Abstract
The metabolites of stored, chilled chicken meat were analyzed using liquid chromatograph-mass spectrometry and metabolomics. The results showed significant differences in the metabolites of chicken meat stored at 4°C for 0 D and meat stored for longer periods of 2 D, 4 D, 6 D, and 10 D, when analyzed based on a variable of importance >2 and P < 0.05. These changed metabolites included amino acids, amines, nucleosides, nucleotides, carbohydrates, organic acids, and other substances. The data from this study provide a holistic understanding of food quality changes in chicken meat during deterioration in storage.
Key words: chilled chicken, metabolomics, quality
Introduction
Metabolomics is the study of small metabolites that weigh less than 1,500 Da (Bover-Cid et al., 2000). In recent years, metabolomics has been widely used in research on disease diagnosis (Janeckova et al., 2015), plants (Schwahn et al., 2014), microbial metabolism (Lechtenfeld et al., 2015), breeding (Słowińska et al., 2018), and the environment (Kouremenos et al., 2014). The development of metabolomics in food science is in its early stage but has progressed rapidly to become the technology of choice for solving problems in food science.
Metabolomics has been employed to monitor real-time, dynamic changes in metabolites in meat during storage and during different processing conditions (Castro-Puyana et al., 2017). This approach provides an important theoretical basis to explain the mechanism underlying changes in meat quality and to improve food storage technology. Lana (Lana et al., 2015) used metabolomics and bioinformatics analysis to monitor changes in beef quality stored at 1°C for 0, 1, 10, 17, and 44 D, and they found that glutamic acid, serine, and arginine could be used as parameters or indicators of meat flavor. Subbaraj (Subbaraj et al., 2016) employed hydrophilic interaction liquid chromatography–mass spectrometry metabolomics to study the color change stability of meat at different maturation levels, storage times, and storage and packaging methods.
Nuclear magnetic resonance technology plays an important role in metabolomics. Wang (Wang et al., 2016) used 600-MHz H-1 nuclear magnetic resonance spectroscopy to analyze the quality of duck meat. Shumilina (Shumilina et al., 2016) applied the technology to evaluate and quantify quality changes in stored salmon byproducts. Warner (Warner et al., 2015) used a combined, nontargeted, metabolomics technique that included 1H and targeted 31P nuclear magnetic resonance and liquid chromatography coupled with a high-performance liquid chromatography (HPLC) photodiode array and HPLC with tandem mass spectrometry to investigate changes in energy metabolism in the long and lumbar muscles of lamb meat during rapid cooling and other treatment conditions. Gas chromatography–mass spectrometry has been used to reveal flavor formation in ham (Shi et al., 2019). In addition, Muroya (Muroya et al., 2014) used capillary electrophoresis–time of flight mass spectrometry to determine key compounds associated with pork quality.
The metabolic and quality changes in chilled chicken meat are highly complex processes. Current research on this topic focuses on analyzing physiological and biochemical indexes related to metabolism (e.g., total volatile base nitrogen, pH). Thus far, few studies have concentrated on the metabolomics of cold, fresh meat. Thus, the dynamic changes in metabolic components and the mechanisms underlying chicken meat corruption during storage remain unclear.
This study assessed chilled chicken meat obtained from a local market. The chicken packaged by food fresh-keeping bags was stored in a refrigerator (4°C) and sampled at 5 storage time points (0, 2, 4, 6, and 10 D). The meat then was subjected to a nontargeted, metabolomic study using liquid chromatography–mass spectrometry. The key metabolites and metabolic pathways related to changes in meat quality were screened and identified via data analysis. The biological significance of these metabolites was systematically interpreted to explain metabolic changes caused by microorganisms and endogenous proteases in chilled chicken meat. This article thus provides a scientific basis for better quality control and storage efficiency of meat products.
Materials and methods
Materials
All meat used in this study came from 50-day-old, broiler hens (Ross 308 white breed) from Qingyuan Chicken Fam (Guangdong, China). Live chickens were kept in the same conditions and fed the same feed, then killed in a municipal abattoir, regardless of weight (approximate average live weight of 2.5 ± 0.3 kg). After slaughter, chickens were butchered and transported within 30 min to Carrefour Supermarket (Wan Guo Plaza, Haizhu District, Guangzhou, China) via cold chain (4 ± 1°C) transportation. Pieces of chicken breasts from the same batch were chosen randomly, placed in an icebox, and transported to the laboratory within 10 min. Once at the lab, all chicken breast pieces were divided into 5 groups under sterile conditions. Each group contained 10 replicates (biological repeat samples) weighing 25 g each. Finally, each sample was sealed in a plastic bag and stored at 4°C. The entire process from slaughter to sample storage took no longer than 1 h. The meat samples that were analyzed the same day as the date of purchase were labeled as 0 D (control group). The remaining samples were divided into 4 experimental groups (2 D, 4 D, 6 D, and 10 D) based on storage time.
Methods
Sample Preparation
The samples were treated as described by Dasenaki et al. (Dasenaki et al., 2016), with minor modifications. 200 μL of water and 800 μL of methanol and acetonitrile (1:1 v/v) were added to 100 mg of samples to disrupt and extract the metabolites. After vortexing and ultrasound sonicating twice for 30 min, the mixtures were stored at −20°C for 1 h. After centrifugation at 14,000 × g for 15 min at 4°C, the supernatant was subjected to vacuum evaporation. Before mass spectrometric measurement, samples were reconstituted in 100 μL of acetonitrile and water (1:1 v/v). After vortexing and centrifuging, all samples were stored for further analysis.
Liquid Chromatography–Tandem Mass Spectrometry Conditions
The liquid chromatography–tandem mass spectrometry analysis was performed using an ultra-HPLC system (Agilent 1290 Infinity LC, Agilent Technologies, Santa Clara, CA) connected to a quadruple time-of-flight mass spectrometer (Triple TOF 6600, AB SCIEX, USA). Hydrophilic interaction liquid chromatography separation was conducted using 2 columns: an ACQUIY UPLC BEH Amide (2.1 mm × 100 mm, 1.7 μm) and ACQUIY UPLC HSS T3 (2.1 mm × 100 mm, 1.8 μm) column (Waters, USA). Each sample (2 μL) was injected at a flow rate of 300 μL/min.
Mobile phases consisted of buffer A (25 mM NH4Ac and 25 mM NH4OH in water) and B (acetonitrile). A linear gradient of buffer B was 85% B for 1 min; 65% B for 12 min; 40% B for 3 min; and finally, 85% B for 5 min. During analysis, samples were maintained in an autosampler at 4°C.
Electrospray ionization (ESI) source conditions on the triple time-of-flight were set as follows: Ion Source Gas 1 and Gas 2 at 60, curtain gas at 30, and source temperature of 600°C. The instrument was set to acquire over the m/z range of 60–1,000 Da, with time-of-flight mass spectrometry scan and m/z range of 25–1,000 Da for the product ion scan. The accumulation time for the time-of-flight mass spectrometry scan was set at 0.20 s/spectra and the product ion scan at 0.05 s/spectra. The unit resolution was selected for precursor ion selection, and the collision energy was fixed at 35 ± 15 eV. The declustering potential was set as ±60 V in positive and negative modes.
For HSS T3 column, the mobile phase consisted of solvent A (0.1% formic acid) and B (0.5 mM of ammonium fluoride in water) in ESI + mode. In ESI mode, the mobile phase consisted of solvent A (0.1% formic acid–acetonitrile) and solvent B (acetonitrile). The gradient was set at 99:1 (A:B) for 1.5 min initially, changed linearly to 1:99 (A:B), and maintained for 3.5 min. The conditions were returned to the starting conditions within 0.1 min and then kept for 20 min. Most conditions were the same as previously described for mass spectrometry, unless Gas1 was set at 40, Gas2 at 80, temperature at 650°C, and IonSpray voltage floating at ±5000 V.
Data Quality Assessment
The stability of the analysis can reflect the reliability of the result. To minimize errors and enhance data accuracy, in each experiment, every 5 samples were set as quality control samples. Large overlaps in the sample quality control spectra indicate good stability and therefore high reliability of the experimental data.
Data Processing and Analysis
After the original data were converted into mzML format using Proteo Wizard, a series of operations including peak alignment, retention time correction, and peak area extraction were conducted using XCMS software. The ion peak of missing data (>50%) was deleted from the XCMS data, and the Perato scaling method was used for normalization (Want et al., 2010). After that, multivariate and univariate statistical analyses, including unsupervised principal component analysis, supervised partial least squares discriminant analysis (PLS-DA), fold-change, t test (P < 0.05), and one-way ANOVA at P < 0.01 (Benton et al., 2015), were conducted using Metabo Analyst 3.0 software. A boxplot was used to reflect significant differences between dynamic changes in metabolites. The metabolic pathway was analyzed according to the Kyoto Encyclopedia of Genes and Genomes database.
Results and discussion
Quality Control of Experiment
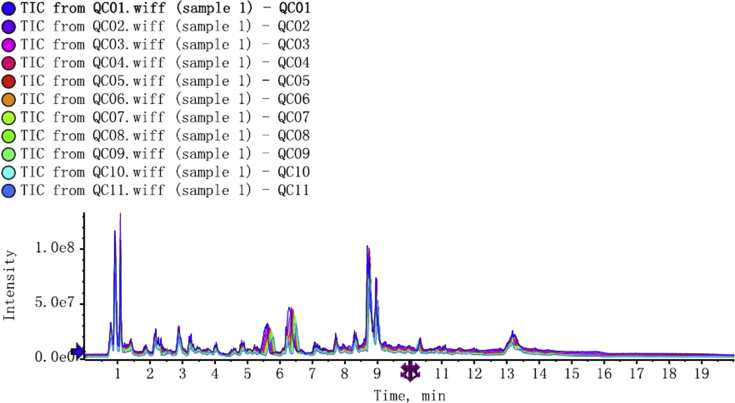
Figure 1 shows the ultra-HPLC Q-TOF mass spectrometry spectra of 11 quality control samples and their overlaps, represented by different colors. In the spectra, the horizontal coordinate represents the retention time, and the vertical coordinate represents the response intensity. Only the overlap of the hydrophilic interaction liquid chromatography cation is shown. The overlapping peaks suggest high stability of the detection system and good reliability of the experimental data.
Figure 1.
Positive-ionization-mode total ion current spectra of quality control samples and their hydrophilic interaction chromatography overlap.
Principal Component Analysis of Chilled Chicken Meat Tissue Samples
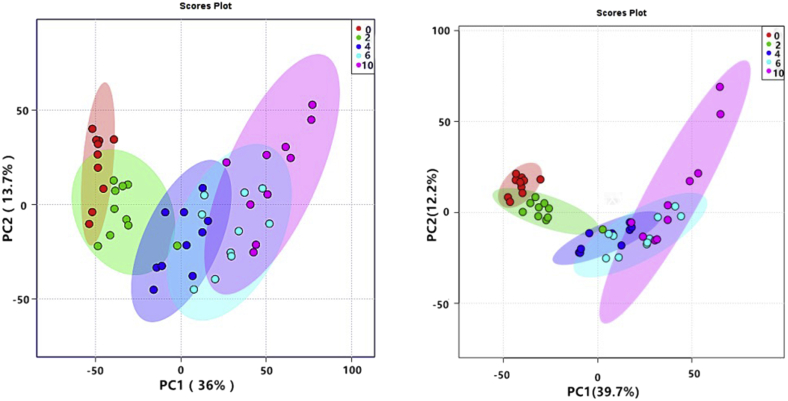
Figures 2 and 3 depict the principal component analysis results for the chilled chicken meat samples. Results were obtained based on the positive and negative ionization mode data from the BEH Amide and HSS T3 columns, respectively. Each dot represents a sample. Dots with the same color are samples from the same group (i.e., the same storage period), and the distance between each dot represents the difference or similarity between metabolites in the samples. As shown in Figure 2, the data obtained by the BEH Amide column in both ESI+ and ESI− modes of the 5 groups of samples were clustered, with good separations into 5 groups based on the number of storage days.
Figure 2.
Principal component analysis of chilled chicken meat samples based on positive and negative ion modes of the hydrophilic interaction chromatography column.
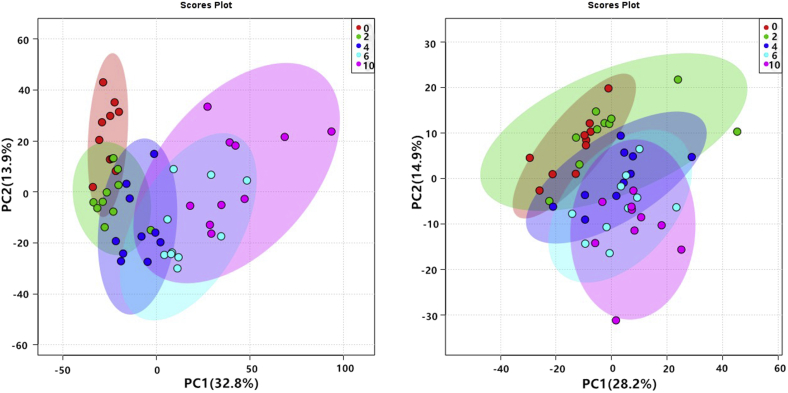
Figure 3.
Principal component analysis of chilled chicken meat samples based on the positive and negative ion modes of the HSS T3 column.
The principal component analysis shows that the 0-day group could be distinguished from the 2-day group and that groups with longer storage had better differentiation from the 0-day group. This result indicates that the overall change tendency of metabolites kept increasing with the increase in chilled storage time. In addition, the longer the storage period, the larger the distance between each sample in the control group. However, the data obtained by the HSS T3 column (Figure 3) were relatively dispersed among samples in the same group. Despite some overlaps in both the PC1 and PC2 diagrams, the separation between the samples in the positive ionization modes was obvious, whereas that between samples in the negative ionization mode was not. The overall distribution of dots in the PC1 chart was chronological, and the dots of the 4 experimental groups were farther away from those of the control. These data indicate that the metabolites in chilled chicken meat tend to change over time, which may be caused by decomposition during storage.
Screening and Identifying Different Metabolites
To identify differences between the metabolites in each chilled chicken meat samples, PLS-DA models for these samples were compared, respectively. Table 1 lists the evaluation parameters (R2, Q2) obtained from the PLS-DA models after being verified by 10 interactional cycles. When R2 values were closer to 1 and Q2 values greater than 0.6, the models established by both BEH Amide and HSS T3 column data were reliable, indicating that the models have good predictive ability.
Table 1.
Evaluation parameters obtained from PLS-DA models.
| Mode | HILIC |
HSS T3 |
||||
|---|---|---|---|---|---|---|
| PC | R2 (cum) | Q2 (cum) | PC | R2 (cum) | Q2 (cum) | |
| ESI+ | 4 | 0.99865 | 0.93448 | 5 | 0.99981 | 0.8924 |
| ESI− | 5 | 0.99992 | 0.94174 | 4 | 0.99557 | 0.65937 |
Abbreviations: ESI, electrospray ionization; HILIC, hydrophilic interaction liquid chromatography; PLS-DA, partial least squares discriminant analysis.
R2: represents the model interpretation rate; Q2: represents the prediction ability of the model; The closer R2 and Q2 are to 1, the more stable and reliable the model is.
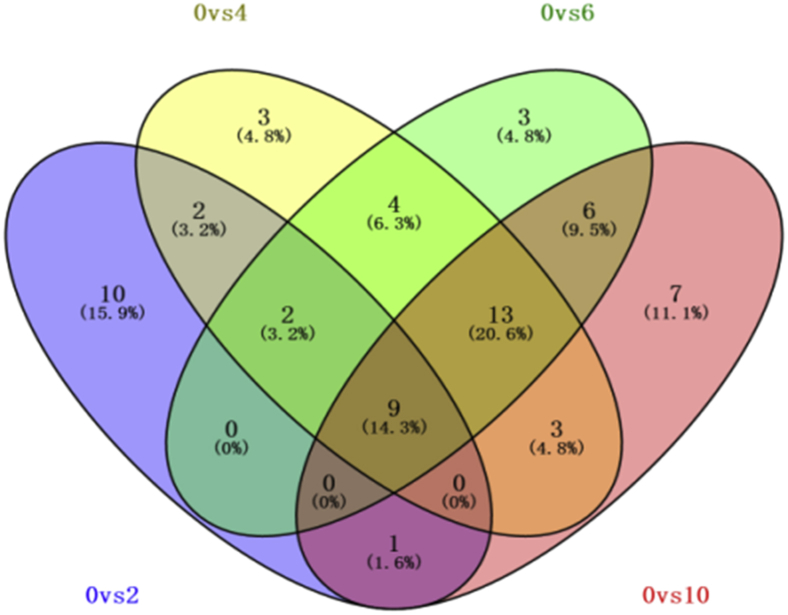
According to the PLS-DA models, a variable importance (VIP) variable >2 was used as a standard value to screen differences between groups. Differences between 2 groups that satisfied both VIP > 2 and P < 0.05 were considered significant. Finally, the different metabolites were compared and identified using a self-built database and the Human Metabolome Database, as shown in the Supplementary Tables 1–4. Figure 4 shows a Venn diagram illustrating the distribution of different metabolites in samples stored for 0, 2, 4, 6, and 10 D.
Figure 4.
Venn diagram showing distribution of metabolites in different samples.
Analysis of Different Metabolites in Chilled Chicken Meat Samples at Different Storage Periods
A total of 23,868 molecular features were extracted from all samples (Table 2). The peak intensity of each feature was obtained, and 175 metabolites were annotated. Using principal component analysis, partial least squares discriminant analysis, and hierarchical clustering analysis, 63 distinct differential metabolites were identified, including amino acids, sugars, amines, nucleosides, nucleotides, and organic acids.
Table 2.
Numbers of peaks.
| Mode of ESI | HILIC | HSS T3 |
|---|---|---|
| Positive | 11,816 | 5,132 |
| Negative | 5,733 | 1,187 |
Abbreviations: ESI, electrospray ionization; HILIC, hydrophilic interaction liquid chromatography.
To verify whether significant differences in metabolites occurred over storage time, a one-way ANOVA was employed to observe differences in metabolite levels. Additionally, a boxplot analysis was used to analyze relative content changes during each of the 5 storage periods. The horizontal coordinate represents the storage days, and the vertical coordinate represents the peak strength of metabolites.
Amino Acids
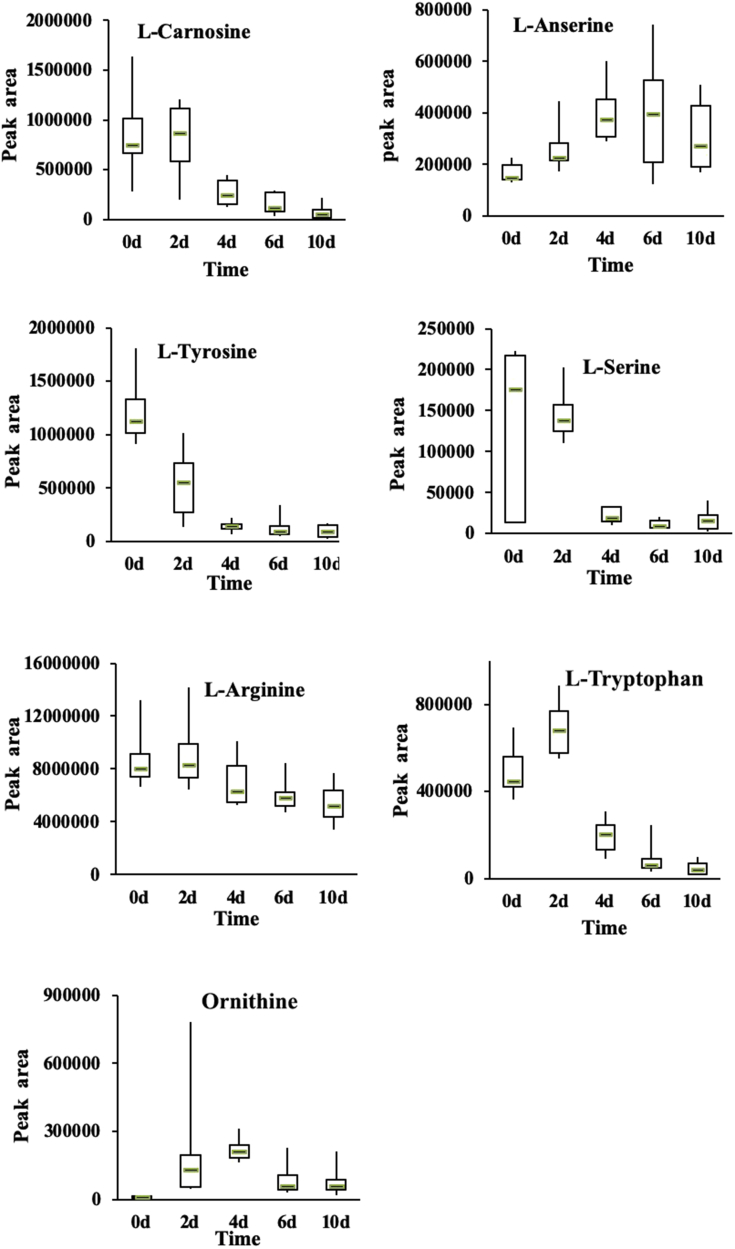
During refrigeration, microorganisms can decompose proteins in chicken meat into various intermediate products, such as small molecular peptides and free amino acids (Wang et al., 2017). We found that the contents of small-molecule peptides L-carnosine and L-anserine differed significantly across storage periods (P < 0.01). These small peptides greatly influenced the quality of the meat during cold storage.
Carnosine is a potential active oxygen scavenger with antioxidant capacity, and it affects the stability of meat color via inhibiting fat peroxidation and protein damage (Xiao et al., 2018). With increasing storage time, myoglobin in the tissue of chilled chicken meat gradually converts to high iron-containing isoform, causing the meat color to turn dark brown (Ramanathan et al., 2011). Carnosine can also effectively inhibit fat oxidation and formation of high-iron myoglobin, thus preserving meat color (D'Astous-Page et al., 2017, Sundekilde et al., 2017). Peptides in goose muscle peptide have similar abilities.
Figure 5 shows the box diagram representing the interval distribution between 25 and 75%. The horizontal line in the middle of the box represents the median. The relative contents of carnosine increased as storage time increased from 0 to 2 D and that it declined after 2 D, which might associate with gradual color changes in chilled chicken meat during storage. In addition, the content of anserine increased during the first 0 to 6 D but declined thereafter, suggesting a relationship between anserine and quality of chilled chicken meat.
Figure 5.
Relative changes of amino acids in chilled chicken meat samples during chilled storage.
Free amino acids are important components of nonprotein nitrogen and an important flavor and flavor precursor substance in chicken meat. Microbial and endogenous enzymes degraded and decarboxylated free amino acids to form bioamines or other secondary metabolites (Bover-Cid et al., 2000, Martuscelli et al., 2009). Many free amino acids are closely related to meat flavor and can be used as indicators of quality and freshness (Leggio et al., 2012). Free amino acids detected in the study included L-tyrosine, L-arginine, L-tryptophan, L-serine, D-proline, L-histidine, L-pyroglutamic acid, and ornithine. We found that as storage time increased, the quality of the chilled chicken meat gradually changed from fresh to sub-fresh to rotten. Accordingly, some amino acids differed across different storage periods (Figure 5). L-carnosine, L-tyrosine, L-serine, and L-arginine decreased with increased storage days. However, L-tryptophan and ornithine increased between 0 and 2 D of storage and between 0 and 4 D of storage, respectively, after which they both decreased. The actions of endogenous proteases and microorganisms are associated with free amino acid generation (Yang et al., 2016, Oh et al., 2019, Wickramasinghe et al., 2019).
Bioamines
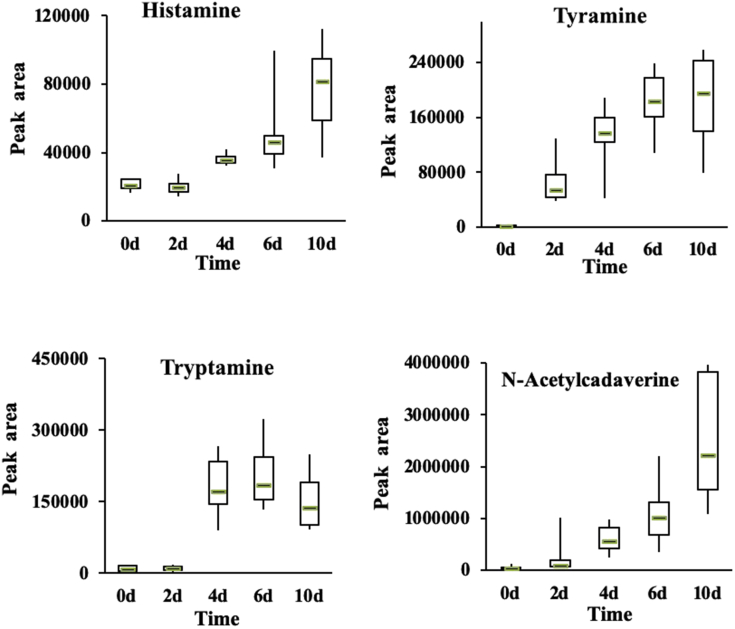
Bioamines are bioactive, low–molecular weight compounds found in many different food products, such as aquatic and meat products. A small amount of bioamines are sufficient to maintain proper physiological function of organisms, but excessive amounts can lead to adverse effects on human health (Alvarez and Moreno-Arribas, 2014, Tofalo et al., 2016, Papageorgiou et al., 2018). Bioamines are formed during food storage when microbial enzymes decarboxylate food proteins or amino acids (Halász et al., 1994, Lorenzo et al., 2017). Bioamines also are closely linked to bacterial growth. Because bioamine content can indirectly reflect changes in bacterial content, it can also be used to measure food deterioration and shelf life (Suzzi and Gardini, 2003, Lázaro et al., 2015). The bioamines include histamine, cadaverine, tyramine, putrescine, and tryptamine, and the precursors are histidine, lysine, tyrosine, ornithine, and tryptophan (Santos, 1996, Onal, 2007).
Among all bioamines, histamine poses the highest potential threat to human health, followed by tyramine. We found that tryptamine decreased at storage day 10, but the relative contents of histamine, tyramine, and N-acetyl cadaverine increased as the storage days increased (Figure 6). The contents of histamine, tyramine, and N-acetyl cadaverine were low during the first 0 to 2 D of storage but increased rapidly on day 4. Compared with the 0-day group, the fold-change increased the most for histamine and tyramine at 10 D of storage (87.15 and 122.67, respectively).
Figure 6.
Relative changes of bioamines in chilled chicken meat samples during chilled storage.
It has been reported that Pseudomonas, Proteus, and Lactobacillus species in chilled meat have strong decarboxylation capacity decarboxylate for converting amino acids into bioamines (Santos, 1996, Wen et al., 2018). Aeromonas salmonicida 35, Pseudomonas fluorescens H5, and Pseudomonas fragi H8 isolated from chilled chicken meat can hydrolyze the protein in chilled chicken meat, increase the total volatile base nitrogen, and generate alcohols, aldehydes, ketones, and sulfur compounds during storage (Wang et al., 2017). Further study is needed to determine whether other spoilage organisms, including Myroides, can decarboxylate amino acids into bioamines in chilled chicken meat.
Nucleosides and Nucleotides
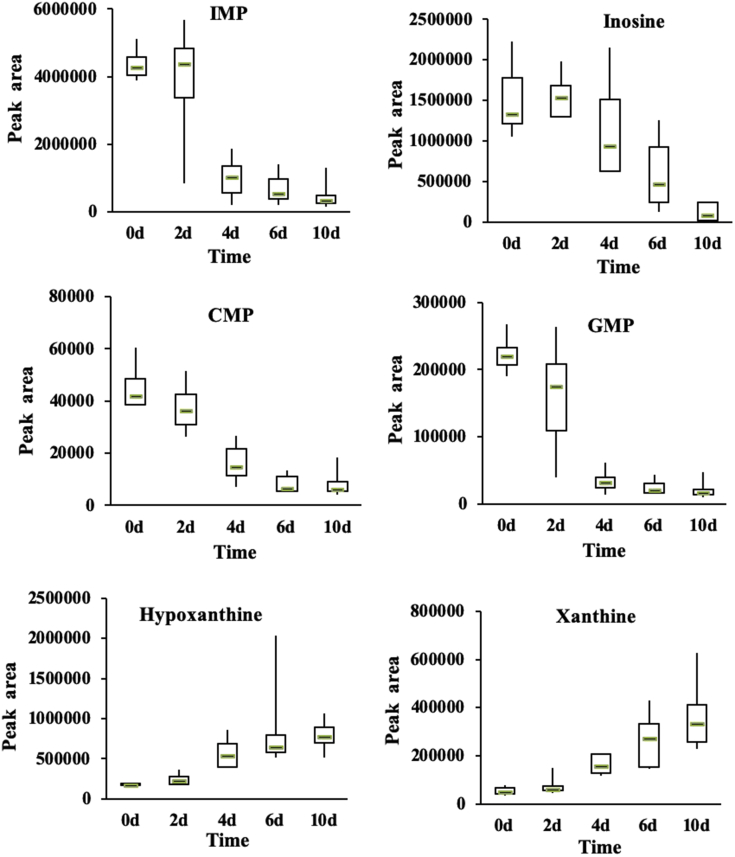
Nucleosides and nucleotides have many physiological functions, including directly participating in the metabolism of other substances through different biochemical processes. Changes in amino acids and other compounds during storage can affect the freshness and taste of meat (Ryu et al., 2009). Nucleotides also contribute to freshness, particularly adenosine-5′-triphosphate (ATP) (Dannert and Pearson, 1967). During storage, ATP in meat can be degraded by various enzymes into a series of related products that deteriorate meat flavor and color (Li et al., 2017).
Products associated with ATP include inosine 5′-monophosphate (IMP) and its precursors ATP, adenosine-5′-diphosphate (ADP), and adenosine monophosphate (AMP), as well as the degradation products inosine (H x R), hypoxanthine (Hx), adenosine, xanthine (Xt), and adenine (Howgate, 2006). IMP is the main flavor component in meat and fish; however, it is unstable in muscles and could be further degraded into H x R and Hx. Among the degradation products of IMP, inosine and Hx have a bitter taste and play an important role in flavor changes of meat during storage (Hong et al., 2017, Li et al., 2017). Therefore, IMP has become an important indicator to measure the freshness of meat.
We found that the relative contents of most nucleotides were low (fold-change <1) and significantly different among samples from different groups (different days of storage; P < 0.001) as shown in Figure 7. As storage days increased, the contents of cytidine 5′-monophosphate and guanosine-5′-monophosphate gradually declined, with the largest slope occurring between 2 and 4 D. By contrast, the relative contents of IMP and H x R increased during the first 2 D of storage, indicating good quality, but then dropped dramatically during days 4 to 6 of storage. This observation is consistent with previously reported data on sensory evaluation showing that the quality of chilled chicken worsened during storage (Ye et al., 2015). After slaughter, ATP in meat can be degraded gradually into ADP, AMP, IMP, H x R, and Hx. Hypoxanthine continues to oxidize into Xt (Qiu et al., 2015). In our study, the relative contents of Hx and Xt gradually increased (Figure 7). The overall increase of Xt was greater than that of Hx. This finding indicates that degradation of IMP and oxidization of Hx to Xt gradually increased with increasing storage days and that the umami taste and freshness of the chilled chicken meat gradually decreased. IMP thus has become an important index for measuring the umami taste of meat (Lee et al., 2011, Masic and Yeomans, 2014). According to the trends of IMP and other nucleotides observed, the highest possible shelf life of chilled chicken is 6 D. For best flavor, chilled chicken meat should be consumed within 4 D.
Figure 7.
Relative changes of nucleosides and nucleotides in chilled chicken meat samples during chilled storage. Abbreviations: CMP, cytidine 5′-monophosphate; GMP, guanosine-5′-monophosphate; IMP, inosine 5′-monophosphate.
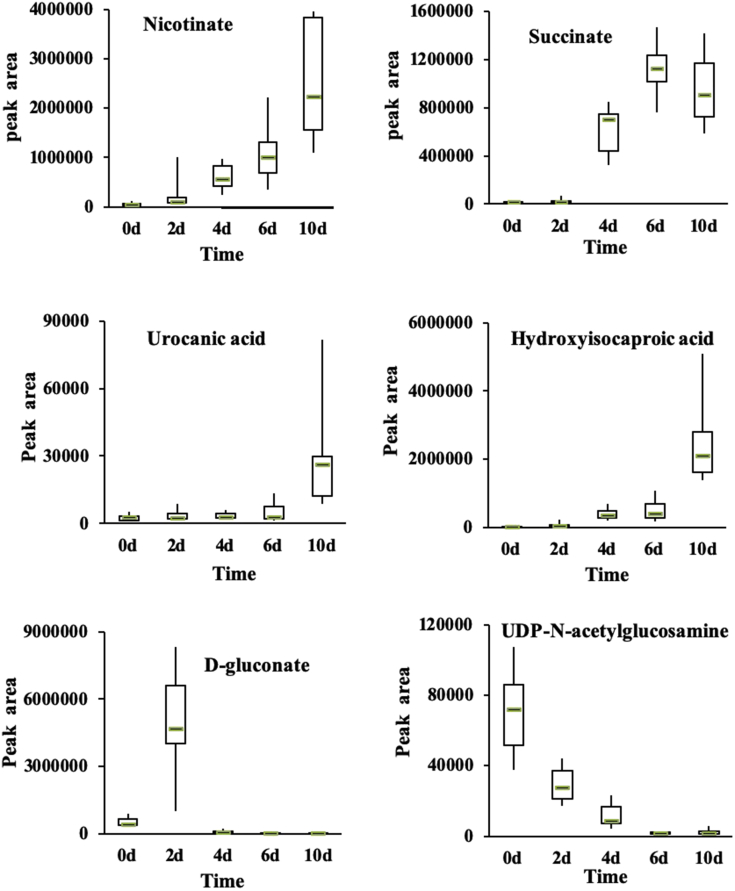
Sugars and Organic Acids
Organic acids detected in this study were urocanic, succinate, nicotinate, 5-amino pentanoic, R-3-hydroxy butyric, hydroxyisocaproic, γ-aminobutyric, and DL-3-phenyllyl acids. As shown in Figure 8, the contents of nicotinate, succinate, urocanic acid, and hydroxyisocaproic acid increased with increasing numbers of storage days. Urocanic acid and hydroxyisocaproic acid decreased during days 0 to 6, but then substantially increased at day 10 (P < 0.01). Succinate and its derivatives influence color stability and flavor of meat (Ramanathan et al., 2011). We found that although the relative content of succinate on other storage days declined, it remained higher than it did on day 0. The content of UDP-N-acetylglucosamine gradually decreased with increasing numbers of storage days (Figure 8). Additionally, the relative content of D-gluconate peaks at days 0 to 2. This finding may be because the sugar in the meat largely degraded during the first 2 D of storage and fully depleted after 4 D.
Figure 8.
Relative changes of carbohydrates and organic acids in chilled chicken meat samples during chilled storage.
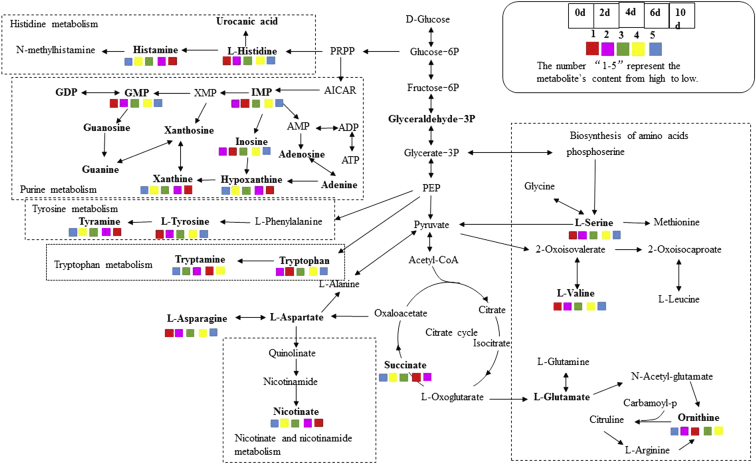
Analysis of Metabolic Pathway of Metabolites Produced During Storage of Chilled Chicken
To better reflect the relationship between metabolite changes in chilled chicken during storage, the biosynthetic pathways of involved metabolites were drawn (Figure 9). We focused on the purine and bioamine pathways, which relate to meat flavor and safety, respectively. We found that the metabolism-related products in the purine metabolic pathway showed complex processes, rather than gradually increasing or decreasing. However, the metabolomic profiles in this study included a mixture of metabolites from microorganisms and chicken, and it is difficult to separate chicken metabolites from microorganism metabolites for pathway analysis. The contents of IMP peaks after 10 D of storage (Figure 9), which greatly affected the freshness of chilled chicken. In the metabolic pathway for bioamines, the contents of histidine, tyrosine, and tryptophan gradually decreased, whereas histamine, tyramine, and tryptamine increased. Fraqueza (Fraqueza et al., 2012) found that tyramine increases significantly (P < 0.05) in turkey meat during storage in modified atmosphere packaging. They point out that bioamines maintain a close association with Pseudomonas and Enterobacteriaceae spp. Lysine, tyrosine, and histidine are the main precursors of bioamines in sausage; thus, their contents increase considerably during storage because of microbial-mediated proteolysis (Rabie et al., 2014). According to Wen (Wen et al., 2018), Pseudomonas is the dominant bacteria in chilled chicken meat during initial storage, followed by Shewanellaceae over time. Experiments confirm that Pseudomonas produces histamine in fish, whereas Shewanellaceae is the sole producer of cadaverine (Liu et al., 2018).
Figure 9.
Metabolic pathways of important metabolites in chilled chicken meat samples during chilled storage. Abbreviations: ADP, adenosine-5 Q4 0-diphosphate; AICAR, 5-aminoimidazole-4-carboxamide ribonucleoside; AMP, adenosine monophosphate; ATP, adenosine-50-triphosphate; GDP, guanosine diphosphate; GMP, guanosine-5′-monophosphate; IMP, inosine 5′-monophosphate; PEP, pyruvate phosphate; PRPP, 5-phosphoribosyl-1-pyrophosphate; XMP, xanthosine monophosphate.
Conclusion
We obtained 63 different metabolites from our screening, using VIP>2 and P < 0.05 as criteria. Most amino acids decreased over chilled storage time, whereas amines such as histamine, tyramine, tryptamine, and N-acetylcadaverine increased. These changes were large compared with those of the control. In addition, we detected the nucleoside and nucleotide precursor and degradation products of IMP and IMP. Changes in IMP were consistent with changes in meat quality. Other organic acids and sugars also showed an upward or downward trend with increased storage days that closely aligned with changes in meat quality.
Acknowledgments
This study was supported by the university characteristic innovation project of Guangdong province (No. KA1548811) and the project of Guangzhou Science and Technology Innovation Committee (No. 201704020191).
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2019.11.070.
Supplementary data
References
- Alvarez M.A., Moreno-Arribas M.V. The problem of biogenic amines in fermented foods and?the use of potential biogenic amine-degrading microorganisms as a solution. Trends Food Sci. Technol. 2014;39:146–155. [Google Scholar]
- Benton H.P., Ivanisevic J., Mahieu N.G., Kurczy M.E., Johnson C.H., Franco L., Rinehart D., Valentine E., Gowda H., Ubhi B.K., Tautenhahn R., Gieschen A., Fields M.W., Patti G.J., Siuzdak G. Autonomous metabolomics for rapid metabolite identification in global profiling. Anal. Chem. 2015;87:884–891. doi: 10.1021/ac5025649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bover-Cid S., Izquierdo-Pulido M., Vidal-Carou M.C. Influence of hygienic quality of raw materials on biogenic amine production during ripening and storage of dry fermented sausages. J. Food Prot. 2000;63:1544–1550. doi: 10.4315/0362-028x-63.11.1544. [DOI] [PubMed] [Google Scholar]
- Castro-Puyana M., Pérez-Míguez R., Montero L., Herrero M. Application of mass spectrometry-based metabolomics approaches for food safety, quality and traceability. Trac Trends Anal. Chem. 2017;93:102–118. [Google Scholar]
- D'Astous-Page J., Gariepy C., Blouin R., Cliche S., Sullivan B., Fortin F., Palin M.-F. Carnosine content in the porcine longissimus thoracis muscle and its association with meat quality attributes and carnosine-related gene expression. Meat Sci. 2017;124:84–94. doi: 10.1016/j.meatsci.2016.11.004. [DOI] [PubMed] [Google Scholar]
- Dannert R.D., Pearson A.M. Concentration of inosine 5′-monophosphate in meat. J. Food Sci. 1967;32:49–52. [Google Scholar]
- Dasenaki M.E., Michali C.S., Thomaidis N.S. Analysis of 76 veterinary pharmaceuticals from 13 classes including aminoglycosides in bovine muscle by hydrophilic interaction liquid chromatography–tandem mass spectrometry. J. Chromatogr. A. 2016;1452:67–80. doi: 10.1016/j.chroma.2016.05.031. [DOI] [PubMed] [Google Scholar]
- Fraqueza M.J., Alfaia C.M., Barreto A.S. Biogenic amine formation in turkey meat under modified atmosphere packaging with extended shelf life: index of freshness. Poult. Sci. 2012;91:1465–1472. doi: 10.3382/ps.2011-01577. [DOI] [PubMed] [Google Scholar]
- Halász A., Baráth Á, Simon-Sarkadi L., Holzapfel W. Biogenic amines and their production by microorganisms in food. Trends Food Sci. Technol. 1994;5:42–49. [Google Scholar]
- Hong H., Regenstein J.M., Luo Y. The importance of ATP-related compounds for the freshness and flavor of post-mortem fish and shellfish muscle: a review. Crit. Rev. Food Sci. Nutr. 2017;57:1787–1798. doi: 10.1080/10408398.2014.1001489. [DOI] [PubMed] [Google Scholar]
- Howgate P. A review of the kinetics of degradation of inosine monophosphate in some species of fish during chilled storage. Int. J. Food Sci. Technol. 2006;41:341–353. [Google Scholar]
- Janeckova H., Kalivodova A., Najdekr L., Friedecky D., Hron K., Bruheim P., Adam T. Untargeted metabolomic analysis of urine samples in the diagnosis of some inherited metabolic disorders. Biomed. Papers-Olomouc. 2015;159:582–585. doi: 10.5507/bp.2014.048. [DOI] [PubMed] [Google Scholar]
- Kouremenos K.A., Beale D.J., Antti H., Palombo E.A. Liquid chromatography time of flight mass spectrometry based environmental metabolomics for the analysis of Pseudomonas putida bacteria in potable water. J. Chromatogr. B-Analytical Tech. Biomed. Life Sci. 2014;966:179–186. doi: 10.1016/j.jchromb.2014.02.058. [DOI] [PubMed] [Google Scholar]
- Lázaro C.A., Contejúnior C.A., Canto A.C., Monteiro M.L.G., Costalima B., Cruz A.G.D., Mársico E.T., Franco R.M. Biogenic amines as bacterial quality indicators in different poultry meat species. LWT - Food Sci. Technol. 2015;60:15–21. [Google Scholar]
- Lana A., Longo V., Dalmasso A., D'Alessandro A., Bottero M.T., Zolla L. Omics integrating physical techniques: aged Piedmontese meat analysis. Food Chem. 2015;172:731–741. doi: 10.1016/j.foodchem.2014.09.146. [DOI] [PubMed] [Google Scholar]
- Lechtenfeld O.J., Hertkorn N., Shen Y., Witt M., Benner R. Marine sequestration of carbon in bacterial metabolites. Nat. Commun. 2015;6:6711. doi: 10.1038/ncomms7711. [DOI] [PubMed] [Google Scholar]
- Lee H.Y., Kim J.-M., Byun M.-J., Kang K.-S., Kim T.-H., Hong K.-C., Lee K.-T. Structure and polymorphisms of the 5′ regulatory region of porcineadenylate kinase 3-like 1gene and effect on trait of meat quality. Genes & Genomics. 2011;33:147–153. [Google Scholar]
- Leggio A., Belsito E.L., Marco R.D., Liguori A., Siciliano C., Spinella M. Simultaneous extraction and derivatization of amino acids and free fatty acids in meat products. J. Chromatogr. A. 2012;1241:96–102. doi: 10.1016/j.chroma.2012.04.029. [DOI] [PubMed] [Google Scholar]
- Li Q., Zhang L., Lu H., Song S., Luo Y. Comparison of postmortem changes in ATP-related compounds, protein degradation and endogenous enzyme activity of white muscle and dark muscle from common carp (Cyprinus carpio) stored at 4 degrees C. Lwt-Food Sci. Technol. 2017;78:317–324. [Google Scholar]
- Liu X., Huang Z., Jia S., Zhang J., Li K., Luo Y. The roles of bacteria in the biochemical changes of chill-stored bighead carp (Aristichthys nobilis): proteins degradation, biogenic amines accumulation, volatiles production, and nucleotides catabolism. Food Chem. 2018;255:174–181. doi: 10.1016/j.foodchem.2018.02.069. [DOI] [PubMed] [Google Scholar]
- Lorenzo J.M., Munekata P.E.S., Domínguez R. Role of autochthonous starter cultures in the reduction of biogenic amines in traditional meat products. Curr. Opin. Food Sci. 2017;14:61–65. [Google Scholar]
- Martuscelli M., Pittia P., Casamassima L.M., Manetta A.C., Lupieri L., Neri L. Effect of intensity of smoking treatment on the free amino acids and biogenic amines occurrence in dry cured ham. Food Chem. 2009;116:955–962. [Google Scholar]
- Masic U., Yeomans M.R. Umami flavor enhances appetite but also increases satiety. Am. J. Clin. Nutr. 2014;100:532–538. doi: 10.3945/ajcn.113.080929. [DOI] [PubMed] [Google Scholar]
- Muroya S., Oe M., Nakajima I., Ojima K., Chikuni K. CE-TOF MS-based metabolomic profiling revealed characteristic metabolic pathways in postmortem porcine fast and slow type muscles. Meat Sci. 2014;98:726–735. doi: 10.1016/j.meatsci.2014.07.018. [DOI] [PubMed] [Google Scholar]
- Oh H., Lee H.J., Lee J., Jo C., Yoon Y. Identification of microorganisms associated with the quality Improvement of dry-aged beef through microbiome analysis and DNA sequencing, and evaluation of their effects on beef quality. J. Food Sci. 2019;84:2944–2954. doi: 10.1111/1750-3841.14813. [DOI] [PubMed] [Google Scholar]
- Onal A. A review: current analytical methods for the determination of biogenic amines in foods. Food Chem. 2007;103:1475–1486. [Google Scholar]
- Papageorgiou M., Lambropoulou D., Morrison C., Modzinska E., Namiesnik J., Plotka-Wasylka J. Literature update of analytical methods for biogenic amines determination in food and beverages. Trac-Trends Anal. Chem. 2018;98:128–142. [Google Scholar]
- Qiu W., Xie J., Chen S., Qu Y., Song X., Danni W. Changes of ATP-related compounds contents and its degradation pathways in Shrimps during chilled storage. Mod. Food Sci. Technol. 2015;31:103–108+188. [Google Scholar]
- Rabie M.A., Peres C., Malcata F.X. Evolution of amino acids and biogenic amines throughout storage in sausages made of horse, beef and turkey meats. Meat Sci. 2014;96:82–87. doi: 10.1016/j.meatsci.2013.05.042. [DOI] [PubMed] [Google Scholar]
- Ramanathan R., Mancini R.A., Dady G.A. Effects of pyruvate, succinate, and lactate enhancement on beef longissimus raw color. Meat Sci. 2011;88:424–428. doi: 10.1016/j.meatsci.2011.01.021. [DOI] [PubMed] [Google Scholar]
- Ryu K.Y., Shim S.L., Kim W., Jung M.S., Hwang I.M., Kim J.H., Hong C.H., Jung C.H., Kim K.S. Analysis of the seasonal change of the proximate composition and taste components in the Conger Eels (Conger myriaster) J. Korean Soc. Food Sci. Nutr. 2009;38:1069–1075. [Google Scholar]
- Santos M.H.S. Biogenic amines: their importance in foods. Int. J. Food Microbiol. 1996;29:213–231. doi: 10.1016/0168-1605(95)00032-1. [DOI] [PubMed] [Google Scholar]
- Schwahn K., Souza L.P.D., Fernie A.R., Tohge T. Metabolomics-assisted refinement of the pathways of steroidal glycoalkaloid biosynthesis in the tomato clade. J. Integr. Plant Biol. 2014;56:864–875. doi: 10.1111/jipb.12274. [DOI] [PubMed] [Google Scholar]
- Shi Y., Li X., Huang A. A metabolomics-based approach investigates volatile flavor formation and characteristic compounds of the Dahe black pig dry-cured ham. Meat Sci. 2019;158:107904. doi: 10.1016/j.meatsci.2019.107904. [DOI] [PubMed] [Google Scholar]
- Shumilina E., Slizyte R., Mozuraityte R., Dykyy A., Stein T.A., Dikiy A. Quality changes of salmon by-products during storage: assessment and quantification by NMR. Food Chem. 2016;21:803–811. doi: 10.1016/j.foodchem.2016.05.088. [DOI] [PubMed] [Google Scholar]
- Słowińska M., Sallem H., Clench M.R., Ciereszko A. Metabolomic analysis of white and yellow seminal plasma in turkeys (Meleagris gallopavo) Poult. Sci. 2018;97:1059–1065. doi: 10.3382/ps/pex366. [DOI] [PubMed] [Google Scholar]
- Subbaraj A.K., Kim Y.H.B., Fraser K., Farouk M.M. A hydrophilic interaction liquid chromatography–mass spectrometry (HILIC–MS) based metabolomics study on colour stability of ovine meat. Meat Sci. 2016;117:163–172. doi: 10.1016/j.meatsci.2016.02.028. [DOI] [PubMed] [Google Scholar]
- Sundekilde U.K., Rasmussen M.K., Young J.F., Bertram H.C. High resolution magic angle spinning NMR spectroscopy reveals that pectoralis muscle dystrophy in chicken is associated with reduced muscle content of anserine and carnosine. Food Chem. 2017;217:151–154. doi: 10.1016/j.foodchem.2016.08.104. [DOI] [PubMed] [Google Scholar]
- Suzzi G., Gardini F. Biogenic amines in dry fermented sausages: a review. Int. J. Food Microbiol. 2003;88:41–54. doi: 10.1016/s0168-1605(03)00080-1. [DOI] [PubMed] [Google Scholar]
- Tofalo R., Perpetuini G., Schirone M., Suzzi G. Biogenic amines: Toxicology and health effect. In: Caballero B., Finglas P.M., Toldrá F., editors. Encyclopedia of Food and Health. Academic Press; Oxford: 2016. pp. 424–429. [Google Scholar]
- Wang G.Y., Wang H.H., Han Y.W., Xing T., Zhou G.H. Evaluation of the spoilage potential of bacteria isolated from chilled chicken in vitro and in situ. Food Microbiol. 2017;63:139–146. doi: 10.1016/j.fm.2016.11.015. [DOI] [PubMed] [Google Scholar]
- Wang X., Fang C., He J., Dai Q., Fang R. Comparison of the meat metabolite composition of Linwu and Pekin ducks using 600 MHz 1H nuclear magnetic resonance spectroscopy. Poult. Sci. 2016;96:192. doi: 10.3382/ps/pew279. [DOI] [PubMed] [Google Scholar]
- Want E.J., Wilson I.D., Gika H., Theodoridis G., Plumb R.S., Shockcor J., Holmes E., Nicholson J.K. Global metabolic profiling procedures for urine using UPLC-MS. Nat. Protoc. 2010;5:1005–1018. doi: 10.1038/nprot.2010.50. [DOI] [PubMed] [Google Scholar]
- Warner R.D., Jacob R.H., Rosenvold K., Rochfort S., Trenerry C., Plozza T., McDonagh M.B. Altered post-mortem metabolism identified in very fast chilled lamb M. longissimus thoracis et lumborum using metabolomic analysis. Meat Sci. 2015;108:155–164. doi: 10.1016/j.meatsci.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Wen D., Cheng S., Liu Y., Yu Q. Analysis of bacterial community diversity of chilled chicken at different enrichment temperatures using high-throughput Sequencing. Food Sci. 2018;39:156–161. [Google Scholar]
- Wickramasinghe N.N., Ravensdale J., Coorey R., Chandry S.P., Dykes G.A. The predominance of Psychrotrophic Pseudomonads on aerobically stored chilled red meat. Compr. Rev. Food Sci. Food Saf. 2019;18:1622–1635. doi: 10.1111/1541-4337.12483. [DOI] [PubMed] [Google Scholar]
- Xiao S., Zhuang H., Zhou G., Zhang J. Investigation of inhibition of lipid oxidation by L-carnosine using an oxidized-myoglobin-mediated washed fish muscle system. LWT-Food Sci. Technol. 2018;97:703–710. [Google Scholar]
- Yang F., Xia W.-S., Zhang X.-W., Xu Y.-S., Jiang Q.-X. A comparison of endogenous and microbial proteolytic activities during fast fermentation of silver carp inoculated with Lactobacillus plantarum. Food Chem. 2016;207:86–92. doi: 10.1016/j.foodchem.2016.03.049. [DOI] [PubMed] [Google Scholar]
- Ye Z., Xie J., Qiu W., Gao L., Zhu H., Zhao Y., Wang Y., Zhang N. Changes of flavor nucleotides and free amino acid contents in chicken muscle under room temperature and cold storage. Sci. Technol. Food Industry. 2015;36:301–305. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.