Abstract
Maternally derived antibodies (MDA) substantially interfere with active immunity in post-hatch vaccination, although they provide early protection against disease through passive immunity in young chickens. Previously, Newcastle disease virus (NDV) strain TS09-C was demonstrated to be safe and immunogenic as in-ovo vaccine in specific-pathogen-free chickens. Here, we evaluated the safety, protective efficacy, and duration of clinical protection of the TS09-C virus as an in-ovo vaccine for commercial chickens in the presence of Maternally derived antibodies against NDV. This vaccine was safe in commercial chickens and provided at least 80% protection against a virulent NDV challenge for 3 mo, despite inducing a low hemagglutinin-inhibition titer. For commercial chickens, the protective efficacy of the in-ovo vaccination was markedly higher than that of posthatch vaccination, and the cellular immune response might play an important role in the higher protective efficacy of the in-ovo vaccine. The overall results indicate that the maternally derived antibodies against NDV do not significantly interfere with the ability of the in-ovo vaccine strain TS09-C to induce protective cellular immunity.
Key words: Newcastle disease virus, in-ovo vaccine, maternally derived antibody, strain TS09-C
Introduction
Newcastle disease (ND) is one of the most important infectious avian diseases and poses a considerable threat to the poultry industry worldwide. The disease is caused by virulent strains of Newcastle disease virus (NDV) (Alexander and Allan, 1974), which is enzootic in multiple countries in Asia, Europe, Africa, the Middle East, and the Americas (Dimitrov et al., 2016). Vaccination with live vaccines is a common worldwide strategy for ND control. As a consequence of these ND vaccination programs, maternally derived antibodies (MDA) against NDV are universally present in the progeny of vaccinated chicken breeder flocks. Although MDA can prevent clinical disease through passive immunization in early life stages, it can also hinder the immune response in vaccination and lead to inadequate and even invalid protection against disease (Westbury et al., 1984, Niewiesk, 2014, Yosipovich et al., 2015). Thus, there is a need to develop new vaccines or vaccination strategies to overcome MDA interference.
As an attractive immunization approach for chickens, in-ovo vaccination can elicit an appreciable degree of protection by the time of hatching, assist in closing the window in which chickens are susceptible to infection, provide uniform and fast delivery of vaccines, and decrease labor costs through the use of mechanized injectors (Negash et al., 2004, Williams and Zedek, 2010, Peebles, 2018). However, conventional live ND vaccine strains, such as LaSota and V4, are highly lethal for chicken embryos and thus cannot be administered via the in-ovo route (Mast et al., 2006). The NDV antibody-antigen complex vaccine, in which the release of virus is delayed until after hatching, has been developed as an in-ovo vaccine (Haddad et al., 2003). Through replacement of the L gene with that of the Clone-30 strain and insertion of the infectious bursal disease virus VP2 gene, the chimeric NDV strain LaSota is attenuated significantly and has been found to be a safe and effective in-ovo vaccine against both NDV and infectious bursal disease virus. Commercial chickens in-ovo vaccinated with these 2 vaccines acquire protective immunity without interference by the MDA (Kapczynski et al., 2012, Ge et al., 2014).
Previously, we confirmed that NDV strain TS09-C was safe and immunogenic as an in-ovo vaccine for specific-pathogen-free (SPF) chicken embryos (Wen et al., 2017). Here, we evaluated the safety, protective efficacy, and duration of clinical protection of TS09-C virus as an in-ovo vaccine for commercial chickens in the presence of MDA against NDV and compared the protective efficacy between in-ovo and post-hatch vaccination.
Materials and methods
Animals and Ethics Statement
Embryonated eggs from commercial layer (Jianghan) breeders were purchased from a local chicken farm in Hubei, China. Layer breeder chickens were vaccinated with NDV LaSota vaccine 4 times before laying eggs. Chicken embryos were hatched in a contained environment at 37.5°C and a humidity of ∼60% and raised in negative pressure isolators. Animal experiments were approved (Permit number: 39/2017) and supervised by the Institutional Animal Care and Use Committee of the Hubei Academy of Agriculture Sciences.
Viruses
Four NDV strains V4, LaSota, F48E9, and HB0901 were obtained from the pathogen repository bank at the Hubei Academy of Agriculture Sciences (Hubei, China). NDV strain TS09-C was developed through serial passaging of strain V4 in BHK-21 cells. All viruses were propagated in SPF chicken embryos and titrated with 50% egg infectious dose (EID50) assays. The GenBank accession numbers of NDV strain V4, LaSota, F48E9, HB0901, and TS09-C are JX524203, JF950510, MG456905, MH579784, and JX110635, respectively.
Immunization and Challenge Experiments
Three commercial chicken experiments were carried out to evaluate the NDV strain TS09-C as in-ovo vaccine for (1) safety and protective efficacy, (2) duration of clinical protection, and (3) immunogenic comparison with posthatch vaccination. The method of in-ovo vaccination has been described previously (Wen et al., 2017). Briefly, 18-day-old embryonated eggs were cleaned with 70% ethanol. A 1-mm hole was punctured in the top of the eggs, and 103.0 EID50 of virus in a 0.1-mL volume (a safe dose that infected all embryos without causing obvious histopathological lesions) was injected into the amniotic cavity with a 38-mm 23 G needle at a depth of 1 inch. The vaccinated eggs were sealed and hatched in separate hatchers.
Experiment 1
One hundred and five 18-day-old chicken embryos were divided randomly into 3 groups of 35 eggs. Egg yolks collected from 5 eggs in each group were diluted one-fold with normal saline and then used to determine NDV hemagglutinin-inhibition (HI) titers. The remaining 30 chicken embryos in each group were inoculated with 103.0 EID50 of strain TS09-C, V4, or PBS. For each group, the proportions of vaccinated eggs that hatched successfully and survived at 7 D post-hatch (dph) were calculated. Ten birds were weighed at 1 and 14 dph, and the body weight gain at 14 dph was calculated by using the following formula: (mean body weight at 14 dph – mean body weight at 1 dph)/mean body weight at 1 dph. At 7 dph, 3 birds from each group were sacrificed, and the lung and trachea tissues were collected, fixed in 4% paraformaldehyde; paraffin embedded, sectioned, and stained with hematoxylin-eosin; and analyzed under a microscope. At 28 dph, 10 chickens randomly selected from each group were challenged with 104.0 EID50 of virulent NDV strain F48E9 via the intranasal and intraocular (IN/IO) routes in a 0.1-mL volume. Clinical ND signs and the mortality of challenged birds were monitored daily for 14 D. Before challenge, sera were collected from each bird at 1, 7, 14, 21, and 28 dph, and NDV antibody was detected with the HI assay. The antigen used for HI detection was the LaSota strain.
Experiment 2
One hundred and twenty 18-day-old chicken embryos were divided randomly into 2 groups of 60 eggs and in-ovo vaccinated with NDV strain TS09-C (103.0 EID50) or PBS. Ten hatched birds were selected randomly from each group at 10, 30, 60, and 90 dph and challenged with 104.0 EID50 of virulent NDV strain HB0901 via IN/IO routes. The protection rates of chickens at the indicated number of dph were calculated, and HI titers of chickens immediately before challenge were tested.
Experiment 3
One hundred and twenty 18-day-old chicken embryos were divided randomly into 4 groups of 30 eggs. Birds from group 1 and 2 were in-ovo vaccinated with PBS and NDV TS09-C (103.0 EID50), respectively. Birds from groups 3 and 4 were post-hatch vaccinated with 105.0 EID50 of NDV strains TS09-C and LaSota, respectively, at 7 dph. Ten birds selected randomly from each group were challenged with 104.0 EID50 of virulent NDV strain HB0901 via IN/IO routes at 28 dph. Clinical ND signs and the mortality of challenged birds were monitored daily for 14 D. Before challenge, sera were collected from each bird at 1, 7, 14, 21, and 28 dph and detected for NDV antibody with the HI assay. At 14 and 28 dph, 3 birds from each group were sacrificed, and spleen tissues were collected. Single splenic lymphocyte suspensions were prepared by using a chicken splenic lymphocyte separation kit (Solarbio, Beijing, China) and detected for CD3+ CD4+ and CD3+ CD8+ lymphocyte subsets with flow cytometry assay (Li et al., 2016).
Statistical Analysis
Statistical differences in the percentages of body weight gain and splenic lymphocytes between different groups were determined by one-way ANOVA at 5% level of significance, and those in the hatch and survival rates were analyzed by Log Rank test, in GraphPad Prism 5.0 (GraphPad Software, San Diego, CA).
Results and discussion
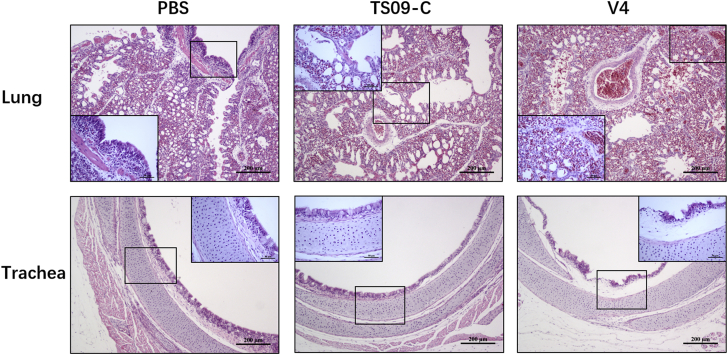
Previously, NDV strain TS09-C was confirmed to be safe as an in-ovo vaccine for SPF chickens, and the safe dose that infected all chicken embryos without causing obvious histopathological lesions was 103.0 EID50 per bird (Wen et al., 2017). In this study, the safety profile of this in-ovo vaccine was further evaluated in commercial chickens with the same dosage. As shown in Table 1, the NDV HI titers of egg yolks from commercial chicken embryos (embryonic day 18) were 6.00–6.40 log2, thus indicating a high level of NDV MDA in the commercial chickens. The hatching and survival rates of birds in the TS09-C group were 93.3%, similar to those of the PBS group but markedly higher than those of the V4 group. The percentage body weight gain in the TS09-C group (239.8%) was higher than that in the PBS group (198.5%). When immunized with a coccidiosis in-ovo vaccine, the birds also showed higher body weight gain than control birds at 14 dph (Sokale et al., 2018), thus suggesting that infection with NDV strain TS09-C, similar to coccidiosis, might increase the cecal weight and intestine length in vaccinated birds. Furthermore, only birds in the V4 group showed severe histopathological lesions in both the lung (congestion and moderate lymphocyte infiltration) and trachea (mucosal epithelial cell necrosis and cilia shedding) tissues at 7 dph (Figure 1). The results confirmed that NDV strain TS09-C could be used safely as in-ovo vaccine for commercial chickens.
Table 1.
The proportion of successful hatching, cumulative survival, and body weight gain of commercial chickens in-ovo vaccinated with different NDV strains.
| Vaccine | Dosage (log10 EID50) | Eggs | HI titer1 (log2) | % Hatched | % Survival2 | % Body weight gain 3 |
|---|---|---|---|---|---|---|
| TS09-C | 3.0 | 30 | 6.20 ± 0.84 | 93.3 (28/30)a | 93.3 (28/30)a | 239.8a |
| V4 | 3.0 | 30 | 6.00 ± 0.70 | 53.3 (16/30)b | 43.3 (13/30)b | 190.8b |
| PBS | - | 30 | 6.40 ± 1.14 | 93.3 (28/30)a | 90.0 (27/30)a | 198.5b |
a,bMeans in a same column with different superscript lowercase alphabets significantly differ (P < 0.05).
Abbreviations: HI, hemagglutinin-inhibition; NDV, Newcastle disease virus; SD, standard deviation.
HI titers of egg yolks collected from 5 commercial chicken embryos in each group at embryonic day 18 are expressed as log2 mean ± SD.
Global survival percentage of commercial chickens at 7 dph.
([Mean body weight at 14 dph – mean body weight at 1 dph]/mean body weight at 1 dph) × 100 (n = 10).
Figure 1.
Histopathological analyses of tissue samples from commercial chickens in-ovo vaccinated with different NDV strains. The lung and trachea samples were collected from in-ovo vaccinated chickens (PBS, TS09-C and V4 group) at 7 dph, fixed in 4% paraformaldehyde, paraffin embedded, sectioned and stained with hematoxylin-eosin, and analyzed under a microscope. Scale bar = 200 μm.
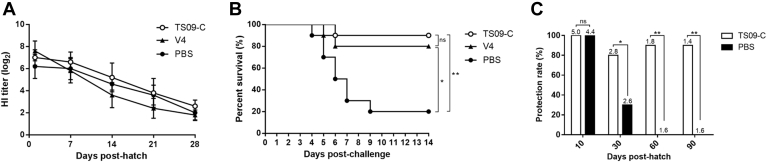
Maternal antibodies against NDV have been confirmed to substantially interfere with the immune response after post-hatch vaccination. To determine whether the MDA against NDV might interfere with the immunogenicity of the in-ovo vaccine, we challenged the in-ovo vaccinated commercial birds with the virulent NDV strain F48E9 (genotype IX, a challenge strain for vaccine efficiency assessment commonly used in China) at 28 dph. After in-ovo vaccination, the mean HI titers in the TS09-C group decreased gradually from 7.0 (1 dph) to 2.6 log2 (28 dph), similar to those in the V4 and PBS groups (Figure 2A). After the challenge with F48E9 virus, commercial birds in the TS09-C and V4 groups showed 90 and 80% survival, respectively, whereas the survival rate of chickens in the PBS group was only 20% (Figure 2B). These data indicated that the in-ovo vaccine strain TS09-C provided better protection against virulent NDV challenge in commercial chickens in the presence of MDA, although a low HI titer was induced. This finding is in agreement with the published data obtained with the antibody-antigen complex and chimeric rLaC30L-VP2 vaccine (Kapczynski et al., 2012, Ge et al., 2014). Commercial birds in-ovo vaccinated with rLaC30L-VP2 also showed 100% survival after virulent NDV challenge at 22 dph, although the HI titer was lower than 3 log2 at that time point.
Figure 2.
Protective efficacy of the in-ovo NDV vaccine strain TS09-C in commercial chickens against challenge with different virulent NDVs. The in-ovo vaccinated commercial chickens were challenged with virulent NDV strain F48E9 at 28 dph (A, B) and virulent strain HB0901 at the indicated dph (C). (A) The NDV-specific hemagglutinin-inhibition (HI) antibody titers of immunized birds before challenge. (B) The survival percentages of challenged birds on different days after challenge. (C) The protection rates of chickens challenged at the indicated dph. The HI titers of chickens immediately before challenge are indicated at the top of each column. Statistical significances in survival between different groups were analyzed by Log Rank test (ns, P > 0.05; *, P < 0.05; **, P < 0.01).
The precise mechanism through which the in-ovo vaccine prevents MDA interference against NDV is unclear. The MDA is present predominantly in the egg yolk (Leslie and Clem, 1969) and is transported into the embryonic circulation at a low rate as early as embryonic day 7 (Kramer and Cho, 1970). The rate of MDA transfer begins to markedly increase by embryonic days 19 to 21 (Kowalczyk et al., 1985). Thus, we speculated that although the MDA against NDV in the egg yolk was high, the MDA in the embryo body was relatively low at embryonic day 18 and could not efficiently hinder the replication of NDV inoculated via the amniotic route, thus resulting in induction of a protective immune response in commercial chickens.
We then evaluated the duration of clinical protection of the in-ovo vaccine strain TS09-C for commercial chickens. The in-ovo–vaccinated birds were challenged with virulent NDV genotype VII strain HB0901 at 10, 30, 60, and 90 dph. The mean HI titers of birds in-ovo vaccinated with TS09-C virus decreased to 2.8 log2 at 30 dph. The protection rates in the PBS group at 10, 30, 60, and 90 dph were 100, 30, 0, and 0%, respectively, whereas those in the TS09-C group were ≥80% (Figure 2C). Therefore, the in-ovo vaccine strain TS09-C provided better protection against genotype VII NDV challenge in commercial birds, and the duration of clinical protection of this vaccine was at least 3 mo.
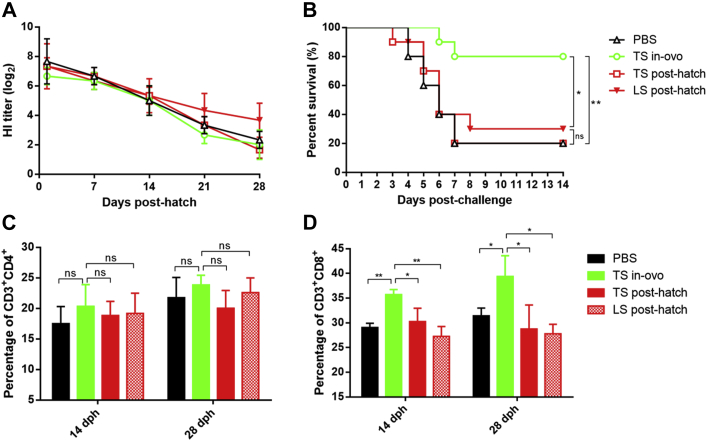
The protective efficacies of the in-ovo and post-hatch vaccination strategies in commercial chickens were compared. As shown in Figure 3A, the TS in-ovo, TS post-hatch, and PBS groups showed similar humoral immune responses, and the HI titers in these groups decreased to ≤2.3 log2 at 28 dph, whereas those in the LS post-hatch group decreased to only 3.7 log2 at 28 dph. The survival rates of challenged birds in the TS in-ovo, TS post-hatch, LS post-hatch, and PBS groups were 80, 20, 30, and 20%, respectively (Figure 3B). These results indicated that the protective efficacy of in-ovo vaccination was markedly higher than that of post-hatch vaccination for commercial chickens.
Figure 3.
Immunogenic comparison of in-ovo and post-hatch vaccination with NDV vaccine strains. Commercial chickens were in-ovo or post-hatch vaccinated with the indicated NDV vaccine strains and challenged with virulent NDV strain HB0901 at 28 dph. (A) The NDV-specific hemagglutinin-inhibition antibody titers of immunized birds before challenge. (B) Survival percentages of birds after challenge with virulent NDV. Statistical significances in survival between different groups were analyzed by Log Rank test. (C, D) Percentages of CD3+ CD8+ and CD3+ CD4+ subsets of the splenic lymphocytes collected from vaccinated birds at the indicated dph. Statistical significance in the percentages of splenic lymphocyte was determined by 2-tailed t-test (ns, P > 0.05; *, P < 0.05; **, P < 0.01).
From 3 animal experiments, we concluded that the TS09-C virus as in-ovo vaccine could provide at least 80% protection for commercial chickens, although the humoral immune response was far lower than the level considered to confer protection (4 log2). The results indicated that other types of immune response might play an important role in the in-ovo vaccine. We then determined the cellular immune response in these 4 groups. The percentages of CD3+ CD4+ splenic lymphocytes were comparable among these 4 groups (Figure 3C). The percentages of CD3+ CD8+ splenic lymphocytes in the PBS, TS post-hatch, and LS post-hatch groups were similar, whereas those in the TS in-ovo group was significantly higher (Figure 3D). Cellular immunity might play a more important role than humoral immunity in the protective efficacy of in-ovo vaccination. To the best of our knowledge, this is the first report on the cellular immunity response to an in-ovo ND vaccine. For the post-hatch ND vaccine rmNA-1, the percentages of both CD4+ and CD8+ splenic lymphocytes in vaccinated birds were significantly higher than those in control birds (Xu et al., 2019). In agreement with our finding, administration of Marek's disease virus strain CVI988 via an in-ovo route has also been found to result in expansion of CD8+ cells (Gimeno et al., 2015). The CD8+ cells mediate the cytotoxic T-lymphocytes response, which is responsible for the clearance of virus during infection.
In summary, the in-ovo NDV vaccine strain TS09-C is safe and immunogenic, and it provides substantial protection in commercial chickens. Our results indicate that the presence of MDA specific for NDV does not significantly interfere with the ability of the in-ovo vaccine to elicit protective cellular immunity against ND.
Acknowledgements
This work was supported by the National Key Research and Development Project of China (2017YFD0501104), the National Natural Science Foundation of China (31873018 and 31670157), the China Agriculture Research System (CARS-41-G13), and the Special Fund for Innovative Technology of Hubei province (2017ABA138, 2019CFA071 and 2019AHB066).
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Alexander D.J., Allan W.H. Newcastle disease virus pathotypes. Avian Pathol. 1974;3:269–278. doi: 10.1080/03079457409353840. [DOI] [PubMed] [Google Scholar]
- Dimitrov K.M., Ramey A.M., Qiu X., Bahl J., Afonso C.L. Temporal, geographic, and host distribution of avian paramyxovirus 1 (Newcastle disease virus) Infect. Genet. Evol. 2016;39:22–34. doi: 10.1016/j.meegid.2016.01.008. [DOI] [PubMed] [Google Scholar]
- Ge J., Wang X., Tian M., Wen Z., Feng Q., Qi X., Gao H., Wang X., Bu Z. Novel in-ovo chimeric recombinant Newcastle disease vaccine protects against both Newcastle disease and infectious bursal disease. Vaccine. 2014;32:1514–1521. doi: 10.1016/j.vaccine.2014.01.020. [DOI] [PubMed] [Google Scholar]
- Gimeno I.M., Faiz N.M., Cortes A.L., Barbosa T., Villalobos T., Pandiri A.R. In ovo vaccination with Turkey Herpesvirus Hastens Maturation of chicken embryo immune responses in specific-pathogen-free chickens. Avian Dis. 2015;59:375–383. doi: 10.1637/11060-031115-Reg.1. [DOI] [PubMed] [Google Scholar]
- Haddad E.E., Martin M., Schaeffer J., Burnes K., Whitfill C. Proceedings of the Western Poultry Conference. 2003. Ovo vaccination against Newcastle disease: field safety evaluation; pp. 34–36. [Google Scholar]
- Kapczynski D.R., Martin A., Haddad E.E., King D.J. Protection from clinical disease against three highly virulent strains of Newcastle disease virus after in ovo application of an antibody-antigen complex vaccine in maternal antibody-positive chickens. Avian Dis. 2012;56:555–560. doi: 10.1637/9980-110311-Reg.1. [DOI] [PubMed] [Google Scholar]
- Kowalczyk K., Daiss J., Halpern J., Roth T.F. Quantitation of maternal-fetal IgG transport in the chicken. Immunology. 1985;54:755–762. [PMC free article] [PubMed] [Google Scholar]
- Kramer T.T., Cho H.C. Transfer of immunoglobulins and antibodies in the hen's egg. Immunology. 1970;19:157–167. [PMC free article] [PubMed] [Google Scholar]
- Leslie G.A., Clem L.W. Phylogen of immunoglobulin structure and function. 3. Immunoglobulins of the chicken. J. Exp. Med. 1969;130:1337–1352. doi: 10.1084/jem.130.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Wang Y., Han Z., Wang Y., Liang S., Jiang L., Hu Y., Kong X., Liu S. Recombinant duck enteritis viruses expressing major structural proteins of the infectious bronchitis virus provide protection against infectious bronchitis in chickens. Antivir. Res. 2016;130:19–26. doi: 10.1016/j.antiviral.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast J., Nanbru C., Decaesstecker M., Lambrecht B., Couvreur B., Meulemans G., van den Berg T. Vaccination of chicken embryos with escape mutants of La Sota Newcastle disease virus induces a protective immune response. Vaccine. 2006;24:1756–1765. doi: 10.1016/j.vaccine.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Negash T., al-Garib S.O., Gruys E. Comparison of in ovo and post-hatch vaccination with particular reference to infectious bursal disease. A. Review. Vet. Q. 2004;26:76–87. doi: 10.1080/01652176.2004.9695170. [DOI] [PubMed] [Google Scholar]
- Niewiesk S. Maternal antibodies: clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front. Immunol. 2014;5:446. doi: 10.3389/fimmu.2014.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles E.D. In ovo applications in poultry: a review. Poult. Sci. 2018;97:2322–2338. doi: 10.3382/ps/pey081. [DOI] [PubMed] [Google Scholar]
- Sokale A.O., Williams C.J., Cummings T.S., Gerard P.D., Bello A., Peebles E.D. Effects of in ovo injection of different doses of coccidiosis vaccine and turn-out times on broiler performance. Poult. Sci. 2018;97:1891–1898. doi: 10.3382/ps/pey028. [DOI] [PubMed] [Google Scholar]
- Wen G., Li L., Yu Q., Wang H., Luo Q., Zhang T., Zhang R., Zhang W., Shao H. Evaluation of a thermostable Newcastle disease virus strain TS09-C as an in-ovo vaccine for chickens. PLoS One. 2017;12:e0172812. doi: 10.1371/journal.pone.0172812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbury H.A., Parsons G., Allan W.H. Comparison of the immunogenicity of Newcastle disease virus strains V4, Hitchner B1 and La Sota in chickens. 2. Tests in chickens with maternal antibody to the virus. Aust. Vet. J. 1984;61:10–13. doi: 10.1111/j.1751-0813.1984.tb07121.x. [DOI] [PubMed] [Google Scholar]
- Williams C.J., Zedek A.S. Comparative field evaluations of in ovo applied technology. Poult. Sci. 2010;89:189–193. doi: 10.3382/ps.2009-00093. [DOI] [PubMed] [Google Scholar]
- Xu X., Xue C., Liu X., Li J., Fei Y., Liu Z., Mu J., Bi Y., Qian J., Yin R., Ding Z. A novel recombinant attenuated Newcastle disease virus expressing H9 subtype hemagglutinin protected chickens from challenge by genotype VII virulent Newcastle disease virus and H9N2 avian influenza virus. Vet. Microbiol. 2019;228:173–180. doi: 10.1016/j.vetmic.2018.11.006. [DOI] [PubMed] [Google Scholar]
- Yosipovich R., Aizenshtein E., Shadmon R., Krispel S., Shuster E., Pitcovski J. Overcoming the susceptibility gap between maternal antibody disappearance and auto-antibody production. Vaccine. 2015;33:472–478. doi: 10.1016/j.vaccine.2014.10.043. [DOI] [PubMed] [Google Scholar]