Abstract
Duchenne muscular dystrophy (DMD) is a muscle degenerative disorder that manifests in early childhood and results in progressive muscle weakness. Physical therapists have long been an important component of the multidisciplinary team caring for people with DMD, providing expertise in areas of disease assessment, contracture management, assistive device prescription, and exercise prescription. Over the last decade, magnetic resonance imaging of muscles in people with DMD has led to an improved understanding of the muscle pathology underlying the clinical manifestations of DMD. Findings from magnetic resonance imaging (MRI) studies in DMD, paired with the clinical expertise of physical therapists, can help guide research that leads to improved physical therapist care for this unique patient population. The 2 main goals of this perspective article are to (1) summarize muscle pathology and disease progression findings from qualitative and quantitative muscle MRI studies in DMD and (2) link MRI findings of muscle pathology to the clinical manifestations observed by physical therapists with discussion of any potential implications of MRI findings on physical therapy management.
Keywords: Muscular Dystroophies, Neuromuscular Diseases, Muscle Weakness
Duchenne muscular dystrophy (DMD) is a progressive muscle wasting disorder resulting in skeletal muscle weakness, cardiac impairment, and respiratory insufficiency.1–3 DMD is caused by mutations in the X chromosome dystrophin gene.4 Dystrophin protein is normally found at the muscle cell sarcolemma linking the cell cytoskeleton to the extracellular matrix; however, dystrophin is absent in cases of DMD and reduced in the milder Becker muscular dystrophy.5 When muscle cells lack dystrophin, they become more susceptible to contraction-induced damage and inflammation, particularly during eccentric contractions.6,7 As regenerative capacity is exhausted, muscle cells die and become replaced by both fibrous and fatty tissue.8
The primary clinical manifestation of DMD is progressive muscle weakness and deterioration of functional abilities. Signs of proximal muscle weakness, including Gower’s maneuver (standing from the floor or a seated position using the arms to climb the thighs) and difficulty climbing stairs, present in early childhood.9 Muscle weakness progresses in a proximal to distal manner leading to loss of independent ambulation in the early teens, loss of overhead reach in the teens, and loss of self-feeding in early adulthood.10 Cardiopulmonary complications, caused by cardiac and respiratory muscle degeneration, are major causes of death, typically in early adulthood.11 In addition to muscle weakness, DMD is often characterized by calf hypertrophy, contracture development, scoliosis, and neuropsychological manifestations.12–15 Therapeutic interventions for DMD include glucocorticosteroids to slow progression of muscle weakness (albeit with side effects like weight gain and osteoporosis) and cardiac medication to manage cardiomyopathy symptoms.1,16–18 Additionally, mutation-specific dystrophin restoration therapies have been conditionally approved in the United States and Europe.19,20
Current care recommendations include physical therapy assessment and management as key components of multidisciplinary care across the lifespan.1,21 Physical therapists, in consultation with the broader care management team, are ideally suited to assess and intervene in issues of musculoskeletal health such as range of motion (ROM) limitations, seating and positioning, and exercise prescription. However, a paucity of evidence-based recommendations exists for physical therapy management in DMD, and many care recommendations cite expert opinions or research performed prior to current medical management rather than contemporary, data-driven findings.1,21 Within the last decade, magnetic resonance imaging (MRI) techniques have become increasingly utilized to understand and quantify muscle disease patterns in DMD to expand understanding of the underlying muscle pathology leading to clinical manifestations of muscle weakness, movement pattern compensations, and functional decline.22–24 An understanding of MRI data, paired with the clinical expertise of physical therapists, may help guide research and lead to improved physical therapist care for this population.
MRI can be either qualitative, producing images for visual inspection, or quantitative, where characteristics of muscle health are objectively measured. T1-weighted MRIs are often used qualitatively in DMD to visualize fatty replacement (hyperintense/bright areas) within darker muscle tissue (Fig. 1A).25 T1-weighted images can be given an ordinal rating that estimates the extent of muscle involvement.26 T2-weighted images can be used to visualize fatty tissue, inflammation, and/or edema, all of which have hyperintense (bright) signal (Fig. 1B). Fat signal can be suppressed to visualize inflammation/edema in isolation (Fig. 1C).27,28
Figure 1.
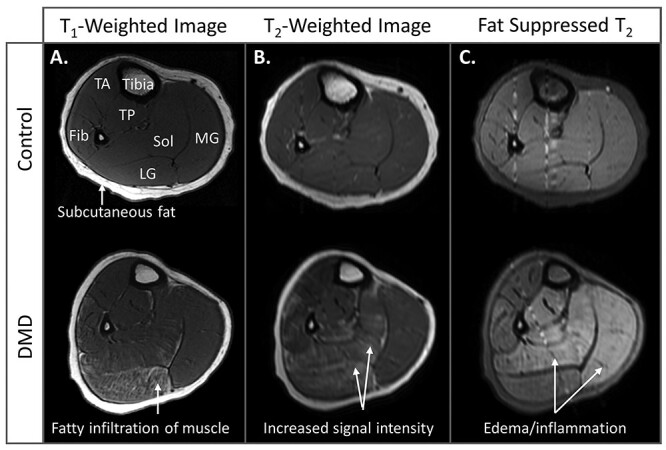
Magnetic resonance imaging (MRI) of qualitative muscle. (A) T1-weighted images of the calf in an unaffected 8-year-old control and a 7-year-old with Duchenne muscular dystrophy (DMD). On T1-weighted images, healthy muscle is homogenous in intensity, and fat appears in bone marrow and the subcutaneous tissue as bright signal intensity. In DMD, T1-weighted contrast highlights the fatty infiltration of muscle. Here, fatty infiltration is visible in the lateral gastrocnemius and soleus. (B) In unaffected controls, T2-weighted images of muscle have similar contrast to T1-weighted images. In DMD, T2-weighted imaging reveals higher signal intensity in the muscle that can be reflective of either inflammation, fat, or both. (C) T2-weighted images with suppressed fat signal can highlight muscle edema well, particularly in this steroid-untreated individual. No increased muscle signal intensity is visible in the control calf, but increased signal intensity is visible in the individual with DMD in the soleus and medial gastrocnemius. The vertical lines in these images are small artifacts from blood vessels. Note: Each of the 3 images from the control and individual with DMD are taken at the same slice location, demonstrating the ability of different image contrasts to highlight different pathologies. Fib = fibularis longus and brevis; LG = lateral gastrocnemius; MG = medial gastrocnemius; Sol = soleus; TA = tibialis anterior; TP = tibialis posterior.
Quantitative MRI measures include muscle size, fatty replacement, and edema. MRIs can be used to determine muscle cross-sectional area or volume, assessing muscle atrophy and growth/hypertrophy. Fatty infiltration can be quantified using chemical shift-encoded MRI (Dixon imaging) or magnetic resonance spectroscopy.25,29 These methods separate the water signal (muscle) and the fat signal (fatty infiltration and subcutaneous fat) to give the muscle’s fat fraction (percent intramuscular fat) (Fig. 2A–C). Fat fraction is considered a biomarker of a muscle’s health, and higher levels of fatty infiltration in a muscle are linked to worsening functional abilities in children and teenagers with DMD.30–32 Another common MRI approach is quantitative T2 imaging (Fig. 2D). MRI T2 reflects both fatty infiltration and inflammation/edema from muscle damage, with higher values when either or both of these processes are present.27,33,34
Figure 2.
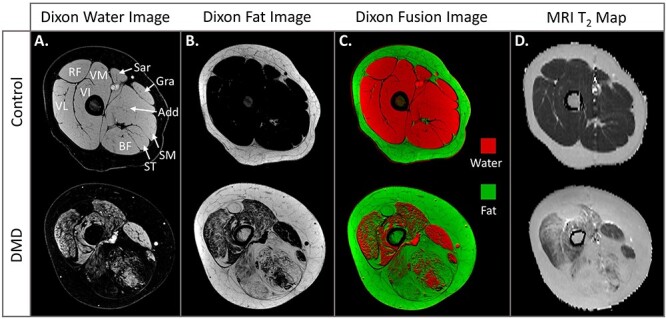
Magnetic resonance imaging (MRI) of quantitative muscle. Chemical shift-encoded imaging of the thigh muscles produces 2 separate images: (A) 1 that only contains signal from water, and (B) 1 that only contains signal from fat. Unaffected muscle, such as that seen in this 9-year-old control, should be comprised nearly entirely of water signal. Fat signal is present in the subcutaneous tissue and in dystrophic muscle, including in the muscles of this 10-year-old with Duchenne muscular dystrophy (DMD). (C) When the water image is digitally colored red and fat image digitally colored green, they can be overlaid (fused) to create a visually informative image. (D) MRI T2 maps display the T2 values of each individual pixel of the MRI. MRI T2 is a value that differs for each tissue, but unaffected muscle has a low T2 value (~30–35 ms) while fatty/edematous muscle has higher T2 values (~35–80 ms). MRI T2 values are well correlated with fat fraction values in DMD. Add = adductor group; BF = biceps femoris long head; Gra = gracilis; RF = rectus femoris; Sar = sartorius; SM = semimembranosus; ST = semitendinosus; VI = vastus intermedius; VL = vastus lateralis; VM = vastus medialis.
The purpose of this article is twofold. First, we summarize muscle pathology and disease progression findings from qualitative and quantitative muscle MRI studies in DMD. Second, we link MRI findings of muscle pathology to clinical observations and discuss any potential implications of MRI findings for physical therapy management. We use representative MRIs collected from 2 institutional review board-approved natural history studies of magnetic resonance biomarkers in DMD to illustrate typical patterns of muscle pathology in 3 major body regions: the trunk, lower extremities, and upper extremities. For each region, we first review MRI findings and second, draw relationships to strength and movement alterations, acknowledging that muscle pathology is only 1 factor that can affect movement. We hope this information provides a foundation of knowledge to physical therapists treating individuals with DMD to facilitate evidence-based treatment and care decisions. Additionally, we hope insights into the underlying muscle pathology can serve as a catalyst for thoughtful development of robust research studies of physical therapist care for this unique patient population.
Axial Muscles
MRI of Axial Musculature
Both clinical and MRI research of axial muscle involvement in DMD are limited, even though these are among the first muscles affected. One study, performed by our group, captured the trunk while imaging respiratory muscles.35 Control participants have little/no axial muscle fatty infiltration (Fig. 3A). In contrast, individuals with DMD have evidence of muscle pathology beginning in early stages of the disease when they are still ambulatory and highly functional (Fig. 3B). In particular, the pectoral muscles, latissimus dorsi, internal oblique, and lumbar paraspinals show the earliest signs of involvement.35 Fatty infiltration of these muscles progresses relentlessly throughout the ambulatory stage followed by involvement of the psoas, quadratus lumborum, external obliques, and rectus abdominis. Within the first few years of losing ambulation, the trunk muscles show near complete replacement by fat, as demonstrated in Figure 3C.
Figure 3.
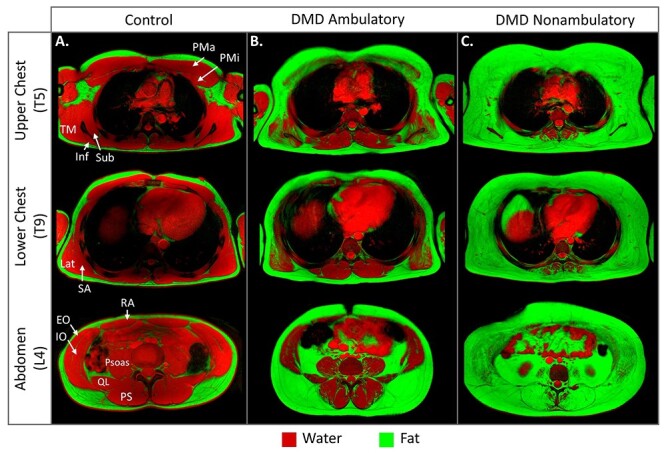
Magnetic resonance imaging (MRI) of abdominal and chest muscle. (A) Axial fat/water fusion images of the trunk at the T5, T9, and L4 vertebral levels reveal little to no fatty infiltration in a control (age = 17 y). (B) Involvement of several muscles is visible in a representative early ambulatory individual with DMD (age = 11 y). (C) Once individuals become non-ambulatory, the majority of the trunk musculature becomes replaced by fatty tissue. This is a 12-year-old, steroid-untreated individual who has been non-ambulatory for approximately 2 y. EO = external oblique; Inf = infraspinatus; IO = internal oblique; Lat = latissimus dorsi; PMa = pectoralis major; PMi = pectoralis minor; PS = paraspinal muscle group; psoas = psoas major; QL = quadratus lumborum; RA = rectus abdominis; SA = serratus anterior; Sub = subscapularis; TM = teres major.
Although respiratory impairment is a later manifestation of DMD, the axially located diaphragm and accessory respiratory muscles are critical for ventilation and respiratory health. The diaphragm is very thin, but a recent study quantified fatty replacement of the thicker diaphragm crura, which attach to the vertebrae.36 The diaphragm showed signs of fatty infiltration in the early-mid teens with a slow progression into the mid-late 20s, and fatty replacement correlated with forced vital capacity. MRI movies and breath-hold images obtained during maximal breathing have shown reduced diaphragm descent during inspiration, primarily in individuals older than 15 years, reduced chest expansion, and smaller lung dimensions.35–37 The external oblique, internal oblique, and rectus abdominis are used for forced expiration and coughing, which are critical for airway clearance. The internal oblique is most affected, followed by the external oblique and rectus abdominis, and fatty infiltration of these muscles is correlated with maximal expiratory pressures.35 The participant in Figure 3C has a forced vital capacity of 93% but a maximal expiratory pressure of only 51% (where >80% is considered within normal limits), which may be a result of the extensive expiratory abdominal muscle degeneration.
Linking MRI Data to Axial Muscle Function
The axial muscles of the trunk are responsible for flexion, extension, lateral movements, and rotary movements related to postural control, stabilization, balance, and reaching. In individuals with DMD, equilibrium/righting reactions and dynamic postural control mechanisms that rely on trunk musculature may contribute to decreased balance.38,39 In other populations with weak trunk muscles, individuals generally attempt to use the arms to stabilize the trunk (i.e. cerebral palsy). As the axial and upper extremity muscles experience disease progression in DMD, these compensations are not viable ways to stabilize the trunk or prevent falling. Given the early involvement of the axial muscles, progressive lower extremity weakness, and other individual factors, fall risk reduction is important for physical therapists to address, particularly in individuals with steroid-induced bone fragility.21
A recent study evaluating trunk muscle performance in young teens with DMD revealed reduced active ROM and maximal joint torques in all directions compared with controls.40 Despite the axial weakness, individuals with DMD compensate for shoulder/scapular weakness during reaching by using increased trunk lateral bending or flexion-extension movements.40 Based on EMG, some individuals require nearly 100% of available arm and trunk muscle capacity to perform reaching tasks with weighted objects. The results of this study mirror the early and widespread muscle involvement captured in MRIs of the trunk (Fig. 3) and shoulder. Given the extensive degeneration of trunk muscles already present when individuals transition to full-time wheelchair use, adequate trunk support should be an important consideration in seating selection. Additionally, pressure relief cushions may become important as loss of trunk musculature limits the ability for independent leaning and repositioning for pressure relief.41
Physical therapists may have a role in the management of respiratory manifestations in DMD. Because respiratory muscles degenerate and weaken over time, respiratory muscle training has been attempted. Although not addressed in the DMD care guidelines, a 2019 Cochrane Review states respiratory muscle training may be beneficial in DMD; however, the certainty of evidence is low, and concerns regarding muscle damage have not been assessed.42 We hypothesize that there may be benefits to carefully prescribed inspiratory and expiratory muscle training in DMD, but this is an area that requires further physical therapy research. DMD care guidelines also indicate that maintaining chest wall mobility may help mitigate respiratory impairments, though there is no direct evidence supporting this statement.17 MRI of the chest wall (Fig. 3C) does suggest that by the non-ambulatory stage, the rib cage becomes surrounded by fibrofatty tissue rather than healthy muscle. The impact of thoracic fibrofatty tissue on chest mobility and respiratory function, as well as interventions to address rib cage stiffness, are interesting areas for future physical therapy research.
Lower Extremity
MRI of Lower Extremity Musculature
MRI investigations of disease progression in DMD have primarily focused on the lower extremity muscles, which are critical for ambulation and affected early in the disease. Given the proximal to distal progression of DMD, the gluteal muscles are among the first lower extremity muscles to display evidence of fatty infiltration, which is visible in the fat/water fusion images in Figure 4.23,43–47 One qualitative MRI study found that 13/14 children ages 1 to 2 years already had evidence of fatty infiltration in the gluteus maximus, and over 90% of 9- to 10-year-olds had >60% fatty replacement.44 The gluteus medius and minimus are also affected early, but quantitative MRI T2 imaging suggests severity is slightly less than the gluteus maximus.23,45 The iliopsoas maintains lower fatty infiltration than the gluteals despite signs of inflammation via elevated MRI T2.23,45
Figure 4.
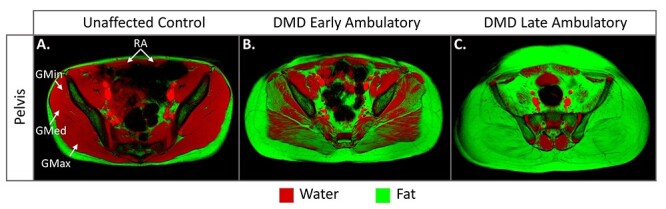
Magnetic resonance imaging (MRI) of pelvic girdle muscle. Fat (green) and water (red) fusion images of axial MRIs of the pelvis. (A) Unaffected controls have little to no visible pelvis muscle fatty infiltration. The individual shown here is 13 y old. (B) Gluteal muscle involvement is present in DMD beginning at a young age and even in individuals with preserved functional abilities. Shown here is an 11-year-old who could rise from the floor in 3.2 seconds (average control time = 1.5 s) and climb stairs in 1.9 seconds without rail support (average control time = 1.2 s). (C) By the late ambulatory phase, many individuals have complete replacement of the gluteal muscles by fat. Representative image from a 16-year-old who lost ambulation 9 months after image acquisition. GMax = gluteus maximus; GMed = gluteus medius; GMin = gluteus minimus; RA = rectus abdominis.
Of the upper leg muscles (Fig. 5A–D), the adductor magnus shows signs of fatty infiltration first, followed by the biceps femoris and quadriceps.47 Within the quadriceps, the rectus femoris is often most affected, while the other 3 component muscles are similarly affected.28,45 Although most MRI studies image children >5 years old, in a qualitative case series, fat was observed in the adductor magnus and quadriceps of 2/5 children <5 years old with DMD.46 Initial increases in fat infiltration in the vastus lateralis are present in most boys ages 5 and 6,48 and a regression analysis estimated biceps femoris fat infiltration as early as 3 to 4 years old.49 In young individuals who do not yet have increased fat in their quadriceps, MRI T2 can be increased, likely from inflammation and muscle damage.50 Interestingly, the sartorius, gracilis, and, to a lesser degree, semitendinosus demonstrate relative sparing (Fig. 5C). MRI measures of muscle volume or cross-sectional area reveal quadriceps atrophy in some, but not all, individuals, while the gracilis and sartorius often hypertrophy.51,52
Figure 5.
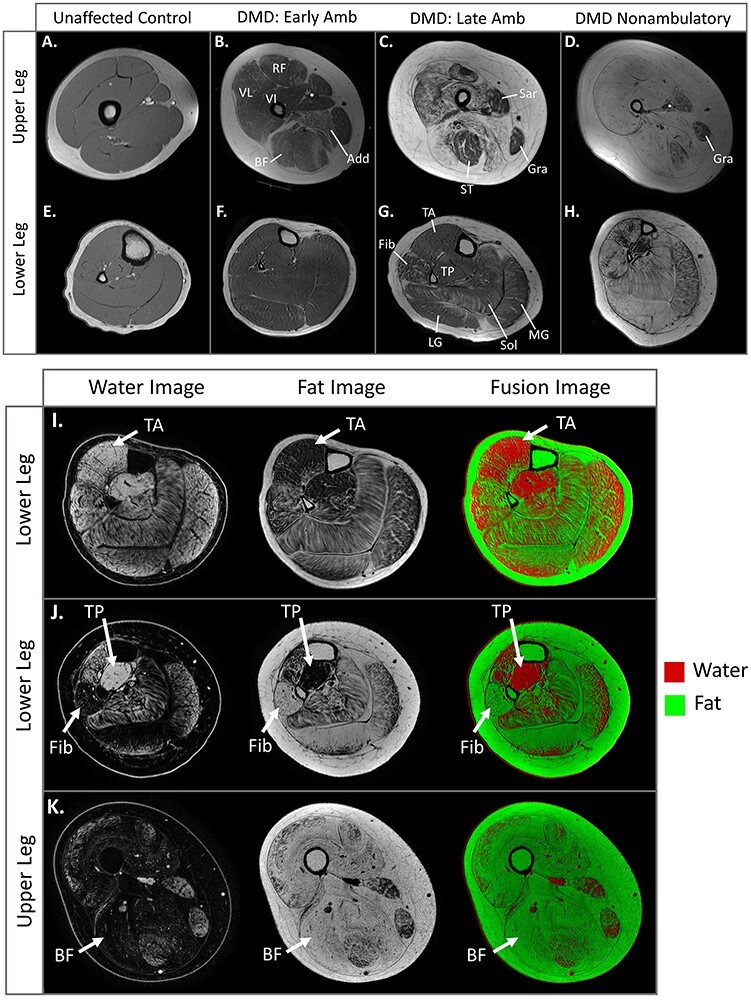
Magnetic resonance imaging (MRI) of upper and lower leg muscle. (A–D) T1-weighted axial MRIs of the upper leg and (E–H) lower leg in an unaffected control and 3 individuals with Duchenne muscular dystrophy (DMD). (A,E) In the unaffected control (age = 12 y), the muscles have a smooth-appearing texture and moderate signal intensity without evidence of fatty infiltration. (B,F) This individual with DMD (age = 11 y, same individual as in Fig. 4B) has visible fatty infiltration of the quadriceps, adductor magnus, and biceps femoris muscles. There is minimal fatty infiltration visible in the gastrocnemius muscles. (C,G) In this 16-year-old who requires 8.2 seconds to traverse 10 m (average control time = 2.7 s), the vastus lateralis (VL) has approximately 50% fat, the adductors (Add) are approaching complete fatty replacement, and the fibularis group and calf muscles have an estimated 40% fat. (D,H) This steroid-untreated individual (age = 12 y, nonambulatory for ~2 y) has nearly complete fatty replacement of muscle with some sparing of the gracilis (Gra) and tibialis posterior (TP). (I–K) Water, fat, and fat/water fusion images highlight patterns of muscle involvement and sparing as well as loss of contractile tissue in the upper and lower legs. (I) Despite plantar-flexion contractures, the tibialis anterior (TA) typically remains less involved than the calf muscles. (J) These images demonstrate dramatic involvement of the fibularis group in stark contrast to sparing of the TP that contributes to equinovarus contractures. (K) Contractures are ubiquitous in the nonambulatory stage, and these images reveal the lack of remaining contractile tissue in this stage. Note the complete replacement of the biceps femoris by fat. Add = adductor; BF = biceps femoris long head; Early amb = early ambulation; Fib = fibularis longus and brevis; late amb = late ambulation; LG = lateral gastrocnemius; RF = rectus femoris; Sar = sartorius; Sol = soleus; ST = semitendinosus; VI = vastus intermedius.
The lower leg muscles demonstrate less pathology than the upper leg muscles at a given age, consistent with the proximal to distal disease pattern (Fig. 5E–H).1,48,52 The fibularis longus/brevis, soleus, and gastrocnemius muscles progress most quickly in the lower leg, while the tibialis anterior progresses at an intermediate rate, and the tibialis posterior is preserved (Fig. 5G).52–54 As with thigh muscles, signs of pathology can be detected prior to fatty infiltration through elevated MRI T2, elevated intracellular sodium, and abnormal spectroscopy markers compared with unaffected individuals.55,56 Calf hypertrophy is a classic sign of DMD, and MRI studies confirm increased contractile area and volume of the triceps surae and occasionally the fibularis longus/brevis and tibialis anterior.51,52,57
Linking MRI Data to Ambulatory Abilities
Gait deviations in the DMD population are well studied and described, and MRI evidence of muscle degeneration can help explain many deviations. One of the first gait deviations noted is increased lumbar lordosis in an attempt to move the line of force behind the hip joint to compensate for gluteal and hip extensor weakness.58–60 This compensation mirrors MRI findings of very early loss of contractile tissue in gluteal and hip extensor muscles. In Figure 4, compared with an unaffected control, the individual with DMD in the early ambulatory stage (defined here as able to rise from the floor and climb stairs) already has substantial fatty infiltration of all 3 gluteal muscles. This image dramatically illustrates that significant muscle degeneration can occur prior to overt functional impairment as this individual was able to climb stairs without a railing in 1.85 sec and run with a flight phase at the time of image acquisition. The early degeneration of the gluteus medius and minimus likely contribute to the characteristic waddling gait and positive Trendelenburg sign.58,61 The pelvic muscles progress rapidly, and by the late ambulatory stage (unable to rise from the floor or climb stairs), these muscles can be completely replaced by fibrofatty tissue (Fig. 4C).
Gait is also impacted by knee extensor weakness, which is consistent with progressive MRI signs of pathology in the quadriceps group (Fig. 5B-C). In the loading response phase of gait, individuals place the foot flat or toes first to reduce knee flexion and maintain the force line in front of the knee joint.60 Attempts to maintain an extensor moment continue in mid- and terminal stance.61 Early involvement of the quadriceps also contributes to reduced eccentric control with squatting or step-down movements, and, combined with hip extensor involvement, leads to a dependence on handrails to ascend/descend stairs. At the ankle, the dorsiflexion moment at initial contact and early stance is decreased, and in-toeing becomes common during stance and swing. These alterations may be due to several factors including a purposeful reduction in knee flexion, limited ankle dorsiflexion ROM, and a preserved tibialis posterior that contributes to ankle inversion (Fig. 5C).60,61
Quantitative muscle MRI measures can give physical therapists an idea of the fat fraction percentage associated with specific gait and ambulatory abilities, helping to guide intervention selection, predict future functional changes, and predict future needs such as wheeled mobility. Individuals who can run with a flight phase average approximately 10% vastus lateralis fat, while those who can run, but without a flight phase, average approximately 20% vastus lateralis fat.30 Individuals first begin losing the ability to ambulate when the quadriceps reaches approximately 40% replacement by fat; however, some individuals are able to use compensatory strategies to continue ambulating even when up to 70% of the quadriceps has been replaced by fat, suggesting many factors play a role in maintenance of ambulation.30 This sentinel event is not age dependent but occurs over a range of ages. Another group estimated that loss of ambulation occurs when fatty replacement of the quadriceps, adductors, and hamstrings reaches 50%.49
Beyond gait, the degeneration of the proximal lower extremity muscles contributes to classic compensatory movements such as Gower’s maneuver to rise from the floor and step-to patterns while ascending or descending stairs. MRI evidence of gluteal and hamstring involvement in children ≤6 years old explains why Gower’s maneuver is often an initial manifestation of DMD.44,50 One MRI study found that gluteus maximus pathology (measured by MRI T2) was well correlated with time to rise from the floor, and loss of this ability was estimated to occur when the gluteus maximus has >75% fatty replacement and the vastus lateralis has approximately 45% replacement.23,30,47
Despite muscle degeneration, muscle weakness, and other individual/environmental factors, individuals with DMD adopt impressive compensatory strategies to maintain ambulatory functional skills. Attempting to correct these postural and movement compensations is typically not appropriate and may be counterproductive. For example, attempting to reduce standing lumbar lordosis in an individual reporting lower back pain during gait may limit the ability to compensate for significant axial and hip muscle weakness and degeneration. Other management strategies may be more appropriate, including positioning recommendations to relieve stress on the lumbar spine when not ambulating and referral to a physician to assess for vertebral fractures. Similarly, physical therapists may mistakenly believe ankle-foot orthoses (AFOs) will help gait. As noted in the DMD care guidelines, the use of AFOs to assist with gait is typically not recommended.1,21 AFOs shift the center of gravity, limiting individuals from using compensatory strategies during functional movement and making tasks such as rising from the floor, walking, and navigating stairs even more challenging.
Clinicians may believe strengthening exercises are appropriate in the early phases of DMD since they do not observe noticeable changes (though pathological changes likely exist), and the literature indicates that young boys may not have reached a plateau in their motor skills. While preliminary evidence suggests sub-maximal exercise using cycling or isometric contractions may be beneficial to individuals with DMD,62–64 caution is required for exercise prescription. Eccentric contractions create a high level of muscle strain and can cause muscle damage in typically healthy individuals.65 The muscles of boys with DMD are more susceptible to this type of contraction-induced muscle damage and injury from eccentric exercise.6 Therefore, as noted in the care guidelines, eccentric muscle training or high-intensity exercise in DMD is not recommended and should be avoided.1
Linking MRI Data to Lower Extremity Contracture Development
DMD care guidelines state a primary focus of physical therapy is prevention and/or management of lower extremity contractures.1,21 Plantar-flexion contractures typically manifest in the ambulatory stage while loss of hip and knee extension ROM and progression of ankle contractures to an equinovarus position often develop primarily after loss of ambulation.13,66,67 Contracture development in DMD is ubiquitous and likely multifactorial, resulting from an interplay between loss of contractile tissue, replacement by fibrofatty tissue, static positioning, agonist/antagonist imbalance, and compensatory movement patterns.67 The pathophysiology of contracture development in DMD is not well understood; however, MRI does offer some clues for understanding the development of lower extremity contractures.
Figure 5I illustrates the well-documented sparing of the tibialis anterior compared with the gastrocnemius and soleus muscles.52–54 Here, plantar-flexion contracture development may be multifactorial, potentially driven by fibrofatty changes in the calf muscles, a preserved tibialis posterior, and compensatory toe walking rather than solely by gross agonist/antagonist muscle imbalance such as preferential dorsiflexor weakness. In Figure 5J, a nonambulatory individual (−40 degree ankle dorsiflexion) demonstrates complete fatty replacement of the fibularis longus/brevis (ankle evertors), degeneration of calf muscles, and selective sparing of the tibialis posterior, likely contributing to the equinovarus deformity typical of the nonambulatory stage. Finally, Figure 5K demonstrates the absence of quadriceps and hamstring contractile tissue in a nonambulatory individual. Static sitting position, limited antigravity quadriceps strength, and fibrosis of the knee flexors are possible contributors to knee flexion contractures.67 The efficacy of stretching and bracing in the presence of significant loss of muscle contractile tissue and replacement by fibrofatty tissue is not well understood (see “Looking Ahead”).
Upper Extremity
MRI of Upper Extremity Musculature
Interest in better understanding upper extremity muscle involvement in DMD has increased as individuals are now thriving long after loss of ambulation; however, limited MRI data exist.24,68–71 Two surprising findings from initial imaging studies are: (1) shoulder girdle muscle involvement is present well before loss of ambulation (some degree of involvement of the shoulder girdle muscles was seen in all 5- to 10-year-old boys in the early ambulatory stage in 1 study),24 and (2) muscle involvement may already be present before any decreases in the commonly utilized Upper Extremity Brooke Scale (UEBS) or Performance of Upper Limb (PUL) assessments are seen.72,73 For reference, the UEBS rates individuals from 1 (able to abduct arms overhead without compensation) to 6 (has no useful hand function), while the 22-item PUL assesses upper extremity tasks, including lifting heavy cans, tracing a path, and picking up coins.
Unaffected controls have little to no fatty infiltration of the upper extremity muscles (Fig. 6A). Imaging has found that in DMD, rotator cuff, deltoid, serratus anterior, and latissimus dorsi muscles are the first muscles affected involved in arm function.24,68 These muscles also attach to the thorax, demonstrating the tight connection between shoulder and axial musculature. Figure 6B represents a highly functional individual with a UEBS score of 1 and nearly perfect PUL (41/42 points). However, MRI images in Figure 6B, as well as in the same individual in Figure 3B, already reveal signs of fatty infiltration in shoulder girdle muscles. In fact, most individuals with UEBS scores of 1 and full PUL scores already have fatty infiltration in the infraspinatus, subscapularis, serratus anterior, teres minor, latissimus dorsi, and the deltoid on MRI.24
Figure 6.
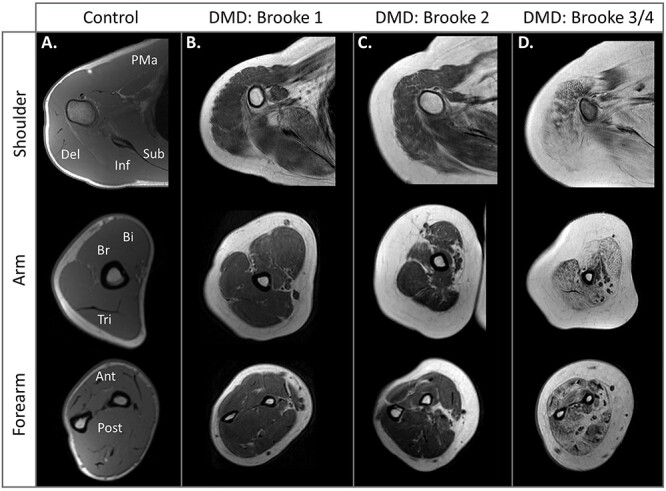
Magnetic resonance imaging (MRI) of upper extremity muscle. T1-weighted images of the shoulder, arm, and forearm. (A) The unaffected control has little to no visible fat in the upper extremity muscles. (B) In this early–ambulatory stage individual (age = 11 y) who can abduct the arms overhead without compensation, the scapular muscles and deltoid already show fatty infiltration. (C) In a late ambulatory individual who can abduct his arms overhead only by using compensatory movements (age = 16 y), progression of shoulder complex muscles is visible, and signs of upper arm muscle involvement are present. (D) Progressive fatty infiltration of the shoulder, arm, and forearm muscles is visible in this steroid-untreated 12-year-old who cannot abduct his arms overhead but can bring his hand to his mouth. Ant = anterior forearm muscle group; Bi = biceps brachii; Br = brachialis; Del = deltoid; Inf = infraspinatus; PMa = pectoralis major; Post = posterior forearm muscle group; Sub = subscapularis; Tri = triceps brachii.
Evidence of fat in the biceps, triceps, and brachialis becomes noticeable typically in the late ambulatory or early nonambulatory years.24 However, some younger individuals show signs of inflammation (increased MRI T2) prior to increased fat in these muscles.68Figure 6C highlights the progression of shoulder girdle muscle fatty infiltration and the initial signs of upper arm muscle degeneration in a late ambulatory individual with a UEBS score of 2 (able to abduct arms overhead only with compensatory movements) and 39/42 on the PUL. The forearm muscles are also being studied with MRI as they are thought to be sensitive to disease progression in later stages when upper arm and shoulder girdle muscles are nearing complete degeneration. Imaging studies reveal the supinator and pronator teres muscles are the first forearm muscles with fatty infiltration, and this may occur even in ambulatory individuals.24 However, disease progression of the forearm muscles is much slower in the ambulatory phase compared with the nonambulatory phase.71 Once individuals become nonambulatory, forearm muscle fatty infiltration increases significantly year to year.69,70Figure 6D is representative of a nonambulatory individual with a UEBS score of 3 to 4 (limited antigravity shoulder strength but able to lift the hand to the mouth) and 22/42 points on the PUL. In this example, the forearm muscles are already significantly affected.
Linking MRI Data to Upper Extremity Function
Physical and occupational therapists are experts in assessment of strength and function; however, the UEBS and PUL assessments may not detect early arm muscle pathology or functional limitations in DMD. Innovative measures using Microsoft Kinect platforms have been developed to quantify the important arm function of reaching. The Reachable Workspace measure found that individuals with DMD with UEBS scores of 1 had similar reaching areas as controls (the trunk was stabilized), but once the wrist was weighted with a 0.5-kg or 1-kg weight, the reachable space was significantly reduced.74 The physical therapist–developed ACTIVE-Seated measure, which does not provide trunk stabilization, found decreased seated reaching volume in individuals with DMD with UEBS scores of 1 compared with controls, likely due to early trunk muscle weakness.75 These results demonstrate an ability to capture functional impairments even in individuals with UEBS scores of 1, and additional clinical assessments of early arm function or strength impairments may be informative for physical therapists.
The shoulder musculature both stabilizes and creates motion at the shoulder girdle complex, and the early and progressive degeneration of the shoulder muscles in DMD impair shoulder girdle/glenohumeral stability as well as mobility. Shoulder pain and stiffness are reported with increasing frequency as individuals with DMD age and may need to be addressed by physical therapists. Arm support systems have been developed with the goal of maximizing functional shoulder and arm movement, active ROM, and reaching ability,76,77 and physical therapists can assist in the trialing and selection of the best arm support systems for an individual’s specific impairments, daily environment, and required tasks. Finally, given the early involvement of axial, shoulder, and arm muscles in DMD, prescribing a walker for gait assistance is typically not appropriate and may increase risk of falls and fatigue.
Maintenance of elbow, forearm, and hand ROM are important goals, particularly in the nonambulatory phase, to allow individuals to optimize independence with self-care, feeding, keyboard/phone use, and powerchair operation. Fibrofatty infiltration of the biceps brachii, with loss of contractile tissue and more frequent positioning of the arms on wheelchair armrests, may contribute to elbow flexion contractures alongside agonist/antagonist imbalance. In fact, MRI quantification of muscle pathology shows the triceps brachii is typically slightly less involved than the biceps brachii in boys and teenagers.68 As noted above, forearm muscles, which control wrist/hand function, progress much slower in the ambulatory phases of the disease; however, limited supination may appear while boys are still walking, potentially due to the faster progressing supinator, pronator teres, and pronator quadratus. The loss of these motions, in combination with elbow contractures, can affect functional activities such as reaching, grooming, self-feeding, and object manipulation (opening containers, turning objects over, opening doors). As with lower extremity contracture management, additional physical therapy research is needed to better understand upper extremity contracture pathophysiology, prevention, and management strategies.
Looking Forward
The natural history of disease progression in DMD has evolved significantly over the last 2 decades, particularly with the widespread use of corticosteroids from a young age. Individuals with DMD are very commonly living into adulthood, but there is limited contemporary scientific literature addressing the physical therapy needs of boys and young men with DMD. MRI of dystrophic muscle compellingly documents the progression of contractile tissue loss, and in this review, we have detailed MRI findings in DMD to facilitate understanding of the compensatory mechanisms used to sustain posture and functional movement.
Many areas of additional physical therapy research in DMD are needed, including some that may benefit from inclusion of imaging measures. One exciting application of MRI to this population is the use of quantitative imaging to assess the safety and efficacy of different exercise intensities and regimens. MRI was recently utilized as a safety measure to assess any increase in skeletal muscle inflammation or edema in a pilot study of a 12-week isometric exercise program.64 Continued pairing of quantitative MRI with high-quality exercise studies in DMD may assist clinicians in better understanding what activities are safe vs damaging. Additionally, as dystrophin-restoration therapies continue to show future promise, physical therapists’ understanding of how partial dystrophin production affects exercise safety will be important.
Another area of limited research is contracture management and prevention. Thus far, studies investigating the benefits of bracing, casting, and stretching for lower extremity contracture management have inconclusive results, particularly in relation to functional improvement.78–81 Differentiating the nature and mechanisms of contractures in this population is critical to better guide clinical decisions in DMD. Randomized clinical trials investigating contracture management and prevention interventions are needed to more definitively support or argue against different management approaches. MRI could be a useful adjunct tool to classify individuals based on muscle pathology and determine if interventions have more/less success at different stages of contractile tissue loss and fibrofatty replacement.
In conclusion, we are opening the door and inviting researchers and clinicians to critically explore the role of physical therapy in DMD using contemporary evidence from imaging studies and high-quality clinical studies as a guide. The challenge before us is to enhance our understanding of DMD and optimize therapy recommendations for families' already demanding daily routines and regimens. Our responsibility as providers is to understand the current literature, including the imaging literature, to inform best practice and shape the future of interventions for this population by studying the effect of therapy interventions at the impairment, activity, and participation levels in people with DMD.
Author Contributions and Acknowledgments
Concept/idea/research design: C.R. Senesac, A.M. Barnard, D.J. Lott, A.T. Harrington, R.J. Willcocks, W.D. Rooney, G.A. Walter, K. Vandenborne
Writing: C.R. Senesac, A.M. Barnard, D.J. Lott, K.S. Nair, R.J. Willcocks, K.L. Zilke, G.A. Walter
Data collection: C.R. Senesac, A.M. Barnard, D.J. Lott, K.S. Nair, A.T. Harrington, R.J. Willcocks, K.L. Zilke, W.D. Rooney, K. Vandenborne
Data analysis: A.M. Barnard, D.J. Lott, A.T. Harrington, R.J. Willcocks, W.D. Rooney, K. Vandenborne
Project management: C.R. Senesac, W.D. Rooney, K. Vandenborne
Fund procurement: K. Vandenborne
Providing participants: C.R. Senesac, K. Vandenborne
Providing facilities/equipment: W.D. Rooney, K. Vandenborne
Providing institutional liaisons: W.D. Rooney, K. Vandenborne
Consultation (including review of manuscript before submitting): A.T. Harrington
The authors acknowledge the participants and their families for their dedication and involvement in research and are appreciative of the MRI technologists who assisted in image acquisition.
Ethics Approval
The original magnetic resonance images included in the figures in this study are images from 2 University of Florida Institutional Review Board-approved and HIPAA-compliant studies utilizing written informed consent and written assent (from minors).
Funding
The magnetic resonance images displayed in the figures of this article were collected as part of 2 studies supported by grant funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (R01-AR056973 and U54-AR052646). One of the first authors (A.M.B.) was supported by a training grant from the National Heart, Lung, and Blood Institute (T32-HL134621). The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest.
Contributor Information
Claudia R Senesac, Department of Physical Therapy, University of Florida, Box 100154, UFHSC, Gainesville, FL 32610-0154 (USA).
Alison M Barnard, Department of Physiology and Functional Genomics, University of Florida.
Donovan J Lott, Department of Physical Therapy, University of Florida.
Kavya S Nair, Department of Physical Therapy, University of Florida.
Ann T Harrington, Center for Rehabilitation, Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania; and Department of Physical Therapy, Arcadia University, Glenside, Pennsylvania.
Rebecca J Willcocks, Department of Physical Therapy, University of Florida.
Kirsten L Zilke, Oregon Health & Science University, Shriners Hospitals for Children, Portland, Oregon.
William D Rooney, Advanced Imaging Research Center, Oregon Health & Science University.
Glenn A Walter, Department of Physiology and Functional Genomics, University of Florida.
Krista Vandenborne, Department of Physical Therapy, University of Florida.
References
- 1. Birnkrant DJ, Bushby K, Bann CM, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018;17:251–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nigro G, Comi LI, Politano L, Bain RJI. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int J Cardiol. 1990;26:271–277. [DOI] [PubMed] [Google Scholar]
- 3. LoMauro A, Romei M, Gandossini S, et al. Evolution of respiratory function in Duchenne muscular dystrophy from childhood to adulthood. Eur Respir J. 2018;51:1701418. [DOI] [PubMed] [Google Scholar]
- 4. Hoffman EP, Brown Jr. RH, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 1987;51:919–928. [DOI] [PubMed] [Google Scholar]
- 5. Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 1993;122:809–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Petrof BJ, Shrager JB, Stedman HH, et al. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci. 1993;90:3710–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Godfrey C, Muses S, McClorey G, et al. How much dystrophin is enough: the physiological consequences of different levels of dystrophin in the mdx mouse. Hum Mol Genet. 2015;24:4225–4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peverelli L, Testolin S, Villa L, et al. Histologic muscular history in steroid-treated and untreated patients with Duchenne dystrophy. Neurology. 2015;85:1886–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mercuri E, Coratti G, Messina S, et al. Revised north star ambulatory assessment for young boys with Duchenne muscular dystrophy. PLOS ONE. 2016;11:e0160195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McDonald CM, Henricson EK, Abresch RT, et al. Long-term effects of glucocorticoids on function, quality of life, and survival in patients with Duchenne muscular dystrophy: a prospective cohort study. The Lancet. 2017;391:451–461. [DOI] [PubMed] [Google Scholar]
- 11. Passamano L, Taglia A, Palladino A, et al. Improvement of survival in Duchenne muscular dystrophy: retrospective analysis of 835 patients. Acta Myol. 2012;31:121–125. [PMC free article] [PubMed] [Google Scholar]
- 12. Kornegay JN, Childers MK, Bogan DJ, et al. The paradox of muscle hypertrophy in muscular dystrophy. Phys Med Rehabil Clin N Am. 2012;23:149–172xii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Choi Y-A, Chun S-M, Kim Y, Shin H-I. Lower extremity joint contracture according to ambulatory status in children with Duchenne muscular dystrophy. BMC Musculoskelet Disord. 2018;19:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Archer JE, Gardner AC, Roper HP, et al. Duchenne muscular dystrophy: the management of scoliosis. J. Spine Surg. 2016;2:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ricotti V, Mandy WPL, Scoto M, et al. Neurodevelopmental, emotional, and behavioural problems in Duchenne muscular dystrophy in relation to underlying dystrophin gene mutations. Dev Med Child Neurol. 2016;58:77–84. [DOI] [PubMed] [Google Scholar]
- 16. McNally EM, Kaltman JR, Benson DW, et al. Contemporary cardiac issues in Duchenne muscular dystrophy. Circulation. 2015;131:1590–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Birnkrant DJ, Bushby K, Bann CM, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018;17:347–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Griggs RC, Miller JP, Greenberg CR, et al. Efficacy and safety of deflazacort vs prednisone and placebo for Duchenne muscular dystrophy. Neurology. 2016;87:2123–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haas M, Vlcek V, Balabanov P, et al. European Medicines Agency review of ataluren for the treatment of ambulant patients aged 5 years and older with Duchenne muscular dystrophy resulting from a nonsense mutation in the dystrophin gene. Neuromuscul Disord. 2015;25:5–13. [DOI] [PubMed] [Google Scholar]
- 20. Aartsma-Rus A, Goemans N. A sequel to the eteplirsen saga: eteplirsen is approved in the United States but was not approved in Europe. Nucleic Acid Ther. 2019;29:13–15. [DOI] [PubMed] [Google Scholar]
- 21. Case LE, Apkon SD, Eagle M, et al. Rehabilitation management of the patient with Duchenne muscular dystrophy. Pediatrics. 2018;142:S17–S33. [DOI] [PubMed] [Google Scholar]
- 22. Mercuri E, Pichiecchio A, Allsop J, et al. Muscle MRI in inherited neuromuscular disorders: past, present, and future. J Magn Reson Imaging. 2007;25:433–440. [DOI] [PubMed] [Google Scholar]
- 23. Kim HK, Laor T, Horn PS, et al. T2 mapping in Duchenne muscular dystrophy: distribution of disease activity and correlation with clinical assessments 1. Radiology. 2010;255:899–908. [DOI] [PubMed] [Google Scholar]
- 24. Brogna C, Cristiano L, Tartaglione T, et al. Functional levels and MRI patterns of muscle involvement in upper limbs in Duchenne muscular dystrophy. PLOS ONE. 2018;13:e0199222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burakiewicz J, Sinclair CDJ, Fischer D, et al. Quantifying fat replacement of muscle by quantitative MRI in muscular dystrophy. J Neurol. 2017;264:2053–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mercuri E, Talim B, Moghadaszadeh B, et al. Clinical and imaging findings in six cases of congenital muscular dystrophy with rigid spine syndrome linked to chromosome 1p (RSMD1). Neuromuscul Disord. 2002;12:631–638. [DOI] [PubMed] [Google Scholar]
- 27. Arpan I, Forbes SC, Lott DJ, et al. T2 mapping provides multiple approaches for the characterization of muscle involvement in neuromuscular diseases: a cross-sectional study of lower leg muscles in 5–15-year-old boys with Duchenne muscular dystrophy. NMR Biomed. 2013;26:320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marden FA, Connolly AM, Siegel MJ, Rubin DA. Compositional analysis of muscle in boys with Duchenne muscular dystrophy using MR imaging. Skeletal Radiol. 2005;34:140–148. [DOI] [PubMed] [Google Scholar]
- 29. Gaeta M, Scribano E, Mileto A, et al. Muscle fat fraction in neuromuscular disorders: dual-echo dual-flip-angle spoiled gradient-recalled MR imaging technique for quantification—a feasibility study. Radiology. 2011;259:487–494. [DOI] [PubMed] [Google Scholar]
- 30. Barnard AM, Willcocks RJ, Finanger EL, et al. Skeletal muscle magnetic resonance biomarkers correlate with function and sentinel events in Duchenne muscular dystrophy. PloS One. 2018;13:e0194283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bonati U, Hafner P, Schädelin S, et al. Quantitative muscle MRI: a powerful surrogate outcome measure in Duchenne muscular dystrophy. Neuromuscul Disord. 2015;25:679–685. [DOI] [PubMed] [Google Scholar]
- 32. Mankodi A, Bishop CA, Auh S, et al. Quantifying disease activity in fatty-infiltrated skeletal muscle by IDEAL-CPMG in Duchenne muscular dystrophy. Neuromuscul Disord. 2016;26:650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maillard SM. Quantitative assessment of MRI T2 relaxation time of thigh muscles in juvenile dermatomyositis. Rheumatology. 2004;43:603–608. [DOI] [PubMed] [Google Scholar]
- 34. Carlier PG. Global T2 versus water T2 in NMR imaging of fatty infiltrated muscles: DIFFERENT methodology, different information and different implications. Neuromuscul Disord. 2014;24:390–392. [DOI] [PubMed] [Google Scholar]
- 35. Barnard AM, Lott DJ, Batra A, et al. Imaging respiratory muscle quality and function in Duchenne muscular dystrophy. J Neurol. 2019;266:2752–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pennati F, Arrigoni F, LoMauro A, et al. Diaphragm involvement in Duchenne muscular dystrophy (DMD): an MRI study. J Magn Reson Imaging. 2020;51:461–471. [DOI] [PubMed] [Google Scholar]
- 37. Mankodi A, Kovacs W, Norato G, et al. Respiratory magnetic resonance imaging biomarkers in Duchenne muscular dystrophy. Ann Clin Transl Neurol. 2017;4:655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. de SCDSC, Fagundes IK, Araújo TB, et al. The relevance of trunk evaluation in Duchenne muscular dystrophy: the segmental assessment of trunk control. Arq Neuropsiquiatr. 2016;74:791–795. [DOI] [PubMed] [Google Scholar]
- 39. Kaya P, Alemdaroğlu İ, Yılmaz Ö, et al. Effect of muscle weakness distribution on balance in neuromuscular disease. Pediatr Int. 2015;57:92–97. [DOI] [PubMed] [Google Scholar]
- 40. Peeters LHC, Kingma I, Dieën JH, Groot IJM. Don’t forget the trunk in Duchenne muscular dystrophy patients: more muscle weakness and compensation than expected. J Neuroengineering Rehabil. 2019;16:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu M, Mineo K, Hanayama K, et al. Practical problems and management of seating through the clinical stages of Duchenne’s muscular dystrophy. Arch Phys Med Rehabil. 2003;84:818–824. [DOI] [PubMed] [Google Scholar]
- 42. Silva IS, Pedrosa R, Azevedo IG, et al. Respiratory muscle training in children and adults with neuromuscular disease. Cochrane Database Syst Rev. 2019;9:CD011711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim HK, Merrow AC, Shiraj S, et al. Analysis of fatty infiltration and inflammation of the pelvic and thigh muscles in boys with Duchenne muscular dystrophy (DMD): grading of disease involvement on MR imaging and correlation with clinical assessments. Pediatr Radiol. 2013;43:1327–1335. [DOI] [PubMed] [Google Scholar]
- 44. Li W, Zheng Y, Zhang W, et al. Progression and variation of fatty infiltration of the thigh muscles in Duchenne muscular dystrophy, a muscle magnetic resonance imaging study. Neuromuscul Disord. 2015;25:375–380. [DOI] [PubMed] [Google Scholar]
- 45. Polavarapu K, Manjunath M, Preethish-Kumar V, et al. Muscle MRI in Duchenne muscular dystrophy: evidence of a distinctive pattern. Neuromuscul Disord. 2016;26:768–774. [DOI] [PubMed] [Google Scholar]
- 46. Pichiecchio A, Alessandrino F, Bortolotto C, et al. Muscle ultrasound elastography and MRI in preschool children with Duchenne muscular dystrophy. Neuromuscul Disord. 2018;28:476–483. [DOI] [PubMed] [Google Scholar]
- 47. Gaeta M, Messina S, Mileto A, et al. Muscle fat-fraction and mapping in Duchenne muscular dystrophy: evaluation of disease distribution and correlation with clinical assessments: preliminary experience. Skeletal Radiol. 2012;41:955–961. [DOI] [PubMed] [Google Scholar]
- 48. Willcocks RJ, Rooney WD, Triplett WT, et al. Multicenter prospective longitudinal study of magnetic resonance biomarkers in a large Duchenne muscular dystrophy cohort. Ann Neurol. 2016;79:535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fischmann A, Hafner P, Gloor M, et al. Quantitative MRI and loss of free ambulation in Duchenne muscular dystrophy. J Neurol. 2013;260:969–974. [DOI] [PubMed] [Google Scholar]
- 50. Forbes SC, Willcocks RJ, Triplett WT, et al. Magnetic resonance imaging and spectroscopy assessment of lower extremity skeletal muscles in boys with Duchenne muscular dystrophy: a multicenter cross sectional study. PLoS ONE. 2014;9:e106435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Godi C, Ambrosi A, Nicastro F, et al. Longitudinal MRI quantification of muscle degeneration in Duchenne muscular dystrophy. Ann Clin Transl Neurol. 2016;3:607–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wokke BH, Bergen JC, Versluis MJ, et al. Quantitative MRI and strength measurements in the assessment of muscle quality in Duchenne muscular dystrophy. Neuromuscul Disord. 2014;24:409–416. [DOI] [PubMed] [Google Scholar]
- 53. Willcocks RJ, Arpan IA, Forbes SC, et al. Longitudinal measurements of MRI-T2 in boys with Duchenne muscular dystrophy: effects of age and disease progression. Neuromuscul. Disord. 2014;24:393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Torriani M, Townsend E, Thomas BJ, et al. Lower leg muscle involvement in Duchenne muscular dystrophy: an MR imaging and spectroscopy study. Skeletal Radiol. 2012;41:437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mankodi A, Azzabou N, Bulea T, et al. Skeletal muscle water T2 as a biomarker of disease status and exercise effects in patients with Duchenne muscular dystrophy. Neuromuscul Disord. 2017;27:705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gerhalter T, Gast LV, Marty B, et al. 23 Na MRI depicts early changes in ion homeostasis in skeletal muscle tissue of patients with Duchenne muscular dystrophy. J Magn Reson Imaging. 2019;50:1103–1113. [DOI] [PubMed] [Google Scholar]
- 57. Vohra RS, Lott D, Mathur S, et al. Magnetic resonance assessment of hypertrophic and pseudo-hypertrophic changes in lower leg muscles of boys with Duchenne muscular dystrophy and their relationship to functional measurements. PloS One. 2015;10:e0128915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. D’Angelo MG, Berti M, Piccinini L, et al. Gait pattern in Duchenne muscular dystrophy. Gait Posture. 2009;29:36–41. [DOI] [PubMed] [Google Scholar]
- 59. Gaudreault N, Gravel D, Nadeau S, et al. Gait patterns comparison of children with Duchenne muscular dystrophy to those of control subjects considering the effect of gait velocity. Gait Posture. 2010;32:342–347. [DOI] [PubMed] [Google Scholar]
- 60. Sutherland DH, Olshen R, Cooper L, et al. The pathomechanics of gait in Duchenne muscular dystrophy. Dev Med Child Neurol. 1981;23:3–22. [DOI] [PubMed] [Google Scholar]
- 61. Doglio L, Pavan E, Pernigotti I, et al. Early signs of gait deviation in Duchenne muscular dystrophy. Eur J Phys Rehabil Med. 2011;47:587–594. [PubMed] [Google Scholar]
- 62. Jansen M, Alfen N, Geurts ACH, Groot IJM. Assisted bicycle training delays functional deterioration in boys with Duchenne muscular dystrophy: the randomized controlled trial no use is disuse. Neurorehabil Neural Repair. 2013;27:816–827. [DOI] [PubMed] [Google Scholar]
- 63. Alemdaroğlu I, Karaduman A, Yilmaz ÖT, Topaloğlu H. Different types of upper extremity exercise training in Duchenne muscular dystrophy: effects on functional performance, strength, endurance, and ambulation. Muscle Nerve. 2015;51:697–705. [DOI] [PubMed] [Google Scholar]
- 64. Lott DJ, Taivassalo T, Park H, et al. Safety and feasibility of strength training in patients with Duchenne muscular dystrophy. Med Sci Sports Exerc. 2019;49:S696. [Google Scholar]
- 65. Douglas J, Pearson S, Ross A, McGuigan M. Eccentric exercise: physiological characteristics and acute responses. Sports Med. 2017;47:663–675. [DOI] [PubMed] [Google Scholar]
- 66. Kiefer M, Bonarrigo K, Quatman-Yates C, et al. Progression of ankle plantarflexion contractures and functional decline in Duchenne muscular dystrophy: implications for physical therapy management. Pediatr Phys Ther. 2019;31:61–66. [DOI] [PubMed] [Google Scholar]
- 67. Skalsky AJ, McDonald CM. Prevention and management of limb contractures in neuromuscular diseases. Phys Med Rehabil Clin N Am. 2012;23:675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Willcocks RJ, Triplett WT, Forbes SC, et al. Magnetic resonance imaging of the proximal upper extremity musculature in boys with Duchenne muscular dystrophy. J Neurol. 2017;264:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ricotti V, Evans MR, Sinclair CD, et al. Upper limb evaluation in Duchenne muscular dystrophy: fat-water quantification by MRI, muscle force and function define endpoints for clinical trials. PloS One. 2016;11:e0162542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hogrel J-Y, Wary C, Moraux A, et al. Longitudinal functional and NMR assessment of upper limbs in Duchenne muscular dystrophy. Neurology. 2016;86:1022–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wary C, Azzabou N, Giraudeau C, et al. Quantitative NMRI and NMRS identify augmented disease progression after loss of ambulation in forearms of boys with Duchenne muscular dystrophy. NMR Biomed. 2015;28:1150–1162. [DOI] [PubMed] [Google Scholar]
- 72. Brooke MH, Fenichel GM, Griggs RC, et al. Duchenne muscular dystrophy: patterns of clinical progression and effects of supportive therapy. Neurology. 1989;39:475–481. [DOI] [PubMed] [Google Scholar]
- 73. Mayhew AG, Coratti G, Mazzone ES, et al. Performance of upper limb module for Duchenne muscular dystrophy. Dev Med Child Neurol. 2020;62:633–639. [DOI] [PubMed] [Google Scholar]
- 74. Han JJ, Kurillo G, Abresch RT, et al. Upper extremity 3-dimensional reachable workspace analysis in dystrophinopathy using Kinect. Muscle Nerve. 2015;52:344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lowes LP, Alfano LN, Crawfis R, et al. Reliability and validity of ACTIVE-seated: an outcome in dystrophinopathy. Muscle Nerve. 2015;52:356–362. [DOI] [PubMed] [Google Scholar]
- 76. Janssen MMHP, Bergsma A, Geurts ACH, Groot IJM. Patterns of decline in upper limb function of boys and men with DMD: an international survey. J Neurol. 2014;261:1269–1288. [DOI] [PubMed] [Google Scholar]
- 77. Heutinck L, Jansen M, Elzen Y, et al. Virtual reality computer gaming with dynamic arm support in boys with Duchenne muscular dystrophy. J Neuromuscul Dis. 2018;5:359–372. [DOI] [PubMed] [Google Scholar]
- 78. Glanzman AM, Flickinger JM, Dholakia KH, et al. Serial casting for the management of ankle contracture in Duchenne muscular dystrophy. Pediatr Phys Ther. 2011;23:275–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Main M, Mercuri E, Haliloglu G, et al. Serial casting of the ankles in Duchenne muscular dystrophy: can it be an alternative to surgery? Neuromuscul Disord. 2007;17:227–230. [DOI] [PubMed] [Google Scholar]
- 80. Nishizawa H, Matsukiyo A, Shiba N, et al. The effect of wearing night splints for one year on the standing motor function of patients with Duchenne muscular dystrophy. J Phys Ther Sci. 2018;30:576–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hyde SA, FlŁytrup I, Glent S, et al. A randomized comparative study of two methods for controlling Tendo Achilles contracture in Duchenne muscular dystrophy. Neuromuscul Disord. 2000;10:257–263. [DOI] [PubMed] [Google Scholar]


