Abstract
This study was conducted to determine the effects of diet with different proportions of ryegrass on breast meat quality of geese. In total, 240 healthy male Yangzhou geese (28-day-old) with similar body weight were divided randomly into 4 diet groups (control group: fed commercial diets; treatment groups I, II, and III: fed ryegrass and commercial diet in the ratios of 1.5:1, 2:1, and 3:1, respectively), the birds being fed from the age of 29 to 70 D. The results shows that the body weights of 70-day-old geese of treatment groups II and III were lower than those in the control group, whereas those of geese of treatment group I were similar to those of the control group. The contents of flavor amino acid and total (essential) amino acids in treatment groups I and II were higher than those in treatment group III (P < 0.05). In addition, grass supplementation reduced saturated fatty acid content and increased that of omega-3 (n-3) polyunsaturated fatty acids, relative to the control group (P < 0.05). Finally, among the 6 minerals analyzed in breast muscle, differences existed in Zn, Se, and Cu contents among the geese fed with different proportions of ryegrass. Zn content of geese from treatment groups II and III was significantly higher than that of those of the control group; Cu content was lower with grass intake and was significantly higher in the control group than in treatment group III; Se content was significantly higher in the control group than in both groups II and III (all at P < 0.05). The results from this study indicated that geese fed with low proportions of ryegrass (1.5:1 or 2:1) showed good growth performance and increased total (essential) amino acid, flavor amino acid, n-3 polyunsaturated fatty acid, and Zn content in meat, which had a certain guiding value for the production of high-quality goose meat under intensive feeding conditions.
Key words: goose, meat quality, ryegrass, nutrient composition
Introduction
Goose (Anas cygnoides) is a traditional grazing animal that is farmed for its nutritious meat for human consumption (Schwartz, 2012). Goose meat contains high-quality protein, low cholesterol (52–76 mg/100 g), and high concentrations of polyunsaturated fatty acid (PUFA) (Cui et al., 2015). Traditionally, small-scale farming of broiler geese involves feeding with bulky grass that reduces growth performance; these feeding methods have become insufficient to meet the increasing demands for goose meat (Wood et al., 2011). However, meat from geese that are not pasture-fed tends to contain fewer nutrients than that of geese supplemented with pasture feeding (Liu et al., 2013). Therefore, commercial diets supplemented with grass can be adopted in intensive breeding systems to meet the increasing demand for goose meat.
Amino acids are central to the nutritional value of meat because they are the main constituents of proteins (Okruszek et al., 2013). High-protein feed can improve amino acid levels during meat production (Guo et al., 2018). The human health effects of the fatty acid composition of meat are controversial (Orellana et al., 2009). Saturated fatty acids (SFA) can elevate the level of plasma cholesterol, which is a risk factor for atherosclerosis (Velasco et al., 2001). In contrast, PUFA are of benefit to human health and well-being, owing to their ability to reduce the risk of thrombosis and arteriosclerosis (Mushi et al., 2010). Therefore, it is desirable to produce meat with a more favorable PUFA-to-SFA ratio. Furthermore, owing to the presence of linolenic acid (C18:3n-3) in grass, the meat of grazers contains relatively high concentrations of omega-3 (n-3) unsaturated fatty acids (O'Sullivan et al., 2004).
In contrast to fatty acid composition, the role of cholesterol is unambiguous. Meat contains cholesterol, which is essential for human health and is a major constituent of animal cells (Chizzolini et al., 1999). Furthermore, various mineral elements are vital nutrients, especially iron, which is associated with an increased livery flavor of meat (Yancey et al., 2006). Selenium plays an important role in several functions including antioxidation, maintaining the immune system, resisting radiation, and promoting growth (Rayman, 2000).
Ryegrass belongs to the family Gramineae, which is also one of the high-quality forage for dairy cattle, beef cattle, sheep, and geese because of its high yield, rich nutrition, and good palatability (Wang et al., 2015). Adding a small amount of ryegrass during the feeding process can reduce carcass fat deposition in geese (Yu et al., 1998). In addition, ryegrass meal pellet feed increases intestinal length and cecum weight in geese (Zhan et al., 2015). However, there are few studies that assessed the meat quality of geese by adding ryegrass in diet.
Therefore, the aim of this study was to elucidate the influence of ryegrass content on body weight (BW) and meat nutrient composition, including the amino acid, fatty acid, and cholesterol content and mineral composition of the breast meat. Our aim is to provide theoretical support for adding the optimum proportion of ryegrass to diet under intensive farming conditions.
Materials and methods
Animals and Experimental Design
All experimental animals were approved by the Institutional Animal Care and Use Committee of the Chinese Academy of Agricultural Sciences, and best effort was taken to minimize the suffering of the animals as per recommendations suggested by the European Commission (Howe, 1997). All the experimental methods were implemented in accordance with the guidelines. A total of 300 male Yangzhou goslings were raised in Yangzhou Tiange goose industry Co. Ltd. (Yangzhou, China), and then, 240 healthy birds of similar BW (775.9 ± 105.78 g; 28-day-old) were randomly divided into 4 groups (control group: fed commercial diets; treatment groups I, II, and III: fed ryegrass and commercial diets in the ratio of 1.5:1, 2:1, and 3:1, respectively), the birds being fed from the age of 29 to 70 D (Table 1). During the experiment, the geese were kept in pens (5 individuals per square meter) that included a playground and pool. The feed and water were given during daytime when the geese were released into an open area outside the house. The geese were exposed to natural lighting and temperature throughout this study. The ingredient and chemical composition of the commercial diets are shown in Table 2. After 12 h of starvation at the age of 70 D, all geese were weighed individually using an electronic scale (CHS-D, Anheng Inc., Shenzhen, China; sensitivity: 1 g), and five geese per group (with BW closest to mean per group weight) were selected. Then, birds were sacrificed by manual exsanguination immediately by anesthetizing them with sodium pentobarbital, and breast meat was taken from the left side of the carcasses after being transferred to the refrigerator and stored at −20°C for further analyses.
Table 1.
Ratio of ryegrass to concentrate for each group.
| Age | Control | Group I | Group II | Group III |
|---|---|---|---|---|
| From 29- to 70-day-old | Commercial diets | Ryegrass:commercial diets = 1.5:1 | Ryegrass:commercial diets = 2:1 | Ryegrass:commercial diets = 3:1 |
Table 2.
Ingredients and nutrient levels of the commercial diet.
| Items | Content |
|---|---|
| Ingredients, % | |
| Corn | 56.0 |
| Soybean meal | 21.0 |
| Wheat bran | 15.0 |
| Premix | 5.0 |
| Bone meal | 3.0 |
| Nutrient levels | |
| Crude protein, % | 17.2 |
| Crude fat, % | 3.7 |
| Crude fiber, % | 5.3 |
| Ca, g/kg | 10.7 |
| Total P, g/kg | 4.8 |
| Lys, g/kg | 7.6 |
| Met, g/kg | 4.4 |
| Apparent ME, MJ/kg | 10.46 |
Premix provided per kilogram of diet: vitamin A, 2000 IU; vitamin D3, 45000U; vitamin E, 300IU; vitamin K3, 20 mg; vitamin B1, 10 mg; vitamin B2, 120 mg; vitamin B6, 20 mg; nicotinic acid, 600 mg; pantothenic acid, 180 mg; folic acid, 10 mg; choline, 7 g; Fe, 1.2 g; Cu, 0.2 g; Mn, 1.9 g; Zn, 1.8 g; I, 10 mg; Se, 6 m.
Amino Acid Analysis
The goose breast samples (2 g) were placed in 20-mL hydrolysis tubes with 16 mL of 6 mol/L HCl. The samples were then subjected to vacuum degassing for 30 min, and the tubes were filled with nitrogen and sealed. The samples were hydrolyzed at 110°C for 24 h, cooled, and transferred nondestructively to 50-mL capacity bottles with deionized water. Next, 1 mL of the hydrolysate was extracted, acids were removed under vacuum, and the samples were dried. Thereafter, 1 mL of water was added, and the tubes were pumped dry, followed by the addition of further 1 mL of water and draining. One microliter of 0.02 mol/L HCl was added, and the samples were allowed to dissolve fully; thereafter, the samples were sifted through a 0.22-μm water-based membrane and separated using an amino acid automatic analyzer (Hitachi L-8900, Hitachi, Tokyo, Japan).
Chromatography was performed at a sampling volume of 20 μL on a 2622#PH ion-exchange column (4.5 mm × 60 mm; Thermo Fisher Scientific, Waltham, MA) at a column temperature of 57°C. Postcolumn derivatization was performed at 135°C, and the flow rate was 0.35 mL/min for the derivative reagent and 0.4 mL/min for the buffer.
Determination of Amino Acid Composition
The amino acid composition of samples is an important index for evaluating protein content. The amino acid score (AAS) was calculated using the formula provided by the Food and Agriculture Organization (FAO)/World Health Organization (WHO) (Eggum, 1991) guidelines. The reference scoring pattern was obtained using the following equation:
The essential amino acid index (EAAI) showed the geometric mean of the ratio of the essential amino acids (EAA) in breast meat proteins relative to the amount in the protein pattern (Okruszek et al., 2013) as follows:
where n is the number of amino acids, p is the concentration of breast meat protein, and s is the pattern protein.
Fatty Acid Analysis
After extracting crude fat from the muscles, 8 mL of a sodium hydroxide methanol solution and defatted zeolite were added. A condensation tube was fixed to the flask containing the sample until the oil droplets disappeared. Reflux speed was controlled at 45 s/drop for 8 min. A proper amount of boron trifluoride–methanol solution was added to the upper part of the condensation tube using a pipette or an automatic liquid feeder. The solution was then boiled for 3 min. Thereafter, a proper amount of isooctane was added to the upper part of the condensation tube. Then, heating was stopped, the condensation tube was removed, and 20 mL of saturated NaCl solution was immediately added before the flask was allowed to cool. The solution was then vigorously shaken for 20 s while adding more saturated NaCl solution to the neck of the flask. Next, 2 mL of the upper isooctane layer was transferred into the test tube and dehydrated by adding an appropriate amount of anhydrous sodium sulfate. The solution was then filtered using a 0.45-μm filter membrane for liquid chromatography–mass spectrometry (MS) analysis (Trace 1310 ISQ, Thermo Fisher Scientific).
Chromatography was performed using a TG-5MS column (30 m × 0.25 mm × 0.25 μm; Thermo Fisher Scientific). The temperature of the solution was maintained at 80°C for 1 min, increased to 200°C at a rate of 10°C/min, and then further increased at a rate of 5°C/min. When the sample temperature reached 250°C, the temperature was increased to 270°C at a rate of 2°C/min and maintained for 3 min. The sample inlet temperature was 290°C, and the carrier flow rate was 1.2 mL/min. For nondiversion injection, the valve opening time was 1 min. Mass spectrometry was performed at an ion source temperature of 280°C and a transmission line temperature of 280°C with a solvent delay time of 5 min.
Cholesterol Analysis
To determine the cholesterol content, 1 g (accurate to 0.001 g) of the sample was weighed and placed in a 50-mL plugged test tube. Next, 10 mL of potassium hydroxide solution (1.0 mol/mL) and 10 mL of absolute ethanol were added, and the solution was mixed thoroughly. The samples were added to a condensation tube and saponified for 1 h at 90°C until the solution was clear. Then, the solution was transferred into a 50-mL separating funnel, and 10 mL of ether was added to the separating funnel. The funnel was shaken gently, and the water layer was poured into the plugged test tube. Next, 10 mL of ether was added into the plugged test tube, which was gently shaken, and the ether layer was transferred to the separating funnel. Thereafter, 10 mL of ether was extracted in the plugged test tube, and the ether layer was moved into a separating funnel. The solution in the separating funnel was washed three times with 15 mL of water. After stratification, the aqueous layer was discarded. The ether layer was dried with 20 g of anhydrous sodium sulfate, and then transferred to a fresh plug tube. After drying with nitrogen, the solution was dissolved in 1 mL of anhydrous ethanol and passed through a 0.45-μm filter membrane for gas chromatography–MS analysis (Agilent 7890a, NYSE: A, Agilent Technologies, Santa Clara, CA).
Mineral Analysis
The content of zinc, iron, magnesium, copper, calcium, and selenium was analyzed in this study. Breast meat samples of approximately 2 g (accurate to 0.001 g) were cut and placed in a digestion tube, and perchloric acid was added. Next, 30 mL of concentrated nitric acid (perchloric acid-to-concentrated nitric acid ratio = 1:4) was added, and the samples were covered. The samples were allowed to digest for 2 h, placed on a heating plate at 120°C for 2 h, and then allowed to cool naturally. After cooling, the tubes were filled to a volume of 50 mL with ultrapure water and incubated for 15 min. Next, 10 mL of solution was measured using an atomic absorption spectrophotometer (PerkinElmer Optima 7300 V ICP, PerkinElmer, Waltham, MA).
Statistical Analysis
Statistical analysis was conducted by one-way analysis of variance using SPSS 19.0 software (SPSS, Inc., Chicago, IL). Differences were considered significant at a value of P < 0.05, and the means within each group were compared using Duncan's test.
Results
Growth Performance
Goose BW was affected by the addition of ryegrass in different proportions. At the age of 70 D, BW was lower in groups that had more grass in their diet (Figure 1). BW did not differ significantly between the control group and group I, but was significantly higher in the control group than in groups II and III (P < 0.05).
Figure 1.
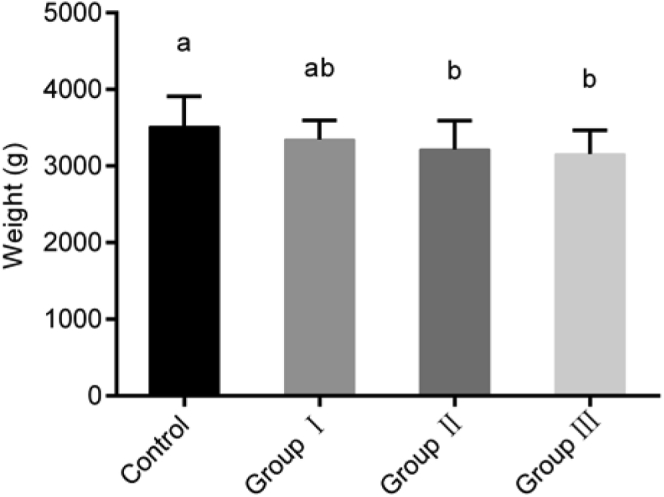
Body weight of Yangzhou geese (70-day-old). The y-axis represents the body weight; the x-axis represents the 4 groups. All values are expressed as means ± SD. Lacking the same letters (a, b) means differences at P < 0.05.
Amino Acid Composition
The amino acid composition of the breast meat is presented in Table 3. The different diets affected the proportions of all amino acids in the meat. In terms of nonessential amino acids, Glu was present in the highest concentration in group II, and this level differed significantly from the levels in the control and other groups (P < 0.05); Glu levels were higher in the control group and group I than in group III (P < 0.05). Asp, which showed the lowest concentration in the control group, differed significantly from that in groups I, II, and III (P < 0.05), and the level in group II was higher than in group III (P < 0.05). Arg, Pro, Ala, and Ser were found in higher concentrations in groups I and II than in group III (P < 0.05), and the level was higher in the control group than in group III (P < 0.05). Gly content did not differ significantly among the 4 groups. In terms of EAA, most amino acids (Leu, Lys, Val, Phe, Ile, Tyr, Thr, and Met) were in lower concentrations in group III than in the other 3 groups (P < 0.05). The concentration of His was lower in the control group and group III than in groups I and II (P < 0.05). Cys was present in the highest concentration in groups I and II, significantly higher than in the control group and group III; its level in the control group was higher than that in group III (P < 0.05). In addition, the concentration of total EAA was higher in the control group and in groups I and II than in group III (P < 0.05). The concentration of total flavor amino acids (FAA) was higher in groups I and II than in the control group and group III (P < 0.05). There were significant differences in the concentration of total amino acids (TAA) between all 4 groups, in the descending order of group II, group I, control group, and group III (P < 0.05).
Table 3.
Amounts of amino acids (±SD) in breast meat (g/100 g of meat).
| Amino acid | Control | Group I | Group II | Group III | P value |
|---|---|---|---|---|---|
| Nonessential amino acid | |||||
| Glu | 3.23 ± 0.15b | 3.21 ± 0.13b | 3.51 ± 0.05a | 2.83 ± 0.02c | <0.01 |
| Asp | 1.46 ± 0.10c | 1.84 ± 0.05a,b | 1.90 ± 0.02a | 1.76 ± 0.03b | <0.01 |
| Arg | 1.39 ± 0.03b | 1.41 ± 0.03a,b | 1.43 ± 0.01a | 1.29 ± 0.01c | <0.01 |
| Pro | 0.70 ± 0.04a | 0.72 ± 0.05a | 0.75 ± 0.03a | 0.51 ± 0.01c | <0.01 |
| Ala | 1.21 ± 0.03b | 1.22 ± 0.05a,b | 1.27 ± 0.03a | 1.15 ± 0.02c | 0.003 |
| Ser | 0.81 ± 0.04b | 0.83 ± 0.02a,b | 0.85 ± 0.01a | 0.74 ± 0.01c | <0.01 |
| Gly | 0.94 ± 0.07 | 1.00 ± 0.09 | 1.02 ± 0.07 | 0.94 ± 0.05 | 0.318 |
| Essential amino acid | |||||
| Leu | 1.72 ± 0.07a | 1.70 ± 0.06a | 1.73 ± 0.03a | 1.58 ± 0.03b | 0.005 |
| Lys | 1.86 ± 0.07a | 1.86 ± 0.06a | 1.91 ± 0.03a | 1.72 ± 0.01b | 0.001 |
| Val | 0.99 ± 0.05a | 0.96 ± 0.03a | 0.97 ± 0.01a | 0.90 ± 0.01b | 0.004 |
| Phe | 1.01 ± 0.10a | 0.98 ± 0.05a | 0.99 ± 0.02a | 0.83 ± 0.01b | 0.003 |
| Ile | 0.97 ± 0.04a | 0.98 ± 0.03a | 1.01 ± 0.02a | 0.92 ± 0.02b | 0.007 |
| Tyr | 0.90 ± 0.09a | 0.89 ± 0.04a | 0.89 ± 0.02a | 0.74 ± 0.01b | 0.002 |
| Thr | 0.98 ± 0.03a | 0.98 ± 0.02a | 1.01 ± 0.02a | 0.92 ± 0.01b | 0.001 |
| His | 0.64 ± 0.07b | 0.91 ± 0.06a | 0.86 ± 0.01a | 0.60 ± 0.01b | <0.01 |
| Met | 0.59 ± 0.03a | 0.62 ± 0.05a | 0.61 ± 0.04a | 0.43 ± 0.02b | <0.01 |
| Cys | 0.35 ± 0.03b | 0.50 ± 0.02a | 0.49 ± 0.02a | 0.29 ± 0.01c | <0.01 |
| ∑ EAA | 10.01 ± 0.53a | 10.36 ± 0.35a | 10.46 ± 0.19a | 8.92 ± 0.09b | <0.01 |
| ∑ FAA | 6.83 ± 0.33b | 7.26 ± 0.20a | 7.69 ± 0.07a | 6.67 ± 0.05b | <0.01 |
| ∑ TAA | 19.74 ± 0.56c | 20.58 ± 0.38b | 21.19 ± 0.21a | 18.13 ± 0.14d | <0.01 |
a–dMeans with different superscripts within the same row differ significantly (P < 0.05).
FAA includes Gly, Glu, Asp, Ala.
Abbreviations: EAA, essential amino acid; FAA, flavor amino acid; TAA, total amino acid.
Amino Acid Nutritional Parameters
The amino acid composition of the samples was used to determine several nutritional parameters of goose meat protein. The AAS and EAAI of breast meat proteins are presented in Table 4. The AAS in all 4 groups was higher than the reference protein. The highest score was 233 in group I for His, whereas the lowest score was 124 in group II for Leu. In addition, the total EAAI ranged from 154 to 161, which is consistent with the FAO/WHO standards.
Table 4.
Values of the essential amino acid score (AAS) and the essential amino acid index (EAAI) of essential amino acids of breast meat proteins.
| Amino acid | Pattern protein: FAO/WHO, 1991 (g/100 g of protein) | Control | Group I | Group II | Group III |
|---|---|---|---|---|---|
| AAS (%) | |||||
| Leu | 6.6 | 132 | 125 | 124 | 132 |
| Lys | 5.8 | 162 | 156 | 155 | 164 |
| Val | 3.5 | 143 | 133 | 131 | 142 |
| Phe+Tyr | 6.3 | 154 | 144 | 141 | 137 |
| Ile | 2.8 | 175 | 170 | 170 | 181 |
| Met+Cys | 2.5 | 190 | 218 | 208 | 159 |
| Thr | 3.4 | 146 | 140 | 140 | 149 |
| His | 1.9 | 171 | 233 | 214 | 174 |
| ∑ EAAI | 32.8 | 158 | 161 | 157 | 154 |
Abbreviations: FAO, Food and Agriculture Organization; WHO, World Health Organization.
Fatty Acid Composition
The fatty acid composition of the breast meat was affected by the amount of ryegrass in the diet (Table 5). The concentration of stearic acid (C18:0) was higher in the control group than in groups I, II, and III (P < 0.05). The concentration of g-linolenic acid (C18:3 n-3) was higher with higher grass intake, and its concentration in groups II and III was significantly higher than that in the control group (P < 0.05). The concentration of docosahexaenoic acid (C22:6 n-3) was also increased by the addition of grass to the diet, and its concentration in group III was higher than that in the control group (P < 0.05). The concentrations of most fatty acids (C14:0, C16:0, C21:0, C23:0, C24:0, C16:1, C18:1 n-9c, C18:1 n-9t, and C18:2) did not differ significantly among the 4 groups. The concentration of SFA was higher in the control group than in groups I, II, and III (P < 0.05). The concentration of monounsaturated fatty acids was not significantly different between the groups, whereas that of PUFA was higher in both groups II and III than in the control group (P < 0.05). Most importantly, with increased grass in the diet, the concentration of omega-6 (n-6) fatty acids was not significantly different between the groups, but the concentration of n-3 fatty acids was higher in groups I, II, and III than in the control group (P < 0.05). The n-6-to-n-3 ratio decreased with increasing ryegrass content in the diet and was lower in groups II and III than in the control group (P < 0.05).
Table 5.
Amounts of fatty acids (±SD) in breast meat (% total fatty acids).
| Fatty acids | Control | Group I | Group II | Group III | P value |
|---|---|---|---|---|---|
| C14:00 | 1.59 ± 0.37 | 2.15 ± 0.58 | 1.49 ± 0.24 | 1.66 ± 0.52 | 0.220 |
| C16:00 | 22.62 ± 1.97 | 23.39 ± 1.38 | 23.98 ± 1.13 | 22.27 ± 0.79 | 0.344 |
| C18:00 | 18.68 ± 1.96a | 13.75 ± 0.35b | 14.24 ± 0.63b | 13.54 ± 0.48b | <0.01 |
| C21:00 | 6.57 ± 0.65 | 6.63 ± 0.45 | 7.17 ± 0.29 | 7.39 ± 0.22 | 0.146 |
| C23:00 | 2.62 ± 1.93 | 1.11 ± 0.19 | 1.08 ± 0.59 | 1.83 ± 1.24 | 0.256 |
| C24:00 | 0.50 ± 0.47 | 0.92 ± 0.09 | 0.79 ± 0.29 | 0.83 ± 0.81 | 0.664 |
| C16:1 n-7 | 1.38 ± 0.38 | 1.78 ± 0.40 | 1.34 ± 0.09 | 1.49 ± 0.35 | 0.275 |
| C18:1 n-9c | 25.33 ± 3.09 | 28.14 ± 0.64 | 26.84 ± 1.11 | 27.37 ± 0.56 | 0.175 |
| C18:1 n-9t | 2.49 ± 0.34 | 2.34 ± 0.28 | 2.30 ± 0.08 | 2.45 ± 0.12 | 0.613 |
| C18:2 n-6 | 16.38 ± 2.71 | 17.09 ± 0.55 | 17.95 ± 0.49 | 18.11 ± 0.36 | 0.324 |
| C18:3 n-3 | 0.81 ± 0.11b | 0.95 ± 0.09a,b | 1.07 ± 0.19a | 1.10 ± 0.15a | 0.040 |
| C22:6 n-3 | 1.04 ± 0.35b | 1.76 ± 0.68a,b | 1.77 ± 0.40a,b | 1.94 ± 0.41a | 0.024 |
| SFA | 52.57 ± 1.57a | 47.94 ± 0.58b | 48.74 ± 1.27b | 47.52 ± 1.05b | <0.01 |
| MUFA | 29.20 ± 3.52 | 32.26 ± 0.90 | 30.47 ± 1.23 | 31.32 ± 0.57 | 0.204 |
| PUFA | 18.23 ± 2.59b | 19.80 ± 0.50a,b | 20.79 ± 0.40a | 21.16 ± 0.79a | 0.048 |
| n-6 | 16.38 ± 2.71 | 17.09 ± 0.55 | 17.95 ± 0.49 | 18.11 ± 0.36 | 0.324 |
| n-3 | 1.85 ± 0.37b | 2.71 ± 0.72a | 2.84 ± 0.30a | 3.05 ± 0.43a | 0.020 |
| n-6/n-3 | 9.18 ± 2.33a | 6.76 ± 2.26a,b | 6.39 ± 0.88b | 6.02 ± 0.72b | 0.025 |
a,bMeans with different superscripts within the same row differ significantly (P < 0.05).
Abbreviations: MUFA, monounsaturated fatty acids; n-6/n-3 = omega-6-to-omega-3 ratio; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids.
Cholesterol Content
The cholesterol content in breast meat is shown in Figure 2. With increased grass in the diet, the cholesterol content in breast meat decreased gradually, but there were no significant differences among the groups.
Figure 2.
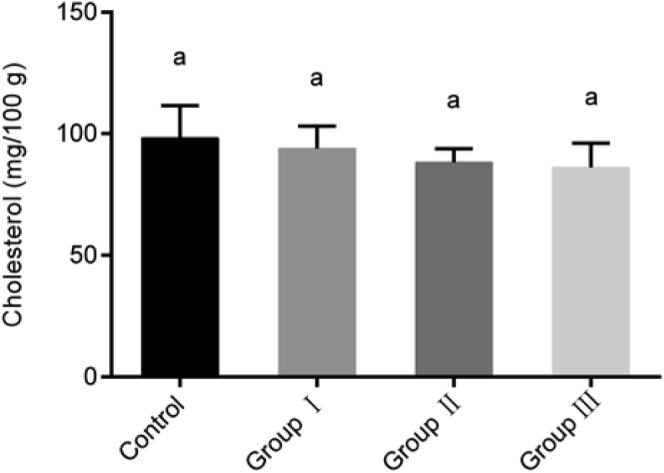
Cholesterol content in the breast meat of geese (70-day-old). The y-axis represents the levels of cholesterol; the x-axis represents the 4 groups. All values are expressed as means ± SD. The same letters (a) means differences were not significant at P > 0.05.
Mineral Composition
The mineral composition of breast meat from each group is shown in Table 6. Mineral element content was not significantly different for iron, magnesium, and calcium. However, zinc content in groups II and III was significantly higher than that in the control group and group I (P < 0.05). Copper content gradually was lower with higher grass intake and was significantly higher in the control group than in group III (P < 0.05). Selenium content in the control group was significantly higher than that in groups II and III (P < 0.05), and its content in group I was significantly higher than in group III (P < 0.05).
Table 6.
Amount of mineral composition (±SD) in breast meat.
| Mineral | Control | Group I | Group II | Group III | P value |
|---|---|---|---|---|---|
| Zinc (mg/100 g) | 18.38 ± 0.94b | 20.09 ± 1.20b | 24.06 ± 3.21a | 23.00 ± 1.16a | 0.001 |
| Iron (mg/100 g) | 54.63 ± 15.08 | 52.82 ± 3.84 | 49.05 ± 0.88 | 48.23 ± 4.04 | 0.173 |
| Magnesium (mg/100 g) | 277.96 ± 35.35 | 265.92 ± 3.53 | 269.04 ± 9.89 | 268.50 ± 4.67 | 0.507 |
| Copper (mg/100 g) | 9.68 ± 2.87a | 9.10 ± 0.31a,b | 7.44 ± 1.44a,b | 7.27 ± 0.24b | 0.014 |
| Calcium (mg/100 g) | 85.91 ± 9.24 | 85.37 ± 10.13 | 101.81 ± 45.9 | 76.87 ± 13.11 | 0.301 |
| Selenium (μg/100 g) | 287.16 ± 6.12a | 278.85 ± 7.73a,b | 229.26 ± 4.72c | 248.02 ± 5.26b,c | 0.014 |
a–cThe means in columns, within main effect, with different superscripts are significantly different at P < 0.05.
Discussion
The Yangzhou goose is of medium size, and its standard weight ranges from 3.2 to 3.5 kg under intensive breeding (Xie et al., 2012). Traditionally, small-scale feeding of geese includes the addition of bulky grass, but geese fed with this diet do not achieve the market standard weight by the age of 70 D owing to the lack of metabolites and proteins in grass, requiring the feeding period to be prolonged by approximately 1 week (Song et al., 2017a). Therefore, it is necessary to supplement diets with moderate volumes of grass for intensive breeding of geese (Torki et al., 2018). In the present study, our analysis of growth performance revealed that the ideal weight at the age of 70 D was achieved in all the groups. We also found that the BW of Yangzhou geese decreased gradually with increased grass intake in the diet. We speculate that this may be due to the low energy content of the grass, which reduces BW gain proportionally with increasing amounts of grass in the diet.
Amino acids are the basic building blocks of animal proteins, and changes in the amino acid composition directly affect the nutritional value of meat (Jiménez-Colmenero et al., 2001). Guo et al., 2018 showed that feeding high-protein diets could increase amino acid content in goose meat. Chen et al., 2010 demonstrated that small amounts of alfalfa meal in the diet could increase amino acid content in rabbit meat, although excessive alfalfa meal was shown to reduce the amino acid content. In the present study, we found parabolic relationships between the contents of EAA, FAA, and TAA in the meat and increase in grass intake in the diet; the maximum contents were reached in group II. This was because adding low volumes of grass improved the absorption and utilization of protein in the feed, whereas with increased grass intake, the protein content and energy in the diet was insufficient, leading to reduced amino acid content and growth performance. In addition, FAA such as Glu, Asp, Ala, and Gly are important precursors of volatile flavor compounds in meat; they are automatically oxidized to produce flavor compounds (Sperling et al., 1978). Our results showed that Glu is a major taste-active component in meat. Furthermore, the nutritional value of proteins mainly depends on the composition and proportion of EAA (Mans and Novelli, 1961). The total EAAI in the analyzed goose meat exceeded the standards of the FAO and WHO (Eggum, 1991) in all the groups.
There has been increased interest in studying the fatty acid profiles of food products in recent years because of their contribution to the development of lifestyle-related diseases (Wood et al., 2008). The consumption of foods with a lower n-6-to-n-3 fatty acid ratio can reduce the risk of developing cardiovascular diseases (Hu et al., 2001). The present study showed that the n-6-to-n-3 ratio in goose meat (ranging from 6.02 to 9.18) was higher than that in beef (2.11) and lamb (1.32), and lower than that in pork (9.22) (Enser et al., 1996). This was not totally unexpected because grazing provides cows and sheep increased concentrations of n-3 PUFA, especially linolenic acid (C18:3n-3) (Ponte et al., 2008). Our results showed that the concentration of linolenic acid gradually increased with higher grass intake. Linolenic acid in grass exists in an esterified form in structural lipids, including galactose lipids in chloroplasts (Enser, 1984). Therefore, we can conclude that the digestive system of geese can digest structural lipids or free linolenic acid from galactose lipids. In addition, analysis of SFA revealed the most significant difference in concentration among the groups for C18:0, which showed a reduced concentration with increased grass intake. Liu and Zhou (2013) also observed that SFA content decreased when geese were fed with grass rather than grain. The PUFA showing the greatest differences in concentration were C18:3 and C22:6, both of which contain double bonds, enabling them to be rapidly oxidized; these are important for flavor development and help to extend the shelf-life of meat (Wood et al., 2004). Therefore, grass feeding helped to improve the flavor of breast meat.
Although cholesterol is an important nutritional factor, excessive cholesterol intake may cause heart problems (Chizzolini et al., 1999). Hence, the recommended maximum daily cholesterol intake is only 300 mg (Jiménez-Colmenero et al., 2001). Cholesterol is influenced by diet, and bovine marrow from grass-fed animals contains an average cholesterol content of 119.6 mg/100 g, while marrow from grain-fed animals contains an average of 150.6 mg/100 g (Kunsman et al., 2010). In our study, increased ryegrass content in the diet caused a gradual reduction in breast meat cholesterol. This may be because ryegrass contains more fiber, which is fermented in the intestine to produce higher concentrations of volatile fatty acids such as acetic acid, propionic acid, and butyric acid. Propionic acid can inhibit the activity of liver HMG-CoA reductase, effectively reducing cholesterol synthesis in the body, thus reducing the cholesterol content in the goose (Roberfroid, 1993). In addition, acetic acid enters the peripheral tissues and inhibits the synthesis of cholesterol (Hara et al., 1998).
Meat is a major source of minerals, especially Cu, Zn, Fe, Mg, Ca, and Se (Mills, 1979). The mineral composition of meat can vary depending on genetic factors, physiological factors such as gender, and environmental factors such as diet (Doyle, 1980). By analysis of mineral elements in goose meat, Song et al. (2017b) determined that Mg and Cu content was higher in geese fed with grass supplemented with grain than in those fed with grass only. In the process of animal agriculture, small concentrations of minerals must be added to the diet to maintain animal health (Jones et al., 1987). Although it remains unclear whether adding ryegrass affects the mineral element content of meat, our study reveals that ryegrass has a major influence on the contents of Zn, Cu, and Se in goose meat.
Conclusions
When the geese were fed with different proportions of ryegrass, their growth and development and amino acid, fatty acid and mineral content in meat would be affected at least in part, but they had little effect on cholesterol content. The diet with ryegrass in the ratio of 1.5:1 or 2:1 would contribute toward good growth performance and increased EAA, FAA, TAA, n-3 fatty acid, and Zn and decreased SFA content in meat, which provided an effective data reference for high-quality goose production under intensive feeding conditions.
Acknowledgments
The authors are grateful to the staff at their laboratory for the assistance they have provided during the study. This work was supported by the Jiangsu Province Agricultural Science and Technology Independent Innovation Fund (CX (17) 2001), Jiangsu Province Science and Technology Support Plan Key Project (BE2017346), Yangzhou University Science and Technology Innovation Fund (X2017699), and National Natural Science Foundation of China (31772583).
References
- Y. U B., Tsai C.C., Hsu J.C., Chiou W.S. Effect of different sources of dietary fibre on growth performance, intestinal morphology and caecal carbohydrases of domestic geese. Br. Poult. Sci. 1998;39:560–567. doi: 10.1080/00071669888773. [DOI] [PubMed] [Google Scholar]
- Chen J.H., Wang C.Z., Yan X.B., Shi Y.H., Wang Y.H., Pan J.L. Effect of alfalfa meal on growth and meat amino acid content of meat rabbit. Acta. Agrestia Sinica. 2010;18:462–468. [Google Scholar]
- Chizzolini R., Zanardi E., Dorigoni V., Ghidini S. Calorific value and cholesterol content of normal and low-fat meat and meat products. Trends Food Sci. Technology. 1999;10:119–128. [Google Scholar]
- Cui L.L., Wang J.F., Xie K.Z., Li A.H., Geng T.Y., Sun L.R., Liu J.Y., Zhao M., Zhang G.X., Dai G.J. Analysis of meat flavor compounds in pedigree and two-strain Yangzhou geese. Poult. Sci. 2015;94:2266–2271. doi: 10.3382/ps/pev179. [DOI] [PubMed] [Google Scholar]
- Doyle J.J. Genetic and nongenetic factors affecting the elemental composition of human and other animal tissues. A review. J. Anim. Sci. 1980;50:1173–1183. doi: 10.2527/jas1980.5061173x. [DOI] [PubMed] [Google Scholar]
- Eggum B. Comments on report of a joint FAO/WHO expert consultation on protein quality evaluation, Rome 1990. Z. Für Ernährungswissenschaft. 1991;30:81–88. doi: 10.1007/BF01610063. [DOI] [PubMed] [Google Scholar]
- Enser M. The chemistry, biochemistry and nutritional importance of animal fats. In: Wiseman J., editor. Fats in Animal Nutrition. Butterworth-Heinemann; Oxford, UK: 1984. pp. 23–51. [Google Scholar]
- Enser M., Hallett K., Hewitt B., Fursey G.A.J., Wood J.D. Fatty acid composition of English beef, lamb and pork at retail. Meat Sci. 1996;42:443–456. doi: 10.1016/0309-1740(95)00037-2. [DOI] [PubMed] [Google Scholar]
- Guo Y., Chen B., Shi T., Wang B., Zhang Y., Zhao X., Wang Y., Dai G., Xie K. Comparison on the contents of amino acids and Inosine Monphosphate in muscle of Yangzhou geese and their Crossbred Combinations. China Anim. Husbandry Vet. Med. 2018 [Google Scholar]
- Hara H., Haga S., Kasai T., Kiriyama S. Fermentation products of sugar-beet fiber by cecal bacteria lower plasma cholesterol concentration in rats. J. Nutr. 1998;128:688–693. doi: 10.1093/jn/128.4.688. [DOI] [PubMed] [Google Scholar]
- Howe K. Animal health and related problems in densely populated livestock areas of the community: proceedings of a workshop held in Brussels 22–23 November 1994. In: Dijkhuizen A.A., Davies G., editors. Vol. 31. 1997. pp. 162–165. (Sponsored by the European Commission Directorate-General F. Preventive Veterinary Medicine). [Google Scholar]
- Hu F., Manson J., Willett W. Types of dietary fat and risk of coronary heart disease: a critical review. J. Am. Coll. Nutr. 2001;20:5–19. doi: 10.1080/07315724.2001.10719008. [DOI] [PubMed] [Google Scholar]
- Jiménezcolmenero F., Carballo J., Cofrades S. Healthier meat and meat products: their role as functional foods. Meat Sci. 2001;59:5–13. doi: 10.1016/s0309-1740(01)00053-5. [DOI] [PubMed] [Google Scholar]
- Jones R.L., Hanson H.C., Ziegler E.L. Mineral composition of feathers from Canada geese (Branta canadensis) fed experimental diets. Curr. Surg. 1987;41:200–202. [Google Scholar]
- Kunsman J.E., Collins M.A., Field R.A., Miller G.J. Cholesterol content of beef bone marrow and mechanically deboned meat. J. Food Sci. 2010;46:1785–1788. [Google Scholar]
- Liu D.C., Zhou X.L., Zhao P.T., Gao M., Han H.Q., Hong-Lian H.U. Effects of increasing non-fiber carbohydrate to neutral detergent fiber ratio on rumen fermentation and microbiota in goats. J. Integr. Agric. 2013;12:319–326. [Google Scholar]
- Liu H.W., Zhou D.W. Influence of pasture intake on meat quality, lipid oxidation, and fatty acid composition of geese. J. Anim. Sci. 2013;91:764–771. doi: 10.2527/jas.2012-5854. [DOI] [PubMed] [Google Scholar]
- Mans R.J., Novelli G.D. Measurement of the incorporation of radioactive amino acids into protein by a filter-paper disk method. Arch. Biochem. Biophys. 1961;94:48–53. [Google Scholar]
- Mills C.F. Trace elements in animals. Philo. Trans. R. Soc. Lond. 1979;288:51. doi: 10.1098/rstb.1979.0090. [DOI] [PubMed] [Google Scholar]
- Mushi D.E., Thomassen M.S., Kifaro G.C., Eik L.O. Fatty acid composition of minced meat, longissimus muscle and omental fat from small East African goats finished on different levels of concentrate supplementation. Meat Sci. 2010;86:337–342. doi: 10.1016/j.meatsci.2010.05.006. [DOI] [PubMed] [Google Scholar]
- O'Sullivan A., O'Sullivan K., Galvin K., Moloney A.P., Troy D.J., Kerry J.P. Influence of concentrate composition and forage type on retail packaged beef quality. J. Anim. Sci. 2004;82:2384. doi: 10.2527/2004.8282384x. [DOI] [PubMed] [Google Scholar]
- Okruszek A., Wołoszyn J., Haraf G., Orkusz A., Wereńska M. Chemical composition and amino acid profiles of goose muscles from native Polish breeds. Poult. Sci. 2013;92:1127–1133. doi: 10.3382/ps.2012-02486. [DOI] [PubMed] [Google Scholar]
- Orellana C., Peña F., García A., Perea J., Martos J., Domenech V., Acero R. Carcass characteristics, fatty acid composition, and meat quality of Criollo Argentino and Braford steers raised on forage in a semi-tropical region of Argentina. Meat Sci. 2009;81:57–64. doi: 10.1016/j.meatsci.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Ponte P.I.P., Rosado C.M.C., Crespo J.P., Crespo D.G., Mourão J.L., Chaveiro-Soares M.A., Brás J.L.A., Mendes I., Gama L.T., Prates J.A.M. Pasture intake improves the performance and meat sensory attributes of free-range broilers. Poult. Sci. 2008;87:71–79. doi: 10.3382/ps.2007-00147. [DOI] [PubMed] [Google Scholar]
- Rayman M.P. The importance of selenium to human health. Lancet. 2000;356:233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- Roberfroid M. Dietary fiber, inulin, and oligofructose: a review comparing their physiological effects. C R. C Crit. Rev. Food Technology. 1993;33:103–148. doi: 10.1080/10408399309527616. [DOI] [PubMed] [Google Scholar]
- Schwartz S. A metagenomic study of diet-dependent interaction between gut microbiota and host in infants reveals differences in immune response. Genome Biol. 2012;13:r32. doi: 10.1186/gb-2012-13-4-r32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C., Wang B., Tan J., Zhu L., Lou D., Cen X. Comparative analysis of the gut microbiota of black bears in China using high-throughput sequencing. Mol. Genet. Genomics. 2017;292:407–414. doi: 10.1007/s00438-016-1282-0. [DOI] [PubMed] [Google Scholar]
- Song Y., Li Y., Zheng S., Dai W., Shen X., Zhang Y., Zhao W., Chang G., Xu Q., Chen G. Effects of forage feeding versus grain feeding on the growth performance and meat quality of Yangzhou geese. Br. Poult. Sci. 2017;58:1–5. doi: 10.1080/00071668.2017.1307942. [DOI] [PubMed] [Google Scholar]
- Sperling R., Furie B.C., Blumenstein M., Keyt B., Furie B. Metal binding properties of gamma-carboxyglutamic acid. Implications for the vitamin K-dependent blood coagulation proteins. J. Biol. Chem. 1978;253:3898–3906. [PubMed] [Google Scholar]
- Torki M., Schokker D., Duijster-Lensing M., Van Krimpen M.M. Effect of nutritional interventions with quercetin, oat hulls, -glucans, lysozyme and fish oil on performance and health status related parameters of broilers chickens. Br. Poult. Sci. 2018;59:579–590. doi: 10.1080/00071668.2018.1496402. [DOI] [PubMed] [Google Scholar]
- Velasco S., Cañeque V., Pérez C., Lauzurica S., Díaz M.T., Huidobro F., Manzanares C., González J. Fatty acid composition of adipose depots of suckling lambs raised under different production systems. Meat Sci. 2001;59:325–333. doi: 10.1016/s0309-1740(01)00135-8. [DOI] [PubMed] [Google Scholar]
- Wang H.Y., Liu Y., Gong S.M., Chen Y.S., Da-Qian H.E. Effects of ryegrass amount on the weight gain,feed utilization rate and slaughter performance of Zhedong white geese. Acta Agriculturae Shanghai. 2015;31:31–34. [Google Scholar]
- Wood J.D., Enser M., Fisher A.V., Nute G.R., Sheard P.R., Richardson R.I., Hughes S.I., Whittington F.M. Fat deposition, fatty acid composition and meat quality: a review. Meat Sci. 2008;78:343–358. doi: 10.1016/j.meatsci.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Wood J.D., Nute G.R., Richardson R.I., Whittington F.M., Southwood O., Plastow G., Mansbridge R., Da C.N., Chang K.C. Effects of breed, diet and muscle on fat deposition and eating quality in pigs. Meat Sci. 2004;67:651–667. doi: 10.1016/j.meatsci.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Wood K.M., Salim H., Mcewen P.L., Mandell I.B., Miller S.P., Swanson K.C. The effect of corn or sorghum dried distillers grains plus solubles on growth performance and carcass characteristics of cross-bred beef steers. Anim. Feed Sci. Technology. 2011;165:23–30. [Google Scholar]
- Xie K.Z., Huang Y.P., Chen X.S., Chen S.Q., Dai G.J., Zhao W.L. Study on the meat production performance and meat quality of Yangzhou geese and its crossed combinations. Chin. J. Anim. Sci. 2012;48:1–6. [Google Scholar]
- Yancey E.J., Grobbel J.P., Dikeman M.E., Smith J.S., Hachmeister K.A., Iv E.C.C., Gadgil P., Milliken G.A., Dressler E.A. Effects of total iron, myoglobin, hemoglobin, and lipid oxidation of uncooked muscles on livery flavor development and volatiles of cooked beef steaks. Meat Sci. 2006;73:680–686. doi: 10.1016/j.meatsci.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Zhan J., Chen X., Liu S., Zhou M., Yang H., Lin M., Liu M., Zhao G. Effects of ryegrass on growth, carcass traits and blood biochemical indices of Yangzhou geese. Acta Prataculturae Sinica. 2015;24:168–175. [Google Scholar]


