TO THE EDITOR
Immune checkpoint blockade (ICB) targeting programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) has become a promising approach as anti-cancer immunotherapy. 1 At first, anti-PD-1 therapy and anti-CTLA-4 therapy were approved for patients with advanced melanoma; since then, anti-PD-1 therapy has been shown to be effective for several solid tumors, such as non-small-cell lung cancer (NSCLC), renal cell carcinoma, urothelial carcinoma, head and neck squamous cell carcinoma (SCC), esophageal SCC, gastric adenocarcinoma, and triple-negative breast carcinoma. 2 , 3 In terms of hematological malignancies, anti-PD-1 therapy showed a high clinical response rate in Hodgkin lymphoma patients, and ICB became a standard therapy for Hodgkin lymphoma. 4 The potential clinical benefit of anti-PD-1 therapy has also been reported in Richter transformation of chronic lymphocytic leukemia and diffuse large B-cell lymphoma. 5 , 6 CTLA-4 is expressed on T-lymphocytes and competitively inhibits the binding of CD28 to costimulatory molecules, such as CD80 and CD86. Alternatively, PD-1 ligands are expressed not only on cancer cells, but also on immune cells. Among immune cells, antigen-presenting cells, such as macrophages and dendritic cells, express high levels of PD-1 ligands. 7 , 8 Regarding the sites where immune checkpoint molecules work, it is now considered that CTLA-4 acts as a negative regulator of the initial activation of T cells in regional lymph nodes, i.e., inhibition at the induction phase, and PD-1 ligands suppress T-cell activation in the tumor microenvironment, i.e., inhibition at the effector phase. 2 However, some studies using animal cancer models have revealed that anti-PD-1/PD-L1 therapy exerts effects at regional lymph nodes. In 2003, Curiel et al. showed that PD-L1 was overexpressed in myeloid dendritic cells (CD11c+ and MHC-class II+) in cancer-bearing mice, and blockade of the PD-1/PD-L1 interaction enhanced dendritic cell-mediated T-cell activation. 9 Recently, Fransen et al. showed that PD-L1 expression was significantly elevated in myeloid cells (CD11b+) in lymph nodes, and anti-PD-1 therapy induced T-cell activation and proliferation in lymph nodes using an animal model. 10 They also demonstrated that the resection of draining lymph nodes completely abrogated anti-tumor immune responses induced by PD-1/PD-L1 blockade therapy. These data indicated that not only CTLA-4 but also PD-1/PD-L1 works as a negative regulator at regional lymph nodes, main sites for the induction of anti-tumor T cells.
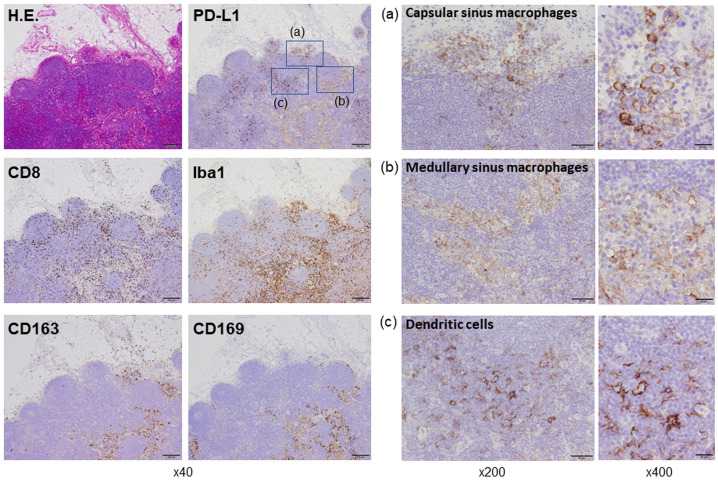
Despite laborious search using PubMed, we have never been able to find an image on PD-L1 expressi in human lymph nodes. However, we recently reported that macrophages detected in the tumor microenvironment and regional lymph nodes of human samples were positive for PD-L1. 11 , 12 Therefore, in the present letter, we would like to introduce our data on PD-L1-positive cells in lymph nodes resected from patients with colon cancer. PD-L1 expression was seen in 4 of 4 cases, and images from a case in which the strongest positive signals were detected are presented (Figure 1). High PD-L1 expression was seen on dendritic cells (Iba1+, CD163-, CD169-), whereas weak to moderate expression was seen on sinus macrophages (Iba1+, CD163+, CD169+). Sinus macrophages are divided into capsular sinus macrophages and medullary sinus macrophages, and PD-L1 expression was seen on both cell types (Figure 1). Lymphocytes in lymph nodes were negative for PD-L1. CD8-positive lymphocytes were detected in both follicle areas and sinus areas, suggesting a direct interaction between T-lymphocytes and antigen-presenting cells (Figure 1). These observations suggest that the efficacy of anti-PD-1/PD-L1 blockade therapy is, at least partially, ascribed to T-lymphocyte activation in regional lymph nodes in cancer patients, as seen in mice models.
Fig. 1.
Histology of the lymph node. Hematoxylin and eosin (H.E.) staining and immunohistochemistry of PD-L1 (clone 22C3, DAKO, Glostrup, Denmark), CD8 (clone C8/144B, Nichirei, Tokyo, Japan), Iba1 (clone NCNP27, WAKO, Tokyo, Japan), CD163 (clone 10D6, Abcam, Cambridge, UK), and CD169 (clone SP216, Abcam Cambridge, UK) are shown. The immunohistochemistry procedures were described in a previous report. 13 Subcapsular and medullary sinus macrophages that were positive for Iba1, CD163, and CD169 were also positive for PD-L1. Dendritic cells that were positive for Iba1 and negative for CD163 and CD169 strongly expressed PD-L1. CD8-positive T-cells were more commonly detected in areas in which antigen-presenting cells were seen.
Many researchers are now trying to identify markers and factors that can be used for prediction of the responsiveness to ICB therapy. In NSCLC and head and neck SCC, higher response rates were seen in cases with PD-L1-positive cancer cells. 14 - 16 In urothelial cancer and breast cancer, higher response rates were seen in cases with PD-L1-positive cancer cells and immune cells in the cancer tissue. 17 , 18 In contrast, the PD-L1 expression status was not associated with the clinical response to anti-PD-1/PD-L1 therapy in some other cancers, including lung SCC, melanoma, and renal cell carcinoma. 17 , 18 Given that there is no study on the correlation between immune activation status in lymph nodes and the efficacy of ICB therapy so far, it would be of great interest to assess the immune activation status or PD-L1 expression in the regional lymph nodes of cancer patients.
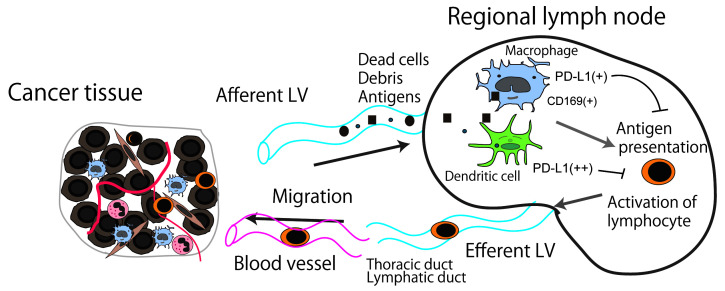
Although dendritic cells, macrophages and B cells can work as antigen-presenting cells, their roles are somewhat different. Dendritic cells primarily present antigen to naïve T cells as an induction phase of immune response, whereas macrophages and B cells present it to antigen-primed T cells as an initiation of the effector phase. 19 In terms of their roles in lymph nodes, dendritic cells play a central role in the priming of naïve T cells to tumor antigens, 20 whereas it has not been fully elucidated regarding roles of macrophages and B cells as antigen-presenting cells. However, several studies revealed that macrophages in lymph nodes can work as antigen-presenting cells in animal studies. In 2011, Asano et al. found that CD169-positive subcapsular sinus macrophages were involved in antigen presentation and the induction of cytotoxic T-lymphocytes. 21 In 2015, Benhard et al. demonstrated that not only dendritic cells, but also CD169-positive sinus macrophages induced anti-cancer immune responses. 22 Strömvall et al. reported that the reduction of CD169 in pre-metastatic regional lymph nodes was associated with lymph node metastasis in a rat model, and they additionally showed that a high expression level of CD169 was a favorable prognostic factor in patients with prostatic cancer. 23 The correlation between a high CD169 expression level in sinus macrophages and a better clinical course has been reported in several solid tumors, including melanoma, colorectal cancer, endometrial cancer, esophageal cancer, and bladder cancer. 13 , 24 - 26 A correlation between anti-cancer immune responses and high CD169 expression has been suggested in these tumors. In addition to dendritic cells, sinus macrophages in regional lymph nodes are closely associated with anti-cancer immune responses since they engulf dead cells and debris from tumor tissues (Figure 2). These antigen-presenting cells express PD-L1 (and potentially PD-L2), which negatively regulates activation of T-lymphocyte. Therefore, their expression of PD-L1 in regional lymph nodes would be worthy of attention in patients who will receive the ICB therapy targeting the interaction of PD-1/PD-L1 interaction, as is the case with tumor tissues.
Fig. 2.
Schema of the anti-cancer immune response associated with regional lymph nodes. Dead cells and debris, including tumor-specific antigens, drain into the lymph node sinus via lymphatic vessels (LVs). Antigen-presenting cells, such as macrophages and dendritic cells, capture and engulf these antigens, then activate the antigen-reactive T-lymphocytes. PD-L1 (and potentially PD-L2 as well) expressed on antigen-presenting cells might negatively regulate T-lymphocyte activation.
ACKNOWLEDGMENTS
This work was supported by Takeda Science Foundation. Paraffin-embedded lymph node samples were prepared from specimens obtained from patients diagnosed as colon cancer and surgically resected at Izumi General Hospital (Izumi, Kagoshima, Japan). Written informed consent was obtained from all patients, and the study design was approved by the review board (#57).
Footnotes
CONFLICT OF INTEREST
All authors have no financial competing interests to declare.
REFERENCES
- 1. Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016; 8: 328rv4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018; 359: 1350-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vaddepally RK, Kharel P, Pandey R, Garje R, Chandra AB. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers (Basel). 2020; 12: 738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu-Monette ZY, Zhou J, Young KH. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood. 2018; 131: 68-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ding W, LaPlant BR, Call TG, et al. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood. 2017; 129: 3419-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith SD, Till BG, Shadman MS, et al. Pembrolizumab with R-CHOP in previously untreated diffuse large B-cell lymphoma: potential for biomarker driven therapy. Br J Haematol.; in press. [DOI] [PubMed] [Google Scholar]
- 7. Yamazaki T, Akiba H, Iwai H, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002; 169: 5538-5545. [DOI] [PubMed] [Google Scholar]
- 8. Ishida M, Iwai Y, Tanaka Y, et al. Differential expression of PD-L1 and PD-L2, ligands for an inhibitory receptor PD-1, in the cells of lymphohematopoietic tissues. Immunol Lett. 2002; 84: 57-62. [DOI] [PubMed] [Google Scholar]
- 9. Curiel TJ, Wei S, Dong H, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003; 9: 562-567. [DOI] [PubMed] [Google Scholar]
- 10. Fransen MF, Schoonderwoerd M, Knopf P, et al. Tumor-draining lymph nodes are pivotal in PD-1/PD-L1 checkpoint therapy. JCI Insight. 2018; 3: e124507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Horlad H, Ma C, Yano H, et al. An IL-27/Stat3 axis induces expression of programmed cell death 1 ligands (PD-L1/2) on infiltrating macrophages in lymphoma. Cancer Sci. 2016; 113: 1696-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Komohara Y, Ohnishi K, Takeya M. Possible functions of CD169-positive sinus macrophages in lymph nodes in anti-tumor immune responses. Cancer Sci. 2017; 108: 290-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakagawa T, Ohnishi K, Kosaki Y, et al. Optimum immunohistochemical procedures for analysis of macrophages in human and mouse formalin fixed paraffin-embedded tissue samples. J Clin Exp Hematop. 2017; 57: 31-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim J, Cho J, Lee MH, Lim JH. Relative Efficacy of Checkpoint Inhibitors for Advanced NSCLC According to Programmed Death-Ligand-1 Expression: A Systematic Review and Network Meta-Analysis. Sci Rep. 2018; 8: 11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. KEYNOTE-189 Investigators. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018; 378: 2078-2092. [DOI] [PubMed] [Google Scholar]
- 16. Ferris RL, Blumenschein G, Jr, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016; 375: 1856-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rui X, Gu TT, Pan HF, Zhang HZ. Evaluation of PD-L1 biomarker for immune checkpoint inhibitor (PD-1/PD-L1 inhibitors) treatments for urothelial carcinoma patients: A meta-analysis. Int Immunopharmacol. 2019; 67: 378-385. [DOI] [PubMed] [Google Scholar]
- 18. Schmid P, Adams S, Rugo HS, et al. IMpassion130 Trial Investigators. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med. 2018; 379: 2108-2121. [DOI] [PubMed] [Google Scholar]
- 19. Berard M, Tough DF. Qualitative differences between naïve and memory T cells. Immunology. 2002; 106: 127-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sánchez-Paulete AR, Teijeira A, Cueto FJ, et al. Antigen cross-presentation and T-cell cross-priming in cancer immunology and immunotherapy. Ann Oncol. 2017; 28(suppl_12): xii44-xii55. [DOI] [PubMed]
- 21. Asano K, Nabeyama A, Miyake Y, et al. CD169-positive macrophages dominate antitumor immunity by crosspresenting dead cell-associated antigens. Immunity. 2011; 34: 85-95. [DOI] [PubMed] [Google Scholar]
- 22. Bernhard CA, Ried C, Kochanek S, Brocker T. CD169 + macrophages are sufficient for priming of CTLs with specificities left out by cross-priming dendritic cells. Proc Natl Acad Sci USA. 2015; 112: 5461-5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Strömvall K, Sundkvist K, Ljungberg B, Halin Bergström S, Bergh A. Reduced number of CD169 + macrophages in pre-metastatic regional lymph nodes is associated with subsequent metastatic disease in an animal model and with poor outcome in prostate cancer patients. Prostate. 2017; 77: 1468-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Asano T, Ohnishi K, Shiota T, et al. CD169-positive sinus macrophages in the lymph nodes determine bladder cancer prognosis. Cancer Sci. 2018; 109: 1723-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saito Y, Ohnishi K, Miyashita A, et al. Prognostic Significance of CD169+ Lymph Node Sinus Macrophages in Patients with Malignant Melanoma. Cancer Immunol Res. 2015; 3: 1356-1363. [DOI] [PubMed] [Google Scholar]
- 26. Takeya H, Shiota T, Yagi T, et al. High CD169 expression in lymph node macrophages predicts a favorable clinical course in patients with esophageal cancer. Pathol Int. 2018; 68: 685-693. [DOI] [PubMed] [Google Scholar]