Abstract
Background:
Individual health behaviors (i.e., eating habits, sedentary lifestyle) are associated with type 2 diabetes (T2D). Health behavior profiles specific to adolescents with T2D have not been described.
Objective:
To identify health behavior profiles in adolescents with T2D and examine how these profiles change over time.
Methods:
Diet (via food frequency questionnaire) and activity behaviors (via 3-day physical activity recall) examined at baseline, 6 and 24 months from participants in the TODAY study were used for this analysis. Latent profile analysis identified profiles of health behaviors within three time points and latent transition probabilities were estimated to examine the change from baseline to 6 months (n=450) and baseline to 24 months (n=415). Multinomial logistic regressions were used to examine if the assigned TODAY treatment group [Metformin (Met), Met + Rosiglitazone (Rosi), or Met + Lifestyle] predicted change in health behavior profiles.
Results:
Three profiles emerged: “most sedentary”, “healthy eaters” and “active and eat most.” At 6 months, 50% of males and 29% of females in the Met + Lifestyle treatment group improved in their health behavior profile. Among males only, the Met + Lifestyle treatment group were more likely to improve their profiles from baseline to 6 months (p=0.01).
Conclusions:
Three health behavior profiles emerged and shifted over time. A high quality, lifestyle intervention had little effect on improving health behavior profiles. Optimizing outcomes in youth with T2D might require more robust and multifaceted interventions beyond family-level lifestyle, including more extensive psychosocial intervention, novel medication regimen or bariatric surgery.
Keywords: adolescence, type 2 diabetes, obesity, health behaviors, profiles
Introduction
As type 2 diabetes mellitus (T2D) continues to increase in adolescents along with the rapid progression of complications related to the disease, including early signs of cardiovascular disease,1-3 it is critical to understand modifiable behaviors as potential targets for future treatment and prevention strategies. Observational studies have found that adolescents with T2D that have better diet quality and higher levels of physical activity have improved metabolic control compared to those that have lower diet quality and poor physical activity.4 Many individual health behaviors (i.e., poor eating habits, sedentary lifestyle) have been linked to insulin resistance and T2D5 but, to our knowledge, the health behavior profiles of adolescents with T2D have not been fully described. Changing lifestyle behaviors in adolescents with T2D has been reported as extremely difficult due to both poor compliance and their lack of readiness for adopting healthy behaviors, especially in older adolescent populations.6 Unfortunately, when changes are made in diet and activity behaviors, the changes most often occur in the first few months (3-6 months) of the intervention and are not sustained one to two years later.4,6 Trials evaluating lifestyle behaviors alone for the treatment of hyperglycemia in adolescent populations do not exist.4 The TODAY study did include lifestyle modification along with pharmacologic therapy; hence, offers the opportunity to examine how patterns of lifestyle behaviors may have changed over the course of treatment. Further, as data on all relevant health behaviors and outcomes existed at 6 and 24 months post intervention, we were able to examine the possible effect of the lifestyle modification both short and long-term.
The Treatment Options for type 2 Diabetes in Adolescents and Youth (TODAY) study, a randomized controlled trial of over 600 adolescents (n=699) with T2D, individual dietary and activity behaviors were documented over 24 months. Kriska et al has previously reported change in specific dietary components (i.e., macronutrients, servings of fruit/vegetables, sweetened beverages, etc.) at baseline, 6 months, and 24 months, and calculated change in these individual components by sex.7 Although dietary intake remained relatively stable in the components examined, a few notable changes did exist. For males, intake of sweetened beverages significantly increased from baseline to 24 months and females were found to decrease their intake of both desserts and dairy over the course of 24 months. Baseline physical activity data have also been previously reported in the TODAY cohort.8 In this study, Adolescents in the TODAY study were found to be less active and reported spending more time being sedentary than the national representative sample of youth. To our knowledge, no one has examined health behavior profiles by combining diet and activity behaviors in adolescents with T2D.
In general population studies, problematic lifestyle choices are commonly found in adolescents, with inadequate moderate-to-vigorous movement, predominance of sedentary choices, and inadequate consumption of fruits and vegetables.9-11 Using a national representative sample of over 9,000 U.S. adolescents, Iannotti et al found three unique health behavior [i.e., diet, physical activity (PA), and sedentary behavior (SB)] profiles: healthful, unhealthful, and typical.12 Almost half of the sample (47.2%) was found to have a “typical” pattern, which corresponded to the lowest consumption of fruits, vegetables, and sweets and moderate levels of PA and SB. Adolescents reporting the “typical” pattern were found to be older with a higher prevalence of overweight/obesity. In a similar study of European adolescents, five health behavior profiles emerged and consisted of a mix of healthy and unhealthy lifestyle behaviors.13 A diet quality index was calculated; higher scores reflective of higher quality dietary habits. Profiles included “unhealthy”, “sedentary”, “inactive, high diet quality”, “active, low diet quality”, and “healthy”. Males most commonly had the pattern characterized by high levels of moderate-to-vigorous PA (MVPA) and low-quality diets, while females had the opposite pattern with low levels of MVPA and high-quality diets.
Using the TODAY study cohort, the first objective of our analysis was to carry out latent profile analysis at three separate time points (baseline, 6 months, and 24 months post-intervention) to identify health behavior profiles within each time point. Next we examined how these profiles changed over time by focusing on profile membership changes from baseline to 6 months and from baseline to 24 months. Lastly, we examined the potential added effect of treatment group to the transition of profile membership to account for possible intervention effects on transitioning from one health behavior profile to another.
Methods
The details of the TODAY study have been previously published.14,15 The main objective of the original study was to examine the relative efficacy of three treatments: 1) Metformin (Met), 2) Met + Rosiglitazone (Rosi), and 3) Met + Lifestyle. Standard education was given to all groups. The TODAY lifestyle program (TLP) was a family-based behavioral lifestyle change intervention that targeted weight loss.16 TLP was developed to give the adolescents with T2D and their families intensive, ongoing support, and training in the behavioral skills needed for weight loss and maintenance. Each family was assigned a Personal Activity and Nutrition Leader (PAL) who met with the family weekly for the first 6-8 months, every other week through 12-16 months, and then monthly to 24-28 months post-enrollment. The first 6-8 months included behavioral change targets to promote weight loss, including increasing PA and an individual calorie intake goal. PALs focused on teaching the families behavioral skills (e.g., self-monitoring, goal-setting, and problem-solving). In the next phase of the TLP, lifestyle maintenance was the focus of the intervention and the behavioral targets were to maintain the PA and calorie goals accomplished in the first phase. The final year of the trial consisted of the PALs meeting with the families monthly. The TLP utilized a modified Traffic Light Diet17 to assist the participants in attaining their calorie goals. PALs worked with the participants to decrease the number of RED foods consumed daily (i.e., high caloric foods, including high fat foods, sugary cereals, and soft drinks) and increase consumption of GREEN foods (low-calorie foods).17 To improve PA among the participants, PALs worked to increase PA to 200 minutes per week (around 3 hours) and decrease sedentary behaviors (use sparingly). A similar traffic light reference guide was used; GREEN (moderate intensity activities like brisk walking), YELLOW (household chores), and RED (watching TV).
Participants
A total of 699 adolescents aged between 10-17 years were enrolled into the study between 2004-2009. Eligibility included a diagnosis of T2D of less than 2 year’s duration, a body mass index (BMI) above the 85th percentile for age and sex, and no known diabetes autoantibodies. A 2-6-month run-in period was used in order to ensure participants could demonstrate knowledge of standard diabetes education, adherence to study procedures, and adequate glycemic control for two consecutive months.
Measures
Diet was assessed at baseline, 6 months, and 24 months using the TODAY food frequency questionnaire (FFQ), a modified version of the SEARCH for Diabetes in Youth FFQ.18 The TODAY FFQ was based on the Kids’ Food Questionnaire which has been previously validated for children eight years and older, including for low-income African American youth.19 Ethnically and regionally diverse foods representative of the TODAY participants were also added.7 The questionnaire was administered by a certified interviewer and scored using the Nutrition Data System for Research (database 3 version 4.005/33, University of Minnesota). The assessment provided a measurement of dietary intake, including servings of specific food groups (i.e., high fat dairy, low fiber cereal, and high fat meat). Food groups were used to create the number of servings of red and green foods for the analysis. Red foods included the total number of servings of the following food groups: sweets, sugar sweetened beverages, high fat dairy, and high fat meat. Green foods included fruit and vegetables, low fat dairy, low fat poultry, and low-fat snacks. The number of servings for each participant was standardized by 1000 kcal. Physical activity and sedentary time were measured using the 3-day physical activity recall (3DPAR), which has been validated as a self-administered questionnaire that assesses activity for the previous 3 days in youth populations.20 The 3DPAR was completed by participants with guidance from a trained interviewer at each of the clinical visits. Data regarding the total amount of moderate-to-vigorous physical activity (hours) and sedentary time (hours) were used for this analysis.
Table 3.
Class Prevalence (%) and Transition Probabilities (%)
| Class Prevalence | Transition Probabilities | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Health Behavior Pattern |
Baseline to 6 months post-intervention |
Baseline to 24 months post-intervention |
|||||||
| Base | 6 mo | 24 mo | Most Sedentary |
Healthy Eaters |
Active and Eat Most |
Most Sedentary |
Healthy Eaters |
Active and Eat Most |
|
| Females | |||||||||
| Most Sedentary | 41.9 | 46.3 | 32.1 | 17.6 | 16.0 | 8.3 | 15.0 | 23.6 | 4.9 |
| Healthy Eaters | 21.4 | 34.5 | 52.1 | 11.2 | 7.0 | 3.2 | 5.6 | 9.7 | 4.5 |
| Active and Eat Most | 36.7 | 19.2 | 15.7 | 17.6 | 11.5 | 7.7 | 11.6 | 18.7 | 6.4 |
| Males | |||||||||
| Most Sedentary | 41.8 | 43.5 | 31.1 | 19.2 | 14.7 | 7.9 | 14.9 | 18.2 | 3.4 |
| Healthy Eaters | 17.5 | 36.2 | 48.7 | 5.7 | 6.2 | 5.7 | 6.8 | 14.2 | 6.1 |
| Active and Eat Most | 40.7 | 20.3 | 20.3 | 18.6 | 15.3 | 6.8 | 9.5 | 16.2 | 10.8 |
Example of interpreting table: A total of 41.9% of females were classified as “most sedentary” at baseline. At 6 months post-intervention; 17.6% remained “most sedentary”, 16.0% transitioned to “healthy eaters”, and 8.3% transitioned to “active and eat most”.
Statistical Analysis
We conducted latent profile analysis (LPA) to identify adolescents’ health behavior profiles. LPA is a commonly used approach to identify subpopulations by shared item response profiles. Using this approach, profiles of individuals in specific latent profiles are expected to be similar to one another with respect to the variables of interest, but different from individuals with other profiles. LPA was conducted using Mplus Version 7.1 to identify health behavior profiles using four indicators of health behaviors (diet using the number of green foods and the number of red foods consumed with each standardized per 1000 kcal per day; physical activity using hours per day of moderate-to-vigorous activity and sedentary time using hours per day of sedentary time from the 3DPAR).
LPA started with a one-class model and the number of profiles increased until the best-fitting model was identified for each time period (baseline, 6 months, and 24 months). Relative fit indices based on information-heuristic criteria included Akaike’s Information Criterion (AIC), the Bayesian Information Criterion (BIC), Consistent Akaike’s Information Criterion (CAIC), and the Approximate Weight of Evidence Criterion (AWE). Additional fit statistics included the Lo-Mendell-Rubin likelihood ratio test (LMR-LRT), the Bayes Factor, and the Correct Model Probability (cmP).
To assess temporal changes in health behavior profiles longitudinally, we examined the probability of transitioning from one profile to another over time. To conduct this analysis, first latent profile membership probabilities were estimated at each time point (baseline, 6 months, and 24 months) and the stability of profiles across time points was assessed. Next, a transition probability was modeled to reflect the probability of transitioning from one latent status from baseline to six months and from baseline to 24 months. Together, these probabilities estimate the amount of change over time in the outcome on the basis of pattern membership. A probabilities matrix showing transitions from 1) baseline to 6 months and 2) baseline to 24 months provides a summary of the change over those two time points in behavior profiles. Thus, we defined an individual’s change into three categories: 1) stable (remained in same pattern at baseline and either 6 months or 24 months), 2) improved (transitioned to a healthier overall pattern of behaviors), and 3) worsened (transitioned to a pattern of behaviors that was worse than the baseline pattern). Unadjusted multinomial logistic regression analyses, stratified by sex, were used to examine if treatment group predicted transitions of health behavior profiles at both 6 months and 24 months post-intervention.
The analyses presented in this paper reflect a secondary analysis of the TODAY cohort and were not incorporated in the power calculations for the parent trial. Formal power calculations for the current analyses would have been of a post hoc nature and were therefore not estimated. Statistical power for LPA is dependent on several factors, such as the number of indicators and the effect sizes associated with the separation between classes,21 the latter of which is difficult to determine on an a priori basis. However, sample sizes found in previous literature22-24 suggest that the sample size of more n=400 subjects will allow us to perform LPA and determine meaningful subgroups within the population.
Results
Of the 699 participants, 642 (92%) completed relevant diet and activity assessments at baseline, 530 (76%) at 6 months, and 453 (65%) at 24 months post-trial. Our analytic cohort consisted of individuals that completed both baseline and 6 months visit (n=450) as well as both baseline and 24 months visit (n=415). There were no significant differences for SES indicators (i.e., gender, age, race, household income, and body mass index) between the full cohort (n=699) and the two sub cohorts. We estimated between one to six models using multiple random perturbations of start values for each model. All models converged and there were no negative indications regarding model identification. The log likelihood values were replicated for all solutions. Model fit indices including the AIC, BIC, and CAIC, and the measures of absolute and relative fit supported the three-class solution across all time periods. Table 1 illustrates the demographic and health behavior means by baseline class for the cohort. The class “most sedentary” had the highest number of hours of sedentary time and the lowest amount of time in MVPA. The “healthy eaters” class had moderate levels of sedentary time and MVPA, and reported eating more green than red foods. Finally, the “active and eat most” class had the lowest number of hours of sedentary time, the highest hours in MVPA, and the highest number of both red and green foods consumed. We did not detect a pattern in this cohort of a group of participants that were active and ate the least number of calories. Table 2 illustrates the individual health behavior averages by time period for the cohort by class.
Table 1.
Demographic Characteristics of Cohort by Assigned Health Behavior Profiles at Baseline.
| Most Sedentary |
Healthy Eaters |
Active & Eat Most |
|
|---|---|---|---|
| n (%) | 205 (42) | 98 (20) | 187 (38) |
| Gender, n (%) | |||
| Female | 131 (42) | 67 (21) | 115 (37) |
| Male | 74 (42) | 31 (17) | 72 (41) |
| Age, n (%) | |||
| ≤ 13 years | 92 (42) | 46 (21) | 81 (37) |
| 14 years | 25 (37) | 12 (18) | 31 (46) |
| 15 years | 35 (41) | 21 (24) | 30 (35) |
| > 15 years | 53 (45) | 19 (16) | 45 (38) |
| Race, n (%) | |||
| Non-Hispanic Black | 58 (37) | 39 (26) | 58 (37) |
| Hispanic | 82 (42) | 37 (19) | 77 (39) |
| Non-Hispanic White | 44 (41) | 19 (18) | 44 (41) |
| Other | 21 (66) | 3 (10) | 8 (24) |
| Household Income, n (%) | |||
| < $24,999 | 66 (37) | 37 (21) | 76 (42) |
| $25,000-$49,999 | 61 (41) | 28 (19) | 60 (40) |
| > $50,000 | 56 (50) | 22 (20) | 34 (30) |
Note: Means are very similar by sex; hence, they are shown as a collective cohort.
Table 2.
Means of Individual Health Behaviors by Assigned Health Behavior Profiles.
| Most Sedentary | Healthy Eaters | Active and Eat Most | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Base | 6 mo | 24 mo | Base | 6 mo | 24 mo | Base | 6 mo | 24 mo | |
| Sedentary Time (hr) | 6.17 | 6.64 | 5.90 | 5.54 | 4.51 | 5.22 | 5.37 | 5.39 | 4.31 |
| MVPA (hr) | 0.39 | 0.44 | 0.06 | 1.69 | 1.68 | 1.00 | 2.45 | 1.83 | 2.37 |
| Red Foods* (#) | 3.21 | 3.08 | 3.27 | 2.63 | 2.26 | 2.89 | 5.82 | 6.01 | 4.96 |
| Green Foods* (#) | 3.32 | 3.37 | 2.81 | 5.47 | 5.86 | 4.87 | 8.13 | 8.64 | 6.83 |
| Total Kcal | 1253 | 1351 | 1336 | 1350 | 1242 | 1328 | 1263 | 1190 | 1186 |
| Macronutrients | |||||||||
| %FAT | 39 | 38 | 38 | 38 | 37 | 37 | 38 | 37 | 37 |
| %CHO | 44 | 46 | 45 | 46 | 46 | 47 | 45 | 46 | 46 |
| %PRO | 18 | 17 | 17 | 18 | 18 | 17 | 18 | 18 | 18 |
standardized by total kcal. Base = baseline, CHO = carbohydrate, and PRO = protein
Note: Means are very similar by sex; hence, they are shown as a collective cohort.
There were numerous transitions in profile members across time in males and females (Table 3). For example, individuals that transitioned from “most sedentary” to “healthy eaters” were categorized as improved because they decreased their screen time and improved their diet. On the other hand, those that transitioned from “healthy eaters” to “most sedentary” were categorized as worsened. Similar proportions of males reported stable profiles of health behaviors at both 6 and 24 months, respectively. Similarly, females had stable profiles at both 6 and 24 months, respectively. The majority of adolescents transitioned into new health behavior profiles, which reflected both improvements and worsening of health behaviors. Figure 1 represents how the cohort transitioned over time (remained stable, improved health behavior pattern, or worsened health behaviors) by sex and treatment group. Figure 1A illustrates transitions from baseline to 6 months post-intervention and Figure 1B from baseline to 24 month post-intervention. Similar transitions were found between baseline and both 6 and 24 months post.
Figure 1.
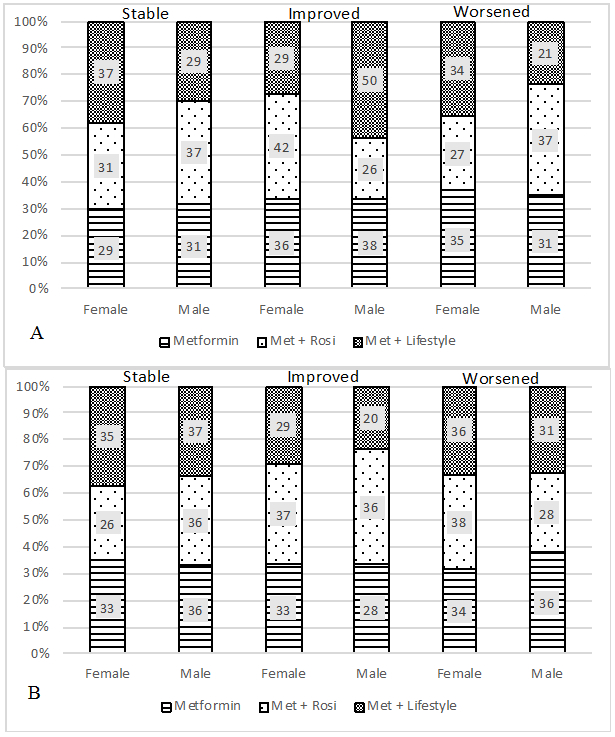
Health Behavior Profiles Change by Sex and Treatment Group at 6 (A) and 24 (B) Months.
In the multinomial regression analysis, compared with the Met + Rosi group, males in the Met + Lifestyle group were more likely to improve their health behavior profiles from baseline to 6 months (p=0.01). However, the change in health behavior profiles did not persist at 24 months. No significant changes were observed for the females at either time point. Figure 2 A (6 months) and B (24 months) illustrate the mean change in each of the four health behaviors according to the transition overtime. For example, individuals in the “improved” transition category at 6 months increased their MVPA by 0.93 hours/day as well as increased consumption of green foods. Sedentary time decreased and red food consumption remained stable. The “worsened” transition category decreased MVPA and green food consumption, as well as increased sedentary time.
Figure 2a.
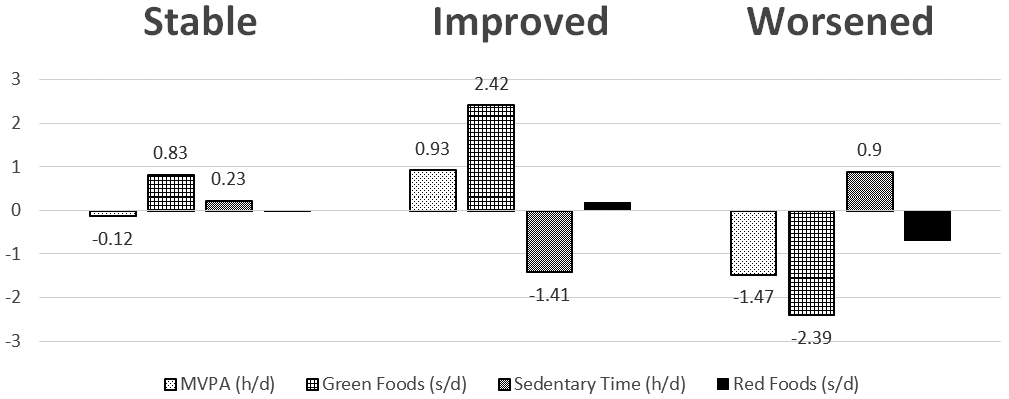
Changes in Health Behavior Profiles by Individual Behaviors at 6 Months
Figure 2b.
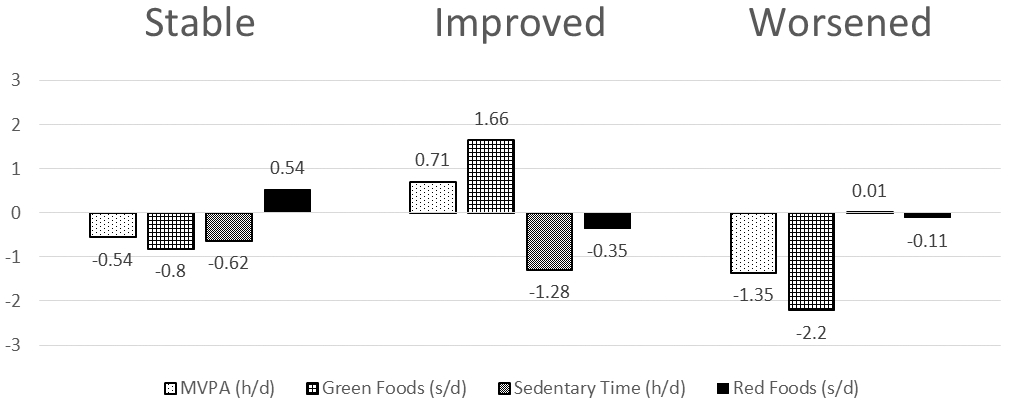
Changes in Health Behavior Profiles by Individual Behaviors at 24 Months
Discussion
In this cross-sectional analysis of health behavior profiles in a cohort of adolescents with T2D, three profiles emerged and pattern membership shifted over time. Overall, the majority of adolescents in the TODAY study did not retain the same health behavior profiles throughout the trial, but rather transitioned profiles as behaviors changed over the course of 6 and 24 months. Males in the Met + Lifestyle group were more likely to transition into an “improved” health behavior pattern compared with the other two treatment groups. This finding regarding the male health behaviors is in line with the original outcomes paper from the TODAY study in regards to time to treatment failure (i.e., elevated glycated hemoglobin level >/=8% over a period of 6 months or persistent metabolomic decompensation). These results showed that males in the Met + Lifestyle group had a lower failure rate (41.3%) after 60 months compared to Met (51.2%); however, the treatment group differences were not statistically significant (p=0.06).25
Our findings are consistent with existing literature regarding the challenges faced when intervening on adolescent’s health behaviors,6 though there are several new pieces of knowledge that can be gleaned from the current analyses. Interventions to date most often report that changes in individual diet and activity behaviors may occur within the first few months (3-6 months) of the intervention, but are not sustained a year or two later.4,6 However, such findings are limited by examining behaviors individually; consideration of an individual’s patterns of both healthy and unhealthy diet and activity may be more sensitive to evaluating change due to intervention. Combinations of change in lifestyle patterns may indeed improve insulin resistance, which could lead to improvements in management of T2D.
The novelty of our analysis is the use of health behavior patterns versus examining changes in only one behavior at a time.5 Adolescents in the TODAY study were found to transition between patterns comprised of healthy and less healthy behaviors over the course of intervention period. This could be possibly due to the adolescent’s readiness to adopt lifestyle behaviors at one data collection interval versus the next. A study examining readiness to adopt healthy lifestyle changes in adolescents with T2D found that only 14% of their participants reported being “ready” to adopt such changes.6 This finding may shed light on the issue that examining behaviors in isolation may be a limitation of existing methods to examine the full impact of lifestyle changes on health outcomes. Using the approach outlined here may more accurately reflect patterns of lifestyle behaviors in a cohort of adolescents with T2D which may be used for the development of more effective interventions within this unique population.
Health behavior data presented in this paper are similar to those reported in other population studies that focus on adolescents with T2D. Our findings are similar to a previous TODAY analysis in which macronutrient intake did not significantly change from baseline to 24 months.7 Regardless of which health behavior pattern an individual was in, their macronutrient composition ranged from 37-39% FAT, 44-47% CHO, and 17-18% PRO. We have added to these findings by finding that daily energy intake (1186-1361kcal) and servings of red and green foods also remained relatively stable over time. As previously reported, TODAY participants were found to engage in unhealthy sedentary behaviors from 4-7 hours per day.8 We found in the current analysis that the main difference in activity levels was that individuals with lower levels of sedentary time (4-5 hours per day) were also found to participate in MVPA for around 2 hours per day versus the most sedentary individuals who participated in almost no MVPA (less than 30 min per day). The largest differences for activity behaviors in the TODAY participants were between those in the “most sedentary” pattern compared with those in the “active and eat most” pattern. Although individuals transitioned between these three profiles over the course of 24 months, the profiles themselves remained stable and were similar at all three time points.
These profiles and how adolescents’ transition from certain health behaviors to others may not reflect health behaviors of all adolescents. Our findings need to be interpreted cautiously as TODAY participants had extreme obesity and all had T2D. Further, to be eligible for TODAY, all adolescents had to first undergo an extensive run-in period during which time completers had a decrease in weight compared with those that did not complete run-in.25 Hence, the baseline behavior profiles most likely already reflected an improvement in lifestyle habits, and may not reflect participants’ initial health behaviors. Thus, participants may have had a tendency to regress to their old behavior profiles during the trial.
Our analysis is not without limitations. Power for these analyses was not considered prospectively for the parent trial, so power calculations for the current analyses would have been of a post hoc nature. The data that we present should therefore be considered exploratory. Health behaviors were measured using food frequency questionnaires for dietary intake and 3-day PAR for physical activity and sedentary time. As both measures are subjective, there may have been under- or over-reporting, especially among obese adolescents,26.26 Further, we only included consumption of red and green foods in our pattern analysis, and did not include yellow foods which may be accounting for the larger variation in intake; hence, total energy intake and macronutrient content were similar across our three health behavior profiles. For example, participants in the “healthy eaters” category consumed the most green foods and least red foods compared to the other two groups; however, total kcal and macronutrient intake were not very different. This group must have consumed a larger number of calories from yellow food which then negated the benefit from the low calorie green foods.
In conclusion, a high quality lifestyle intervention had little effect on improving health behavior profiles. The lifestyle intervention, which was based on then-current research evidence, provided by appropriate and adequately trained personnel, and with ample funding did not achieve sustained changes in behavior. For example, there is now strong evidence that severe obesity is less responsive to behavioral treatment approaches.4,6 Optimizing outcomes in youth with T2D will require more robust and multifaceted interventions beyond family-level lifestyle, including more extensive psychosocial intervention, novel medication regimens, and/or bariatric surgery.
Acknowledgments
We acknowledge the participants and their families who participated in TODAY.
Appendix:
This work was completed with funding from NIDDK and the NIH Office of the Director (OD) through grants U01-DK61212, U01-DK61230, U01-DK61239, U01-DK61242, and U01-DK61254; from the National Center for Research Resources General Clinical Research Centers Program grant numbers M01-RR00036 (Washington University School of Medicine), M01-RR00043-45 (Children’s Hospital Los Angeles), M01-RR00069 (University of Colorado Denver), M01-RR00084 (Children’s Hospital of Pittsburgh), M01-RR01066 (Massachusetts General Hospital), M01-RR00125 (Yale University), and M01-RR14467 (University of Oklahoma Health Sciences Center); and from the NCRR Clinical and Translational Science Awards grant numbers UL1-RR024134 (Children’s Hospital of Philadelphia), UL1-RR024139 (Yale University), UL1-RR024153 (Children’s Hospital of Pittsburgh), UL1-RR024989 (Case Western Reserve University), UL1-RR024992 (Washington University in St Louis), UL1-RR025758 (Massachusetts General Hospital), and UL1-RR025780 (University of Colorado Denver). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The TODAY Study Group thanks the following companies for donations in support of the study’s efforts: Becton, Dickinson and Company; Bristol-Myers Squibb; Eli Lilly and Company; GlaxoSmithKline; LifeScan, Inc.; Pfizer; Sanofi Aventis. We also gratefully acknowledge the participation and guidance of the American Indian partners associated with the clinical center located at the University of Oklahoma Health Sciences Center, including members of the Absentee Shawnee Tribe, Cherokee Nation, Chickasaw Nation, Choctaw Nation of Oklahoma, and Oklahoma City Area Indian Health Service; the opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the respective Tribes and the Indian Health Service.
Materials developed and used for the TODAY standard diabetes education program and the intensive lifestyle intervention program are available to the public at https://today.bsc.gwu.edu/.
The following individuals and institutions constitute the TODAY Study Group (* indicates principal investigator or director):
CLINICAL CENTERS Baylor College of Medicine: S. McKay*, M. Haymond*, B. Anderson, C. Bush, S. Gunn, H. Holden, S.M. Jones, G. Jeha, S. McGirk, S. Thamotharan Case Western Reserve University: L. Cuttler*, E. Abrams, T. Casey, W. Dahms (deceased), C. Ievers-Landis, B. Kaminski, M. Koontz, S. MacLeish, P. McGuigan, S. Narasimhan Children’s Hospital Los Angeles: M. Geffner*, V. Barraza, N. Chang, B. Conrad, D. Dreimane, S. Estrada, L. Fisher, E. Fleury-Milfort, S. Hernandez, B. Hollen, F. Kaufman, E. Law, V. Mansilla, D. Miller, C. Muñoz, R. Ortiz, A. Ward, K. Wexler, Y.K. Xu, P. Yasuda Children's Hospital of Philadelphia: L. Levitt Katz*, R. Berkowitz, S. Boyd, B. Johnson, J. Kaplan, C. Keating, C. Lassiter, T. Lipman, G. McGinley, H. McKnight, B. Schwartzman, S. Willi Children's Hospital of Pittsburgh: S. Arslanian*, F. Bacha, S. Foster, B. Galvin, T. Hannon, A. Kriska, I. Libman, M. Marcus, K. Porter, T. Songer, E. Venditti Columbia University Medical Center: R. Goland*, D. Gallagher, P. Kringas, N. Leibel, D. Ng, M. Ovalles, D. Seidman Joslin Diabetes Center: L. Laffel*, A. Goebel-Fabbri, M. Hall, L. Higgins, J. Keady, M. Malloy, K. Milaszewski, L. Rasbach Massachusetts General Hospital: D.M. Nathan*, A. Angelescu, L. Bissett, C. Ciccarelli, L. Delahanty, V. Goldman, O. Hardy, M. Larkin, L. Levitsky, R. McEachern, D. Norman, D. Nwosu, S. Park-Bennett, D. Richards, N. Sherry, B. Steiner Saint Louis University: S. Tollefsen*, S. Carnes, D. Dempsher, D. Flomo, T. Whelan, B. Wolff State University of New York Upstate Medical University: R. Weinstock*, D. Bowerman, S. Bristol, J. Bulger, J. Hartsig, R. Izquierdo, J. Kearns, R. Saletsky, P. Trief University of Colorado Denver: P. Zeitler* (Steering Committee Chair), N. Abramson, A. Bradhurst, N. Celona-Jacobs, J. Higgins, M.M. Kelsey, G. Klingensmith, K. Nadeau, T. Witten University of Oklahoma Health Sciences Center: K. Copeland* (Steering Committee Vice-Chair), E. Boss, R. Brown, J. Chadwick, L. Chalmers, S. Chernausek, A. Hebensperger, C. Macha, R. Newgent, A. Nordyke, D. Olson, T. Poulsen, L. Pratt, J. Preske, J. Schanuel, S. Sternlof University of Texas Health Science Center at San Antonio: J. Lynch*, N. Amodei, R. Barajas, C. Cody, D. Hale, J. Hernandez, C. Ibarra, E. Morales, S. Rivera, G. Rupert, A. Wauters Washington University in St Louis: N. White*, A. Arbeláez, D. Flomo, J. Jones, T. Jones, M. Sadler, M. Tanner, A. Timpson, R. Welch Yale University: S. Caprio*, M. Grey, C. Guandalini, S. Lavietes, P. Rose, A. Syme, W. Tamborlane
COORDINATING CENTER George Washington University Biostatistics Center: K. Hirst*, S. Edelstein, P. Feit, N. Grover, C. Long, L. Pyle
PROJECT OFFICE National Institute of Diabetes and Digestive and Kidney Diseases: B. Linder*
CENTRAL UNITS Central Blood Laboratory (Northwest Lipid Research Laboratories, University of Washington): S.M. Marcovina*, J. Harting DEXA Reading Center (University of California at San Francisco): J. Shepherd*, B. Fan, L. Marquez, M. Sherman, J. Wang Diet Assessment Center (University of South Carolina): M. Nichols*, E. Mayer-Davis, Y. Liu Echocardiogram Reading Center (Johns Hopkins University): J. Lima*, S Gidding, J. Puccella, E. Ricketts Fundus Photography Reading Center (University of Wisconsin): R. Danis*, A. Domalpally, A. Goulding, S. Neill, P. Vargo Lifestyle Program Core (Washington University): D. Wilfley*, D. Aldrich-Rasche, K. Franklin, C. Massmann, D. O’Brien, J. Patterson, T. Tibbs, D. Van Buren
OTHER Hospital for Sick Children, Toronto: M. Palmert Medstar Research Institute, Washington DC: R. Ratner Texas Tech University Health Sciences Center: D. Dremaine University of Florida: J. Silverstein
References
- 1.Berkowitz RI, Marcus MD, Anderson BJ, et al. Adherence to a lifestyle program for youth with type 2 diabetes and its association with treatment outcome in the TODAY clinical trial. Pediatric diabetes. 2018;19(2):191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinhas-Hamiel O, Zeitler P. Acute and chronic complications of type 2 diabetes mellitus in children and adolescents. Lancet. 2007;369(9575):1823–1831. [DOI] [PubMed] [Google Scholar]
- 3.Prevention CfDCa. National Diabetes Statistics Report, 2017. . Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services;2017. [Google Scholar]
- 4.McGavock J, Dart A, Wicklow B. Lifestyle therapy for the treatment of youth with type 2 diabetes. Curr Diab Rep. 2015;15(1):568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaar JL, Simon SL, Schmiege SJ, Nadeau KJ, Kelsey MM. Adolescent's Health Behaviors and Risk for Insulin Resistance: A Review of the Literature. Curr Diab Rep. 2017;17(7):49. [DOI] [PubMed] [Google Scholar]
- 6.McGavock J, Durksen A, Wicklow B, et al. Determinants of Readiness for Adopting Healthy Lifestyle Behaviors Among Indigenous Adolescents with Type 2 Diabetes in Manitoba, Canada: A Cross-Sectional Study. Obesity (Silver Spring). 2018;26(5):910–915. [DOI] [PubMed] [Google Scholar]
- 7.Kriska A, El Ghormli L, Copeland KC, et al. Impact of lifestyle behavior change on glycemic control in youth with type 2 diabetes. Pediatr Diabetes. 2018;19(1):36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kriska A, Delahanty L, Edelstein S, et al. Sedentary behavior and physical activity in youth with recent onset of type 2 diabetes. Pediatrics. 2013;131(3):e850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kann L, Kinchen S, Shanklin SL, et al. Youth risk behavior surveillance--United States, 2013. MMWR supplements. 2014;63(4):1–168. [PubMed] [Google Scholar]
- 10.Kim SA, Moore LV, Galuska D, et al. Vital signs: fruit and vegetable intake among children - United States, 2003-2010. MMWR Morbidity and mortality weekly report. 2014;63(31):671–676. [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Department of Health and Human Services. Healthy People 2020. http://www.healthypeople.gov/2020. Accessed.
- 12.Iannotti RJ, Wang J. Patterns of physical activity, sedentary behavior, and diet in U.S. adolescents. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2013;53(2):280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ottevaere C, Huybrechts I, Benser J, et al. Clustering patterns of physical activity, sedentary and dietary behavior among European adolescents: The HELENA study. BMC Public Health. 2011;11:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and beta-cell function in TODAY. Diabetes Care. 2013;36(6):1749–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeitler P, Epstein L, Grey M, et al. Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatric diabetes. 2007;8(2):74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Design of a family-based lifestyle intervention for youth with type 2 diabetes: the TODAY study. International journal of obesity (2005). 2010;34(2):217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epstein LH, Paluch RA, Kilanowski CK, Raynor HA. The effect of reinforcement or stimulus control to reduce sedentary behavior in the treatment of pediatric obesity. Health Psychol. 2004;23(4):371–380. [DOI] [PubMed] [Google Scholar]
- 18.Mayer-Davis EJ, Nichols M, Liese AD, et al. Dietary intake among youth with diabetes: the SEARCH for Diabetes in Youth Study. J Am Diet Assoc. 2006;106(5):689–697. [DOI] [PubMed] [Google Scholar]
- 19.Block G MM, Roullet J, Wakimoto P, Crawford P, Block T. Pilot validation of a FFQ for children 8-10 years. Paper presented at: Fourth International Conference on Dietary Assessment Methods2000; Tucson, AZ. [Google Scholar]
- 20.McMurray RG, Ring KB, Treuth MS, et al. Comparison of two approaches to structured physical activity surveys for adolescents. Medicine and science in sports and exercise. 2004;36(12):2135–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tein JY, Coxe S, Cham H. Statistical Power to Detect the Correct Number of Classes in Latent Profile Analysis. Struct Equ Modeling. 2013;20(4):640–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dodd MJ, Cho MH, Cooper BA, et al. Identification of latent classes in patients who are receiving biotherapy based on symptom experience and its effect on functional status and quality of life. Oncol Nurs Forum. 2011;38(1):33–42. [DOI] [PubMed] [Google Scholar]
- 23.Flensborg Damholdt M, Shevlin M, Borghammer P, Larsen L, Ostergaard K. Clinical heterogeneity in Parkinson's disease revisited: a latent profile analysis. Acta Neurol Scand. 2012;125(5):311–318. [DOI] [PubMed] [Google Scholar]
- 24.Guerin E, Fortier M. Motivational Profiles for Physical Activity: Cluster Analysis and Links with Enjoyment. PHEnex Journal. 2012;4(2):1–21. [Google Scholar]
- 25.Zeitler P, Hirst K, Pyle L, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. The New England journal of medicine. 2012;366(24):2247–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins CE, Watson J, Burrows T. Measuring dietary intake in children and adolescents in the context of overweight and obesity. Int J Obes (Lond). 2010;34(7):1103–1115. [DOI] [PubMed] [Google Scholar]


