Abstract
A 47-year-old male with macroglossia presented with dyspnea on effort and chest pain at rest. Cardiac MRI revealed diffuse global subendocardial late gadolinium enhancement below the left ventricular endocardium and a dark blood pool of intracardiac contrast medium. Tongue biopsy revealed amyloid deposition, which was limited in the myocardium. He was diagnosed with primary light chain amyloidosis. His condition was stage I according to the Mayo Clinic staging system. He underwent autologous peripheral blood stem cell transplantation. On Day 10, he developed chest pain and died suddenly on Day 11. Postmortem examination revealed amyloid deposition throughout the heart.
Keywords: cardiac MRI, primary AL amyloidosis, cardiac amyloidosis, autologous peripheral blood stem cell transplant, cardiac biomarker
INTRODUCTION
Primary light chain (AL) amyloidosis can manifest with diverse clinical symptoms due to the deposition of amyloid protein in many organs. 1 The organs/tissues most commonly affected include the kidneys (46%), heart (30%), liver (9%), digestive tract (7%), peripheral nerves (5%), and soft tissue (3%), 1 and the heart is affected relatively frequently. As involvement of the heart is considered to be an important determinant of the prognosis in patients with primary AL amyloidosis, 2 - 8 early diagnosis and treatment of cardiac amyloidosis (CA) are important. 6 Autologous peripheral blood stem cell transplantation (ASCT) is considered an effective treatment method for this condition, 3 , 5 , 8 , 9 but at present, the treatment-related mortality (TRM) is relatively high. 5 - 10 To reduce the TRM, it is necessary to precisely assess the severity of CA and judge the indications for ASCT in each case. 5 , 7 - 11 Magnetic resonance imaging (MRI) may be useful for making an early diagnosis of CA and for assessing its severity.
CASE REPORT
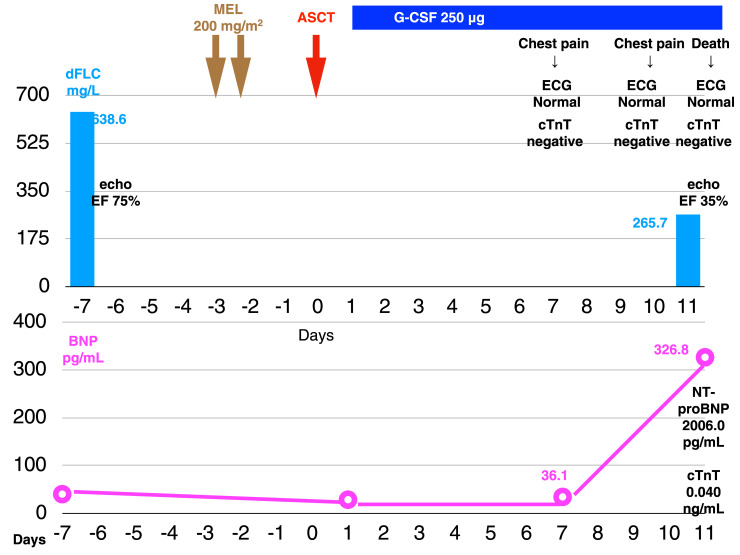
In December of X-2, a 47-year-old male was admitted to a local hospital with cardiogenic shock and was diagnosed with fulminant myocarditis. He was administered intravenous gamma globulin and his condition improved. Myocardial biopsy was not performed and selective coronary arteriography (CAG) revealed no abnormalities. In January of X, he presented with a history of dyspnea on effort and chest pain at rest lasting for several seconds to minutes. Cardiac MRI performed at that time revealed diffuse global subendocardial late gadolinium enhancement (LGE) below the left ventricular endocardium (Fig. 1a). LGE was noted also in the septal wall and the atrium, accompanied by washout (dark blood pool) of the intracardiac contrast material (Fig. 1b). In April, macroglossia was noted (with dental impressions at the edge of the tongue). On tongue biopsy, direct fast scarlet (DFS) 4BS staining was positive, leading to a diagnosis of amyloidosis. In addition, he had numbness at the periphery of the extremities. Myocardial biopsy was carried out, which demonstrated small focal areas of positive DFS 4BS staining in the myocardium. Under a polarizing microscope, the myocardium partially emitted green light. Positive chromatic responses were detected in the cytoplasm of the inflammatory cells within the myocardium. Serum and urine immunofixation assays revealed the M protein of monoclonal Bence Jones Protein (BJP)-κ type. No increase in the plasma cells was found in the bone marrow aspirate and DFS 4BS staining was negative. The test for κ was positive, whereas that for λ was negative. He was diagnosed with primary AL amyloidosis. 12 He visited our department in July of X. The physical examination at that time demonstrated the following: Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0, blood pressure of 119/71 mm Hg, heart rate of 74/min (regular), and mild symmetric peripheral numbness of the extremities (glove and stocking type). There were no signs of autonomic neuropathy. The test results are presented in Table 1. Esophagogastroduodenoscopy and colonoscopy were not performed. Electrocardiography (ECG) (Fig. 2a), echocardiography (Fig. 2b, c), and Holter ECG (data not shown) revealed no abnormalities. According to the criteria for organ failure, only the tongue and peripheral nerves were impaired, and the heart was not judged as an impaired organ (left ventricular inferior wall thickness 11 mm, serum N-terminal pro-brain natriuretic peptide (NT-ProBNP) 263 pg/mL, and serum cardiac troponin T (cTnT) 0.012 ng/mL). 13 According to the Mayo Clinic staging system, his condition was stage I (NT-ProBNP < 332 pg/mL, cTnT < 0.035 ng/mL); 2 According to the Mayo Clinic revised staging system, his condition was stage II (NT-ProBNP < 1800 pg/mL, cTnT < 0.025 ng/mL, involved free light chain minus uninvolved free light chain [dFLC] > 18 mg/dL). 3 Many criteria have been reported for assessing the indications for ASCT. 9 , 14 - 18 He fulfilled all of these criteria and was therefore judged to be suitable for ASCT. Autologous peripheral blood stem cells were collected (2.0 × 106 CD34-positive cells/kg). We considered the amount sufficient for ASCT. 18 , 19 The risk stratified by the melphalan (MEL) dose level was low. 15 , 20 , 21 The MEL dose level was set at 200 mg/m2. ASCT was carried out in August (Fig. 3). On the morning of Day 10 after ASCT, he complained of chest discomfort, but the ECG revealed no abnormalities. On the night of Day 10, he complained of chest discomfort again and the ECG again revealed no abnormalities. On the morning of Day 11, he developed chest discomfort; ECG at this time revealed no abnormalities, whereas echocardiography revealed a decrease in the EF to 35%. He developed cardiopulmonary arrest and was declared dead 50 minutes later. At the time of death, the myocardial troponin T test and Rapicheck test were negative. The serum BNP, NT-ProBNP, and cTnT levels were 326.8 pg/mL, 2006.0 pg/mL, and 0.040 ng/mL, respectively. According to the hematological criteria for judging the responses to treatment, 22 he exhibited a partial response (PR) because the dFLC had decreased by 58.4% to 265.7 mg/mL from 638.6 mg/mL (Fig. 3).
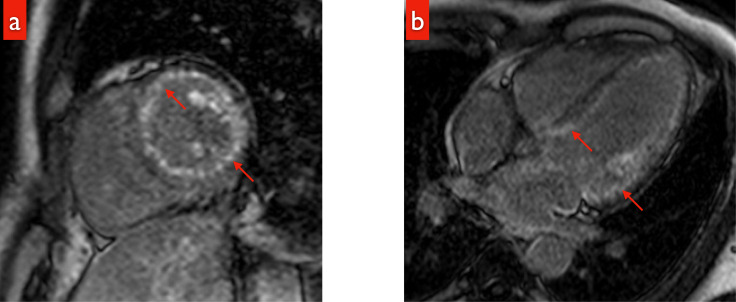
Fig. 1.
a: LGE during cardiac MRI; A sagittal image showing diffuse global subendocardial LGE below the left ventricular endocardium (red arrow).
b: LGE during cardiac MRI: A 4-chamber view showing washout (dark blood pool) of intracardiac contrast material (red arrow).
Table 1.
| Peripheral blood | Biochemistry | Immuno-serological findings | |||||
|---|---|---|---|---|---|---|---|
| WBC | 8700 /μL | TP | 6.9 g/dL | IgG | 596 mg/dL | ||
| Neut | 62.1% | Alb | 4.7 g/dL | IgA | 111 mg/dL | ||
| Ly | 28.6% | T-Bil | 0.5 mg/dL | IgM | 84 mg/dL | ||
| Mono | 5.3% | AST | 22 IU/L | serum β2 MG | 1.8 mg/dL | ||
| Eo | 1.9% | ALT | 29 IU/L | serum IFE | BJP-κ | ||
| Ba | 0.7% | LDH | 208 IU/L | urine IFE | BJP-κ | ||
| RBC | 482 × 104 /μL | ALP | 23 IU/L | serum free κ | 525.0 mg/L | ||
| Hb | 15.2 g/dL | γ-GTP | 18 IU/L | serum free λ | 13.1 mg/L | ||
| Ht | 45.8% | BUN | 16 mg/dL | κ / λ | 40.08 | ||
| MCV | 94.9 fL | Cr | 0.69 mg/dL | dFLC | 511.9 mg/mL | ||
| MCH | 31.6 pg | UA | 7.1 mg/dL | ||||
| Plt | 29.5 × 104 /μL | Ca | 9.4 mg/dL | Urinalysis | |||
| Reti | 1.9% | CRP | 0.2 mg/dL | PH | 7.0 | ||
| protein | (-) | ||||||
| Coagulation | Cardiac muscle marker | glucose | (-) | ||||
| PT | > 100% | CK-MB | 5 ng/mL | OB | (-) | ||
| PT-INR | 0.98 | BNP | 36.5 pg/mL | ||||
| APTT | 27.7 sec | cTnT | 0.012 ng/mL | ||||
| NT-proBNP | 263 pg/mL | ||||||
Abbreviations; WBC, white blood cells; Neut, neutrophil; Ly, lymphocyte; Mono, monocyte; Eo, eosinophil granulocyte; Ba, basophil; RBC, red blood cell; Hb, hemoglobin; Hct, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; Plt, platelet; Reti, reticulocyte; PT, prothrombin time; INR, international normalized ratio; APTT, activation partial thromboplastin time; TP, total protein; Alb, albumin; T-Bil, total -bilirubin; AST, aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; ALP, alkaline phosphatase; γ-GTP, γ-guanosine triphosphate; BUN, blood urea nitrogen; Cr, creatine; UA, uric acid; Ca, calcium; CRP, C-reactive protein; CK-MB, creatine kinase-myoglobin; BNP, brain natriuretic peptide; cTnT, cardiac troponin T; NT-proBNP, N-terminal pro-brain natriuretic peptide; IgG, immunoglobulin G; IgA, immunoglobulin A; IgM, immunoglobulin M; MG, microglobulin; IEP, mmunoelectrophoresis; IFE, mmunofixation electrophoresis; BJP, Bence Jones Protein; dFLC, involved free light chain-uninvolved free light chain; OB, occult blood
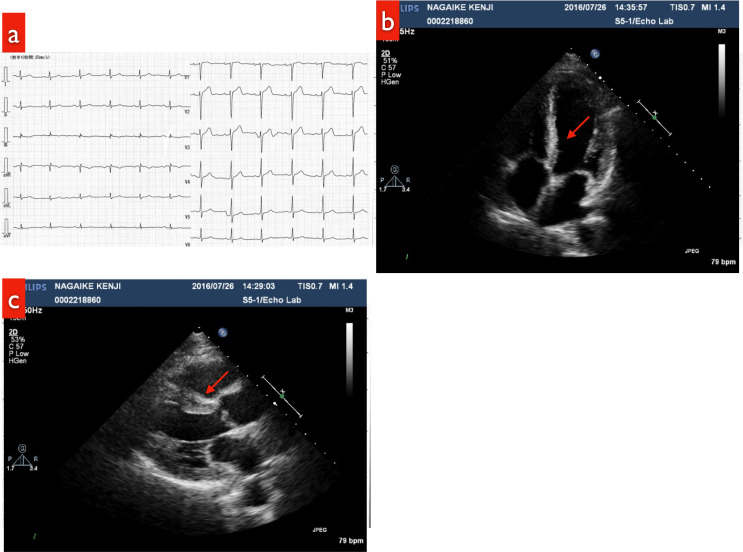
Fig. 2.
a: ECG (at the time of the patient’s visit to our department): no abnormalities
b: Echocardiogram: No abnormalities were noted on parasternal left end long-axial B-mode imaging. There was no hypertrophy of the ventricular septal wall (red arrow).
c: Echocardiogram: No abnormalities were detected in the apical four-chamber view. There was no hypertrophy of the ventricular septal wall (red arrow).
Fig. 3.
Clinical course
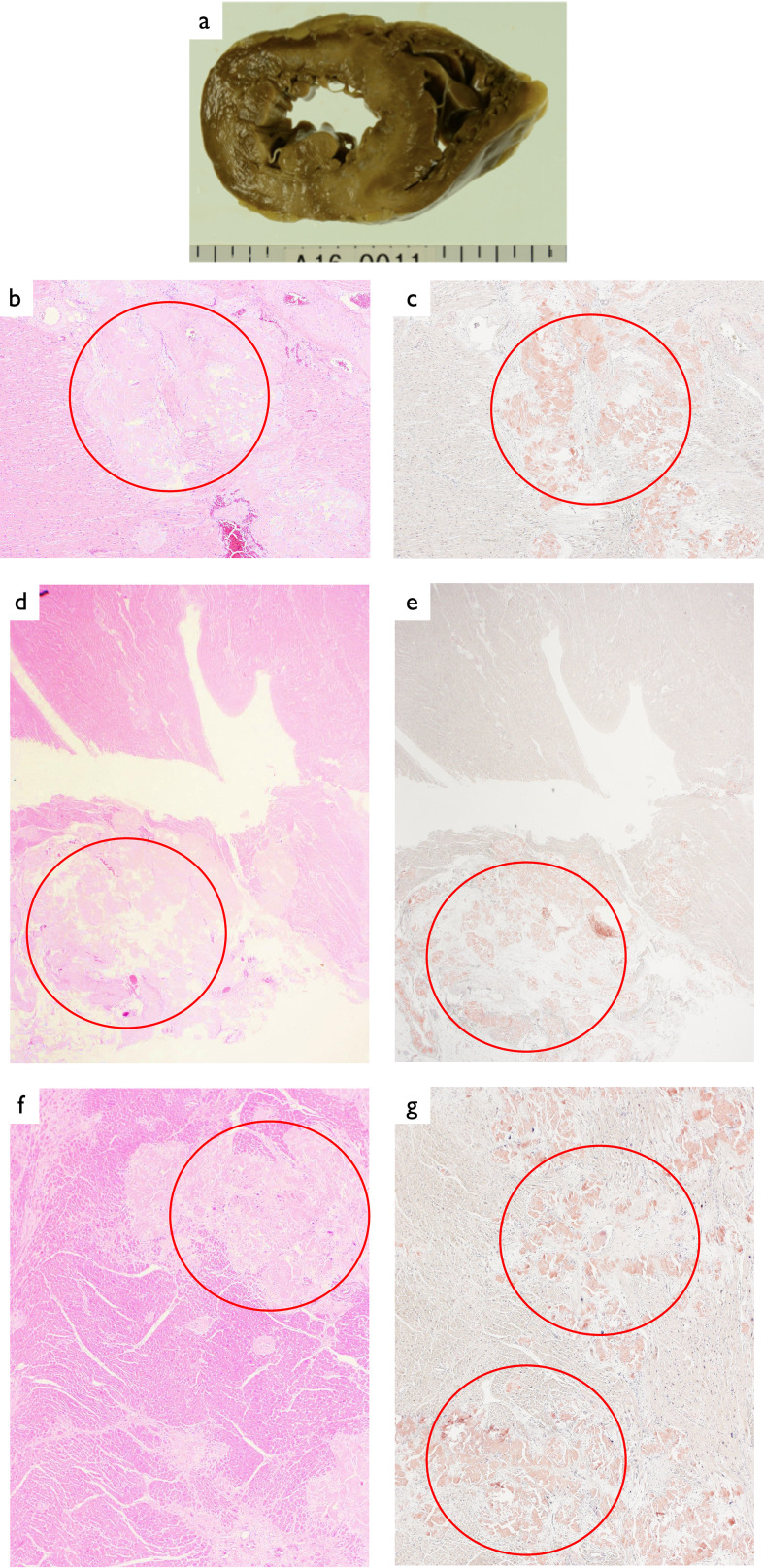
Postmortem examination revealed a heart weight of 350 g. The heart sections were spotty (Fig. 4a) and deposition of weakly acidic homogeneous amorphous material throughout the heart was found on hematoxylin-eosin (HE) staining (left wall, sinoatrial node, and atrioventricular node) (red circles in Fig. 4b, d, f). In addition, DFS 4BS staining was positive (red circles in Fig. 4c, e, g). These findings suggested the severe CA. Amyloid deposition was also noted in multiple organs (data not shown). There was no evidence of myocarditis, infection, or ischemic change.
Fig. 4.
Heart
a: A spotty section.
b: HE staining ×40: Deposition of weakly acidic homogeneous amorphous material was visible.
c: DFS 4BS staining ×40; Positive.
d: Sinoatrial node HE staining ×40: Deposition of weakly acidic homogeneous amorphous material was visible.
e: Sinoatrial node DFS 4S staining ×40; Positive.
f: Atrioventricular node HE staining ×40: Deposition of weakly acidic homogeneous amorphous material was visible.
g: Atrioventricular node DFS 4S staining ×40; Positive.
DISCUSSION
The histopathological findings and clinical course suggest that the patient developed cardiac failure and conduction disturbance as a result of amyloid deposition in the heart, including at the sinoatrial node and atrioventricular node. Death was suspected to be due to acute cardiac failure or fatal arrhythmia due to deterioration of the cardiac condition following ASCT (treatment-related death).
Regarding the prognosis of amyloidosis, it is considered to become poorer as the number of amyloidosis- affected organs increases. 9 In patients with multiple affected organs, if prompt effective treatment is not provided, the survival period is only 1 to 2 years. 4 Symptomatic cardiac failure is associated with a particularly poor prognosis (survival for only 6 months). 4 CA is known to increase the risk of sudden death from fatal arrhythmias. 5 , 6 , 9 CA is also an important determinant of the prognosis in patients with amyloidosis. 2 - 8 Therefore, early diagnosis and prompt therapeutic intervention are important from a stage at which few organs are affected and cardiac failure is absent. 6
ASCT is considered a valid method of treatment for this condition. 3 , 5 , 8 , 9 However, one of the important problems of ASCT is the high TRM (4-24%). 5 - 10 CA is a major cause of TRM in ASCT. 5 - 10 To reduce the TRM associated with ASCT, it is essential to precisely evaluate CA and judge the indications for ASCT in individual cases. 5 , 7 - 11
Many criteria have been reported for assessing the indications for ASCT 9 , 14 - 18 such as those by the Mayo Clinic, 14 Boston University, 15 reliable bodies from the United Kingdom, 16 the Japanese Red Cross Medical Center, 17 and Sapporo Medical University. 18 Our patient fulfilled all of these criteria and was therefore judged as suitable for ASCT. We considered the amount of cells (2.0 × 106 CD34-positive cells/kg) sufficient for ASCT based on the following reports: Hayashi et al. reported that the minimum required amount of cells is 1.5 × 106 CD34-positive cells/kg 18 and Gertz et al. reported that the minimum required amount of cells is 2.0 × 106 CD34-positive cells/kg. 19 He did not fulfill the criteria for reduction of the MEL dose, 15 , 20 , 21 and it was therefore not reduced. However, his death was categorized as treatment-related.
Echocardiography was reported to not be a useful screening tool for CA 2 and myocardial biopsy findings were found to not reflect the extent of amyloid infiltration. 2 Moreover, there is room for improvement of the current criteria for selection of candidates for ASCT. 5 Indeed, evidence of CA was detected in the present case by MRI and myocardial biopsy. In addition, the effectiveness of MRI as a suitable screening modality for CA was previously reported. 7 , 13 , 23 - 25 The MRI findings of CA, in general, include (1) diffuse subendocardial LGE, including on the right ventricular side of the septum, (2) interatrial septal thickening with LGE, and (3) an early decrease in the intracavitary left ventricular blood pool signal on late gadolinium-enhanced MRI. 23 - 25 These findings are useful, with a sensitivity of 93% and specificity of 70%, for the diagnosis of CA, 24 and were observed in the present case (Fig. 1a, b). LGE imaging is the most reliable method for the identification of cardiac involvement in any type of amyloidosis. 25 MRI imaging is a highly reproducible tool that assesses the myocardial morphology, and global and regional heart function. 25 However, these characteristics are nonspecific and differ in their prevalence until the late phases. 25 Thus, there is a risk of false-negatives (shooting error) in contrast cardiac MRI. 25
Currently, there is no report of MRI findings being incorporated into the criteria for assessing the indications for ASCT or those used for MEL dose reduction. It is therefore desirable that new standards incorporating MRI findings be established. In cases in which cardiac MRI suggests amyloid deposition, careful follow-up and strict preventive management are required considering the possibility of sudden death.
Footnotes
CONFLICT OF INTEREST
Disclosure of conflict of interest: None.
REFERENCES
- 1. Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003; 349: 583-596. [DOI] [PubMed] [Google Scholar]
- 2. Dispenzieri A, Gertz MA, Kyle RA, et al. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol. 2004; 22: 3751-3757. [DOI] [PubMed] [Google Scholar]
- 3. Kumar S, Dispenzieri A, Lacy MQ, et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012; 30: 989-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. N Engl J Med. 1997; 337: 898-909. [DOI] [PubMed] [Google Scholar]
- 5. Wechalekar AD, Gillmore JD, Bird J, et al. BCSH Committee. Guidelines on the management of AL amyloidosis. Br J Haematol. 2015; 168: 186-206. [DOI] [PubMed] [Google Scholar]
- 6. D’Souza A, Dispenzieri A, Wirk B, et al. Improved outcomes after autologous hematopoietic cell transplantation for light chain amyloidosis: A Center for International Blood and Marrow Transplant Research Study. J Clin Oncol. 2015; 33: 3741-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tsai SB, Seldin DC, Quillen K, et al. High-dose melphalan and stem cell transplantation for patients with AL amyloidosis: trends in treatment-related mortality over the past 17 years at a single referral center. Blood. 2012; 120: 4445-4446. [DOI] [PubMed] [Google Scholar]
- 8. Jimenez-Zepeda VH, Franke N, Reece DE, et al. Autologous stem cell transplant is an effective therapy for carefully selected patients with AL amyloidosis: experience of a single institution. Br J Haematol. 2014; 164: 722-728. [DOI] [PubMed] [Google Scholar]
- 9. Sher T, Dispenzieri A, Gertz MA. Evolution of hematopoietic cell transplantation for immunoglobulin light chain amyloidosis. Biol Blood Marrow Transplant. 2016; 22: 796-801. [DOI] [PubMed] [Google Scholar]
- 10. Gertz MA, Lacy MQ, Dispenzieri A, et al. Refinement in patient selection to reduce treatment-related mortality from autologous stem cell transplantation in amyloidosis. Bone Marrow Transplant. 2013; 48: 557-561. [DOI] [PubMed] [Google Scholar]
- 11. Girnius S, Seldin DC, Meier-Ewert HK, et al. Safety and efficacy of high-dose melphalan and auto-SCT in patients with AL amyloidosis and cardiac involvement. Bone Marrow Transplant. 2014; 49: 434-439. [DOI] [PubMed] [Google Scholar]
- 12. Gertz MA, Comenzo R, Falk RH, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18-22 April 2004. Am J Hematol. 2005; 79: 319-328. [DOI] [PubMed] [Google Scholar]
- 13. Gillmore JD, Wechalekar A, Bird J, et al. BCSH Committee. Guidelines on the diagnosis and investigation of AL amyloidosis. Br J Haematol. 2015; 168: 207-218. [DOI] [PubMed] [Google Scholar]
- 14. Dispenzieri A, Lacy MQ, Kyle RA, et al. Eligibility for hematopoietic stem-cell transplantation for primary systemic amyloidosis is a favorable prognostic factor for survival. J Clin Oncol. 2001; 19: 3350-3356. [DOI] [PubMed] [Google Scholar]
- 15. Skinner M, Sanchorawala V, Seldin DC, et al. High-dose melphalan and autologous stem-cell transplantation in patients with AL amyloidosis: an 8-year study. Ann Intern Med. 2004; 140: 85-93. [DOI] [PubMed] [Google Scholar]
- 16. Wechalekar AD, Hawkins PN, Gillmore JD. Perspectives in treatment of AL amyloidosis. Br J Haematol. 2008; 140: 365-377. [DOI] [PubMed] [Google Scholar]
- 17. Tsukada N, Ikeda M, Shingaki S, et al. High-dose melphalan and autologous stem cell transplantation for systemic light-chain amyloidosis: a single institution retrospective analysis of 40 cases. Int J Hematol. 2016; 103: 299-305. [DOI] [PubMed] [Google Scholar]
- 18. Hayashi T, Ikeda H, Igarashi T, et al. Autologous stem cell transplantation for AL amyloidosis: adjustment of melphalan dose by factors including BNP. Int J Hematol. 2014; 100: 554-558. [DOI] [PubMed] [Google Scholar]
- 19. Gertz MA, Lacy MQ, Dispenzieri A, et al. Trends in day 100 and 2-year survival after auto-SCT for AL amyloidosis: outcomes before and after 2006. Bone Marrow Transplant. 2011; 46: 970-975. [DOI] [PubMed] [Google Scholar]
- 20. Perfetti V, Siena S, Palladini G, et al. Long-term results of a risk-adapted approach to melphalan conditioning in autologous peripheral blood stem cell transplantation for primary (AL) amyloidosis. Haematologica. 2006; 91: 1635-1643. [PubMed] [Google Scholar]
- 21. Comenzo RL, Gertz MA. Autologous stem cell transplantation for primary systemic amyloidosis. Blood. 2002; 99: 4276-4282. [DOI] [PubMed] [Google Scholar]
- 22. Comenzo RL, Reece D, Palladini G, et al. Consensus guidelines for the conduct and reporting of clinical trials in systemic light-chain amyloidosis. Leukemia. 2012; 26: 2317-2325. [DOI] [PubMed] [Google Scholar]
- 23. Lin L, Li X, Feng J, et al. The prognostic value of T1 mapping and late gadolinium enhancement cardiovascular magnetic resonance imaging in patients with light chain amyloidosis. J Cardiovasc Magn Reson. 2018; 20: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Donnelly JP, Hanna M. Cardiac amyloidosis: an update on diagnosis and treatment. Cleve Clin J Med. 2017; 84(suppl 3): 12-26. [DOI] [PubMed] [Google Scholar]
- 25. Oda S, Utsunomiya D, Nakaura T, et al. Role of noninvasive diagnostic imaging in cardiac amyloidosis: A review. Cardiovascular Imaging Asia. 2018; 2: 97-106. [Google Scholar]