liquid hydrocarbon (C5+) fuels including gasoline (C5–11), jet fuel (C8–16), and diesel (C10–20) play an instrumental role in the global energy supply chain and are wildly used as transportation fuels around the world. Besides, alcohols such as methanol, ethanol, and other higher alcohols (C2+OH) are clean and multipurpose fuels.4,10,11 In both academic research and industrial practices, great progress has been made in the synthesis of C1 molecules such as methanol (CH3OH) from CO2 hydrogenation. Currently, the largest plant, Carbon Recycling International (CRI)’s CO2-to-renewable-methanol plant in Iceland, is capable of producing 4000 t/year of methanol by converting about 5500 t/year of CO2.12 However, direct reduction of CO2 to C2+ products is still a grand challenge due to the lack of efficient catalysts with high stability, as the activity of C–C coupling is low and the formation of byproduct water can easily deactivate the various catalysts for CO2 conversion.2,13−15 Syngas (CO/H2) and CH3OH are the most important C1 platform molecules, and their conversions to value-added products via the Fischer–Tropsch synthesis (FTS) and methanol to hydrocarbons (MTH) processes, respectively, were extensively applied in industry.16−19 Therefore, combining the reverse water–gas shift (RWGS) with FTS and combining high-temperature methanol synthesis with MTH over bifunctional/multifunctional catalysts are two efficient strategies for direct CO2 hydrogenation to C2+ hydrocarbons including liquid hydrocarbons. Moreover, the synthesis of C2+ alcohols is even more challenging than C2+ hydrocarbons either in CO2 or CO hydrogenation, which requires more precise control of the C–C coupling.20−22
In this Outlook, we will discuss recent developments in designing heterogeneous catalysts for direct CO2 hydrogenation to liquid fuels including methanol, liquid hydrocarbons, and higher alcohols, focusing on new structures, smart design, and exciting performance of novel materials. One efficient route of C5+ hydrocarbons synthesis is based on the methanol intermediate. Advances in methanol synthesis will strongly promote the development of this process and the understanding of related reaction mechanisms. Therefore, we mainly introduce the work of highly selective conversion of CO2 to CH3OH, as there have been many excellent recent reviews on methanol synthesis via heterogeneous catalysis.12,23−27
CO2 Hydrogenation to Methanol
During CO2 hydrogenation, the formation of the undesired CO through RWGS is a competitive reaction to methanol synthesis. Decreasing the reaction temperature favors the exothermic methanol synthesis reaction, and raising the space velocity decreases the CO selectivity because of the lower RWGS reaction rate.28 However, the low temperature and/or high space velocity usually result in low single pass CO2 conversion. Thus, it remains a great challenge to simultaneously obtain high CO2 conversion and high methanol selectivity. Very recently, significant progress has been made in developing more efficient catalysts for CO2 hydrogenation to CH3OH, including metal-supported catalysts, bimetallic systems, and reducible metal oxides.
Industrial methanol production from CO2-containing syngas uses the well-known Cu-ZnO-Al2O3 catalysts. Currently, CH3OH synthesis from catalytic CO2 hydrogenation has been implemented at the pilot-plant level by Lurgi, Mitsui, CRI, among others.12,29 These processes mainly used modified Cu-ZnO-Al2O3 catalysts and were carried out under conditions similar to syngas-based methanol synthesis. Therefore, supported copper materials have attracted much attention and have been extensively investigated for CO2 hydrogenation to CH3OH. The methanol synthesis reaction is known to show strong support effects. Using a suitable support material can enhance CH3OH selectivity, attributed to structural, electronic, and chemical promotional effects. For example, CH3OH selectivity is usually high (>70%) at 200–260 °C when ZrO2 is used as the support.30−32 Recently, several works have clarified the origin of the promotional effect of the ZrO2 support that is unknown before. Larmier et al. confirmed that the Cu/ZrO2 interface can promote the conversion of the formate intermediates to methanol by combined experimental and computational investigations.30 Tada et al. found that the interfacial sites on Cu/a-ZrO2 (a-: amorphous) are favored for methanol production compared to those on tetragonal and monoclinic ZrO2 supported Cu.33 Additionally, the oxygen vacancies on tetragonal ZrO2 were proposed to play a crucial role in enhancing the activity of methanol formation by stabilizing the Cu+ active sites adjacent to them.32 Lam et al. suggested that the surface Lewis acid Zr(IV) sites with Cu particles in the vicinity are responsible for improving CH3OH activity and selectivity on the Cu/ZrO2-based catalysts.31 Apart from conventional metal oxides, researchers recently also explored other supports such as TiO2 nanotubes34 and Mg–Al layered double hydroxide (LDH)35 to promote methanol formation.
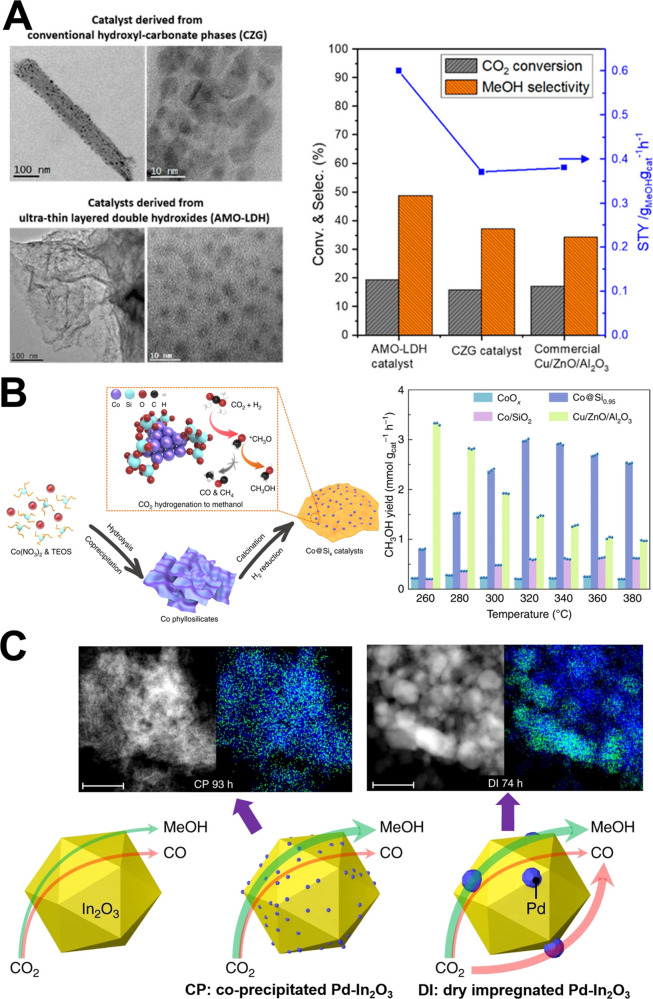
To simultaneously improve the intrinsic activity and catalyst stability, researchers directly used Cu–Zn-based LDH as the precursor to synthesize an efficient methanol synthesis catalyst with a confined structure, in which the active metallic Cu phase is highly dispersed and partially embedded in the remaining oxide matrix.36−39 Li et al. prepared ultrathin Cu–Zn–Ga LDH nanosheets by the aqueous miscible organic solvent treatment method and further increased Cu surface areas and dispersion of the resulting Cu-based catalysts (Figure 1A).37 The CH3OH space time yield (STY) is 0.59 gMeOH gcat–1 h–1 with selectivity of ∼49% at CO2 conversion of ∼20%. On the other hand, scientists also tried to use silica and metal–organic frameworks to confine small Cu nanoparticles and developed highly efficient Cu-based catalysts for CO2 hydrogenation.40−44 For example, Liu et al. synthesized three-dimensional porous Cu@ZrO2 framework catalysts using the Cu@UiO-66 precursor, which displays CH3OH STY of 0.796 gMeOH gcat–1 h–1 and a long-term stability for 105 h with a selectivity of 78.8% and conversion of 13.1% at 260 °C, 4.5 MPa, 21 600 mL g–1 h–1 and H2/CO2 = 3.43
Figure 1.
(A) TEM images of Cu–Zn–Ga catalysts derived from conventional hydroxyl-carbonate phases and ultrathin LDH (left) as well as their performance (right).37 Reaction conditions: 270 °C, 4.5 MPa, 18 000 mL g–1 h–1, and H2/CO2 = 3. (B) Synthesis and catalysis strategies (left) as well as CO2 hydrogenation performance (right) of Co@Six catalysts.48 Reaction conditions: 260–380 °C, 2.0 MPa, 6000 mL g–1 h–1 and H2/CO2 = 3. (C) HAADF-STEM images and corresponding EDX maps (top) of indium (blue) and palladium (green) for the spent coprecipitated (CP, left) and dry impregnated (DI, right) Pd–In2O3 catalysts with 0.75 wt % Pd as well as illustration for the distinct role of Pd in equilibrated CP and DI catalysts with pure In2O3 as a reference.84 The thickness of the arrows qualitatively suggests the methanol and CO formation rates. Reprinted with permission from refs (37), (48), and (84). Copyright 2018 American Chemical Society and Copyright 2019 and 2020 Springer-Nature.
Recently, several groups have developed novel cobalt (Co)-based catalytic systems that display a promising performance for methanol synthesis from CO2 hydrogenation.45−48 Co catalysts are widely used and extensively studied for FTS and can easily catalyze the CO2 methanation reaction.2,17,46,49−52 The high performance for CH3OH synthesis over Co-based catalysts was attributed to the formation of a new active phase, rather than the conventional metallic Co phase. Li et al. reported MnOx nanoparticles supported on a mesoporous Co3O4 for methanol production at low pressure (0.1–0.6 MPa).45 They showed the importance of the CoO phase and revealed the strong interaction between MnOx nanoparticles and Co@CoO core–shell grains, which enhances the performance of CO2 hydrogenation to CH3OH. Lian et al. ascribed the increased CH3OH selectivity to the oxygen defects on the surface of Co@Co3O4 core–shell active species supported on the nitrogen-doped carbon material, which is derived from zeolitic imidazolate framework precursors.47 Wang et al. also inferred that the cobalt oxide phase on silica supported Co catalyst with Co–O–SiOn linkages via Co phyllosilicates could suppress the CO and CH4 formation and promote methanol production (Figure 1B).48 This novel Co catalyst shows CH3OH STY of 0.096 gMeOH gcat–1 h–1 (3.0 mmol gcat–1 h–1) with a selectivity of 70.5% at 8.6% conversion at 320 °C. Because the H2 splitting ability of cobalt oxide is lower than the metallic Co phase, the activities of these Co-based methanol synthesis catalysts are to be further enhanced. Moreover, the mechanism of this new active site inhibiting the CO2 methanation and RWGS reactions needs to be further clarified.
Various bimetallic materials including Pd–Cu,53−55 Pd–Ga,56,57 Pd–In,58−60 Pd–Zn,61 Ni–Ga,62−66 In–Rh,67 In–Cu,68,69 In–Co,70 In–Ni,71,72 and Ni–Cu55,73 have also been examined for methanol production from CO2 hydrogenation. Among these catalysts, some non-noble metal-based bimetallics were designed for efficient CO2 hydrogenation to CH3OH at low pressure. Compared with the benchmark Cu-ZnO-Al2O3, Ni5Ga3 intermetallic compounds exhibited a higher CH3OH synthesis activity and a much lower CO selectivity at ambient pressure.62 The Ga-rich and Ni-rich sites are responsible for methanol synthesis and RWGS, respectively, and poisoning of the Ni sites by adsorbed CO suppress the RWGS reaction. Gallo et al. further suggested that the formation of an amorphous Ga2O3 shell over the metallic Ni5Ga3 during the catalysis after low temperature reduction promoted CO2 activation.63 The Ni3.5In5.3Al/SiO2 catalyst with 15% metal loading via a phyllosilicate precursor also shows a higher conversion and a better stability, while yielding lower CH3OH selectivity than the conventional Cu-ZnO-Al2O3 catalyst at ambient pressure.72 The optimized metal composition and well-dispersed metal particles can be achieved by partial decomposition of the phyllosilicate, which enhances the activity for CO2 hydrogenation. For the GaPd2/SiO2 system, a much higher intrinsic activity and CH3OH selectivity than Cu-ZnO-Al2O3 have been observed at above 200 °C and atmospheric pressure.57 However, under high reaction pressure (above 3.0 MPa), the performance advantage of the bimetallic catalyst is not obvious, even lower than conventional Cu-based catalysts.
In recent years, reducible oxides have received considerable attention due to their excellent performance with high CH3OH selectivity in a wide range of temperatures (200–320 °C). Nearly 100% selectivity can be attained over cubic In2O3 nanomaterial and In2O3 supported on monoclinic ZrO2 at CO2 conversions of less 5.5% under 300 °C, 5.0 MPa, 20 000 mL g–1 h–1 and H2/CO2 = 4.74 The surface oxygen vacancies surrounded by indium atoms are considered as the active sites for CO2 activation and hydrogen splitting, and methanol formation from CO2 hydrogenation follows the cycle between generation and annihilation of vacancies.75,76 Researchers have clearly demonstrated the structure sensitivity of the In2O3 catalyst in terms of both the phase and the exposed facet by combined computational and experimental studies.77,78 Dang et al. reported a successful work of computer-aided rational design of more efficient In2O3 catalysts for CO2 hydrogenation to CH3OH.78 On the basis of density functional theory (DFT) calculations, they designed and synthesized a highly efficient hexagonal In2O3 catalyst with a high proportion of the exposed {104} surface. CH3OH selectivity is as high as 92.4% with a CO2 conversion of more than 17% under 300 °C, 5.0 MPa, 9000 mL gcat–1 h–1, H2/CO2 = 6. For the In2O3/ZrO2 catalyst system, the ZrO2 support remarkably boosted the activity of the In2O3 catalyst and prevented its sintering.74,79 It was suggested that synergic effects between In2O3 and ZrO2 carriers favorably tuned CH3OH selectivity by changing the reaction pathway.80 Frei et al. also investigated the electronic, geometric, and interfacial phenomena related to the peculiar promotional effects of the monoclinic ZrO2 carrier on In2O3.81 Less-pronounced lattice mismatching between In2O3 and ZrO2 favors the formation of more surface oxygen vacancies on In2O3, which is beneficial for methanol synthesis.
Because of the lower H2 splitting ability of In2O3 compared with metal catalyst, palladium (Pd) was introduced to enhance H2 activation and facilitate oxygen vacancy formation and thereby substantially promote the activity of In2O3.82,83 CO2 conversion over In2O3 supported highly dispersed Pd nanocatalyst reached above 20% with CH3OH selectivity of ∼70% and STY up to 0.89 gMeOH gcat–1 h–1,83 which is about 2–5 times higher than pure In2O3 under similar reaction conditions. Moreover, the incorporation of Pd atoms in the In2O3 matrix forming low-nuclearity Pd clusters can simultaneously increase the activity, selectivity and long-term stability (Figure 1C).84 Different from dry impregnated Pd–In2O3, this nanostructure can effectively avoid Pd clustering and minimize In2O3 sintering; thus, CH3OH STY dropped slightly from 1.01 to 0.96 gMeOH gcat–1 h–1 after time-on-stream of 500 h at 280 °C, 5.0 MPa, 48 000 mL g–1 h–1, and H2/CO2 = 4. The addition of Pt, Au, Cu, Co, or Ni can also benefit the generation of active hydrogen species.69,85−89 For the Auδ+-In2O3–x catalyst, the strong Au–In2O3 interaction is responsible for the promising methanol synthesis performance.89 Shi et al. found that the Cu11In9 phase is formed by controlled reduction of CuO-In2O3 mixed oxides, and the synergy between Cu11In9 and In2O3 gives a CH3OH STY of 0.2 gMeOH gcat–1 h–1 with a selectivity of 80.5% at CO2 conversion of 11.4% under 280 °C, 3.0 MPa, 7500 mL g–1 h–1, and H2/CO2 = 3.69 Bavykina et al. combined Co and In2O3 to prepare In@Co catalysts, which significantly enhanced methanol productivity due to the formation of Co-supported In2O3–x films. The CH3OH STY is as high as 0.86 gMeOH gcat–1 h–1 with above 80% selectivity and ∼12% conversion at 300 °C, 5.0 MPa, 27 500 mL g–1 h–1, and H2/CO2 = 4.86
The ZnO-ZrO2 solid solution is another reducible oxide system efficient for methanol synthesis from CO2 hydrogenation. It gives a CH3OH STY of 0.73 gMeOH gcat–1 h–1 at CH3OH selectivity of 86% and CO2 conversion of 10% with high stability over 500 h on stream under 320 °C, 5.0 MPa, 24 000 mL g–1 h–1 and H2/CO2 = 3.90 Under similar reaction conditions, a high selectivity of more than 80% is obtained over CdZrOx and GaZrOx solid-solution catalysts with a conversion of 12.4% and 4.3%, respectively.91 The ZnO-ZrO2 solid solution catalyst also exhibits high resistance to sulfur-containing species in the feed gas up to 50 ppm, which makes the catalyst industrially viable in methanol production processes.90
Compared with Cu-based catalysts, the biggest advantage of the reducible metal oxides (In2O3-based oxides or ZnO-ZrO2 solid solution catalysts) is that it can effectively inhibit the undesired RWGS reaction even if the reaction temperature is as high as 320 °C. However, additional works are needed to further clarify the origin of this promotional effect by identifying the active sites and understanding the reaction mechanisms for both methanol and CO formations, which will promote the rational design of more efficient CO2-to-methanol oxide catalysts. In2O3 is very suitable as a model catalyst to study the structure–activity relationship and the reaction mechanism because it can achieve efficient methanol synthesis from CO2 hydrogenation by itself without using any promoters and supports. This is one of the reasons why In2O3 has received such great attention for the CO2 hydrogenation reactions.
CO2 Hydrogenation to Liquid Hydrocarbons
As CO2 can be easily converted to CO via RWGS, modified CO-FTS catalysts were widely used for direct CO2 hydrogenation to long-chain (C5+) hydrocarbons. Methane and gaseous hydrocarbons (C2–4) are also formed through this classical CO-FTS reaction. Compared with CO-FTS, more H2 is usually needed in CO2-based FTS, and the concentration of the CO intermediates is also lower during CO2 hydrogenation, which results in a higher H/C ratio on the catalyst surface. As CO2 can be directly hydrogenated to CH4, the high H/C ratio favors methane formation and leads to a decrease in the chain growth probability. Therefore, CO2–FTS catalysts to be developed should have high activity for both RWGS and FTS reactions but inhibit the CO2 methanation reaction. Iron (Fe)- and Co-based catalysts are industrially adopted CO-FTS catalysts, though Co has little activity for RWGS, and thus a second component is needed for CO2 conversion to CO. The combined selectivity of CH4 and light hydrocarbons are usually very high (>55%), but the introduction of promoters such as Cu, Mo, and alkali metals to the Co catalyst enhances the selective formation of C5+ hydrocarbons from CO2 hydrogenation.92,93 For example, the optimized Na–Co–Mo/SiO2–TiO2 catalyst shows the C5+ selectivity of 27.3% with CH4 selectivity of 40% at CO2 conversion of 26.9% under the very mild reaction condition of 200 °C and 0.1 MPa.92 Nevertheless, Fe catalysts seem to be more suitable for direct CO2 conversion because it has high RWGS reactivity but relatively lower CO2 methanation activity. Albrecht et al. prepared a bare Fe2O3 catalyst using the cellulose-templated synthesis method, which achieves C5+ hydrocarbons selectivity of 36% at CO2 conversion of 40% at 350 °C and 1.5 MPa.94 Additionally, the selectivities of C2–4 hydrocarbons (olefin/paraffin (o/p) ratio = 2.7) and CH4 are 36% and 12%, respectively.
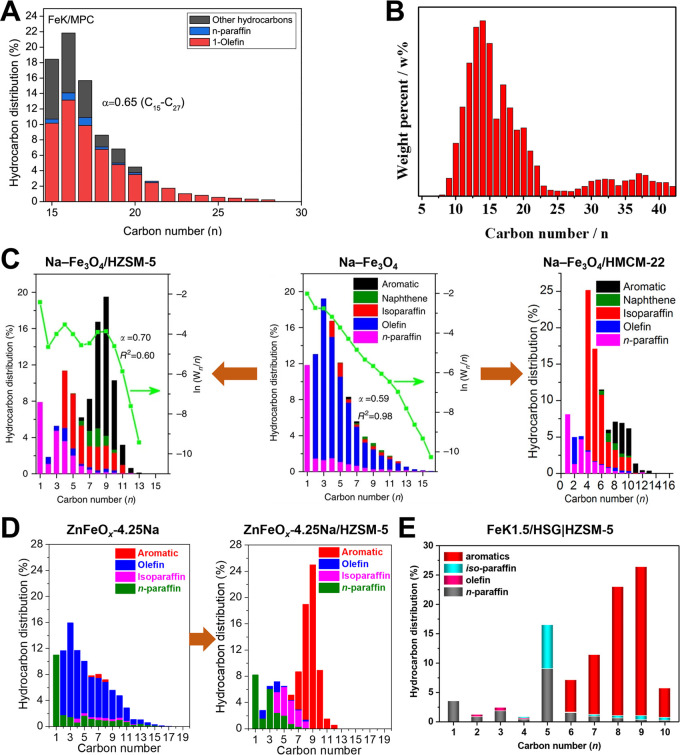
To decrease CH4 selectivity and enhance chain growth ability of Fe catalysts, promoters such as K, Na, Cu, Zn, Mn, and/or Ce are introduced. Simultaneously, some uniquely structured Fe catalysts were fabricated with improved activity and stability. Hwang et al. synthesized a well-defined mesoporous carbon (MPC) supported Fe–K catalyst, and the weak metal–support interaction favored the formation of iron carbide (Fe7C3), which is the active site for CO-FTS.95 The C5+ hydrocarbon selectivity over Fe–K/MPC reaches 44.5% with a CH4 selectivity of 15.4% and chain growth probability (C15–27) of 0.65 at 300 °C and 2.5 MPa (Figure 2A). Liu et al. chose modified ZIF-8 as the precursor to synthesize the Fe–Zn–K catalyst with a hollow sphere structure, which gives the Fe catalyst a high stability under harsh reaction conditions (320 °C and 3.0 MPa).96 The introduction of Cu as a promoter facilitates the reduction of ferric oxide and enhances CO2 adsorption, which can increase conversion and C5+ selectivity as well as decrease CH4 selectivity.97 Delafossite (CuFeO2) derived Fe–Cu catalysts give C5+ hydrocarbon selectivity as high as 66.3% among all hydrocarbons with 31% of C2–4 (o/p = 7.3) and only 2.7% of methane under 300 °C, 1.0 MPa, 1800 mL g–1 h–1, and H2/CO2 = 3.98 The presence of Cu+ species in CuFeO2 promotes the reduction of the Fe catalyst and thus promotes the formation of the FTS active phase (χ-Fe5C2). As shown in Figure 2B, apart from gasoline and diesel range hydrocarbons, the liquid products also contain about 15 wt % of waxes. To the best of our knowledge, there have been no reports on wax (C21+) production directly from CO2. Therefore, CuFeO2 shows an unprecedented chain growth ability in the CO2–FTS reaction, which can be suitable for CO2 hydrogenation to wax. For spinel ZnFe2O4 derived Zn- and Na-modulated Fe catalysts, Zn serves as the structural promoter to improve the dispersion of Fe species.99,100 Gasoline and diesel range hydrocarbons can also be obtained over the reduced ZnFe2O4.100 Under the same reaction condition, C5+ selectivity over the Fe–Zn catalyst is lower than the Fe–Cu catalyst (58.5% vs 66.3%) with much higher CO2 conversion (34.0% vs 17.3%) and lower CO selectivity (11.7% vs 31.7%).98,100 K-promoted Fe–Co/Al2O3 with Co/(Co+Fe) = 0.17 also enables CO2 hydrogenation to liquid hydrocarbons with jet fuel-range α-olefins as the main products.101
Figure 2.
Detail hydrocarbon product distribution obtained over (A) well-defined mesoporous carbon (MPC) supported Fe–K in the range of C15–27,95 (B) Fe–Cu,98 (C) Na–Fe3O4 (middle),105 Na–Fe3O4/HZMS-5 (left),105 and Na–Fe3O4/HMCM-22 (right),109 (D) ZnFeOx-4.25Na (left) and ZnFeOx-4.25Na/HZSM-5 (right) under the reaction conditions of 320 °C, 3.0 MPa, H2/CO2 = 3, and 4000 mL g–1 h–1,107 as well as (E) FeK1.5/HSG|HZSM-5 (SiO2/Al2O3 molar ratio of HZSM-5 = 50) catalysts during the CO2 hydrogenation reaction.110 Reprinted with permission from refs (95), (98), (105), (107) , (109), and (110). Copyright 2017 and 2020 Elsevier, Copyright 2017 Springer-Nature, and Copyright 2018 and 2019 American Chemical Society.
The excellent performance for the synthesis of heavier hydrocarbons using these Co or Fe catalysts is usually related to the presence of alkaline metals (K or Na) because these promoters can enhance the RWGS reaction while suppressing the methanation reaction.100,102−104 Additionally, the high temperature applied for traditional Fe-based FTS favors the endothermic RWGS reaction, and the traditional Co-based catalyst usually operates at a lower temperature of 180–240 °C. Therefore, compared to catalysts with metallic Co as the active site, it is easier to obtain heavier hydrocarbons from CO2, and the product distribution is closer to that derived from CO-FTS over the Fe catalyst with iron carbides as active sites at a higher reaction temperature (300 °C).
However, the CO2 hydrogenation product distribution over the modified Fe-based FTS catalysts still follows the well-known Anderson–Schulz–Flory (ASF) distribution because CO2- and CO-FTS have similar reaction mechanisms. In addition, the hydrocarbons are mainly olefins and normal-paraffins (n-paraffins) for Fe-based FTS (Figure 2).105−107 Recently, Wei et al. combine Fe catalysts with HZSM-5 zeolites to significantly increase the fraction of high-octane gasoline-range isoparaffins and aromatics in the CO2 hydrogenation product (Figure 2C).105 The generated olefins on Na–Fe3O4 diffuse to the acid sites of zeolites and are then converted to C5–11 isoparaffins and aromatics via oligomerization, isomerization, and aromatization reactions. As a result, the selectivity of C5–11 hydrocarbons among all hydrocarbons is up to 73% with 7.9% methane at CO2 conversion of 33.6% and CO selectivity of 14.2% under 320 °C, 3.0 MPa, 4000 mL g–1 h–1, and H2/CO2 = 3. Moreover, the isoparaffin/aromatic ratio in C5–11 hydrocarbons over this bifunctional catalyst system containing Fe-based oxides and zeolites can be tuned by regulating the types, structures, and properties of zeolites.105,108 HMCM-22 zeolite with a unique pore network and appropriate Brønsted acid density and strength enables the formation of branched hydrocarbons with superior selectivity (Figure 2C, ∼35% in all hydrocarbons).109 Recent studies also demonstrate that Na-ZnFeOx/HZSM-5 comprised of Na modified ZnFeOx and hierarchical nanocrystalline HZSM-5 aggregated with an appropriate density of Brønsted acid sites can realize the highly efficient synthesis of aromatics from CO2 hydrogenation (Figure 2D).107 The selectivity of gasoline-range hydrocarbons over this composite catalyst reaches as high as 83.7%, and aromatics selectivity is up to 75.6%. Of course, the enhanced performance of Fe catalysts for CO2 hydrogenation to light olefin intermediates is also favorable for the synthesis of liquid hydrocarbons. For example, Wang et al. synthesized potassium-promoted iron using honeycomb-structured graphene (HSG) as the support and achieved an exceptionally high activity in hydrogenating CO2 to lower olefins, which results in a high liquid hydrocarbons selectivity of 92% and aromatics STY of 11.8 μmolCO2 gcat–1 s–1 at 340 °C, 2.0 MPa, and 26 000 mL g–1 h–1 when coupled with the HZSM-5 zeolite (Figure 2E).110
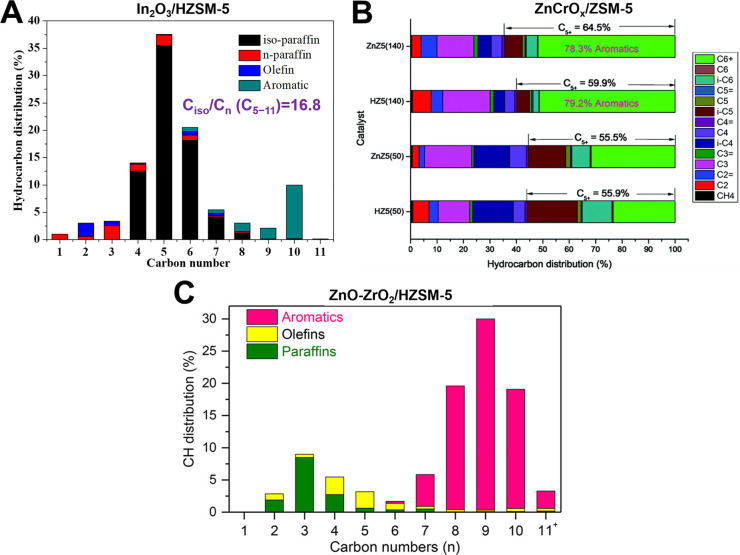
Although the selectivity of hydrocarbons among CO2 hydrogenation products can be increased by coupling olefination with aromatization, the ratio of isoparaffins to n-paraffins (iso/n) is no more than 3, leading to a low octane number.109 It was found that the reducible indium oxides in combination with HZSM-5 zeolites enable direct CO2 conversion into gasoline-range hydrocarbons with a very high iso/n ratio of 16.8 through the formation of methanol as the reaction intermediate (Figure 3A).111 The C5+ selectivity among all hydrocarbons is as high as 78.6% with only 1% CH4 at CO2 conversion of 13.1%. Because the C–C coupling from methanol occurs on zeolites at high temperature (>320 °C), HZSM-5 combined with other active components, which can achieve highly selective CO2 hydrogenation to methanol at above 320 °C, is also suitable for direct liquid hydrocarbons production from CO2 hydrogenation via bifunctional catalysis. The C5+ hydrocarbons selectivity of 70–80% with CH4 selectivity of 0.3–2% is obtained over various bifunctional catalysts containing HZSM-5 zeolites and ZnAlOx, ZnZrOx, ZnCrOx, ZnGaOx, or Cr2O3 oxides (Figure 3B,C).112−117 Similar to the CO2 activation mechanism on In2O3, the activity and formation rates of the methanol intermediate are dominated by the amount of oxygen vacancies on these oxide surfaces. Additionally, many studies have demonstrated that the C5–C11 isoparaffin/aromatic ratio depends on the nature of oxides and zeolites and the proximity of the two components.113,115 For the oxide/zeolite catalyst system, the CO2 conversion is still low (<20%), and the selectivity of byproduct CO is usually >30%. As mentioned above, the introduction of highly dispersed metals can improve the CO2-to-methanl performance of In2O3. This strategy can help to increase the bifunctional catalytic activity for CO2 hydrogenation to liquid hydrocarbons via the methanol intermediates and reduce CO selectivity, while the origin of the enhancement effect of the bifunctional catalysis needs further studies. In addition, the introduction of CO in the feed gas can inhibit the undesired RWGS reaction.111,116 For example, the CO selectivity over Cr2O3/HZSM-5 decreased to only 11.4% with the addition of 5.42 vol % CO into the feed gas.116 Inspired by the CO2 hydrogenation to gasoline process over the multifunctional catalyst comprised of Fe3O4, Fe5C2, and zeolite acid sites, the introduction of iron carbide active sites into oxide/zeolite bifunctional catalysts to promote the further conversion of the generated CO may also be an effective strategy to decrease CO selectivity.
Figure 3.
CO2 hydrogenation hydrocarbon product distribution over (A) In2O3/HZSM-5 under reaction conditions of 340 °C, 3.0 MPa, H2/CO2 = 3, and 9000 mL g–1 h–1,111 (B) composite catalyst containing ZnCrOx and various HZSM-5 zeolites (with and without Zn-exchange) at 330 °C, 5.0 MPa, H2/CO2 = 3, and 2000 mL g–1 h–1,114 and (C) ZnO-ZrO2/HZSM-5 (reaction conditions: 340 °C, 4.0 MPa, H2/CO2 = 3, and 7200 mL g–1 h–1).115 Reprinted with permission from refs (111), (114), and (115). Copyright 2017 Springer-Nature, Copyright 2019 Royal Society of Chemistry, and Copyright 2020 American Chemical Society.
CO2 Hydrogenation to Higher Alcohols
The selective conversion of CO2 and H2 into higher alcohols remains a much greater challenge than that into C2+ hydrocarbon products due to the existence of many parallel and consecutive reactions and usually higher energy barriers for CO insertion compared with CHx hydrogenation.21 Noble metal (Au, Pd)-based catalysts were developed for direct synthesis of ethanol from CO2 hydrogenation with high selectivity in a batch reactor.118−120 Han’s group reported water promoted CO2 hydrogenation to C2–4 alcohols, and the selectivity over Pt/Co3O4 is up to 88.1% at 220 °C and 8.0 MPa in the water/1,3-dimethyl-2-imidazolidinone mixed solvent.120 Recently, non-noble metal-based catalysts were found to also enable highly efficient liquid-phase ethanol synthesis from direct CO2 hydrogenation. The Co–Al LDH derived CoAlOx catalyst gives an ethanol selectivity of 92.1% and an ethanol time yield of 0.444 mmol g–1 h–1 at 140 °C.10 In addition, the introduction of nickel species into the CoAlOx catalyst accelerates the formation of the relatively stable CHx intermediates, which enables Co0.52Ni0.48AlOx to achieve excellent performance for the selective conversion of CO2 to ethanol.121
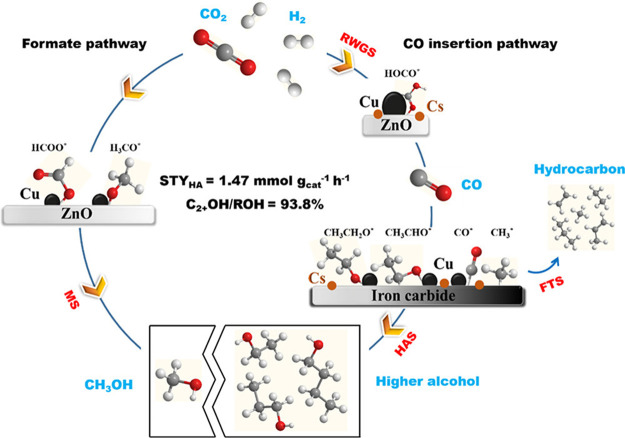
In an autoclave reactor, very high C2+OH selectivity (especially for ethanol) was usually obtained over the above-mentioned catalysts at relatively low reaction temperatures (140–220 °C). Some researchers have recently explored a new strategy to fabricate practical catalysts for direct CO2 hydrogenation to C2+OH in a fixed-bed reactor at a relatively high reaction temperature (>250 °C).22,122−124 Gong’s group developed TiO2 nanorods supported Rh–Fe–Li catalysts for higher alcohol synthesis (HAS) from CO2 hydrogenation and demonstrated that high density of hydroxyl groups on the support is responsible for the highly selective formation of ethanol.124 Rh–Fe–Li/TiO2 displays a high ethanol selectivity of 32% among all carbon-containing products at around 15% CO2 conversion at 250 °C and 3.0 MPa. Zhang et al. found that supported Co2C catalysts enable 62.8% ethanol selectivity in the alcohol distribution at CO2 conversion of around 18% at 250 °C and 5.0 MPa.123 They suggested that CO can form via RWGS at Co2C sites and then insert into the CHx intermediates to produce ethanol. Xu et al. reported Cs-modified Cu–Fe–Zn catalysts for HAS from CO2 hydrogenation, which exhibits a CO2 conversion of 36.6%, C2+OH selectivity of 19.8%, 93.8% C2+OH fraction in the alcohol distribution, and C2+OH STY of 73.4 mg gcat–1 h–1 at 330 °C and 5.0 MPa.22 It was suggested that Fe7C3 and Cu are responsible for CO dissociation to form CHx species and CO nondissociative adsorption, respectively, and a good balance between them enables Cs3 wt %-Cu0.8-Fe1.0-Zn1.0 catalysts to have the best performance among the catalysts with different metal compositions (Figure 4). Although the high C2+OH fraction in the alcohol distribution seems to be easily obtained over these modified FTS catalysts, the alcohol selectivity among all products is very low (<40%), and the main products are still hydrocarbons and CO.
Figure 4.
Reaction pathways of CO2 hydrogenation over the Cs–Cu–Fe-Zn catalyst. Reprinted with permission from ref (22). Copyright 2020 American Chemical Society.
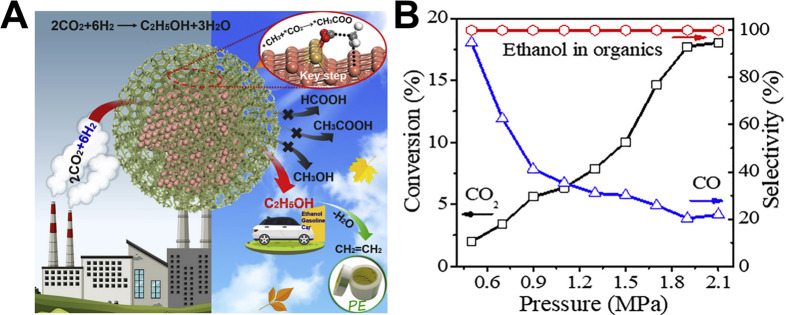
Very recently, Ding’s group reported the Cu@Na-Beta catalyst with Cu nanoparticles enclosed in the Na-Beta zeolite crystal particles that enables highly selective conversion of CO2 to ethanol in a fixed-bed reactor due to the synergistic effects among irregular Cu nanoparticles and surrounding of zeolitic frameworks (Figure 5A).125 Ethanol and CO are the only products, and no hydrocarbons and methanol are formed. The ethanol yield is up to ∼14% with CO selectivity of 21% at CO2 conversion of ∼18% at 2.1 MPa (Figure 5B). They also found that the CH3COO* species are the most important intermediates for ethanol production from CO2, which are formed by bonding between CO2* and CH3* at the step sites of Cu nanoparticles. It is usually difficult to obtain C2+ products at a single Cu site. The subtlety of this work is the use of 3D zeolitic frameworks to confine and regulate Cu nanoparticles in unique shapes to achieve precise C–C coupling on the Cu surface.
Figure 5.
(A) The CO2 hydrogenation to ethanol reaction process over Cu@Na-Beta catalyst. (B) The influence of reaction pressure on CO2 hydrogenation performance of Cu@Na-Beta. Reaction condition: 300 °C, 12 000 mL g–1 h–1, and H2/CO2 = 3.125 Reprinted with permission from ref (125). Copyright 2020 Elsevier.
In the field of CO hydrogenation, new routes using multifunctional catalysts have been developed for higher alcohol synthesis (HAS) from syngas with high selectivity. Sun’s group combined Co–Mn oxides (FTS catalyst) with Cu–Zn–Al–Zr oxides (methanol synthesis catalyst) and markedly increased the oxygenates selectivity to 58.1 wt % with the C2+OH fraction of 92.0 wt %.21 The Cu–Zn–Al–Zr oxides could provide more CHxO* species and selectively promote the CO* (or CHxO*) insertion reaction, which benefits the oxygenates formation. A similar strategy by using multifunctional catalysts composed of Mo-based sulfides and Zn–Cr–Al oxides has been reported for HAS.126 The alcohol selectivity was enhanced to 60.4% with >72.7% C2+OH selectivity in alcohols. Wang’s group proposed relay catalysis strategy using a three-component catalytic system that enables syngas-to-methanol/dimethyl ether (DME), methanol/DME carbonylation, and acetic acid hydrogenation reactions in one reactor.20,127 Ethanol selectivity reaches 64% at a CO conversion of around 10%. Inspired by these effective strategies, the development of a new catalyst with different functions for CO2 hydrogenation with high alcohol selectivity is highly attractive but also very challenging.
Summary and Perspective
In the past few decades, heterogeneous CO2 hydrogenation to methanol via thermal catalysis has received great attention and has seen enormous progress. As a result, various highly efficient and novel catalysts (such as Cu, Co, bimetallic systems, and reducible metal oxides) have been developed with their active sites revealed and their reaction mechanisms understood to some extent. Considering the catalytic performance, catalyst cost, scale-up preparation feasibility, and other factors, Cu-based catalysts still hold the greatest prospect for large-scale industrial applications of methanol synthesis from pure CO2. Very recently, more researchers are considering the direct synthesis of value-added C2+ liquid products from CO2 hydrogenation. Direct CO2 hydrogenation to oxygenates possesses a better atom economy and also a higher efficiency in hydrogen utilization than hydrocarbon production.3 On the other hand, worldwide consumption of liquid hydrocarbon fuels is greater, due to their higher energy content. Currently, high selectivity of gasoline fuels (above 75%) can be achieved over Na–Fe/HZSM-5 and In2O3/HZSM-5 bifunctional catalyst systems albeit via very different reaction mechanisms. The modified FTS catalysts are expected to be more suitable for the synthesis of heavier hydrocarbons (such as jet fuels and diesel) and C2+OH alcohols from CO2 due to the great C–C coupling ability. For CO2 hydrogenation to liquid hydrocarbons over Fe-based catalysts, better understanding of the iron carbide active sites is required for further improving the stability and decreasing the selectivity of light hydrocarbons as well as the industrial implementation of this process. To further enhance the performance of the Co-based CO2–FTS catalyst, it is necessary to introduce a highly active low-temperature RWGS site. For the production of higher alcohols, catalyst development is still at a very early stage, where the problem of low alcohol yield remains to be solved. In addition to learning from the new strategy using multifunctional catalysts in HAS from syngas, using the confinement or modulation effects of zeolites on the reactive centers is an effective strategy to increase the selectivity of higher alcohols in CO2 hydrogenation.
If the CO2 source is coal- or biomass-combustion flue gas, the CO2 conversion process becomes more complicated due to the copresence of CO, O2, SOx, and/or NOx.128,129 Cost associated with cleaning the flue gas especially via deep desulfurization will decrease the economic viability of CO2 conversion.130 In this regard, the ZnO-ZrO2 catalyst mentioned above is more suitable due to its excellent sulfur tolerance. Although little attention has been paid to the effect of sulfur-containing molecules on the CO2 hydrogenation reaction, it can be speculated that other oxide catalyst systems with the oxygen vacancy as the active site also have good sulfur resistance owing to their similar reaction mechanism. Therefore, for CO2 sources from flue gas, partially reduced oxides and oxides/zeolites bifunctional catalysts are industrially more relevant for methanol production and liquid hydrocarbons synthesis via the methanol intermediate, respectively. The In2O3-based catalysts have received especially great attention due to their high performance and relatively simple active site structures. In addition, these catalyst systems usually show excellent selectivity and high stability, although their single-pass CO2 conversions are usually much lower than those over supported metal catalysts (such as Cu- and Fe-based catalysts) and are generally below 20% even with noble metal modifiers and at relatively high reaction temperature (>280 °C). A very high recycle ratio of the unconverted gas is needed in industrial applications of CO2 hydrogenation, which may decrease its energy efficiency and economic value. Thus, it is necessary to further increase the amount of oxygen vacancies and enhance the H2 splitting ability to improve the intrinsic activity of these oxide catalysts.
Recent years have seen emerging experimental and computational technologies for more efficient search and design of catalysts and other materials. Experimental technologies such as high-throughput catalyst synthesis and performance evaluation, 3D printing, and in situ characterizations and monitoring are increasingly being employed for the rapid discovery of novel catalysts and materials.131−133 On the other hand, a similar array of computational technologies including high-throughput and automated computational simulations and reaction modeling coupled with machine learning algorithms also start to enable the theoretical understanding and prediction of new catalysts.134−136 The applicability of the above experimental and computational technologies in designing industrially relevant heterogeneous catalysts vary due to their greater complexity and stringent requirements. Nevertheless, these new technologies hold great potential in revolutionizing the way that industrial catalysts have been traditionally developed, and thus great progress can be expected in this research. Apart from further developing more efficient catalysts for milder reaction conditions, we should also pay sufficient attention to reactor design and optimization. For example, a membrane reactor can shift the equilibrium-limited CO2 hydrogenation to liquid fuels by selective and continuous in situ removal of the byproduct water, leading to a substantial increase in CO2 conversion as well as the yield of liquid fuels.137−139
Acknowledgments
We thank Dr. Alexander van der Made, Dr. Alexander Petrus van Bavel, and Dr. Joost Smits from Shell Global Solutions International B.V. for helpful discussions. P.G. acknowledges funding support from Strategic Priority Research Program of the Chinese Academy of Sciences (XDA21090204), the National Natural Science Foundation of China (21773286, U1832162), Youth Innovation Promotion Association CAS (2018330), the “Frontier Science” program of Shell Global Solutions International B.V. (PT65197, CW373032), and Shanghai Rising-Star Program, China (19QA1409900).
Author Contributions
# These authors contributed equally: P.G., L.Z., and S.L.
The authors declare no competing financial interest.
References
- Sakakura T.; Choi J. C.; Yasuda H. Transformation of carbon dioxide. Chem. Rev. 2007, 107 (6), 2365–2387. 10.1021/cr068357u. [DOI] [PubMed] [Google Scholar]
- Zhou W.; Cheng K.; Kang J. C.; Zhou C.; Subramanian V.; Zhang Q. H.; Wang Y. New horizon in C1 chemistry: breaking the selectivity limitation in transformation of syngas and hydrogenation of CO2 into hydrocarbon chemicals and fuels. Chem. Soc. Rev. 2019, 48 (12), 3193–3228. 10.1039/C8CS00502H. [DOI] [PubMed] [Google Scholar]
- Leonzio G. State of art and perspectives about the production of methanol, dimethyl ether and syngas by carbon dioxide hydrogenation. J. CO2 Util. 2018, 27, 326–354. 10.1016/j.jcou.2018.08.005. [DOI] [Google Scholar]
- Shih C. F.; Zhang T.; Li J.; Bai C. Powering the Future with Liquid Sunshine. Joule 2018, 2 (10), 1925–1949. 10.1016/j.joule.2018.08.016. [DOI] [Google Scholar]
- Buttler A.; Spliethoff H. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: A review. Renewable Sustainable Energy Rev. 2018, 82, 2440–2454. 10.1016/j.rser.2017.09.003. [DOI] [Google Scholar]
- Varone A.; Ferrari M. Power to liquid and power to gas: An option for the German Energiewende. Renewable Sustainable Energy Rev. 2015, 45, 207–218. 10.1016/j.rser.2015.01.049. [DOI] [Google Scholar]
- Deutz S.; Bongartz D.; Heuser B.; Kätelhön A.; Schulze Langenhorst L.; Omari A.; Walters M.; Klankermayer J.; Leitner W.; Mitsos A.; Pischinger S.; Bardow A. Cleaner production of cleaner fuels: wind-to-wheel – environmental assessment of CO2-based oxymethylene ether as a drop-in fuel. Energy Environ. Sci. 2018, 11 (2), 331–343. 10.1039/C7EE01657C. [DOI] [Google Scholar]
- Bos M. J.; Kersten S. R. A.; Brilman D. W. F. Wind power to methanol: Renewable methanol production using electricity, electrolysis of water and CO2 air capture. Appl. Energy 2020, 264, 114672. 10.1016/j.apenergy.2020.114672. [DOI] [Google Scholar]
- Zhang C. D.; Gao R. X.; Jun K. W.; Kim S. K.; Hwang S. M.; Park H. G.; Guan G. F. Direct conversion of carbon dioxide to liquid fuels and synthetic natural gas using renewable power: Techno-economic analysis. J. CO2 Util. 2019, 34, 293–302. 10.1016/j.jcou.2019.07.005. [DOI] [Google Scholar]
- Wang L.; Wang L.; Zhang J.; Liu X.; Wang H.; Zhang W.; Yang Q.; Ma J.; Dong X.; Yoo S. J.; Kim J.-G.; Meng X.; Xiao F.-S. Selective Hydrogenation of CO2 to Ethanol over Cobalt Catalysts. Angew. Chem., Int. Ed. 2018, 57 (21), 6104–6108. 10.1002/anie.201800729. [DOI] [PubMed] [Google Scholar]
- Stangeland K.; Li H. L.; Yu Z. X. Thermodynamic Analysis of Chemical and Phase Equilibria in CO2 Hydrogenation to Methanol, Dimethyl Ether, and Higher Alcohols. Ind. Eng. Chem. Res. 2018, 57 (11), 4081–4094. 10.1021/acs.iecr.7b04866. [DOI] [Google Scholar]
- Zhong J.; Yang X.; Wu Z.; Liang B.; Huang Y.; Zhang T. State of the art and perspectives in heterogeneous catalysis of CO2 hydrogenation to methanol. Chem. Soc. Rev. 2020, 49 (5), 1385–1413. 10.1039/C9CS00614A. [DOI] [PubMed] [Google Scholar]
- Guo L. S.; Sun J.; Ge Q. J.; Tsubaki N. Recent advances in direct catalytic hydrogenation of carbon dioxide to valuable C2+ hydrocarbons. J. Mater. Chem. A 2018, 6 (46), 23244–23262. 10.1039/C8TA05377D. [DOI] [Google Scholar]
- Yang H. Y.; Zhang C.; Gao P.; Wang H.; Li X. P.; Zhong L. S.; Wei W.; Sun Y. H. A review of the catalytic hydrogenation of carbon dioxide into value-added hydrocarbons. Catal. Sci. Technol. 2017, 7 (20), 4580–4598. 10.1039/C7CY01403A. [DOI] [Google Scholar]
- Li J. C.; Wang L. G.; Cao Y.; Zhang C. J.; He P.; Li H. Q. Recent advances on the reduction of CO2 to important C2+ oxygenated chemicals and fuels. Chin. J. Chem. Eng. 2018, 26 (11), 2266–2279. 10.1016/j.cjche.2018.07.008. [DOI] [Google Scholar]
- Bao J.; Yang G. H.; Yoneyama Y.; Tsubaki N. Significant Advances in C1 Catalysis: Highly Efficient Catalysts and Catalytic Reactions. ACS Catal. 2019, 9 (4), 3026–3053. 10.1021/acscatal.8b03924. [DOI] [Google Scholar]
- Khodakov A. Y.; Chu W.; Fongarland P. Advances in the development of novel cobalt Fischer–Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels. Chem. Rev. 2007, 107 (5), 1692–1744. 10.1021/cr050972v. [DOI] [PubMed] [Google Scholar]
- Ilias S.; Bhan A. Mechanism of the Catalytic Conversion of Methanol to Hydrocarbons. ACS Catal. 2013, 3 (1), 18–31. 10.1021/cs3006583. [DOI] [Google Scholar]
- Olsbye U.; Svelle S.; Bjorgen M.; Beato P.; Janssens T. V. W.; Joensen F.; Bordiga S.; Lillerud K. P. Conversion of Methanol to Hydrocarbons: How Zeolite Cavity and Pore Size Controls Product Selectivity. Angew. Chem., Int. Ed. 2012, 51 (24), 5810–5831. 10.1002/anie.201103657. [DOI] [PubMed] [Google Scholar]
- Kang J.; He S.; Zhou W.; Shen Z.; Li Y.; Chen M.; Zhang Q.; Wang Y. Single-pass transformation of syngas into ethanol with high selectivity by triple tandem catalysis. Nat. Commun. 2020, 11 (1), 827. 10.1038/s41467-020-14672-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. J.; Qi X. Z.; Wang X. X.; Xia L.; Wang C. Q.; Yu F.; Wang H.; Li S. G.; Zhong L. S.; Sun Y. H. Direct Production of Higher Oxygenates by Syngas Conversion over a Multifunctional Catalyst. Angew. Chem., Int. Ed. 2019, 58 (14), 4627–4631. 10.1002/anie.201814611. [DOI] [PubMed] [Google Scholar]
- Xu D.; Ding M.; Hong X.; Liu G.; Tsang S. C. E. Selective C2+ Alcohol Synthesis from Direct CO2 Hydrogenation over a Cs-Promoted Cu-Fe-Zn Catalyst. ACS Catal. 2020, 10, 5250–5260. 10.1021/acscatal.0c01184. [DOI] [Google Scholar]
- Jiang X.; Nie X.; Guo X.; Song C.; Chen J. G. Recent Advances in Carbon Dioxide Hydrogenation to Methanol via Heterogeneous Catalysis. Chem. Rev. 2020, 120, 7984. 10.1021/acs.chemrev.9b00723. [DOI] [PubMed] [Google Scholar]
- Ye R. P.; Ding J.; Gong W.; Argyle M. D.; Zhong Q.; Wang Y.; Russell C. K.; Xu Z.; Russell A. G.; Li Q.; Fan M.; Yao Y. G. CO2 hydrogenation to high-value products via heterogeneous catalysis. Nat. Commun. 2019, 10 (1), 5698. 10.1038/s41467-019-13638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guil-Lopez R.; Mota N.; Llorente J.; Millan E.; Pawelec B.; Fierro J. L. G.; Navarro R. M. Methanol Synthesis from CO2: A Review of the Latest Developments in Heterogeneous Catalysis. Materials 2019, 12 (23), 3902. 10.3390/ma12233902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Din I. U.; Shaharun M. S.; Alotaibi M. A.; Alharthi A. I.; Naeem A. Recent developments on heterogeneous catalytic CO2 reduction to methanol. J. CO2 Util. 2019, 34, 20–33. 10.1016/j.jcou.2019.05.036. [DOI] [Google Scholar]
- Dang S. S.; Yang H. Y.; Gao P.; Wang H.; Li X. P.; Wei W.; Sun Y. H. A review of research progress on heterogeneous catalysts for methanol synthesis from carbon dioxide hydrogenation. Catal. Today 2019, 330, 61–75. 10.1016/j.cattod.2018.04.021. [DOI] [Google Scholar]
- Martin O.; Mondelli C.; Curulla-Ferre D.; Drouilly C.; Hauert R.; Perez-Ramirez J. Zinc-Rich Copper Catalysts Promoted by Gold for Methanol Synthesis. ACS Catal. 2015, 5 (9), 5607–5616. 10.1021/acscatal.5b00877. [DOI] [Google Scholar]
- Olah G. A.; Mathew T.; Goeppert A.; Prakash G. K. S. Difference and Significance of Regenerative Versus Renewable Carbon Fuels and Products. Top. Catal. 2018, 61 (7–8), 522–529. 10.1007/s11244-018-0964-8. [DOI] [Google Scholar]
- Larmier K.; Liao W. C.; Tada S.; Lam E.; Verel R.; Bansode A.; Urakawa A.; Comas-Vives A.; Coperet C. CO2-to-Methanol Hydrogenation on Zirconia-Supported Copper Nanoparticles: Reaction Intermediates and the Role of the Metal-Support Interface. Angew. Chem., Int. Ed. 2017, 56 (9), 2318–2323. 10.1002/anie.201610166. [DOI] [PubMed] [Google Scholar]
- Lam E.; Larmier K.; Wolf P.; Tada S.; Safonova O. V.; Coperet C. Isolated Zr Surface Sites on Silica Promote Hydrogenation of CO2 to CH3OH in Supported Cu Catalysts. J. Am. Chem. Soc. 2018, 140 (33), 10530–10535. 10.1021/jacs.8b05595. [DOI] [PubMed] [Google Scholar]
- Samson K.; Sliwa M.; Socha R. P.; Gora-Marek K.; Mucha D.; Rutkowska-Zbik D.; Paul J. F.; Ruggiero-Mikolajczyk M.; Grabowski R.; Sloczynski J. Influence of ZrO2 Structure and Copper Electronic State on Activity of Cu/ZrO2 Catalysts in Methanol Synthesis from CO2. ACS Catal. 2014, 4 (10), 3730–3741. 10.1021/cs500979c. [DOI] [Google Scholar]
- Tada S.; Kayamori S.; Honma T.; Kamei H.; Nariyuki A.; Kon K.; Toyao T.; Shimizu K.; Satokawa S. Design of Interfacial Sites between Cu and Amorphous ZrO2 Dedicated to CO2-to-Methanol Hydrogenation. ACS Catal. 2018, 8 (9), 7809–7819. 10.1021/acscatal.8b01396. [DOI] [Google Scholar]
- Shi Z. S.; Tan Q. Q.; Wu D. F. Enhanced CO2 hydrogenation to methanol over TiO2 nanotubes-supported CuO-ZnO-CeO2 catalyst. Appl. Catal., A 2019, 581, 58–66. 10.1016/j.apcata.2019.05.019. [DOI] [Google Scholar]
- Fang X.; Men Y. H.; Wu F.; Zhao Q. H.; Singh R.; Xiao P.; Du T.; Webley P. A. Improved methanol yield and selectivity from CO2 hydrogenation using a novel Cu-ZnO-ZrO2 catalyst supported on Mg-Al layered double hydroxide (LDH). J. CO2 Util. 2019, 29, 57–64. 10.1016/j.jcou.2018.11.006. [DOI] [Google Scholar]
- Kuhl S.; Tarasov A.; Zander S.; Kasatkin I.; Behrens M. Cu- Based Catalyst Resulting from a Cu, Zn, Al Hydrotalcite- Like Compound: A Microstructural, Thermoanalytical, and In Situ XAS Study. Chem. - Eur. J. 2014, 20 (13), 3782–3792. 10.1002/chem.201302599. [DOI] [PubMed] [Google Scholar]
- Li M. M. J.; Chen C. P.; Ayvali T.; Suo H. R.; Zheng J. W.; Teixeira I. F.; Ye L.; Zou H. B.; O’Hare D.; Tsang S. C. E. CO2 Hydrogenation to Methanol over Catalysts Derived from Single Cationic Layer CuZnGa LDH Precursors. ACS Catal. 2018, 8 (5), 4390–4401. 10.1021/acscatal.8b00474. [DOI] [Google Scholar]
- Kuhl S.; Schumann J.; Kasatkin I.; Havecker M.; Schlogl R.; Behrens M. Ternary and quaternary Cr or Ga-containing ex-LDH catalysts-Influence of the additional oxides onto the microstructure and activity of Cu/ZnAl2O4 catalysts. Catal. Today 2015, 246, 92–100. 10.1016/j.cattod.2014.08.029. [DOI] [Google Scholar]
- Gao P.; Zhong L.; Zhang L.; Wang H.; Zhao N.; Wei W.; Sun Y. Yttrium oxide modified Cu/ZnO/Al2O3 catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol. Catal. Sci. Technol. 2015, 5, 4365–4377. 10.1039/C5CY00372E. [DOI] [Google Scholar]
- Yang H.; Gao P.; Zhang C.; Zhong L.; Li X.; Wang S.; Wang H.; Wei W.; Sun Y. Core–shell structured Cu@m-SiO2 and Cu/ZnO@m-SiO2 catalysts for methanol synthesis from CO2 hydrogenation. Catal. Commun. 2016, 84, 56–60. 10.1016/j.catcom.2016.06.010. [DOI] [Google Scholar]
- Mureddu M.; Ferrara F.; Pettinau A. Highly efficient CuO/ZnO/ZrO2@SBA-15 nanocatalysts for methanol synthesis from the catalytic hydrogenation of CO2. Appl. Catal., B 2019, 258, 117941. 10.1016/j.apcatb.2019.117941. [DOI] [Google Scholar]
- An B.; Zhang J.; Cheng K.; Ji P.; Wang C.; Lin W. Confinement of Ultrasmall Cu/ZnOx Nanoparticles in Metal-Organic Frameworks for Selective Methanol Synthesis from Catalytic Hydrogenation of CO2. J. Am. Chem. Soc. 2017, 139 (10), 3834–3840. 10.1021/jacs.7b00058. [DOI] [PubMed] [Google Scholar]
- Liu T. K.; Hong X. L.; Liu G. L. In Situ Generation of the Cu@3D-ZrOx Framework Catalyst for Selective Methanol Synthesis from CO2/H2. ACS Catal. 2020, 10 (1), 93–102. 10.1021/acscatal.9b03738. [DOI] [Google Scholar]
- Hu B.; Yin Y. Z.; Zhong Z. X.; Wu D. D.; Liu G. L.; Hong X. L. Cu@ZIF-8 derived inverse ZnO/Cu catalyst with sub-5 nm ZnO for efficient CO2 hydrogenation to methanol. Catal. Sci. Technol. 2019, 9 (10), 2673–2681. 10.1039/C8CY02546K. [DOI] [Google Scholar]
- Li C. S.; Melaet G.; Ralston W. T.; An K.; Brooks C.; Ye Y. F.; Liu Y. S.; Zhu J. F.; Guo J. H.; Alayoglu S.; Somorjai G. A. High-performance hybrid oxide catalyst of manganese and cobalt for low-pressure methanol synthesis. Nat. Commun. 2015, 6, 6538. 10.1038/ncomms7538. [DOI] [PubMed] [Google Scholar]
- Stangeland K.; Kalai D. Y.; Ding Y.; Yu Z. X. Mesoporous manganese-cobalt oxide spinel catalysts for CO2 hydrogenation to methanol. J. CO2 Util. 2019, 32, 146–154. 10.1016/j.jcou.2019.04.018. [DOI] [Google Scholar]
- Lian Y.; Fang T. F.; Zhang Y. H.; Liu B.; Li J. L. Hydrogenation of CO2 to alcohol species over Co@Co3O4/C-N catalysts. J. Catal. 2019, 379, 46–51. 10.1016/j.jcat.2019.09.018. [DOI] [Google Scholar]
- Wang L.; Guan E.; Wang Y.; Wang L.; Gong Z.; Cui Y.; Meng X.; Gates B. C.; Xiao F. S. Silica accelerates the selective hydrogenation of CO2 to methanol on cobalt catalysts. Nat. Commun. 2020, 11 (1), 1033. 10.1038/s41467-020-14817-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M.; Pokhrel S.; Thome A.; Zielasek V.; Gesing T. M.; Roessner F.; Madler L.; Baumer M. Highly active Co-Al2O3-based catalysts for CO2 methanation with very low platinum promotion prepared by double flame spray pyrolysis. Catal. Sci. Technol. 2016, 6 (20), 7449–7460. 10.1039/C6CY01252C. [DOI] [Google Scholar]
- Melaet G.; Ralston W. T.; Li C. S.; Alayoglu S.; An K.; Musselwhite N.; Kalkan B.; Somorjai G. A. Evidence of Highly Active Cobalt Oxide Catalyst for the Fischer–Tropsch Synthesis and CO2 Hydrogenation. J. Am. Chem. Soc. 2014, 136 (6), 2260–2263. 10.1021/ja412447q. [DOI] [PubMed] [Google Scholar]
- Wang W.; Wang S. P.; Ma X. B.; Gong J. L. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40 (7), 3703–3727. 10.1039/c1cs15008a. [DOI] [PubMed] [Google Scholar]
- Iglesia E. Design, synthesis, and use of cobalt-based Fischer–Tropsch synthesis catalysts. Appl. Catal., A 1997, 161 (1–2), 59–78. 10.1016/S0926-860X(97)00186-5. [DOI] [Google Scholar]
- Nie X. W.; Jiang X.; Wang H. Z.; Luo W. J.; Janik M. J.; Chen Y. G.; Guo X. W.; Song C. S. Mechanistic Understanding of Alloy Effect and Water Promotion for Pd-Cu Bimetallic Catalysts in CO2 Hydrogenation to Methanol. ACS Catal. 2018, 8 (6), 4873–4892. 10.1021/acscatal.7b04150. [DOI] [Google Scholar]
- Liu L. N.; Fan F.; Jiang Z.; Gao X. F.; Wei J. J.; Fang T. Mechanistic Study of Pd-Cu Bimetallic Catalysts for Methanol Synthesis from CO2 Hydrogenation. J. Phys. Chem. C 2017, 121 (47), 26287–26299. 10.1021/acs.jpcc.7b06166. [DOI] [Google Scholar]
- Alvarez-Garcia A.; Florez E.; Moreno A.; Jimenez-Orozco C. CO2 activation on small Cu-Ni and Cu-Pd bimetallic clusters. Mol. Catal. 2020, 484, 110733. 10.1016/j.mcat.2019.110733. [DOI] [Google Scholar]
- Garcia-Trenco A.; White E. R.; Regoutz A.; Payne D. J.; Shaffer M. S. P.; Williams C. K. Pd2Ga-Based Colloids as Highly Active Catalysts for the Hydrogenation of CO2 to Methanol. ACS Catal. 2017, 7 (2), 1186–1196. 10.1021/acscatal.6b02928. [DOI] [Google Scholar]
- Fiordaliso E. M.; Sharafutdinov I.; Carvalho H. W. P.; Grunwaldt J. D.; Hansen T. W.; Chorkendorff I.; Wagner J. B.; Damsgaard C. D. Intermetallic GaPd2 Nanoparticles on SiO2 for Low-Pressure CO2 Hydrogenation to Methanol: Catalytic Performance and In Situ Characterization. ACS Catal. 2015, 5 (10), 5827–5836. 10.1021/acscatal.5b01271. [DOI] [Google Scholar]
- Garcia-Trenco A.; Regoutz A.; White E. R.; Payne D. J.; Shaffer M. S. P.; Williams C. K. PdIn intermetallic nanoparticles for the Hydrogenation of CO2 to Methanol. Appl. Catal., B 2018, 220, 9–18. 10.1016/j.apcatb.2017.07.069. [DOI] [Google Scholar]
- Snider J. L.; Streibel V.; Hubert M. A.; Choksi T. S.; Valle E.; Upham D. C.; Schumann J.; Duyar M. S.; Gallo A.; Abild-Pedersen F.; Jaramillo T. F. Revealing the Synergy between Oxide and Alloy Phases on the Performance of Bimetallic In-Pd Catalysts for CO2 Hydrogenation to Methanol. ACS Catal. 2019, 9 (4), 3399–3412. 10.1021/acscatal.8b04848. [DOI] [Google Scholar]
- Wu P. P.; Yang B. Intermetallic PdIn catalyst for CO2 hydrogenation to methanol: mechanistic studies with a combined DFT and microkinetic modeling method. Catal. Sci. Technol. 2019, 9 (21), 6102–6113. 10.1039/C9CY01242G. [DOI] [Google Scholar]
- Li X. L.; Liu G. L.; Xu D.; Hong X. L.; Tsang S. C. E. Confinement of subnanometric PdZn at a defect enriched ZnO/ZIF-8 interface for efficient and selective CO2 hydrogenation to methanol. J. Mater. Chem. A 2019, 7 (41), 23878–23885. 10.1039/C9TA03410B. [DOI] [Google Scholar]
- Studt F.; Sharafutdinov I.; Abild-Pedersen F.; Elkjaer C. F.; Hummelshoj J. S.; Dahl S.; Chorkendorff I.; Norskov J. K. Discovery of a Ni-Ga catalyst for carbon dioxide reduction to methanol. Nat. Chem. 2014, 6 (4), 320–324. 10.1038/nchem.1873. [DOI] [PubMed] [Google Scholar]
- Gallo A.; Snider J. L.; Sokaras D.; Nordlund D.; Kroll T.; Ogasawara H.; Kovarik L.; Duyar M. S.; Jaramillo T. F. Ni5Ga3 catalysts for CO2 reduction to methanol: Exploring the role of Ga surface oxidation/reduction on catalytic activity. Appl. Catal., B 2020, 267, 118369. 10.1016/j.apcatb.2019.118369. [DOI] [Google Scholar]
- Tang Q. L.; Ji W. C.; Russell C. K.; Cheng Z. W.; Zhang Y. L.; Fan M. H.; Shen Z. M. Understanding the catalytic mechanisms of CO2 hydrogenation to methanol on unsupported and supported Ga-Ni clusters. Appl. Energy 2019, 253, 113623. 10.1016/j.apenergy.2019.113623. [DOI] [Google Scholar]
- Tang Q. L.; Shen Z. M.; Russell C. K.; Fan M. H. Thermodynamic and Kinetic Study on Carbon Dioxide Hydrogenation to Methanol over a Ga3Ni5(111) Surface: The Effects of Step Edge. J. Phys. Chem. C 2018, 122 (1), 315–330. 10.1021/acs.jpcc.7b08232. [DOI] [Google Scholar]
- Sharafutdinov I.; Elkjaer C. F.; de Carvalho H. W. P.; Gardini D.; Chiarello G. L.; Damsgaard C. D.; Wagner J. B.; Grunwaldt J. D.; Dahl S.; Chorkendorff I. Intermetallic compounds of Ni and Ga as catalysts for the synthesis of methanol. J. Catal. 2014, 320, 77–88. 10.1016/j.jcat.2014.09.025. [DOI] [Google Scholar]
- Li M. M.; Zou H.; Zheng J.; Wu T. S.; Chan T. S.; Soo Y. L.; Wu X. P.; Gong X. Q.; Chen T.; Roy K.; Held G.; Tsang S. C. E. Methanol Synthesis at a Wide Range of H2/CO2 Ratios over a Rh-In Bimetallic Catalyst. Angew. Chem., Int. Ed. 2020, 10.1002/anie.202000841. [DOI] [PubMed] [Google Scholar]
- Shi Z. S.; Tan Q. Q.; Wu D. F. A novel Core-Shell structured CuIn@SiO2 catalyst for CO2 hydrogenation to methanol. AIChE J. 2019, 65 (3), 1047–1058. 10.1002/aic.16490. [DOI] [Google Scholar]
- Shi Z. S.; Tan Q. Q.; Tian C.; Pan Y.; Sun X. W.; Zhang J. X.; Wu D. F. CO2 hydrogenation to methanol over Cu-In intermetallic catalysts: Effect of reduction temperature. J. Catal. 2019, 379, 78–89. 10.1016/j.jcat.2019.09.024. [DOI] [Google Scholar]
- Pustovarenko A.; Dikhtiarenko A.; Bavykina A.; Gevers L.; Ramírez A.; Russkikh A.; Telalovic S.; Aguilar A.; Hazemann J.-L.; Ould-Chikh S.; Gascon J. Metal–Organic Framework-Derived Synthesis of Cobalt Indium Catalysts for the Hydrogenation of CO2 to Methanol. ACS Catal. 2020, 10 (9), 5064–5076. 10.1021/acscatal.0c00449. [DOI] [Google Scholar]
- Richard A. R.; Fan M. The effect of lanthanide promoters on NiInAl/SiO2 catalyst for methanol synthesis. Fuel 2018, 222, 513–522. 10.1016/j.fuel.2018.02.185. [DOI] [Google Scholar]
- Richard A. R.; Fan M. H. Low-Pressure Hydrogenation of CO2 to CH3OH Using Ni-In-Al/SiO2 Catalyst Synthesized via a Phyllosilicate Precursor. ACS Catal. 2017, 7 (9), 5679–5692. 10.1021/acscatal.7b00848. [DOI] [Google Scholar]
- Zhao F. Z.; Gong M.; Cao K.; Zhang Y. H.; Li J. L.; Chen R. Atomic Layer Deposition of Ni on Cu Nanoparticles for Methanol Synthesis from CO2 Hydrogenation. ChemCatChem 2017, 9 (19), 3772–3778. 10.1002/cctc.201700622. [DOI] [Google Scholar]
- Martin O.; Martin A. J.; Mondelli C.; Mitchell S.; Segawa T. F.; Hauert R.; Drouilly C.; Curulla-Ferre D.; Perez-Ramirez J. Indium Oxide as a Superior Catalyst for Methanol Synthesis by CO2 Hydrogenation. Angew. Chem., Int. Ed. 2016, 55 (21), 6261–6265. 10.1002/anie.201600943. [DOI] [PubMed] [Google Scholar]
- Ye J. Y.; Liu C. J.; Mei D. H.; Ge Q. F. Active Oxygen Vacancy Site for Methanol Synthesis from CO2 Hydrogenation on In2O3(110): A DFT Study. ACS Catal. 2013, 3 (6), 1296–1306. 10.1021/cs400132a. [DOI] [Google Scholar]
- Frei M. S.; Capdevila-Cortada M.; Garcia-Muelas R.; Mondelli C.; Lopez N.; Stewart J. A.; Ferre D. C.; Perez-Ramirez J. Mechanism and microkinetics of methanol synthesis via CO2 hydrogenation on indium oxide. J. Catal. 2018, 361, 313–321. 10.1016/j.jcat.2018.03.014. [DOI] [Google Scholar]
- Wang J. Y.; Liu C. Y.; Senftle T. P.; Zhu J.; Zhang G. H.; Guo X. W.; Song C. S. Variation in the In2O3 Crystal Phase Alters Catalytic Performance toward the Reverse Water Gas Shift Reaction. ACS Catal. 2020, 10 (5), 3264–3273. 10.1021/acscatal.9b04239. [DOI] [Google Scholar]
- Dang S.; Qin B.; Yang Y.; Wang H.; Cai J.; Han Y.; Li S.; Gao P.; Sun Y. Rationally designed indium oxide catalysts for CO2 hydrogenation to methanol with high activity and selectivity. Sci. Adv. 2020, 6 (25), eaaz2060. 10.1126/sciadv.aaz2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang S. S.; Gao P.; Liu Z. Y.; Chen X. Q.; Yang C. G.; Wang H.; Zhong L. S.; Li S. G.; Sun Y. H. Role of zirconium in direct CO2 hydrogenation to lower olefins on oxide/zeolite bifunctional catalysts. J. Catal. 2018, 364, 382–393. 10.1016/j.jcat.2018.06.010. [DOI] [Google Scholar]
- Chen T. Y.; Cao C. X.; Chen T. B.; Ding X. X.; Huang H.; Shen L.; Cao X. Y.; Zhu M. H.; Xu J.; Gao J.; Han Y. F. Unraveling Highly Tunable Selectivity in CO2 Hydrogenation over Bimetallic In-Zr Oxide Catalysts. ACS Catal. 2019, 9 (9), 8785–8797. 10.1021/acscatal.9b01869. [DOI] [Google Scholar]
- Frei M. S.; Mondelli C.; Cesarini A.; Krumeich F.; Hauert R.; Stewart J. A.; Ferre D. C.; Perez-Ramirez J. Role of Zirconia in Indium Oxide-Catalyzed CO2 Hydrogenation to Methanol. ACS Catal. 2020, 10 (2), 1133–1145. 10.1021/acscatal.9b03305. [DOI] [Google Scholar]
- Jiang H. X.; Lin J.; Wu X. H.; Wang W. Y.; Chen Y. F.; Zhang M. H. Efficient hydrogenation of CO2 to methanol over Pd/In2O3/SBA-15 catalysts. J. CO2 Util. 2020, 36, 33–39. 10.1016/j.jcou.2019.10.013. [DOI] [Google Scholar]
- Rui N.; Wang Z. Y.; Sun K. H.; Ye J. Y.; Ge Q. F.; Liu C. J. CO2 hydrogenation to methanol over Pd/In2O3: effects of Pd and oxygen vacancy. Appl. Catal., B 2017, 218, 488–497. 10.1016/j.apcatb.2017.06.069. [DOI] [Google Scholar]
- Frei M. S.; Mondelli C.; Garcia-Muelas R.; Kley K. S.; Puertolas B.; Lopez N.; Safonova O. V.; Stewart J. A.; Curulla Ferre D.; Perez-Ramirez J. Atomic-scale engineering of indium oxide promotion by palladium for methanol production via CO2 hydrogenation. Nat. Commun. 2019, 10 (1), 3377. 10.1038/s41467-019-11349-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L. B.; Shen X. C.; Pan Y. B.; Peng Z. M. Synergy between active sites of Cu-In-Zr-O catalyst in CO2 hydrogenation to methanol. J. Catal. 2019, 372, 74–85. 10.1016/j.jcat.2019.02.021. [DOI] [Google Scholar]
- Bavykina A.; Yarulina I.; Al Abdulghani A. J.; Gevers L.; Hedhili M. N.; Miao X. H.; Galilea A. R.; Pustovarenko A.; Dikhtiarenko A.; Cadiau A.; Aguilar-Tapia A.; Hazemann J. L.; Kozlov S. M.; Oud-Chikh S.; Cavallo L.; Gascon J. Turning a Methanation Co Catalyst into an In-Co Methanol Producer. ACS Catal. 2019, 9 (8), 6910–6918. 10.1021/acscatal.9b01638. [DOI] [Google Scholar]
- Jia X.; Sun K.; Wang J.; Shen C.; Liu C.-j. Selective hydrogenation of CO2 to methanol over Ni/In2O3 catalyst. J. Energy Chem. 2020, 50, 409–415. 10.1016/j.jechem.2020.03.083. [DOI] [Google Scholar]
- Han Z.; Tang C.; Wang J.; Li L.; Li C. Atomically dispersed Ptn+ species as highly active sites in Pt/In2O3 catalysts for methanol synthesis from CO2 hydrogenation. J. Catal. 2020, 10.1016/j.jcat.2020.06.018. [DOI] [Google Scholar]
- Rui N.; Zhang F.; Sun K.; Liu Z.; Xu W.; Stavitski E.; Senanayake S. D.; Rodriguez J. A.; Liu C.-J. Hydrogenation of CO2 to Methanol on a Auδ+–In2O3-x Catalyst. ACS Catal. 2020, 10.1021/acscatal.0c02120. [DOI] [Google Scholar]
- Wang J.; Li G.; Li Z.; Tang C.; Feng Z.; An H.; Liu H.; Liu T.; Li C. A highly selective and stable ZnO-ZrO2 solid solution catalyst for CO2 hydrogenation to methanol. Sci. Adv. 2017, 3 (10), e1701290. 10.1126/sciadv.1701290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Tang C.; Li G.; Han Z.; Li Z.; Liu H.; Cheng F.; Li C. High-Performance MaZrOx (Ma = Cd, Ga) Solid-Solution Catalysts for CO2 Hydrogenation to Methanol. ACS Catal. 2019, 9 (11), 10253–10259. 10.1021/acscatal.9b03449. [DOI] [Google Scholar]
- Owen R. E.; Plucinski P.; Mattia D.; Torrente-Murciano L.; Ting V. P.; Jones M. D. Effect of support of Co-Na-Mo catalysts on the direct conversion of CO2 to hydrocarbons. J. CO2 Util. 2016, 16, 97–103. 10.1016/j.jcou.2016.06.009. [DOI] [Google Scholar]
- Shi Z. B.; Yang H. Y.; Gao P.; Chen X. Q.; Liu H. J.; Zhong L. S.; Wang H.; Wei W.; Sun Y. H. Effect of alkali metals on the performance of CoCu/TiO2 catalysts for CO2 hydrogenation to long-chain hydrocarbons. Chin. J. Catal. 2018, 39 (8), 1294–1302. 10.1016/S1872-2067(18)63086-4. [DOI] [Google Scholar]
- Albrecht M.; Rodemerck U.; Schneider M.; Bröring M.; Baabe D.; Kondratenko E. V. Unexpectedly efficient CO2 hydrogenation to higher hydrocarbons over non-doped Fe2O3. Appl. Catal., B 2017, 204, 119–126. 10.1016/j.apcatb.2016.11.017. [DOI] [Google Scholar]
- Hwang S. M.; Zhang C. D.; Han S. J.; Park H. G.; Kim Y. T.; Yang S.; Jun K. W.; Kim S. K. Mesoporous carbon as an effective support for Fe catalyst for CO2 hydrogenation to liquid hydrocarbons. J. CO2 Util. 2020, 37, 65–73. 10.1016/j.jcou.2019.11.025. [DOI] [Google Scholar]
- Liu J. H.; Sun Y. W.; Jiang X.; Zhang A. F.; Song C. S.; Guo X. W. Pyrolyzing ZIF-8 to N-doped porous carbon facilitated by iron and potassium for CO2 hydrogenation to value-added hydrocarbons. J. CO2 Util. 2018, 25, 120–127. 10.1016/j.jcou.2018.03.015. [DOI] [Google Scholar]
- Liu J. H.; Zhang A. F.; Jiang X.; Liu M.; Sun Y. W.; Song C. S.; Guo X. W. Selective CO2 Hydrogenation to Hydrocarbons on Cu-Promoted Fe-Based Catalysts: Dependence on Cu-Fe Interaction. ACS Sustainable Chem. Eng. 2018, 6 (8), 10182–10190. 10.1021/acssuschemeng.8b01491. [DOI] [Google Scholar]
- Choi Y. H.; Jang Y. J.; Park H.; Kim W. Y.; Lee Y. H.; Choi S. H.; Lee J. S. Carbon dioxide Fischer–Tropsch synthesis: A new path to carbon-neutral fuels. Appl. Catal., B 2017, 202, 605–610. 10.1016/j.apcatb.2016.09.072. [DOI] [Google Scholar]
- Zhai P.; Xu C.; Gao R.; Liu X.; Li M. Z.; Li W. Z.; Fu X. P.; Jia C. J.; Xie J. L.; Zhao M.; Wang X. P.; Li Y. W.; Zhang Q. W.; Wen X. D.; Ma D. Highly Tunable Selectivity for Syngas-Derived Alkenes over Zinc and Sodium-Modulated Fe5C2 Catalyst. Angew. Chem., Int. Ed. 2016, 55 (34), 9902–9907. 10.1002/anie.201603556. [DOI] [PubMed] [Google Scholar]
- Choi Y. H.; Ra E. C.; Kim E. H.; Kim K. Y.; Jang Y. J.; Kang K. N.; Choi S. H.; Jang J. H.; Lee J. S. Sodium-Containing Spinel Zinc Ferrite as a Catalyst Precursor for the Selective Synthesis of Liquid Hydrocarbon Fuels. ChemSusChem 2017, 10 (23), 4764–4770. 10.1002/cssc.201701437. [DOI] [PubMed] [Google Scholar]
- Satthawong R.; Koizumi N.; Song C. S.; Prasassarakich P. Bimetallic Fe-Co catalysts for CO2 hydrogenation to higher hydrocarbons. J. CO2 Util. 2013, 3–4, 102–106. 10.1016/j.jcou.2013.10.002. [DOI] [Google Scholar]
- Amoyal M.; Vidruk-Nehemya R.; Landau M. V.; Herskowitz M. Effect of potassium on the active phases of Fe catalysts for carbon dioxide conversion to liquid fuels through hydrogenation. J. Catal. 2017, 348, 29–39. 10.1016/j.jcat.2017.01.020. [DOI] [Google Scholar]
- Liu J. H.; Zhang A. F.; Jiang X.; Liu M.; Zhu J.; Song C. S.; Guo X. W. Direct Transformation of Carbon Dioxide to Value-Added Hydrocarbons by Physical Mixtures of Fe5C2 and K-Modified Al2O3. Ind. Eng. Chem. Res. 2018, 57 (28), 9120–9126. 10.1021/acs.iecr.8b02017. [DOI] [Google Scholar]
- Wei J.; Sun J.; Wen Z. Y.; Fang C. Y.; Ge Q. J.; Xu H. Y. New insights into the effect of sodium on Fe3O4-based nanocatalysts for CO2 hydrogenation to light olefins. Catal. Sci. Technol. 2016, 6 (13), 4786–4793. 10.1039/C6CY00160B. [DOI] [Google Scholar]
- Wei J.; Ge Q.; Yao R.; Wen Z.; Fang C.; Guo L.; Xu H.; Sun J. Directly converting CO2 into a gasoline fuel. Nat. Commun. 2017, 8, 15174. 10.1038/ncomms15174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; Wu T.; Lin J.; Ji Y.; Yan S.; Pei Y.; Xie S.; Zong B.; Qiao M. Iron–Potassium on Single-Walled Carbon Nanotubes as Efficient Catalyst for CO2 Hydrogenation to Heavy Olefins. ACS Catal. 2020, 10, 6389–6401. 10.1021/acscatal.0c00810. [DOI] [Google Scholar]
- Cui X.; Gao P.; Li S. G.; Yang C. G.; Liu Z. Y.; Wang H.; Zhong L. S.; Sun Y. H. Selective Production of Aromatics Directly from Carbon Dioxide Hydrogenation. ACS Catal. 2019, 9 (5), 3866–3876. 10.1021/acscatal.9b00640. [DOI] [Google Scholar]
- Xu Y. B.; Shi C. M.; Liu B.; Wang T.; Zheng J.; Li W. P.; Liu D. P.; Liu X. H. Selective production of aromatics from CO2. Catal. Sci. Technol. 2019, 9 (3), 593–610. 10.1039/C8CY02024H. [DOI] [Google Scholar]
- Wei J.; Yao R. W.; Ge Q. J.; Wen Z. Y.; Ji X. W.; Fang C. Y.; Zhang J. X.; Xu H. Y.; Sun J. Catalytic Hydrogenation of CO2 to Isoparaffins over Fe-Based Multifunctional Catalysts. ACS Catal. 2018, 8 (11), 9958–9967. 10.1021/acscatal.8b02267. [DOI] [Google Scholar]
- Wang S. W.; Wu T. J.; Lin J.; Tian J.; Ji Y. S.; Pei Y.; Yan S. R.; Qiao M. H.; Xu H. L.; Zong B. N. FeK on 3D Graphene-Zeolite Tandem Catalyst with High Efficiency and Versatility in Direct CO2 Conversion to Aromatics. ACS Sustainable. ACS Sustainable Chem. Eng. 2019, 7 (21), 17825–17833. 10.1021/acssuschemeng.9b04328. [DOI] [Google Scholar]
- Gao P.; Li S.; Bu X.; Dang S.; Liu Z.; Wang H.; Zhong L.; Qiu M.; Yang C.; Cai J.; Wei W.; Sun Y. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst. Nat. Chem. 2017, 9 (10), 1019–1024. 10.1038/nchem.2794. [DOI] [PubMed] [Google Scholar]
- Ni Y.; Chen Z.; Fu Y.; Liu Y.; Zhu W.; Liu Z. Selective conversion of CO2 and H2 into aromatics. Nat. Commun. 2018, 9 (1), 3457. 10.1038/s41467-018-05880-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. L.; Qu Y. Z.; Wang J. J.; Liu H. L.; Li M. R.; Miao S.; Li C. Highly Selective Conversion of Carbon Dioxide to Aromatics over Tandem Catalysts. Joule 2019, 3 (2), 570–583. 10.1016/j.joule.2018.10.027. [DOI] [Google Scholar]
- Zhang J.; Zhang M.; Chen S.; Wang X.; Zhou Z.; Wu Y.; Zhang T.; Yang G.; Han Y.; Tan Y. Hydrogenation of CO2 into aromatics over a ZnCrOx-zeolite composite catalyst. Chem. Commun. 2019, 55 (7), 973–976. 10.1039/C8CC09019J. [DOI] [PubMed] [Google Scholar]
- Zhou C.; Shi J. Q.; Zhou W.; Cheng K.; Zhang Q. H.; Kang J. C.; Wang Y. Highly Active ZnO-ZrO2 Aerogels Integrated with H-ZSM-5 for Aromatics Synthesis from Carbon Dioxide. ACS Catal. 2020, 10 (1), 302–310. 10.1021/acscatal.9b04309. [DOI] [Google Scholar]
- Wang Y.; Tan L.; Tan M.; Zhang P.; Fang Y.; Yoneyama Y.; Yang G.; Tsubaki N. Rationally Designing Bifunctional Catalysts as an Efficient Strategy To Boost CO2 Hydrogenation Producing Value-Added Aromatics. ACS Catal. 2019, 9, 895–901. 10.1021/acscatal.8b01344. [DOI] [Google Scholar]
- Zhang X. B.; Zhang A. F.; Jiang X.; Zhu J.; Liu J. H.; Li J. J.; Zhang G. H.; Song C. S.; Guo X. W. Utilization of CO2 for aromatics production over ZnO/ZrO2-ZSM-5 tandem catalyst. J. CO2 Util. 2019, 29, 140–145. 10.1016/j.jcou.2018.12.002. [DOI] [Google Scholar]
- Wang D.; Bi Q. Y.; Yin G. H.; Zhao W. L.; Huang F. Q.; Xie X. M.; Jiang M. H. Direct synthesis of ethanol via CO2 hydrogenation using supported gold catalysts. Chem. Commun. 2016, 52 (99), 14226–14229. 10.1039/C6CC08161D. [DOI] [PubMed] [Google Scholar]
- Bai S.; Shao Q.; Wang P.; Dai Q.; Wang X.; Huang X. Highly Active and Selective Hydrogenation of CO2 to Ethanol by Ordered Pd-Cu Nanoparticles. J. Am. Chem. Soc. 2017, 139 (20), 6827–6830. 10.1021/jacs.7b03101. [DOI] [PubMed] [Google Scholar]
- He Z.; Qian Q.; Ma J.; Meng Q.; Zhou H.; Song J.; Liu Z.; Han B. Water-Enhanced Synthesis of Higher Alcohols from CO2 Hydrogenation over a Pt/Co3O4 Catalyst under Milder Conditions. Angew. Chem., Int. Ed. 2016, 55, 737–741. 10.1002/anie.201507585. [DOI] [PubMed] [Google Scholar]
- Wang L. X.; He S. X.; Wang L.; Lei Y.; Meng X. J.; Xiao F. S. Cobalt-Nickel Catalysts for Selective Hydrogenation of Carbon Dioxide into Ethanol. ACS Catal. 2019, 9 (12), 11335–11340. 10.1021/acscatal.9b04187. [DOI] [Google Scholar]
- Liu B.; Ouyang B.; Zhang Y. H.; Lv K. L.; Li Q.; Ding Y. B.; Li J. L. Effects of mesoporous structure and Pt promoter on the activity of Co-based catalysts in low-temperature CO2 hydrogenation for higher alcohol synthesis. J. Catal. 2018, 366, 91–97. 10.1016/j.jcat.2018.07.019. [DOI] [Google Scholar]
- Zhang S. N.; Liu X. F.; Shao Z. L.; Wang H.; Sun Y. H. Direct CO2 hydrogenation to ethanol over supported Co2C catalysts: Studies on support effects and mechanism. J. Catal. 2020, 382, 86–96. 10.1016/j.jcat.2019.11.038. [DOI] [Google Scholar]
- Yang C. S.; Mu R. T.; Wang G. S.; Song J. M.; Tian H.; Zhao Z. J.; Gong J. L. Hydroxyl-mediated ethanol selectivity of CO2 hydrogenation. Chem. Sci. 2019, 10 (11), 3161–3167. 10.1039/C8SC05608K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L.; Shi T.; Gu J.; Cui Y.; Zhang Z.; Yang C.; Chen T.; Lin M.; Wang P.; Xue N.; Peng L.; Guo X.; Zhu Y.; Chen Z.; Ding W. CO2 Hydrogenation to Ethanol over Cu@Na-Beta. Chem. 2020, 10.1016/j.chempr.2020.07.001. [DOI] [Google Scholar]
- Luan X.; Ren Z.; Dai X.; Zhang X.; Yong J.; Yang Y.; Zhao H.; Cui M.; Nie F.; Huang X. Selective Conversion of Syngas into Higher Alcohols via a Reaction-Coupling Strategy on Multifunctional Relay Catalysts. ACS Catal. 2020, 10 (4), 2419–2430. 10.1021/acscatal.9b04111. [DOI] [Google Scholar]
- Zhou W.; Kang J. C.; Cheng K.; He S.; Shi J. Q.; Zhou C.; Zhang Q. H.; Chen J. C.; Peng L. M.; Chen M. S.; Wang Y. Direct Conversion of Syngas into Methyl Acetate, Ethanol, and Ethylene by Relay Catalysis via the Intermediate Dimethyl Ether. Angew. Chem., Int. Ed. 2018, 57 (37), 12012–12016. 10.1002/anie.201807113. [DOI] [PubMed] [Google Scholar]
- MacDowell N.; Florin N.; Buchard A.; Hallett J.; Galindo A.; Jackson G.; Adjiman C. S.; Williams C. K.; Shah N.; Fennell P. An overview of CO2 capture technologies. Energy Environ. Sci. 2010, 3 (11), 1645–1669. 10.1039/c004106h. [DOI] [Google Scholar]
- Goeppert A.; Czaun M.; Jones J. P.; Prakash G. K. S.; Olah G. A. Recycling of carbon dioxide to methanol and derived products - closing the loop. Chem. Soc. Rev. 2014, 43 (23), 7995–8048. 10.1039/C4CS00122B. [DOI] [PubMed] [Google Scholar]
- Alvarez A.; Bansode A.; Urakawa A.; Bavykina A. V.; Wezendonk T. A.; Makkee M.; Gascon J.; Kapteijn F. Challenges in the Greener Production of Formates/Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 Hydrogenation Processes. Chem. Rev. 2017, 117 (14), 9804–9838. 10.1021/acs.chemrev.6b00816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor D. P.; Roch L. M.; Saikin S. K.; Kreisbeck C.; Sheberla D.; Montoya J. H.; Dwaraknath S.; Aykol M.; Ortiz C.; Tribukait H.; Amador-Bedolla C.; Brabec C. J.; Maruyama B.; Persson K. A.; Aspuru-Guzik A. Accelerating the discovery of materials for clean energy in the era of smart automation. Nat. Rev. Mater. 2018, 3 (5), 5–20. 10.1038/s41578-018-0005-z. [DOI] [Google Scholar]
- Raccuglia P.; Elbert K. C.; Adler P. D. F.; Falk C.; Wenny M. B.; Mollo A.; Zeller M.; Friedler S. A.; Schrier J.; Norquist A. J. Machine-learning-assisted materials discovery using failed experiments. Nature 2016, 533 (7601), 73–76. 10.1038/nature17439. [DOI] [PubMed] [Google Scholar]
- Corma A.; Diaz-Cabanas M. J.; Jorda J. L.; Martinez C.; Moliner M. High-throughput synthesis and catalytic properties of a molecular sieve with 18-and 10-member rings. Nature 2006, 443 (7113), 842–845. 10.1038/nature05238. [DOI] [PubMed] [Google Scholar]
- Tran K.; Ulissi Z. W. Active learning across intermetallics to guide discovery of electrocatalysts for CO2 reduction and H2 evolution. Nat. Catal. 2018, 1 (9), 696–703. 10.1038/s41929-018-0142-1. [DOI] [Google Scholar]
- Boyd P. G.; Chidambaram A.; Garcia-Diez E.; Ireland C. P.; Daff T. D.; Bounds R.; Gladysiak A.; Schouwink P.; Moosavi S. M.; Maroto-Valer M. M.; Reimer J. A.; Navarro J. A. R.; Woo T. K.; Garcia S.; Stylianou K. C.; Smit B. Data-driven design of metal-organic frameworks for wet flue gas CO2 capture. Nature 2019, 576 (7786), 253–256. 10.1038/s41586-019-1798-7. [DOI] [PubMed] [Google Scholar]
- Zhong M.; Tran K.; Min Y. M.; Wang C. H.; Wang Z. Y.; Dinh C. T.; De Luna P.; Yu Z. Q.; Rasouli A. S.; Brodersen P.; Sun S.; Voznyy O.; Tan C. S.; Askerka M.; Che F. L.; Liu M.; Seifitokaldani A.; Pang Y. J.; Lo S. C.; Ip A.; Ulissi Z.; Sargent E. H. Accelerated discovery of CO2 electrocatalysts using active machine learning. Nature 2020, 581 (7807), 178–183. 10.1038/s41586-020-2242-8. [DOI] [PubMed] [Google Scholar]
- Li H.; Qiu C.; Ren S.; Dong Q.; Zhang S.; Zhou F.; Liang X.; Wang J.; Li S.; Yu M. Na(+)-gated water-conducting nanochannels for boosting CO2 conversion to liquid fuels. Science 2020, 367 (6478), 667–671. 10.1126/science.aaz6053. [DOI] [PubMed] [Google Scholar]
- Saeidi S.; Amin N. A. S.; Rahimpour M. R. Hydrogenation of CO2 to value-added products-A review and potential future developments. J. CO2 Util. 2014, 5, 66–81. 10.1016/j.jcou.2013.12.005. [DOI] [Google Scholar]
- Tran T. V.; Nguyen L. P.; Nguyen T. H.; Dang T. T.; Ngo P. T.; Nguyen D. A. Application of NaA Membrane Reactor for Methanol Synthesis in CO2 Hydrogenation at Low Pressure. Int. Int. J. Chem. React. Eng. 2018, 16 (4), 20170046. 10.1515/ijcre-2017-0046. [DOI] [Google Scholar]