Abstract
Background
Treatment for glioblastoma (GBM) remains an unmet need in medicine. Novel therapies that address GBM complexity and heterogeneity in particular are warranted. To this end, we target 4 tumor-associated receptors at a time that span virtually all of the GBM microenvironment including bulk tumor cells, infiltrating tumor cells, neovasculature, and tumor-infiltrating cells with one pharmaceutical agent delivering a cytotoxic load.
Methods
We engineered multivalent ligand-based vector proteins termed QUAD with an ability to bind to 4 of the following GBM-associated receptors: IL-13RA2, EphA2, EphA3, and EphB2. We conjugated QUAD with a modified bacterial toxin PE38QQR and tested it in vitro and in vivo.
Results
The QUAD variants preserved functional characteristics of the respective ligands for the 4 receptors. The QUAD 3.0 variant conjugate was highly cytotoxic to GBM cells, but it was nontoxic in mice, and the conjugate exhibited strong antitumor effect in a dog with spontaneous GBM.
Conclusion
The QUAD addresses, to a large extent, the issues of intra- and intertumoral heterogeneity and, at the same time, it targets several pathophysiologically important tumor compartments in GBM through multiple receptors overexpressed in tumors allowing for what we call “molecular resection.” QUAD-based targeted agents warrant further pre- and clinical development.
Keywords: drug conjugate, GBM, multivalent targeted protein, tumor heterogeneity
Key Points.
GBM heterogeneity can be addressed by employing multivalent vectors targeting several receptors at once.
Multivalent protein that targets IL-13RA2, EphA2, EphA3, and EphB2 receptors can be produced and can safely and effectively deliver cytotoxic load to tumors.
Importance of the Study.
GBM is a complex and heterogeneous tumor that is sustained by a dynamic, locally spreading oncogenic microenvironment. Specific targeting of GBM using tumor-associated antigens is promising although a drawback of single antigen targeting has been either insufficient coverage of the tumor and/or targeted antigen loss. It is therefore imperative that therapies address tumor heterogeneity as proposed by us more than a decade ago. We have demonstrated that a multivalent vector protein, QUAD, can target the following 4 receptors: IL-13RA2, EphA2, EphA3, and EphB2 at once. The combined expression of these receptors is almost 100% of the tumor and its microenvironment and spans tumor cells, tumor-initiating cells, neovasculature, infiltrating cells and tumor cells of monocytic origin. Our data demonstrate that QUAD can be conjugated with a toxin and that the conjugate instills potent cytotoxic effects selectively to tumor cells in vitro and in vivo. We anticipate that the QUAD conjugate will be further developed for human clinical trials.
Glioblastoma (GBM) accounts for 57.3% of all gliomas and 14.6% of all primary brain tumors.1 Despite the standard treatment of surgery, chemotherapy, radiation therapy, and recently introduced tumor-treating fields (TTFs), an overall median survival of patients with GBM up to 20.9 months have been reported.2 The challenges in treating GBM include resistance to chemo and radiation therapy, presence of the blood–brain barrier (BBB), location of the tumor, infiltrative nature of the tumor,3 and genetic, molecular, and functional heterogeneity4,5 of the tumor and its microenvironment.6
Tumor-associated antigens (TAA) offer an array of therapeutic opportunities that allow effectors to be targeted only to tumor cells and spare the normal cells of the CNS7; many of these TAA are plasma membrane receptors. Several of the receptors that have been used to selectively target glioma include interleukin-13 receptor α2 (IL-13RA2),8 erythropoietin-producing human hepatocellular (Eph) receptors,9,10 epidermal growth factor receptor,11 and transferrin receptor,12 among others. Some of the experimental therapeutic approaches used include vaccines,13 immune checkpoint inhibitors,14 chimeric antigen receptor (CAR T-cells),15 viral and gene therapies16 and ADC.7,12 However, all of these targetable receptors are overexpressed in less than 100% of patients with GBM and much less than all supportive cells of the tumor microenvironment. We have been advocating to introduce combination therapies in order to obtain more comprehensive and longer-lasting responses.17 Some of these therapies would eventually be delivered using convection-enhanced delivery (CED), which has shown to be an efficient, minimally invasive method to bypass the BBB and administer therapeutic agents directly to the tumor.12,18 Newly engineered catheters optimize drug delivery by preventing infusate refluxes, utilize multiple tips or hollow fibers especially designed to deliver therapy to most of the tumor volume.17,19,20 Importantly, a real-time monitoring of infusion helps to ascertain proper drug delivery while the deliveries are shortened. Also of high importance, the nonenhancing tumors can be infused.19
IL-13RA2 was found to be a glioma TAA, and the expression of the receptor is negligible in normal cells of the CNS.8 Over 30 variations of targeted therapies have utilized IL-13RA2 as a single target in GBM.7,15 IL-13RA2 is overexpressed in up to 75% of GBM patients21 and is associated with higher-grade glioma, poor patient prognosis, and cells exhibiting a mesenchymal phenotype.22,23 Furthermore, elimination of IL-13RA2-positive cells within a GBM microenvironment has shown to render the tumor less tumorogenic.24 Glioma cells may express up to 4,000,000 IL-13RA2 sites per GBM cell.25 Thus, IL-13RA2 remains an attractive target for anti-GBM therapies.
The other targets that we identified in GBM are the Eph receptors, the largest family of eukaryotic receptor tyrosine kinase receptors.9,10,26 They bind to the ligand ephrin (Eph family receptor interacting proteins) As and Bs and are internalized within minutes of ligand binding and ensuing clustering.9,27 For example, EphA2 receptor is overexpressed in 60% of GBM tumors with increased expressions with higher grades of glioma.10,21,27,28 Recent reports have also associated EphA2 with invasive behavior of glioma stem-like cells in vivo.29,30 The EphA2 receptor is also expressed in tumor neovasculture.31 Of interest, we noticed that IL-13RA2 and EphA2 overexpression is only partially overlapping, making more than 90% of GBM enriched at least in one of the receptors.9,21 Similarly to EphA2, EphA3 is another TAA that is overexpressed in up to 60% of GBM patients.9 The expression of EphA3 spans not just glioma cells but the infiltrating tumor cells, tumor-initiating cells, invasive rings, and niches within the tumor blood vessels.9,32,33 A ligand-bacterial cytotoxin conjugate has effectively targeted EphA3 receptor–positive GBM cells.9 Again, the expression of EphA2 and EphA3 is only partially overlapping. Additionally, the EphB2 receptor has been shown specifically overexpressed in GBM and important in regulating proliferation, invasion, and migration of GBM cells.34,35 Considering the patterns of overexpression for EphA2, EphA3, and EphB2 receptors along with IL-13RA2, we stipulate that combinatorial targeting of these receptors would effectively address tumor heterogeneity as close to 100% of the tumor microenvironment could be targeted this way (Figure 1). This development follows our initial proposal to target IL-13RA2 and EphA2 with a cocktail of targeted cytotoxins17 or, EphA2, EphA3, and EphB2 with one cytotoxin.23 Also, antigen loss due to single-target therapy would be in all likelihood avoided while targeting as many as 4 receptors at a time.36
Figure 1.
IL-13RA2, EphA2, and EphA3 receptors cover conjointly nearly 100% of the heterogeneous glioblastoma (GBM) microenvironment. Immunofluorescent staining of IL-13RA2 (purple), EphA2 (red), EphA3 (green) and nucleus (4′,6-diamidino-2-phenylindole [DAPI], blue) in sections of 2 GBM specimens.
Of high interest, dogs develop spontaneous gliomas like humans with prominent overexpression of IL-13RA2, EphA2, and EphA3, and serve as an excellent model to test for targeted therapies.37–39 A phase I clinical trial in dogs with spontaneous gliomas using a cocktail of ligand-bacterial cytotoxin conjugates that target IL-13RA2 and EphA2 receptors has shown overall meaningful clinical responses. No dose-limiting toxicity (DLT) was found up to 3.2 µg/mL dose. The cocktail was able to produce several exceptional objective responses (up to 95% tumor shrinkage), restore the quality of life in canine patients, and extend overall survival38 (manuscript submitted).
Materials and Methods
Here, we utilize a ligand-based protein, QUAD that is able to bind to all the following 4 receptors: IL-13RA2, EphA2, EphA3, and EphB2 at once. QUAD is composed of IL-13.E13K (a modified version of the IL-13 ligand that more selectively binds the IL-13RA2 vs. the physiological receptor IL-13RA1/IL-4RA)40 and ephrin A5 that binds to EphA2, EphA3, and EphB2 receptors.9 Our data demonstrate that QUAD can be conjugated to a modified version of Pseudomonas exotoxin A (PE), PE38QQR,9,10 and is highly cytotoxic to GBM. It is a prototype of a single pharmaceutical off-the-shelf agent, which could be even applied without receptor status verification in GBM patients.
Cell Lines and Reagents
U-251 MG and human umbilical vein endothelial cells (HUVECs) were received from American Type Culture Collection (ATCC) and cultured as recommended by ATCC. G48a cells were isolated from a human primary high-grade astrocytoma in our laboratory.41 BTCOE 4795 and BTCOE 4525 were obtained from GBM patients within 20 min of resection, validated and cultured as described previously7 (Wake Forest IRB protocol #8427). They were authenticated back to the patient tumor by IDEXX Bioanalytics. Canine GBM cells, G06-A, were gift from Dr. Peter Dickinson at UC Davis.
Design, Expression, and Purification of QUAD Variants and PE38QQR
Genes for IL-13.E13K,40 human IgG1 (cloned in-house), and eA59 were cloned into pMIB V5 His A vector (Thermo Fischer). The QUAD DNA were optimized for the production in insect cells.42 High Five cells (Thermo Fischer) were transfected with the QUAD plasmids using Cellfectin II reagent (Thermo Fischer) according to the manufacturer’s protocol. Media from cells selected with blasticidin were collected over time. Proteins were isolated using HiTrap Protein G HP in AKTA (GE) fast protein liquid chromatography (FPLC) system. PE38QQR was produced in our laboratory as described previously.9,10
ELISA
Human recombinant IL-13RA2, EphA2, EphA3, and EphB2 proteins (Sino Biologicals) were used to coat the ELISA plates. ELISA was performed as described previously.43 Peroxidase-conjugated Anti-Human IgG antibody (Jackson ImmunoResearch Inc.) was used, and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) (Sigma Aldrich) was used as a detection agent. Absorbance values (405 nm) were plotted using the GraphPad prism software.
Flow Cytometry
Cells were detached with Versene (Invitrogen) counted and resuspended in 100 µL phosphate buffered saline (PBS) containing 1% bovine serum albumin (BSA). Cells were blocked with PBS/1% BSA for 1 h on ice. Two micrograms of QUAD 3.0, EphA3 antibody (house made),9 EphA2, EphB2 (R&D Systems), or IL-13RA2 (Biolegend) was added and incubated for 2 h on ice with occasional mixing. Cells incubated with isotype antibody served as controls. Cells were washed with PBS/1% BSA prior to the addition of Alexa-Fluor-labeled secondary antibodies. Cells were incubated on ice for an additional 1 h with occasional mixing. After washing with PBS/1% BSA, cells were postfixed with 10% buffered formalin. Cells were analyzed on an Accuri 6 flow cytometer. Data were analyzed using the FCS Express software (DeNovo Software).
Cultured Cells Doubling Time
Cells were plated in duplicate in 6-well dishes. Cells were counted daily for 7 days. The population doubling time was calculated during log phase growth using the equation (t2−t1)/3.32 × (log n2−log n1), where t = time and n = number of cells.
EphA2 and EphA3 Downregulation Assays and Western Blots
The downregulation of EphA2 and EphA3 was investigated as described previously.43 Western blots for testing the presence of receptors were also performed as described previously.9 Three independent experiments were performed for each QUAD version and the EphA2 and EphA3 receptors. Density of immunoreactive bands was measured on an AI600 RGB imager (GE). Data from 3 independent experiments were normalized to the corresponding β-actin and analyzed in Prism GraphPad.
Chemical Conjugation, Purification, and Cell Viability Assays
Chemical conjugation of the QUAD variants with PE38QQR was performed according to the protocol described previously.9,10 In particular, QUAD 2.0 version and PE38QQR were modified using succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC) and succinimidyl 3-(2-pyridyldithio)propionate) (SDPD)/tris(2-carboxyethyl)phosphine (TCEP), which modifies the lysines to reactive maleimide and thiol derivatives, respectively. QUAD 3.0 version was designed to have a cysteine at the C-terminal end of the molecule so that it provides a reactive thiol group for conjugation without any chemical modifications of the QUAD 3.0 itself. PE38QQR was derivatized using SMCC to form a maleimide derivative and combined with QUAD 3.0 to form stable thioether bonds. The conjugated proteins were purified from their unconjugated counterparts using sizing exclusion chromatography with HiPrep 16/60 Sephacryl S-200 HR (GE) column. Cell viability assays were performed using Thiazolyl blue tetrazolium bromide MTT assay (GoldBio) according to the manufacturer’s protocol in technical quadruplicates. Cell viability was measured 72 h after addition of the conjugate and vehicle control. The desired concentrations of the conjugates were prepared in PBS/0.1% BSA solutions. The controls indicate the cells that were treated with the PBS/0.1% BSA vehicle control. The doubling time of the cell lines is as follows: 24 h for U-251 and HUVEC cells, 25 h for BTCOE 4525, 22 h for BTCOE 4795, and 22 h for G48a cells. The BTCOE 4525 and BTCOE 4795 cells were cultured in serum-free conditions with EGF and FGF.
Toxicity
In order to investigate the toxicity of the QUAD 3.0-PE38QQR conjugate, 0.1 µg, 0.5 µg, and 1.0 µg of the conjugate in 5 µL was intracranially injected in C57BL/6 mice. The conjugate was stereotactically injected 2.0-mm deep into the caudate putamen at a rate of 1 µL/min. The mice were monitored for pain, neurological symptoms, regular physical and grooming activities and weighed daily for 2 weeks after the completion of the surgery.
Hematoxylin and Eosin Staining of Mouse Brain
Mouse brains were fixed in 10% buffered formalin prior to embedding in paraffin. Coronal sections of 5 micron were affixed slides. H & E staining was performed by the Tumor Tissue and Pathology Shared Resource Core of the Comprehensive Center. Whole slides were digitally scanned, and images were captured using the OlyVia Software V 2.8 (Olympus)
QUAD 3.0-PE38QQR Infusion in Canine Spontaneous GBM Model
A dog with a large mass (6.84 cm3) of spontaneous GBM in left parietotemporal region was treated with one cycle infusion of QUAD 3.0-PE38QQR over 2.25 h under real-time MRI monitoring. The CED treatment was performed in July 2019, and the coverage with infusate was 71% of T2/FLAIR tumor volume.
Statistical Analysis
The following nonparametric tests were performed: Mann–Whitney test, 2-tailed, and Kolmogorov–Smirnov test.
Results
Overexpressed IL-13RA2, EphA2, EphA3, and EphB2 Receptors Cover GBM Microenvironment
TAA IL-13RA2 is highly overexpressed in GBM tumors but not in normal brain.8,21–23 We and others have also shown that primary brain tumors overexpress EphA2, EphA3, and EphB2 receptors,9 in addition to IL-13RA2 (Figure 1). Moreover, at least one of these 4 receptors is overexpressed in a patient, but in vast majority of cases, at least 2 are in a nonoverlapping manner as previously reported in more detail.9 Hence, by targeting all 4 receptors at once using the QUAD, we are able to target glioma cells in almost all of the patient population while sparing normal brain (Table 1).
Table 1.
Distribution of the 4 Pharmaceutically Targetable Receptors Within the GBM Microenvironment
| Tumor Compartment | IL-13RA2 | EphA2 | EphA3 | EphB2 |
|---|---|---|---|---|
| Differentiated tumor cells | + | + | + | + |
| Infiltrating tumor cells | + | + | + | + |
| Glioma-stem like cells | + | + | + | + |
| Neovascular | − | + | − | − |
| Tumor-infiltrating cells | − | + | + | − |
| Linked to survival | + | + | + | + |
| Normal brain | − | − | − | − |
QUAD Variants Were Produced in Insect Cells
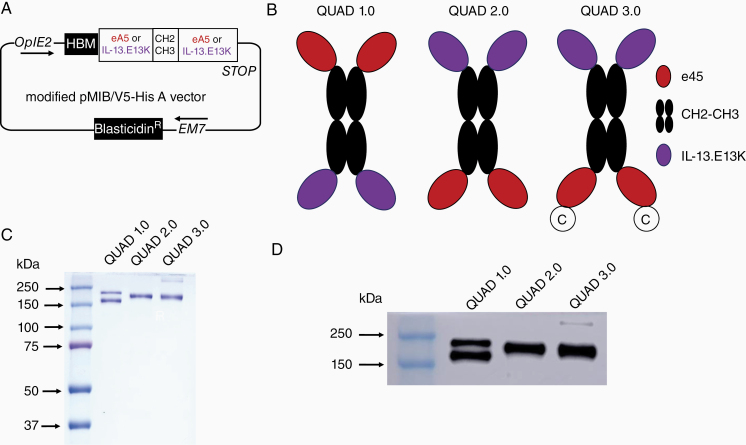
Three different variants of the QUAD recombinant protein were produced. These variants contain a human IgG1 CH2-CH3 Fc region as a scaffold, ligand ephrinA5 (eA5) that binds to receptors EphA2, EphA3, and EphB2,9 and a IL-13.E13K ligand containing a glutamic acid to lysine modification in the 13th amino acid of the IL-13 ligand that makes it more specific to IL-13RA2.40 The QUAD variant DNAs were cloned into a pMIB/V5-His A vector (Figure 2A). QUAD variants were designed as shown in Figure 2B. Both Sf9 (Spodoptera frugiperda) and High Five insect cells (Trichoplusia ni, BTI-TN-5B1-4) were used to produce the QUAD proteins (Figure 2C). Using FPLC, these proteins were isolated from the insect cell media (Figure 2D) and purified to homogeneity. While QUAD 1.0 produced a characteristic double band in SDS–PAGE and western blot analyses, by reversing the ligand sequence from N-terminal to C-terminal, a single-banded protein was produced as QUAD 2.0 (Figure 2C and 2D). Furthermore, adding a Cysteine to the C-terminal end for QUAD 3.0 still produced a single-band protein in SDS–PAGE and western blot analyses (Figure 2C and 2D). A QUAD 3.1 variant was also generated, which is a QUAD 3.0, but its gene was fully optimized for protein production in insect cells for higher yields of protein. Due to higher protein expression, High Five cells in suspension cultures were ultimately used for protein expression.
Figure 2.
Cloning, production, and purification of QUAD variants. (A) The genes encoding the 3 QUAD variants were cloned into a modified pMIB/V5-His A vector. (B) Schemata of the QUAD variants: QUAD 1.0, ephrinA5 (eA5) - CH2CH3—IL-13.E13K; QUAD 2.0, IL-13.E13K - CH2CH3- eA5; QUAD 3.0, IL-13.E13K- CH2CH3- eA5 with Cysteine at the C-terminal end of the protein. (C) SDS–PAGE of QUAD variants purified from the media. (D) Western blots (anti-Human IgG) of purified QUAD 1.0, QUAD 2.0, and QUAD 3.0.
QUAD Variants Are Functional
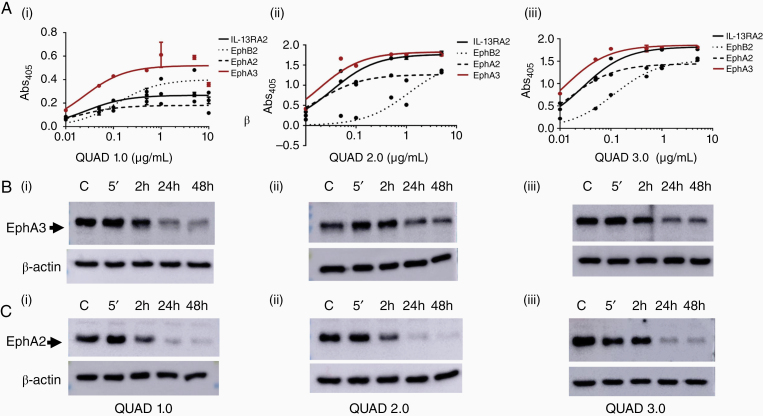
The functionality of the QUAD variants was tested with ELISA and western blot analyses for downregulation of the Eph receptors. ELISA results showed that the QUAD variants retain their ligand properties and bound effectively to all IL-13RA2, EphA2, EphA3, and EphB2, receptors with pM range KD values (Table 2, Figure 3A). The affinity of QUAD variants is comparable to, if not better, than some of the antibody–drug conjugates (ADC) currently approved by the FDA. For instance, the KD value of Kadcyla to HER-2 receptor is 1.08 nM,44 that of Adcetris to CD30 is 2.48 nM,45 and that of Besponsa to CD22 is 120–150 pM.46 The KD values of QUAD variants effectively demonstrate that they bind better than most of the FDA approved drugs. Among the 4 receptors tested, all the QUAD variants bound the least to EphB2 receptor. While the KD values for both QUAD 1.0 and QUAD 3.0 binding to EphB2 were still less than 1 nM, that for QUAD 2.0 was the highest among the 3 variants.
Table 2.
KD Values of QUAD Variants Binding to the IL-13RA2, EphA2, EphA3, and EphB2 Receptors
| Protein | QUAD 1.0 | QUAD 3.0 | QUAD 3.0 |
|---|---|---|---|
| Receptor | |||
| IL-13RA2 | 167 pM | 285 pM | 224 pM |
| EphB2 | 917 pM | 4200 pM | 716 pM |
| EphA2 | 83 pM | 131 pM | 123 pM |
| EphA3 | 167 pM | 208 pM | 166 pM |
Figure 3.
QUAD variants retain the functional properties of their individual ligands. (A) QUADs binding to the targeted receptors. ELISA of QUAD 1.0 (i), QUAD 2.0 (ii), and QUAD 3.0 (iii) binding to IL-13RA2, EphB2, EphA2, and EphA3 receptors. (B) and (C) Downregulation of the EphA3 and EphA2 receptors by the QUADs. Western blot of the EphA3 and EphA2 receptors upon exposure to 1 µg/mL of individual QUADs in U-251 GBM cells.
The internalization of the receptor is important as it allows the cytotoxic agent conjugated with the ligand to enter tumor cells to deliver its cytotoxic load.9,10,40 The eA5 ligand of high concentrations of the QUAD variants downregulated both the EphA3 and EphA2 receptors as these receptors were internalized when exposed to QUAD versions 1.0–3.0 (Figure 3B and 3C; Supplementary Figure 1). It appears that the EphA2 receptor was downregulated more efficiently that the EphA3 receptor, which may be related to the fact that the EphA3 receptor is also significantly expressed intracellularly and thus unavailable for binding to a targeted ligand (Supplementary Figure 1 and ref. 9) Importantly, ABC toxin-based cytotoxins to which PE belongs, require minutes to attach to cells, which lead to irreversible cell death.47 Eph receptors have been shown to be recycled back/re-expressed to plasma membrane,7 providing a therapeutic opportunity for multiple intermittent treatment options. IL-13RA2 plasma membrane levels, on the other hand, are not affected by the ligand-induced internalization at all.48
QUAD Variants Conjugation With PE38QQR and the Binding of a QUAD 3.0-PE38QQR Conjugate to the Receptors
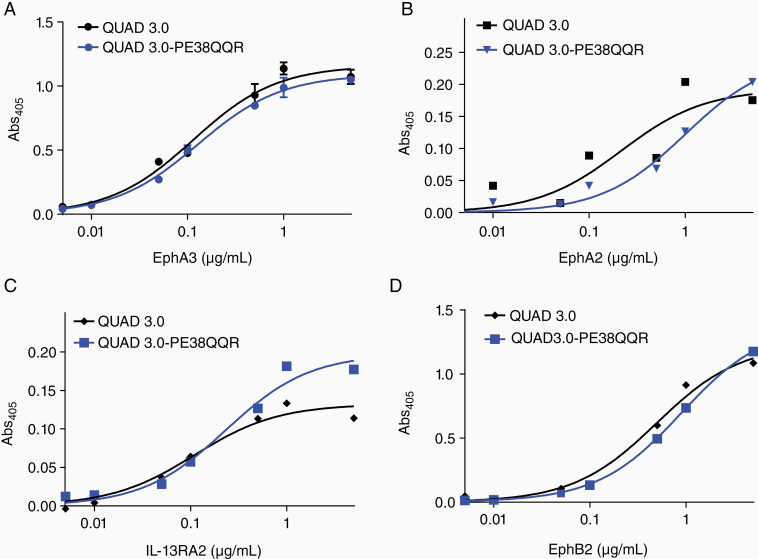
PE38QQR is a modified version of the PE that lacks the toxin’s receptor-binding domain. When conjugated to a receptor ligand, it is internalized into a cell and can deliver its cytotoxic load.9,10 We conjugated QUAD variants successfully to PE38QQR, as described.9,10 We have focused on the QUAD 3.0-PE38QQR conjugate as QUAD 3.0 does not require any chemical modification for conjugation. The purified QUAD3.0-PE38QQR conjugate bound all 4 receptors with similar affinities as the unconjugated QUAD 3.0 protein (Figure 4).
Figure 4.
QUAD 3.0-PE38QQR conjugate retains the binding ability to the 4 targeted receptors. Comparative binding of QUAD 3.0 and QUAD 3.0-PE38QQR conjugate to (A) EphA3, (B) EphA2, (C) IL-13RA2, and (D) EphB2 receptors.
QUAD-PE38QQR Is Cytotoxic to GBM Cells
QUAD-PE38QQR conjugates were tested on established and patient-derived GBM cells and normal human cells for cytotoxicity (Figure 5). These cells express the targeted receptors at various levels (Supplementary Figure 2). We observed that both QUAD 1.0-PE38QQR (Figure 5A), and QUAD 2.0-PE38QQR (Figure 5B) conjugates were cytotoxic to established U-251 GBM cells. Assuming a 1:1 conjugation of the QUAD with the PE38QQR, the IC50 values of the QUAD 1.0-PE38QQR and QUAD 2.0-PE38QQR conjugates are around the range of 1 nM. Similarly, QUAD 3.0-PE38QQR conjugate was also cytotoxic to U-251 cells with IC50 value of 1 × 10−10M (Figure 5C (i)). Because conjugation of the QUAD 3.0 with the PE38QQR does not involve any chemical modification of QUAD 3.0, we used QUAD 3.0-PE38QQR to test for its cytotoxic activity. QUAD 3.0-PE38QQR was highly cytotoxic to established glioma cell lines U-251 and G48a (Figure 5C (ii)). QUAD 3.0-PE38QQR was also cytotoxic to patient-derived glioma cells (Figure 5C (iii–iv)), but not to normal HUVEC (Figure 5C (v)). Light microscopy revealed few mostly necrotic cells remaining after the treatment with QUAD-PE38QQR (Figure 5D). Also, the canine GBM cells were recognized by QUAD 3.0 by flow cytometry (Figure 5E (i)), and they were killed potently by QUAD-PE38QQR (Figure 5E (ii)). We then tested the behavior of the QUAD 3.0-PE38QQR in vivo.
Figure 5.
QUAD conjugates affect cell viability in established and low-passage patient-derived glioblastoma (GBM) cell lines. U-251 GBM cells were treated with (A) QUAD 1.0-PE38QQR and (B) QUAD 2.0-PE38QQR. (C) established ((i) U251, and (ii) G48a), low-passage patient-derived ((iii) BTCOE 4525 and (iv) BTCOE 4795), and (v) normal (HUVEC) cells treated with QUAD 3.0-PE38QQR conjugate. The results were obtained from quadruplicates using an MTT assay. (D) Light microscopy of U-251 MG cells treated with either vehicle or 100nM QUAD 3.0-PE38QQR for 72h. (E) Flow cytometry of QUAD-3.0 binding to canine G06-A GBM cells (i) and cytotoxicity of QUAD 3.0-PE38QQR on G06-A GBM cells (ii).
QUAD-PE38QQR Is Safe in Mice
We used QUAD 3.0-PE38QQR to test for toxicity in mice. Intracranial injections of QUAD 3.0-PE38QQR in C57BL/6 mice at different concentrations: 0.1, 0.5, and 1.0 µg of the conjugate in 5 µL per mouse did not produce any signs of neuro-toxicity or significant changes in weight (Supplementary Figure 3). The mice showed no changes in grooming patterns and behaved normally in both the control and treated groups. The H&E staining of the injected brains and the vicinity of the needle tips were free of necrosis and other signs of potential toxicity (Supplementary Figure 3). Thus, the QUAD 3.0-PE38QQR is safe in C57BL/6 mice.
Treatment of Spontaneous Canine GBM With QUAD-PE38QQR
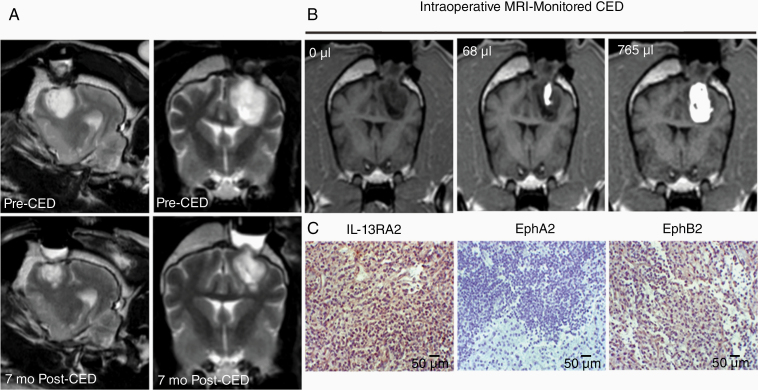
Canine gliomas demonstrate remarkable similarities with human disease thus providing a faithful large animal model to investigate the efficacy of potential antitherapeutic agents in GBM.37 A dog with spontaneous GBM was treated with QUAD 3.0-PE38QQR. The treatment lasted for 2.25 h; 3 catheters were used with infusion rates of 1–20 µL/min. The dose chosen, 1.6 µg/mL, was the second highest used in a Phase I trial with a cocktail of IL-13RA2- and EphA2-targeted cytotoxins in which we did not reach a DLT.38 There were no signs of any toxicity related to treatment with QUAD 3.0-PE38QQR at this dose. Furthermore, repeat MRI of the dog showed ~60% less tumor volume at 7 months post-treatment (Figure 6A), and the dog is alive a year after a single treatment. For comparison, surgical treatment alone offers ~2 months of life in these dogs, and the mean overall survival in the cocktail trial was 224 days.38 The CED was performed under real-time MRI monitoring with a single infusion of QUAD 3.0-PE38QQR (Figure 6B). The immunohistochemistry (IHC)-confirmed GBM was positive for IL-13RA2 and EphA3 and negative for the EphA2 receptor in the biopsied area (Figure 6C). This naturally represents situation to be encountered in the clinic when not all 4 receptors might be present for the targeting entity. Thus, the QUAD 3.0-PE38QQR is showing highly promising antitumor activity in vivo as a single pharmaceutical agent, which will be further evaluated.
Figure 6.
Single-cycle treatment of a dog with glioblastoma (GBM) using QUAD 3.0-PE38QQR. (A) The tumor was almost 60% smaller at 7 months follow-up (earlier were not possible due to the military service of the owners). (B) Tumor coverage with the QUAD-PE38QQR infusate. (C) Preoperative staining for the presence of targeted receptors by IHC. The scale bars correspond to 50 µm.
Discussion
We utilized a ligand-based protein, QUAD that is able to bind to 4 TAA receptors at once. QUAD is engineered to contain human IgG regions CH2 and CH3 as a scaffold, IL-13.E13K, which binds to IL-13RA2, and ephrin A5, which binds to the EphA2, EphA3, and EphB2 receptors. We have designed different variants of the QUAD, one of which contains a cysteine at the C-terminal end of the protein making it ready for conjugation without any chemical modification of the protein, QUAD 3.0. QUAD 3.0 preserves its expected biological activities in binding to the respective receptors and inducing molecular changes. QUAD 3.0 was successfully conjugated to a modified version of PE, PE38QQR. The conjugate is highly cytotoxic to both human and canine GBM cells. PE or its conjugates have also been reported to cause immunogenic cell death rendering the tumor immunologically active.49 This opens another treatment opportunity to potentially administer immunotherapies that could enhance the therapeutic effects of the conjugate. This opportunity to induce additional therapeutic effect through immune responses in yet untreated patients can be at least partially lost with surgical intervention implemented first. Moreover, surgery may promote local infiltration by tumor cells in an animal model.50
The advantages of using a multivalent protein-bacterial cytotoxin conjugate-like QUAD to target GBM are multifold: (a) selective targeting of only glioma and its supportive cells within the heterogeneous tumor microenvironment can be achieved and (b) not only the glioma bulk tumor cells, but the critically important tumor initiating, infiltrative, immune, and vascular components of the heterogeneous tumor microenvironment that not only sustains GBM, but is responsible for its invasive and therapy resistant can be targeted.
One of the challenges in treating GBM with TAA-targeted therapies is tumor heterogeneity as none of the targetable receptors/antigens is overexpressed in 100% of tumor cells specifically and at sufficient levels.7,9 Furthermore, there is both intrapatient and interpatient heterogeneity in GBM TAA expression. In order to address tumor heterogeneity in GBM, aggressive profiling of GBM specimens has been warranted leading to studies that have adopted strategies to devise personalized therapy for GBM patients. One such example is a recently published trial of Glioma Actively Personalized Vaccine Consortium (GAPVAC).51 Based on the immunopeptidomes and transcriptomes of individual GBM patients, APVAC-1 and APVAC-2 vaccines were designed that were derived from a premanufactured library of unmutated antigens and targeted neoepitopes respectively. While APVAC-1 vaccines elicited immune responses of CD8+ and CD4+ T cells,51 the clinical effectiveness of the approach remains to be established.
The other challenge is the loss of antigens in response to treatment that gives rise to acquired resistance to therapy.36 Hypothetically, using combinatorial therapies that target more than one TAA at a given time is more attractive and potentially efficient way to not only deliver cytotoxic effects selectively to various compartments of tumors, but also to override resistance to therapy and address tumor heterogeneity.17,23 While IL-13RA2 is an excellent target with studies showing significant regression of tumors after the targeting of IL-13RA2, reports have also suggested that the tumor loses IL-13RA2 over time describing a phenomenon called, “antigen loss or exhaustion” when the receptor is targeted alone.52 Although antigen loss of IL-13RA2 may have its own benefits,24 therapies that mitigate antigen loss are warranted.38,52
The last 2 decades of research have established that IL-13RA2, EphA2, EphA3, and EphB2 receptors are attractive targets in GBM. These receptors in addition to being present in tumor cells are also found in the supportive GBM microenvironment that not only sustains the tumor but also renders it aggressive (Table 1). This is based on the studies analyzing the databases such as REMBRANDT and TCGA, using gene micro array and TMAs, IHC and immunofluorescence (IF) staining, Western immunoblotting, gene knockdowns, and other cellular and molecular techniques.
In summary, our data demonstrate that a multivalent vector protein, QUAD that targets multiple receptors in GBM can be produced, purified, and conjugated to a bacterial toxin load. Additionally, we demonstrate that the conjugate is highly effective in preclinical evaluation. Thus, this potent, single pharmaceutical off-the-shelf agent will be further developed preclinically in canine spontaneous glioma model38 and subsequently examined in patients with malignant gliomas.
Supplementary Material
Acknowledgments
We would like to thank Yue Huang and Mary Mobley for experimental assistance. We acknowledge the core laboratories of the Wake Forest Baptist Comprehensive Cancer Center. Namely, Cellular Imaging Shared Resource, Tumor Tissue and Pathology Shared Resource, Proteomics and Metabolomics Shared Resource, Clinical and Translational Science Institute and Biostatistics Shared Resource. The National Cancer Institute’s Cancer Center Support Grant award number P30CA012197 supported the core laboratories. We appreciate the support of Dr. James Wood, Ms. Tammy Sexton, Ms. Lena Moretz, Ms. Ashley Davis, Ms. Stephanie Rideout, Dr. Mark Lively, Dr. Jingyun Lee, and Mr. Ken Grant. The content of the paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
This work was previously presented in part at the 2018 Annual Meeting of the Society for Neuro-Oncology: Sharma P, Herpai D, Rossmeisl J, Tatter S, Debinski W. EXTH-29. Multi-receptor targeting in GBM. Neuro Oncol. 2018;20(suppl 6):vi91–vi91. doi:10.1093/neuonc/noy148.378.
Funding
This research has been funded by the National Cancer Institute [P01 NCI CA207206 and R01 CA74145 to W.D.].
Conflict of interest statement. The subject of the current investigation is covered by patents obtained or applied for and owned by the Wake Forest University. W.D. is a Scientific Advisor to WPD Pharmaceuticals, Inc. and a consultant to VoltMed, Inc.
Authorship Statement. P.S., P.S., D.H., and W.D. designed the study. P.S., P.S., and D.H. performed the experiments. P.S., P.S., D.H., and W.D. performed the data analysis and produced the figures. P.S., W.D. wrote the manuscript. J.R. and S.T. generated canine data (J.R.) and provided advice and guidance for the project. W.D. provided overall leadership and supervision of the project and invented the idea of 4 receptors targeting.
References
- 1. Ostrom QT, Cioffi G, Gittleman H, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 2019;21:v1–v100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wen PY, Weller M, Lee EQ, et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22:1073–1113. 10.1093/neuonc/noaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Debinski W, Slagle B, Gibo DM, Powers SK, Gillespie GY. Expression of a restrictive receptor for interleukin 13 is associated with glial transformation. J Neurooncol. 2000;48(2):103–111. [DOI] [PubMed] [Google Scholar]
- 5. atel AP, Tirosh I, Trombetta JJ, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344(6190):1396–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vartanian A, Singh SK, Agnihotri S, et al. GBM’s multifaceted landscape: highlighting regional and microenvironmental heterogeneity. Neuro Oncol. 2014;16(9):1167–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sharma P, Debinski W. Receptor-targeted glial brain tumor therapies. Int J Mol Sci. 2018;19(11):3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Debinski W, Gibo DM, Hulet SW, Connor JR, Gillespie GY. Receptor for interleukin 13 is a marker and therapeutic target for human high-grade gliomas. Clin Cancer Res. 1999;5(5):985–990. [PubMed] [Google Scholar]
- 9. Ferluga S, Tomé CM, Herpai DM, D’Agostino R, Debinski W. Simultaneous targeting of Eph receptors in glioblastoma. Oncotarget. 2016;7(37):59860–59876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wykosky J, Gibo DM, Debinski W. A novel, potent, and specific ephrinA1-based cytotoxin against EphA2 receptor expressing tumor cells. Mol Cancer Ther. 2007;6(12 Pt 1):3208–3218. [DOI] [PubMed] [Google Scholar]
- 11. Sampson JH, Akabani G, Archer GE, et al. Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors. Neuro Oncol. 2008;10(3):320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Laske DW, Youle RJ, Oldfield EH. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat Med. 1997;3(12):1362–1368. [DOI] [PubMed] [Google Scholar]
- 13. Okada H, Kalinski P, Ueda R, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29(3):330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao J, Chen AX, Gartrell RD, et al. , Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat Med. 2019;25(3):462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brown CE, Alizadeh D, Starr R, et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375(26):2561–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chiocca EA, Abbed KM, Tatter S, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10(5):958–966. [DOI] [PubMed] [Google Scholar]
- 17. Debinski W. Drug cocktails for effective treatment of glioblastoma multiforme. Expert Rev Neurother. 2008;8(4):515–517. [DOI] [PubMed] [Google Scholar]
- 18. Debinski W, Tatter SB. Convection-enhanced delivery for the treatment of brain tumors. Expert Rev Neurother. 2009;9(10):1519–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vogelbaum MA, Brewer C, Barnett GH, et al. First-in-human evaluation of the Cleveland Multiport Catheter for convection-enhanced delivery of topotecan in recurrent high-grade glioma: results of pilot trial 1. J Neurosurg. 2019;130(2):476–485. doi: 10.3171/2017.10.JNS171845 [DOI] [PubMed] [Google Scholar]
- 20. Elenes EY, Rylander CG Elenes EY, et al. Maximizing local access to therapeutic deliveries in glioblastoma. Part ii: arborizing catheter for convection-enhanced delivery in tissue phantoms. In: De Vleeschouwer S, ed. Glioblastoma. Brisbane, AU: Codon Publications; 2017. Chapter 18. PMID: 29251864. [PubMed] [Google Scholar]
- 21. Wykosky J, Gibo DM, Stanton C, Debinski W. Interleukin-13 receptor alpha 2, EphA2, and Fos-related antigen 1 as molecular denominators of high-grade astrocytomas and specific targets for combinatorial therapy. Clin Cancer Res. 2008;14(1):199–208. [DOI] [PubMed] [Google Scholar]
- 22. Brown CE, Warden CD, Starr R, et al. Glioma IL13Rα2 is associated with mesenchymal signature gene expression and poor patient prognosis. PLoS One. 2013;8(10):e77769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Debinski W, Priebe W, Tatter SB. Maximizing local access to therapeutic deliveries in glioblastoma. Part I: targeted cytotoxic therapy. In: De Vleeschouwer S, ed. Glioblastoma. Brisbane, AU: Codon Publications; 2017. Chapter 17. PMID: 29251864. [PubMed] [Google Scholar]
- 24. Nguyen V, Conyers JM, Zhu D, et al. IL-13Rα2-targeted therapy escapees: biologic and therapeutic implications. Transl Oncol. 2011;4(6):390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mintz A, Gibo DM, Slagle-Webb B, Christensen ND, Debinski W. IL-13Ralpha2 is a glioma-restricted receptor for interleukin-13. Neoplasia. 2002;4(5):388–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lisabeth EM, Falivelli G, Pasquale EB. Eph receptor signaling and ephrins. Cold Spring Harb Perspect Biol. 2013;5(9):a009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wykosky J, Debinski W. The EphA2 receptor and ephrinA1 ligand in solid tumors: function and therapeutic targeting. Mol Cancer Res. 2008;6(12):1795–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wykosky J, Gibo DM, Stanton C, Debinski W. EphA2 as a novel molecular marker and target in glioblastoma multiforme. Mol Cancer Res. 2005;3(10):541–551. [DOI] [PubMed] [Google Scholar]
- 29. Miao H, Gale NW, Guo H, et al. EphA2 promotes infiltrative invasion of glioma stem cells in vivo through cross-talk with Akt and regulates stem cell properties. Oncogene. 2015;34(5):558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Binda E, Visioli A, Giani F, et al. The EphA2 receptor drives self-renewal and tumorigenicity in stem-like tumor-propagating cells from human glioblastomas. Cancer Cell. 2012;22(6):765–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hatano M, Eguchi J, Tatsumi T, et al. EphA2 as a glioma-associated antigen: a novel target for glioma vaccines. Neoplasia. 2005;7(8):717–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Day BW, Stringer BW, Al-Ejeh F, et al. EphA3 maintains tumorigenicity and is a therapeutic target in glioblastoma multiforme. Cancer Cell. 2013;23(2):238–248. [DOI] [PubMed] [Google Scholar]
- 33. Qazi MA, Vora P, Venugopal C, et al. Cotargeting ephrin receptor tyrosine kinases A2 and A3 in cancer stem cells reduces growth of recurrent glioblastoma. Cancer Res. 2018;78(17):5023–5037. [DOI] [PubMed] [Google Scholar]
- 34. Wang SD, Rath P, Lal B, et al. EphB2 receptor controls proliferation/migration dichotomy of glioblastoma by interacting with focal adhesion kinase. Oncogene. 2012;31(50):5132–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nakada M, Niska JA, Miyamori H, et al. The phosphorylation of EphB2 receptor regulates migration and invasion of human glioma cells. Cancer Res. 2004;64(9):3179–3185. [DOI] [PubMed] [Google Scholar]
- 36. Debinski W. When better still might not be good enough. Transl Cancer Res. 2017;6(suppl 7):S1244–S1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Debinski W, Dickinson P, Rossmeisl JH, Robertson J, Gibo DM. New agents for targeting of IL-13RA2 expressed in primary human and canine brain tumors. PLoS One. 2013;8(10):e77719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rossmeisl JH, Herpai D, Quigley M, et al. Phase I trial of convection-enhanced delivery of IL13RA2 and EPHA2 receptor targeted cytotoxins in dogs with spontaneous intracranial gliomas. Neuro Oncol. 2020. doi: 10.1093/neuonc/noaa196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dickinson PJ, LeCouteur RA, Higgins RJ, et al. Canine spontaneous glioma: a translational model system for convection-enhanced delivery. Neuro Oncol. 2010;12(9):928–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Debinski W, Gibo DM, Obiri NI, Kealiher A, Puri RK. Novel anti-brain tumor cytotoxins specific for cancer cells. Nat Biotechnol. 1998;16(5):449–453. [DOI] [PubMed] [Google Scholar]
- 41. Debinski W, Gibo DM. Fos-related antigen 1 modulates malignant features of glioma cells. Mol Cancer Res. 2005;3(4):237–249. [DOI] [PubMed] [Google Scholar]
- 42. Athey J, Alexaki A, Osipova E, et al. A new and updated resource for codon usage tables. BMC Bioinformatics. 2017;18(1):391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ferluga S, Hantgan R, Goldgur Y, Himanen JP, Nikolov DB, Debinski W. Biological and structural characterization of glycosylation on ephrin-A1, a preferred ligand for EphA2 receptor tyrosine kinase. J Biol Chem. 2013;288(25):18448–18457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Leighton JK. MEMORANDUM Kadcyla (Ado-Trastuzumab Emtansine) 2013. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/125427Orig1s000PharmR.pdf. Accessed May 19, 2019.
- 45. Review Report Pharmaceuticals and Medical Devices Agency 2013. https://www.pmda.go.jp/files/000209916.pdf. Accessed May 19, 2019.
- 46. Center for drug evaluation and research application number: 761040Orig1s000 Multi-discipline Review Summary Review Office Director Cross Discipline Team Leader Review Clinical Review Non-Clinical Review Statistical Review Clinical Pharmacology Review https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/761040Orig1s000MultidisciplineR.pdf. Accessed May 19, 2019.
- 47. Liu TF, Cai J, Gibo DM, Debinski W. Reoxygenation of hypoxic glioblastoma multiforme cells potentiates the killing effect of an interleukin-13-based cytotoxin. Clin Cancer Res. 2009;15(1):160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sonawane P, Choi YA, Pandya H, et al. Novel molecular multilevel targeted antitumor agents. Cancer Transl Med. 2017;3(3):69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Leshem Y, King EM, Mazor R, Reiter Y, Pastan I. SS1P immunotoxin induces markers of immunogenic cell death and enhances the effect of the CTLA-4 blockade in AE17M mouse mesothelioma tumors. Toxins (Basel). 2018;10(11):470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Okolie O, Bago JR, Schmid RS, et al. Reactive astrocytes potentiate tumor aggressiveness in a murine glioma resection and recurrence model. Neuro Oncol. 2016;18(12):1622–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hilf N, Kuttruff-Coqui S, Frenzel K, et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565(7738):240–245. [DOI] [PubMed] [Google Scholar]
- 52. Hegde M, Mukherjee M, Grada Z, et al. Tandem CAR T cells targeting HER2 and IL13Ralpha2 mitigate tumor antigen escape. J Clin Invest. 2016;126:3036–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.