Abstract
While arabinoxylans (AX), an important dietary fiber fraction of wheat-based broiler diets, are known for exerting antinutritional effects in the gastrointestinal (GI) tract of broilers, the prebiotic potential of arabinoxylan-oligosaccharides (AXOS) is also well-documented. However, inconsistent performance responses as well as the effectiveness of low amounts of AXOS used in diets of previously conducted experiments put into question the classical prebiotic route being the sole mode of action of AXOS. The objective of this study was to investigate the effects of dietary AXOS addition on the rate of AX digestion in the gastrointestinal tract of broilers as a function of broiler age to gain more insight into the mode of action of these oligosaccharides. A feeding trial was performed on 480 one-day-old chicks (Ross 308) receiving a wheat-based diet supplemented with or without 0.50% AXOS, containing no endoxylanases. Digesta samples from ileum and caeca and fecal samples were analyzed for AX content, AX digestibility, intestinal viscosity, and microbial AX-degrading enzyme activities at 6 different ages (day 5, 10, 15, 21, 28, 35). Chicks fed from hatching with 0.50% AXOS demonstrated a higher ileal viscosity (P < 0.05). Also higher levels of AX solubilization and fermentation compared to control birds at 10 D were observed. This was noted by the higher total tract AX digestibility of water-extractable AX (WE-AX) and total AX (TOT-AX) at this age (P < 0.05). Although no significant difference in AX-degrading enzyme activities was observed among the dietary treatments, AXOS supplementation in young broilers was shown to stimulate or “kick-start” dietary AX digestion, thereby speeding up the development of a fiber-fermenting microbiome in the young broiler. This stimulation effect of AXOS could enable greater functional value to be extracted from dietary fiber in broiler feeds.
Key words: arabinoxylan-oligosaccharides, arabinoxylan degradation, broiler age, dietary fiber
Introduction
Dietary components are the predisposing factors regulating the microbial, digestive, and health status of the broiler and hence broiler performance outcomes. Thus, the nutritional quality of the dietary ingredients is of critical importance in formulating broiler feeds (Keerqin et al., 2017, Kheravii et al., 2018). In European countries, wheat is a major source of energy in poultry diets. However, the high variability in the nutritional value of wheat is still a big concern for feed manufacturers. A major factor related to the nutritional value of wheat is the non-starch carbohydrate content (Austin et al., 1999, Gebruers et al., 2010). Arabinoxylans (AX) are the main non-starch carbohydrates in wheat and form the major constituents of the cell wall polysaccharides in wheat grains. Owing to the wide variety of substituents and substitution patterns on the AX chain, heterogeneous AX structures are present in varying proportions in the different layers of a wheat kernel, each structure carrying its own specific physicochemical and antinutritional properties into the gastrointestinal (GI) tract of broilers.
Rigid wheat cell wall structures built of water-unextractable arabinoxylans (WU-AX) enclose highly valuable nutrients, which, in combination with the viscous nature of the high molecular weight water-extractable arabinoxylans (WE-AX), hamper efficient nutrient absorption and digestion in the GI tract, thereby impairing broiler performance (Annison and Choct, 1991, Bedford and Classen, 1992). In addition to its antinutritive behavior, AX is also an important part of the dietary fiber fraction in wheat-based poultry diets. Depending on their physicochemical properties, more specifically their solubility and extractability, AX can be rapidly or more slowly fermented by the intestinal microbial community of the broiler (Verspreet et al., 2016). Especially the AX-derived oligosaccharides (AXOS) are known to be well fermented by the intestinal microbiota and able to induce beneficial effects on the microbial and health status of the broiler (Courtin et al., 2008a, Morgan et al., 2019). However, these oligomers do not occur naturally in a sound wheat kernel and therefore need to be obtained through hydrolysis of AX by β-1,4-endoxylanases (E.C. 3.2.1.8, endoxylanases).
The use of such endoxylanases in broiler feed formulations has become common practice (Aftab and Bedford, 2018). In addition to the alleviation of the adverse effects of viscous wheat AX on the physiological processes and the microbial community in the GI tract of broilers, the positive impact of these endoxylanases can also be explained by their ability to generate prebiotic AXOS from the dietary fiber AX (Courtin et al., 2008a, Lee et al., 2017). The beneficial effects of these AXOS on performance characteristics and the overall health status of broilers are already well documented and have been intensively investigated over the past 15 years. AXOS are well-known for their prebiotic potential across species (Courtin et al., 2008b, Geraylou et al., 2012, Yacoubi et al., 2017, Ribeiro et al., 2018). They will selectively stimulate the growth of the health-related microbial species, for example, Bifidobacteria, and increase the short-chain fatty acid (SCFA) production in the hindgut. In broilers, this has been shown to reduce the possibility of a pathogenic challenge (e.g., Salmonella enteritidis) (Eeckhaut et al., 2008). As shown in studies of Courtin et al. (2008a) and Morgan et al. (2019), relatively low amounts of AXOS added to broiler diets can act as substrate for saccharolytic microbiota, which are known to obtain their energy from such carbohydrates, thereby resulting in the production of SCFA. However, the size of performance responses observed when AXOS was added in the diets could not be solely explained by the low amount of energy yielded from the SCFA produced upon AXOS fermentation (Józefiak et al., 2004, Ribeiro et al., 2018). In addition, it is doubtful whether the low amounts of AXOS added in the diets would generate a meaningful amount of SCFA to be able to stimulate associated microbiota-modulated effects such as gut hormone responses. Therefore, the prebiotic function of AXOS in its classical sense as a quantitatively acting substrate for fermentation and subsequent SCFA production as the sole mechanism of action can be questioned (Gibson et al., 2017, Aftab and Bedford, 2018, Ribeiro et al., 2018).
Consequently, the possible effects of AXOS, whether added directly to the diet or generated indirectly by endoxylanases supplementation, need to be clarified. Moreover, while the effects of AXOS on health status, broiler performance, and pathogenic control have been studied intensively, so far no studies have investigated the contribution and the age-related benefits of AXOS toward AX digestion in the GI tract of broilers.
The objective of this study is to assess the effects of dietary AXOS addition on AX digestion in the different sections of the GI tract of broilers as a function of broiler age. In this way, more insights into the versatile mode of action of AXOS and the degradation products of AX formed upon endoxylanase addition in particular and dietary fiber sources in general will be provided.
Material and methods
Animal Ethics
The animal trial performed during this study was approved by the Ethical Committee for experimental use of animals of the KU Leuven under accession number P213/2015. Care for the animals was taken, and the appropriate EU guidelines (Council Directive 86/909/EEC) were followed during the animal experiment.
Dietary Treatments, Birds, and Husbandry
The composition of the basal wheat-based diet used in this study is identical to that described in the study by Bautil et al. (2019) (Supplementary Table 1). Broilers received either this control feed (CTRL) or the control feed supplemented with AXOS. For the AXOS diet, 0.63% wt/wt of an AXOS preparation, purified from wheat bran as described by Swennen et al. (2006) and consisting for 79.0% wt/wt of polymeric arabinose and xylose, was added to the CTRL diet to obtain a net addition of AXOS of 0.50%. The added wheat bran AXOS had an average degree of polymerization of 4.6 and an average degree of arabinose substitution of 0.22 (Swennen et al., 2006). An inert indigestible marker, titanium dioxide (TiO2), was added to both diets (5 g/kg feed). A phytase (Quantum Blue; AB Vista, Marlborough, UK) and coccidiostat (Sacox; Huvepharma, Antwerp, Belgium), but no endoxylanase preparation, was mixed into the diets. These diet formulations were maintained during the entire trial (35 D). During the starter period, feed was given to the broilers in a crumble form, and from the start of the grower period onward (11 D), feed pellets were provided until slaughter age.
A total of 480 one-day-old male broilers (Ross 308) were purchased from a commercial hatchery (Belgabroed nv, Merksplas, Belgium). Upon arrival, chicks were evenly distributed over floor pens, which were each randomly assigned to the CTRL or AXOS dietary treatment. No wood shavings were applied as bedding material in the floor pens because these shavings contain heteroxylan that can interfere with the quantification of the wheat AX content in the digesta samples afterward. Broilers received water and feed ad libitum and were kept under conventional conditions of lighting, heating, and ventilation. The light program maintained during the trial was as follows: during the first 7 D, broilers were raised on a light schedule of 23 h of light to 1 h of darkness (23L:1D). This schedule was adapted to 18L:6D after 7 D of age (Aviagen, 2014). Housing temperature was initially set at 34°C and was gradually reduced to 19°C at 28 D of age and kept at this temperature until the end of the experiment. Three to 5 D in advance to the sampling, broilers were selected randomly from the floor pens and placed in 18 digestibility cages (9 cages per dietary treatment). As very young broilers produce limited amounts of digesta material, the number of birds sampled varied with age to have sufficient material for chemical analyses. In concreto, 6, 5, 4, 4, 3, and 2 broilers for every dietary treatment were put in digestibility cages at day 2, 6, 11, 16, 23, and 29, respectively. These cages had a wire floor, a feed trough at the front, a drinking cup at the rear, and a plastic tray underneath the cage for excreta collection. Sampling of broilers took place when chicks were 5, 10, 15, 21, 28, and 35 D old.
Data and Sample Collection
Broiler's Performance
Body weight gain and feed intake were measured while the birds were in cages over short periods of time (9 repeats per dietary treatment). Feed conversion ratio was calculated by dividing the total feed consumed per cage at a particular short test period by the weight gain during this respective test period and corrected for mortality. Mortality was recorded daily.
Collection of Digesta Samples
According to broiler age (day 5, 10, 15, 21, 28, and 35) 6, 5, 4, 4, 3, and 2 broilers, respectively, were euthanized by electronarcosis followed by decapitation for digesta collection. Digesta contents of each part of the GI tract (except for the proventriculus) were collected by gently finger stripping each GI tract segment and subsequently frozen at −20°C for future analysis. Digesta samples were weighed individually per chick, but digesta were pooled in a falcon tube per digestibility cage, finally resulting in 9 pools of digesta samples per dietary treatment for each sampling day. Weights of the empty gizzard, pancreas, small intestine, and caeca were also recorded for each chick individually. Fecal samples were collected from the plastic tray underneath the cage and stored at −20°C. Before analysis, digesta and fecal samples were freeze-dried and homogenized.
Chemical Analyses
Moisture Content
To determine the dry matter (DM) content of feed and digesta samples, feed moisture content was assessed after 15 h of drying at 130°C in an air oven. Moisture contents of digesta samples were assessed after drying in an air oven using the temperature scheme as previously described by Bautil et al. (2019).
Arabinoxylan Content
Analysis of WE-AX and total arabinoxylan (TOT-AX) content was performed on feed samples and pooled, freeze-dried, and homogenized digesta samples from the ileum, caeca, and feces. Quantification of the total amount of arabinose and xylose (after hydrolysis) in these samples was performed by gas chromatography according to the method previously described by Bautil et al. (2019). In short, for the determination of TOT-AX content, samples were hydrolyzed in an acidic environment to yield monosaccharides. Subsequently, the resulting monosaccharides were reduced with sodium borohydride and acetylated with acetic anhydride according to the procedure of Englyst and Cummings (1984). Determination of the WE-AX content in the aqueous extracts of the digesta samples was preceded by two inactivation steps to prevent endoxylanase activity in the samples. The inactivation of the endoxylanases was accomplished by first heating the sample in 80% v/v ethanol/water (95°C) before extraction and secondly using 10 mL of a potassium chloride/hydrogen chloride (KCl-HCl) buffer (20 mM, pH 3.0) during the extraction. The hydrolysis, reduction, and acetylation of these aqueous extracts were performed in the same manner as described above. TOT-AX and WE-AX content of feed and digesta samples were calculated as 0.88 (i.e., conversion factor to account for the release of water when pentoses are incorporated in the polymers) times the sum of the arabinose and xylose content. The total arabinose-to-xylose ratio (A/X) and the A/X ratio of the aqueous extracts were calculated by dividing the arabinose by the xylose content of the samples themselves and their aqueous extracts. AXOS is analyzed and characterized as part of the WE-AX fraction because a clear distinction between both AX fractions cannot be made in feed and digesta samples. AX content is expressed on DM base (g/kg DM samples).
Grain-Associated Endoxylanase Activity
The Xylazyme AX method (Megazyme, Bray, Ireland) was used to measure the grain-associated endoxylanase activity in the experimental diets. This grain-associated endoxylanase activity was determined using a similar procedure as the one described in detail by Bautil et al. (2019). In short, aqueous feed extracts were made using a 10.0-mL sodium acetate buffer (25 mM) at pH 5.0, prior to endoxylanase activity measurement. For the activity measurement, an azurine cross-linked AX tablet (Megazyme, Bray, Ireland) was added to the aqueous extract (1 mL) and incubated for 6 h. The reaction was stopped by adding 10.0 mL of 1.0% w/v Tris(hydroxymethyl)aminomethane aqueous solution and immediately filtered afterward. Extinction values of the extracts were measured at 590 nm against a control (Ultraspec II UV/vis spectrophotometer; Pharmacia Biotech, Uppsala, Sweden). Endoxylanase activities are expressed as endoxylanase activity units (EU) per g of DM. One unit is defined as the amount of endoxylanase activity needed to yield a corrected extinction value of 1.0 per hour of incubation under the conditions of the assay.
Microbial Endoxylanase and Arabinofuranosidase Activity
The procedures for measuring microbial endoxylanase and arabinofuranosidase activities in ileal and caecal samples were optimized and described by Bautil et al. (2019). In short, supernatants of ileal and caecal samples were collected immediately after sampling by centrifugation at 21,000 g and stored at −80°C until further analysis. For analysis of endoxylanase and arabinofuranosidase activities, an azurine cross-linked AX tablet and a solution of p-nitrophenyl-α-L-arabinofuranoside, prepared in a McIlvaine buffer at pH 6.5 containing 0.02% sodium azide, were used as a substrate for the enzyme activity assays, respectively. Endoxylanase and arabinofuranosidase activities were expressed as EU per g of DM of digesta and as nanokatal per g DM of digesta, respectively. One EU is defined as the amount of endoxylanase activity needed to yield a corrected extinction value of 1.0 per hour of incubation under the conditions of the assay. One nanokatal is defined as the amount of arabinofuranosidase activity that yields 1 μmol of p-nitrophenol formed per minute under the conditions of the assay.
Extract viscosity
Aqueous extracts of feed samples were prepared by suspending samples (1.0 g passed through a 1-mm screen) in 4.0 mL of KCl-HCl buffer (20 mM) at pH 3.0. Suspensions were incubated for 30 min at 40°C in an incubation shaker and subsequently centrifuged during 10 min at 2,800 g and 2 min at 21,000 g. The viscosity of 500 μL supernatant was measured using a Brookfield DV-II+ viscometer (Brookfield Engineering Laboratories Inc., Stoughton, MA) with a CP40 cone and a constant shear rate of 750 s−1 at 37°C. Viscosity values were expressed in centipoise (cP).
Digesta viscosity
Immediately after sampling, ileal contents (2.0 g) were centrifuged (15 min, 21,000 g; Himac CT15RE centrifuge, Hitachi, Japan), after which the supernatant was kept on ice until measurement. The viscosity of 500 μL supernatant was measured using a Brookfield DV-II+ viscometer (Brookfield Engineering Laboratories Inc.) with a CP40 cone and a constant shear rate of 90 s−1. Measurements were carried out at 37°C.
Quantification of TiO2
The amount of the indigestible marker TiO2 in feed and digesta samples was quantified using a downscaled method of Short et al. (1996) with modifications proposed by Myers et al. (2004), similar to the procedure previously described in detail by Bautil et al. (2019). In short, 0.1 g of dried digesta or feed underwent digestion in concentrated sulphuric acid in the presence of copper reaction catalyst, whereafter 30% w/v hydrogen peroxide solution was added, allowing the TiO2 to be precipitated. Subsequently, precipitates were removed by filtration, whereafter extinction values were measured at 410 nm against a control of deionized water.
Arabinoxylan Digestibility
Digestibility of TOT-AX and WE-AX (%) at the level of the ileum, caeca, and feces were calculated according to the following formula of Smeets et al. (2015):
where TiO2feed and TiO2digesta are the measured TiO2 concentrations (g/kg DM) in the diet and digesta, respectively, and AXfeed and AXdigesta are the AX concentrations (TOT-AX and WE-AX) (g/kg DM) in the diet and digesta, respectively.
The meaning of TOT-AX and WE-AX is described in detail by Bautil et al. (2019). In short, hydrolysis of ingested dietary WU-AX by microbial or exogenously added endoxylanases will result in solubilization of the WU-AX. As a result of generation of enzyme-solubilized WE-AX, WE-AX substrates will accumulate along the GI tract, thereby increasing the amount of WE-AX in the GI tract relative to the amount of WE-AX that was initially present in the diet. This solubilization yields negative WE-AX digestibility coefficients in the data. Further hydrolysis and microbial fermentation of dietary WE-AX will finally result in positive TOT-AX digestibility coefficients.
Statistical Analysis
The experimental unit for the performance and intestinal viscosity data was a digestibility cage of 6, 5, 4, 4, 3, and 2 broilers at day 5, 10, 15, 21, 28, and 35, respectively. In total, 9 replicate cages were used in the animal trial for every dietary treatment. Digesta samples were pooled for broilers coming from one digestibility cage. AX content and microbial enzyme activities in the different parts of the GI tract were analyzed in these pooled digesta samples and this for 5 to 6 replicate pools out of the nine replicate pools (N = 5–6). Performance data and data resulting from the chemical analyses were subjected to two-way ANOVA using the fit model platform of the JMP pro 14 software (SAS Institute Inc., Cary, NC) where age, dietary treatment (CTRL vs. AXOS), and the age × dietary treatment interaction were used as model effects. Significantly different means were identified by the post-hoc Tukey's honestly significant difference test. Unless otherwise stated, mean differences were considered significant if a P value ≤ 0.05 was present. A P value ≤ 0.1 was interpreted as a strong tendency in the data.
All results of the chemical analyses are expressed on a DM base. Chemical analyses of feed samples were carried out in triplicate as a minimum. At least 5 replicates of pooled digesta samples (pooled digesta being the experimental unit) were measured for the statistical analysis of AX content and digestibility and TiO2 concentrations, while for the statistical analysis of the performance and intestinal viscosity data, 9 replicates were used (digestibility cage being the experimental unit).
Results
Diet Arabinoxylan Content, Extract Viscosity, and Grain-Associated Endoxylanase Activity
Analysis of AX concentrations in the starter and grower diets confirmed the presence of added AXOS in the AXOS-supplemented diets in comparison with the control (CTRL) diets (Table 1). The TOT-AX A/X and the A/X of the aqueous extract of the two experimental diets differed significantly: a lower TOT-AX A/X was observed in the pelleted feed of the AXOS diet than that in the pelleted CTRL diet, which is consistent with supplementation of AXOS with a low A/X in the AXOS diets. In accordance with expectations, no significant differences were found in both the AXOS and CTRL diets for DM content (%) (P = 0.06), grain-associated endoxylanase activity (EU/g DM feed) (P = 0.11), and TiO2 concentrations (g/kg DM feed) (P = 0.892) (Table 1). A similar extract viscosity was observed for the CTRL and AXOS feeds (P = 0.13), even though more WE-AX was present in diets supplemented with AXOS. The extract viscosity of the wheat variety used in the diets was quite low (1.08 ± 0.02 cP).
Table 1.
Total arabinoxylan (TOT-AX) and water-extractable arabinoxylan (WE-AX) content (g/kg DM), TOT-AX arabinose (A) to xylose (X) ratio and the A to X ratio of the aqueous extracts, noncellulose polymeric glucose content (g/kg DM), TiO2 content (g/kg DM), grain-associated endoxylanase activity (EU/g DM), and extract viscosity (cP) of the crumble and pelleted grower-like feed for control (CTRL) and 0.50% AXOS-supplemented (AXOS) diets.
| Variables1 | Crumble grower-like feed |
Pelleted grower-like feed |
P value | ||
|---|---|---|---|---|---|
| CTRL | AXOS | CTRL | AXOS | ||
| Dry matter (w/w %) | 88.2 ± 0.14 | 88.7 ± 0.27 | 88.2 ± 0.08 | 88.4 ± 0.03 | 0.061 |
| AX content (g/kg DM) | |||||
| TOT-AX | 52.3 ± 2.56b | 60.5 ± 1.64a,b | 54.1 ± 1.13a,b | 60.8 ± 0.81a | 0.014 |
| WE-AX | 2.86 ± 0.06b | 6.80 ± 0.04a | 2.99 ± 0.04b | 6.74 ± 0.04a | <0.001 |
| TOT-AX A/X | 0.81 ± 0.07a,b | 0.68 ± 0.05a,b | 0.81 ± 0.02a | 0.66 ± 0.03b | 0.025 |
| A/X of aqueous extract | 1.28 ± 0.04a | 0.55 ± 0.05b | 1.18 ± 0.01a | 0.54 ± 0.01b | 0.004 |
| Noncellulose polymeric glucose content (g/kg DM) | 485 ± 15.1 | 511 ± 8.8 | 501 ± 29.4 | 556 ± 27.4 | 0.225 |
| TiO2 content (g/kg DM) | 6.60 ± 0.16 | 6.48 ± 0.13 | 6.36 ± 0.36 | 6.59 ± 0.25 | 0.892 |
| Grain-associated endoxylanase activity (EU/g DM) | 0.34 ± 0.01 | 0.35 ± 0.01 | 0.31 ± 0.01 | 0.36 ± 0.02 | 0.110 |
| Extract viscosity (cP) | 1.03 ± 0.01 | 1.01 ± 0.01 | 1.02 ± 0.01 | 1.01 ± 0.01 | 0.130 |
a,bDifferent superscript lowercase alphabets within a row means significantly different (P < 0.05) from each other.
Abbreviation: AXOS, arabinoxylan-oligosaccharides.
Given parameters were analyzed in triplicate for each diet (N = 3).
Broiler Performance and GI Tract Weights
As shown in Supplementary Table 2, adding AXOS to a wheat-based broiler diet had no impact on feed intake, body weight gain, and feed conversion ratio recorded during the very short periods that the broilers resided in the digestibility cages. However, the broilers subjected to the AXOS diet had significantly higher proportional caecal weights (P = 0.002) and contents (P = 0.001) than their control counterparts (Table 2). Proportional weights of the gizzard, empty intestines, and pancreas did not differ between the diets (data not shown).
Table 2.
Proportional weights (g/100 g body weight [BW]) of the full caeca and caecal content measured at different broiler ages (day 5, 10, 15, 21, 28, 35) for broilers fed a control (CTRL) and a 0.50% AXOS-supplemented (AXOS) wheat-based diet.
| Parameters (g/100 g BW)1 | Proportional weight caeca | Proportional weight caecal content |
|---|---|---|
| Main effects | ||
| Age (D) | ||
| 5 | 1.19a | 0.55a |
| 10 | 0.96b | 0.47a,b |
| 15 | 0.73c | 0.34b,c |
| 21 | 0.66c | 0.37b,c |
| 28 | 0.62c,d | 0.33c |
| 35 | 0.49d | 0.27c |
| Diet | ||
| AXOS | 0.91y | 0.46y |
| CTRL | 0.81z | 0.38z |
| Pooled SEM | 0.02 | 0.01 |
| P values | ||
| Age | <0.001 | <0.001 |
| Diet | 0.002 | 0.001 |
| Age × Diet | NS | NS |
a–dDifferent superscripts within the column “Age (D)” have significant age differences for each parameter (main effect of age, P < 0.05).
y,zDifferent superscripts within the column “Diet” have significant dietary treatment differences for each parameter (main effect of diet, P < 0.05).
Abbreviations: AXOS, arabinoxylan-oligosaccharides; NS, not significant.
Means from at least 15 different individual broilers at the 6 different broiler ages (N = 15).
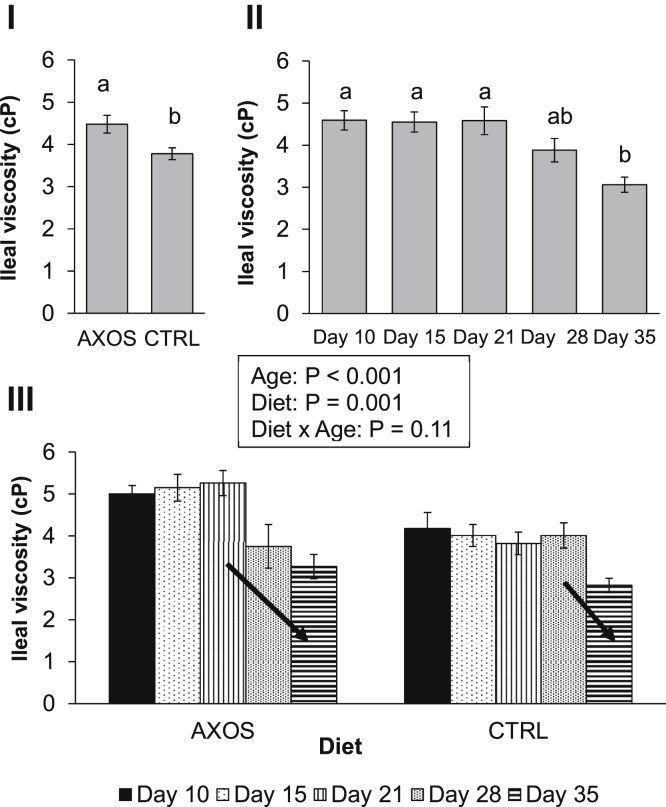
Ileal Viscosity
As shown in Figure 1, values for the ileal viscosity were quite low at all broiler ages. AXOS-fed broilers showed a higher ileal viscosity than control-fed broilers at all broiler ages (P = 0.001; Figure 1 (I)). Regardless of dietary treatment, a decline in ileal viscosity was observed after 21 D of age (P < 0.001; Figure 1 (II)). However, when having a closer look at the average ileal viscosity values at one particular age for both AXOS- and CTRL-fed broilers, a very remarkable result was noticed. The ileal viscosity profile of broilers fed the AXOS diet tended to decrease at a younger age (after 21 D) than broilers fed the control wheat-based diets, where this decrease was observed after 28 D of age (diet × age, P = 0.11; Figure 1 (III)). Although this interaction was not statistically significant at a P < 0.05 threshold, this observation might be of importance in a broader biological context.
Figure 1.
Ileal viscosity (cP) for broilers fed a control (CTRL) and a 0.50% arabinoxylan-oligosaccharides supplemented (AXOS) wheat-based diet at 5 different broiler ages (day 10, 15, 21, 28, and 35). In I and II, the main effect of diet and broiler age is illustrated, respectively. Differing small letters a and b indicate a significant difference in ileal viscosity between dietary treatments (I) and broiler ages (II) (P < 0.05). Arrows indicate a different decline in ileal viscosity means for broilers fed a CTRL and an AXOS diet at one particular age (III).
AX Content and Digestibility at Different Sections of the Hindgut
Ileum
Table 3 shows the TOT- and WE-AX content at the level of the ileum. Providing AXOS in the broiler's diet significantly increased the contents of WE-AX and TOT-AX at the level of the ileum (P < 0.001 and P = 0.005, respectively). Addition of highly fermentable AXOS in the feed increased the digestibility coefficient for WE-AX (P < 0.001), but not for TOT-AX (P = 0.50) (Table 4). Broilers fed the AXOS diet had significantly lower A/X in the ileum than broilers fed the CTRL diet (P < 0.001) (Table 5). No increased microbial AX-degrading activities were measured upon AXOS addition (Supplementary Table 3).
Table 3.
Total arabinoxylan (TOT-AX) and water-extractable arabinoxylan (WE-AX) content (g/kg DM) at the level of the ileum and feces analyzed at 6 different broiler ages for broilers fed a control (CTRL) and a 0.50% AXOS-supplemented (AXOS) wheat-based diet.
| Content (g/kg DM) 1 | TOT-AX |
WE-AX |
||
|---|---|---|---|---|
| Ileum | Feces | Ileum | Feces | |
| Main effects | ||||
| Age (D) | ||||
| 5 | 175.4a | 177.8a | 25.8b | 27.7a |
| 10 | 176.9a | 166.9a,b | 35.8a | 19.1b |
| 15 | 179.4a | 177.6a | 36.2a | 25.4a |
| 21 | 151.2b,c | 156.1b | 34.7a | 18.7b |
| 28 | 166.4a,b | 160.9b | 30.2a,b | 19.1b |
| 35 | 132.8c | 166.6a,b | 27.2b | 18.0b |
| Diet | ||||
| AXOS | 169.8y | 168.8 | 36.2y | 24.2y |
| CTRL | 159.1z | 166.9 | 27.2z | 18.4z |
| Pooled SEM | 2.7 | 1.7 | 1.0 | 0.8 |
| P values | ||||
| Age | <0.001 | <0.001 | <0.001 | <0.001 |
| Diet | 0.005 | NS | <0.001 | <0.001 |
| Diet × Age | NS | NS | NS | NS |
a–cDifferent superscripts within the column “Age (D)” have significant age differences for each parameter (main effect of age, P < 0.05).
y,zDifferent superscripts within the column “Diet” have significant dietary treatment differences for each parameter (main effect of diet, P < 0.05).
Abbreviations: AXOS, arabinoxylan-oligosaccharides; NS, not significant.
Reported values are means of 10 to 12 replicates (N = 10-12) for the main effect of age and 25 to 30 replicates (N = 25–30) for the main effect of diet.
Table 4.
Total arabinoxylan (TOT-AX) and water-extractable arabinoxylan (WE-AX) digestibility (%) at the level of the ileum and caeca analyzed at 6 different broiler ages (day 5, 10, 15, 21, 28, and 35) for broilers fed a control (CTRL) and a 0.50% AXOS-supplemented (AXOS) wheat-based diet.
| Variables (%)1 | TOT-AX digestibility |
WE-AX digestibility |
||
|---|---|---|---|---|
| Ileum | Caeca | Ileum | Caeca | |
| Main effects | ||||
| Age (D) | ||||
| 5 | −10.66b | ND | −112.4d | ND |
| 10 | 1.87a,b | −133.4c | −168.1a–c | −209.0c |
| 15 | −1.17b | −35.58b | −172.3b,c | −188.5b |
| 21 | 14.44a | 21.77a,b | −150.3a | −114.5a,b |
| 28 | −1.03b | 66.57a | −140.3c,d | −20.93a |
| 35 | 14.35a | 61.87a | −114.4a,b | 48.55a |
| Diet | ||||
| AXOS | 3.77 | −1.69 | −85.2y | −27.82y |
| CTRL | 2.14 | −5.82 | −200.8z | −166.1z |
| Pooled SEM | 1.73 | 13.97 | 9.26 | 25.49 |
| P values | ||||
| Age | <0.001 | <0.001 | 0.008 | <0.001 |
| Diet | NS | NS | <0.001 | 0.001 |
| Diet × Age | NS | NS | NS | NS |
a–dDifferent superscripts within the column “Age (D)” have significant age differences for each parameter (main effect of age, P < 0.05).
y,zDifferent superscripts within the column “Diet” have significant dietary treatment differences for each parameter (main effect of diet, P < 0.05).
Abbreviations: AXOS, arabinoxylan-oligosaccharides; ND, not determined; NS, not significant.
Reported values are means of 10 to 12 replicates (N = 10-12) for the main effect of age and 25 to 30 replicates (N = 25–30) for the main effect of diet.
Table 5.
Total arabinose (A) to xylose (X) ratio and the A to X ratio of the aqueous extracts at the level of the ileum, caeca, and feces analyzed at 6 different broiler ages (day 5, 10, 15, 21, 28, and 35) for broilers fed a control (CTRL) and 0.50% AXOS-supplemented (AXOS) wheat-based diet.
| Variables1 | Total A to X ratio |
A to X ratio of the aqueous extract |
||||
|---|---|---|---|---|---|---|
| Ileum | Caeca | Feces | Ileum | Caeca | Feces | |
| Main effects | ||||||
| Age (D) | ||||||
| 5 | 0.80a,b | 0.18c | 0.79a,b | 0.81a | 0.44b | 0.40c |
| 10 | 0.80a,b | 0.19c | 0.82a | 0.73b | 0.66a | 0.70a |
| 15 | 0.79a,b | 0.29b,c | 0.75b | 0.72b | 0.65a,b | 0.64b |
| 21 | 0.85a,b | 0.37a–c | 0.79a | 0.73b | 0.64a,b | 0.71a |
| 28 | 0.78b | 0.45a,b | 0.77b | 0.74b | 0.72a | 0.61b |
| 35 | 0.86a | 0.55a | 0.77b | 0.72b | 0.76a | 0.64b |
| Diet | ||||||
| AXOS | 0.78z | 0.32 | 0.76z | 0.66z | 0.59z | 0.55z |
| CTRL | 0.84y | 0.35 | 0.80y | 0.82y | 0.70y | 0.68y |
| Pooled SEM | 0.01 | 0.03 | 0.01 | 0.01 | 0.03 | 0.02 |
| P values | ||||||
| Age | 0.005 | <0.001 | 0.01 | <0.001 | 0.002 | <0.001 |
| Diet | <0.001 | NS | <0.001 | <0.001 | 0.025 | <0.001 |
| Diet × Age | NS | NS | NS | 0.004 | NS | 0.01 |
a–cDifferent superscripts within the column “Age (D)” have significant age differences for each parameter (main effect of age, P < 0.05).
y,zDifferent superscripts within the column “Diet” have significant dietary treatment differences for each parameter (main effect of diet, P < 0.05).
Abbreviations: AXOS, arabinoxylan-oligosaccharides; NS, not significant.
Reported values of the substitution ratio A to X are means of 10-12 replicates (N = 10–12) for the main effect of age and 25 to 30 replicates (N = 25–30) for the main effect of diet.
Caeca
The TOT-AX and WE-AX contents at the level of the caeca are presented in Table 6. Similar to what was observed for ileal WE-AX content, caecal WE-AX content was significantly higher in the caecal digesta of broilers fed the AXOS supplemented diet than in those of broilers fed the CTRL diet (P = 0.049). In addition to WE-AX content, a higher TOT-AX content at the level of the caeca for AXOS-fed birds than that for their CTRL counterparts was observed, although this difference in content was only significant during the first 10 D as a result of the interaction effect between broiler age and diet (P = 0.048; Table 6). AXOS-fed birds were better at fermenting WE-AX in the caeca, as indicated by the consistently significantly higher WE-AX digestibility coefficient at every broiler age (P = 0.001; Table 4). No significant difference was observed in the corresponding TOT-AX digestibility coefficients (P = 0.91; Table 4). No interaction effect between broiler age and diet for caecal AX digestibility coefficients was seen (Table 4).
Table 6.
Total arabinoxylan (TOT-AX) and water-extractable arabinoxylan (WE-AX) content (g/kg DM) at the level of the caeca analyzed at 6 different broiler ages for broilers fed a control (CTRL) and a 0.50% AXOS-supplemented (AXOS) wheat-based diet.
| Content (g/kg DM)1 | Caeca |
|||
|---|---|---|---|---|
| TOT-AX |
WE-AX |
|||
| CTRL | AXOS | CTRL | AXOS | |
| Age (D) | ||||
| 5 D | 65.5a,z | 75.7a,y | 7.2a,z | 6.4a,y |
| 10 D | 43.7a,z | 87.7a,y | 4.8a,b,z | 8.2a,b,y |
| 15 D | 25.7b | 23.0b | 3.6a–c,z | 5.3a–c,y |
| 21 D | 19.0b | 21.0b | 3.7b,c,z | 4.3b,c,y |
| 28 D | 10.2b | 16.7b | 2.4c,z | 2.7c,y |
| 35 D | 29.0b | 24.3b | 2.4c,z | 3.9c,y |
| Pooled SEM | 4.3 | 6.0 | 0.4 | 0.5 |
| P values | ||||
| Age | <0.001 | <0.001 | ||
| Diet | 0.049 | 0.049 | ||
| Diet × Age | 0.048 | NS | ||
a–cDifferent superscripts within a column have significant age differences for each parameter (main effect of age, P < 0.05).
y,zDifferent superscripts within a row have significant dietary treatment differences for each parameter (main effect of diet, P < 0.05). If superscripts y and z are not presented at every broiler age, this marks the presence of a significant dietary treatment difference at a particular age (interaction effect diet × age, P < 0.05).
Abbreviations: AXOS, arabinoxylan-oligosaccharides; NS, not significant.
Reported values for TOT-AX and WE-AX content are means of 5-6 replicates of pooled caecal samples (N = 5–6).
The A/X ratio of the caecal extracts was significantly lower for birds fed the AXOS diet than that for the CTRL birds, which indicates the presence of less complex AX polymers in the caeca of AXOS-fed birds (P = 0.025). For the TOT-AX A/X, no difference between dietary treatments was observed (Table 5). No increased microbial AX-degrading activities were measured upon AXOS addition (Supplementary Table 3).
Feces
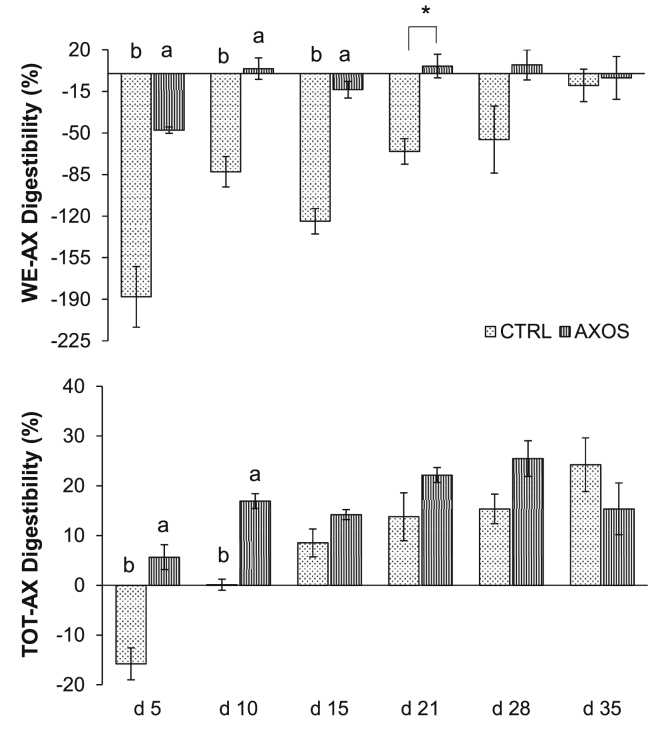
TOT-AX and WE-AX contents at the level of the feces are represented in Table 3. Fecal WE-AX content was significantly higher for broilers fed the AXOS diet (P < 0.001). However, fecal TOT-AX was not affected by dietary treatment. In contrast to this unaffected TOT-AX content, the digestibility coefficients of both WE-AX and TOT-AX improved significantly with AXOS in the broiler feed (P = 0.001 and P < 0.001 for WE-AX and TOT-AX digestibility, respectively). Moreover, this increase in digestibility coefficient was only present at very young broiler ages (interaction age × diet, P < 0.05). As shown in Figure 2, the microbiota of broilers fed the AXOS diet showed a higher TOT-AX fermenting capacity than those of their CTRL counterparts, indicated by the significantly higher WE-AX and TOT-AX digestibility coefficients for AXOS broilers younger than 15 D of age (P = 0.009 and P = 0.04 for WE-AX and TOT-AX digestibility, respectively). An enhanced fermentation of AX in the hindgut was hence observed when AXOS was present in the starter diet. Providing highly fermentable AXOS in the diet resulted in high total tract AX digestibilities at young ages, which persisted over time, while CTRL broilers showed a slowly developing and linearly increasing AX digestion profile as a function of broiler age (P < 0.0001) (Figure 2).
Figure 2.
Water-extractable arabinoxylan (WE-AX) and total arabinoxylan (TOT-AX) digestibility (%) at the level of the feces analyzed at 6 different broiler ages (day 5, 10, 15, 21, 28, and 35) for broilers fed a control (CTRL) and a 0.50% arabinoxylan-oligosaccharides supplemented (AXOS) wheat-based diet. Lowercase alphabets a,b and asterisk (*) denote significant differences between dietary treatment means at a particular age (interaction effect between diet × age, P ≤ 0.05 and P ≤ 0.10, respectively).
Lower A/X ratios were found in the feces of AXOS-fed birds from 10 D of age onward (interaction age × diet, P = 0.01) (Table 5).
Discussion
Prior to this study, we questioned if the previously observed beneficial effects of AXOS on broiler's health and performance (Courtin et al., 2008a, Courtin et al., 2008b, Eeckhaut et al., 2008) are exclusively the result of selective fermentation by host microorganisms, as their supplementation at the dosages used would generate only a relative low amount of SCFA in the broiler's hindgut. In search for an answer, we set out to investigate AXOS-associated and additive attributes next to the health benefits conferred by AXOS as a dietary substrate that is selectively used by host microorganisms (Gibson et al., 2017). In concreto, we aimed to investigate the effect of 0.50% bran AXOS addition on the age-related AX digestion profile in broilers fed wheat-based diets in this study.
Alteration of Intestinal Viscosity when AXOS is Added in Broiler Feeds
Ileal viscosity of AXOS-fed broilers was increased compared with that of broilers fed a CTRL diet. This increase in intestinal viscosity in AXOS-fed broilers was not expected, given the low dosage and nonviscous nature of the added AXOS (very low average degree of arabinose substitution and average degree of polymerization) (Courtin and Delcour, 1998, Broekaert et al., 2011) and the similar extract viscosities measured for both experimental diets. In addition, as AXOS is already in part fermented by the ileal microbiota (Morgan et al., 2019), we can assume that relatively little accumulation of AXOS in the ileal WE-AX fraction took place.
Hence, given the ability of small intestinal microbiota to solubilize WU-AX into WE-AX at young broiler ages already (Bautil et al., 2019) and that AXOS-fed birds had significantly higher WE-AX contents at the level of the ileum than control birds, the data show that conversion of WU-AX into high and low molecular weight solubilized WE-AX by the ileal microbiota occurred to a greater extent when 0.50% AXOS was added in a wheat-based diet. This increased solubilization was, however, not linked to a significantly augmented secretion of AX-hydrolyzing endoxylanases and arabinofuranosidases in the lumen by the ileal microbiota when AXOS was provided in the feed. Some caution is required, however, when interpreting these enzyme activity results. Measurements were only done on the supernatant of digesta fractions, thereby losing part of the AX-hydrolyzing enzymes associated with the microbial community.
Only a possible increase in endoxylanase and arabinofuranosidase activities could not be responsible for an increased conversion of WU-AX in WE-AX in the ileum of AXOS-fed broilers. Indeed, in previous studies in which prebiotic oligosaccharides were added to experimental diets, an increased secretion of GI peptides such as glucagon-like peptide-1 (GLP-1) and peptide tyrosine tyrosine (PYY), which are able to slow down GI transit, was observed (Delzenne et al., 2007, Neyrinck et al., 2012, Singh et al., 2012). By delaying the transit time in the GI tract, the microbiota could spend more time attacking and hydrolyzing the dietary fiber fraction of the diet (Delzenne et al., 2007, Hooda et al., 2011, Neyrinck et al., 2012, Singh et al., 2012, Müller et al., 2018). In addition, diets containing large amounts of soluble dietary fiber, for example, viscous WE-AX, are known to slow down gut transit, thereby increasing nutrient digestibility (Hooda et al., 2011, Kheravii et al., 2018, Müller et al., 2018). Our data for AXOS-fed broilers suggest that similar phenomena could be taking place. Furthermore, the observation that a higher TOT-AX content and hence bulk mass was present at the ileum indicates that small intestinal transit time was indeed slowed down and supports the hypothesis that, in broilers fed the AXOS diet, a longer exposure time of the dietary wheat AX toward the microbiota was probably established.
Alteration of AX Content and Digestion Profiles in the Hindgut when AXOS is Added in Feeds
Greater quantities of both the WE-AX and TOT-AX substrates flowed into the caeca of young broilers fed the AXOS diet than in those fed the CTRL diet, probably because of the increased precaecal modification of the wheat AX by the AXOS receiving ileal microbiota. This higher preceacal digestion was also visible in the general lower A/X ratios of the AX polymers found at the level of the ileum and caeca in broilers fed the AXOS diet. These less-complex substrates can stimulate AX fermentation more strongly (Van Craeyveld et al., 2008, Damen et al., 2011). A higher digestibility of WE-AX, resulting from more WU-AX solubilization and subsequent WE-AX fermentation, was indeed observed for AXOS-supplemented broilers at the level of the ileum and caeca. The increased WE-AX and TOT-AX digestibility confirms the presence of a microbial modulating effect in the hindgut sections upon AXOS addition. As 0.50% AXOS was administered immediately after hatch, it likely established a quicker emergence of a microbial community that had a higher capacity for fermentation of the dietary wheat AX. The higher proportional caecal weights that were measured for broilers fed the AXOS diet seem to support this, as increased fermentation is known to increase the cell wall density and epithelial cell proliferation of the caeca (Campbell et al., 1997, Pan et al., 2009).
The Kick-Starter Effect of AXOS on the Hindgut Microbiota
As demonstrated by the total tract digestibility coefficients (Figure 2), the AXOS diet resulted in a greater ability for the intestinal microbiota to solubilize the dietary WU-AX toward WE-AX and to ferment this pool of solubilized WE-AX, especially in young broilers. A clear stimulating effect of AXOS on the dietary fiber–degrading capacity of the hindgut microbiota was visible. Providing a highly fermentable dietary fiber source, having structural similarities with the main fiber source in the diet, seems to train and imprint the metabolic activity of colonizing microbiota in the hindgut, enabling these bacteria to start hydrolyzing and fermenting the dietary wheat AX. It was demonstrated by de Vries et al. (2016) that adding an extra source of dietary fiber, that is, 6% β-glucan, to the diet of pigs can alter digestion and fermentation of other dietary fiber sources. Furthermore, recent work of Bedford and Apajalahti (2018) suggested that prebiotic oligosaccharides, in their study formed in the GI tract of broilers through the addition of endoxylanases in the feed, upregulate the AX-fermentation pathways of the microbiota and hence trigger their dietary fiber degradation capacity. Our AX digestibility results hence seem to provide proof for these hypotheses by showing that highly fermentable AXOS are able to kick-start the hydrolysis and fermentation activities of the young microbiota toward the more recalcitrant fiber source of the diet, which in this case is the dietary fiber AX.
This kick-starter effect of AXOS on the total tract AX digestion and fermentation at young ages could hence be explained by the combination of two concomitant occurring phenomena: the quicker emergence of a young microbiome displaying a greater AX-degrading ability and a microbial-modulated effect of slowing down the GI transit time which allowed a greater precaecal modification of the dietary AX, as previously discussed. To reinforce the occurrence of these phenomena in AXOS-fed broilers, additional microbial analyses should be performed, as well as measurements of gastrointestinal transit times, especially in young broilers.
With this kick-starter effect, we can also explain the faster and steeper decline in the ileal viscosity as a function of broiler age for AXOS-fed birds. As previously described by Bautil et al. (2019) and Fischer (2003), viscosity will initially increase due to solubilization of WU-AX into viscous WE-AX by the ileal microbiota up until broilers are 21 to 28 D old. The increase in fermentative capacity together with an adaptation of the ileal microbiota toward these viscous WE-AX will finally result in a decrease in viscosity toward slaughter age. As this decline in viscosity in broilers subjected to the AXOS treatment tended to decrease at a younger age (21 D) compared with the CTRL broilers (28 D), it can be assumed that these AXOS had stimulated the solubilizing and fermentative abilities of the ileal microbiome toward the dietary wheat AX substrates.
This kick-starter benefit conveyed by AXOS did not persist over time. This is not illogical as Bautil et al. (2019) showed that the capacity of the hindgut microbiota to solubilize and ferment the AX substrates present in the CTRL diet in the GI tract for broilers increases linearly as a function of broiler age. The broilers fed the CTRL diet did effectively “catch up” as the birds aged, thereby explaining the relative minor differences between the two treatments for AX digestibility coefficients at the level of the feces in older birds.
Hence, this study proves once more that the first ingested feed and the composition of the diet is one of the most important factors in modulating the early microbial colonization of the GI tract, thereby regulating the nutrient recovery along the GI tract and GI tract integrity (Lu et al., 2003, Pedroso et al., 2005, Torok et al., 2013). More specifically, it emphasizes the significant impact that adding a dietary fiber source to young bird rations has on the complex interaction between the microbiota and dietary components. Almost all parameters analyzed in this study corroborate the proposed kick-starter effect of AXOS. The view of these AXOS (degree of polymerization < 10), as being prebiotics in the classical sense, needs to be reconsidered. It was demonstrated that these oligosaccharides can form a signaling agent for microbiota to speed up the development of their fiber-degrading capacity and slow down the GI transit time. To the best of our knowledge, we are the first to report that this phenomenon can occur in the GI tract of broilers despite their short lifetime of 5 to 6 wk.
As AXOS can also be generated in situ when endoxylanases are added to broiler diets (Courtin et al., 2008a, Cowieson and Masey O'Neill, 2013, Morgan et al., 2019), these feed enzymes could exert a similar kick-starter effect on the intestinal microbiota, thereby giving the intestinal microbiome a boost in dietary fiber hydrolysis and fermentation. Additional mechanistic actions should therefore be considered, when the mode of action of feed enzymes or other dietary components are subject of research.
Conclusion
Investigating the additional modes of action of dietary fiber supplementation in the form of fermentable AXOS in wheat-based broiler diets revealed that these AXOS boosted total tract wheat AX digestion in young broilers. The impact of AXOS on transit time, the stimulation of the fiber-degrading capacity of the existing microbiome, or the emergence of a microbial community with enhanced fiber-degrading capacity could be at the basis of this kick-starter effect. It enables greater functional value to be extracted from dietary fiber in broiler feed.
Acknowledgements
The diligent technical assistance of Jens Lesuisse, Seline Schallier, and Concong Li is gratefully acknowledged.
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2019.12.041
Supplementary data
References
- Aftab U., Bedford M.R. The use of NSP enzymes in poultry nutrition: myths and realities. Worlds Poult. Sci. J. 2018;74:277–286. [Google Scholar]
- Annison G., Choct M. Anti-nutritive activities of cereal non-starch polysaccharides in broiler diets and strategies minimizing their effects. Worlds Poult. Sci. J. 1991;47:232–242. [Google Scholar]
- Austin S.C., Wiseman J., Chesson A. Influence of non-starch polysaccharides structure on the Metabolisable energy of U.K. Wheat fed to poultry. J. Cereal Sci. 1999;29:77–88. [Google Scholar]
- Aviagen . Aviagen Incorporated; Huntsville, AL: 2014. Ross 308 Broiler Management Guide. [Google Scholar]
- Bautil A., Verspreet J., Buyse J., Goos P., Bedford M.R., Courtin C.M. Age-related arabinoxylan hydrolysis and fermentation in the gastrointestinal tract of broiler fed wheat-based diets. Poult. Sci. 2019;98:4606–4621. doi: 10.3382/ps/pez159. [DOI] [PubMed] [Google Scholar]
- Bedford M.R., Classen H.L. Reduction of intestinal viscosity through manipulation of dietary rye and pentosanase concentration is effected through changes in the carbohydrate composition of the intestinal aqueous phase and results in improved growth rate and food conversion efficiency of broiler chicks. J. Nutr. 1992;122:560–569. doi: 10.1093/jn/122.3.560. [DOI] [PubMed] [Google Scholar]
- Bedford M.R., Apajalahti J.H.A. Poultry Science Association 107th Annual Meeting; San Antonio, TX: 2018. Exposure of a Broiler to a Xylanase for 35d Increases the Capacity of Cecal Microbiome to Ferment Soluble Xylan. Proc. E-Supplement 1. 98–99. [Google Scholar]
- Broekaert W.F., Courtin C.M., Verbeke K., Van de Wiele T., Verstraete W., Delcour J.A. Prebiotic and other health-related effects of cereal-derived arabinoxylans, arabinoxylan-oligosaccharides, and xylooligosaccharides. Crit. Rev. Food Sci. Nutr. 2011;51:178–194. doi: 10.1080/10408390903044768. [DOI] [PubMed] [Google Scholar]
- Campbell J.M., Fahey J.G.C., Wolf B.W. Selected indigestible oligosaccharides Affect large bowel mass, Cecal and fecal short-chain fatty acids, pH and Microflora in rats. J. Nutr. 1997;127:130–136. doi: 10.1093/jn/127.1.130. [DOI] [PubMed] [Google Scholar]
- Courtin C.M., Delcour J.A. Physicochemical and Bread-Making properties of low molecular weight wheat-derived arabinoxylans. J. Agric. Food Chem. 1998;46:4066–4073. [Google Scholar]
- Courtin C.M., Broekaert W.F., Swennen K., Lescroart O., Onagbesan O., Buyse J., Decuypere E., Van de Wiele T., Marzorati M., Verstraete W., Huyghebaert G., Delcour J.A. Dietary inclusion of wheat bran arabinoxylooligosaccharides induces beneficial nutritional effects in chickens. Cereal Chem. 2008;85:607–613. [Google Scholar]
- Courtin C.M., Swennen K., Broekaert W.F., Swennen Q., Buyse J., Decuypere E., Michiels C.W., De Ketelaere B., Delcour J.A. Effects of dietary inclusion of xylooligo- saccharides, arabinoxylooligosaccha- rides and soluble arabinoxylan on the microbial composition of caecal contents of chickens. J. Sci. Food Agric. 2008;88:2517–2522. [Google Scholar]
- Cowieson A.J., Masey O'Neill H.V. Effects of exogenous xylanase on performance, nutrient digestibility and caecal thermal profiles of broilers given wheat-based diets. Br. Poult. Sci. 2013;54:346–354. doi: 10.1080/00071668.2013.780200. [DOI] [PubMed] [Google Scholar]
- Damen B., Verspreet J., Pollet A., Broekaert W.F., Delcour J.A., Courtin C.M. Prebiotic effects and intestinal fermentation of cereal arabinoxylans and arabinoxylan oligosaccharides in rats depend strongly on their structural properties and joint presence. Mol. Nutr. Food Res. 2011;55:1862–1874. doi: 10.1002/mnfr.201100377. [DOI] [PubMed] [Google Scholar]
- de Vries S., Gerrits W.J.J., Kabel M.A., Vasanthan T., Zijlstra R.T. β-Glucans and Resistant starch alter the fermentation of recalcitrant fibers in growing pigs. PLoS One. 2016;11:e0167624. doi: 10.1371/journal.pone.0167624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delzenne N.M., Cani P.D., Daubioul C., Neyrinck A.M. Impact of inulin and oligofructose on gastrointestinal peptides. Br. J. Nutr. 2007;93:S157–S161. doi: 10.1079/bjn20041342. [DOI] [PubMed] [Google Scholar]
- Eeckhaut V., Van Immerseel F., Dewulf J., Pasmans F., Haesebrouck F., Ducatelle R., Courtin C.M., Delcour J.A., Broekaert W.F. Arabinoxylooligosaccharides from wheat bran Inhibit Salmonella colonization in broiler chickens. Poult. Sci. 2008;87:2329–2334. doi: 10.3382/ps.2008-00193. [DOI] [PubMed] [Google Scholar]
- Englyst H.N., Cummings J.H. Simplified method for the measurement of total non-starch polysaccharides by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst. 1984;109:937–942. doi: 10.1039/an9820700307. [DOI] [PubMed] [Google Scholar]
- Fischer E.N. PhD Thesis. University of Saskatchewan; Saskatoon, SK, Canada: 2003. Interrelationship of Diet Fibre and Endoxylanase with Bacteria of the Chicken Gut. [Google Scholar]
- Gebruers K., Dornez E., Bedõ Z., Rakszegi M., Frás A., Boros D., Courtin C.M., Delcour J.A. Environment and Genotype effects on the content of dietary fiber and its components in wheat in the HEALTHGRAIN Diversity screen. J. Agric. Food Chem. 2010;58:9353–9361. doi: 10.1021/jf100447g. [DOI] [PubMed] [Google Scholar]
- Geraylou Z., Souffreau C., Rurangwa E., D'Hondt S., Callewaert L., Courtin C.M., Delcour J.A., Buyse J., Ollevier F. Effects of arabinoxylan-oligosaccharides (AXOS) on juvenile Siberian sturgeon (Acipenser baerii) performance, immune responses and gastrointestinal microbial community. Fish Shellfish Immunol. 2012;33:718–724. doi: 10.1016/j.fsi.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J., Scott K., Stanton C., Swanson K.S., Cani P.D., Verbeke K., Reid G. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14:491. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- Hooda S., Metzler-Zebeli B.U., Vasanthan T., Zijlstra R.T. Effects of viscosity and fermentability of dietary fibre on nutrient digestibility and digesta characteristics in ileal-cannulated grower pigs. Br. J. Nutr. 2011;106:664–674. doi: 10.1017/S0007114511000985. [DOI] [PubMed] [Google Scholar]
- Józefiak D., Rutkowski A., Martin S.A. Carbohydrate fermentation in the avian ceca: a review. Anim. Feed Sci. Technol. 2004;113:1–15. [Google Scholar]
- Keerqin C., Morgan N.K., Wu S.B., Swick R.A., Choct M. Dietary inclusion of arabinoxylo-oligosaccharides in response to broilers challenged with subclinical necrotic enteritis. Br. Poult. Sci. 2017;58:418–424. doi: 10.1080/00071668.2017.1327705. [DOI] [PubMed] [Google Scholar]
- Kheravii S.K., Morgan N.K., Swick R.A., Choct M., Wu S.B. Roles of dietary fibre and ingredient particle size in broiler nutrition. Worlds Poult. Sci. J. 2018;74:301–316. [Google Scholar]
- Lee S.A., Apajalahti J., Vienola K., González-Ortiz G., Fontes C.M.G.A., Bedford M.R. Age and dietary xylanase supplementation affects ileal sugar residues and short chain fatty acid concentration in the ileum and caecum of broiler chickens. Anim. Feed Sci. Technol. 2017;234:29–42. [Google Scholar]
- Lu J., Idris U., Harmon B., Hofacre C., Maurer J.J., Lee M.D. Diversity and Succession of the intestinal bacterial community of the Maturing broiler chicken. Appl. Environ. Microbiol. 2003;69:6816–6824. doi: 10.1128/AEM.69.11.6816-6824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan N.K., Keerqin C., Wallace A., Wu S.-B., Choct M. Effect of arabinoxylo-oligosaccharides and arabinoxylans on net energy and nutrient utilization in broilers. Anim. Nutr. 2019;5:56–62. doi: 10.1016/j.aninu.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Canfora E.E., Blaak E.E. Gastrointestinal transit time, glucose Homeostasis and metabolic health: Modulation by dietary fibers. Nutrients. 2018;10:275. doi: 10.3390/nu10030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers W.D., Ludden P.A., Nayigihugu V., Hess B.W. Technical Note: a procedure for the preparation and quantitative analysis of samples for titanium dioxide1. J. Anim. Sci. 2004;82:179–183. doi: 10.2527/2004.821179x. [DOI] [PubMed] [Google Scholar]
- Neyrinck A.M., Van Hée V.F., Piront N., De Backer F., Toussaint O., Cani P.D., Delzenne N.M. Wheat-derived arabinoxylan oligosaccharides with prebiotic effect increase satietogenic gut peptides and reduce metabolic endotoxemia in diet-induced obese mice. Nutr. Diabetes. 2012;2:e28. doi: 10.1038/nutd.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X.D., Chen F.Q., Wu T.X., Tang H.G., Zhao Z.Y. Prebiotic oligosaccharides change the concentrations of short-chain fatty acids and the microbial population of mouse bowel. J. Zhejiang Univ. Sci. B. 2009;10:258–263. doi: 10.1631/jzus.B0820261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedroso A.A., Menten J.F.M., Lambais M.R. The structure of bacterial community in the intestines of Newly hatched Chicks1. J. Appl. Poult. Res. 2005;14:232–237. [Google Scholar]
- Ribeiro T., Cardoso V., Ferreira L.M.A., Lordelo M.M.S., Coelho E., Moreira A.S.P., Domingues M.R.M., Coimbra M.A., Bedford M.R., Fontes C.M.G.A. Xylo-oligosaccharides display a prebiotic activity when used to supplement wheat or corn-based diets for broilers. Poult. Sci. 2018;97:4330–4341. doi: 10.3382/ps/pey336. [DOI] [PubMed] [Google Scholar]
- Short F.J., Gorton P., Wiseman J., Boorman K.N. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed Sci. Technol. 1996;59:215–221. [Google Scholar]
- Singh A., O’Neill H.V.M., Ghosh T.K., Bedford M.R., Haldar S. Effects of xylanase supplementation on performance, total volatile fatty acids and selected bacterial population in caeca, metabolic indices and peptide YY concentrations in serum of broiler chickens fed energy restricted maize–soybean based diets. Anim. Feed Sci. Technol. 2012;177:194–203. [Google Scholar]
- Smeets N., Nuyens F., Van Campenhout L., Delezie E., Pannecoucque J., Niewold T. Relationship between wheat characteristics and nutrient digestibility in broilers: comparison between total collection and marker (titanium dioxide) technique. Poult. Sci. 2015;94:1584–1591. doi: 10.3382/ps/pev116. [DOI] [PubMed] [Google Scholar]
- Swennen K., Courtin C.M., Lindemans G.C., Delcour J.A. Large-scale production and characterisation of wheat bran arabinoxylooligosaccharides. J. Sci. Food Agric. 2006;86:1722–1731. [Google Scholar]
- Torok V.A., Dyson C., McKay A., Ophel-Keller K. Quantitative molecular assays for evaluating changes in broiler gut microbiota linked with diet and performance. Anim. Prod. Sci. 2013;53:1260–1268. [Google Scholar]
- Van Craeyveld V., Swennen K., Dornez E., Van de Wiele T., Marzorati M., Verstraete W., Delaedt Y., Onagbesan O., Decuypere E., Buyse J., De Ketelaere B., Broekaert W.F., Delcour J.A., Courtin C.M. Structurally different wheat-derived arabinoxylooligosaccharides have different prebiotic and fermentation properties in rats. J. Nutr. 2008;138:2348–2355. doi: 10.3945/jn.108.094367. [DOI] [PubMed] [Google Scholar]
- Verspreet J., Damen B., Broekaert W.F., Verbeke K., Delcour J.A., Courtin C.M. A critical look at prebiotics within the dietary fiber Concept. Annu. Rev. Food Sci. Technol. 2016;7:167–190. doi: 10.1146/annurev-food-081315-032749. [DOI] [PubMed] [Google Scholar]
- Yacoubi N., Saulnier L., Bonnin E., Devillard E., Eeckhaut V., Rhayat L., Ducatelle R., Van Immerseel F. Short-chain arabinoxylans prepared from enzymatically treated wheat grain exert prebiotic effects during the broiler starter period. Poult. Sci. 2017;97:412–424. doi: 10.3382/ps/pex297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.