Abstract
In this study, the aim was to investigate effects of chronic heat stress (CHS) on the mRNA levels of proinflammatory cytokines (interleukin [IL]-6, IL-8, IL-1β, and tumor necrosis factor alpha [TNF-α]), toll-like receptors (TLR2 and TLR4), heat shock proteins (Hsp70, heat shock transcription factor [HSF]-1, and HSF3) and antioxidant enzymes (catalase, glutathione peroxidase, NADPH oxidase, and superoxide-dismutase) in the jejunal mucosae of broiler chickens subjected to thermal manipulation (TM) during embryogenesis. TM was carried out at 39°C and 65% relative humidity (RH) for 18 h daily from embryonic days 10 to 18. Control group was incubated at 37.8°C and 56% RH. CHS was induced by raising the temperature to 35°C for 7 D throughout posthatch days 28 to 35. On post-hatch-day 28 (day zero of CHS) and after 1, 3, 5, and 7 D of CHS, the jejunal mucosae were collected from both groups to evaluate the mRNA levels by real-time reverse transcription-PCR analysis. On day zero of CHS, the mRNA levels of antioxidant enzymes, TLRs, HSF3, IL-1β, and TNF-α were not significantly different between TM and control groups, while the levels of IL-6, IL-8, and HSF1 were lower and the level of Hsp70 was higher in TM. However, during CHS, the mRNA levels of antioxidant enzymes, IL-1β, TNF-α, TLR4, and HSF1 were significantly lower in TM than in controls, while the levels of TLR2 and IL-8 were significantly higher in TM than in controls. In addition, TM led to significant increase of mRNA levels of IL-6 and HSF3 after 1 D and Hsp70 after 3 D of CHS and to significant decrease of mRNA levels of IL-6 after 3 and 5 D, HSF3 after 7 D, and Hsp70 after 5 D of CHS. Results of this study suggest that TM led to altered posthatch antioxidant, immunological, and Hsp response to CHS in the jejunal mucosae of broiler chickens, probably indicating that TM may mitigate the adverse effects of CHS.
Key words: thermal manipulation, chronic heat stress, immunity, antioxidant, heat shock protein
Introduction
In the recent decades, the modern meat-type broiler chicken breeds, Gallus gallus domesticus, had been selected for commercial purposes, such as fast-growth and rapid meat mass production (Havenstein et al., 2003). This broiler chicken breeds have higher metabolic rates than their predecessors, resulting in increased body heat production, which increases the severity of heat stress under high environmental temperatures (Settar et al., 1999, Quinteiro-Filho et al., 2010). Heat stress has a significant impact on the systems of broiler chickens' bodies because it severely affected the growth, metabolism, digestive system, and the general physiology of the birds (Lara and Rostagno, 2013) and caused changes in the proportions of circulating leucocyte components and blood chemistry (Abbas et al., 2017). Heat stress may lead to intestinal damage because it was reported to decrease jejunal weight and length, villus height, and increase intestinal crypt depth in broilers (Garriga et al., 2006, Quinteiro-Filho et al., 2010, Song et al., 2014, Abdelqader and Al-Fataftah, 2016, He et al., 2018). Moreover, heat stress affects the activities of digestive enzymes and reduces the digestibility of carbohydrates, lipids, and proteins (Hai et al., 2000, Routman et al., 2003, de Souza et al., 2016, Yi et al., 2016, Song et al., 2017, Xiaofang et al., 2018).
Heat stress induces inflammatory response and oxidative stress and increases the expression of several heat shock proteins (HSP) in the tissues of broiler chickens (Altan et al., 2000, Altan et al., 2003, Mujahid et al., 2005, Lin et al., 2006; Yang et al., 2010; Lara and Rostagno, 2013, Huang et al., 2015). HSP are upregulated in the cells exposed to any stress conditions (Kregel, 2002). HSP upregulation is a normal response to protect the cells and cellular proteins during the exposure to heat and other stress factors (Fulda et al., 2010). One of the most studied HSP is the Hsp70, which is very important for cellular viability during stressful environments (Song and King, 2015). HSP expression is induced by heat shock transcription factors (HSF), such as HSF1 and HSF3 (Morimoto, 1998). On the other hand, the inflammatory response can be induced by the increased levels of low-molecular-weight signaling proteins called proinflammatory cytokines, such as IL-1β, IL-8, IL-6, and tumor necrosis factor alpha (TNF-α) (Kaiser and Stäheli, 2014). The proinflammatory cytokines are produced during stressful conditions and are very important to regulate the acute phase response, which is an innate response that regenerate injured tissues (Kushner, 1993). Heat stress was also found to increase the expression of toll-like receptor 4, a pathogen recognition receptor that is an important protein in the innate immunity (Huang, 2017).
Heat stress leads to high levels of reactive oxygen species (ROS). This induced oxidative stress alter the activity of several antioxidant enzymes (Ray et al., 2012). NADPH oxidase (NOX) is a membrane-bound protein complex that works as an oxygen sensor (Kirkman and Gaetani, 1984). The main activity of NOX is to transfer electrons to oxygen molecules from NADPH, which results in (O2−) superoxide production (Kirkman and Gaetani, 1984). The produced O2− superoxide is then transformed to the molecular oxygen (O2) or to hydrogen peroxide (H2O2), and this reaction is catalyzed by superoxide-dismutase (SOD) (Surai, 2015, Ighodaro and Akinloye, 2017). Finally, the produced H2O2 is transformed to O2 and water molecule through catalase and glutathione peroxidase (GPX) enzymes (Dunning et al., 2013). Several studies have shown that heat stress increased the activities and mRNA levels of the antioxidant enzymes (NOX, SOD, catalase, and GPX) in broiler chickens (Altan et al., 2003, Lin et al., 2006, Yang et al., 2010, Huang et al., 2015, Kikusato et al., 2015; Rimoldi et al., 2015, Del Vesco et al., 2017).
Thermal manipulation (TM) during broiler embryogenesis, by increasing or decreasing the incubation temperature, was suggested to enhance heat tolerance during posthatch life (Piestun et al., 2008, Molenaar et al., 2010, Al-Zghoul et al., 2013, Al-Zghoul et al., 2015b, Al-Rukibat et al., 2017). Heat-tolerance improvement was achieved by the modulation of thyroid hormones, which are major players in the basal metabolic rate regulation, and HSP levels (Al-Zghoul et al., 2013, Al-Zghoul et al., 2015a, Al-Zghoul et al., 2015b, Al-Zghoul et al., 2015c, Al-Rukibat et al., 2017). Previously, TM was reported to increase Hsp70, HSF3, IL-1β, TNF-α, IL-6, TLR2, and TLR4 and to decrease catalase, GPX, SOD, and NOX expression in broiler chickens during acute heat stress (Al-Zghoul et al., 2018, Al-Zghoul et al., 2019). During chronic heat stress, chickens subjected to TM had higher SOD activity and glutathione levels and lower levels of lipid peroxidation (Vinoth et al., 2015). Furthermore, Vinoth et al. (2015) reported that layer breeders that were subjected to TM possessed higher nonenzymatic antioxidants activity in their semen.
The effect of TM on the status of oxidative stress, immune, and heat shock genes during posthatch exposure to acute heat stress had been evaluated. However, the effect of posthatch chronic heat stress on the expression of antioxidant enzymes and inflammatory and heat shock genes in thermally manipulated broiler chickens is not clear. Thus, the primary objective of the present study is to evaluate the impacts of chronic heat stress on the mRNA levels of HSP (Hsp70, HSF1, and HSF3), proinflammatory cytokines (IL-6, IL-8, IL-1β, and TNF-α), toll-like receptors (TLR) 2 and 4, and antioxidant enzymes (NOX, GPX, SOD, and catalase) in broiler chickens subjected to TM.
Materials and methods
All experiment procedures were approved by Jordan University of Science and Technology's Animal Care and Use Committee (JUST-ACUC).
Experimental Population and Incubation
Six hundred fertile eggs from a Cobb breeder were purchased from certified suppliers in Madaba, Jordan. The procured eggs were checked for any breakage, and abnormal eggs were excluded. The proper eggs were incubated using commercial Type-I HS-SF incubators (Barcelona, Spain). The eggs were divided into 2 treatment groups: the control group and the thermally manipulated (TM) group. The eggs of the control group were kept under the standard incubation conditions (37.8°C and 56% relative humidity [RH]) throughout the incubation period, whereas those of the TM group were exposed to cyclic increased incubation temperature at 39°C and 65% RH for 18 h/D from ED 10 to 18. Through candling, the eggs were checked to remove the infertile eggs and the eggs containing dead embryos on the seventh day of incubation.
Hatching Management and Rearing
On the hatching day, the hatched chicks were kept until feather drying, then the 1-day old chicks were transferred to JUST Animal House where the rearing and chronic heat stress were carried out. The chicks were randomly distributed into groups of tens per each cage pen. The chicks were kept under 33°C ± 1°C room temperature during the first week, then the room temperature was gradually lowered to 24°C by the end of the third week. During the posthatch days 24 to 35, the room temperature was maintained at 21°C. During the whole field experiment period, water and feed were provided to the chicks ad libitum. The chicks were provided with Newcastle disease vaccine on posthatch days 8 and 20 and infectious bursal disease vaccine on posthatch day 15.
Chronic Heat Stress and Samples Collection
On the posthatch day 28, 80 chickens were randomly selected from TM and control groups and transported to a new room where the chronic heat stress was achieved. CHS was carried out by rising the room temperature to 35°C for a duration of 7 D. After 0, 1, 3, 5, and 7 D of CHS, 5 chicks were randomly chosen from each treatment group and humanely euthanized. Jejunal mucosae samples were collected in tubes filled with TRI Reagent (Zymo Research, Irvine, CA) and snap-frozen using liquid nitrogen. Then, the frozen jejunal mucosae samples were transported to the laboratory and stored at −80°C for subsequent molecular analysis.
RNA Extraction and cDNA Synthesis
Total RNA was extracted from jejunal mucosae samples using Direct-Zol RNA MiniPrep (Zymo Research, Irvine, CA) with TRI Reagent (Zymo Research, Irvine, CA) according to manufacturer procedure. Using Biotek PowerWave XS2 Spectrophotometer (BioTek Instruments, Inc., Winooski, VR), RNA concentrations were measured. From each sample, 2 μg of total RNA were used in the reverse transcription reaction using the SuperScript IV VILO Master Mix (Invitrogen, Thermo Fisher Scientific, Wilmington, DE).
Real-Time Quantitative Reverse Transcription-PCR Analysis and Relative mRNA Quantitation
The real-time quantitative reverse transcription-PCR analysis was carried out using QuantiFast SYBR Green PCR Kit (Qiagen Corp., Valencia, CA) on a Rotor-Gene Q MDx 5 plex instrument (Qiagen Corp., Valencia, CA). Briefly, the 20-μL reaction mix was prepared using 10 μL of master mix, 1.2 μL of forward primer (12 pmol), 1.2 μL of reverse primer (12 pmol), 1 μL of sample cDNA, and 6.6 μL of nuclease-free water. The parameters of PCR cycles included the following phases: hold at 95°C for 5 min—40 cycles at 95°C for 10 s followed by 30 s at 55°C and 72°C for 10 s—with final melting at 95°C for 20 s. Detection of fluorescence emission occurred during the extension step. The 28S ribosomal RNA and glyceraldehyde-3-phosphate dehydrogenase were used as internal controls to which the fold changes in gene expression were normalized. Triplicates from each cDNA library were analyzed, and the single target amplification specificity was approved by the melting curve. Relative quantitation was calculated automatically.
The primer sequences that were used in this study are 28S rRNA; F-(5′-CCTGAATCCCGAGGTTAACTATT-3′) and R-(5′-GAGGTGCGGCTTATCATCTATC-3′), GAPDG; F-(5′-ACTGTCAAGGCTGAGAACGG-3′) and R-(5′-CATTTGATGTTGCTGGGGTC-3′), IL-1β; F-(5′-GGGCATCAAGGGCTACAA-3′) and R-(5′-CTGTCCAGGCGGTAGAAGA-3′), IL-6; F-(5′-GCGAGAACAGCATGGAGATG-3′) and R-(5′-GTAGGTCTGAAAGGCGAACAG-3′), IL-8; F-(5′-CTGCGGTGCCAGTGCAT TA-3′) and R-(5′-AGCACACCTCTCTTCCATC C-3′), TNF-α; F-(5′-GACAGCCTATGCCAACAAGTA-3′) and R-(5′-GAATTAAGCAACAG CCAGCTATG-3′), TLR2; F-(5′-AACCCACA GTTCTCCATCATC-3′) and R-(5′-TTTCAGA CTTCCAGGCTCATAC-3′), TLR4; F-(5′-GG AGTTGAGAGTGCTTCGTATTA-3′) and R-(5′-AGCAGGTAAGGAAGGAGAGA-3′), GPX; F-(5′-GATTACACCCAG CTCAACCA-3′) and R-(5′-GCTTGAGGC A GTTGAGGAT-3′), superoxide dismutase; F-(5′-CTGACCTGCCTTACGACTATG-3′) and R-(5′-CGCCTCTTTGTATTTCTCCTCT-3′), NOX; F-(5′-CCAGACCAACTTAGAGGAACAC-3′) and R-(5′-TCTGGGAAA G GCTCAGTAGTA-3′), catalase; F-(5′-GAAGCAG AGAGGTTCCCATTTA-3′) and R-(5′-CAT ACGCCATCTGTTCTACCTC-3′), HSF1; F-(5′-CAGCGTGTCCAGCATAAAGA-3′) and R-(5′-TGAGCTTATT GACCACCTTCTG-3′), HSF3; F-(5′-GAGTTCCAGCACCCTTTCTT-3′) and R-(5′-TCTTTCCACAGGGCCTTATT T-3′), Hsp70; F-(5′-GGATGAAGCCAACAGAGA TAGG-3′) and R-(5′-TCTGCTTGTGCTCATACTCTTC-3′).
Statistical Analysis
All statistical analyses were conducted using IBM SPSS Statistics 24.0 (IBM software, Chicago, IL). The folds of mRNA levels of proinflammatory cytokines (IL-6, IL-8, IL-1β, and TNF-α), TLR2 and TLR4, HSP (Hsp70, HSF1, and HSF3), and antioxidant enzymes (catalase, GPX, NOX, and SOD) were expressed as means ± standard deviation. Two-way ANOVA followed by Bonferroni test was used to compare the mRNA fold changes between TM vs. control groups and within treatment groups (day 0 of CHS vs. day 1, 3, 5, and 7 after CHS). Parametric differences were considered statistically significant at P < 0.05.
Results
Effect of TM on the Intestinal mRNA Levels of Antioxidant Enzymes, Proinflammatory Cytokines, TLR, and HSP on Posthatch Day 28 (day 0 of CHS)
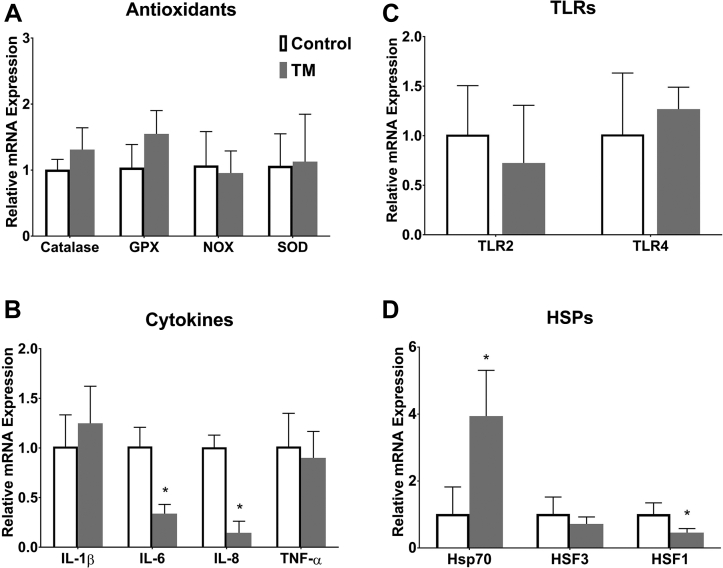
Figures 1A–1D represents effects of TM on the intestinal mRNA levels of antioxidant enzymes (catalase, GPX, NOX, and SOD), proinflammatory cytokines (IL-6, IL-8, IL-1β, and TNF-α), TLR2 and TLR4, and HSP (Hsp70, HSF1, and HSF3) on posthatch day 28 (day 0 of CHS). TM did not lead to significant changes in the intestinal mRNA levels of antioxidant enzymes, TLR, IL-1β, TNF-α, and HSF3. However, TM led to significantly lower mRNA levels of IL-6, IL-8, and HSF1 and to significantly higher mRNA level of Hsp70 than the control group.
Figure 1.
The intestinal mRNA levels of (A) antioxidant enzymes (catalase, glutathione peroxidase [GPX], NADPH oxidase [NOX], and superoxide-dismutase [SOD]), (B) proinflammatory cytokines (interleukin [IL]-1β, IL-6, IL-8, and tumor necrosis factor alpha [TNF-α]), (C) toll-like receptors (TLR2 and TLR4), and (D) heat shock proteins (Hsp70, heat shock transcription factor [HSF]-1, and HSF3) on posthatch day 28 (day 0 of chronic heat stress [CHS]) in broiler chickens subjected to thermal manipulation (TM) during embryogenesis (n = 5). The values in the chart indicates folds of mRNA level in the control groups. *Mean ± standard deviation is significantly different compared to control (P < 0.05).
Effects of CHS on the Intestinal mRNA Levels of Antioxidant Enzymes in Broiler Chickens Subjected to TM
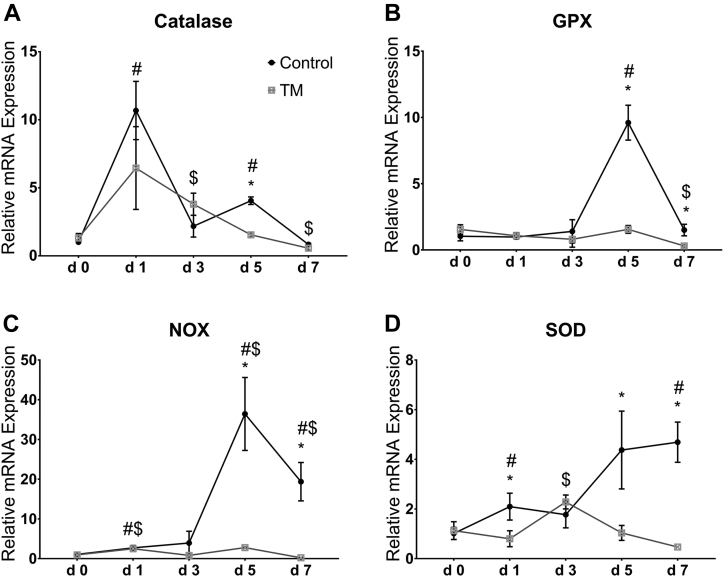
Effects of CHS on the intestinal mRNA levels of antioxidant enzymes (catalase, GPX, NOX, and SOD) in broiler chickens subjected to TM is shown in Figure 2A–D.
Figure 2.
Effect of chronic heat stress (CHS) (at 35°C) on the intestinal mRNA levels of catalase (A), glutathione peroxidase (GPX) (B), NADPH oxidase (NOX) (C), and superoxide-dismutase (SOD) (D) in control and thermal manipulation (TM) broiler chickens (n = 5). The values in the chart indicates folds of mRNA level in the control group day 0. *Within same day, mean ± standard deviation (SD) of TM is significantly different compared to control (P < 0.05). #Within control group, mean ± SD of day 1, 3, 5, or 7 is significantly different compared to day 0 (P < 0.05). $Within the TM group, mean ± SD of day 1, 3, 5, or 7 is significantly different compared to day 0 (P < 0.05).
Catalase
During CHS, the mRNA level of catalase was significantly higher after 1 and 5 D than at the day 0 of CHS in control group, while in the TM group, the mRNA level of catalase was significantly higher after 3 D and significantly lower after 7 D than that at day 0 of CHS (Figure 2A). After 5 D of CHS, the mRNA level of catalase was significantly lower in TM than that in the control group.
Glutathione Peroxidase
No significant change was observed in the mRNA level of GPX during CHS in the TM group (Figure 2B). However, the mRNA level of GPX was significantly increased after 5 D compared with that at day 0 of CHS in the control group. Furthermore, the mRNA level of GPX was significantly lower after 5 and 7 D of CHS in TM group than that in control.
NADPH Oxidase
The mRNA level of NOX was significantly higher after 1 and 5 D than at day 0 of CHS in both groups, but only in the control group, the level remained significantly increased on day 7 of CHS (Figure 2C). The mRNA level of NOX was significantly lower in TM group than that in control after 5 and 7 D of CHS.
Superoxide-dismutase
After days 1 and 7 of CHS, there was significant increases in the mRNA levels of SOD compared with those at day 0 of CHS in the control group (Figure 2D). In the TM group, the mRNA level of SOD was significantly increased after 3 D of CHS compared with that at day 0 of CHS. The mRNA level of SOD was significantly lower in the TM group than that in control after 1, 5, and 7 D of CHS.
Effects of CHS on the Intestinal mRNA Levels of Proinflammatory Cytokines and TLR in Broiler Chickens Subjected to TM
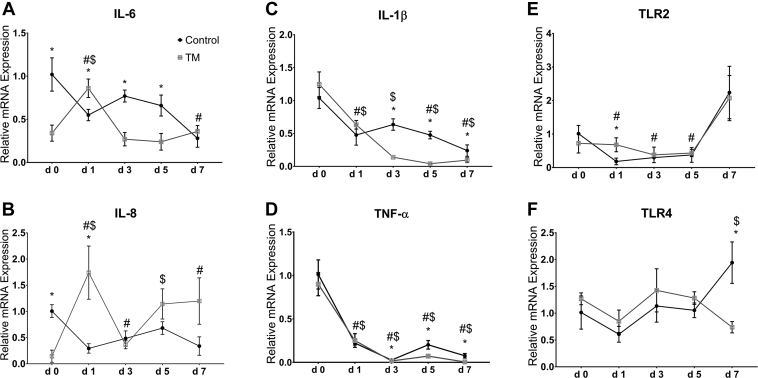
Figure 3A–F represents the effects of CHS on the intestinal mRNA levels of proinflammatory cytokines and TLR in broiler chickens subjected to TM.
Figure 3.
Effect of chronic heat stress (CHS) (at 35°C) on the intestinal mRNA levels of interleukin (IL)-6 (A), IL-8 (B), IL-1β (C), tumor necrosis factor alpha (TNF-α) (D), toll-like receptor (TLR)-2 (E), and TLR4 (F) in control and thermal manipulation (TM) broiler chickens (n = 5). The values in the chart indicate folds of mRNA level in the control group day 0. *Within same day, mean ± standard deviation (SD) of TM is significantly different compared to control (P < 0.05). #Within the control group, mean ± SD of day 1, 3, 5, or 7 is significantly different compared to day 0 (P < 0.05). $Within the TM group, mean ± SD of day 1, 3, 5, or 7 is significantly different compared to day 0 (P < 0.05).
Interleukin-6
In the control group, CHS significantly decreased the mRNA levels of IL-6 after 1 and 7 D compared with those at day 0, whereas in the TM group, CHS significantly increased the levels of IL-6 after 1 D compared with those at day 0 (Figure 3A). The mRNA level of IL-6 was significantly higher after 1 D and significantly lower after 3 and 5 D of CHS in TM than that in the control group.
Interleukin-8
In the control group, CHS significantly decreased the mRNA levels of IL-8 after 1, 3, and 7 D compared with those at day 0, whereas in the TM group, CHS significantly increased the levels of IL-8 after 1 and 5 D compared with those at day 0 (Figure 3B). The mRNA level of IL-8 was significantly higher in the TM group than that in the control after 1 D of CHS.
Interleukin-1β
In the control group, CHS significantly decreased the mRNA levels of IL-1β after 1, 5, and 7 D compared with those at day 0, whereas in the TM group, CHS significantly decreased the levels of IL-1β after all time intervals compared with those at day 0 (Figure 3C). The mRNA level of IL-1β was significantly lower in the TM group than that in the control after 3, 5, and 7 D of CHS.
TNF-α
In both control and TM groups, CHS significantly decreased the mRNA levels of TNF-α after all studied days compared with those at day 0 (Figure 3D). After 3, 5, and 7 D of CHS, the mRNA level of TNF-α was significantly lower in the TM group than that in control.
Toll-Like Receptor 2
In the control group, CHS significantly decreased the mRNA level of TLR2 after 1, 3, and 5 D compared with day 0, whereas in the TM group, CHS did not significantly change TLR2 mRNA level compared with that in day 0 (Figure 3E). However, TLR2 mRNA level was significantly higher in the TM group than in the control group after 1 D of CHS.
Toll-Like Receptor 4
In the control group, CHS did not significantly change the mRNA level of TLR4 compared with that at day 0, but in the TM group, CHS significantly decreased the levels of TLR4 after 7 D compared with that at day 0 (Figure 3F). The mRNA level of TLR4 was significantly lower in the TM group than in the control group after 7 D of CHS.
Effects of CHS on the Intestinal mRNA Levels of Heat-Shock Proteins in Broiler Chickens Subjected to TM
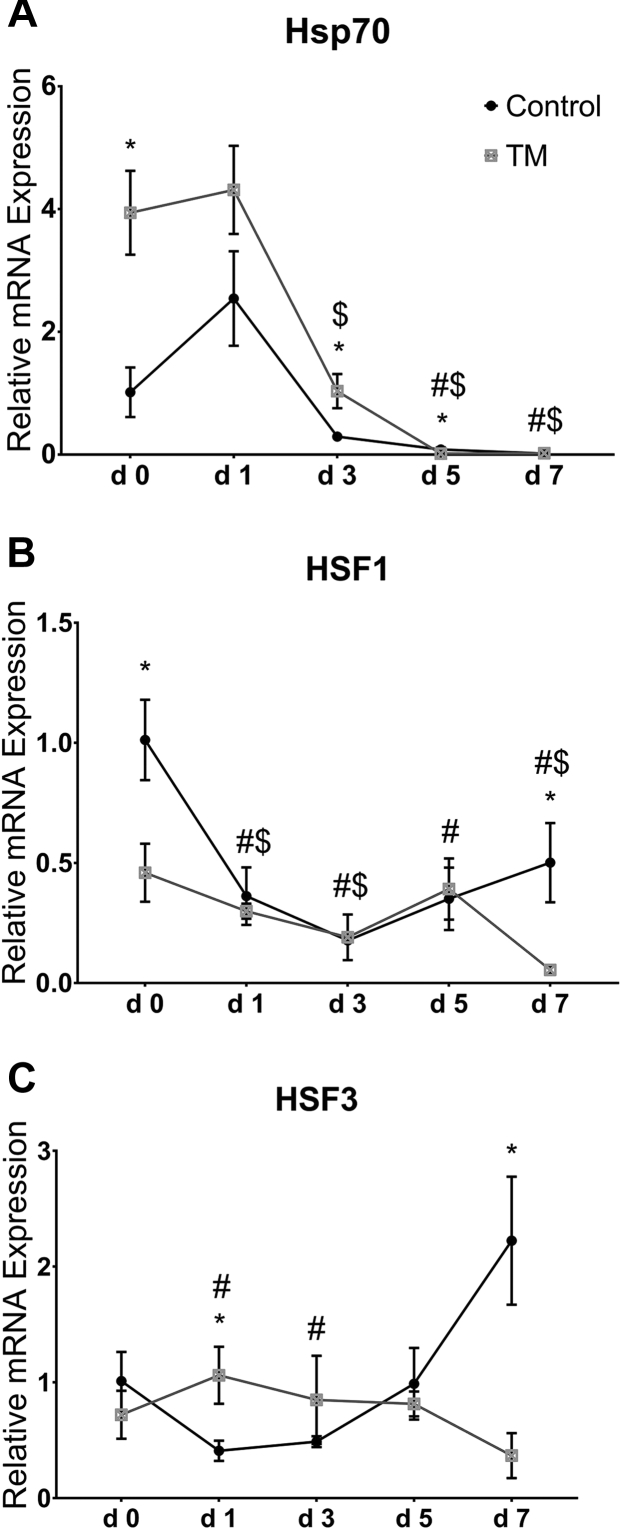
Figure 4A–C represents the effects of CHS on the intestinal mRNA levels of Hsp in broiler chickens subjected to TM.
Figure 4.
Effect of chronic heat stress (CHS) (at 35°C) on the intestinal mRNA levels of heat shock protein 70 (Hsp70) (A), heat shock transcription factor (HSF)-1 (B) and HSF3 (C) in control and thermal manipulation (TM) broiler chickens (n = 5). The values in the chart indicates folds of mRNA level in the control group day 0. *Within same day, mean ± SD of TM is significantly different compared to control (P < 0.05). #Within the control group, mean ± standard deviation (SD) of day 1, 3, 5, or 7 is significantly different compared to day 0 (P < 0.05). $Within the TM group, mean ± SD of day 1, 3, 5, or 7 is significantly different compared to day 0 (P < 0.05).
Heat Shock Protein 70
In the control group, CHS significantly decreased the mRNA level of Hsp70 after 5 and 7 D compared with that in day 0, while in the TM group, CHS significantly decreased the mRNA levels of Hsp70 after 3, 5, and 7 D compared with those at day 0 (Figure 4A). The mRNA level of Hsp70 was significantly higher after 3 D and significantly lower after 5 D of CHS in TM than that in the control group.
Heat Shock Transcription Factor 1
In the control group, CHS significantly decreased the mRNA levels of HSF1 after all time intervals compared with those in day 0, whereas in the TM group, CHS significantly decreased the mRNA levels of HSF1 after 1, 3, and 7 D compared with those at day 0 (Figure 4B). After 7 D of CHS, the mRNA level of HSF1 was significantly lower in the TM group than that in the control group.
Heat Shock Transcription Factor 3
In the control group, CHS significantly decreased the mRNA levels of HSF3 after 1 and 3 D compared with those in day 0, but in the TM group, CHS did not significantly change the mRNA level of HSF3 with respect to day 0 (Figure 4C). The mRNA level of HSF3 was significantly higher in the TM group than that in the control group after 1 D of CHS; however, after 7 D of CHS, the mRNA levels of HSF3 was significantly lower in TM chicks than those in control chicks.
Discussion
The present study aimed in evaluating effects of TM during embryogenesis (39°C and 65% RH for 18 h daily during ED 10-18) on the mRNA levels of antioxidant enzymes, proinflammatory cytokines, TLR, and HSP in the jejunal mucosae of broiler chickens exposed to chronic heat stress (35°C for 7 D).
In the present study, on posthatch day 28 (day 0 of CHS), there was no significant difference in the mRNA levels of antioxidant enzymes between TM and control groups. In the other hand, after CHS, there was a significant increase in the mRNA levels of antioxidant enzymes in TM and control groups; however, the TM group possessed lower mRNA levels than the control group. Similarly, it was previously found that TM led to lower mRNA levels of NOX, SOD, catalase, and GPX during posthatch acute heat stress in broiler chickens (Al-Zghoul et al., 2019). It was reported that elevated superoxide (O2-) level was associated with the increased NOX expression during heat stress in vitro, and it was suggested that NOX was the source of elevated ROS during high environmental temperatures (Kikusato et al., 2015). Thus, the current results suggest that the reduced NOX expression in the TM chickens ameliorate the heat-induced oxidative stress because the NOX-induced ROS production is decreased. Acute and chronic heat stress were shown to increase the activity and mRNA expression of antioxidant enzymes in broiler chickens as a protective response to minimize or prevent oxidative stress (Altan et al., 2003, Yang et al., 2010, Hao and Gu, 2014, Rimoldi et al., 2015). Therefore, the lower mRNA levels of antioxidant enzymes in TM groups as shown in the present study may suggest that heat stress was ameliorated and did not induce oxidative stress in TM chickens.
In the present study, TM had a higher basal mRNA level of Hsp70 and a lower basal mRNA level of HSF1 on posthatch day 28 (day 0 of CHS). However, CHS decreased jejunal Hsp70, HSF1, and HSF3 mRNA levels in both TM and control groups, but TM group possessed higher mRNA levels of HSF3 and Hsp70 on the first days of CHS, then, the levels of Hsp70, HSF1, and HSF3 were declined compared with those in the control group. Previously, it was shown that TM has a long-lasting impact on the expression of HSF1, HSF3, and Hsp70, which was associated with improved heat-tolerance acquisition in the broiler chickens (Al-Zghoul et al., 2013, Al-Zghoul et al., 2019). Such altered expression dynamics might be a result of epigenetic modifications that occurred as a response to TM during embryogenesis. Previously, it has been reported that TM affected the DNA methylation on the promoter of Hsp70, which, in turn, modify the expression of this protein during posthatch life (Vinoth et al., 2018). The reduced expression of Hsp70, HSF1, and HSF3 in the present study may indicate that TM chicks were more heat tolerant because the control chicks were still responding to heat stress and had higher HSP mRNA levels. Likewise, it was found previously that the expression of HSP were decreased after CHS in TM chickens (Vinoth et al., 2015).
The proinflammatory cytokines, including IL-8, IL-6, TNF-α, and IL-1β, have an important role in innate and acquired immunity, and they are major players in the regulation of the acute phase response (Wigley and Kaiser, 2003, Giansanti et al., 2006). Proinflammatory cytokines play a critical role in wound healing and regeneration of tissue injury (Rennekampff et al., 2000, Streetz et al., 2000, Bosch et al., 2002, Wigley and Kaiser, 2003, Eming et al., 2007, Crouser et al., 2009, Eming et al., 2009, McFarland-Mancini et al., 2010, Welc et al., 2013, Phillips et al., 2015). In vertebrates, several studies had reported that heat stress increases the expression of proinflammatory cytokines (Leon, 2007, Heled et al., 2013, Prakasam et al., 2013; Cheng et al., 2015, Varasteh et al., 2015, Al-Zghoul et al., 2019). In the present study, TM led to lower basal mRNA levels of IL-8 and IL-6, but during CHS exposure, TM chicks possessed higher IL-6 and IL-8 mRNA levels after 1 D and lower IL-1β, IL-6, and TNF-α mRNA levels after 3, 5, and 7 D of CHS than the control group. In contrast, it was previously found that TM led to increased basal mRNA level of IL-6 in the spleen and liver of broiler chickens; furthermore, the expression of proinflammatory cytokines IL-1β, TNF-α, and IL-6 were higher in TM chicks after the exposure to acute heat stress (Al-Zghoul et al., 2019). This contradiction might be due to the difference in tissues and organs evaluated and different stress conditions.
The increased IL-6 level after 1 D of CHS in TM chicks might be associated with the high level of HSF3 on the same day for these chicks. Previously, it has been reported that HSF3 is a transcription factor that activates the expression of both Hsp70 and IL-6 in chickens during heat stress; thus, IL-6 was suggested to work as a heat-shock gene (Prakasam, et al., 2013). However, the lower levels of proinflammatory cytokines after 3, 5, and 7 D of CHS in the TM group could be explained that this group was more tolerant to the heat stress than the control group because higher levels of proinflammatory cytokines indicate inflammation response, which may occur due to heat-induced tissue damage.
In the present study, at posthatch day 28 (day 0 of CHS), TM did not affect the basal mRNA levels of TLRs. However, in chicks subjected to CHS, the TLR2 mRNA level was higher after 1 D, and the mRNA level of TLR4 was lower after 7 D of CHS in TM groups than that in controls. Formerly, it was reported that acute heat stress increased the expression of TLR2 and TLR4 in broiler chickens (Huang, 2017, Al-Zghoul et al., 2019). Moreover, it was shown that TM did not affect the basal mRNA levels of TLR2 and TLR4 in broiler chickens in thermoneutral conditions, while during acute heat stress, TM resulted in enhanced TLR2 and TLR4 expression (Al-Zghoul et al., 2019). TLR are a part of the innate immunity, and they are called pathogen-recognition receptors; their function is to recognize certain pathogen-associated signals (such as lipopolysaccharide) and danger-associated molecular pattern (DAMPs) and subsequently induce inflammation (McCarthy et al., 2013). DAMP are the molecules that get out of the cells because of membrane damage (such as Hsp70, which also could be released by several tissues during stress) and work as ligands to TLR2 and TLR4 (Asea et al., 2002; Calderwood et al., 2007). The binding of Hsp70 to TLR2 and TLR4 results in the activation of signal transduction pathways that lead to the induction of IL-6 expression (Asea et al., 2002). This might explain the simultaneous increased mRNA levels of TLR2 and IL-6 after 1 D of CHS in the TM group. Moreover, in the control group, the increased mRNA level of TLR4 on day 7 of CHS may suggest that CHS induced inflammatory response; however, this was not observed in the TM group.
Conclusion
The results of the present study suggest that TM led to altered posthatch antioxidant, immunological, and HSP response to chronic heat stress in the jejunal mucosae of broiler chickens, probably indicating that TM mitigated CHS and improved heat-tolerance acquisition.
Acknowledgments
Authors would like to express their deep appreciation and thanks to the Deanship of Research/Jordan University of Science & Technology for its financial support of this work (grant#: 44/2019). The authors would also like to thank Eng. Ibrahim Alsukhni and Miss Amneh Tarkhan for their excellent technical assistance and valuable comments.
Conflict of Interest: Authors declare that they have no conflict of interest.
References
- Abbas G., Sultan M., Ahsan H., Haq N. Effect of dietary inclusion of sodium bicarbonate on blood profile of caged layers during summer. Pak. J. Agri. Sci. 2017;54:443–450. [Google Scholar]
- Abdelqader A., Al-Fataftah A. Effect of dietary butyric acid on performance, intestinal morphology, microflora composition and intestinal recovery of heat-stressed broilers. Livest. Sci. 2016;183:78–83. [Google Scholar]
- Al-Rukibat R.K., Al-Zghoul M.B., Hananeh W.M., Al-Natour M.Q., Abu-Basha E.A. Thermal manipulation during late embryogenesis: effect on body weight and temperature, thyroid hormones, and differential white blood cell counts in broiler chickens. Poult. Sci. 2017;96:234–240. doi: 10.3382/ps/pew298. [DOI] [PubMed] [Google Scholar]
- Al-Zghoul M.B., Dalab A.E., Ababneh M.M., Jawasreh K.I., Al Busadah K.A., Ismail Z.B. Thermal manipulation during chicken embryogenesis results in enhanced Hsp70 gene expression and the acquisition of thermotolerance. Res. Vet. Sci. 2013;95:502–507. doi: 10.1016/j.rvsc.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Al-Zghoul M.B., Dalab A.E., Yahya I.E., Althnaian T.A., Al-Ramadan S.Y., Ali A.M., Albokhadaim I.F., El-Bahr S.M., Al Busadah K.A., Hannon K.M. Thermal manipulation during broiler chicken embryogenesis: effect on mRNA expressions of Hsp108, Hsp70, Hsp47 and Hsf-3 during subsequent post-hatch thermal challenge. Res. Vet. Sci. 2015;103:211–217. doi: 10.1016/j.rvsc.2015.10.015. [DOI] [PubMed] [Google Scholar]
- Al-Zghoul M.B., El-Bahr S.M., Al-Rukibat R.K., Dalab A.E., Althnaian T.A., Al-Ramadan S.Y. Biochemical and molecular investigation of thermal manipulation protocols during broiler embryogenesis and subsequent thermal challenge. BMC Vet. Res. 2015;11:292. doi: 10.1186/s12917-015-0609-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Zghoul M.B., Ismail Z.B., Dalab A.E., Al-Ramadan A., Althnaian T.A., Al-Ramadan S.Y., Ali A.M., Albokhadaim I.F., Al Busadah K.A., Eljarah A., Jawasreh K.I., Hannon K.M. Hsp90, Hsp60 and HSF-1 genes expression in muscle, heart and brain of thermally manipulated broiler chicken. Res. Vet. Sci. 2015;99:105–111. doi: 10.1016/j.rvsc.2014.12.014. [DOI] [PubMed] [Google Scholar]
- Al-Zghoul M.B., Saleh K.M., Ababneh M.M.K. Effects of pre-hatch thermal manipulation and post-hatch acute heat stress on the mRNA expression of interleukin-6 and genes involved in its induction pathways in 2 broiler chicken breeds. Poult. Sci. 2018;98:1805–1819. doi: 10.3382/ps/pey499. [DOI] [PubMed] [Google Scholar]
- Al-Zghoul M.B., Sukker H., Ababneh M.M. Effect of thermal manipulation of broilers embryos on the response to heat-induced oxidative stress. Poult. Sci. 2019;98:991–1001. doi: 10.3382/ps/pey379. [DOI] [PubMed] [Google Scholar]
- Altan O., Altan A., Oguz I., Pabuccuoglu A., Konyalioglu S. Effects of heat stress on growth, some blood variables and lipid oxidation in broilers exposed to high temperature at an early age. Br. Poult. Sci. 2000;41:489–493. doi: 10.1080/713654965. [DOI] [PubMed] [Google Scholar]
- Altan Ö, Pabuçcuoğlu A., Altan A., Konyalioğlu S., Bayraktar H. Effect of heat stress on oxidative stress, lipid peroxidation and some stress parameters in broilers. Br. Poult. Sci. 2003;44:545–550. doi: 10.1080/00071660310001618334. [DOI] [PubMed] [Google Scholar]
- Asea A., Rehli M., Kabingu E., Boch J.A., Bare O., Auron P.E., Stevenson M.A., Calderwood S.K. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- Bosch I., Xhaja K., Estevez L., Raines G., Melichar H., Warke R.V., Fournier M.V., Ennis F.A., Rothman A.L. Increased production of interleukin-8 in primary human monocytes and in human epithelial and endothelial cell lines after dengue virus challenge. J. Virol. 2002;76:5588–5597. doi: 10.1128/JVI.76.11.5588-5597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood S.K., Mambula S.S., Gray P.J., Theriault J.R. Extracellular heat shock proteins in cell signaling. FEBS Lett. 2007;581:3689–3694. doi: 10.1016/j.febslet.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Cheng C.Y., Tu W.L., Wang S.H., Tang P.C., Chen C.F., Chen H.H., Lee Y.P., Chen S.E., Huang S.Y. Annotation of differential gene expression in small yellow follicles of a broiler-type strain of Taiwan country chickens in response to acute heat stress. PLoS One. 2015;10:e0143418. doi: 10.1371/journal.pone.0143418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouser E.D., Shao G., Julian M.W., Macre J.E., Shadel G.S., Tridandapani S., Huang Q., Wewers M.D. Monocyte activation by necrotic cells is promoted by mitochondrial proteins and formyl peptide receptors. Crit. Care Med. 2009;37:2000. doi: 10.1097/CCM.0b013e3181a001ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza L.F.A., Espinha L.P., de Almeida E.A., Lunedo R., Furlan R.L., Macari M. How heat stress (continuous or cyclical) interferes with nutrient digestibility, energy and nitrogen balances and performance in broilers. Livest. Sci. 2016;192:39–43. [Google Scholar]
- Del Vesco A.P., Khatlab A.S., Goes E.S.R., Utsunomiya K.S., Vieira J.S., Oliveira Neto A.R., Gasparino E. Age-related oxidative stress and antioxidant capacity in heat-stressed broilers. Animal. 2017;11:1783–1790. doi: 10.1017/S1751731117000386. [DOI] [PubMed] [Google Scholar]
- Dunning S., Ur Rehman A., Tiebosch M.H., Hannivoort R.A., Haijer F.W., Woudenberg J., van den Heuvel F.A., Buist-Homan M., Faber K.N., Moshage H. Glutathione and antioxidant enzymes serve complementary roles in protecting activated hepatic stellate cells against hydrogen peroxide-induced cell death. Biochim. Biophys. Acta. 2013;1832:2027–2034. doi: 10.1016/j.bbadis.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Eming S.A., Krieg T., Davidson J.M. Inflammation in wound repair: molecular and cellular mechanisms. J. Invest. Dermatol. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- Eming S.A., Hammerschmidt M., Krieg T., Roers A. Interrelation of immunity and tissue repair or regeneration. Semin. Cell. Dev. Biol. 2009;20:517–527. doi: 10.1016/j.semcdb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Fulda S., Gorman A.M., Hori O., Samali A. Cellular stress responses: cell survival and cell death. Int. J. Cell. Biol. 2010;2010:214074. doi: 10.1155/2010/214074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garriga C., Hunter R.R., Amat C., Planas J.M., Mitchell M.A., Moreto M. Heat stress increases apical glucose transport in the chicken jejunum. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R195–R201. doi: 10.1152/ajpregu.00393.2005. [DOI] [PubMed] [Google Scholar]
- Giansanti F., Giardi M., Botti D. Avian cytokines-an overview. Curr. Pharm. Des. 2006;12:3083–3099. doi: 10.2174/138161206777947542. [DOI] [PubMed] [Google Scholar]
- Hai L., Rong D., Zhang Z.Y. The effect of thermal environment on the digestion of broilers. J. Anim. Physiol. Anim. Nutr. 2000;83:57–64. [Google Scholar]
- Hao Y., Gu X.H. Effects of heat shock protein 90 expression on pectoralis major oxidation in broilers exposed to acute heat stress. Poult. Sci. 2014;93:2709–2717. doi: 10.3382/ps.2014-03993. [DOI] [PubMed] [Google Scholar]
- Havenstein G.B., Ferket P.R., Qureshi M.A. Growth, livability, and feed conversion of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003;82:1500–1508. doi: 10.1093/ps/82.10.1500. [DOI] [PubMed] [Google Scholar]
- He X., Lu Z., Ma B., Zhang L., Li J., Jiang Y., Zhou G., Gao F. Effects of chronic heat exposure on growth performance, intestinal epithelial histology, appetite-related hormones and genes expression in broilers. J. Sci. Food Agric. 2018;98:4471–4478. doi: 10.1002/jsfa.8971. [DOI] [PubMed] [Google Scholar]
- Heled Y., Fleischmann C., Epstein Y. Cytokines and their role in hyperthermia and heat stroke. J. Basic Clin. Physiol. Pharmacol. 2013;24:85–96. doi: 10.1515/jbcpp-2012-0040. [DOI] [PubMed] [Google Scholar]
- Huang C., Jiao H., Song Z., Zhao J., Wang X., Lin H. Heat stress impairs mitochondria functions and induces oxidative injury in broiler chickens. J. Anim. Sci. 2015;93:2144–2153. doi: 10.2527/jas.2014-8739. [DOI] [PubMed] [Google Scholar]
- Huang S. Upregulation of TLR4 mRNA expression levels in broiler chickens under acute heat stress. Rev. Bras. Cienc. Avic. 2017;19:87–94. [Google Scholar]
- Ighodaro O., Akinloye O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2017;54:287–293. [Google Scholar]
- Kaiser P., Stäheli P. Avian Immunology. 2nd ed. Elsevier; Cambridge, MA: 2014. Avian Cytokines and Chemokines; pp. 189–204. [Google Scholar]
- Kikusato M., Yoshida H., Furukawa K., Toyomizu M. Effect of heat stress-induced production of mitochondrial reactive oxygen species on NADPH oxidase and heme oxygenase-1 mRNA levels in avian muscle cells. J. Therm. Biol. 2015;52:8–13. doi: 10.1016/j.jtherbio.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Kirkman H.N., Gaetani G.F. Catalase: a tetrameric enzyme with four tightly bound molecules of NADPH. Proc. Natl. Acad. Sci. U S A. 1984;81:4343–4347. doi: 10.1073/pnas.81.14.4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregel K.C. Invited review: heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J. Appl. Physiol. 2002;92:2177–2186. doi: 10.1152/japplphysiol.01267.2001. [DOI] [PubMed] [Google Scholar]
- Kushner I. Regulation of the acute phase response by cytokines. Perspect. Biol. Med. 1993;36:611–622. doi: 10.1353/pbm.1993.0004. [DOI] [PubMed] [Google Scholar]
- Lara L.J., Rostagno M.H. Impact of heat stress on poultry production. Animals (Basel) 2013;3:356–369. doi: 10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon L.R. Heat stroke and cytokines. Prog. Brain Res. 2007;162:481–524. doi: 10.1016/S0079-6123(06)62024-4. [DOI] [PubMed] [Google Scholar]
- Lin H., Decuypere E., Buyse J. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2006;144:11–17. doi: 10.1016/j.cbpa.2006.01.032. [DOI] [PubMed] [Google Scholar]
- McCarthy C.G., Goulopoulou S., Wenceslau C.F., Spitler K., Matsumoto T., Webb R.C. Toll-like receptors and damage-associated molecular patterns: novel links between inflammation and hypertension. Am. J. Physiol. Heart Circ. Physiol. 2013;306:H184–H196. doi: 10.1152/ajpheart.00328.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland-Mancini M.M., Funk H.M., Paluch A.M., Zhou M., Giridhar P.V., Mercer C.A., Kozma S.C., Drew A.F. Differences in wound healing in mice with deficiency of IL-6 versus IL-6 receptor. J. Immunol. 2010;184:7219–7228. doi: 10.4049/jimmunol.0901929. [DOI] [PubMed] [Google Scholar]
- Molenaar R., Reijrink I.A.M., Meijerhof R., Van den Brand H. Meeting embryonic requirements of broilers throughout incubation: a review. Rev. Bras. Cienc. Avic. 2010;12:137–148. [Google Scholar]
- Morimoto R.I. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- Mujahid A., Yoshiki Y., Akiba Y., Toyomizu M. Superoxide radical production in chicken skeletal muscle induced by acute heat stress. Poult. Sci. 2005;84:307–314. doi: 10.1093/ps/84.2.307. [DOI] [PubMed] [Google Scholar]
- Phillips N.A., Welc S.S., Wallet S.M., King M.A., Clanton T.L. Protection of intestinal injury during heat stroke in mice by interleukin-6 pretreatment. J. Physiol. 2015;593:739–752. doi: 10.1113/jphysiol.2014.283416. discussion 753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piestun Y., Shinder D., Ruzal M., Halevy O., Brake J., Yahav S. Thermal manipulations during broiler embryogenesis: effect on the acquisition of thermotolerance. Poult. Sci. 2008;87:1516–1525. doi: 10.3382/ps.2008-00030. [DOI] [PubMed] [Google Scholar]
- Prakasam R., Fujimoto M., Takii R., Hayashida N., Takaki E., Tan K., Wu F., Inouye S., Nakai A. Chicken IL-6 is a heat-shock gene. FEBS Lett. 2013;587:3541–3547. doi: 10.1016/j.febslet.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Quinteiro-Filho W.M., Ribeiro A., Ferraz-de-Paula V., Pinheiro M.L., Sakai M., Sa L.R., Ferreira A.J., Palermo-Neto J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010;89:1905–1914. doi: 10.3382/ps.2010-00812. [DOI] [PubMed] [Google Scholar]
- Ray P.D., Huang B.W., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennekampff H.-O., Hansbrough J.F., Kiessig V., Doré C., Sticherling M., Schröder J.-M. Bioactive interleukin-8 is expressed in wounds and enhances wound healing. J. Surg. Res. 2000;93:41–54. doi: 10.1006/jsre.2000.5892. [DOI] [PubMed] [Google Scholar]
- Rimoldi S., Lasagna E., Sarti F.M., Marelli S.P., Cozzi M.C., Bernardini G., Terova G. Expression profile of six stress-related genes and productive performances of fast and slow growing broiler strains reared under heat stress conditions. Meta Gene. 2015;6:17–25. doi: 10.1016/j.mgene.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routman K.S., Yoshida L., Frizzas de Lima A.C., Macari M., Pizauro J.M., Jr. Intestinal and pancreas enzyme activity of broilers exposed to thermal stress. Rev. Bras. Cienc. Avic. 2003;5:23–27. [Google Scholar]
- Settar P., Yalcin S., Turkmut L., Ozkan S., Cahanar A. Season by genotype interaction related to broiler growth rate and heat tolerance. Poult. Sci. 1999;78:1353–1358. doi: 10.1093/ps/78.10.1353. [DOI] [PubMed] [Google Scholar]
- Song D., King A. Effects of heat stress on broiler meat quality. Worlds Poult. Sci. J. 2015;71:701–709. [Google Scholar]
- Song J., Xiao K., Ke Y.L., Jiao L.F., Hu C.H., Diao Q.Y., Shi B., Zou X.T. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult. Sci. 2014;93:581–588. doi: 10.3382/ps.2013-03455. [DOI] [PubMed] [Google Scholar]
- Song Z.H., Cheng K., Zheng X.C., Ahmad H., Zhang L.L., Wang T. Effects of dietary supplementation with enzymatically treated Artemisia annua on growth performance, intestinal morphology, digestive enzyme activities, immunity, and antioxidant capacity of heat-stressed broilers. Poult. Sci. 2017;97:430–437. doi: 10.3382/ps/pex312. [DOI] [PubMed] [Google Scholar]
- Streetz K.L., Luedde T., Manns M.P., Trautwein C. Interleukin 6 and liver regeneration. Gut. 2000;47:309–312. doi: 10.1136/gut.47.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surai P. Antioxidant systems in poultry biology: superoxide dismutase. J. Ani. Res. Nutr. 2015;1 [Google Scholar]
- Varasteh S., Braber S., Akbari P., Garssen J., Fink-Gremmels J. Differences in susceptibility to heat stress along the chicken intestine and the protective effects of galacto-oligosaccharides. PLoS One. 2015;10:e0138975. doi: 10.1371/journal.pone.0138975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinoth A., Thirunalasundari T., Tharian J.A., Shanmugam M., Rajkumar U. Effect of thermal manipulation during embryogenesis on liver heat shock protein expression in chronic heat stressed colored broiler chickens. J. Therm. Biol. 2015;53:162–171. doi: 10.1016/j.jtherbio.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Vinoth A., Thirunalasundari T., Shanmugam M., Uthrakumar A., Suji S., Rajkumar U. Evaluation of DNA methylation and mRNA expression of heat shock proteins in thermal manipulated chicken. Cell Stress Chaperones. 2018;23:235–252. doi: 10.1007/s12192-017-0837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welc S.S., Clanton T.L., Dineen S.M., Leon L.R. Heat stroke activates a stress-induced cytokine response in skeletal muscle. J. Appl. Physiol. 2013;115:1126–1137. doi: 10.1152/japplphysiol.00636.2013. [DOI] [PubMed] [Google Scholar]
- Wigley P., Kaiser P. Avian cytokines in health and disease. Rev. Bras. Cienc. Avic. 2003;5:1–14. [Google Scholar]
- Xiaofang H., Lu Z., Ma B., Zhang L., Li J., Jiang Y., Zhou G., Gao F. Chronic heat stress damages small intestinal epithelium associated with AMPK pathway in broilers. J. Agric. Food Chem. 2018;66:7301–7309. doi: 10.1021/acs.jafc.8b02145. [DOI] [PubMed] [Google Scholar]
- Yang L., Tan G.Y., Fu Y.Q., Feng J.H., Zhang M.H. Effects of acute heat stress and subsequent stress removal on function of hepatic mitochondrial respiration, ROS production and lipid peroxidation in broiler chickens. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2010;151:204–208. doi: 10.1016/j.cbpc.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Yi D., Hou Y., Tan L., Liao M., Xie J., Wang L., Ding B., Yang Y., Gong J. N-acetylcysteine improves the growth performance and intestinal function in the heat-stressed broilers. Anim. Feed Sci. Technol. 2016;220:83–92. [Google Scholar]