Abstract
In documents, maternal betaine modulates hypothalamic cholesterol metabolism in chicken posthatchings, but it remains unclear whether this effect can be passed on by generations. In present study, eggs were injected with saline or betaine at 2.5 mg/egg, and the hatchlings (F1) were raised under the same condition until sexual maturation. Both the control group and the betaine group used artificial insemination to collect sperm from their cockerels. Fertilized eggs were incubated, and the hatchlings of the following generation (F2) were raised up to 64 D of age. F2 cockerels in betaine group showed significantly (P < 0.05) lower body weight, which was associated with significantly decreased (P < 0.05) hypothalamic content of total cholesterol and cholesterol ester. Concordantly, hypothalamic expression of cholesterol biosynthetic genes, SREBP2 and HMGCR, were significantly downregulated (P < 0.05), together with cholesterol conversion-related and excretion-related genes, CYP46A1 and ABCA1. These changes coincided with a significant downregulation in mRNA expression of regulatory neuropeptides including brain-derived neurotrophic factor, neuropeptide Y, and corticotropin-releasing hormone. Moreover, genes involved in methyl transfer cycle were also modified. Betaine homocysteine methyltransferase (P < 0.05) was downregulated, yet DNA methyltransferase1 tended to be upregulated (P = 0.06). S-adenosyl methionine/S-adenosylhomocysteine ratio was higher in the hypothalamus of betaine-treated F2 cockerels, which was associated with significantly modified CpG methylation on the promoter of those affected genes. These results suggested that betaine might regulate central cholesterol metabolism and hypothalamic expression of genes related to brain function by altering promoter DNA methylation in F2 cockerels.
Key words: betaine, cholesterol, chicken, DNA methylation, hypothalamus
Introduction
The main sources of betaine in animal organism are dietary intake and choline oxidation (Lever et al., 2010). Under the catalysis of betaine homocysteine methyltransferase (BHMT), betaine acts as a methyl donor to convert homocysteine into methionine (Kulis et al., 2012). Methionine then transfers its methyl group to S-adenosylmethionine (SAM), the universal methyl donor for various methylation reactions catalyzed by various methyltransferases (Mudd et al., 2012).
Betaine is essential for embryonic and fetal development. Prenatal betaine supplementation modulated hepatic cholesterol metabolism in new born piglets (Cai et al., 2014) and newly hatched chicks (Hu et al., 2015), which involved modifications of epigenetic marks, including DNA and histone methylation, on the promoter of key cholesterol metabolic genes. It was well-documented that the effects of prenatal nutrition could be transmitted through generations (Aiken et al., 2016). For example, protein deficiency during pregnancy in F0 female rats caused changes in glucose homeostasis and hepatic transcriptome through 3 generations of rat (Hoile et al., 2011). Also, feeding methyl-enriched diet exclusively to F0 boars significantly changed the carcass traits, gene expression and DNA methylation in F2 generation (Braunschweig et al., 2012). Recently, we reported that in ovo administration of betaine modulated hypothalamic expression of cholesterol metabolism genes in chicken (Idriss et al., 2017). However, it remains unknown whether this effect will continue to be transmitted to subsequent generations of chickens.
Cholesterol plays an important role for neuronal physiological function. Cholesterol, a precursor of steroid hormones, is an important structural component of cell membrane and myelin sheath and a material for synapse and dendrite formation (Goritz et al., 2005). Cholesterol depletion in neurons impairs synaptic vesicle exocytosis, which suppresses neuronal activity and neurotransmission (Linetti et al., 2010). Because the blood–brain barrier prevents lipoprotein from being absorbed from the peripheral circulation to the brain, brain cholesterol is mainly derived from ab initio synthesis (Zhang and Liu, 2015). Cholesterol balance in the brain is achieved by coordinated regulation of biosynthesis, storage, and excretion. Sterol response element binding protein 2 (SREBP2) is a major transcription factor that activates cholesterol biosynthesis genes, including the rate-limiting enzyme 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGCR) (Sharpe and Brown, 2013). Cholesterol 24-hydroxylase (CYP46A1) converts cholesterol to 24S-hydroxycholesterol (Goyal et al., 2014) that can cross blood–brain barrier much faster than cholesterol itself for excretion (Lange et al., 1995, Meaney et al., 2002). In addition, low-density lipoprotein receptor mediates cholesterol inflow, while ABC transporters, including ATP-binding cassette subfamily A member 1 (ABCA1), act as cholesterol efflux pumps to remove cholesterol from cells (Gelissen et al., 2006, Vaughan et al., 2009).
Numerous studies in mammals had shown that brain cholesterol homeostasis was closely related to various physiological functions (Suzuki et al., 2010, Fukui et al., 2015). In fact, many neurodegenerative diseases, including Huntington's disease, Alzheimer's disease. and Parkinson's disease, were associated with deficiencies in brain cholesterol metabolism (Petrov et al., 2016a, Wang et al., 2015). Hypothalamus plays an important role in regulating growth, energy balance, and stress response through a variety of neuropeptides or neurotransmitters (Naseem and Heald, 1987). It was reported that brain-derived neurotrophic factor (BDNF) activated cholesterol de novo synthesis in the brain (Petrov et al., 2016b). Although hypothalamic neuropeptide Y (NPY) and corticotropin-releasing hormone (CRH) were key regulatory factors in avian metabolism-related function such as feed intake (Zhang et al., 2015), growth performance (Kalra et al., 1991, Blankenship et al., 2016), and stress response (Khan et al., 2015). However, evidences linking hypothalamic cholesterol metabolism and the expression of these functional neuropeptides are scarce in avian species.
The present study was aimed to investigate the effect of prenatal betaine exposure on hypothalamic cholesterol content and the expression of cholesterol metabolic genes in chicken offspring. Meanwhile, hypothalamic expression of BDNF, NPY, and CRH was also detected to link with the expression of cholesterol metabolic genes. Furthermore, CpG methylation status on the promoter sequences of affected gene was analyzed to provide more evidences for the methyl donor effect of prenatal betaine.
Materials and methods
Ethics Approval
The experimental protocol was approved by the Animal Ethics Committee of Nanjing Agricultural University, with the project number 2012CB124703. Animal care and handling were in compliance with regulations of AVMA Guidelines for the Euthanasia of Animals: 2013 Edition. The sampling procedures complied with the “Guidelines on Ethical Treatment of Experimental Animals'’ (2006) No. 398 set by the Ministry of Science and Technology, China.
Animals and Treatment
One hundred and forty fertilized eggs (42.1 ± 0.11 g) laid by Rugao yellow breeders were obtained from Poultry Institute of Yangzhou, Jiangsu, China. Before incubation, eggs were randomly divided into control and betaine groups (70 in each group) and were injected with 100 μL of saline or betaine (2.5 mg per egg, B2629, Sigma-Aldrich), as previously described (Haussmann et al., 2012). All the newly hatched chicks (F1) were raised under the same condition complied with the Feeding Management Regulations of local breeds of Rugao Chickens. All birds were kept in cages with 2 birds per cage according to groups. All cages are arranged in parallel groups to avoid spatial errors. The stock density was about 30 kg/m2. The room temperature range was 35°C∼37°C in the first week, then eventually changed to 21°C with 3°C/week steps. The relative humidity was maintained at 40∼60%. Chickens were weighed every week. After 120 D of age, 8 laying hens from the control and the betaine-treated group were randomly selected, respectively. Following artificial insemination, 5 fertilized eggs were obtained from each 8 female. After hatching, the offsprings were randomly divided into 2 corresponding treatment with 6 replicates and 6 birds per replicate (2 birds per cage). In each group, 2 birds were randomly selected from each replicate for sampling. After hatching, second generation (F2) chicks were raised under the same standard condition up to 64 D of age when all the chicks were weighed and sacrificed. Plasma was separated from the jugular vein blood with heparinized treatment by centrifugation at 3,000 × g. Hypothalami were dissected according to a previous publication (Yuan et al., 2009) and rapidly frozen in liquid nitrogen, then stored at −70°C for further analysis.
Total Cholesterol, Free Cholesterol, and Cholesterol Ester Content
The contents of total cholesterol (Tch, E1015, Applygen Technologies, Inc. Beijing, China) and free cholesterol (E1016, Applygen Technologies, Inc. Beijing, China) in hypothalamus were determined according to the manufacturer's instructions. Cholesterol ester content was calculated by subtracting the free cholesterol content from the total Tch content.
Serum Concentration of Total Cholesterol, High-Density Lipoprotein-Cholesterol, and Low-Density Lipoprotein-Cholesterol
Serum concentration of total cholesterol was measured using a commercial cholesterol assay kit (E1005, Applygen Technologies, Inc. Beijing, China). Serum concentrations of high-density lipoprotein-cholesterol (HDL-C, 0063280, Beijing BHKT Clinical Reagent Co., China) and low-density lipoprotein-cholesterol (LDL-C, 006340, Beijing BHKT Clinical Reagent Co., China) were measured with respective assay kits.
Hypothalamus Content of SAM and S-adenosylhomocysteine
Hypothalamic content of SAM and S-adenosylhomocysteine (SAH) was determined according to a previous publication (She et al., 1994) with some modifications. Briefly, hypothalamic tissue extracts were prepared using 0.4 mol/L perchloric acid (1:1, v/v) and then the extracts were centrifuged at 10,000 rpm for 20 min at 4°C. The supernatants were neutralized with KOH (2 mmol/L) and filtered through 0.22 μm membrane filters. Ultraperformance liquid chromatography (UPLC) was performed with a reverse-phase column (ACQUITY UPLC BEH C18 1.7 μm 2.1 × 150 mm column, Waters, Milford, MA). The column temperature was set at 35°C, and the wavelength UV monitor was set at 254 nm. A mobile phase (pH 3.0) that consisted of 40 mmol/L NH4H2PO4, 8 mmol/L 1-heptanesulfonic acid sodium salt, and 18% methanol was used; the flow rate was maintained at 0.61 mL/min by an ACQUITY UPLC H-Class system (Waters). Calibration curves were prepared by a 6-point standard (1, 0.5, 0.25, 0.125, 0.0625, and 0.03125 μmol/L) of SAM and SAH mixture in 0.4 mmol/L perchloric acid.
Total RNA Isolation and Quantitative Real-Time PCR, Protein Extraction, and Western Blotting, Methylated DNA Immune Precipitation Analysis
Total RNA, genomic DNA, and total protein were isolated from ground chicken hypothalamic samples, and Methylated DNA Immune Precipitation (MeDIP) assay was hired to observe the CpG methylation status on the promoter sequences of corresponding genes. Detailed protocols are the same as those described in our previous article (Idriss et al., 2017). To be clear, all of the primers used for quantitative real-time PCR are listed in the first part of Table 1, and the primers used for MeDIP assay are marked with “- promoter” tails.
Table 1.
Nucleotide sequences of specific primers.
| Target genes | GenBank accession No. | Primer sequences (5′ to 3′) | PCR products (bp) |
|---|---|---|---|
| SREBP2 | XM_416222 | F:CCCAGAACAGCAAGCAAGG R:GCGAGGACAGGAAAGAGAGTG |
108 |
| HMGCR | NM_204485.1 | F:TTGGATAGAGGGAAGAGGGAAG R: CTCGTAGTTGTATTCGGTAA |
130 |
| LDLR | NM_204452.1 | F: CCACCATTTGGCAGAGGAA R: ACCGCAGTCAGACCAGAAGAG |
86 |
| ABCA1 | NM_204145.2 | F: TCCTCTGGCTTAGACTTGA R: CTCGTAGTTGTATTCGGTAA |
130 |
| CYP46A1 | NM_001252027.1 | F: CATGCCGCGTATGACCACAT R: GCCATTCCCCAGAAACCTCA |
291 |
| BHMT | XM_414685.3 | F: TCTTCCTGAATTTCCCTT R: TGAACATCCCATCTAGTGA |
157 |
| DNMT1 | NM_206952.1 | F: CGAGTGGGACGGCTTCTT R: AGGCGATAGGTGTCAGGGA |
144 |
| DNMT3A | NM_001024832.1 | F: GGAGCACCCTTTGTTTATCG R: GGTATCCGTCGTCATCGTATTG |
89 |
| β-actin | L08165.1 | F:TGCGTGACATCAAGGAGAAG R:TGCCAGGGTACATTGTGGTA | 300 |
| HMGCR-promoter | NC_006127.3 | F: GGACTCAGGGTCTAAAG R: ACAAACATTGCTCACAG |
114 |
| SREBP2-promoter | NC_006088.3 | F: CAAGGAGATCCGCAAGGG R: GCCGCATCGGCTGAAAA |
131 |
| CYP46A1-promoter | NC_006092.4 | F: ACGAAGCATTAAAGCGCTGG R:CGAATCCCCTGACTTCACCG | 82 |
| ABCA1-promoter | NC_006127.4 | F: CAGACAGGTCAGCAGCCAG R:GAAAACAAACGGTGGGGCTG | 154 |
| BDNF-promoter | NM_001031616.1 | F: TTAGTGGCGAAGGTTCAGGC R: TCCCTGGAGTACCACTGCTT |
247 |
| NPY-promoter | P28673 | F: GTGGCACTGGTGGACTGTT R: ACACCTCGAGAGTTACCTGC |
185 |
| CRH-promoter | NM_001123031.1 | F: ATCCGTAATGTGCAGCCACT R:CGTACCCCTCCCTTTAAGCC |
151 |
Statistical Analysis
The data were calculated, and comparisons were performed with SPSS 18.0 for windows. Independent-samples t test applied to examine statistical significance. The results were shown as means ± SEM. The differences were considered statistically significant when P < 0.05.
Results
Body Weight, Serum Concentration, and Hypothalamic Content of Cholesterol
The body weight of 64-day-old cockerels (F2) derived from in ovo betaine-injected hens was significantly lower (P < 0.05) than their control counterparts. Also, in ovo betaine injection significantly decreased the serum concentration of total cholesterol and HDL-C (P < 0.05) in F2 cockerels, whereas LDL-C did not differ between betaine and control groups (Table 2). Moreover, the hypothalamic contents of total and free cholesterol (P < 0.01), as well as cholesterol ester (P < 0.05), were significantly lower in betaine group (Table 2).
Table 2.
Body weight and total cholesterol, HDL-C concentration in the serum, with hypothalamic content of total, free, and esterified cholesterol in chicken.
| Parameters | Control | Betaine | P-value |
|---|---|---|---|
| BW (g) | 653.3 ± 21.9 | 585.5 ± 21.7 | 0.04 |
| Serum concentration | |||
| Tch (mmol/L) | 3.70 ± 0.09 | 3.33 ± 0.10 | 0.01 |
| HDL-C (mmol/L) | 2.53 ± 0.05 | 2.26 ± 0.04 | 0.002 |
| Hypothalamic content | |||
| Tch (mg/g) | 20.82 ± 0.77 | 17.73 ± 0.62 | 0.006 |
| Free cho (mg/g) | 11.28 ± 0.32 | 9.93 ± 0.23 | 0.006 |
| Cho Ester (mg/g) | 9.63 ± 0.71 | 7.26 ± 0.50 | 0.04 |
Values are means ± SEM. N = 11
Abbreviations: BW, body weight; Cho Ester, cholesterol ester (N = 10); Free cho, free cholesterol; HDL-C, high-density lipoprotein; Tch, total cholesterol.
Hypothalamic Expression of Cholesterol Metabolic Genes
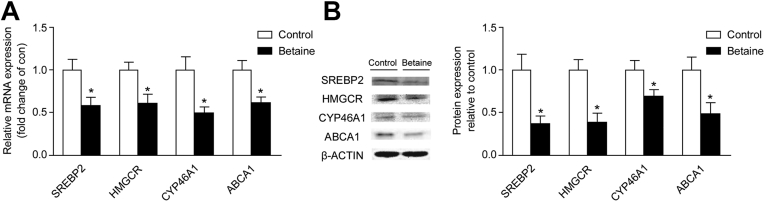
Hypothalamic cholesterol metabolic genes including SREBP2, HMGCR, CYP46A1, and ABCA1 are significantly decreased (P < 0.05) in F2 cockerels derived from in ovo betaine-injected hens both in mRNA transcription (Figure 1A) and protein content (Figure 1B).
Figure 1.
Effect of in ovo injection of betaine for second generation (F2) on hypothalamic expression of cholesterol metabolic genes in chickens. (A) Hypothalamic mRNA expression of SREBP2 (P = 0.03), HMGCR (P = 0.03), CYP46A1 (P = 0.02), and ABCA1 (P = 0.02). (B) Protein expression of SREBP2 (P = 0.04), HMGCR (P = 0.01), CYP46A1 (P = 0.05), and ABCA1 (P = 0.03). Values are means ± SEM, *P < 0.05, compared with control (n = 6). SREBP2, sterol response element binding protein 2; HMGCR, 3-hydroxy-3-methyl-glutaryl-CoA reductase; CYP46A1, cholesterol 24-hydroxylase; ABCA1, ATP-binding cassette subfamily A member 1.
Hypothalamic mRNA Expression of Neuropeptide Genes
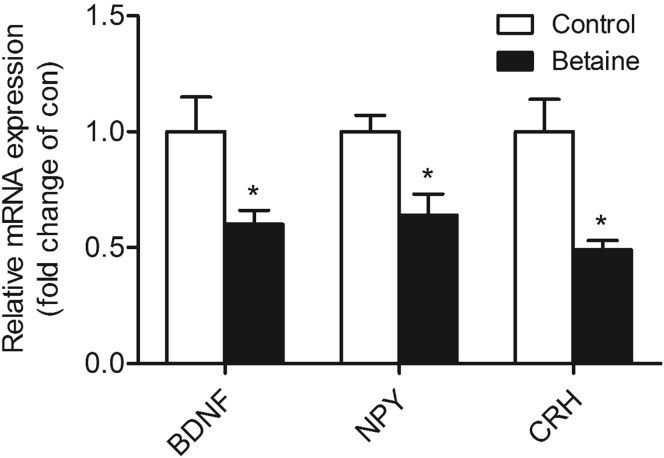
In ovo betaine injection significantly decreased (P < 0.05) the mRNA abundance of BDNF, NPY, and CRH in the hypothalamus of F2 cockerels (Figure 2).
Figure 2.
Effect of in ovo injection of betaine for second generation (F2) on hypothalamic genes expression of neuropeptides. Hypothalamic mRNA expression of BDNF (P = 0.04), NPY (P = 0.01), and CRH (P = 0.01). Values are means ± SEM, *P < 0.05, compared with control (n = 6). BDNF, brain-derived neurotrophic factor; NPY, neuropeptide Y; CRH, corticotropin-releasing hormone.
Hypothalamic SAM and SAH Contents and Expression of Methyl Transfer Genes
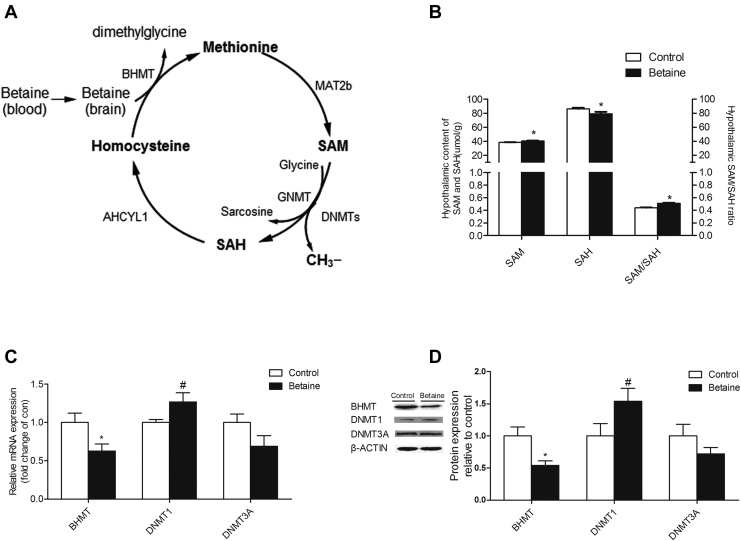
Betaine is involved in the methionine cycle (Figure 3A) that is critical for the production of SAM, a universal methyl donor. In ovo betaine injection significantly increased (P < 0.05), the hypothalamic content of SAM, while decreased that of SAH (P = 0.05), in F2 cockerels, leading to higher (P < 0.05) ratio of SAM/SAH (Figure 3B). Also, in ovo betaine injection significantly decreased (P < 0.05) the expression of BHMT both in mRNA and protein levels, whereas DNMT1 mRNA tended to be increased (P < 0.06). No significant alterations were observed for DNMT3A in either mRNA or protein level (Figure 3C and 3D).
Figure 3.
Effect of in ovo injection of betaine for second generation (F2) on hypothalamic genes expression involved in methyl transfer. (A) Diagram of methyl transfer cycle. (B) Hypothalamic content of SAM (P = 0.02) and SAH (P = 0.05). (C) Hypothalamic mRNA expression of BHMT (P = 0.01), DNMT1 (P = 0.053) and DNMT3A. (D) Hypothalamic protein expression of BHMT (P = 0.004), DNMT1 (P = 0.064), and DNMT3A. Values are means ± SEM, *P < 0.05, **P < 0.01, #P < 0.06, compared with control (n = 6). BHMT, betaine homocysteine methyltransferase; DNMT1, DNA methyltransferase 1; DNMT3A, DNA methyltransferase 3A; SAM, S-adenosylmethionine.
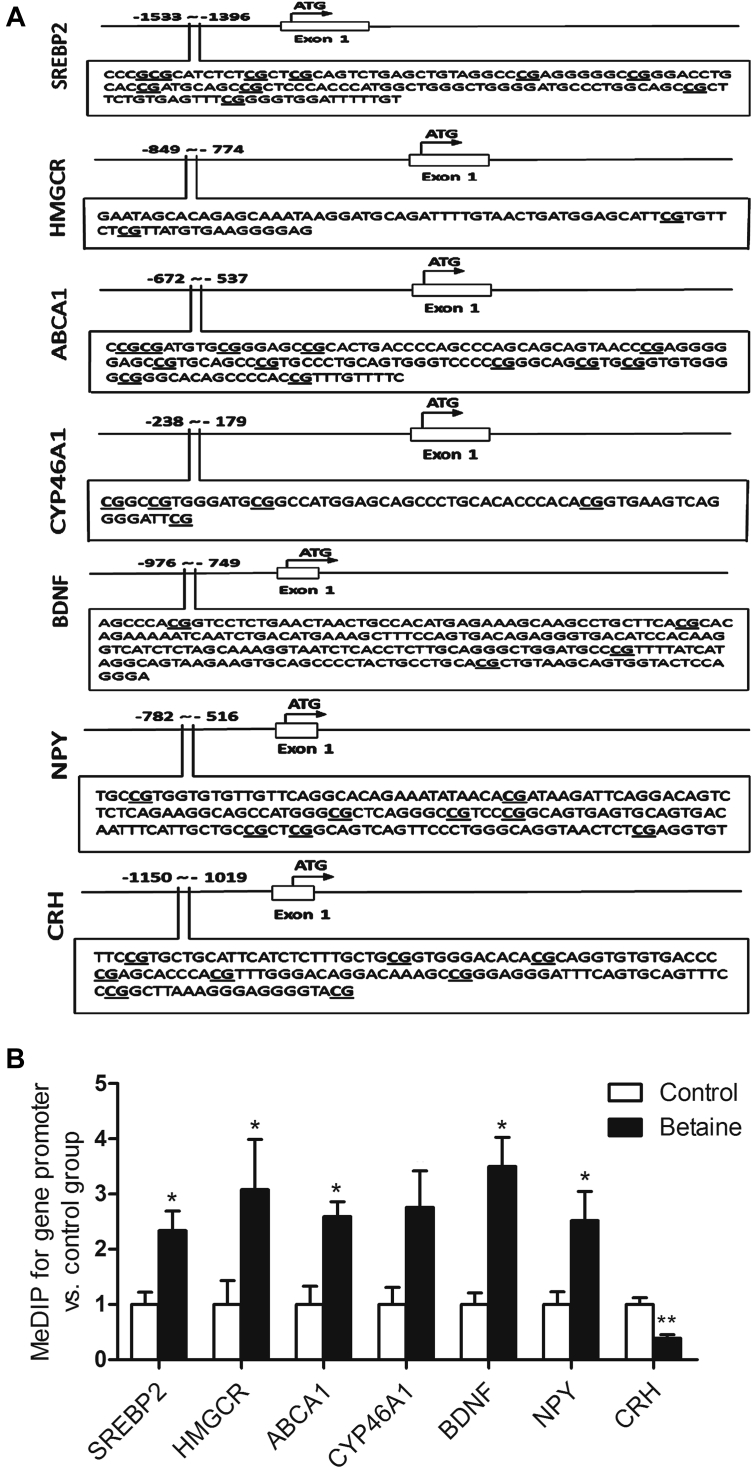
DNA Methylation Status on the Promoter of Modified Genes
The detected promoter regions of chicken SREBP2, HMGCR, BDNF, NPY, and CRH genes contain different numbers of CpG sites (Figure 4A). The methylation status of these regions was determined with MeDIP analysis (Figure 4B). As the figure shown, promoters of SREBP2, HMGCR, and ABCA1, as well as BDNF and NPY genes, were significantly hypermethylated (P < 0.05) in betaine-treated group, which coincided with lower mRNA abundances detected for these 5 genes. The detected CpG sites in the sequence of CYP46A1 promoter tended to be hypermethylated, which was associated with significant downregulation of CYP46A1 mRNA in betaine-treated group. However, CRH gene, which was significantly downregulated in mRNA level, was detected to show lower CpG methylation status in the promoter region in the hypothalamus of betaine-treated chickens.
Figure 4.
Methylation status on the promoter of cholesterol metabolism-related genes and neuropeptides in the hypothalamus of chickens for second generation (F2) in chicken. (A) Schematic diagram showing CpG sites among the promoter sequences of SREBP2, HMGCR, ABCA1, CYP46A1, BDNF, NPY and CRH genes, determined in present study. (B) Methylation ratio of determined sequences in present study on the promoter of SREBP2 (P = 0.04), HMGCR (P = 0.02), ABCA1 (P = 0.04), CYP46A1 (P = 0.053), BDNF (P = 0.01), NPY (P = 0.04) and CRH (P = 0.01) genes. Values are means ± SEM, *P < 0.05, **P < 0.01, compared with control (n = 4). BDNF, brain-derived neurotrophic factor; CRH, corticotropin-releasing hormone; SREBP2, sterol response element binding protein 2; HMGCR, 3-hydroxy-3-methyl-glutaryl-CoA reductase; CYP46A1, cholesterol 24-hydroxylase; ABCA1, ATP-binding cassette subfamily A member 1.
Discussion
Intergeneration programming is an impact of consequences of an environmental offense such as nutrient exposure and environmental stressors through the generations. Intergenerational effects are limited to those who are directly exposed to environmental stressor (Skinner 2008). While, transgenerations based on whether or not influence individuals in the latter generations are promptly exposed to the agents (Daxinger and Whitelaw, 2012). Transgenerational effects can be continuous into F2 generation, where the F0 mother experiences suboptimal diet throughout her pregnancy (Catherine et al., 2016). In this preliminary research, we showed first time in ovo administration of betaine associated with intergeneration for F1 offspring growth, as well as in other studies such as dietary supplementation with methyl donors reduced body weight in chicken (Paternain et al., 2016). Our results were agreed with betaine supplementation significantly decreasing body weight and total cholesterol in broilers (Hu et al., 2015). In addition, we elucidated that first effect of lower cholesterol content in the hypothalamus was related to the lower body weight, which was in agreement with recent finding that an increase level of methyl donor during prenatal stage significantly downregulated the body weight of offspring in female rat (Giudicelli et al., 2013). Other studies documented that methyl donor supplementation increased of fetus weight in rat (Oster et al., 2016) and birth weight in swine (Jia et al., 2015), because low maternal methyl donor associated with the decreased birth weight. Negative transgenerational effects about glucose metabolism in F1 and F2 offspring were reported in reduction of protein's diet model (Pinheiro et al., 2008). However, the influence effect of the multigenerational transmission of phenotypes was still unclear (Szyf, 2015). According to multiple interplay factors among environment, metabolism, and epigenetic, it was suggested to engage in such nongenetic transgenerational inheritance (Daxinger and Whitelaw, 2012). Recent publications had demonstrated that prenatal caffeine administration induced an augmented susceptibility to metabolic conditions with variances of lipid metabolic phenotypes in mature (F1) of intrauterine growth delaying rats and hypothalamic pituitary adrenal (HPA) axis associated with neuroendocrine metabolic programming (Luo et al., 2014).
Serum lipid is necessary index on lipid metabolism. In present research, we found that decreased concentration of total cholesterol and HDL-C in betaine-treated group, which was agreed with the other reported findings in chicken (Dixon et al., 2016, Leng et al., 2016). Cholesterol is synthesized de novo from acetyl CoA in the brain. Usually, cholesterol is transited from neurons throughout the embryogenesis to oligodendrocytes during postnatal myelination and to mainly astrocytes in the mature animal (Saher and Stumpf, 2015). Cholesterol homeostasis is highly related to the combination between astrocytes and neurons, in which astrocytes are main producers while neurons are responsible for cholesterol uptake, storage, turnover, and secretion (Pfrieger and Ungerer, 2011). In our current study, it was not possible to figure out brain cholesterol metabolism in cellular level. Here, we could only reveal the overall expression of cholesterol metabolic genes in the chicken hypothalamus. It was well understood that SREBP2 was a significant transcriptional factor that switched on the expression of HMGCR genes expression (Horton, 1998). We found SREBP2 and HMGCR were downregulated in both mRNA and protein levels in the hypothalamus of the betaine-exposed group, which appeared to be the direct cause for repressed transcription of HMGCR genes in betaine-treated chickens. Our result was in line with downregulation of SREBP2 of the betaine-exposed group in pig (Cai et al., 2014) and decreased HMGCR gene expression of betaine supplementation group in chicken (Leng et al., 2016). The downregulated mRNA and protein expression of ABCA1 and CYP46A1 might indicate lowering of sterol efflux and conversion which led to lower cholesterol content, which was agreed with downregulation of ABCA1 levels in neurons reduced effluxes of cholesterol (Minagawa et al., 2009).
Principally, SAM plays important role for epigenetic regulation because of support methyl indicator to assess the methylated status of general and specific genes (Caudill et al., 2001). We found augmented significant change of SAM contents and SAM/SAH ratio, which was in line with exogenous glycine-treated group increased levels of SAM and SAM/SAH and decreased SAH levels in brain of chick (Miller et al., 2006).
Brain-derived neurotrophic factor BDNF was released by terminals of cortical neuron in the stratum which was implicated not only in synaptic plasticity and cell survival but also in induction of cholesterol synthesis in postsynaptic neuron (Leoni and Caccia, 2015). In addition, the upregulation of BDNF against methylation status was agreed with the study that reported methionine supplementation was able to increase DNA methylation and reduce BDNF mRNA expression in rat brain (Parrish et al., 2015). Here, we found that addition of methyl donors, betaine, had a similar effect on DNA methylation status on BDNF promoters and the mRNA expression levels among the chicken offsprings. Even within more generations, the maternal effect of betaine on hypothalamic transcription of BDNF is similar to our previous work (Idriss et al., 2018). These findings, including associated with the modification of the cholesterol metabolism in chicken brain, could be a new insight of methyl donor effect on BDNF mRNA transcription.
Hypothalamus contains higher concentration of neuropeptides which regulates energy utilization via modulation of activated fat deposition and metabolism (Naveilhan et al., 1998). Our result showed that downregulation of NPY gene expression in betaine expose group might lead to lower levels of fat deposition regulating the lipid metabolism and energy intake. Additionally, data about NPY mRNA level was agreed with our results that suggested epigenetic modifications could contribute to the altered gene expression of the NPY and POMC in hypothalami leading to development of obesity in rats (Mahmood et al., 2013). Otherwise, a significant upregulation of NPY mRNA expression was observed in our previous chicken model (Idriss et al., 2018). The maternal effect of betaine on hypothalamic transcription of NPY was obviously reversed in the different generations.
Corticotropin releasing hormone is a stress high-related hormone released by neurosecretory cells in the hypothalamus (Cho et al., 2015). In many other studies, CRH was functional to suppress cholesterol efflux via decreased expression of ABCA1 (Cho et al., 2015). Based on our animal model, we hypothesis that CRH could contribute to the betaine transgenerational effect on brain cholesterol metabolism regulation. Epigenetic modification was observed in prenatal stress model, in which the CRH promoter in the amygdala of rat offspring was demethylated (Xu et al., 2014). Our result was inconsistent with of CRH mRNA, while agreed with increased CRH gene expression in those reported phenomena (Xu et al., 2014). According to our previous work (Idriss et al., 2018), the maternal effect of betaine on hypothalamic transcription of CRH, like BDNF, was sustained through generations. Otherwise, more experimental evidences are really needed to explain these interesting findings.
In ovo administration of betaine modified the expression of cholesterol metabolic genes in hypothalamus of chicken offspring. Meanwhile, the expression of one-carbon metabolic and methyl transfer genes was also modulated. Betaine homocysteine methyltransferase is a major enzyme that converts homocysteine to methionine using betaine as substrate (Pajares and Perez-Sala, 2006). Previous studies in our lab had revealed that BHMT could be downregulated with maternal betaine supplementation in pig (Cai et al., 2014). Here, we found the same pattern in chickens. Moreover, DNMT1, the major DNA methyltransferase responsible for the maintenance of CpG methylation status, was also activated in both mRNA and protein level in betaine treated group. The changes of DNMT1 in DNA methylation patterns could lead to global hypomethylation and regional hypermethylation, particularly in the promoter regions of certain genes (Kovacheva et al., 2007). In our result, we found an increase of DNMT1 but a decrease of DNMT3A under the condition of betaine treatment. Honestly, it remained a mystery how precisely happened in one-carbon cycle in the context of the present experiment.
Conclusion
Taken together, here we showed in a preliminary study that in ovo injection of betaine-modulated reduction of growth and cholesterol levels in the chicken F2. These phenotypic alterations were associated with modified expression of cholesterol homeostasis in the hypothalamus. Modified DNA methylation on the promoter of cholesterol metabolic and neuropeptide genes might contribute, at least in part, to the repression of cholesterol metabolic genes caused by prenatal betaine exposure. These results provided a new evidence for the offspring programming effects of betaine on the cholesterol homeostasis, as well as the brain development and function in hens.
Acknowledgments
The present study was supported by, the National Natural Science Foundation of China (31672512), Special Fund for Agro-scientific Research in the Public Interest (201003011), the Fundamental Research Funds for the Central Universities (KYZ201212), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Conflict of Interest Statement: The author did not provide any conflict of interest statement.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2019.12.040
Supplementary data
References
- Aiken C.E., Tarry-Adkins J.L., Penfold N.C., Dearden L., Ozanne S.E. Decreased ovarian reserve, dysregulation of mitochondrial biogenesis, and increased lipid peroxidation in female mouse offspring exposed to an obesogenic maternal diet. FASEB J. 2016;30:1548–1556. doi: 10.1096/fj.15-280800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship K., Gilley A., Piekarski A., Orlowski S., Greene E., Bottje W., Anthony N., Dridi S. Differential expression of feeding-related hypothalamic neuropeptides in the first generation of quails divergently selected for low or high feed efficiency. Neuropeptides. 2016;58:31–40. doi: 10.1016/j.npep.2015.12.007. [DOI] [PubMed] [Google Scholar]
- Braunschweig M., Jagannathan V., Gutzwiller A., Bee G. Investigations on transgenerational epigenetic response down the male line in F2 pigs. PLoS One. 2012;7:e30583. doi: 10.1371/journal.pone.0030583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D., Jia Y., Lu J., Yuan M., Sui S., Song H., Zhao R. Maternal dietary betaine supplementation modifies hepatic expression of cholesterol metabolic genes via epigenetic mechanisms in newborn piglets. Br. J. Nutr. 2014;112:1459–1468. doi: 10.1017/S0007114514002402. [DOI] [PubMed] [Google Scholar]
- Catherine N.L., Gonzalez A., Boyle M., Sheehan D., Jack S.M., Hougham K.A., McCandless L., MacMillan H.L., Waddell C. Improving children's health and development in British Columbia through nurse home visiting: a randomized controlled trial protocol. BMC Health Serv. Res. 2016;16:349. doi: 10.1186/s12913-016-1594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudill M.A., Wang J.C., Melnyk S., Pogribny I.P., Jernigan S., Collins M.D., Santos-Guzman J., Swendseid M.E., Cogger E.A., James S.J. Intracellular S-adenosylhomocysteine concentrations predict global DNA hypomethylation in tissues of methyl-deficient cystathionine beta-synthase heterozygous mice. J. Nutr. 2001;131:2811–2818. doi: 10.1093/jn/131.11.2811. [DOI] [PubMed] [Google Scholar]
- Cho W., Kang J.L., Park Y.M. Corticotropin-releasing hormone (CRH) promotes Macrophage Foam cell formation via reduced expression of ATP binding Cassette Transporter-1 (ABCA1) PLoS One. 2015;10:e0130587. doi: 10.1371/journal.pone.0130587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daxinger L., Whitelaw E. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat. Rev. Genet. 2012;13:153–162. doi: 10.1038/nrg3188. [DOI] [PubMed] [Google Scholar]
- Dixon L.M., Sparks N.H., Rutherford K.M. Early experiences matter: a review of the effects of prenatal environment on offspring characteristics in poultry. Poult. Sci. 2016;95:489–499. doi: 10.3382/ps/pev343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui K., Ferris H.A., Kahn C.R. Effect of cholesterol reduction on receptor signaling in neurons. J. Biol. Chem. 2015;290:26383–26392. doi: 10.1074/jbc.M115.664367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelissen I.C., Harris M., Rye K.A., Quinn C., Brown A.J., Kockx M., Cartland S., Packianathan M., Kritharides L., Jessup W. ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arterioscler., Thromb., Vasc. Biol. 2006;26:534–540. doi: 10.1161/01.ATV.0000200082.58536.e1. [DOI] [PubMed] [Google Scholar]
- Giudicelli F., Brabant A.L., Grit I., Parnet P., Amarger V. Excess of methyl donor in the perinatal period reduces postnatal leptin secretion in rat and interacts with the effect of protein content in diet. PLoS One. 2013;8:e68268. doi: 10.1371/journal.pone.0068268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goritz C., Mauch D.H., Pfrieger F.W. Multiple mechanisms mediate cholesterol-induced synaptogenesis in a CNS neuron. Mol. Cell. Neurosci. 2005;29:190–201. doi: 10.1016/j.mcn.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Goyal S., Xiao Y., Porter N.A., Xu L., Guengerich F.P. Oxidation of 7-dehydrocholesterol and desmosterol by human cytochrome P450 46A1. J. Lipid Res. 2014;55:1933–1943. doi: 10.1194/jlr.M051508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussmann M.F., Longenecker A.S., Marchetto N.M., Juliano S.A., Bowden R.M. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc. Biol. Sci. 2012;279:1447–1456. doi: 10.1098/rspb.2011.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoile S.P., Lillycrop K.A., Thomas N.A., Hanson M.A., Burdge G.C. Dietary protein restriction during F0 pregnancy in rats induces transgenerational changes in the hepatic transcriptome in female offspring. PLoS One. 2011;6:e21668. doi: 10.1371/journal.pone.0021668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton A.M., Jr. Development of a short-form screening index for severity of brain damage in older children. Appl. Neuropsychol. 1998;5:48–50. doi: 10.1207/s15324826an0501_7. [DOI] [PubMed] [Google Scholar]
- Hu Y., Sun Q., Li X., Wang M., Cai D., Zhao R. In Ovo injection of betaine affects hepatic cholesterol metabolism through epigenetic gene regulation in newly hatched chicks. PLoS One. 2015;10:e0122643. doi: 10.1371/journal.pone.0122643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idriss A.A., Hu Y., Sun Q., Jia L., Jia Y., Omer N.A., Abobaker H., Zhao R. Prenatal betaine exposure modulates hypothalamic expression of cholesterol metabolic genes in cockerels through modifications of DNA methylation. Poult. Sci. 2017;96:1715–1724. doi: 10.3382/ps/pew437. [DOI] [PubMed] [Google Scholar]
- Idriss A.A., Hu Y., Hou Z., Hu Y., Sun Q., Omer N.A., Abobaker H., Ni Y., Zhao R. Dietary betaine supplementation in hens modulates hypothalamic expression of cholesterol metabolic genes in F1 cockerels through modification of DNA methylation. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 2018;217:14–20. doi: 10.1016/j.cbpb.2017.12.001. [DOI] [PubMed] [Google Scholar]
- Jia A.F., Feng J.H., Zhang M.H., Chang Y., Li Z.Y., Hu C.H., Zhen L., Zhang S.S., Peng Q.Q. Effects of immunological challenge induced by lipopolysaccharide on skeletal muscle fiber type conversion of piglets. J. Anim. Sci. 2015;93:5194–5203. doi: 10.2527/jas.2015-9391. [DOI] [PubMed] [Google Scholar]
- Kalra S.P., Dube M.G., Sahu A., Phelps C.P., Kalra P.S. Neuropeptide Y secretion increases in the paraventricular nucleus in association with increased appetite for food. Proc. Natl. Acad. Sci. U. S. A. 1991;88:10931–10935. doi: 10.1073/pnas.88.23.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.S., Shigeoka C., Takahara Y., Matsuda S., Tachibana T. Ontogeny of the corticotrophin-releasing hormone system in slow- and fast-growing chicks (Gallus gallus) Physiol. Behav. 2015;151:38–45. doi: 10.1016/j.physbeh.2015.06.033. [DOI] [PubMed] [Google Scholar]
- Kovacheva V.P., Mellott T.J., Davison J.M., Wagner N., Lopez-Coviella I., Schnitzler A.C., Blusztajn J.K. Gestational choline deficiency causes global and Igf2 gene DNA hypermethylation by up-regulation of Dnmt1 expression. J. Biol. Chem. 2007;282:31777–31788. doi: 10.1074/jbc.M705539200. [DOI] [PubMed] [Google Scholar]
- Kulis M., Heath S., Bibikova M., Queiros A.C., Navarro A., Clot G., Martinez-Trillos A., Castellano G., Brun-Heath I., Pinyol M., Barberan-Soler S., Papasaikas P., Jares P., Bea S., Rico D., Ecker S., Rubio M., Royo R., Ho V., Klotzle B., Hernandez L., Conde L., Lopez-Guerra M., Colomer D., Villamor N., Aymerich M., Rozman M., Bayes M., Gut M., Gelpi J.L., Orozco M., Fan J.B., Quesada V., Puente X.S., Pisano D.G., Valencia A., Lopez-Guillermo A., Gut I., Lopez-Otin C., Campo E., Martin-Subero J.I. Epigenomic analysis detects widespread gene-body DNA hypomethylation in chronic lymphocytic leukemia. Nat. Genet. 2012;44:1236–1242. doi: 10.1038/ng.2443. [DOI] [PubMed] [Google Scholar]
- Lange Y., Ye J., Strebel F. Movement of 25-hydroxycholesterol from the plasma membrane to the rough endoplasmic reticulum in cultured hepatoma cells. J. Lipid Res. 1995;36:1092–1097. [PubMed] [Google Scholar]
- Leng Z., Fu Q., Yang X., Ding L., Wen C., Zhou Y. Increased fatty acid beta-oxidation as a possible mechanism for fat-reducing effect of betaine in broilers. Anim. Sci. J. 2016;87:1005–1010. doi: 10.1111/asj.12524. [DOI] [PubMed] [Google Scholar]
- Leoni V., Caccia C. The impairment of cholesterol metabolism in Huntington disease. Biochim. Biophys. Acta. 2015;1851:1095–1105. doi: 10.1016/j.bbalip.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Lever M., George P.M., Slow S., Elmslie J.L., Shand B.I., Scott R.S., Chambers S.T. Fibrates plus betaine: a winning combination? N. Z. Med. J. 2010;123:74–78. [PubMed] [Google Scholar]
- Linetti A., Fratangeli A., Taverna E., Valnegri P., Francolini M., Cappello V., Matteoli M., Passafaro M., Rosa P. Cholesterol reduction impairs exocytosis of synaptic vesicles. J. Cell Sci. 2010;123:595–605. doi: 10.1242/jcs.060681. [DOI] [PubMed] [Google Scholar]
- Luo H., Deng Z., Liu L., Shen L., Kou H., He Z., Ping J., Xu D., Ma L., Chen L., Wang H. Prenatal caffeine ingestion induces transgenerational neuroendocrine metabolic programming alteration in second generation rats. Toxicol. App. Pharmacol. 2014;274:383–392. doi: 10.1016/j.taap.2013.11.020. [DOI] [PubMed] [Google Scholar]
- Mahmood S., Smiraglia D.J., Srinivasan M., Patel M.S. Epigenetic changes in hypothalamic appetite regulatory genes may underlie the developmental programming for obesity in rat neonates subjected to a high-carbohydrate dietary modification. J. Dev. Orig. Health Dis. 2013;4:479–490. doi: 10.1017/S2040174413000238. [DOI] [PubMed] [Google Scholar]
- Meaney S., Bodin K., Diczfalusy U., Bjorkhem I. On the rate of translocation in vitro and kinetics in vivo of the major oxysterols in human circulation: critical importance of the position of the oxygen function. J. Lipid Res. 2002;43:2130–2135. doi: 10.1194/jlr.m200293-jlr200. [DOI] [PubMed] [Google Scholar]
- Miller R.R., Jr., Hay C.M., Striegnitz T.R., Honsey L.E., Coykendall C.E., Blacquiere K.D. Exogenous glycine partially attenuates homocysteine-induced apoptosis and membrane peroxidation in chick embryos. Comparative biochemistry and physiology. Toxicol. Pharmacol.: CBP. 2006;144:25–33. doi: 10.1016/j.cbpc.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Minagawa H., Gong J.S., Jung C.G., Watanabe A., Lund-Katz S., Phillips M.C., Saito H., Michikawa M. Mechanism underlying apolipoprotein E (ApoE) isoform-dependent lipid efflux from neural cells in culture. J. Neurosci. Res. 2009;87:2498–2508. doi: 10.1002/jnr.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd S.H., Wagner C., Luka Z., Stabler S.P., Allen R.H., Schroer R., Wood T., Wang J., Wong L.J. Two patients with hepatic mtDNA depletion syndromes and marked elevations of S-adenosylmethionine and methionine. Mol. Genet. Metab. 2012;105:228–236. doi: 10.1016/j.ymgme.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseem S.M., Heald F.P. Cytotoxicity of cholesterol oxides and their effects on cholesterol metabolism in cultured human aortic smooth muscle cells. Biochem. Int. 1987;14:71–84. [PubMed] [Google Scholar]
- Naveilhan P., Neveu I., Arenas E., Ernfors P. Complementary and overlapping expression of Y1, Y2 and Y5 receptors in the developing and adult mouse nervous system. Neuroscience. 1998;87:289–302. doi: 10.1016/s0306-4522(98)00141-9. [DOI] [PubMed] [Google Scholar]
- Oster M., Nuchchanart W., Trakooljul N., Murani E., Zeyner A., Wirthgen E., Hoeflich A., Ponsuksili S., Wimmers K. Methylating micronutrient supplementation during pregnancy influences foetal hepatic gene expression and IGF signalling and increases foetal weight. Eur. J. Nutr. 2016;55:1717–1727. doi: 10.1007/s00394-015-0990-2. [DOI] [PubMed] [Google Scholar]
- Pajares M.A., Perez-Sala D. Betaine homocysteine S-methyltransferase: just a regulator of homocysteine metabolism? Cell Mol. Life Sci. 2006;63:2792–2803. doi: 10.1007/s00018-006-6249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish R.R., Buckingham S.C., Mascia K.L., Johnson J.J., Matyjasik M.M., Lockhart R.M., Lubin F.D. Methionine increases BDNF DNA methylation and improves memory in epilepsy. Ann. Clin. Transl. Neurol. 2015;2:401–416. doi: 10.1002/acn3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternain L., Martisova E., Campion J., Martinez J.A., Ramirez M.J., Milagro F.I. Methyl donor supplementation in rats reverses the deleterious effect of maternal separation on depression-like behaviour. Behav. Brain Res. 2016;299:51–58. doi: 10.1016/j.bbr.2015.11.031. [DOI] [PubMed] [Google Scholar]
- Petrov A.M., Kasimov M.R., Zefirov A.L. Brain cholesterol metabolism and its Defects: Linkage to neurodegenerative diseases and synaptic Dysfunction. Acta Naturae. 2016;8:58–73. [PMC free article] [PubMed] [Google Scholar]
- Petrov D., Luque M., Pedros I., Ettcheto M., Abad S., Pallas M., Verdaguer E., Auladell C., Folch J., Camins A. Evaluation of the role of JNK1 in the Hippocampus in an experimental model of Familial Alzheimer's disease. Mol. Neurobiol. 2016;53:6183–6193. doi: 10.1007/s12035-015-9522-6. [DOI] [PubMed] [Google Scholar]
- Pfrieger F.W., Ungerer N. Cholesterol metabolism in neurons and astrocytes. Prog. Lipid Res. 2011;50:357–371. doi: 10.1016/j.plipres.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Pinheiro A.R., Salvucci I.D., Aguila M.B., Mandarim-de-Lacerda C.A. Protein restriction during gestation and/or lactation causes adverse transgenerational effects on biometry and glucose metabolism in F1 and F2 progenies of rats. Clin. Sci. (Lond) 2008;114:381–392. doi: 10.1042/CS20070302. [DOI] [PubMed] [Google Scholar]
- Saher G., Stumpf S.K. Cholesterol in myelin biogenesis and hypomyelinating disorders. Biochim. Biophys. Acta. 2015;1851:1083–1094. doi: 10.1016/j.bbalip.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Sharpe L.J., Brown A.J. Controlling cholesterol synthesis beyond 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) J. Biol. Chem. 2013;288:18707–18715. doi: 10.1074/jbc.R113.479808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She Q.B., Nagao I., Hayakawa T., Tsuge H. A simple HPLC method for the determination of S-adenosylmethionine and S-adenosylhomocysteine in rat tissues: the effect of vitamin B6 deficiency on these concentrations in rat liver. Biochem. Biophys. Res. Commun. 1994;205:1748–1754. doi: 10.1006/bbrc.1994.2871. [DOI] [PubMed] [Google Scholar]
- Skinner M.K. What is an epigenetic transgenerational phenotype? F3 or F2. Reprod. Toxicol. 2008;25:2–6. doi: 10.1016/j.reprotox.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R., Lee K., Jing E., Biddinger S.B., McDonald J.G., Montine T.J., Craft S., Kahn C.R. Diabetes and insulin in regulation of brain cholesterol metabolism. Cell Metab. 2010;12:567–579. doi: 10.1016/j.cmet.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyf M. Nongenetic inheritance and transgenerational epigenetics. Trends Mol. Med. 2015;21:134–144. doi: 10.1016/j.molmed.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Vaughan A.M., Tang C., Oram J.F. ABCA1 mutants reveal an interdependency between lipid export function, apoA-I binding activity, and Janus kinase 2 activation. J. Lipid Res. 2009;50:285–292. doi: 10.1194/jlr.M800366-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Li X.J., Hu Y.S., Cheng Q., Wang C.F., Xiao Q., Liu J., Ma J.F., Zhou H.Y., Pan J., Tan Y.Y., Wang Y., Chen S.D. Mortality from Parkinson's disease in China: findings from a five-year follow up study in Shanghai. The Canadian journal of neurological sciences. Can J. Neurol. Sci. 2015;42:242–247. doi: 10.1017/cjn.2015.49. [DOI] [PubMed] [Google Scholar]
- Xu L., Sun Y., Gao L., Cai Y.Y., Shi S.X. Prenatal restraint stress is associated with demethylation of corticotrophin releasing hormone (CRH) promoter and enhances CRH transcriptional responses to stress in adolescent rats. Neurochem. Res. 2014;39:1193–1198. doi: 10.1007/s11064-014-1296-0. [DOI] [PubMed] [Google Scholar]
- Yuan L., Ni Y., Barth S., Wang Y., Grossmann R., Zhao R. Layer and broiler chicks exhibit similar hypothalamic expression of orexigenic neuropeptides but distinct expression of genes related to energy homeostasis and obesity. Brain Res. 2009;1273:18–28. doi: 10.1016/j.brainres.2009.03.052. [DOI] [PubMed] [Google Scholar]
- Zhang J., Liu Q. Cholesterol metabolism and homeostasis in the brain. Protein Cell. 2015;6:254–264. doi: 10.1007/s13238-014-0131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Bai S., Liu D., Cline M.A., Gilbert E.R. Neuropeptide Y promotes adipogenesis in chicken adipose cells in vitro. Comp. Biochem. Physiol. A, Mol. Integr. Physiol. 2015;181:62–70. doi: 10.1016/j.cbpa.2014.11.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.