Abstract
This study was conducted to investigate the effects of replacing inorganic trace minerals (ITM) with organic trace minerals (OTM; complexed glycinates) on reproductive performance, blood profiles, and antioxidant status in broiler breeders. A total of 648, 23-week-old healthy broiler breeders (ZhenNing), with similar body weight (1.40 ± 0.002 kg), were randomly divided into 4 groups with 6 replicates in each group (27 hens/replicate) and fed the respective experimental diets for 14 wk (including 2 wk for adaptation). The experimental treatments consisted of T1: Cont., commercially recommended levels of ITM (Cu, Zn, Fe, and Mn sulfates); T2: Mix, half trace minerals (TM) were provided from ITM and half from OTM (glycinates); T3: M-OTM, TM were provided from glycinates and reduced to 70% of T1; T4: L-OTM, TM were provided from glycinates and reduced to 50% of T1. The results showed that commercial level of inorganic trace minerals replaced by low-dose complexed glycinates (T3 and T4) exhibited no significant effects on laying performance, 50% ITM replaced by complexed glycinates (T2) numerically improved laying rate by 1.23% than cont. treatment (T1). Broiler breeders fed complexed glycinates tended to produce more qualified eggs (P = 0.05) in T3, with better yolk color (P < 0.01) and eggshell thickness (P = 0.05) in T2 treatment. Replacement of low-dose complexed glycinates reduced fertilization rate (P < 0.01), while it did not affect hatchability. There were no significant differences in serum reproductive hormones such as estrogen and progesterone among the treatments. Serum total protein, albumin, and phosphorus were increased respectively with the replacement of ITM by low-dose OTM from complexed glycinates (P < 0.05). Total liver antioxidant capacity in M-OTM and L-OTM treatment was higher than that of Cont. and Mix treatments (P < 0.01). In conclusion, replacement of high levels of ITM by lower levels of OTM in the form of complexed glycinates is beneficial for egg quality and liver antioxidant status in broiler breeders during the peak laying period.
Key words: antioxidant status, broiler breeder, blood profile, complexed glycinates, production performance
Introduction
Trace minerals are indispensable in the diet of breeder birds due to their critical role in eggshell formation, embryonic development (Leeson, 2005, Richards et al., 2010), and other vital biochemical processes (Bao et al., 2007, Richards et al., 2010). Trace element, such as copper (Cu), is an essential component of many enzyme systems and serves as a cofactor for cytochrome oxidase and superoxide dismutase (Swiątkiewicz et al., 2014). Moreover, it plays a key role in regulation of lysyl oxidase activity (Jensen, 2000); thus, its deficiency may lead to malabsorption syndrome due to damage in connective tissues of digestive system (Dermauw et al., 2013). Iron (Fe) is another essential micro element, involved in cellular respiration, cell proliferation, and oxygen transport (Milanovic et al., 2008). Manganese (Mn) is an essential component of metalloenzymes and involved in metabolism of glucose, fatty acid, and amino acid (Crowley et al., 2000), and adequate Mn intake is crucial for cellular energy production, which is required for all systems of the body to work properly. The function of zinc (Zn) is associated with immunomodulation in birds as a cofactor of different enzymes and hormones (Kidd et al., 1996). In most cases, trace minerals (TM) are supplemented in the diet due to their deficiency in feed ingredients (Leeson, 2005) and they are mainly obtained from inorganic compounds: sulfates, oxides, chlorides, and phosphates due to cost and availability at a commercial level (Nollet et al., 2007). Inorganic trace minerals (ITM) are unstable and rapidly dissociate in gastrointestinal tract and interact with other compounds leading to their loss before absorption (Aksu et al., 2011). Owing to low digestibility of ITM, higher levels of dietary ITM are supplemented to meet the requirements of birds (Yan and Waldroup, 2006, Mezes et al., 2012), which eventually increase the cost of production and environmental problems. These problems could be overcome by substituting ITM with organic trace minerals (OTM). Organic trace minerals are either chelated or complexed form of minerals with organic compounds such as amino acid(s), protein, or organic acid. OTM are more stable due to their organically bound structure with better digestion and absorption in intestine (Ammerman et al., 1998), which in turn increase their bioavailability and assimilability (Bhoyar, 2015) and consequently reduce the fecal and urinary excretion (Wang et al., 2019b).
Glycinate minerals complex are preferred organic source of TM due to their good stability, palatability, and electrical neutrality (Zhang et al., 2017). Glycine being the lowest molecular weight amino acid favors the stability of chelate compounds and avoids the release of TM in the stomach and intestine (Kulkarni et al., 2011). Glycine chelate of iron (Fe-Gly) and Zn (Zn-Gly) are most studied glycinate TM than others. Absorption efficiency of Fe-Gly is 2X higher than ferrous sulfate (FeSO4) (Layrisse et al., 2000). Fe-Gly reduced the oxidative stress and faecal Fe excretion in broilers (Bao et al., 2007, Ma et al., 2012), improved immunological and hematological parameters of weanling pigs (Feng et al., 2007), and well absorbed and utilized in rats (Pineda and Ashmead 2001) than FeSO4. Similarly, Zn-Gly showed better results than zinc sulfate (ZnSO4) (Ma et al., 2011). Zn-Gly improves intestinal absorption of Zn which is positively linked with growth performance of broilers (dos Santos et al., 2010, Feng et al., 2010, Ma et al., 2011). Zn-Gly showed significantly higher Zn retention in the muscle, liver, and albumen than ZnSO4 at the same dose rate with positive effects on reproductive performance of broilers breeders (Zhang et al., 2017). Zn-Gly supplementation significantly improved the intestinal maturity (Ma et al., 2011), which is related to the better growth performance of broilers.
Based on the hypothesis that organic minerals are biologically more available, the trend on the use of inorganic mineral has been shifted to organic minerals. In literature, several studies reported the superior efficacy and stability of organically complexed or chelated trace minerals in comparison with inorganic forms (Aksu et al., 2011, Yang et al., 2011, Manangi et al., 2012; Wang et al., 2019a, Wang et al., 2019b). El-Husseiny et al. (2012) reported that partial replacement of ITM with OTM enhanced the growth, and carcass characteristics with improved tibial and liver TM retention accompanied with reduced excretion in broilers. In another study, replacement of ITM with OTM improved the egg weight with significant reduction in egg loss without compromising the feed efficiency and eggshell quality in older laying hens (Maciel et al., 2010). Similarly, the results of Wang et al., 2019a, Wang et al., 2019b stated that replacement of ITM with organically bound trace minerals enhanced the mineral retention in tissues and beneficial for productive and reproductive performance of broiler breeder with reduced mineral excretion. However, conflicting results are reported in other studies which demonstrated no significant difference on organic and inorganic trace minerals feeding (Nollet et al., 2007, Yang et al., 2011). These contradictions may be due to different reasons including basal level of trace mineral, chemical structure, source of organic trace mineral used, and duration of feeding trial.
Most of the available literature studies concentrate on the glycinate complex of single TM and limited experiments are conducted on simultaneous use of glycinate complex of different trace elements in broiler breeders. Therefore, the present study was designed to investigate the effect of substitution of ITM (Cu, Fe, Mn, and Zn) with complexed glycinates on reproductive performance, egg quality, blood, and antioxidant profile in broiler breeders at peak period of laying.
Materials and methods
Birds and Management
All the experimental procedures and protocol used during this experiment were approved by the Animal Science College of Zhejiang University (Hangzhou, China), on the care and use of experimental animals. A total of 648, 23-week-old healthy broiler breeders of native dual-purpose breed ZhenNing (Liu et al., 2019), with uniform body weight (1.40 ± 0.002 kg), were used in the feeding trial that lasted for 14 wk (including 2 wk for adaptation). Experimental birds were divided into 4 groups (Cont., Mix, M-TOM, and L-TOM); each group consists of 6 replicates (27 hens/replicate) and 3 hens were kept in a cage (cage dimensions: 50 × 50 × 50 cm3). Feed and water were provided ad libitum, and temperature (25°C), humidity (50–60%), and light (light/dark 16:8 h) were artificially controlled throughout the experiment. Artificial insemination was carried out every 5 D to maintain the fertilization. Massage collection technique (Gee and Sexton 1979) was used to get semen from male birds (1-year-old) of same breed and its quality was ensured by using sperm quality analyzer (McDaniel et al., 1998).
Diets and Experimental Group
The basal diet was formulated to meet nutrient requirement of broiler breeder according to recommendations of National Research Council, 1994 to achieve the actual production needs, modifications were done according to NY/T 33-2004 (2004) (Table 1). The supplemental doses and sources of mineral premix are presented in Table 2. (1) Cont., commercially recommended levels of ITM (Cu, Zn, Fe, and Mn sulfates); (2) Mix, half TM were provided from inorganic source and half from organic source (glycinates by BASF Animal Nutrition, Germany); (3) M-OTM, TM were provided from glycinates and reduced to 70% of the Cont.; (4) L-OTM, TM were provided from glycinates and reduced to 50% of the Cont. The feed samples of treatments were subjected to microwave digestion and analyzed the content of Cu, Zn, Fe, and Mn by flame atomic absorption spectrophotometer (Thermo Scientific S Series, Thermo Fisher Scientific Inc.), and the analyzed data were included in Table 2.
Table 1.
Ingredient and nutrient composition of basal diet.
| Ingredients (%) | Nutrient (%) | ||
|---|---|---|---|
| Corn | 63.50 | ME4(MJ/kg) | 11.41 |
| Soybean meal | 19.00 | CP | 16.73 |
| Fish meal | 2.50 | EE | 4.08 |
| Wheat bran | 1.50 | CF | 3.29 |
| Soybean oil | 1.60 | Lys | 0.84 |
| Shell powder | 7.50 | Met | 0.39 |
| Limestone | 1.80 | Ca | 3.88 |
| CaHPO4 | 1.10 | TP | 0.61 |
| NaCl | 0.20 | ||
| NaHCO₃ | 0.30 | ||
| Met | 0.10 | ||
| Choline (50%) | 0.10 | ||
| Titanium dioxide | 0.30 | ||
| Vitamin premix1 | 0.20 | ||
| Trace element premix2 | 0.30 | ||
| Phytase3 | + | ||
| Total | 100.00 | ||
Provided per kilogram of diet: VA 8000IU, VD 1600 IU, VE 5 mg, VK 0.5 mg, VB1 0.8 mg, VB2 2.5 mg, VB5 2.2 mg, VB3 20 mg, VB6 3 mg, VB7 0.1 mg, VB9 0.25 mg, VB12 0.004 mg.
Premix according to the experimental design.
BASF SE, Natuphos E 10,000, 50 g/t feed, which was premixed with vitamin and trace mineral premixes before feed preparation.
ME based on calculated values; others are measured values.
Table 2.
Experimental treatments and levels of trace minerals (mg/kg).
| mg/kg | Cont. | Mix | M-OTM | L-OTM |
|---|---|---|---|---|
| Supplemental | ||||
| Cu | 8.0 | 8.0 | 5.6 | 4.0 |
| Fe | 60.0 | 60.0 | 42.0 | 30.0 |
| Zn | 80.0 | 80.0 | 56.0 | 40.0 |
| Mn | 60.0 | 60.0 | 42.0 | 30.0 |
| Analyzed | ||||
| Cu | 22.0 | 22.5 | 20.8 | 18.3 |
| Fe | 331.5 | 337.3 | 320.7 | 305.7 |
| Zn | 165.3 | 164.6 | 140.2 | 123.4 |
| Mn | 147.8 | 153.5 | 136.9 | 124.6 |
Cont.: inorganic minerals (sulfates salts), Mix: 50% inorganic + 50% glycinates.
Glycinates minerals were used in both M-OTM and L-OTM, and inclusion level was reduced to 70% and 50%, respectively.
Samples Collection and Measurement
Laying Performance
During the feeding trial, daily eggs production (number and weight), no. of qualified eggs, cracked eggs, soft-shelled eggs, and mortality for each replicate were recorded. Feed consumption was recorded on a weekly basis, and daily feed intake (g/bird/day) and feed to egg ratio (kg of feed/kg of egg) were calculated at the end of trial.
Reproductive Performance
Twenty qualified eggs per replicate (total 480) were randomly collected at the third, sixth, ninth, and 12th wk, respectively, for hatching (total 1,920). Incubation was done in a commercial incubator with automatic egg turning at 37°C and 65 to 75% relative humidity (Liu et al., 2019). On day 19, no. of fertile eggs was recorded and transferred to hatcher to find the hatchability of fertilized eggs. To determine the levels of reproductive hormones (E2; estradiol and P4; progesterone), blood samples were collected from the wing vein of 3 birds per replicate at 8:00 am (blood samples were collected from all birds in a very short duration) on the last day of experiment and Automatic Biochemical Analyzer (AU5421, Olympus Crop., Japan) was used for this purpose (Wang et al., 2019a, Wang et al., 2019b).
Egg Quality
At the 12th wk, 6 eggs per replicate (total 144) were collected to measure egg quality indexes (egg weight, eggshell strength, yolk color, eggshell thickness, albumen height, and Haugh unit) using a digital egg tester (DET-6000, Nabel Co., Ltd., Kyoto, Japan) according to the methods of Xiao et al. (2014) and Yilmaz et al. (2015).
Blood Profiles and Antioxidant Profile
On the last day of experiment, 3 hens per replicate (total 72) were selected randomly for blood collection from the wing vein and serum was extracted by centrifugation of blood at 630 × g for 15 min using TDL-80-2B centrifuge (Shanghai Anting Scientific Instrument Factory, China) and stored at −20°C until further analysis. Total protein, albumin, alkaline phosphatase, uric acid, serum glucose, calcium, and phosphorus were analyzed using Automatic Biochemical Analyzer (AU5421, Olympus Crop., Japan). Ceruloplasmin and hemoglobin were analyzed using ELISA kits (Jining Bioengineering Institute, Shanghai, China).
Liver tissue samples were collected and immediately washed with cold PBS followed by storage in liquid nitrogen until further analysis. Antioxidant status was evaluated on the basis of enzymatic activities of following enzymes in liver and serum samples: glutathione peroxidase (GSH-Px), total superoxide dismutase (T-SOD), Cu/Zn superoxide dismutase (Cu/Zn-SOD), Mn superoxide dismutase (Mn-SOD), and concentration of malondialdehyde (MDA) by using biochemical kits according to the instructions of manufacturer (Nanjing Jiancheng Biological Engineering Institute, China).
Statistical Analysis
All the data collected from this trial were subjected to one-way ANOVA techniques using SPSS 23.0, in which dietary treatments were served as independent variables and one dietary treatment as the experimental unit, while each replicate was served as the statistical unit. Data were expressed as the mean ± SEM. A significant level of P < 0.05 and extremely significant level of P < 0.01 were used to find significant difference between treatments through the LSD test.
Results
Production Performance
The data concerning production performance of broiler breeders fed different sources of TM were summarized on a triweekly basis (Table 3). No significant (P > 0.05) difference was observed by feeding different sources of TM or by reduced levels of organic TM than conventional levels on overall (1–12 wk) laying rate, feed intake, and feed to egg ratio during this trial. Similarly, no significant (P > 0.05) effect was found on any production performance parameters during different intervals of trial, except on feed intake during 7 to 9 wk of period. Although there was no significant effect of dietary treatments on production performance of broiler breeders, the results of L-OTM were numerically comparable with control group and the best performance regarding laying rate and feed to egg ratio was observed in Mix group (50% ITM and OTM).
Table 3.
Effects of inorganic trace minerals replaced by complexed glycinates on production performance.
| Parameters | Treatments |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| Cont. | Mix | M-OTM | L-OTM | |||
| 1–3 wk | ||||||
| Laying rate (%) | 79.14 | 79.69 | 78.06 | 78.00 | 0.662 | 0.780 |
| Feed intake (g/bids/D) | 97.54 | 96.20 | 96.21 | 95.48 | 0.376 | 0.281 |
| Feed to egg ratio | 3.19 | 3.16 | 3.20 | 3.18 | 0.032 | 0.970 |
| 4–6 wk | ||||||
| Laying rate (%) | 77.90 | 79.95 | 76.18 | 76.90 | 0.881 | 0.484 |
| Feed intake (g/bids/D) | 103.47 | 103.00 | 102.30 | 102.51 | 0.267 | 0.432 |
| Feed to egg ratio | 3.25 | 3.45 | 3.35 | 3.29 | 0.068 | 0.784 |
| 7–9 wk | ||||||
| Laying rate (%) | 74.83a,b | 75.63a | 69.39b | 75.33a | 1.032 | 0.095 |
| Feed intake (g/bids/D) | 104.44a | 104.30a | 103.28b | 104.44a | 0.143 | 0.002 |
| Feed to egg ratio | 3.27a,b | 3.26a | 3.57b | 3.38a,b | 0.049 | 0.078 |
| 10–12 wk | ||||||
| Laying rate (%) | 68.21 | 69.73 | 68.07 | 69.61 | 0.898 | 0.883 |
| Feed intake (g/bids/D) | 100.00a | 99.89a,b | 99.95a,b | 99.77b | 0.038 | 0.155 |
| Feed to egg ratio | 3.42 | 3.33 | 3.49 | 3.35 | 0.057 | 0.776 |
| 1–12 wk | ||||||
| Laying rate (%) | 75.02 | 76.25 | 72.92 | 74.96 | 0.711 | 0.444 |
| Feed intake (g/bids/D) | 101.36 | 100.85 | 100.44 | 100.55 | 0.171 | 0.226 |
| Feed to egg ratio | 3.28 | 3.30 | 3.40 | 3.30 | 0.036 | 0.635 |
a,bValues within a row with different superscript letters are significantly different (P < 0.05). Data are presented as mean ± SEM, n = 6.
Cont.: inorganic minerals (sulfates salts), Mix: 50% inorganic + 50% glycinates.
Glycinates minerals were used in both M-OTM and L-OTM, and inclusion level was reduced to 70 and 50%, respectively.
Reproductive Performance
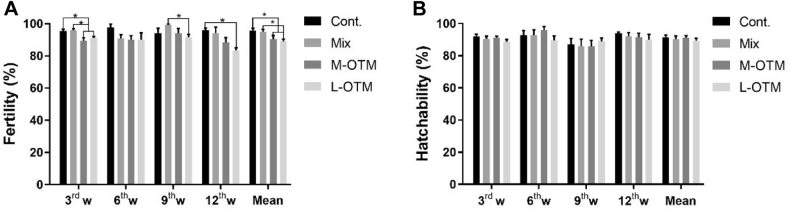
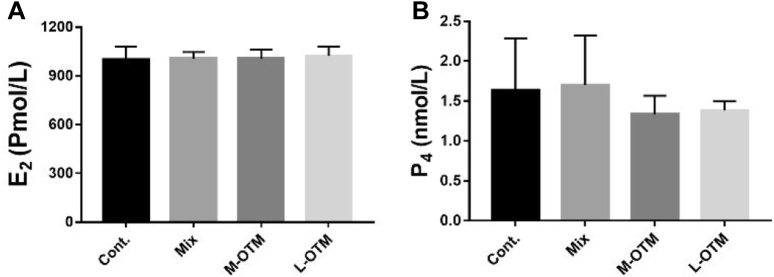
Fertilization rate (%) of control group was higher (P < 0.05) than feeding lower levels of TM in the form of glycinate complexes, but no difference (P > 0.05) was found between control and Mix group at different weeks and during whole experimental period; however, hatchability rate (%) remained unaffected (P > 0.05). Highest performance concerning fertility and hatchability was observed in the control group (Figure 1; A and B). Similarly, levels of reproductive hormones were not changed significantly (P > 0.05) by dietary treatments in this trial (Figure 2; A and B).
Figure 1.
Effects of inorganic trace minerals replaced by complexed glycinates on fertility% (A) and hatchability% (B). Data are presented as mean ± SEM, n = 6. An asterisk * indicates the significant difference between 2 different treatments where P < 0.05.
Figure 2.
Effects of inorganic trace minerals replaced by complexed glycinates on reproductive hormones; serum estradiol E2 (A) and progesterone P4 (B). Data are presented as mean ± SEM, n = 6.
Egg Quality
The glycinate TM presented positive effects on some egg quality traits (Table 4). Significant improvement in the rate of qualified eggs (P = 0.05), yolk color index (P < 0.01), and eggshell thickness (P < 0.05) was found in the experimental group. Highest percentage of qualified eggs was observed in the M-OTM group, while better yolk color was found in group Mix (P < 0.05). However, highest values of Haugh unit and albumin height were exhibited by the M-OTM group.
Table 4.
Effects of inorganic trace minerals replaced by complexed glycinates on egg quality.
| Parameters | Treatments |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| Cont. | Mix | M-OTM | L-OTM | |||
| Rate of qualified eggs (%) | 65.31a,b | 63.04b | 68.91a | 64.78a,b | 0.798 | 0.054 |
| Egg weight (g) | 45.87 | 46.16 | 45.47 | 45.45 | 0.301 | 0.829 |
| Eggshell weight (g) | 5.85b | 6.09a,b | 6.13a | 5.93a,b | 0.048 | 0.124 |
| Yolk color (YCF) | 7.92b | 8.58a | 8.25a,b | 8.50a | 0.082 | 0.009 |
| Eggshell strength (Kgf) | 4.22 | 4.31 | 4.20 | 4.56 | 0.077 | 0.343 |
| Eggshell thickness (mm) | 0.33b | 0.35a | 0.34a | 0.35a | 0.003 | 0.023 |
| Albumen height (mm) | 4.80 | 4.68 | 4.93 | 4.75 | 0.089 | 0.808 |
| Haugh unit | 72.65 | 71.68 | 73.67 | 72.88 | 0.669 | 0.796 |
a,bValues within a row with different superscript letters are significantly different (P < 0.05). Data are presented as mean ± SEM, n = 6.
Cont.: inorganic minerals (sulfates salts), Mix: 50% inorganic + 50% glycinates.
Glycinates minerals were used in both M-OTM and L-OTM, and inclusion level was reduced to 70 and 50%, respectively.
Blood Profile
The overall analysis of blood profiles was shown in Table 5. The substitution of recommended ITM with low-dose glycinate TM (M-OTM) significantly improves the levels of albumin (P < 0.05) and phosphorus (P < 0.01); similarly, highest (P < 0.01) level of total protein was found at reduced level of TM (L-OTM). Similar trend was found in other components of blood profiles, but the difference was non-significant (P > 0.05) among the treatments.
Table 5.
Effects of inorganic trace minerals replaced by complexed glycinates on the blood profiles.
| Parameters | Treatments |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| Cont. | Mix | M-OTM | L-OTM | |||
| Total protein (g/L) | 57.66b | 57.02b | 63.25a,b | 70.33a | 1.660 | 0.006 |
| Albumin (g/L) | 21.03b | 22.48a,b | 23.75a | 23.71a | 0.398 | 0.036 |
| ALP (U/L) | 691.50 | 644.83 | 455.75 | 584.00 | 64.298 | 0.624 |
| Ca (mmol/L) | 5.01 | 5.07 | 5.28 | 5.58 | 0.127 | 0.402 |
| Glu (mmol/L) | 12.83 | 12.57 | 12.61 | 13.47 | 0.190 | 0.333 |
| P (mmol) | 1.42c | 1.62b,c | 1.91a | 1.84a,b | 0.061 | 0.008 |
| Uric acid (μmol/L) | 208.42b | 217.67a,b | 266.33a | 231.33a,b | 11.797 | 0.340 |
| Ceruloplasmin (U/L) | 159.73 | 160.50 | 298.85 | 266.69 | 27.462 | 0.158 |
| Hemoglobin (U/L) | 317.03 | 264.12 | 545.83 | 468.92 | 57.597 | 0.287 |
a,b,cValues within a row with different superscript letters are significantly different (P < 0.05). Data are presented as mean ± SEM, n= 6.
Cont.: inorganic minerals (sulfates salts), Mix: 50% inorganic + 50% glycinates.
Glycinates minerals were used in both M-OTM and L-OTM, and inclusion level was reduced to 70% and 50%, respectively.
Antioxidant Status
As shown in Table 6, no significant (P > 0.05) effect of dietary treatments was found on antioxidant indexes, except total antioxidative capacity of the liver which was significantly high (P < 0.001) in broilers fed on M-OTM and L-OTM diet. Improved activities of superoxide dismutase and Mn-SOD in the serum were found in the group L-OTM. Similarly, better activities of serum GSH-Px and Cu/Zn-SOD were exhibited by the group M-OTM. Correspondingly, improved levels of antioxidant enzymes were found in the liver tissues of broilers fed on glycinate TM than ITM.
Table 6.
Effects of inorganic trace minerals replaced by complexed glycinates on antioxidant status in the serum and liver.
| Parameters | Treatments |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| Cont. | Mix | M-OTM | L-OTM | |||
| Serum | ||||||
| T-AOC (U/mL) | 10.52 | 11.97 | 10.32 | 11.16 | 0.679 | 0.846 |
| GSH-Px(U/mL) | 16.72a,b | 18.43a,b | 19.75a | 16.08b | 0.632 | 0.155 |
| MDA (nmol/mL) | 5.98 | 6.46 | 5.64 | 4.70 | 0.553 | 0.742 |
| T-SOD (U/mL) | 338.87 | 332.87 | 355.03 | 356.70 | 5.133 | 0.279 |
| Cu/Zn-SOD (U/mL) | 302.31a,b | 298.27b | 316.90a | 307.74a,b | 3.055 | 0.149 |
| Mn-SOD (U/mL) | 36.56 | 34.60 | 38.14 | 48.96 | 3.787 | 0.585 |
| Liver | ||||||
| T-AOC (U/mL) | 0.59b | 0.52b | 1.95a | 1.35c | 0.153 | <0.001 |
| GSH-Px (U/mL) | 49.08 | 62.30 | 47.69 | 50.64 | 3.904 | 0.560 |
| MDA (nmol/mL) | 0.40 | 0.38 | 0.35 | 0.40 | 0.027 | 0.915 |
| T-SOD (U/mL) | 11.77 | 11.26 | 13.03 | 13.87 | 0.502 | 0.249 |
| Cu/Zn-SOD (U/mL) | 10.06 | 8.96 | 10.61 | 10.93 | 0.409 | 0.354 |
| Mn-SOD (U/mL) | 1.96 | 2.31 | 2.42 | 2.93 | 0.214 | 0.474 |
a,bValues within a row with different superscript letters are significantly different (P < 0.05). Data are presented as mean ± SEM, n = 6.
Cont.: inorganic minerals (sulfates salts), Mix: 50% inorganic + 50% glycinates. Glycinates minerals were used in both M-OTM and L-OTM, and inclusion level was reduced to 70% and 50%, respectively.
Discussion
The aim of the present study was to compare the effect of ITM substituted with glycinate OTM on productive and reproductive performance with some other parameters in broiler breeders. The results showed no significant difference in the production performance of broilers by reducing the levels of OTM in the form glycinate complexes. No significant difference (P > 0.05) was found in overall rate of eggs production by 50% reduction of the levels of TM when provided as glycinate complexes than the control group. However, best results were obtained by feeding diet containing 50% OTM and ITM. These findings support the idea that OTM have the potential to enhance the production performance of boiler breeders. Our findings are also supported by Swiatkiewicz and Koreleski (2008) because they found no effects of replacing ITM (Zn and Mn oxides) with metal-amino acid complexes on laying performance parameters. In another study, no significant effect was found by 50% replacement of ITM (Zn, Mn, and Cu) with organic sources (Maciel et al., 2010) on egg production and feed conversion ratio in laying hens. Alternatively, significant increase in laying performance was found by Klecker et al. (2002), by replacing 20 or 40% Mn and Zn from inorganic to organic chelates form. The difference in the structure of OTM, age and breed of birds, and duration of feeding trial along with other factors might be the reason of contradiction in the finding of different studies (Yenice et al., 2015). Similarly, overall (1–12 wk) feed intake was not affected by dietary treatments as found in our previous trial (Wang et al., 2019a). Our findings are also supported by Leeson and Caston (2008); they found no difference in feed intake by ITM (Fe, Cu, Zn, and Mn) compared with reduced levels of OTM.
Results showed negative effect of reducing TM levels on the rate of fertilization because it was significantly reduced by lowering TM levels. However, hatchability and reproductive hormones (E2 and P4) were not affected significantly by dietary treatments. Positive relationship was detected between fertility and levels of TM, and lowest rate of fertilization was found at 50% reduction of TM in the form of glycinate complexes. Similarly, level of progesterone was also reduced by reducing TM levels. From present results, it appears that reduced levels of OTM are not adequate to completely achieve the TM's reproductive requirements of broiler breeders, as TM have very critical role in reproduction. Because minerals also influence the secretions of hormonal (Peters and Mahan, 2008) which are essentially required for proper follicle development and pregnancy maintenance (Sakumoto et al., 2014), for example, Cu has vital role in synthesis and maintenance of the proper level of follicle-stimulating hormone in the serum (Rajeswari and Swaminathan, 2014), whereas Zn is the component of special type of proteins which are involved in genetic expression of reproductive hormone (Tapiero and Tew, 2003). Mn has a role in steroid hormones synthesis as it is involved in the metabolism of cholesterol (Xie et al., 2014) and its deficiency reduced the reproductive hormones in layer hens (Yang, 2008). However, OTM has a positive effect on reproductive performance when provided in sufficient amount, as OTM enhance the no. of fertilized oocytes in heifers (Lamb et al., 2008), increase the rate of conceptions per services in beef cattle (Stanton et al., 2000), and also improve the reproductive performance in sows (Peters and Mahan, 2008) as compared to feeding on ITM.
As far as the egg quality parameters are concerned, these were not affected by the dietary treatments except yolk color index, which was better in group fed 50% OTM and ITM and eggshell thickness in all experimental groups than the control group. TM have critical importance for eggshell because they are involved in its formation through different ways. Eggshell and its membranes have high contents of Cu and varying levels of Zn and Mn. Similarly, Zn and Mn are the components of carbonic anhydrase which involved in calcium metabolism (Richards, 1997, Leeson, 2009). It has been reported that deficiency of Cu could be the reason of improper egg weight, eggshell structure, pigments, and thin albumen portion (Leeson, 2009). In the present study, highest egg weight was found in 50% OTM and ITM group and it decreased as the levels of TM reduced. Comparable trend was found by Wang et al. (2019a) and Gheisari et al. (2011) in egg weight of broiler breeders and laying hens, respectively, by feeding OTM than ITM. Maciel et al. (2010) also reported that 50% replacement of ITM with OTM has a positive effect on egg weight, without affecting egg production or eggshell quality. But, Favero et al. (2013) found significant improvement in eggshell weight and thickness by replacing ITM with OTM.
It is well recognized that hematobiochemical parameters are auspicious indicators of health and performance (Ghasemi et al., 2013), and under this experiment, blood biochemical parameters indicated that broiler breeders performed better by feeding reduced levels of TM in the form of glycinate mineral complexes. Highest concentrations of total protein, serum calcium, and glucose were found in broilers by feeding low levels (50%) of glycinate complexes than ITM. Similarly, better contents of phosphorus, ceruloplasmin, and hemoglobin were also observed in broilers fed on low levels (70%) of OTM as compared to ITM. In accordance with the current findings, the results of other experiments have found that organic Zn in guinea pigs (Shinde et al., 2006) and Cu in broilers (Mondal et al., 2007) had no significant effect on plasma glucose concentrations. In general, present findings agree with previous results (Wang et al., 2007; Feng et al., 2010). Similarly, under the present study, organic form of TM positively affected the calcium and phosphorus levels in the serum, but the difference was non-significant, as reported in previous studies (Idowu et al., 2011, Yenice et al., 2015). Organic form of TM might reduce the formation of free TM ions at the intestinal level, thereby reducing the chance of insoluble calcium compound formation with other TM which ultimately increase the serum calcium level. In addition, during this trial, higher blood glucose and hemoglobin levels were found in OTM groups. This increase might be due to their higher availability of TM in the form of glycinate complexes than ITM, which ultimately enhance the biochemical processed in the body (Richards et al., 2010) which subsequently increase the blood glucose and hemoglobin.
As for the antioxidant status, broilers fed on lower levels of OTM or 50% OTM and ITM were under lower oxidant stress status than fed on ITM as depicted from the results of the present study. Because most of the indexes studied to examine the antioxidant status were better in OTM group. The activities of glutathione peroxidase and SOD, MDA concentration and T-AOC capacity could be used to represent the antioxidant status in animals. Glutathione peroxidase and SOD are directly involved in inactivation of reactive oxygen species, GSH-Px convert hydrogen peroxide to water while SOD has a vital role in defeating oxygen-free radicals (Zinnuroglu et al., 2012). TM such as Zn, Cu, and Mn are essential cofactors of SOD (Aksu et al., 2010), and it has been reported that SOD works efficiently if cofactors are available in a suitable amount (Underwood and Suttle, 1999, Ma et al., 2011). It is worth mentioning that higher activities of SOD in broilers fed on glycinate mineral complexes showed their protective role in oxidative stress, or glycinate mineral complexes might enhance the availability of TM which reduce the accumulation of reactive oxygen species. Similarly, Cu/Zn-SOD and Mn-SOD levels were also higher in OTM groups. Zn as a component of Cu/Zn-SOD protects proteins and enzymes from radical attacks, and second, Zn prevents the free radicals' formation from other metals (Fe and Cu) (Kucuk, 2008). It is also demonstrated in previous studies that broiler breeders showed better antioxidant status by feeding organic Zn as compared to inorganic form (Zhang et al., 2017) and reduced the level of serum and liver MDA (Sahin et al., 2005, Sun et al., 2012). In line with the present findings, it is suggested by other studies that broiler breeders show better antioxidant status by feeding OTM compared with ITM (Wang et al., 2019a). Based on the current trial results, it could be concluded that glycinate TM even at lower levels have the potential to meet the broiler requirements for antioxidative defense.
Conclusion
Replacement of commercially recommended levels of ITM by lower levels of OTM in the form of complexed glycinates is beneficial for egg quality and liver antioxidant status in broiler breeders during the peak laying period. Medium dose of OTM (70% glycinate mineral complex) is enough to cope with oxidative stress with better blood profile, but for maximum production and better reproductive performance, mix TM of 50% OTM and ITM is the best option in this regard.
Acknowledgments
This study was financially supported by BASF SEA Pte Ltd. and Three Agricultural and Six-Party Research Cooperation Project of Zhejiang Province, China (No. CTZB-F180706LWZ-SNY1). The authors acknowledge the great sustain from the Ningbo Zhenning Animal Husbandry Ltd. and Lei Lu for the technical support in sample collection.
References
- Aksu D., Aksu T., Ozsoy B., Baytok E. The effects of replacing inorganic with a lower level of organically complexed minerals (Cu, Zn and Mn) in broiler diets on lipid peroxidation and antioxidant. Asian Australas. J. Anim. Sci. 2010;23:1066–1072. [Google Scholar]
- Aksu T., Özsoy B., Aksu D.S., Yörük M.A., Gül M. The effects of lower levels of organically complexed zinc, copper and manganese in broiler diets on performance, mineral concentration of tibia and mineral excretion. Kafkas Univ. Vet. Fak. Derg. 2011;17:141–146. [Google Scholar]
- Ammerman C.B., Henry P.R., Miles R.D. Supplemental organically bound mineral compounds in livestock nutrition. In: Garnsworthy P.C., Wiseman J., editors. Recent Advances in Animal Nutrition. University Press; Nottingham: 1998. pp. 67–97. [Google Scholar]
- Bao Y.M., Choct M., Iji P.A., Brucrton K. Effect of organically complexed copper, iron, manganese and zinc on broiler performance, mineral excretion and accumulation in tissues. J. Appl. Poult. Res. 2007;16:448–455. [Google Scholar]
- Bhoyar A. High quality trace minerals support improved breeder hen longevity. Int. Hatch. Pract. 2015;29:25–27. [Google Scholar]
- Crowley J.D., Traynor D.A., Weatherburn D.C. Enzymes and proteins containing manganese: an overview. Met. Ions Biol. Syst. 2000;37:209–278. [PubMed] [Google Scholar]
- Dermauw V., Yisehak K., Dierenfeld E.S., Laing G.D., Buyse J., Wuyts B., Janssens G.P.J. Effects of trace element supplementation on apparent nutrient digestibility and utilisation in grass-fed zebu (Bos indicus) cattle. Livest. Sci. 2013;155:255–261. [Google Scholar]
- dos Santos T.T., Corzo A., Kidd M.T., McDaniel C.D., Filho R.A.T., Araujo L.F. Influence of in ovo inoculation with various nutrients and egg size on broiler performance. J. Appl. Poult. Res. 2010;19:1–12. [Google Scholar]
- El-Husseiny O.M., Hashish S.M., Ali R.A., Arafa S.A., Abd El-Samee L.D., Olemy A.A. Effects of feeding organic zinc, manganese and copper on broiler growth carcass characteristics, bone quality and mineral content in bone, liver and excreta. Int. J. Poult. Sci. 2012;11:368–377. [Google Scholar]
- Favero A., Vieira S.L., Angel C.R., Bess F., Cemin H.S., Ward T.L. Reproductive performance of Cobb 500 breeder hens fed diets supplemented with zinc, manganese, and copper from inorganic and amino acid-complexed sources. J. Appl. Poult. Res. 2013;22:80–91. doi: 10.3382/ps.2012-02670. [DOI] [PubMed] [Google Scholar]
- Feng J., Ma W.Q., Xu Z.R., Wang Y.Z., Liu J.X. Effects of iron glycine chelate on growth, haematological and immunological characteristics in weaning pigs. Anim. Feed Sci. Technol. 2007;134:261–272. [Google Scholar]
- Feng J., Ma W.Q., Niu H.H., Wu X.M., Wang Y., Feng J. Effects of zinc glycine chelate on growth, hematological, and immunological characteristics in broilers. Biol. Trace Elem. Res. 2010;133:203–211. doi: 10.1007/s12011-009-8431-9. [DOI] [PubMed] [Google Scholar]
- Gee G.F., Sexton T.J. National Audubon Society; Fort Collins, CO: 1979. Artificial insemination of cranes with frozen semen; pp. 89–94. [Google Scholar]
- Ghasemi H.A., Kazemi-Bonchenari M., Khaltabadi-Farahani A.H., Khodaei-Motlagh M. The effect of feeding rations with different ratios of concentrate to alfalfa hay on blood hematological and biochemical parameters of farmed ostriches (Struthio camelus) Trop. Anim. Health Prod. 2013;45:1635–1640. doi: 10.1007/s11250-013-0409-0. [DOI] [PubMed] [Google Scholar]
- Gheisari A.A., Sanei A., Samie A., Gheisari M.M., Toghyani M. Effect of diets supplemented with different levels of manganese, zinc, and copper from their organic or inorganic sources on egg production and quality characteristics in laying hens. Biol. Trace Elem. Res. 2011;142:557–571. doi: 10.1007/s12011-010-8779-x. [DOI] [PubMed] [Google Scholar]
- Idowu O.M.O., Ajuwon R.O., Oso A.O., Akinloye O.A. Effects of zinc supplementation on laying performance, serum chemistry and Zn residue in tibia bone, liver, excreta and eggshell of laying hens. Int. J. Poult. Sci. 2011;10:225–230. [Google Scholar]
- Jensen J.T. Gastrointestinal abnormalities and involvement in systemic mastocytosis. Hematol. Oncol. Clin. North Am. 2000;14:579–623. doi: 10.1016/s0889-8588(05)70298-7. [DOI] [PubMed] [Google Scholar]
- Kidd M.T., Ferket P.R., Qureshi M.A. Zinc metabolism with special reference to its role in immunity. World Poult. Sci. J. 1996;52:309–324. [Google Scholar]
- Klecker D., Zeman L., Jelinek P., Bunesova A. Effect of manganese and zinc chelates on the quality of eggs. Acta Univ. Agric. Silvic. Mendel. Brun. 2002;50:59–68. [Google Scholar]
- Kucuk O. Zinc in a combination with magnesium helps reducing negative effects of heat stress in quails. Biol. Trace Elem. Res. 2008;123:144–153. doi: 10.1007/s12011-007-8083-6. [DOI] [PubMed] [Google Scholar]
- Kulkarni R.C., Shrivastava H.P., Mandal A.B., Deo C., Deshpande K.Y., Singh R., Bhanja S.K. Assessment of growth performance, immune response and mineral retention in colour broilers as influenced by dietary iron. Anim. Feed Sci. Technol. 2011;11:81–90. [Google Scholar]
- Lamb G.C., Brown D.R., Larson J.E., Dahlen C.R., DiLorenzo N., Arthington J.D. Effect of organic or inorganic trace mineral supplementation on follicular response, ovulation, and embryo production in superovulated Angus heifers. Anim. Reprod. Sci. 2008;106:221–231. doi: 10.1016/j.anireprosci.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Layrisse M., García-Casal M.N., Solano L., Baron M.A., Arguello F., Llovera D., Ramirez J., Leets I., Tropper E. Iron bioavailability in humans from breakfasts enriched with iron bis-glycine chelate, phytates and polyphenols. J. Nutr. 2000;130:2195–2199. doi: 10.1093/jn/130.9.2195. [DOI] [PubMed] [Google Scholar]
- Leeson S. Copper metabolism and dietary needs. World Poult. Sci. J. 2009;65:353–366. [Google Scholar]
- Leeson S., Caston L. Using minimal supplements of trace minerals as a method of reducing trace mineral content of poultry manure. Anim. Feed Sci. Technol. 2008;142:339–347. [Google Scholar]
- Leeson S. Trace mineral requirements of poultry–validity of the NRC recommendations. In: Taylor-Pickard J.A., Tucker L.A., editors. Redefining Mineral Nutrition. Nottingham University Press; Nottingham: 2005. pp. 107–117. [Google Scholar]
- Liu K., Cao H., Dong X., Liu H., Wen Y., Mao H., Lu L., Yin Z. Polymorphisms of pro-opiomelanocortin gene and the association with reproduction traits in chickens. Anim. Repro. Sci. 2019;210:106–196. doi: 10.1016/j.anireprosci.2019.106196. [DOI] [PubMed] [Google Scholar]
- Maciel M.P., Saraiva E.P., Aguiar E.D.F., Ribeiro P.A.P., Passos D.P., Silga J.B. Effect of using organic microminerals on performance and external quality of eggs of commercial laying hens at the end of laying. Rev. Bra. Zootec. 2010;39:344–348. [Google Scholar]
- Ma W., Niu H., Wang Y., Feng J. Effects of zinc glycine chelate on oxidative stress, contents of trace elements, and intestinal morphology in broilers. Biol. Trace Elem. Res. 2011;142:546–556. doi: 10.1007/s12011-010-8824-9. [DOI] [PubMed] [Google Scholar]
- Ma W.Q., Sun H., Zhou Y., Wu J., Feng J. Effects of iron Glycine chelate on growth, tissue mineral concentrations, Fecal mineral excretion and liver antioxidant enzyme activities in broilers. Biol. Trace. Elem. Res. 2012;149:204–211. doi: 10.1007/s12011-012-9418-5. [DOI] [PubMed] [Google Scholar]
- Manangi M.K., Vazquez-Anon M., Richards J.D., Carter S., Buresh R.E., Christensen K.D. Impact of feeding lower levels of chelated trace minerals versus industry levels of inorganic trace minerals on broiler performance, yield, footpad health, and litter mineral concentration. J. Appl. Poult. Res. 2012;21:881–890. [Google Scholar]
- McDaniel C.D., Hannah J.L., Parker H.M., Smith T.W., Schultz C.D., Zumwalt C.D. Use of a sperm analyzer for evaluating broiler breeder males. 1. Effects of altering sperm quality and quantity on the sperm motility index. Poult. Sci. 1998;77:888–893. doi: 10.1093/ps/77.6.888. [DOI] [PubMed] [Google Scholar]
- Mezes M., Erdelyi M., Balogh K. Deposition of organic trace metal complexes as feed additives in farm animals. Eur. Chem. Bull. 2012;1:410–413. [Google Scholar]
- Milanovic S., Lazarevic M., Jokic Z., Jovanovic I., Pešut O., Kirovski D., Marinkovic D. The influence of organic and inorganic Fe supplementation on red blood picture, immune response and quantity of iron in organs of broiler chickens. Acta Vet. 2008;58:179–189. [Google Scholar]
- Mondal M.K., Das T.K., Biswas P., Samanta C.C., Bairagi B. Influence of dietary inorganic and organic copper salt and level of soybean oil on plasma lipids, metabolites and mineral balance of broiler chickens. Anim. Feed Sci. Technol. 2007;139:212–233. [Google Scholar]
- National Research Council . Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. 9th rev. ed. [Google Scholar]
- Nollet L., Van der klis J.D., Lensing M., Spring P. The effect of replacing inorganic with organic trace minerals in broiler diets on productive performance and mineral excretion. J. Appl. Poult. Res. 2007;16:592–597. [Google Scholar]
- Peters J., Mahan D. Effects of dietary organic and inorganic trace mineral levels on sow reproductive performances and daily mineral intakes over six parities. J. Anim. Sci. 2008;86:2247–2260. doi: 10.2527/jas.2007-0431. [DOI] [PubMed] [Google Scholar]
- Pineda O., Ashmead H.D. Effectiveness of treatment of iron deficiency anaemia in infants and young children with ferrous bisglycinate chelate. J. Nutr. 2001;17:381–384. doi: 10.1016/s0899-9007(01)00519-6. [DOI] [PubMed] [Google Scholar]
- Rajeswari S., Swaminathan S. Role of copper in health and diseases. Int. J. Curr. Sci. 2014;10:94–107. [Google Scholar]
- Richards M.P. Trace mineral metabolism in the avian embryo. Poult. Sci. 1997;76:152–164. doi: 10.1093/ps/76.1.152. [DOI] [PubMed] [Google Scholar]
- Richards J.D., Zhao J., Harrell R.J., Atwell C.A., Dibner J.J. Trace mineral nutrition in poultry and swine. Asian-Australas. J. Anim. Sci. 2010;23:1527–1534. [Google Scholar]
- Sahin K., Smith M.O., Onderci M., Sahin N., Gursu M.F., Kucuk O. Supplementation of zinc from organic or inorganic source improves performance and antioxidant status of heat-distressed quail. Poult. Sci. 2005;84:882–887. doi: 10.1093/ps/84.6.882. [DOI] [PubMed] [Google Scholar]
- Sakumoto R., Hayashi K.G., Takahashi T. Different expression of PGE synthase, PGF receptor, TNF, Fas and oxytocin in the bovine corpus luteum of the estrous cycle and pregnancy. Reprod. Biol. 2014;14:115–121. doi: 10.1016/j.repbio.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Shinde P., Dass R.S., Garg A.K., Chaturvedi V.K., Kumar R. Effect of zinc supplementation from different sources on growth, nutrient digestibility, blood metabolic profile, and immune response of male Guinea pigs. Biol. Trace. Elem. Res. 2006;112:247–262. doi: 10.1385/BTER:112:3:247. [DOI] [PubMed] [Google Scholar]
- Stanton T.L., Whittier J.C., Geary T.W., Kimberling C.V., Johnson A.B. Effects of trace mineral supplementation on cow-calf performance, reproduction, and immune function. Pro. Anim. Sci. 2000;16:121–127. [Google Scholar]
- Sun Q.J., Guo Y.M., Ma S.D., Yuan J.M., An S.Y., Li J.H. Dietary mineral sources altered lipid and antioxidant profiles in broiler breeders and post hatch growth of their offsprings. Biol. Trace Elem. Res. 2012;145:318–324. doi: 10.1007/s12011-011-9196-5. [DOI] [PubMed] [Google Scholar]
- Swiatkiewicz S., Koreleski J. The effect of zinc and manganese source in the diet for laying hens on eggshell and bones quality. Vet. Med. 2008;53:555–563. [Google Scholar]
- Swiątkiewicz S., Arczewska-Włosek A., Józefiak D. The efficacy of organic minerals in poultry nutrition: review and implications of recent studies. World Poult. Sci. J. 2014;70:475–486. [Google Scholar]
- Tapiero H., Tew K.D. Trace elements in human physiology and pathology: zinc and metallothioneins. Biomed. Pharmacother. 2003;57:399–411. doi: 10.1016/s0753-3322(03)00081-7. [DOI] [PubMed] [Google Scholar]
- Underwood E.J., Suttle N. 3rd ed. CAB Int; Wallingford: 1999. The Mineral Nutrition of Livestock. [Google Scholar]
- Wang G., Liu L.J., Tao W.J., P Xiao Z., Pei X., Liu B.J., Wang M.Q., Lin G., Ao T.Y. Effects of replacing inorganic trace minerals with organic trace minerals on the production performance, blood profiles, and antioxidant status of broiler breeders. Poult. Sci. 2019;98:2888–28958. doi: 10.3382/ps/pez035. [DOI] [PubMed] [Google Scholar]
- Wang G., Liu L., Wang Z., Pei X., Tao W., Xiao Z., Liu B., Wang M., Lin G., Ao T. Comparison of inorganic and organically bound trace minerals on tissue mineral Deposition and Fecal excretion in broiler breeders. Biol. Trace. Elem. Res. 2019;189:224–232. doi: 10.1007/s12011-018-1460-5. [DOI] [PubMed] [Google Scholar]
- Wang Z., Cerrate S., Coto C., Yan F., Waldroup P.W. Evaluation of Mintrex® Copper as a Source of Copper in Broiler Diets. Inte. J. Poult. Sci. 2007;6:308–313. [Google Scholar]
- Xiao J.F., Zhang Y.N., Wu S.G., Zhang H.J., Yue H.Y., Qi G.H. Manganese supplementation enhances the synthesis of glycosaminoglycan in eggshell membrane: a strategy to improve eggshell quality in laying hens. Poult. Sci. 2014;93:380–388. doi: 10.3382/ps.2013-03354. [DOI] [PubMed] [Google Scholar]
- Xie J.J., Tian C.H., Zhu Y.W., Zhang L.Y., Lu L., Luo X.G. Effects of inorganic and organic manganese supplementation on gonadotropin-releasing hormone-I and folliclestimulating hormone expression and reproductive performance of broiler breeder hens. Poult. Sci. 2014;93:959–969. doi: 10.3382/ps.2013-03598. [DOI] [PubMed] [Google Scholar]
- Yan F., Waldroup P.W. Evaluation of Mintrex® manganese as a source of manganese for young broilers. Int. J. Poult. Sci. 2006;5:708–713. [Google Scholar]
- Yang Y. Adjustment of nutrition on the reproduction of poultry. J. Shanxi Agric. Univ. 2008;39:239–242. [Google Scholar]
- Yang X.J., Sun X.X., Li C.Y., Wu X.H., Yao J.H. Effects of copper, iron, zinc, and manganese supplementation in a corn and soybean meal diet on the growth performance, meat quality, and immune responses of broiler chickens. J. Appl. Poult. Res. 2011;20:263–871. [Google Scholar]
- Yenice E., Cengizhan M., Meltem G., Zafer A., Tunca M. Effects of organic and inorganic forms of manganese, zinc, copper, and Chromium on bioavailability of these minerals and calcium in late-Phase laying hens. Biol. Trace. Elem. Res. 2015;167:300–307. doi: 10.1007/s12011-015-0313-8. [DOI] [PubMed] [Google Scholar]
- Yilmaz D.B., Sozcu A., Aipek U. Effects of supplementary mineral amino acid chelate (ZnAA-MnAA) on the laying performance, egg quality and some blood parameters of late laying period layer hens. Kafkas. Univ. Vet. Fakult. Dergisi. 2015;21:155–162. [Google Scholar]
- Zhang L., Wang Y., Xiao X., Wang J., Wang Q., Li K., Guo T., Zhan X. Effects of zinc glycinate on productive and reproductive performance, zinc concentration and antioxidant status in broiler breeders. Biol. Trace Elem. Res. 2017;178:320–326. doi: 10.1007/s12011-016-0928-4. [DOI] [PubMed] [Google Scholar]
- Zinnuroglu M., Dincel A.S., Kosova F., Sepici V., Karatas G.K. Prospective evaluation of free radicals and antioxidant activity following 6-month risedronate treatment in patients with postmenopausal osteoporosis. Rheumatol. Int. 2012;32:875–880. doi: 10.1007/s00296-010-1708-7. [DOI] [PubMed] [Google Scholar]