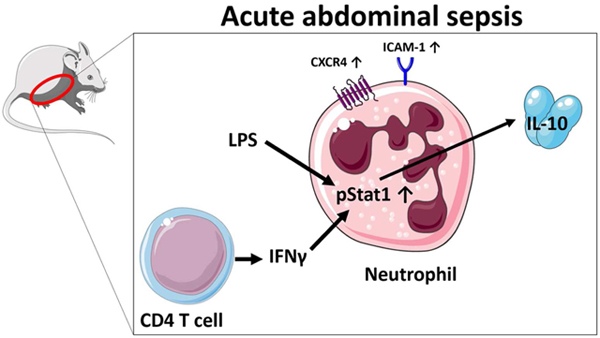
Abstract
The disease burden of sepsis continues to increase, with intraabdominal contamination being a significant source of infection. Sepsis is a syndrome involving both an increase in systemic inflammation as well as a regulatory component. We have previously demonstrated that neutrophils are significant IL-10 producers in the abdomen during sepsis. Here, we sought to further characterize these neutrophils and elucidate potential underlying mechanisms resulting in IL-10 generation. Using transcriptional reporter mice, we observed that IL-10 producing neutrophils were activated, non-apoptotic, and expressed C-X-C chemokine receptor type 4-expressing. Further, we observed that active Signal Transducer and Activator of Transcription 1 expression was significantly increased in IL-10 producing versus non-IL-10 producing neutrophils. During sepsis, IFN-γ blockade lead to a decrease of neutrophil IL-10 production, while peritoneal CD4 T cells were found to be the most numerous acute producers of IFN-γ. Altogether, this report demonstrates that during sepsis, mature neutrophils can potentially dampen local inflammation by IL-10 production and this can be orchestrated by CD4 T cells through an IFN-γ dependent manner.
Keywords: Sepsis, Neutrophil, IL-10, STAT-1, IFN-γ, T cell
Graphical abstract
Introduction
Although the mortality rate of sepsis in recent years is unchanged, case numbers continue to climb [1]. International studies demonstrate the abdomen as a significant source of infection for sepsis [2]. The syndrome of sepsis is composed of a potent inflammatory reaction, concurrent with a immune suppressive mechanisms [3]. The initial inflammatory response is needed to decrease the host’s bacterial burden, whereas immune regulation prevents exaggerated inflammation that can lead to early life-threatening organ dysfunction and death [3]. Neutrophils have a crucial function in the initial phase after sepsis [4] in that they are one of the first cells to migrate into infected compartments to eradicate pathogens. However, this activation must be balanced, as excessive inflammation can lead to collateral damage by uncontrolled reactive oxygen species (ROS) production by which healthy tissue gets harmed [4].
The production of cytokine IL-10 is an important mechanism in the prevention of excessive inflammation by suppressing both innate and adaptive immune cell functions [5]. In a previous murine study, we demonstrated that at the site of infection, neutrophils are the predominate cell producing IL-10 [6]. Currently, the phenotype of IL-10-producing neutrophils remains unclear. Here, we sought to identify a receptor pattern that would lend to better understanding of their activation state. Two adhesion molecules were utilized to address this question. First, the upregulation of ICAM-1 can indicate maturity of a neutrophil and is associated with enhanced effector functions such as enhanced phagocytosis and reactive oxygen species (ROS) production [7]. Secondly, L-Selectin shedding from neutrophil plasma membrane is considered to indicate neutrophil activation or partial activation (priming) [8]. As we wanted to assess maturity, it is noted that L-Selectinlow/negtive neutrophils are not considered senescent neutrophils [4,9]. A previous report has used these two molecules and demonstrated that the L-SelectinlowICAM-1+ subtype showed increased activation [10,11]. Additionally, we sought to elucidate the determinants of IL-10 generation.
Material and Methods
Animal models
We used B6(Cg)-IL10tm1.1Karp/J (VertX) mice (Jackson Laboratories, Bar Harbor, ME) in order to assess IL-10 production. These mice express green fluorescent protein (GFP) downstream of the IL-10 gene, allowing for analysis by flow cytometry while maintaining viability. The production of IL-10 is not impaired by this genetic modification [12] and the half-life of the produced GFP is greater than 24h [13]. All animal experiments were performed under protocols approved by the Institutional Animal Care and Use Committee of the University of Cincinnati (IACUC protocol no: 08-09-19-01).
Cecal ligation and puncture
The induction of polymicrobial sepsis was induced by cecal ligation and puncture (CLP) as described previously [14]. Briefly, mice were anesthetized using 2.5% isoflurane in oxygen via face mask. The abdominal area was shaved, disinfected with povidone-iodine and a 1 cm incision was made to access the cecum. Different severities of sepsis were established by 33% ligation, 1x puncture 25-gauge needle (mild), 50% ligation, 1x puncture 25-gauge needle (moderate) and 66% ligation, 1x puncture 22-gauge needle (severe). The cecum was replaced intraabdominally, and the incision closed in a two-layer suture. The mice were resuscitated with 1 ml of 0.9% saline solution (Hospira, Lake Forest, IL) and placed on a heating pad for one hour. Cells were harvested after 24h by intraperitoneal wash. To neutralize IFN-γ ,100 μg anti-IFN-γ-Ab (clone: XMG1.2, BioXCell, Lebanon, NH) was administered intraperitoneally 24h prior to the induction of sepsis[15].
LPS stimulation of neutrophils in cell culture
Bone marrow of both femur and tibia was flushed and 2 million cells per well were incubated at 37°C and 5% CO2 for 24h in the presence of Lipopolysaccharide (LPS) (Escherichia coli 0111:B4, Sigma-Aldrich, St. Louis, MO, USA).
Intracellular and surface labeling of cells using flow cytometry
After harvest cells were enumerated using cell counter (Beckman Coulter, Brea, CA), 1 million cells were incubated with a cell viability dye LIVE/DEAD™ Cell Vitality Assay Kit (Thermo Fisher Scientific, Waltham, MA), washed and afterwards labeled for flow cytometry. All labeling included Fc-receptor blockage prior to labeling using CD16/CD32 (Mouse BD Fc Block™) (clone 2.4G2 (RUO), BD Pharmingen, San Jose, CA, USA) and 5% rat serum (Invitrogen, Carlsbad, CA). To assess apoptosis, AnnexinV was used to label cells in Annexin V Binding Buffer BD Pharmingen, San Jose, CA, USA). After labeling, cells were immediately analyzed using flow cytometry. For intracellular labeling of neutrophils, cells were fixed with 1% paraformaldehyde and permeabilized using cold methanol 90% as previously described [14].
To analyze intracellular IFN-γ expression in lymphoid cells in vivo, mice were pre-treated with i.p. injection of 250 μg protein transport inhibitor brefeldin A (BFA, Sigma-Aldrich, St. Louis, MO) 30min prior to inducing CLP, as described previously [16]. For intracellular labeling of lymphoid cells, the Mouse Foxp3 Buffer Set (BD Pharmingen, San Diego, CA) was used according to the manufacturer instructions.
The following fluorescent-labeled antibodies were used for surface and intracellular labeling: Ly6G (clone: 1A-8), Ly6C (clone: AL-21), CD4 (clone RM4-5), ICAM-1(CD54) (clone: 3E2), L-Selectin (CD62L) (clone: MEL-14) and CD44 (clone: IM7) all from BD Biosciences, San Jose, CA and CXCR4 (clone: L276F12), CD8 (clone: 53-6.7) as well as pStat1 (clone: A15158B), IFN-γ (clone: XMG1.2), NK1.1 (clone: PK136) and AnnexinV all from BioLegend, San Diego, CA. Flow cytometry acquisition and analysis were performed on an Attune® NxT™ Acoustic Focusing Cytometer (Thermo Fisher Scientific, Waltham, MA). Neutrophil subsets were characterized using L-Selectin (CD62L) and ICAM-1 (CD54). The L-Selectin+/ICAM-1- subtype was considered immature, while the L-Selectin+/ICAM-1+ and L-Selectin-/ICAM-1+ subtypes were considered mature [7,8]. T-cell subsets were identified as follows: naive (CD44-/CD62L+), central memory (CD44+/CD62L+), and effector memory (CD44+/CD62L-) [17,18].
Statistical analyses
For statistical analysis of the data GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA) was used. Outliers were identified using the ROUT method (Q=1%) and removed. Groups were tested for normality using the Shapiro-Wilk and the DÀgostino and Pearson normality test. If the normality test was passed, differences were analyzed using a two tailed Student’s t test comparison of two groups or one-way ANOVA with Tukey post-hoc analysis for comparisons of more than two groups. If the normality test was not passed, differences were analyzed using a Mann-Whitney test to compare two groups or Kruskal-Wallis test with Dunn`s multiple-comparison test analysis for comparisons of more than two groups. Individual data points are depicted, as well as mean ± standard deviation of the mean. A p value of ≤0.05 was considered statistically significant.
Results
Activated, but non-apoptotic neutrophils were the main producers of IL-10
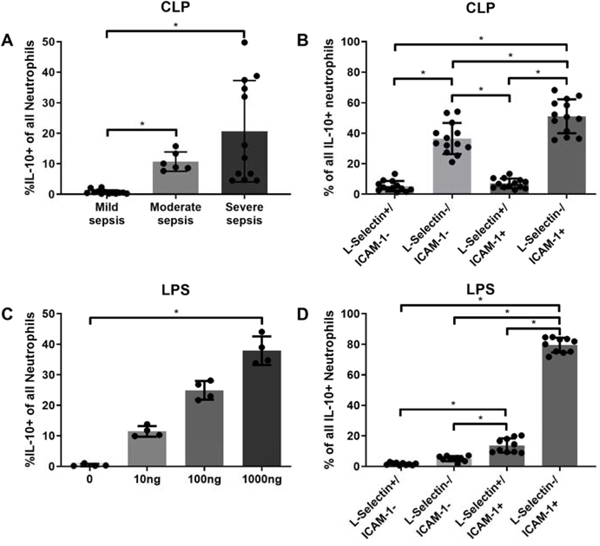
Previously, it was reported that during peritoneal sepsis, local IL-10 production was mainly facilitated by neutrophils. However, it remains unclear whether all neutrophils produced IL-10 or only certain subsets. We postulated that neutrophil IL-10 expression varies depending on the stimulus and that these neutrophils may express a distinct characterizing phenotype. Our data showed a significant increase in the proportion of IL-10 producing neutrophils, positively associated with sepsis severity (Fig. 1A). A similar association was observed in vitro with increasing dosages of LPS (Fig.1B). Interestingly, both IL-10 positive and negative neutrophils were overwhelmingly non-apoptotic in vivo (data not shown). The L-Selectin-/ICAM-1+, activated subtype represented the highest proportion in both experiments (Fig. 1B,D). Thus, neutrophil IL-10 production was dependent upon both sepsis severity and LPS-dose dependent and the L-Selectin-/ICAM-1+ phenotype was predominant of the different subtypes.
Figure 1: Acute IL-10 production of intraperitoneal neutrophils is associated with injury severity in vivo and LPS dosage in vitro.
Peritoneal neutrophils were harvested: A and B) 24 h after cecal ligation and puncture (CLP). Bone marrow derived neutrophils were harvested: C and D) after 24 hours LPS stimulation. The enumeration and characterization were conducted as described in material and methods. Data are expressed as means ± SD. *p < 0.05.
Neutrophils producing IL-10 have higher CXCR4 expression compared to non-producers
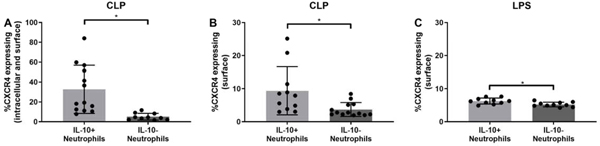
A recent study examined how extracellular ubiquitin, which binds to CXCR4, influences IL-10 production in macrophages from healthy individuals [19]. The authors demonstrated that macrophages produced less IL-10 when CXCR4 was blocked before stimulating them with ubiquitin compared to stimulation without blockage [19], which assumes that CXCR4 regulates IL-10 production. We therefore hypothesized the expression of CXCR4 on IL-10 producing neutrophils is higher as in non-producers. As the receptor can be expressed on the surface or become internalized, neutrophils were subjected to both total and surface labeling. Overall, a higher proportion of IL-10 producing neutrophils showed increased expression of total CXCR4 (Fig 2A). Additionally, this was true of surface CXCR4 surface labeling both 24 hours of sepsis induction and LPS injection (Figs. 2B,D). Altogether, these data demonstrate that peritoneal IL-10 producing neutrophils have higher expression of CXCR4 and ICAM-1, lower expression of CD62L, and are non-apoptotic.
Figure 2: CXCR4 expression is increased in IL-10 producing neutrophils compared to IL-non-producing neutrophils both in vivo and in vitro.
Peritoneal neutrophils of IL-10 GFP reporter mice were harvested and processed: A and B) 24 h after CLP. Bone marrow derived LPS stimulated neutrophils were harvested: C) 24 h post stimulation. Total and surface CXCR4 expression was determined as described in material nad methods. Data are expressed as means ± SD. *p < 0.05.
Neutrophils IL-10 production is augmented by the IFN-γ/Stat1-axis
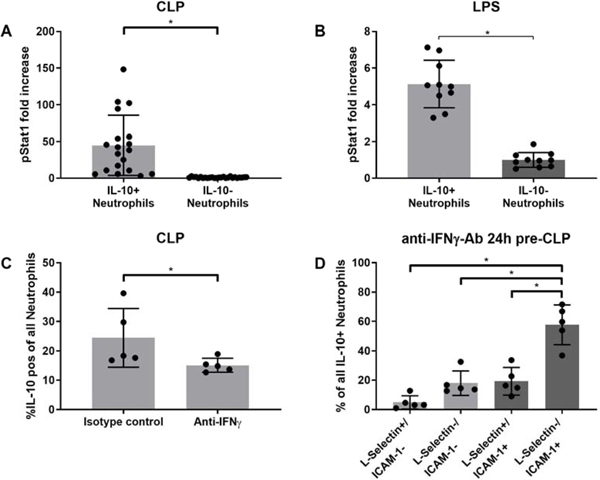
The transcriptional regulation of IL-10 has been demonstrated to be vitally dependent on active Stat3, and to a slightly lesser extend on active Stat1 [20]. Here, we hypothesized that neutrophil expression of IL-10 is driven by the IFN-γ/Stat1-axis, as Stat1. To assess the role of Stat1 in neutrophil IL-10 production, we determined activated phosphorylated Stat1 expression in vivo and in vitro. For a better evaluation the results were normalized, which demonstrated an increase in pStat1 expression in IL-10 positive neutrophils as compared to IL-10 negative neutrophils 24h after both sepsis induction (Fig. 3A) and LPS stimulation (Fig. 3B). To assess whether IFN-γ in the peritoneal cavity drives IL-10 production in neutrophils we examined whether there were detectable changes in the production when IFN-γ is neutralized. After an anti-IFN-γ-Ab was administered i.p. 24h prior to the injury, the percentage of neutrophils producing IL-10 decreased significantly (Fig. 3C). We additionally characterized the IL-10 producing neutrophils using L-Selectin and ICAM-1. As in the untreated neutrophils (Fig. 1B) the L-Selectin-/ICAM-1+ subtype was the main subtype within all IL-10 positive neutrophils (Fig. 3D). Neutrophils producing IL-10 showed increased expression of pStat in vivo and in vitro, IFN-γ disruption decreased IL-10 expression and remaining IL-10 positive neutrophils were mainly found to be L-Selectin-/ICAM-1+.
Figure 3: The IFN-γ / active STAT-1 signaling pathway is associated with neutrophil IL-10 generation.
Peritoneal neutrophils of IL-10 GFP reporter mice were harvested and processed: A) 24 h after CLP while bone marrow derived, LPS-stimulated neutrophils were harvested: B) 24 h post stimulation. Active STAT-1 was determine as described in the materials and methods. To assess IFN-γ dependency of IL-10 production 100 μg anti-IFN-γ-Ab was pre-administered 24 hours prior to CLP surgeries. C) The proportion neutrophils generating IL-10 from mice treated with isotype or IFN-γ-neutralizing antibodies was determined as described. D) With IFN-γ blockade, IL-10 producing neutrophils are characterized as described in the material and methods. Data are expressed as means ± SD. *p < 0.05.
CD4 T cells are the most prevalent IFN-γ producers
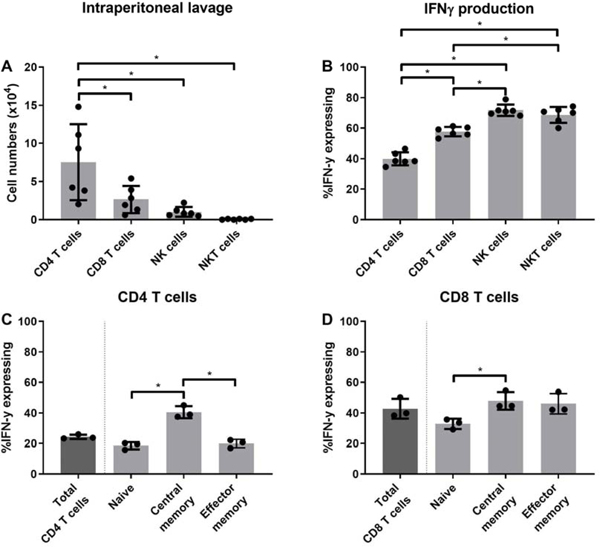
In previous work, we observed that during acute sepsis, peritoneal IFN-γ levels were elevated using the murine model of CLP [21]. We have also demonstrated that mortality can be increased in sepsis in IFN-γ KO mice [22]. These data suggest IFN-γ can play a key role during sepsis, but the predominate cell that produced IFN-γ during acute sepsis has not been elucidated. Here, we observed that of the leukocytes producing IFN-γ, CD4 T cell numbers were most prevalent, followed by CD8 T cells, NK cells and NKT cells (Fig. 4A). Although NK and NKT proportionally produced more IFN-γ than CD8 or CD4 T cells (Fig. 4B), their total numbers were significantly smaller than CD4 or CD8 T cells (Fig. 4A). Next, we further phenotyped which CD4 and CD8 T cells produced IFN-γ. For CD4 T cells, IFN-γ production was proportionately highest in the central memory subtype (Fig. 4C). For CD8 T cells, we observed a similar trend, albeit not significant. Altogether, these data suggest that CD4 central memory cells are significant producers of IFN-γ production.
Figure 4: CD4 T cells are the most prevalent IFN-γ producers during acute sepsis.
Twenty-four hours prior to the CLP surgeries, 250 μg of Brefeldin A were injected into wild type C57BL/6J mice. Peritoneal cells were harvested 3 hours after CLP with intracellular labeling for IFN-γ expression conducted as described in material and methods. As described in the material and methods, A) Leukocyte numbers were determined. B) Leukocyte proportions analyzed, C) CD4 phenotype and D) CD8 phenotype was determined for IFN-γ expression. Data are expressed as means ± SD. *p < 0.05.
Discussion
With this study we sought to characterize the IL-10 producing intraperitoneal neutrophils in the acute onset of abdominal sepsis and to determine the mechanisms behind IL-10 production. We demonstrated the injury-severity dependent nature of IL-10 production and IL-10 producing neutrophil mainly expressed a mature non-apoptotic L-Selectin-/ICAM-1+ phenotype and increased CXCR4 expression, dependent on the IFN-γ/Stat1 signaling pathway. These results are similar in vivo and in vitro indicating they are a result of stimulation by bacteria and not a general reaction to upregulation of inflammation. Lastly, we observed that central memory CD44+/CD62L+ CD4 T cells are the main producers of IFN-γ. We therefore conclude that at the acute onset of abdominal sepsis CD4 T cells and bacterial stimuli promote the production of IL-10 in an IFN-γ and LPS dependent manner in peritoneal mature neutrophils.
Several authors have suggested that neutrophils undergo apoptosis quickly after pathogen clearance to prevent excessive inflammation, with immunosuppressive capabilities attributed to either newly recruited and immature neutrophils or to impaired-functioning neutrophils influenced by various stimuli in septic setting [23]. However, the characterization of neutrophils as mainly L-Selectin-/ICAM-1+, and the fact that the majority of IL-10 positive neutrophils are not apoptotic suggest that the IL-10 producing neutrophils are senescent, as this pattern is associated with potent anti-inflammatory functionality [4,7]. Neutrophils producing IL-10 in vitro and in vivo expressed CXCR4, which suggests that the immunosuppressive effect of these cells is mediating inflammation not only locally but also potentially systemically in lymph nodes and bone marrow, as CXCR4 expression is associated with the reverse migration from inflamed tissue to lymph nodes modulating lymphocyte proliferation [24]. Moreover, CXCR4 activation might regulate neutrophil IL-10 production, as this was found in macrophages [19].
We confirmed IL-10 production is also regulated by active Stat1 in this murine CLP model. It is of note that this was the case for both in vivo and in vitro, indicating the process to be driven by bacteria and not by general immune activation. It was shown that Stat1 is part of the signaling pathway of the IL-10 receptors [20]. Therefore, Stat1 activation might be part of a positive feedback loop, as neutrophils are known to express IL-10 receptors [25]. The activation of the interferon-α-receptor by IFN-α or IFN-β as well as the activation of the interferon-γ-receptor by IFN-γ leads to the phosphorylation and therefore activation of Stat1 [26]. Our data suggests pStat1 upregulation is driven by IFN-γ, as the production of IL-10 was displayed to be IFN-γ dependent and all IL-10 producing neutrophils showed increased expression of pStat1. Interestingly, IL-10 producing neutrophils after IFN-γ blockage were mainly L-Selectin-/ICAM-1+, indicating that IFN-γ does not change the activation pattern of the neutrophils.
In previous work it has been determined that CD3 KO mice have a higher CLP induced mortality then WT mice [22]. Moreover, IFN-γ KO mice [22] as well as IFN-γR KO mice [27] showed increased mortality in murine abdominal sepsis. Interestingly a recent study in rats demonstrated the intraperitoneal administration of Interleukin-15 one hour post sepsis induction increased the total numbers of T cells and NK cells and IFN-γ levels, which lead to a reduced mortality [28]. In a murine Toxoplasma gondii infection model CD4+ cells were found vitally important to attenuate inflammation [15]. If a glucocorticoid auto-feedback loop did not lower CD4+ Th1 hyperactivity with increased IFN-γ production, mortality was significantly increased [15]. Our data suggests that CD4 T cells orchestrate the immunosuppressive release of IL-10 in neutrophils in the peritoneum by the release of IFN-γ. These studies [15,28] implicate that IFN-γ release by peritoneal CD4+ T cells potentially orchestrates a the environment site of inflammation during sepsis.
We chose our 3h post-CLP timepoint because human septic shock studies showed a significant decrease in circulating T cells and NK cells already at the time of admission [29]. Interestingly, in total numbers we found CD4 T cells to be the main producers of IFN-γ, with central memory CD44+/CD62L+ cells being the largest subset. Effector memory T cells are sought to migrate to inflamed tissue to exert immediate effector functions, whereas central memory T cells are suggested to home to secondary lymphoid tissue to proliferate and differentiate into effector memory T cells [17]. Memory T cells are considered antigen-experienced [17], which suggests that the peritoneal T cells found here might have previously encountered the respective antigens in the gut. As sepsis is followed by rapid T cell apoptosis with CD44hi/+/ CD62Lhi/+ central memory being largely spared [30,31], this would altogether allow a concurrent proinflammatory response concurrently balanced by a down-stream inflammation resolving response.
In conclusion, we found the acute (24h) production of IL-10 by neutrophils to be dependent on sepsis severity. The IL-10 producing neutrophils revealed a non-apoptotic mature phenotype and expressed CXCR4 indicating potential reverse migration functionality. The upregulation of Stat1 in IL-10 producing neutrophils indicated an IFN-γ-promoted induction of IL-10, which was confirmed in an IFN-γ blockage model. Quantitatively CD4 T cells were found to be the main producers of IFN-γ in the very acute phase of sepsis (3h). We propose CD4 T cells as orchestrators of the neutrophil IL-10 production in the acute phase of sepsis; potentially preventing an exaggerated immune activation. We believe that it is important to further elucidate this likely beneficial effect in the acute phase of sepsis. It may present a target for the modulation of the peritoneal immune response during abdominal sepsis. The translation of these findings to humans has to be conducted carefully as human neutrophils seem to express IL-10 only after being primed [32] and may react differently.
Highlights.
During sepsis, IL-10pos neutrophils are activated, non-apoptotic and express CXCR4
Active STAT-1 expression is significantly increased in IL-10 producing neutrophils
IFN-γ blockade leads to a decrease of neutrophil IL-10 production during sepsis
Peritoneal CD4 T cells are the most numerous producers of IFN-γ in acute sepsis
Acknowledgements
The authors would like to thank Holly Goetzman and Lisa England for their great help conducting the experiments and assistance with the surgeries.
Funding:
This work was supported by funding from the Deutsche Forschungsgemeinschaft (German Research Foundation) (BE 7016/1-1) (C.C.B.) and the National Institute of General Medical Sciences (T32 GM08478) (C.E.S.)
Footnotes
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hajj J, Blaine N, Salavaci J, Jacoby D, The “Centrality of Sepsis”: A Review on Incidence, Mortality, and Cost of Care, Healthcare (Basel) 6 (2018). 10.3390/healthcare6030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Muresan MG, Balmos IA, Badea I, Santini A, Abdominal Sepsis: An Update, J Crit Care Med (Targu Mures) 4 (2018) 120–125. 10.2478/jccm-2018-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL, Sepsis and septic shock, Nat Rev Dis Primers 2 (2016) 16045 10.1038/nrdp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mortaz E, Alipoor SD, Adcock IM, Mumby S, Koenderman L, Update on Neutrophil Function in Severe Inflammation, Frontiers in immunology 9 (2018) 2171 10.3389/fimmu.2018.02171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Oberholzer A, Oberholzer C, Moldawer LL, Interleukin-10: A complex role in the pathogenesis of sepsis syndromes and its potential as an anti-inflammatory drug, Critical care medicine 30 (2002) S58–S63. [PubMed] [Google Scholar]
- [6].Kasten KR, Muenzer JT, Caldwell CC, Neutrophils are significant producers of IL-10 during sepsis, Biochem Biophys Res Commun 393 (2010) 28–31. 10.1016/j.bbrc.2010.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Woodfin A, Beyrau M, Voisin MB, Ma B, Whiteford JR, Hordijk PL, Hogg N, Nourshargh S, ICAM-1-expressing neutrophils exhibit enhanced effector functions in murine models of endotoxemia, Blood 127 (2016) 898–907. 10.1182/blood-2015-08-664995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ivetic A, Hoskins Green HL, Hart SJ, L-selectin: A Major Regulator of Leukocyte Adhesion, Migration and Signaling, Frontiers in immunology 10 (2019) 1068 10.3389/fimmu.2019.01068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kamp VM, Pillay J, Lammers JW, Pickkers P, Ulfman LH, Koenderman L, Human suppressive neutrophils CD16bright/CD62Ldim exhibit decreased adhesion, Journal of leukocyte biology 92 (2012) 1011–1020. 10.1189/jlb.0612273. [DOI] [PubMed] [Google Scholar]
- [10].Eruslanov EB, Phenotype and function of tumor-associated neutrophils and their subsets in early-stage human lung cancer, Cancer immunology, immunotherapy : CII 66 (2017) 997–1006. 10.1007/s00262-017-1976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hayashi F, Means TK, Luster AD, Toll-like receptors stimulate human neutrophil function, Blood 102 (2003) 2660–2669. 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- [12].Madan R, Demircik F, Surianarayanan S, Allen JL, Divanovic S, Trompette A, Yogev N, Gu Y, Khodoun M, Hildeman D, Boespflug N, Fogolin MB, Grobe L, Greweling M, Finkelman FD, Cardin R, Mohrs M, Muller W, Waisman A, Roers A, Karp CL, Nonredundant roles for B cell-derived IL-10 in immune counter-regulation, Journal of immunology 183 (2009) 2312–2320. 10.4049/jimmunol.0900185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Corish P, Tyler-Smith C, Attenuation of green fluorescent protein half-life in mammalian cells, Protein Eng 12 (1999) 1035–1040. 10.1093/protein/12.12.1035. [DOI] [PubMed] [Google Scholar]
- [14].Tschop J, Kasten KR, Nogueiras R, Goetzman HS, Cave CM, England LG, Dattilo J, Lentsch AB, Tschop MH, Caldwell CC, The cannabinoid receptor 2 is critical for the host response to sepsis, Journal of immunology 183 (2009) 499–505. 10.4049/jimmunol.0900203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kugler DG, Mittelstadt PR, Ashwell JD, Sher A, Jankovic D, CD4+ T cells are trigger and target of the glucocorticoid response that prevents lethal immunopathology in toxoplasma infection, The Journal of experimental medicine 210 (2013) 1919–1927. 10.1084/jem.20122300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Xia BT, Beckmann N, Winer LK, Pugh AM, Pritts TA, Nomellini V, Gulbins E, Caldwell CC, Amitriptyline Reduces Inflammation and Mortality in a Murine Model of Sepsis, Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 52 (2019) 565–579. 10.33594/000000040. [DOI] [PubMed] [Google Scholar]
- [17].Sallusto F, Geginat J, Lanzavecchia A, Central memory and effector memory T cell subsets: function, generation, and maintenance, Annual review of immunology 22 (2004) 745–763. 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- [18].Budd RC, Cerottini JC, Horvath C, Bron C, Pedrazzini T, Howe RC, MacDonald HR, Distinction of virgin and memory T lymphocytes. Stable acquisition of the Pgp-1 glycoprotein concomitant with antigenic stimulation, Journal of immunology 138 (1987) 3120–3129. [PubMed] [Google Scholar]
- [19].Barrera-Vargas A, Gomez-Martin D, Carmona-Rivera C, Merayo-Chalico J, Torres-Ruiz J, Manna Z, Hasni S, Alcocer-Varela J, Kaplan MJ, Differential ubiquitination in NETs regulates macrophage responses in systemic lupus erythematosus, Ann Rheum Dis 77 (2018) 944–950. 10.1136/annrheumdis-2017-212617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG, Regulation and functions of the IL-10 family of cytokines in inflammation and disease, Annual review of immunology 29 (2011) 71–109. 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- [21].Kasten KR, Prakash PS, Unsinger J, Goetzman HS, England LG, Cave CM, Seitz AP, Mazuski CN, Zhou TT, Morre M, Hotchkiss RS, Hildeman DA, Caldwell CC, Interleukin-7 (IL-7) treatment accelerates neutrophil recruitment through gamma delta T-cell IL-17 production in a murine model of sepsis, Infection and immunity 78 (2010) 4714–4722. 10.1128/IAI.00456-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Martignoni A, Tschop J, Goetzman HS, Choi LG, Reid MD, Johannigman JA, Lentsch AB, Caldwell CC, CD4-expressing cells are early mediators of the innate immune system during sepsis, Shock 29 (2008) 591–597. 10.1097/shk.0b013e318157f427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Leliefeld PH, Wessels CM, Leenen LP, Koenderman L, Pillay J, The role of neutrophils in immune dysfunction during severe inflammation, Critical care 20 (2016) 73 10.1186/s13054016-1250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hampton HR, Bailey J, Tomura M, Brink R, Chtanova T, Microbe-dependent lymphatic migration of neutrophils modulates lymphocyte proliferation in lymph nodes, Nature communications 6 (2015) 7139 10.1038/ncomms8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bazzoni F, Tamassia N, Rossato M, Cassatella MA, Understanding the molecular mechanisms of the multifaceted IL-10-mediated anti-inflammatory response: lessons from neutrophils, Eur J Immunol 40 (2010) 2360–2368. 10.1002/eji.200940294. [DOI] [PubMed] [Google Scholar]
- [26].Michalska A, Blaszczyk K, Wesoly J, Bluyssen HAR, A Positive Feedback Amplifier Circuit That Regulates Interferon (IFN)-Stimulated Gene Expression and Controls Type I and Type II IFN Responses, Frontiers in immunology 9 (2018) 1135 10.3389/fimmu.2018.01135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zantl N, Uebe A, Neumann B, Wagner H, Siewert JR, Holzmann B, Heidecke CD, Pfeffer K, Essential role of gamma interferon in survival of colon ascendens stent peritonitis, a novel murine model of abdominal sepsis, Infection and immunity 66 (1998) 2300–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhao X, Qi H, Zhou J, Xu S, Gao Y, Treatment with Recombinant Interleukin-15 (IL-15) Increases the Number of T Cells and Natural Killer (NK) Cells and Levels of Interferon-gamma (IFN-gamma) in a Rat Model of Sepsis, Med Sci Monit 25 (2019) 4450–4456. 10.12659/MSM.914026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Venet F, Davin F, Guignant C, Larue A, Cazalis MA, Darbon R, Allombert C, Mougin B, Malcus C, Poitevin-Later F, Lepape A, Monneret G, Early assessment of leukocyte alterations at diagnosis of septic shock, Shock 34 (2010) 358–363. 10.1097/SHK.0b013e3181dc0977. [DOI] [PubMed] [Google Scholar]
- [30].Kasten KR, Tschop J, Adediran SG, Hildeman DA, Caldwell CC, T cells are potent early mediators of the host response to sepsis, Shock 34 (2010) 327–336. 10.1097/SHK.0b013e3181e14c2e. [DOI] [PubMed] [Google Scholar]
- [31].McDunn JE, Turnbull IR, Polpitiya AD, Tong A, MacMillan SK, Osborne DF, Hotchkiss RS, Colonna M, Cobb JP, Splenic CD4+ T cells have a distinct transcriptional response six hours after the onset of sepsis, J Am Coll Surg 203 (2006) 365–375. 10.1016/j.jamcollsurg.2006.05.304. [DOI] [PubMed] [Google Scholar]
- [32].Lewkowicz N, Mycko MP, Przygodzka P, Cwiklinska H, Cichalewska M, Matysiak M, Selmaj K, Lewkowicz P, Induction of human IL-10-producing neutrophils by LPS-stimulated Treg cells and IL-10, Mucosal immunology 9 (2016) 364–378. 10.1038/mi.2015.66. [DOI] [PubMed] [Google Scholar]