Abstract
Brooding behavior, a common characteristic of native breeds of the domestic chicken, is marked by elevated prolactin (PRL) levels, which is necessary for incubation and connected with changes in hypothalamic–pituitary–gonadal axis activity. Evidence indicates the serotoninergic system is a potent modulator of PRL secretion. The objective of this study is to investigate whether blocking serotonin synthesis with parachlorophenylalanine (PCPA) prevents incubation behavior in native Polish crested chickens. In addition, we examined the effect of PCPA on the gene expression of the gonadal and lactotrophic axes. Birds were stimulated to broodiness by artificial eggs in nests. At 34 wk of age (April: spring period), the hens were divided into 2 groups (14 hens in each group): control and PCPA-treated (50 mg/kg BW) group. After 5 wk of treatment, the artificial eggs were removed from the nests. Egg production, incubation activity, and levels of plasma ovarian steroids progesterone (P4), testosterone (T), estradiol (E2), and PRL were examined. At the end of the experiment (45 wk of age, June: summer period), ovarian characteristics and mRNA gene expression of gonadal (gonadotropin-releasing hormone [GnRH] I, luteinizing hormone [LH] ß, follicle-stimulating hormone [FSH] ß) and lactotrophic (vasoactive intestinal peptide [VIP], PRL) axes were measured by quantitative real-time PCR. Incubation activity was observed in the hens of both groups but with lower frequency in PCPA-treated birds. Moreover, the PCPA group had a higher cumulative egg production than the controls. During the first six and 8 wk of the experiment, levels of P4 and E2, respectively, were similar in both groups, but all concentrations increased in the PCPA-treated hens after this period. In addition, increased GnRH-I, LHß, and FSHß and decreased VIP mRNA expression was observed in the PCPA group compared with the controls. There were no differences in PRL mRNA expression, the PRL level, and ovarian morphometry between the 2 groups. These results indicate that blockage of serotonin synthesis by PCPA does not effectively prevent incubation in native Polish crested chickens. However, treatment with PCPA increased gonadal axis activity and improved reproductive performance.
Key words: reproduction, native breed, incubation, lactotrophic axis, prolactin
Introduction
Maternal behavior in poultry has 2 phases: incubation and brooding. In chickens, incubation is restricted to the female and is associated with cessation of egg laying, reduction in food and water intake, and reduction in social interaction (Romanov et al., 2002, Sharp, 2009). Environmental conditions, such as the presence of eggs and nests, are factors that encourage incubation (Zadworny et al., 1989, Richard-Yris et al., 1998).
In high-producing, commercial hybrids of laying hens, genetic selection for egg production persistency has greatly minimized maternal behavior. Nevertheless, this instinct is still noted in indigenous breeds of hens (Zadworny et al., 1989, Eltayeb et al., 2010, Jiang et al., 2010, Geng et al., 2014, Namken et al., 2017), which are recommended for organic and small-scale farming. However, egg-laying pause caused by egg incubation can limit economic profits. Because of this, it is important to know if broodiness behavior can be regulated in this particular population.
Polish crested chickens are a native breed with the characteristic huge bouffant crest of feathers that raises their visual qualities. After many years of breeding work, they were restituted. In the spring, persistent nesting is observed in some females; therefore, they can serve as a model for regulation of incubation behavior.
In birds, it is well established that the hypothalamic neuropeptide gonadotropin-releasing hormone (GnRH) activates reproduction by promotion of gonadotropin (luteinizing hormone [LH]; follicle-stimulating hormone [FSH]) synthesis and pituitary gland release. Gonadotropins stimulate gametogenesis and sex steroid synthesis in the gonad (GnRH- FSH and LH, gonadal axis) (Ubuca et al., 2013). Initiation of incubation behavior is predominantly connected with increased neuroendocrine activity of the hypothalamic vasoactive intestinal peptide (VIP), which is prolactin (PRL)-releasing factor from the pituitary lactotropes (VIP-PRL, lactotrophic axes) (El Halawani et al., 1996, El Halawani et al., 1984, El Halawani et al., 1980; Vleck and Patrick, 1999, Freeman et al., 2000). The elevated circulating level of PRL (hyperprolactinemia) is the characteristic of the incubation stage of reproduction (Lea et al., 1982, El Halawani et al., 2000) and are associated with antigonadotropin and antigonadal actions (Bluhm et al., 1983, Zadworny et al., 1988, Rozenboim et al., 2004, Kosonsiriluk et al., 2008), resulting in ovarian regression and cessation of egg production.
Elevated PRL levels increase nesting activity and maintain incubation behavior (Prakobsaeng et al., 2011). Both systems are additionally modulated by the monoaminergic pathway involving dopamine (DA) and serotonin (5-hydroxytryptamine [5-HT]) (Chaiseha and El Halawani, 2005). In the turkey, two subtypes of 5-HT receptors have been found with a dual role in the regulation of PRL secretion (Chaiseha et al., 1998, Bakken et al., 2014).
Serotonin concentrations may be modulated by tryptophan hydroxylase, the initial and rate-limiting enzyme in 5-HT biosynthesis (Jequier et al., 1967). Pharmacological drugs such as parachlorophenylalanine (PCPA) specifically reduce levels of 5-HT in the brain through an irreversible inhibition of tryptophan hydroxylase (Berger et al., 1989, Park et al., 1994). Pharmacologically induced changes in the metabolism of 5-HT in the central nervous system have been shown to modify reproductive performance in turkey hens with heat-induced hyperprolactinemia (Rozenboim et al., 2004) and, in moderate levels, alter egg production in hens after retinal photoreceptor photostimulation by green light (Mobarkey et al., 2013). Moreover, treating aging roosters by PCPA increases semen quality variables by changes of gonadal and lactotrophic axis gene expression (Avital-Cohen et al., 2015).
Currently, pharmacological methods used to restore the hypothalamic–pituitary–ovarian axis in incubating fowls have mainly focused on turkeys (El Halawani et al., 1995, El Halawani et al., 1983, Millam et al., 1980, Guémené and Etches, 1989), the studies of which have shown inconclusive results. Administration of PCPA to nest-deprived broody turkeys did not alter the resumption of nesting (El Halawani et al., 1980). On the contrary, treatment of incubating turkeys with PCPA caused reinitiation of ovulatory cycles (El Halawani et al., 1983) or was ineffective in resumption of egg production (Guémené and Etches, 1989). Furthermore, subcutaneous injection or oral administration of pimozide (DA receptor–blocking agent) resulted in positive results in frequency of nest visits and daily egg production (Millam et al., 1980). In broody chickens, resumption of egg production was stimulated by intramuscular injection of clomiphene citrate (an antiestrogen) alone or after bromocriptine (CB154) administration (Bedrak et al., 1983). To date, there have been no reports regarding 5-HT synthesis modulation in regulating incubation behavior in domestic chickens. Therefore, we hypothesize that PCPA treatment of native chickens will prevent incubation behavior by alterations in the expression of the gonadal and lactotrophic axes.
Thus, it seems reasonable to undertake an additional investigation attributable to orchestration of incubation behavior in native chicken breeds. The objective of this study was (1) to investigate whether blockade of serotonin synthesis by PCPA might prevent the expression of incubation behavior in native Polish crested chickens and (2) to examine the effect of PCPA treatment on gonadal and lactotrophic axis gene expression.
Materials and methods
Experimental Animals and Management
The study was carried out on 28 native Polish crested chickens (CP-11 strain). The birds were reared and managed at the Research and Education Center of the Faculty of Animal Sciences of the Agricultural University in Krakow, Poland. The native Polish crested chicken is a native, local breed not selected for egg production traits. The birds were kept in 2 groups (1 male:14 females; harem mating system) on litter inside the experimental building. The chickens were individually marked for identification purposes. Two pens (200 × 250 cm; stocking density: 3 birds/m2) with 6 nests for individual laying control were used. Each nest box contained chopped straw as nesting material and 6 artificial eggs to synchronize and encourage incubation behavior. The pens were connected with runs (stocking density: 1.25 birds/m2) situated outside the building. The natural and artificial lighting schedule was used, 16 h of light and 8 h of dark (16L:8D; lights on from 05.00–21.00 h). The birds had free access to food and water. The commercial layer-breeder mixture with 16.0% CP/kg and 11.3 MJ MEN/kg was used in feeding.
Experimental Design
All the procedures were approved by the First Local Ethical Committee on Animal Testing of the Jagiellonian University in Krakow, Poland.
At 34 wk of age (April: spring period), the hens were subjected to experimental treatments. The PCPA group received PCPA (Sigma, St. Louis, MO) orally in gelatin capsules (50 mg/kg body weight) daily for 3 consecutive days, and the second group (control) received empty gelatin capsules (Adox Sp. z o.o., Antoninów, Poland). The dose of PCPA was chosen based on the published data. The birds in the experimental group were individually weighed to determine the PCPA dose for each hen. The capsules were gelatin based and had the following dimensions: approximately 18 mm in length and 6.35 mm in width. The theoretical volume of the capsules was 370 mg. The capsules were certified for use in human medicine. Parachlorophenylalanine treatment was repeated every other week (6 treatments) using the same procedure until hens were 44 wk of age (June: summer period). Five week after experimental initiation, the artificial eggs were removed from the nests for 1 wk and then placed back in again.
Egg production and nesting activity were recorded daily. A bird that was found for 5 consecutive days in the nest without laying an egg was considered incubating.
Measurement Parameters
Heparinized blood samples were drawn on a weekly basis from a brachial vein for determination of plasma PRL and progesterone (P4), estradiol (E2), and testosterone (T) concentrations. The samples were centrifuged at 1,500 × g for 10 min, and plasma was stored at −20°C until assay could be performed. The experiment was terminated after 11 wk at 45 wk of age. Nonincubating hens (n = 5) were selected at random and euthanized by decapitation. Within 15 min after slaughter, the hypothalamus and pituitary were dissected from the brain. The entire hypothalamus was removed as per the landmarks of the optic chiasm rostrally and the mammillary bodies caudally (Xu et al., 2011, Mobarkey et al., 2013, Avital-Cohen et al., 2015). The entire hypothalamus and pituitary were placed into RNAlater (Sigma-Aldrich, St. Louis, MO) for future real-time (RT) PCR analysis. The ovary was isolated. The following compartments were distinguished in the ovary: stroma with follicles < 1 mm in diameter, white follicles (> 1–4 mm), yellowish follicles (> 4–8 mm), yellow follicles (> 8–36 mm), and atretic follicles.
Hormone Analysis
Ovarian steroid hormones were measured in a single assay by ELISA as per a previously described protocol (Nash et al., 2000), which was validated for plasma from laying domestic chickens (Mobarkey et al., 2013). Steroid hormones were extracted from 0.5 mL of plasma with 5 mL of diethyl ether. Recovery after extraction was 90% for T, P4, and E2. Dilutions of the primary antibody and tracer were 1:320,000 and 1:320; 1:160,000 and 1:160; and 1:5,000 and 1:50 for T, P4, and E2, respectively. All samples were analyzed in duplicate, and for every other plate, a separate standard curve was determined. Minimal detectable doses were < 0.03 ng/mL, < 0.002 ng/mL, and < 0.03 ng/mL for T, P4, and E2, respectively. The intra-assay CV was 5%. Cross-reactivity of anti-T (ICN 647381, Irvine, CA) was determined with the use of dihydrotestosterone, androstenedione, dehydroepiandrosterone, E2, P4, and corticosterone. Only dihydrotestosterone had more than 5% cross-reactivity (55.3%). Cross-reactivity of anti-E2 (ICN 614051, Irvine, CA) was determined with the use of estrone, estriol, dihydrotestosterone, androstenedione, dehydroepiandrosterone, testosterone, P4, and corticosterone and was found to be less than 2%. As per the manufacturer's data, cross-reactivity of anti-P4 (A1405, AbKem Iberia S.L., Vigo, Spain) with gonadal and nongonadal steroids was less than 5%. Plasma PRL was assayed by competitive ELISA with the use of biotinylated PRL tracers as per a previously described method (Rochester et al., 2008). The assay of plasma PRL levels in native Polish crested chickens was validated. Pooled plasma samples of native Polish crested chickens produced a dose–response curve that paralleled a chicken PRL standard curve. The minimal detectable dose was < 0.09 ng/mL. Absorbance at 405 nm was read in a Tecan Sunrise microplate reader (Tecan Group Ltd., Männedorf, Switzerland). Pooled extracted plasma samples were run in duplicate in each plate as an internal quality control. The intra-assay CV was 7%. Plasma concentration of all hormones was determined in one analysis to avoid interassay variation.
RNA Isolation and RT-PCR Analysis
Total RNA was extracted from tissues using the TRI Reagent (Sigma-Aldrich, St. Louis, MO) as per the manufacturer's protocol. Total RNA (2 μg) was reverse transcribed using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Reverse transcriptase reaction mixtures were performed in a volume of 20 μL including the random primers, dNTP mix, and MultiScribe Reverse Transcriptase. The reaction was conducted in a thermocycler (Mastercycler Gradient, Eppendorf, Hamburg, Germany) and performed at 25°C for 5 min, 37°C for 120 min, and 85°C for 5 min. The cDNA was stored at −20°C. The duplex RT quantitative PCRs were carried out for GnRH-I, LHß, FSHß, VIP, and PRL using a 96-well StepOne Plus thermocycler (Applied Biosystems, Foster City, CA) in a volume of 10 μL containing 5 μL of TaqMan Gene Expression Master Mix (Applied Biosystems), 0.5 μL of TaqMan Gene Expression Assays with specific TaqMan MGB-probe and one pair of primers (Table 1), 0.5 μL of Eukaryotic 18S rRNA Endogenous Control (pair of primers and TaqMan probe-labeled VIC/TAMRA, cat # 4310893E, amplicon size: 187 bp; Applied Biosystems), 2 μL of water, and 2 μL of cDNA (10 × diluted samples after the reverse transcription). Amplifications included an initial denaturation step at 50°C for 2 min and 95°C for 10 min and 40 PCR cycles at 95°C for 15 s and then at 60°C for 1 min. Each sample was run in duplicate. Water was used as a negative control in all reactions. The 2−ΔΔCt method was used to calculate relative expression of analyzed genes after normalization to 18S rRNA and calibration to expression of the control group.
Table 1.
TaqMan probe sequences or assay ID and size of amplicons generated by a guantitative real-time PCR (qRT-PCR) assay for chicken GnRH-I, VIP, LHß, FSHß, and PRL.
| Gene | Genbank accession no. | Primers/TaqMan probe (FAM5′→3′ NFQ) or assay ID | Amplicon (bp) |
|---|---|---|---|
| GnRH-I | NM_001080877.1 | Assay ID: Gg03361359_ml | 77 |
| VIP | NM_001177309.1 | Assay ID: Gg03339726_ml | 89 |
| LHß | HQ872606.1 | Forward: 5′-GTGTCGCCCCATAAACGTAA-3′ Reverse: 5′-TGGTGGTCACAGCCATACAT-3′ GTGGAGAAGGACGGATGC |
71 |
| FSHß | NM_204257.1 | Assay ID: Gg03364284_ml | 71 |
| PRL |
J04614.1 NM_205466.2 |
Assay ID:Gg03349351_ml | 85 |
Abbreviations: FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone; PRL, prolactin; VIP, vasoactive intestinal peptide. FAM, FAM reported dye; NFQ, nonfluorescent quencher.
Statistical Analysis
All variables were examined for normality and homogeneity of variance using the Shapiro–Wilk test of normality. The significance of differences between means was analyzed using the Student t test or the Mann–Whitney U test. Hormonal data were analyzed on the model based on repeated measurement. P-values less than 0.05 were considered to be statistically significant. Data are presented as means ± standard errors of the mean. For statistical analysis, the experimental unit was the individual hen. Calculations were performed using SigmaPlot (Systat Software Inc., San Jose, CA).
Results
Incubation Activity and Egg Production
Incubation activity was noted in both groups of native Polish crested chickens (Figure 1). The percentage of incubating hens ranged from 0 to 28.6% in the control group and 0 to 21.4% in the PCPA group, depending on the time of the experiment. Both groups increased incubation activity substantially within 1 wk after introduction of artificial eggs, but the proportion of PCPA-treated hens incubating quickly leveled out, whereas control proportions continued to rise to higher levels. Four week after experiment initiation, the proportion of incubating hens stabilized in both groups, but in the PCPA-treated hens, incubation was 7.2% lower for 3 consecutive weeks than in control hens. After the artificial eggs were removed, incubation activity decreased in both groups. Two week after the artificial eggs were removed, incubation activity ranged from 7.1 to 21.4% in control hens but was not observed in the PCPA-treated hens throughout the remainder of the experimental period.
Figure 1.
Percentage of incubating native Polish crested chickens (n = 14) during the period of 11 wk from April (spring) to June (summer). Hens were stimulated to incubation by artificial eggs in nests and treated with PCPA or left untreated (control). Arrows indicate PCPA treatment. Data are means. PCPA, parachlorophenylalanine.
Treatment with PCPA increased (P < 0.05) egg production traits. The weekly egg laying ratio was observed to be 5 times higher in the PCPA group throughout the duration of the experimental period (week 1, 4, 5, 8, and 10; Figure 2). After artificial egg introduction, the weekly egg laying ratio in the control group slowly decreased from 41.8 to 22.5%, the lowest in the entire experimental period, 5 wk later. At the same time period, variations in laying ratio from 31.6 to 45.9% were noticed in the PCPA group. In the second half of the experiment, after the artificial eggs were removed from the nests, egg production increased in both groups, and there was a more stable egg laying pattern in the control group. Cumulative egg production was 15% (72 eggs) higher (P < 0.05) in the PCPA group than in the control group (Figure 3).
Figure 2.
Egg laying ratio of native Polish crested chickens (n = 14) during the period of 11 wk from April (spring) to June (summer). Hens were stimulated to incubation by artificial eggs in nests and treated with PCPA or left untreated (control). Arrows indicate PCPA treatment. Data are means ± SEM. Values with different letters are significantly different between the groups at each time point of the experiment (P < 0.05). PCPA, parachlorophenylalanine; SEM, standard error of the mean.
Figure 3.
Cumulative egg production of native Polish crested chickens (n = 14) during the period of 11 wk from April (spring) to June (summer). Hens were stimulated to incubation by artificial eggs in nests and treated with PCPA or left untreated (control). Data are means ± SEM. Values with different letters are significantly different (P < 0.05). PCPA, parachlorophenylalanine; SEM, standard error of the mean.
Plasma Ovarian Steroid and PRL Concentrations
During the first 5 wk, plasma P4, T, and E2 concentrations were similar in the PCPA and control groups (Figure 4 A to C, respectively). In the second half of the experiment, after artificial eggs were removed from the nest, increased P4, T, and E2 concentrations were noted in the PCPA group compared with the controls.
Figure 4.
Plasma progesterone (A), testosterone (B), and estradiol (C) of native Polish crested chickens (n = 14) during the period of 11 wk from April (spring) to June (summer). Hens were stimulated to incubation by artificial eggs in nests and treated with PCPA or left untreated (control). Arrows indicate PCPA treatment. Data are means ± SEM. Values with different letters are significantly different between the groups at each time point of the experiment (P < 0.05). PCPA, parachlorophenylalanine; SEM, standard error of the mean.
There were no significant differences in plasma PRL levels between the groups except during the first week after treatment, during which increases in the PCPA group were found (Figure 5). In the control group, high variability in PRL concentration was noted.
Figure 5.
Plasma prolactin of native Polish crested chickens (n = 14) during the period of 11 wk from April (spring) to June (summer). Hens were stimulated to incubation by artificial eggs in nests and treated with PCPA or left untreated (control). Arrows indicate PCPA treatment. Data are means ± SEM. Values with different letters are significantly different between the groups at each time point of the experiment (P < 0.05). PCPA, parachlorophenylalanine; SEM, standard error of the mean.
Expression of the Gonadal and Lactotrophic Axis Genes
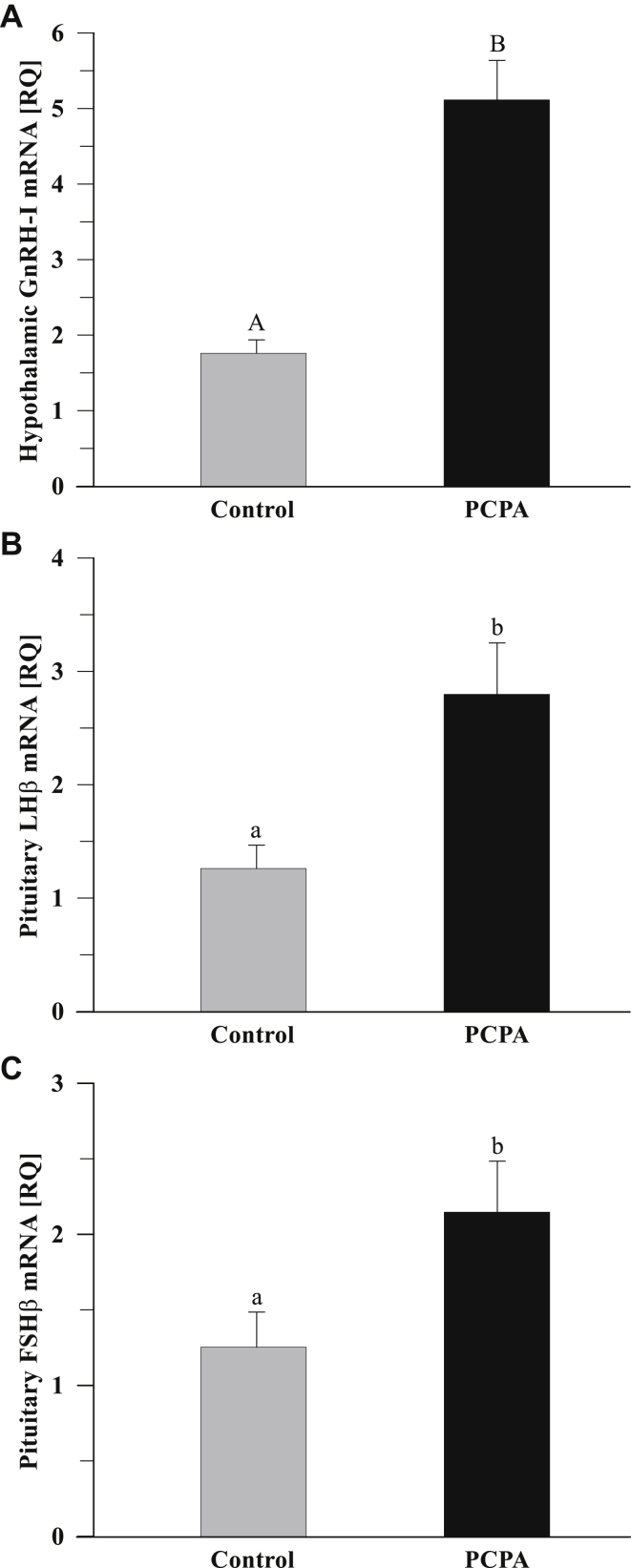
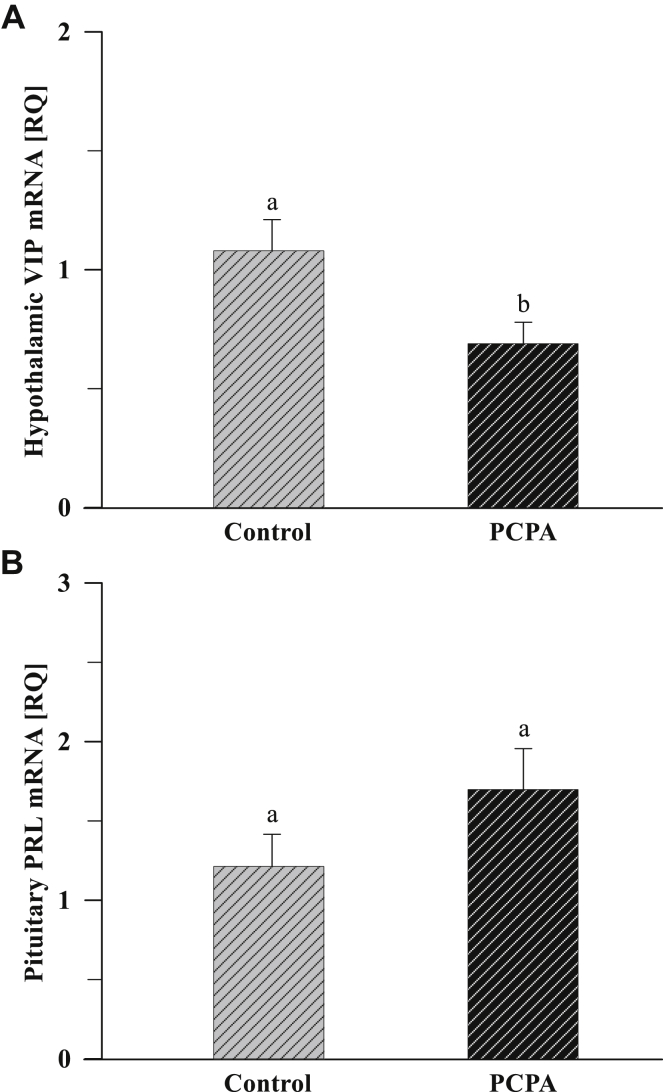
Treatment with PCPA increased (P < 0.001; P < 0.05; P < 0.05) hypothalamic GnRH-I, pituitary LHß, and pituitary FSHß mRNA expression compared with the controls (Figure 6 A to C, respectively). In hens treated with PCPA, decreased hypothalamic VIP (Figure 7 A) mRNA expression was noted (P < 0.05). There were no differences in pituitary PRL gene expression between the PCPA and control groups (Figure 7 B).
Figure 6.
The abundance of hypothalamic GnRH-I (A), pituitary LHß (B), and FSHß (C) subunit transcripts in native Polish crested chickens (n = 5) determined by a quantitative real-time PCR analysis. mRNA expression levels were normalized to 18S rRNA as an endogenous control, calibrated to expression in the control group, and calculated using the 2−ΔΔCT method. Hens were stimulated to incubation by artificial eggs in nests and treated with PCPA or left untreated (control). Data are means of relative quantity (RQ) ± SEM. Values with different letters are significantly different (a,b: P < 0.05; A,B: P < 0.001). GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone; FSH, follicle-stimulating hormone; PCPA, parachlorophenylalanine; SEM, standard error of the mean.
Figure 7.
The abundance of hypothalamic VIP (A) and pituitary PRL (B) transcripts in native Polish crested chickens (n = 5) determined by a quantitative real-time PCR analysis. mRNA expression levels were normalized to 18S rRNA as an endogenous control, calibrated to expression in the control group, and calculated using the 2−ΔΔCT method. Hens were stimulated to incubation by artificial eggs in nests and treated with PCPA or left untreated (Control). Data are means of relative quantity (RQ) ± SEM. Values with different letters are significantly different (a,b: P < 0.05). VIP, vasoactive intestinal peptide; PRL, prolactin; PCPA, parachlorophenylalanine; SEM, standard error of the mean.
Ovarian Characteristics
Parachlorophenylalanine administration had no effect on ovarian follicle number (Table 2), ovarian and stroma weight, or weight of follicles (Table 3).
Table 2.
Effect of parachlorophenylalanine (PCPA) treatment on the number of follicles (mean ± SEM) in the ovary of native Polish crested chickens (5 chickens/group).
| Follicles | Diameter | Group |
|
|---|---|---|---|
| Control | PCPA | ||
| White | (1–4 mm) | 34.83 ± 5.68 | 41.67 ± 7.86 |
| Yellowish | (4–8 mm) | 14.33 ± 3.01 | 16.17 ± 1.89 |
| Yellow | (8–12 mm) | 0.50 ± 0.22 | 0.83 ± 0.17 |
| (12–18 mm) | 0.63 ± 0.21 | 0.83 ± 0.17 | |
| (18–24 mm) | 0.83 ± 0.17 | 0.83 ± 0.17 | |
| (24–30 mm) | 1.67 ± 0.33 | 2.17 ± 0.17 | |
| Atretic | 7.83 ± 2.21 | 7.50 ± 0.85 | |
Abbreviation: SEM, standard error of the mean.
Table 3.
Effect of parachlorophenylalanine (PCPA) treatment on the weight of the ovary, weight of the stroma, total weight of the group of ovarian follicles, and average weight of the follicle (mean ± SEM) in native Polish crested chickens (5 chickens/group).
| Items | Diameter | Group |
|
|---|---|---|---|
| Control | PCPA | ||
| Weight of the ovary (g) | 29.12 ± 4.94 | 37.65 ± 1.78 | |
| Weight of the stroma (g) | 2.89 ± 0.36 | 3.34 ± 0.22 | |
| Weight of the group of follicles (g) | |||
| White | (1–4 mm) | 0.41 ± 0.06 | 0.51 ± 0.10 |
| Yellowish | (4–8 mm) | 1.24 ± 0.24 | 1.16 ± 0.21 |
| Yellow | (8–12 mm) | 0.18 ± 0.09 | 0.40 ± 0.11 |
| (12–18 mm) | 0.71 ± 0.23 | 1.51 ± 0.27 | |
| (18–24 mm) | 3.74 ± 0.22 | 4.15 ± 0.32 | |
| (24–30 mm) | 18.45 ± 4.44 | 24.12 ± 1.66 | |
| Atretic | 0.24 ± 0.07 | 0.89 ± 0.21 | |
| Weight of the follicle (mg) | |||
| White | (1–4 mm) | 12.4 ± 1.82 | 12.63 ± 1.02 |
| Yellowish | (4–8 mm) | 91.38 ± 7.80 | 72.34 ± 5.63 |
| Yellow | (8–12 mm) | 180.00 ± 88.05 | 439.17 ± 101.59 |
| (12–18 mm) | 707.83 ± 226.20 | 1,512.33 ± 372.56 | |
| (18–24 mm) | 3,154.50 ± 664.95 | 4,133.83 ± 871.04 | |
| (24–30 mm) | 10,642.67 ± 818.71 | 10,893.86 ± 453.66 | |
| Atretic | 30.42 ± 2.70 | 119.84 ± 59.38 | |
Abbreviation: SEM, standard error of the mean.
Discussion
The occurrence of brooding instinct in native chickens can limit profits in small-scale farming. In such production systems, the egg collection from nests often occurs at a low frequency. In the present study, synchronization and stimulation of brooding behavior in native Polish crested chickens was carried out in the spring period by using artificial eggs in nests. Treatment of hens with PCPA, an inhibitor of 5-HT synthesis (Berger et al., 1989, Park et al., 1994), was ineffective in preventing incubation behavior. However, positive modulation of incubation activity frequency was observed in PCPA-treated hens, especially in the period after removal of artificial eggs, and higher cumulative egg production was noted. Furthermore, the PCPA-treated hens showed increased hypothalamic GnRH-I, pituitary LHß, and FSHß mRNA levels and decreased hypothalamic VIP mRNA concentrations compared with the untreated control hens. Despite this, no differences were found in pituitary PRL mRNA abundance and circulating PRL levels. These results are partially in agreement with the previous study reported by El Halawani et al., (1980) in incubating turkeys; these authors showed that PCPA treatment of turkeys, temporarily deprived of nests, suppressed circulating PRL levels regardless of the hen's continued incubation. The PCPA effect was dose dependent, but its effective dose was lower than that used in the present study. However, contrary to the current results, later studies by El Halawani et al., (1983) observed that treatment of incubating turkeys by PCPA reduced nesting frequencies, decreased circulating PRL levels, stimulated gonadotropin secretion, and enhanced gonadal development. Moreover, PCPA treatment was more effective than nest deprivation, and reinitiating of egg production was also observed.
The limited success of the present investigation is consistent with research by Guémené and Etches (1989), who demonstrated a positive effect of PCPA by reducing circulating PRL levels in brooding turkeys but did not restore hypothalamic–pituitary–gonadal axis activity to a physiological state in laying birds. On the other hand, Rozenboim et al., (2004) showed that PCPA treatment of turkeys with heat stress–induced hyperprolactinemia lowered circulating PRL levels, reduced nesting frequencies, and decreased the expression of incubation behavior.
Of note, the present study, contrary to previously discussed reports, showed no effect of PCPA treatment on circulating PRL levels. However, high individual variability in this hormone level was observed owing to the high levels noted in incubating hens. Another reason PCPA showed no effect on plasma PRL levels may be due to the strong stimulation of incubation behavior from artificial eggs in nests. The control of PRL secretion involves the interaction of external stimuli with endocrine mechanisms. In incubating birds, a brood patch develops, which has a tactile stimulatory effect from the eggs on the hypothalamus, stimulating PRL secretion (Book et al., 1991). Anesthesia applied to the brood patch in incubating ducks (Anas platyrhynchos) suppressed circulating PRL levels (Hall and Goldsmith, 1983). In our experiment, the brood patch structure was observed in all incubating hens, so the peripheral nervous input can modulate multiple levels of hypothalamus–pituitary response mechanism. It has been suggested that in the native Thai chicken, nesting activity stimulates PRL secretion by activating dopaminergic input, which in turn affects VIP-PRL axis secretion (Prakobsaeng et al., 2011). Moreover, Namken et al., (2017) reported that the presence of eggs or chicks during incubation or the brooding period positively affects the number of vasoactive intestinal peptide – immunoreactive (VIP-ir) neurons within the different areas of the hypothalamus (nucleus inferioris and infundibuli hypothalami). Changes in the number of VIP-ir neurons within these areas are directly correlated to plasma PRL concentrations across the reproductive stages in birds (Kosonsiriluk et al., 2008). Thus, it seems probable that in native Polish crested chickens, protecting eggs during incubation appears to override the facilitator effect of increased gonadal axis activity, preventing hens from starting a new reproductive cycle. Stimuli from the artificial eggs may promote or sustain elevated PRL levels.
Nest and egg deprivation is a procedure traditionally used to disrupt incubation behavior expression in commercial flocks of turkeys. In the present study, artificial eggs were removed after 5 wk of incubation. It is known that incubation of infertile eggs persists for a more prolonged period than that required to hatch chicks (Sharp, 2009). It is noteworthy that in PCPA-treated hens, there was no return to incubation as opposed to the observations in domestic hens, where motivation to renest is maintained for up to a week after nest deprivation (Richard-Yris et al., 1998). The present results suggest that treating native Polish crested chickens against serotonin biosynthesis, along with the destruction of the nest, effectively decreased motivation to incubate, even in environmental conditions propitious to brooding. These observations are consistent with restoration of the gonadal axis after PCPA treatment as demonstrated by increased GnRH-I, LHß, and FSHß mRNA expression and circulating levels of P4 and E2. The upregulation of the gonadal axis and downregulation of the VIP was noted, but despite expectations, these findings were not connected with alterations in PRL mRNA expression and circulating PRL concentrations. These findings are partially in accordance with the observations made by Mobarkey et al., (2013) in broiler breeder hens photostimulated with green light and by Avital-Cohen et al., (2015) in aging broiler breeder roosters. In both studies, PCPA treatment positively stimulated the bird's reproductive potential and increased gonadal axis expression while decreasing lactotrophic axis gene expression.
Previous results, taken together with these findings, may argue that 5-HT affects hypothalamic–pituitary–gonadal axis activity directly through suppression of GnRH-I synthesis and LH ( Hall et al., 1986) and FSH secretion. Similarly, 5-HT decreased GnRH-I and LH release in female rats after sexual maturity (Arias et al., 1990; Moguilevsky and Wuttke, 2001). Moreover, serotonin may act indirectly through DA and VIP as mediators, and it may inhibit or facilitate PRL secretion depending on the 5-HT receptor subtype stimulation (Chaiseha et al., 2010). In female turkeys, PRL appears to be regulated by stimulatory 5-HT2A/2C and inhibitory 5-HT1A receptor subtypes residing on DA neurons (Chaiseha et al., 1998, Bakken et al., 2014). However, as per the study by Bakken et al., (2014), changes in circulating PRL levels during the reproductive cycle are more dependent on the abundance of inhibitory 5-HT1A receptors, whose expression changes across the reproductive stages. In birds, it is well established that the VIP acts directly on the pituitary as a key releasing factor of PRL during the reproductive period. Active immunization against the VIP decreased PRL concentration in turkey hens during the first and second cycle of reproduction (El Halawani et al., 2000) as well as in birds with heat-induced hyperprolactinemia (Rozenboim et al., 2004). In the study by Ahn et al., (2001), VIP immunoneutralization of turkey hens resulted in decreased PRL mRNA expression followed by increased pituitary LHβ and FSHβ mRNA expression. These findings are further supported by studies in broiler breeder hens showing changes in PRL mRNA expression (Mobarkey et al., 2013) and in aging roosters as decreased VIP/PRL gene expression is associated with VIP immunoneutralization (Avital-Cohen et al., 2015). In reproductively active (Mauro et al., 1992) and naturally hyperprolactinemic incubating turkey hens, upregulation of the hypothalamic VIP was noted (Mauro et al., 1989). Therefore, it may be suggested that in the present study, functional changes in the VIP in response to PCPA appear to be related to transcript-level modification and do not affect protein expression.
Furthermore, the stimulatory effect of the VIP on PRL synthesis and release is mediated by receptors in the pituitary gland (Rozenboim and El Halawani, 1993, Chaiseha et al., 2010), and their abundance correlates with circulating PRL levels during the different reproductive stages in turkeys (Youngren et al., 1996, Chaiseha et al., 2004). Thus, it seems plausible that lack of differences in pituitary PRL mRNA expression between the control and PCPA-treated hens could be also partly attributable to pituitary VIP receptor–level stability during the reproductive cycle in native Polish crested chickens.
In summary, the pharmacologically induced changes of 5-HT synthesis by PCPA treatment positively modulates egg production in native Polish crested chickens, and a lower number of hens showed incubation behavior. Moreover, PCPA has been shown to modify the gonadal axis and, partially, gene expression of the lactotrophic axis without changes in ovary morphology. These data confirm previous reports on turkey and broiler breeder hens that PCPA treatment increases reproduction potential and show, for the first time, that it is also effective in native Polish crested chickens. It cannot be excluded that a higher dose of PCPA might fully restore egg production potential in hens. Further investigation using different doses of PCPA should be conducted. In addition, the mechanism governing 5-HT action in native breeds of hens has not been completely elucidated; therefore, further studies are warranted.
Acknowledgments
This study was financially supported by the Ministry of Science and Higher Education (SUB 215- D205) in Poland. The publication fee was covered by the Society for Biology of Reproduction in Poland.
References
- Ahn J., You S., Kim H., Chaiseha Y., El Halawani M. Effects of active immunization with inhibin α subunit on reproductive characteristics of Turkey hens. Biol. Reprod. 2001;65:1594–1600. doi: 10.1095/biolreprod65.5.1594. [DOI] [PubMed] [Google Scholar]
- Arias P., Szwarcfarb B., de Rondina D.C., Carbone S., Sverdlik R., Moguilevsky J.A. In vivo and in vitro studies on the effect of the serotorinergic system on luteinizing hormone and luteinizing hormone-releasing hormone secretion in prepubertal and peripubertal female rats. Brain Res. 1990;523:57–61. doi: 10.1016/0006-8993(90)91634-s. [DOI] [PubMed] [Google Scholar]
- Avital-Cohen N., Heiblum R., Rosenstrauch A., Chaiseha Y., Mobarkey N., Gumułka M., Rozenboim I. Role of the serotonergic axis in the reproductive failure associated with aging broiler breeder roosters. Domest. Anim. Endocrinol. 2015;53:42–51. doi: 10.1016/j.domaniend.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Bakken T., Kang S.W., Kosonsiriluk S., Kuwayama T., Chaiseha Y., El Halawani M.E. Differential roles of hypothalamic serotonin receptor subtypes in the regulation of prolactin secretion in the Turkey hen. Acta Histochem. 2014;116:131–137. doi: 10.1016/j.acthis.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Bedrak E., Harvey S., Robinzon B. Pharmacological disruption of broodiness in white rock domestic fowls. Br. Poult. Sci. 1983;24:573–579. doi: 10.1080/00071668308416777. [DOI] [PubMed] [Google Scholar]
- Berger U.V., Grzanna R., Molliver M.E. Depletion of serotonin using p-chlorophenylalanine (PCPA) and reserpine protects against the neurotoxic effects of p-chloroamphetamine (PCA) in the brain. Exp. Neurol. 1989;103:111–115. doi: 10.1016/0014-4886(89)90071-x. [DOI] [PubMed] [Google Scholar]
- Bluhm C.K., Phillips R.E., Burke W.H. Serum levels of luteinizing hormone, prolactin, estradiol and progesterone in laying and nonlaying mallards (Anas platyrhynchos) Biol. Reprod. 1983;28:295–305. doi: 10.1095/biolreprod28.2.295. [DOI] [PubMed] [Google Scholar]
- Book C.M., Millam J.R., Guinan M.J., Kitchell R.L. Brood patch innervation and its role in the onset of incubation in the Turkey hen. Physiol. Behav. 1991;50:281–285. doi: 10.1016/0031-9384(91)90067-x. [DOI] [PubMed] [Google Scholar]
- Chaiseha Y., El Halawani M.E. Neuroendocrinology of the female Turkey reproductive cycle. J. Poult. Sci. 2005;42:87–100. [Google Scholar]
- Chaiseha Y., Kang S.W., Leclerc B., Kosonsiriluk S., Sartsoongnoen N., El Halawani M.E. Serotonin receptor subtypes influence prolactin secretion in the Turkey. Gen. Comp. Endocrinol. 2010;165:170–175. doi: 10.1016/j.ygcen.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Chaiseha Y., Tong Z., Youngren O.M., El Halawani M.E. Transcriptional changes in hypothalamic vasoactive intestinal peptide during a photo-induced reproductive cycle in the Turkey. J. Mol. Endocrinol. 1998;21:267–275. doi: 10.1677/jme.0.0210267. [DOI] [PubMed] [Google Scholar]
- Chaiseha Y., Youngren O.M., El Halawani M.E. Expression of vasoactive intestinal peptide receptor messenger RNA in the hypothalamus and pituitary throughout the Turkey reproductive cycle. Biol. Reprod. 2004;70:593–599. doi: 10.1095/biolreprod.103.022715. [DOI] [PubMed] [Google Scholar]
- El Halawani M.E., Burke W.H., Dennison P.T. Effects of p-Chlorophenylalanine on the rise in serum prolactin associated with nesting in broody turkeys. Biol. Reprod. 1980;23:815–819. doi: 10.1095/biolreprod23.4.815. [DOI] [PubMed] [Google Scholar]
- El Halawani M.E., Burke W.H., Millam J.R., Fehrer S.C., Hargis B.M. Regulation of prolactin and its role in gallinaceous bird reproduction. J. Exp. Zool. 1984;232:521–529. doi: 10.1002/jez.1402320319. [DOI] [PubMed] [Google Scholar]
- El Halawani M.E., Pitts G.R., Sun S., Silsby J.L., Sivanandan V. Active immunization against vasoactive intestinal peptide prevents photo-induced prolactin secretion in turkeys. Gen. Comp. Endocrinol. 1996;104:76–83. doi: 10.1006/gcen.1996.0143. [DOI] [PubMed] [Google Scholar]
- El Halawani M.E., Silsby J.L., Fehrer S.C., Behnke E.J. Reinitiation of ovulatory cycles in incubating female turkeys by an inhibitor of serotonin synthesis, P-chlorophenylalanine. Biol. Reprod. 1983;28:221–228. doi: 10.1095/biolreprod28.1.221. [DOI] [PubMed] [Google Scholar]
- El Halawani M.E., Silsby J.L., Mauro L.J. Vasoactive intestinal peptide is a hypothalamic prolactin-releasing neuropeptide in the Turkey (Meleagris gallopavo) Gen. Comp. Endocrinol. 1990;78:66–73. doi: 10.1016/0016-6480(90)90048-q. [DOI] [PubMed] [Google Scholar]
- El Halawani M.E., Silsby J.L., Rozenboim I. Increased egg production by active immunization against vasoactive intestinal peptide in the Turkey (Meleagris gallopavo) Biol. Reprod. 1995;52:179–183. doi: 10.1095/biolreprod52.1.179. [DOI] [PubMed] [Google Scholar]
- El Halawani M.E., Whiting S.E., Silsby J.L., Pitts G.R., Chaiseha Y. Active immunization with vasoactive intestinal peptide in Turkey hens. Poult. Sci. 2000;79:349–354. doi: 10.1093/ps/79.3.349. [DOI] [PubMed] [Google Scholar]
- Eltayeb N.M., Wani C.E., Yousif I.A. Assessment of broodiness and its influence on production performance and plasma prolactin level in native chicken of the Sudan. Asian J. Poult. Sci. 2010;4:1–6. [Google Scholar]
- Freeman M.E., Kanyicska B., Lerant A., Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol. Rev. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- Geng A.L., Xu S.F., Zhang Y., Zhang J., Chu Q., Liu H.G. Effects of photoperiod on broodiness, egg-laying and endocrine responses in native laying hens. Br. Poult. Sci. 2014;55:264–269. doi: 10.1080/00071668.2013.878782. [DOI] [PubMed] [Google Scholar]
- Guémené D., Etches R.J. Endocronological and behavioural effects of p-chlorophenylalanine (PCPA) oral administration to broody Turkey hens (Meleagris gallopavo) Reprod. Nutr. Dev. 1989;29:469–476. doi: 10.1051/rnd:19890408. [DOI] [PubMed] [Google Scholar]
- Hall T.R., Cheung A., Harvey S. Serotoninergic inhibition of LH secretion in the domestic fowl. J. Endocrinol. 1986;110:239–244. doi: 10.1677/joe.0.1100239. [DOI] [PubMed] [Google Scholar]
- Hall M.R., Goldsmith A.R. Factors affecting prolactin secretion during breeding and incubation in the domestic duck (Anas platyrhynchos) Gen. Comp. Endocrinol. 1983;49:270–276. doi: 10.1016/0016-6480(83)90144-2. [DOI] [PubMed] [Google Scholar]
- Jequier E., Lovenberg W., Sjoerdsma A. Tryptophan hydroxylase inhibition : depletes the mechanism by which p-chlorophenylalanine depletes rat brain serotonin. Mol. Pharmacol. 1967;3:274–278. [PubMed] [Google Scholar]
- Jiang R.S., Chen X.Y., Geng Z.Y. Broodiness, egg production, and correlations between broody traits in an indigenous chicken breed. Poult. Sci. 2010;89:1094–1096. doi: 10.3382/ps.2009-00621. [DOI] [PubMed] [Google Scholar]
- Kosonsiriluk S., Sartsoongnoen N., anong Chaiyachet O., Prakobsaeng N., Songserm T., Rozenboim I., El Halawani M., Chaiseha Y. Vasoactive intestinal peptide and its role in continuous and seasonal reproduction in birds. Gen. Comp. Endocrinol. 2008;159:88–97. doi: 10.1016/j.ygcen.2008.07.024. [DOI] [PubMed] [Google Scholar]
- Lea R.W., Sharp P.J., Chadwick A. Daily variations in the concentrations of plasma prolactin in broody bantams. Gen. Comp. Endocrinol. 1982;48:275–284. doi: 10.1016/0016-6480(82)90027-2. [DOI] [PubMed] [Google Scholar]
- Mauro L.J., Elde R.P., Youngren O.M., Phillips R.E., Halawani M.E.E. Alterations in hypothalamic vasoactive intestinal peptide-like immunoreactivity are associated with reproduction and prolactin release in the female Turkey (Meleagris gallopavo) Endocrinology. 1989;125:1795–1804. doi: 10.1210/endo-125-4-1795. [DOI] [PubMed] [Google Scholar]
- Mauro L.J., Youngren O.M., Proudman J.A., Phillips R.E., El Halawani M.E. Effects of reproductive status, ovariectomy, and photoperiod on vasoactive intestinal peptide in the female Turkey hypothalamus. Gen. Comp. Endocrinol. 1992;87:481–493. doi: 10.1016/0016-6480(92)90056-p. [DOI] [PubMed] [Google Scholar]
- Millam J.R., Burke W.H., El Halawani M.E., Ogren L.A. Preventing broodiness in Turkey hens with a dopamine receptor blocking agent. Poult. Sci. 1980;59:1126–1131. doi: 10.3382/ps.0591126. [DOI] [PubMed] [Google Scholar]
- Mobarkey N., Avital N., Heiblum R., Rozenboim I. The effect of parachlorophenylalanine and active immunization against vasoactive intestinal peptide on reproductive activities of broiler breeder hens photostimulated with green light. Biol. Reprod. 2013;88(4):83. doi: 10.1095/biolreprod.112.103697. [DOI] [PubMed] [Google Scholar]
- Moguilevsky J.A., Wuttke W. Changes in the control of gonadotrophin secretion by neurotransmitters during sexual development in rats. Exp. Clin. Endocrinol. Diabetes. 2001;109:188–195. doi: 10.1055/s-2001-15105. [DOI] [PubMed] [Google Scholar]
- Namken S., Sinpru P., Kamkrathok B., Sartsoongnoen N., Chaiseha Y. Role of vasoactive intestinal peptide during the transition from incubation behavior to rearing behavior in the female native Thai chicken. Poult. Sci. 2017;96:3768–3774. doi: 10.3382/ps/pex180. [DOI] [PubMed] [Google Scholar]
- Nash J.P., Davail-Cuisset B., Bhattacharyya S., Suter H.C., Le Menn F., Kime D.E. An enzyme linked immunosorbant assay (ELISA) for testosterone, estradiol, and 17,20β-dihydroxy-4-pregenen-3-one using acetylcholinesterase as tracer: Application to measurement of diel patterns in rainbow trout (Oncorhynchus mykiss) Fish Physiol. Biochem. 2000;22:355–363. [Google Scholar]
- Park D.H., Stone D.M., Baker H., Kim K.S., Joh T.H. Early induction of rat brain tryptophan hydroxylase (TPH) mRNA following parachlorophenylalanine (PCPA) treatment. Brain Res. Mol. Brain Res. 1994;22:20–28. doi: 10.1016/0169-328x(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Prakobsaeng N., Sartsoongnoen N., Kosonsiriluk S., Chaiyachet O.A., Chokchaloemwong D., Rozenboim I., El Halawani M., Porter T.E., Chaiseha Y. Changes in vasoactive intestinal peptide and tyrosine hydroxylase immunoreactivity in the brain of nest-deprived native Thai hen. Gen. Comp. Endocrinol. 2011;171:189–196. doi: 10.1016/j.ygcen.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Richard-Yris M.A., Guémené D., Lea R.W., Sharp P.J., Bédécarrats G., Forasté M., Wauters A.M. Behaviour and hormone concentrations in nest deprived and renesting hens. Br. Poult. Sci. 1998;39:309–317. doi: 10.1080/00071669888836. [DOI] [PubMed] [Google Scholar]
- Rochester J.R., Heiblum R., Rozenboim I., Millam J.R. Post-hatch oral estrogen exposure reduces oviduct and egg mass and alters nest-building behavior in adult zebra finches (Taeniopygia guttata) Physiol. Behav. 2008;95:370–380. doi: 10.1016/j.physbeh.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Romanov M.N., Talbot R.T., Wilson P.W., Sharp P.J. Genetic control of incubation behavior in the domestic hen. Poult. Sci. 2002;81:928–931. doi: 10.1093/ps/81.7.928. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., El Halawani M.E. Characterization of vasoactive intestinal peptide pituitary membrane receptors in Turkey hens during different stages of reproduction. Biol. Reprod. 1993;48:1129–1134. doi: 10.1095/biolreprod48.5.1129. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Mobarky N., Heiblum R., Chaiseha Y., Kang S.W., Biran I., Rosenstrauch A., Sklan D., El Halawani M.E. The role of prolactin in reproductive failure associated with heat stress in the domestic Turkey. Biol. Reprod. 2004;71:1208–1213. doi: 10.1095/biolreprod.104.028167. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Tabibzadeh C., Silsby J.L., El Halawani M.E. Effect of ovine prolactin administration on hypothalamic vasoactive intestinal peptide (VIP), gonadotropin releasing hormone I and II content, and anterior pituitary VIP receptors in laying Turkey hens. Biol. Reprod. 1993;48:1246–1250. doi: 10.1095/biolreprod48.6.1246. [DOI] [PubMed] [Google Scholar]
- Sharp P.J. Broodiness and broody control. In: Hocking P.M., editor. Biology of Breeding Poultry. Vol. 29. CABI; Wallingford, UK: 2009. pp. 181–205. (Poult. Sci. Symp. Ser.). [Google Scholar]
- Ubuka T., Bentley G.E., Tsutsui K. Neuroendocrine regulation of gonadotropin secretion in seasonally breeding birds. Front. Neurosci. 2013;7:1–38. doi: 10.3389/fnins.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleck C.M., Patrick D.J. Effects of vasoactive intestinal peptide on prolactin secretion in three species of passerine birds. Gen. Comp. Endocrinol. 1999;113:146–154. doi: 10.1006/gcen.1998.7191. [DOI] [PubMed] [Google Scholar]
- Xu P., Siegel P.B., Denbow D.M. Genetic selection for body weight in chickens has altered responses of the brain’s AMPK system to food intake regulation effect of ghrelin, but not obestatin. Behav. Brain Res. 2011;221:216–226. doi: 10.1016/j.bbr.2011.02.034. [DOI] [PubMed] [Google Scholar]
- Youngren O., Chaiseha Y., Phillips R., El Halawani M. Vasoactive intestinal peptide concentrations in Turkey hypophysial portal blood differ across the reproductive cycle. Gen. Comp. Endocrinol. 1996;103:323–330. doi: 10.1006/gcen.1996.0128. [DOI] [PubMed] [Google Scholar]
- Zadworny D., Shimada K., Ishida H., Sumi C., Sato K. Changes in plasma levels of prolactin and estradiol, nutrient intake, and time spent nesting during the incubation phase of broodiness in the Chabo hen (Japanese bantam) Gen. Comp. Endocrinol. 1988;71:406–441. doi: 10.1016/0016-6480(88)90269-9. [DOI] [PubMed] [Google Scholar]
- Zadworny D., Shimada K., Ishida H., Sato K. Gonadotropin-stimulated estradiol production in small ovarian follicles of the hen is suppressed by physiological concentrations of prolactin in vitro. Gen. Comp. Endocrinol. 1989;74:468–473. doi: 10.1016/s0016-6480(89)80044-9. [DOI] [PubMed] [Google Scholar]