Abstract
Effects of dietary available phosphorus (aP) and Ca levels and an Escherichia coli 6-phytase supplementation were studied in Lohmann LSL-Lite hens from 25 to 37 wk of age. Eighty-four hens were used in a completely randomized design with 7 treatments. The treatments were a positive control (PC) diet with 0.45% aP, 3.70% Ca, and 0.16% Na from 25 to 28 wk and 0.38% aP, 3.73% Ca, and 0.15% Na from 29 to 37 wk; a negative control (NC) diet, similar to the PC diet, with 0.22% aP, 3.00% Ca, and 0.13% Na from 25 to 28 wk and 0.19% aP, 3.02% Ca, and 0.13% Na from 29 to 37 wk; the NC diets supplemented with phytase at 150 (NC + 150), 300 (NC + 300), 600 (NC + 600), or 1,200 (NC + 1,200) phytase unit (FTU)/kg; and the PC diet supplemented with phytase at 1,200 (PC + 1,200) FTU/kg. Hen performance, eggshell, and bone quality were measured on a 4-wk basis. Bone breaking strength and ash and apparent ileal digestibility (AID) of P and Ca were determined at 37 wk. One- and 2-way ANOVA were conducted, and Tukey's range test was used to compare multiple means where P ≤ 0.05. No differences in hen performance, eggshell quality, bone breaking strength, bone ash, and P digestibility were observed between the PC and the NC treatments. The NC hens had lower cortical (P < 0.001) and trabecular + medullary bone mineral density (P = 0.004) and total bone mineral content (P < 0.001) than the PC hens. The PC + 1,200 increased cortical bone mineral density (P < 0.001). The reductions of aP and Ca in the NC diet were not deficient for performance but had a minor impact on bone mineralization. The NC + 600 and NC + 1,200 increased AID of P (P = 0.024), and all phytase treatments except the NC + 150 increased AID of Ca (P = 0.010) compared with the NC diet.
Key words: phytase, eggshell, bone, P and Ca digestibility, laying hen
Introduction
Phytate (a salt complex of myo-inositol-1,2,3,4,5,6-hexakisdihydrogenphosphate) is a P storage compound in plants (Pallauf and Rimbach, 1997), which accounts for approximately two-thirds of the total P in plant ingredients (Ravindran et al., 1995). Phytate is not well used by chickens as a source of P because of limited endogenous phytase in the digestive tract (Maenz and Classen, 1998) and owing to P and Ca inhibition of phytate degradation at common mineral application rates (Sommerfeld et al., 2018b). Low availability of P in plant-based diets leads to inorganic P supplementation of poultry diets. Owing to P being one of the most expensive nutrients in poultry diets (Naves et al., 2016), increasing inorganic P in the diet, increases feed cost. Exogenous phytase has been used in poultry diets to liberate P and other minerals such as Ca, reduce P pollution in poultry excreta (Selle and Ravindran, 2007, Zyla et al., 2012), and reduce feed cost (Ponnuvel et al., 2014). Commercial phytase products differ in terms of type (3- or 6-phytase), sources (microbial source from which they are derived), characteristics (optimal pH, thermostability, ability to liberate P from the phytate-P complex), and catalytic and biochemical properties (Menezes-Blackburn et al., 2015). The difference in these characteristics and properties can impact the activity of phytase in the digestive tract of birds (Onyango et al., 2005). Research studies on phytase use are well documented in broilers (Hamdi et al., 2015, Sommerfeld et al., 2018b, Gautier et al., 2018, Leyva-Jimenez et al., 2019), but very little work has been carried out on laying hens.
Phytase has the potential to liberate both P and Ca (Selle and Ravindran, 2007), and it is unlikely to result in a response on digestibility of these minerals if dietary requirements for available P (aP) and Ca are being met. Phytase supplementation in the diet containing 0.25% aP and 3.80% Ca did not cause negative effects on egg production and specific gravity (Hughes et al., 2008), ileal digestibility of protein and bone mineralization (Hughes et al., 2009). Similarly, adding phytase to diets having 0.4 or 0.3% aP with 4.0% Ca did not increase egg production, eggshell quality, and bone mineral density (BMD) (Punna and Roland, 1999). Further reductions of both aP and Ca levels lower than the actual requirements in laying hen diets may allow us to observe the effect of phytase and better understand changes in bone mineralization in laying hens. The objective of the present study was to determine the effects of phytase supplementation in diets with substantially reduced aP and Ca on eggshell and bone quality and ileal digestibility of P and Ca in laying hens from 25 to 37 wk of age. We hypothesized that eggshell and bone quality would decrease in hens fed with a reduced aP and Ca levels in the negative control (NC) diet and that phytase supplementation would increase eggshell and bone quality to the level of the positive control (PC) diet.
Materials and methods
All animal care procedures were approved by the Animal Care and Use Committee for Livestock of the University of Alberta and followed principles established by the Canadian Council on Animal Care (Canadian Council on Animal Care, 2009).
Animals and Housing
Eighty-four Lohmann LSL-Lite laying hens aged 20 wk were randomly selected from the Poultry Unit flock of the University of Alberta. Each hen was weighed, wing-banded before placement, and housed in individual cages in a two-tier battery (L x W; 50 × 50 cm; 43 cm high at the front and 33 cm high at the back) located in an environmentally controlled facility. Each replicate cage was equipped with one external feed trough (L x W x D; 50 × 15 × 10 cm), 2 automatic water nipples, and external egg tray receiver (L x W x H; 50 × 12 × 2.5 cm). During the pre-experimental period from 20 to 25 wk of age, all the hens were fed with the same diet. At 25 wk of age, hens were weighed and randomly assigned to 1 of the 7 dietary treatments with 12 replicate cages of 1 hen per treatment in a completely randomized design. Standard management practices were followed as per the primary breeder management guide (Lohmann Tierzucht, 2012). The photoperiod was 16:8D, and the room temperature was maintained at approximately 21°C throughout the experiment. Birds were checked and eggs were collected and recorded twice daily. Feed and water were provided ad libitum throughout the experiment. The experimental period lasted 12 wk from 25 to 37 wk of age.
Experimental Diets
The corn–soy–canola meal–based experimental diets provided approximately 2,800 kcal/kg ME (Table 1). Seven experimental diets were fed as mash in 2 dietary phases: start-lay diets from 25 to 28 wk of age and layer phase I diets from 29 to 37 wk of age. Diet 1 was a PC diet with 0.45% aP, 3.70% Ca, and 0.16% Na from 25 to 28 wk of age and 0.38% aP, 3.73% Ca, and 0.15% Na from 29 to 37 wk of age. The PC diet was formulated to meet or exceed nutrient recommendations (Lohmann Tierzucht, 2012). Diet 2 was a NC, diet similar to the PC diet but reduced to 0.22% aP, 3.00% Ca, and Na 0.13% from 25 to 28 wk of age and to 0.19% aP, 3.02% Ca, and 0.13% Na from 29 to 37 wk of age. The reduction level of aP and Ca in the NC diet in this study were intended to provide aP and Ca levels lower than the respective requirements. Diets 3, 4, 5, and 6 were the NC diets supplemented with phytase at 150 (NC + 150), 300 (NC + 300), 600 (NC + 600), and 1,200 (NC + 1,200) phytase unit (FTU)/kg, respectively. Diet 7 was the PC diet supplemented with phytase at 1,200 FTU/kg (PC + 1,200). Because 300 FTU phytase/kg is the common commercial level used in diets for laying hens, the NC + 600, the NC + 1,200, and the PC + 1,200 were included to investigate high dose effects of phytase. To observe an extraphosphoric effect, the highest dose of 1,200 FTU/kg was also supplemented to the PC diet. The phytase used in this study was a thermotolerant, enhanced Escherichia coli 6-phytase produced in Trichoderma reesei (Quantum Blue, AB Vista; Marlborough, UK). Phytase activity is reported in FTU; one FTU is the amount of enzyme that liberates 1 μmol of inorganic orthophosphate per minute from sodium phytate at 37°C and pH 5.5 (Engelen et al., 1994). A single batch of each of the the PC and NC diets were mixed and subdivided to subsequently mix the respective experimental diets. The phytase product at 30, 60, 120, 240, and 240 g/tonne were added on top of the respective experimental diets. Feed phytase activities were analyzed by Enzyme Services Consultancy (Ystrad Mynach, UK) after feed sample extraction. Quantiplate ELISA Kits specific for Quantum Blue were used for quantification of the enzyme activity (Envirologix method AP181 with some modifications; designated Enzyme Services Consultancy Standard Analytical Method SAM099). The analyzed Ca, total P, and phytase activities are presented in Table 2. Celite (an indigestible marker; Celite Corp., Lompoc, CA) was used at 2% of the experimental diets, added on top of each diet. The Celite-containing diets were fed for 2 wk at the end of the trial from 35 to 37 wk of age. Feed samples were collected at the time of mixing, grounded, and stored at −20°C for further analysis.
Table 1.
The ingredients and nutrient composition of positive control and negative control diets fed to laying hens from 25–37 wk of age.
| 25–28 wk |
29–37 wk |
|||
|---|---|---|---|---|
| PC1 | NC2 | PC1 | NC2 | |
| Ingredients (%) | ||||
| Corn | 58.52 | 61.03 | 59.63 | 62.04 |
| Soybean meal | 18.96 | 18.96 | 16.47 | 16.47 |
| Canola meal | 10.00 | 10.00 | 12.00 | 12.00 |
| Canola oil | 1.44 | 1.44 | 0.96 | 0.96 |
| Calcium carbonate | 8.41 | 7.23 | 8.67 | 7.35 |
| Dicalcium phosphate | 1.76 | 0.50 | 1.37 | 0.33 |
| Salt | 0.32 | 0.24 | 0.29 | 0.24 |
| DL-Methionine | 0.10 | 0.10 | 0.10 | 0.10 |
| Vitamin-mineral premix3 | 0.50 | 0.50 | 0.50 | 0.50 |
| Phytase4 (g/kg) | Variable5 | Variable5 | Variable5 | Variable5 |
| Calculated nutrient composition (%) | ||||
| ME (kcal/kg) | 2,800 | 2,885 | 2,775 | 2,857 |
| Crude protein | 18.00 | 18.20 | 17.60 | 17.80 |
| Calcium | 3.70 | 3.00 | 3.73 | 3.02 |
| Available phosphorus | 0.45 | 0.22 | 0.38 | 0.19 |
| Phytate phosphorus | 0.25 | 0.26 | 0.26 | 0.27 |
| Total phosphorus | 0.70 | 0.48 | 0.64 | 0.46 |
| Sodium | 0.16 | 0.13 | 0.15 | 0.13 |
PC = positive control diet; PC diet was mixed as a single batch and subdivided into the PC and PC + phytase treatments.
NC = negative control diet; NC diet was mixed as a single batch and subdivided into the NC and various NC + phytase treatments.
Vitamin–mineral premix (units per kilogram of feed): vitamin A, 12,500 IU; vitamin D3, 3,125 IU; vitamin E, 40 IU; vitamin K (menadione), 2.5 mg; riboflavin, 7.5 mg; D-pantothenic acid, 12.5 mg; vitamin B12, 0.01875 mg; pyridoxine, 5 mg; thiamine, 2.55 mg; folic acid, 0.625 mg; niacin, 37.5 mg; biotin, 0.15 mg; iodine, 1.65 mg; copper, 15 mg; iron, 80 mg; selenium, 0.3 mg; manganese, 88 mg; zinc, 100 mg.
Quantum Blue phytase (5,000 FTU/g of premix; AB Vista, Marlborough, UK).
Quantum Blue phytase was added on top of the NC diet at 0.03 (150 FTU/kg), 0.06 (300 FTU/kg), 0.12 g/kg (600 FTU/kg) or 0.24 (1,200 FTU/kg) g/kg for the NC phytase-containing diets, or on top of the PC diet at 0.24 g/kg (1,200 FTU/kg) for the PC phytase-containing diet.
Table 2.
| Diet3 |
|||||||
|---|---|---|---|---|---|---|---|
| PC | NC | NC + 150 | NC + 300 | NC + 600 | NC + 1,200 | PC + 1,200 | |
| Calcium (% of the diet, as-fed basis) | |||||||
| 25–28 wk | 4.64 (0.06) | 3.22 (0.10) | 2.74 (0.15) | 2.52 (0.11) | 3.64 (0.07) | 3.16 (0.07) | 4.13 (0.17) |
| 29–34 wk | 4.22 (0.08) | 4.10 (0.04) | 3.90 (0.09) | 4.32 (0.07) | 3.47 (0.03) | 3.30 (0.09) | 4.37 (0.13) |
| 35–37 wk4 | 4.35 (0.12) | 2.38 (0.11) | 3.18 (0.12) | 2.94 (0.13) | 2.98 (0.08) | 2.90 (0.12) | 4.09 (0.11) |
| Total phosphorus (% of the diet, as-fed basis) | |||||||
| 25–28 wk | 0.63 (0.03) | 0.48 (0.01) | 0.46 (0.01) | 0.45 (0.03) | 0.49 (0.01) | 0.47 (0.03) | 0.60 (0.01) |
| 29–34 wk | 0.58 (0.01) | 0.45 (0.03) | 0.46 (0.01) | 0.44 (0.01) | 0.41 (0.03) | 0.47 (0.01) | 0.61 (0.03) |
| 35–37 wk4 | 0.59 (0.04) | 0.31 (0.01) | 0.45 (0.03) | 0.38 (0.01) | 0.41 (0.04) | 0.43 (0.02) | 0.50 (0.03) |
| Phytase activity5 (FTU/kg) | |||||||
| 25–28 wk | <50 | <50 | 267 (13) | 421 (50) | 875 (0) | 2,000 (0) | 1,820 (91) |
| 29–37 wk | <50 | <50 | 254 (33) | 442 (35) | 743 (0) | 1,540 (231) | 1,570 (173) |
Feed provided in mash form.
Means of 2 replicate feed samples, standard deviations are given in parentheses. Phytase activity in the PC and NC diets were lower than the detection level, therefore means and standard deviations were not calculated.
PC, a positive control diet, nutritionally complete diet containing 0.45% aP, 3.70% Ca, and 0.16% Na (25–28 wk) and 0.38% aP, 3.73% Ca, and 0.15% Na (29–37 wk); NC, a negative control diet, similar to the PC diet but having 0.22% aP, 3.00% Ca, and 0.13% Na (25–28 wk) and 0.19% aP, 3.02% Ca, and 0.13% Na (29–37 wk); NC + 300, NC + 600, and NC + 1,200, the NC diet supplemented with 300, 600, or 1,200 FTU Quantum Blue phytase (AB Vista, Marlborough, UK)/kg, respectively; and PC + 1,200, the PC diet supplemented with 1,200 FTU Quantum Blue phytase/kg.
Value for each diet was used to calculate AID of P and Ca for each respective treatment.
Analyzed by Enzyme Services Consultancy (Ystrad Mynach, UK) using Quantiplate ELISA Kits for Quantum Blue (Envirologix method AP181 with some modifications; designated Enzyme Services Consultancy Standard Analytical Method SAM099).
Laying Performance
Individual body weights were measured on a 4-week basis from 25 to 37 wk of age. Feed intake (FI) was recorded during each period to calculate feed conversion ratio (FCR; kg of FI per dozen eggs) on a cage basis. Eggs were collected twice daily at 9:30 am and 3:00 pm. Daily egg production was recorded to calculate total egg number at the end of the trial. Hen-day egg production was calculated for each 4-week period. At 4-week intervals, individual fresh egg weight was recorded.
Eggshell Quality
At 4-week intervals, eggs were collected from each hen for 2 consecutive days; one egg was used for determination of egg specific gravity (SG; g/cm3), the other for determination of eggshell thickness (mm). Eggs were kept at room temperature overnight (18 h) in the same room as the saline solutions before the determination of eggshell quality. Specific gravity (n = 12 per treatment) was measured by flotation using 11 sequential saline solutions ranging from 1.060 to 1.110 in increments of 0.005 (Holder and Bradford, 1979, Wu et al., 2007). The saline solutions were calibrated before each test. For the eggshell thickness measurement (n = 6 per treatment), a 1 cm × 1 cm square was marked and cut at 6 different locations (broad-end, narrow-end, and 4 points around the equator) on each egg. The thickness (without membrane) of each eggshell square was measured using a digital micrometer gauge (Mitutoyo, Japan), and the mean value was calculated for each egg.
At 37 wk of age, an additional egg from each hen was collected to determine eggshell breaking strength (n = 12 per treatment). The breaking force (the force required to break the egg) was measured using an Instron Materials tester (Model 4411, Instron Corp., Canton, MA) with Bluehill 2 software, version 2.29, and a 200 N static load cell as described by Bello and Korver (2019).
Bone Characteristics
Bone Mineralization
At 4-week intervals, 6 hens per treatment were randomly selected at the beginning of the trial for in vivo bone scanning of the shank (tarsometatarsus) at each age. The birds were anaesthetized with Rompun (2 mg/kg BW of a 20 mg/mL solution) with Ketamine (20 mg/kg of BW of 100 mg/mL) to ensure the birds remained motionless during approximately 20 min needed to conduct the scan (Korver et al., 2004). The right shank was scanned at 25% of the length of the shank from the proximal end to determine the total, cortical, and trabecular + medullary BMD and cross-sectional areas by quantitative computed tomography (QCT) using a Stratec Norland XCT (XCT Research SA, Norland Corp., Fort Atkinson, WI) scanner with a 50 kV x-ray tube (Saunders-Blades and Korver, 2015). The threshold used in this study was 400 mg/cm3 to separate the cortical and subcortical bone from the trabecular bone (Korver et al., 2004). The total term was the weighted average of both the cortical and trabecular + medullary bone measures and reflected the density or area of each bone compartment. Cortical BMD was the outer shell of the bone that was determined to have a density of >500 mg/cm3 (Saunders-Blades et al., 2009). Owing to the limitation of the current QCT to distinguish between the trabecular and the medullary bone (Korver et al., 2004), bone in the trabecular space was assumed to include the medullary bone. Bone mineral content (BMC; mg/mm) represents the amount of bone mineral in mg contained in a 1-mm-thick longitudinal section of the bone and was calculated as BMD multiplied by the cross-sectional area (Saunders-Blades et al., 2009).
Bone Breaking Strength
At the end of the experiment (37 wk of age), all 84 hens were euthanized by cervical dislocation and the right femurs were removed. Femurs were cleaned out of soft tissue except for the cartilage caps and kept at −20°C for subsequent determinations of bone breaking strength (BBS) and ash. The frozen right femurs were thawed at 4°C for 24 h. Each femur was marked at the proximal 25%, the midpoint, and distal 25% (25, 50, and 75% from the proximal epiphysis of the length of the femur, respectively) determined using a digital caliper (Model CD-8″C, Mitutoyo Corp., Japan) before BBS and ash determination. Bone breaking strength was measured as described by Riczu et al. (2004) using an Instron Materials Tester (Model 4411, Instron Corp., Canton, MA) with Bluehill 2 software, version 2.29, and a 500 N static load cell.
Bone Ash
After BBS measurement, each femur was cut at 25 and 75% from the proximal epiphysis of the length of the bone using a Dremel tool (Model 200, Racine, WI) to separate the proximal end (25%), mid-diaphysis (50%), and distal end (25%). Each bone segment was oven-dried (Despatch Oven Co., Minneapolis, MN) at 100°C for 48 h and subsequently ashed in a muffle furnace (30400 Thermolyne Furnace, Dubuque, IA) at 500°C for 48 h as described by Bello et al. (2014). The ash was weighed to determine the ash content (in g) and percent ash of each segment. Total dry bone weight and total ash content were calculated by summation of the 3 dry bone weights and ash content weights, respectively.
Phosphorus and Calcium Digestibility Assays
At 37 wk of age, the ileum was removed from 84 birds after euthanasia. Digesta from the distal part of the ileum (defined as the posterior one-third of the section between Meckel's diverticulum and 2 cm anterior to the ileocecal junction) of each bird were collected separately by gentle squeezing and immediately frozen at −20°C until further analysis. Feed and digesta samples were analyzed for acid insoluble ash (AIA; Scott and Boldaji, 1997), P (method 935.13; AOAC, 1990) and Ca (method 964.06; AOAC, 1990) concentrations for determination of apparent ileal digestibility (AID) of P and Ca, respectively, as described by Bello and Korver (2019).
Calculation of Phosphorus and Calcium Digestibility
During the digestibility determination period from 35 to 37 wk of age, the analyzed AIA, total P, and Ca concentrations from the Celite-containing diets and digesta for each respective diet were used to calculate AID of P or Ca on a dry matter basis using the following equation:
where AIAdiet was the initial AIA concentration in the diet; Mineraldiet was the initial dietary concentration of the mineral (i.e. P or Ca); AIAdigesta was the concentration of AIA in digesta; and Mineraldigesta was the respective concentration of the mineral in digesta.
Statistical Analysis
The cage (individual hen) was considered the experimental unit for all measures, with 12 replicate cages per treatment. One-way ANOVA was conducted using the MIXED procedure of SAS (SAS Institute Inc., 2012) for total egg number, eggshell breaking strength, BBS, dry bone weight, bone ash content, percent bone ash, and AID of P and Ca. Two-way ANOVA was used to determine the effect of diet, age, and their interaction using the MIXED procedure of SAS (SAS Institute Inc., 2012) for FI, FCR, egg production, egg weight, SG, eggshell thickness, and BMD, bone cross-sectional area, and BMC of the total, cortical, and trabecular + medullary bone tissues. Two-way ANOVA was also conducted using the MIXED and the HPMIXED procedures of SAS (SAS Institute Inc., 2012) for BW. All data were tested for normality and normality of residuals using UNIVARIATE procedure. Because egg production is percentage data and did not fit a normal distribution, arcsine transformation was used before statistical analysis. Body weight was used as a covariate for determination of bone traits. Means were separated using the LSMEANS statement. Tukey's range test was applied to compare multiple mean comparisons. The linear, quadratic, and cubic effects of phytase supplementation among the NC treatments were analyzed for AID of P and Ca using a polynomial regression to describe the shape of the response to increasing doses of phytase supplementation in the NC diet. The Proc IML procedure of SAS (SAS Institute Inc., 2012) was used to generate the polynomial coefficients for unequal interval of phytase doses. Statistical significance was considered when P ≤ 0.05. Trends were reported where 0.05 < P ≤ 0.10. Values are presented as least squares means (LSM) with the respective standard errors of the mean.
Results
Analyzed Ca, P, and Phytase Activity
The analyzed Ca levels were higher than the formulated levels, but overall, they were consistently higher across the dietary treatments from 25 to 34 wk, except the NC + 150 and NC + 300 from 25 to 28 wk (Table 2). From 35 to 37 wk, the NC diet had lower analyzed Ca than the expected (2.38 vs 3.02%), whereas the PC and PC + 1,200 had higher analyzed Ca (4.35 and 4.09%, respectively) than the planned level. The analyzed total P of the NC and NC supplemented with phytase treatments were in the expected range except low levels in the NC and NC + 300 diets (0.31 and 0.38%, respectively) from 35 to 37 wk. The analyzed total P of the PC from 29 to 34 and from 35 to 37 wk were slightly lower (0.58 and 0.59%, respectively) than the targeted level. The analyzed total P of the PC + 1,200 was lower (0.50%) than that calculated from 35 to 37 wk. Overall, feed phytase activities were higher than the planned levels but were consistent with the assumed stepwise increase of phytase activity among treatments.
Hen Performance
Body weight, FI, FCR, and egg production were not affected by the diet × age interaction or diet main effect (Table 3). Feed intake at 37 wk of age tended to be higher than at 33 wk of age (P = 0.080). Feed conversion ratio increased with hen age (P < 0.001). Hen-day egg production, total egg number, or egg weight were not affected by dietary treatments. Hens at 29 wk of age had lower egg weight (59.3 ± 0.48 g) than at 33 wk (61.5 ± 0.48 g) and 37 wk of age (61.5 ± 0.52 g; P = 0.002).
Table 3.
Main effects of diet and age on performance in hens fed with different dietary aP and Ca levels and phytase supplementation from 25–37 wk of age.
| Body weight1,2 (kg) |
Feed intake1 (g/day per hen) |
Feed conversion ratio1 (kg feed/dozen eggs) |
Hen-day egg production1,3 (%) |
Total egg number1,4 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LSM5 | SEM6 | LSM5 | SEM6 | LSM5 | SEM6 | LSM5 | SEM6 | LSM5 | SEM6 | |
| Diet7 | ||||||||||
| PC | 1.64 | 0.01 | 108 | 1.29 | 1.27 | 0.02 | 98.2 | 0.51 | 82.6 | 0.63 |
| NC | 1.62 | 0.02 | 110 | 1.46 | 1.31 | 0.02 | 97.6 | 0.75 | 81.9 | 0.58 |
| NC + 150 | 1.65 | 0.02 | 111 | 1.13 | 1.29 | 0.01 | 98.9 | 0.31 | 83.3 | 0.39 |
| NC + 300 | 1.65 | 0.02 | 109 | 1.03 | 1.27 | 0.01 | 99.0 | 0.30 | 83.4 | 0.36 |
| NC + 600 | 1.59 | 0.02 | 107 | 1.26 | 1.26 | 0.02 | 98.5 | 0.39 | 82.8 | 0.39 |
| NC + 1,200 | 1.65 | 0.02 | 110 | 1.02 | 1.29 | 0.01 | 97.4 | 0.67 | 82.0 | 0.79 |
| PC + 1,200 | 1.61 | 0.02 | 108 | 1.36 | 1.27 | 0.02 | 98.4 | 0.50 | 82.8 | 0.41 |
| Age | ||||||||||
| 25–29 wk | 1.62 | 0.01 | 109 | 0.92 | 1.23c | 0.01 | 98.1 | 0.39 | - | - |
| 29–33 wk | 1.62 | 0.01 | 108 | 0.59 | 1.27b | 0.01 | 98.4 | 0.33 | - | - |
| 33–37 wk | 1.62 | 0.01 | 111 | 0.87 | 1.34a | 0.01 | 98.4 | 0.29 | - | - |
| 37 wk | 1.66 | 0.02 | - | - | - | - | - | - | - | - |
| Source of variation | Prob > F | |||||||||
| Diet | 0.104 | 0.309 | 0.328 | 0.439 | 0.334 | |||||
| Age | 0.198 | 0.080 | <0.001 | 0.924 | - | |||||
| Diet × age | 0.999 | 0.998 | 0.999 | 0.947 | - | |||||
a-cMeans within column with no common superscript differ significantly (P ≤ 0.05).
Means of 12 replicates of 1 hen for each treatment.
Age means for body weight were measured at the beginning of each specific age range (25, 29, 33, and 37 wk of age).
Arcsine transformation was used before statistical analysis; egg production data are presented as original values in percent.
Total egg produced throughout 84 D of experiment.
LSM = least squares mean.
SEM = standard error of the mean.
PC, a positive control diet, nutritionally complete diet containing 0.45% aP, 3.70% Ca, and 0.16% Na (25–28 wk) and 0.38% aP, 3.73% Ca, and 0.15% Na (29–37 wk); NC, a negative control diet, similar to the PC diet but having 0.22% aP, 3.00% Ca, and 0.13% Na (25–28 wk) and 0.19% aP, 3.02% Ca, and 0.13% Na (29–37 wk); NC + 150, NC + 300, NC + 600, and NC + 1,200, the NC diet supplemented with 150, 300, 600, or 1,200 FTU Quantum Blue phytase (AB Vista, Marlborough, UK)/kg, respectively; and PC + 1,200, the PC diet supplemented with 1,200 FTU Quantum Blue phytase/kg.
Eggshell Quality
There were no diet × age interactions for egg SG or eggshell thickness. The NC + 1,200 had greater egg SG than the NC (1.090 ± 0.001 vs 1.088 ± 0.001 g/cm3; P = 0.004). At 33 wk of age, egg SG was higher (1.090 ± 0.001 g/cm3) than at 29 wk (1.088 ± 0.001 g/cm3) and 37 wk (1.089 ± 0.001 g/cm3; P = 0.001). Eggshell thickness was not affected by diet (0.36 ± 0.002 mm; P = 0.105). However, eggshell thickness at 37 wk of age tended to be higher than at the other ages (P = 0.072). No differences were observed among dietary treatments for eggshell breaking strength at 37 wk of age (5.06 ± 0.20 kg-force; P = 0.402).
Bone Characteristics
No diet × age interactions were observed for in vivo BMD (Table 4), bone cross-sectional area (Table 5), or BMC (Table 6) of the total, cortical, and trabecular + medullary bone tissues. However, the PC hens had greater total BMD than those of the NC, the NC + 300, the NC + 600, and the NC + 1,200 hens (P < 0.001; Table 4). The PC hens also had higher cortical BMD than the NC, the NC + 300, and the NC + 600 hens (P < 0.001). A decrease in trabecular + medullary BMD was observed in the NC, the NC + 150, and the PC + 1,200 relative to the PC treatment (P = 0.004). At 25 wk of age, total BMD was higher than at 33 and 37 wk of age (P < 0.001).
Table 4.
Main effects of diet and age on in vivo shank mineral density in the proximal metaphysis in hens fed with different dietary aP and Ca levels and phytase supplementation from 25–37 wk of age1.
| Bone mineral density (mg/cm3) |
||||||
|---|---|---|---|---|---|---|
| Total2 |
Cortical3 |
Trabecular + medullary4 |
||||
| LSM5 | SEM6 | LSM5 | SEM6 | LSM5 | SEM6 | |
| Diet7 | ||||||
| PC | 513a | 7.34 | 981a | 3.38 | 65.4a | 4.88 |
| NC | 462b,c,d | 8.54 | 966b,c | 3.34 | 49.1b | 1.92 |
| NC + 150 | 480a,b,c | 7.69 | 976a,b | 4.79 | 48.1b | 1.46 |
| NC + 300 | 456c,d | 3.17 | 963b,c | 2.48 | 51.0a,b | 1.64 |
| NC + 600 | 448d | 3.44 | 955c | 3.55 | 54.0a,b | 1.34 |
| NC + 1,200 | 479b | 6.68 | 972a,b,c | 6.99 | 55.3a,b | 2.54 |
| PC + 1,200 | 495a,b | 8.46 | 984a | 3.06 | 48.8b | 1.47 |
| Age | ||||||
| 25 wk | 495a | 4.65 | 975 | 3.10 | 54.8 | 1.76 |
| 29 wk | 477a,b | 5.11 | 974 | 3.06 | 53.4 | 1.93 |
| 33 wk | 469b | 5.40 | 969 | 3.32 | 52.3 | 1.91 |
| 37 wk | 464b | 5.34 | 965 | 3.14 | 51.8 | 1.85 |
| Source of variation | Prob > F | |||||
| Diet | <0.001 | <0.001 | 0.004 | |||
| Age | <0.001 | 0.105 | 0.676 | |||
| Diet × age | 0.999 | 0.887 | 0.986 | |||
| Body weight8 | 0.145 | 0.002 | 0.606 | |||
a-dMeans within column with no common superscript differ significantly (P < 0.05).
Means of 6 replicates of 1 hen for each treatment.
Total term was the weighted average of both the cortical and trabecular + medullary bone measures.
Cortical bone was the outer shell of the bone that was determined to have a density >500 mg/cm3.
Trabecular + medullary bone were assume to present in the trabecular space.
LSM = least squares mean.
SEM = standard error of the mean.
PC, a positive control diet, nutritionally complete diet containing 0.45% aP, 3.70% Ca, and 0.16% Na (25–28 wk) and 0.38% aP, 3.73% Ca, and 0.15% Na (29–37 wk); NC, a negative control diet, similar to the PC diet but having 0.22% aP, 3.00% Ca, and 0.13% Na (25–28 wk) and 0.19% aP, 3.02% Ca, and 0.13% Na (29–37 wk); NC + 150, NC + 300, NC + 600, and NC + 1,200, the NC diet supplemented with 150, 300, 600, or 1,200 FTU QuantumTM Blue phytase (AB Vista, Marlborough, UK)/kg, respectively; and PC + 1,200, the PC diet supplemented with 1,200 FTU QuantumTM Blue phytase/kg.
Body weight was used as a covariate.
Table 5.
Main effects of diet and age on in vivo shank cross-sectional area in the proximal metaphysis in hens fed different dietary aP and Ca levels, and phytase supplementation from 25–37 wk of age1.
| Bone cross-sectional area (mm2) |
||||||
|---|---|---|---|---|---|---|
| Total2 |
Cortical3 |
Trabecular + medullary4 |
||||
| LSM5 | SEM6 | LSM5 | SEM6 | LSM5 | SEM6 | |
| Diet7 | ||||||
| PC | 26.5c | 0.21 | 12.5a | 0.15 | 12.9c | 0.23 |
| NC | 28.1a | 0.41 | 12.1a,b | 0.08 | 14.8a,b | 0.46 |
| NC + 150 | 26.7a,b,c | 0.48 | 12.0a,b,c | 0.12 | 13.6a,b,c | 0.40 |
| NC + 300 | 27.5a,b | 0.24 | 11.7b,c | 0.12 | 14.8a | 0.21 |
| NC + 600 | 27.3a,b,c | 0.26 | 11.5c | 0.14 | 14.7a | 0.17 |
| NC + 1,200 | 26.4b,c | 0.30 | 11.8b,c | 0.13 | 13.6b,c | 0.21 |
| PC + 1,200 | 26.2b,c | 0.44 | 12.1a,b,c | 0.14 | 13.1b,c | 0.38 |
| Age | ||||||
| 25 wk | 26.7 | 0.27 | 12.3a | 0.11 | 13.3b | 0.21 |
| 29 wk | 26.9 | 0.25 | 11.9b | 0.10 | 13.9a,b | 0.22 |
| 33 wk | 27.2 | 0.26 | 11.9b | 0.09 | 14.2a | 0.25 |
| 37 wk | 27.1 | 0.27 | 11.7b | 0.09 | 14.3a | 0.26 |
| Source of variation | Prob > F | |||||
| Diet | 0.003 | <0.001 | <0.001 | |||
| Age | 0.621 | <0.001 | 0.010 | |||
| Diet × age | 0.999 | 0.999 | 0.999 | |||
| Body weight8 | <0.001 | <0.001 | <0.001 | |||
a-cMeans within column with no common superscript differ significantly (P ≤ 0.05).
Means of 6 replicates of 1 hen for each treatment.
Total term was the weighted average of both the cortical and trabecular + medullary bone measures.
Cortical bone was the outer shell of the bone that was determined to have a density >500 mg/cm3.
Trabecular + medullary bone were assumed to be present in the trabecular space.
LSM = least squares mean.
SEM = standard error of the mean.
PC, a positive control diet, nutritionally complete diet containing 0.45% aP, 3.70% Ca, and 0.16% Na (25–28 wk) and 0.38% aP, 3.73% Ca, and 0.15% Na (29–37 wk); NC, a negative control diet, similar to the PC diet but having 0.22% aP, 3.00% Ca, and 0.13% Na (25–28 wk) and 0.19% aP, 3.02% Ca, and 0.13% Na (29–37 wk); NC + 150, NC + 300, NC + 600, and NC + 1,200, the NC diet supplemented with 150, 300, 600, or 1,200 FTU Quantum Blue phytase (AB Vista, Marlborough, UK)/kg, respectively; and PC + 1,200, the PC diet supplemented with 1,200 FTU Quantum Blue phytase/kg.
Body weight was used as a covariate.
Table 6.
Main effects of diet and age on in vivo shank mineral content in the proximal metaphysis in hens fed different dietary aP and Ca levels, and phytase supplementation from 25–37 wk of age1.
| Bone mineral content2 (mg/mm) |
||||||
|---|---|---|---|---|---|---|
| Total3 |
Cortical4 |
Trabecular + medullary5 |
||||
| LSM6 | SEM7 | LSM6 | SEM7 | LSM6 | SEM7 | |
| Diet8 | ||||||
| PC | 13.6a | 0.21 | 12.3a | 0.17 | 0.83a,b | 0.05 |
| NC | 12.9b | 0.09 | 11.7a,b,c | 0.10 | 0.72a,b,c | 0.03 |
| NC + 150 | 12.8b,c | 0.13 | 11.7a,b,c | 0.12 | 0.65b,c | 0.02 |
| NC + 300 | 12.4b,c | 0.14 | 11.3c,d | 0.12 | 0.75a,b | 0.02 |
| NC + 600 | 12.2c | 0.16 | 11.0d | 0.15 | 0.79a | 0.02 |
| NC + 1,200 | 12.7a,b,c | 0.22 | 11.5b,c,d | 0.18 | 0.75a,b,c | 0.04 |
| PC + 1,200 | 13.0a,b | 0.16 | 11.9a,b | 0.14 | 0.64c | 0.03 |
| Age | ||||||
| 25 wk | 13.2a | 0.13 | 12.1a | 0.11 | 0.72 | 0.02 |
| 29 wk | 12.8a,b | 0.12 | 11.7a,b | 0.10 | 0.74 | 0.02 |
| 33 wk | 12.7b | 0.12 | 11.6b | 0.10 | 0.74 | 0.02 |
| 37 wk | 12.5b | 0.12 | 11.3b | 0.11 | 0.73 | 0.02 |
| Source of variation | Prob > F | |||||
| Diet | <0.001 | <0.001 | <0.001 | |||
| Age | 0.002 | <0.001 | 0.978 | |||
| Diet × age | 0.999 | 0.999 | 0.997 | |||
| Body weight9 | <0.001 | <0.001 | 0.007 | |||
a-dMeans within column with no common superscript differ significantly (P ≤ 0.05).
Means of 6 replicates of 1 hen for each treatment.
Bone mineral content was calculated as bone mineral density multiplied by the bone cross-sectional area and is the amount of bone mineral contained in a 1 mm linear section of the scanned region of the bone.
Total term was the weighted average of both the cortical and trabecular + medullary bone measures.
Cortical bone was the outer shell of the bone that was determined to have a density >500 mg/cm3.
Trabecular + medullary bone were assumed to be present in the trabecular space.
LSM = least squares mean.
SEM = standard error of the mean.
PC, a positive control diet, nutritionally complete diet containing 0.45% aP, 3.70% Ca, and 0.16% Na (25–28 wk) and 0.38% aP, 3.73% Ca, and 0.15% Na (29–37 wk); NC, a negative control diet, similar to the PC diet but having 0.22% aP, 3.00% Ca, and 0.13% Na (25–28 wk) and 0.19% aP, 3.02% Ca, and 0.13% Na (29–37 wk); NC + 150, NC + 300, NC + 600, and NC + 1,200, the NC diet supplemented with 150, 300, 600, or 1,200 FTU Quantum Blue phytase (AB Vista, Marlborough, UK)/kg, respectively; and PC + 1,200, the PC diet supplemented with 1,200 FTU Quantum Blue phytase/kg.
Body weight was used as a covariate.
The NC had higher total bone cross-sectional area than the PC, the NC + 1,200, and the PC + 1,200 (P = 0.003; Table 5). Total bone cross-sectional area in the NC + 300 was greater than in the PC. The PC had higher cortical bone cross-sectional area than the NC + 300, the NC + 600, and the NC + 1,200 (P < 0.001). Trabecular + medullary bone cross-sectional area in trabecular space in the NC + 300 and the NC + 600 was greater than the PC, the NC + 1,200, and the PC + 1,200 (P < 0.001). At 25 wk of age, cortical bone cross-sectional area was higher than the other ages (P < 0.001). However, trabecular + medullary bone cross-sectional area at 25 wk of age was lower than at 33 and 37 wk of age (P = 0.010).
The PC had greater total BMC than the other treatments except NC + 1,200 and the PC + 1,200 (P < 0.001; Table 6). Cortical BMC was higher in the PC than the NC + 300, the NC + 600, and the NC + 1,200 treatments (P < 0.001). The NC + 600 had greater trabecular + medullary BMC than the NC + 150 and the PC + 1,200 (P < 0.001). Total and cortical BMC decreased with hen age (P = 0.002 and P < 0.001, respectively). At 37 wk of age, there were no differences among treatments in BBS (20.06 ± 0.47 kg-force; P = 0.446). Dry bone weight, bone ash content and the percent ash of the proximal, mid-bone and distal segments, or total were not affected by dietary treatments at 37 wk of age.
Apparent Ileal Digestibility of Phosphorus and Calcium
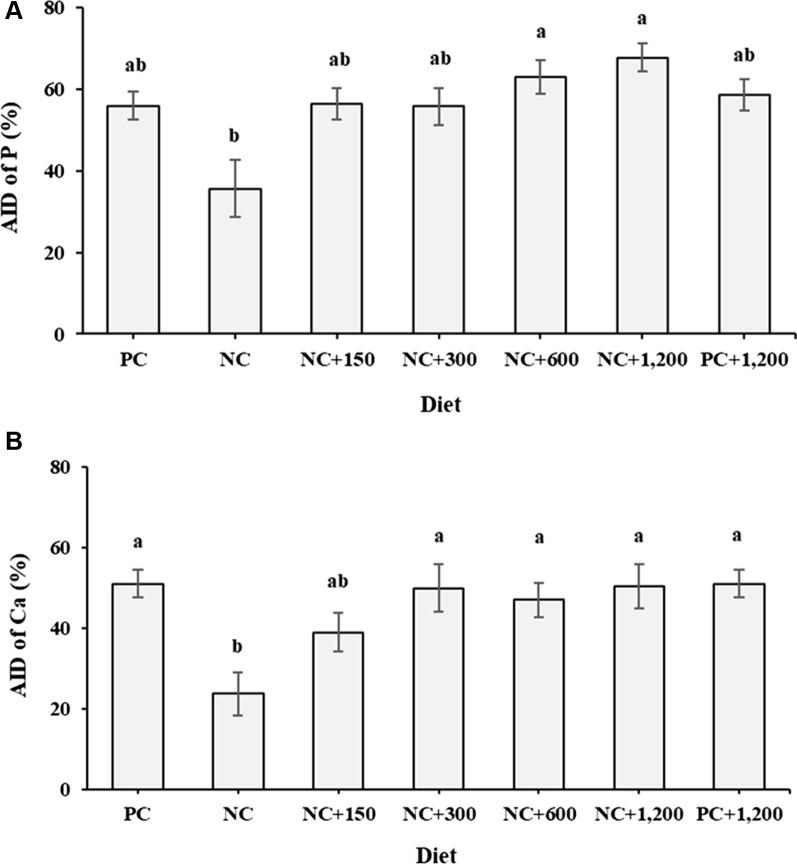
At 37 wk of age, the AID of P in the NC hens was lower than in the NC + 600 and the NC + 1,200 hens (P = 0.024; Figure 1A). Hens fed the NC diet had lower AID of Ca than the other treatments except the NC + 150 (P = 0.010; Figure 1B). There was a quadratic (P < 0.001) response in AID of P (Y = 39.11 + 0.07X – 0.0004X2; R2 = 0.323) with the supplementation of exogenous phytase (150, 300, 600, and 1,200 FTU/kg) to the NC diet. The quadratic (P < 0.008) response was also observed for the AID of Ca (Y = 25.78 + 0.06X – 0.0004X2; R2 = 0.169) with increasing doses of phytase supplementation.
Figure 1.
Effect of dietary aP and Ca and phytase supplementation on (A) apparent ileal digestibility (AID) of P (P = 0.024; n = 84) and (B) AID of Ca (P = 0.010; n = 84) at 37 wk of age. Apparent ileal digestibility of P and Ca was determined from 35 to 37 wk of age. A positive control (PC) diet, nutritionally complete diet containing 0.45% aP, 3.70% Ca, and 0.16% Na (25–28 wk) and 0.38% aP, 3.73% Ca, and 0.15% Na (29–37 wk); NC, a negative control diet, similar to the PC diet but having 0.22% aP, 3.00% Ca, and 0.13% Na (25–28 wk) and 0.19% aP, 3.02% Ca, and 0.13% Na (29–37 wk); NC + 150, NC + 300, NC + 600, and NC + 1,200, the NC diet supplemented with 150, 300, 600, or 1,200 FTU Quantum Blue phytase (AB Vista, Marlborough, UK)/kg, respectively; and PC + 1,200, the PC diet supplemented with 1,200 FTU Quantum Blue phytase/kg. Data are presented as least squares means with the respective standard errors of the mean. a,bMeans within treatment with no common superscript differ significantly (P ≤ 0.05).
Discussion
From 25 to 34 wk of age, the analyzed Ca levels were 5 to 24% higher than the formulated, whereas the analyzed total P levels were slightly lower than the formulated but were in the expected range. High Ca in the diet may have contributed inconsistent results to hen performance. However, the Ca and total P in the PC treatment were still higher than the NC and NC plus phytase treatments, the analyzed values still allow us to draw valid conclusions from our results. During the mineral digestibility period from 35 to 37 wk of age, the analyzed Ca and total P were in the expected range except the NC treatment. Although the analyzed Ca and total P in the NC diet were 21 and 33% lower, respectively, than formulated, this did not interfere with our interpretation. Having low analyzed Ca and total P in the NC diet during the mineral digestibility period allowed us to observe not only negative effects in the NC hens but also the efficacy of phytase.
At 37 wk of age, the BW of the NC hens (1.65 ± 0.04 kg) was within the range between 1.58 and 1.71 kg, and egg production in the NC treatment (98.12 ± 0.93%) was higher relative to the management guide (Lohmann Tierzucht, 2012). The average daily aP intake of the NC hens in each period was approximately halved relative to the PC hens (240 vs. 490 and 210 vs. 410 mg/day per hen from 25 to 28 and 29 to 37 wk of age, respectively). Although aP intake of the NC hens was lower than the NRC recommendation of 250 mg/day per hen (NRC, 1994), hens were still performing well and no mortality was observed, indicating that aP and Ca levels in the NC diet apparently met the actual requirements for maintenance of performance. Hens were able to maintain BW throughout the 12-week experiment without any symptoms of P or Ca deficiency, whereas bone demineralization in NC hens suggests the initial stages of a mineral shortage. This could be because the NC diet contained slightly low Ca during the last 2 wk of the experiment. Egg production remained relatively constant from 25 to 37 wk of age but daily FI and FCR increased with hen age. This could be because hens gradually increased egg size from 29 to 37 wk of age.
Actively laying hens require Ca to form amorphous calcium carbonate (Rodriguez-Navarro et al., 2015) and calcium phosphate during eggshell calcification (Murakami et al., 2007). During mid-production, reducing dietary Ca to 3.60% (Bello and Korver, 2019), 3.35% (Kaur et al., 2013), or 3.25% (Rama Rao et al., 2003) did not decrease eggshell quality in White Leghorn layers. Dietary Ca at 3.22, 4.10, and 2.38% at each period was sufficient to maintain the eggshell quality of hens throughout this study. Others had reported decreased eggshell thickness and breaking strength when fed 3% Ca in older Lohmann LSL-Lite layers aged 74 to 81 wk (Akbari Moghaddam Kakhki et al., 2019), possibly due to Ca absorption being inefficient in aged laying hens (Bronner, 1987, Pelicia et al., 2009). Although 1,200 FTU phytase/kg in the NC diet increased SG, it did not increase the eggshell thickness or breaking strength relative to the NC diet. The lack of a consistent effect of phytase on eggshell quality might be because of the lack of adverse effects of the NC diet. To observe a response to phytase, it is necessary for Ca or P be subsequently released from phytate to contribute to meet the requirement of the hen. If the dietary levels of Ca and aP are already adequate, there can be no response to the liberated minerals because the hen has no further need for them. We would be able to see the effect of phytase to restore eggshell quality if the levels of aP and Ca in the NC diet were reduced lower than the actual requirements over a longer period without any further bone demineralization when adverse effects in the NC hens are found. Typically, eggshell quality decreases as hens age (Al-Batshan et al., 1994, Machal and Simeonovova, 2002), but this was not observed in the present study. This may be because the increase in medullary bone in bone trabecular space (trabecular + medullary) from 25 to 37 wk of age was sufficient to support eggshell quality. In addition, hens in this flock were young and the experiment lasted only 12 wk.
Although the NC diet provided sufficient Ca for performance and eggshell quality, it did not appear to be sufficient to maintain bone mineralization as shown in the tarsometatarsus QCT results. From 35 to 37 wk of age, 2.38% Ca in the NC diet may have contributed to the negative effect on bone quality. However, femur BBS and ash content results were not consistent with the tarsometatarsus QCT data. First, the tarsometatarsus has proportionally lower structural and medullary bone volumes and ash content than the femur in laying hens (Taylor and Moore, 1954). Therefore the tarsometatarsus may be more sensitive to changes than the femur. Second, BBS and ash include the total (whole) bone whereas QCT was used to measure a single point at 25% of the length of the tarsometatarsus from the proximal end. In addition, the NC hens had begun to mobilize bone mineral but not to the point where it decreased BBS or overall ash content. Reducing aP from 0.25 to 0.12% with adequate Ca at 3.50% did not decrease BBS and ash content in Hy-Line W36 hens (Martinez Rojas et al., 2018). Providing sufficient level of Ca (3.51 g Ca/day per hen or 32.5 g Ca/kg diet) did not decrease serum Ca and BBS and ash in laying hens, whereas serum alkaline phosphatase and bone resorption were increased (Keshavarz and Nakajima, 1993, Leeson et al., 1993, Rama Rao et al., 2003), indicating that bone depletion occurred even though hens were able to maintain BBS. Total BMC and cortical, trabecular + medullary, and total BMD were lower in the NC hens than in the PC hens, indicating that the NC hens started mobilizing bone mineral to support eggshell formation, maintain egg production and performance. Laying hens are able to physiologically adapt to P- and Ca-reduced diets and maintain performance, depending on the degree of nutrient deficiency (Boling et al., 2000, Nie et al., 2013, Geraldo et al., 2014) and varying with strains (Hughes et al., 2009). Phytase supplementation to the NC diets did not fully restore total BMD and BMC to the level of the PC treatment. Total Ca levels in layer diets are beyond those of broilers in which negative impact with increased Ca level on phytate degradation were shown (Sommerfeld et al., 2018b). In addition, when P and Ca move to the higher pH environment of the lower gastrointestinal tract, P and Ca can form insoluble salts of calcium phosphate (Nelson and Kirby, 1987, Tamim et al., 2004, Hamdi et al., 2015, Sommerfeld et al., 2018b) and subsequently impair P and Ca absorption. High dietary Ca caused high pH in the gastrointestinal tract (Nelson and Kirby, 1987) and therefore decreased phytase efficiency (Van der Klis et al., 1997, Sommerfeld et al., 2018b). These may explain why phytase supplementation in the NC diets did not consistently restore total BMD and BMC to the level of the PC treatment. Bone quality in hens fed high dietary Ca (4.2%) was not affected when supplemented with phytase at 300, 600, or 1,200 FTU phytase/kg (Fernandez et al., 2019). The results for each measure of the NC + 600, NC + 1,200, and PC + 1,200 treatments did not differ from the commercial recommendation level at 300 FTU phytase/kg in layer diets, possibly because both the NC and the PC diets contained sufficient or even excess P and Ca relative to the actual needs of hens over this short supplementation period. It is also assumed that individual response on the selected bone measurement varies, and more replicates and a longer feeding period might be needed to determine the phytase effect on bones in older birds like laying hens.
As expected, structural bone decreased as the hens aged. The bone cross-sectional area in the trabecular space (trabecular + medullary bone) increased from 25 to 37 wk of age, reflecting an age-related increase in the accumulation of medullary bone. In actively laying hens, cortical and trabecular bone tissues can be mobilized but not formed, whereas medullary bone can be deposited and mobilized (Fleming et al., 1998a, Whitehead, 2004, Fleming, 2008, Kerschnitzki et al., 2014). In a longer lasting trial, structural bone volume in the proximal tarsometatarsus of layers decreased from 15 to 25 wk, remained constant from 25 to 50 wk, and then decreased at 70 wk of age in hens fed with Ca- and P-adequate diet (Fleming et al., 1998b). Whereas hens maintained structural bone from 30 to 70 wk of age in moderate reductions in dietary Ca and aP (Bello and Korver, 2019). However, structural bone decreased from 25 to 33 wk of age in Ca- and aP-reduced diets in our study, suggesting that greater reductions of Ca and aP in diet impaired bone quality in the short term. Monitoring bone changes using QCT allowed us to keep the hens alive and follow up individual hens throughout the 12-week experiment period, which are some advantages of the QCT technique (Korver, 2004, Korver et al., 2004). Because of limited bird numbers, we measured BBS and ash at the end of trial only, thus limiting the opportunity to determine age effects on bone changes using traditional methods. Each segment or total of bone ash provided the same results, suggesting that femur ash determination for one segment would be sufficient for the determination of bone ash in the short-term in laying hens.
The bone architecture and structure and the metabolic behaviors in physiological processes are associated with different bone regions because the trabecular bone is concentrated at the proximal (Bello and Korver, 2019) and distal ends (Sullivan et al., 2017), whereas the cortical and medullary bone are concentrated in the mid-diaphysis (Kerschnitzki et al., 2014, Bello and Korver, 2019). Although different bone regions have different bone architecture and structure, for bone development of broilers and ducks, the epiphyseal bone mineralization was greater than at the diaphyseal region because the epiphyseal ends are responsible for linear growth and chondrocyte replication in the growth plate (Applegate and Lilburn, 2002, Van Wyhe et al., 2012). In the mouse, mRNA expression of alkaline phosphatase and osteocalcin, 2 key bone formation markers, was greater in the metaphyseal than the diaphyseal regions (Li et al., 2017). These authors also reported that the bone remodeling process in the tibia metaphysis was higher than in the diaphysis, indicating that different locations of the long bones have different properties and metabolic activity. In White egg-laying hens (30–70 wk), the pattern of bone mineralization at the proximal metaphysis was as same as at the mid-diaphysis (Bello and Korver, 2019). This is the main reason why bone QCT measurement in the present study was conducted at only 25% of the length of the shank from the proximal end.
Laying hens are able to increase dietary P absorption via the upregulation of the Na–P IIb transporter in the duodenum when fed with a P-deficient diet (Nie et al., 2013). The AID of P in the NC hens did not differ from the PC hens, implying that the 0.31% analyzed total P in the NC diet apparently met the actual requirement of P in laying hens at this age. Phytase supplementation to the NC diet at 600 and 1,200 FTU/kg increased P digestibility relative to the NC diet. It is possible that high levels of dietary phytase increased inositol hexaphosphate degradation to inositol heptaphosphate and lower esters (Sommerfeld et al., 2018a), thereby more P was available to absorb in the small intestine (Angel et al., 2002) compared with the diet without or low level of phytase. Reducing dietary aP increased the pH at the end of the small intestine phase in an in vitro broiler digestion assay (Farhadi et al., 2019). Increased small intestine pH accelerated precipitation of mineral-phytate complexes (Tamim et al., 2004, Walk et al., 2012), and high dosages of exogenous phytase alleviated such adverse effects and increased P solubility in the small intestine phase in the in vitro digestion assay (Farhadi et al., 2019). We assumed that P digestibility in laying hens would show a similar response to high doses of phytase as in broilers as demonstrated by others when supplementation of 5,000 FTU phytase/kg in a P-reduced laying hen diet decreased phytate P content in digesta and increased P digestibility relative to 500 FTU phytase/kg (Gao et al., 2013).
Laying hens responded to a Ca-deficient diet by increasing the intestinal expression of mRNA for the Ca transporter CaBP-D28K (Ieda et al., 1999) to increase Ca absorption from the diet (Pelicia et al., 2009). However, because both the dietary Ca level and the AID of Ca were lower in the NC group than in the PC group, less Ca would have been absorbed from the diet. It seemed that NC hens did not upregulate dietary Ca absorption but instead mobilized bone Ca. The analyzed Ca and total P in the NC diet during the digestibility determination period were 2.38 and 0.31%, respectively, and maintained at a Ca:total P ratio of around 8:1, which was wider than the recommended Ca:total P ratio of 6:1 (Lohmann Tierzucht, 2012). Low Ca and total P and the widening of the Ca:total P in the diet elevated Ca excretion in the kidney (Rao and Roland, 1990). The widening of the Ca:total P ratio in the NC diet in the present study may have increased the formation of insoluble Ca phosphate and decreased the solubility of Ca in the digestive tract. Subsequently, Ca cannot be absorbed in the small intestine and then be excreted (Rao et al., 1990, Keshavarz and Nakajima, 1993), which may explain the decrease in AID of Ca in the NC hens. Because of this, the NC hens then started to mobilize bone to support eggshell formation and production. Phytase supplementation to the NC diet at 300, 600, and 1,200 FTU/kg increased AID of Ca. These phytase levels were able to liberate Ca from Ca-phytate complexes (Selle et al., 2009, Humer et al., 2015). However, phytase supplementation at 150 FTU/kg did not increase AID of Ca. It is assumed that 150 FTU/kg was too low a supplementation rate to reduce inositol hexaphosphate to an extent that could increase Ca digestibility.
Overall, the reduction of aP and Ca in the NC diet did not cause any adverse effects on performance, production, and eggshell quality in laying hens over the 12 wk of the study. The current recommendations for Ca and aP provided by the primary breeders are likely substantially higher than actually required by hens. From a bone biology standpoint, the NC hens started mobilizing bone at the end of the trial, resulting in decreased BMD and BMC; however, this did not appear to cause osteoporosis or other bone problems. The increase of P and Ca digestibility by phytase supplementation resulted in some but inconsistent increases in bone measures. The implications of longer term feeding of the NC diet to determine the efficacy of phytase in laying hens should be studied further. Short-term phytase studies should be designed with further reductions in Ca and aP to adequately asses the efficacy of phytases, while minimizing the risk of severe bone issues.
Acknowledgments
The authors would like to acknowledge the technical assistance of Kerry Nadeau, the help of the research team in Doug Korver's lab and the staff of the Poultry Research Centre, University of Alberta, Edmonton, AB, Canada. The authors also acknowledge AB Vista, Marlborough, UK for funding this study.
Conflict of Interest Statement: No conflicts of interest to report.
References
- Akbari Moghaddam Kakhki R., Heuthorst T., Mills A., Neijat M., Kiarie E. Interactive effects of calcium and top-dressed 25-hydroxy vitamin D3 on egg production, egg shell quality, and bones attributes in aged Lohmann LSL-lite layers. Poult. Sci. 2019;98:1254–1262. doi: 10.3382/ps/pey446. [DOI] [PubMed] [Google Scholar]
- Al-Batshan H.A., Scheideler S.E., Black B.L., Garlich J.D., Anderson K.E. Duodenal calcium uptake, femur ash, and eggshell quality decline with age and increase following molt. Poult. Sci. 1994;73:1590–1596. doi: 10.3382/ps.0731590. [DOI] [PubMed] [Google Scholar]
- Angel R., Tamim N.M., Applegate T.J., Dhandu A.S., Ellestad L.E. Phytic acid chemistry: influence on phytin-phosphorus availability and phytase efficacy. J. Appl. Poult. Res. 2002;11:471–480. [Google Scholar]
- AOAC . 15th ed. Association of Official Analytical Chemists; Washington, DC: 1990. Official Methods of Analysis. [Google Scholar]
- Applegate T., Lilburn M. Growth of the femur and tibia of a commercial broiler line. Poult. Sci. 2002;81:1289–1294. doi: 10.1093/ps/81.9.1289. [DOI] [PubMed] [Google Scholar]
- Bello A., Bricka R.M., Gerard P.D., Peebles E.D. Effects of commercial in ovo injection of 25-hydroxycholecalciferol on broiler bone development and mineralization on days 0 and 21 posthatch. Poult. Sci. 2014;93:1053–1058. doi: 10.3382/ps.2013-03608. [DOI] [PubMed] [Google Scholar]
- Bello A., Korver D.R. Long-term effects of Buttiauxella sp. phytase on performance, eggshell quality, apparent ileal Ca and P digestibility, and bone properties of white egg layers. Poult. Sci. 2019;98:4848–4859. doi: 10.3382/ps/pez220. [DOI] [PubMed] [Google Scholar]
- Boling S.D., Douglas M.W., Shirley R.B., Parsons C.M., Koelkebeck K.W. The effects of various dietary levels of phytase and available phosphorus on performance of laying hens. Poult. Sci. 2000;79:535–538. doi: 10.1093/ps/79.4.535. [DOI] [PubMed] [Google Scholar]
- Bronner F. Intestinal calcium absorption: mechanisms and applications. J. Nutr. 1987;117:1347–1352. doi: 10.1093/jn/117.8.1347. [DOI] [PubMed] [Google Scholar]
- Canadian Council on Animal Care . Canadian Council on Animal Care; Ottawa, ON: 2009. CCAC Guidelines on: The Care and Use of Farm Animals in Research, Teaching and Testing. [Google Scholar]
- Engelen A.J., van der Heeft F.C., Randsdorp P.H., Smit E.L. Simple and rapid determination of phytase activity. J. AOAC Int. 1994;77:760–764. [PubMed] [Google Scholar]
- Farhadi D., Karimi A., Sadeghi A.A., Rostamzadeh J., Bedford M.R. Effect of a high dose of exogenous phytase and supplementary myo-inositol on mineral solubility of broiler digesta and diets subjected to in vitro digestion assay. Poult. Sci. 2019;98:3870–3883. doi: 10.3382/ps/pez104. [DOI] [PubMed] [Google Scholar]
- Fernandez S.R., Charraga S., Avila-Gonzalez E. Evaluation of a new generation phytase on phytate phosphorus release for egg production and tibia strength in hens fed a corn-soybean meal diet. Poult. Sci. 2019;98:2087–2093. doi: 10.3382/ps/pey558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming R.H., McCormack H.A., McTeir L., Whitehead C.C. Medullary bone and humeral breaking strength in laying hens. Res. Vet. Sci. 1998;64:63–67. doi: 10.1016/s0034-5288(98)90117-5. [DOI] [PubMed] [Google Scholar]
- Fleming R.H., McCormack H.A., Whitehead C.C. Bone structure and strength at different ages in laying hens and effects of dietary particulate limestone, vitamin K and ascorbic acid. Br. Poult. Sci. 1998;39:434–440. doi: 10.1080/00071669889024. [DOI] [PubMed] [Google Scholar]
- Fleming R.H. Nutritional factors affecting poultry bone health. Proc. Nutr. Soc. 2008;67:177–183. doi: 10.1017/S0029665108007015. [DOI] [PubMed] [Google Scholar]
- Gao C.Q., Ji C., Zhao L.H., Zhang J.Y., Ma Q.G. Phytase transgenic corn in nutrition of laying hens: residual phytase activity and phytate phosphorus content in the gastrointestinal tract. Poult. Sci. 2013;92:2923–2929. doi: 10.3382/ps.2013-03226. [DOI] [PubMed] [Google Scholar]
- Gautier A.E., Walk C.L., Dilger R.N. Effects of a high level of phytase on broiler performance, bone ash, phosphorus utilization, and phytate dephosphorylation to inositol. Poult. Sci. 2018;97:211–218. doi: 10.3382/ps/pex291. [DOI] [PubMed] [Google Scholar]
- Geraldo A., Aurora Gomes K.R., Fassani E.J., Bertechini A.G., Simao S.D., Nogueira F.S. Carbohydrase and phytase supplementation in diets for semi-heavy laying hens. Acta Sci. Anim. Sci. 2014;36:285–290. [Google Scholar]
- Hamdi M., Lopez-Verge S., Manzanilla E.G., Barroeta A.C., Perez J.F. Effect of different levels of calcium and phosphorus and their interaction on the performance of young broilers. Poult. Sci. 2015;94:2144–2151. doi: 10.3382/ps/pev177. [DOI] [PubMed] [Google Scholar]
- Holder D.P., Bradford M.V. Relationship of specific gravity of chicken eggs to number of cracked eggs observed and percent shell. Poult. Sci. 1979;58:250–251. [Google Scholar]
- Hughes A.L., Dahiya J.P., Wyatt C.L., Classen H.L. The efficacy of Quantum phytase in a forty-week production trial using White Leghorn laying hens fed corn-soybean meal-based diets. Poult. Sci. 2008;87:1156–1161. doi: 10.3382/ps.2007-00505. [DOI] [PubMed] [Google Scholar]
- Hughes A.L., Dahiya J.P., Wyatt C.L., Classen H.L. Effect of Quantum phytase on nutrient digestibility and bone ash in White Leghorn laying hens fed corn-soybean meal-based diets. Poult. Sci. 2009;88:1191–1198. doi: 10.3382/ps.2008-00233. [DOI] [PubMed] [Google Scholar]
- Humer E., Schwarz C., Schedle K. Phytate in pig and poultry nutrition. J. Anim. Physiol. Anim. Nutr. 2015;99:605–625. doi: 10.1111/jpn.12258. [DOI] [PubMed] [Google Scholar]
- Ieda T., Saito N., Shimada K. Effect of low calcium diet on messenger ribonucleic acid levels of calbindin-D28K of intestine and shell gland in laying hens in relation to egg shell quality. Jpn. Poult. Sci. 1999;36:295–303. [Google Scholar]
- Kaur R., Rathgeber B.M., Thompson K.L., MacIsaac J. Uterine fluid proteins and egg quality characteristics for 2 commercial and 2 heritage laying hen lines in response to manipulation of dietary calcium and vitamin D3. Poult. Sci. 2013;92:2419–2432. doi: 10.3382/ps.2012-02983. [DOI] [PubMed] [Google Scholar]
- Kerschnitzki M., Zander T., Zaslansky P., Fratzl P., Shahar R., Wagermaier W. Rapid alterations of avian medullary bone material during the daily egg-laying cycle. Bone. 2014;69:109–117. doi: 10.1016/j.bone.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Keshavarz K., Nakajima S. Re-evaluation of calcium and phosphorus requirements of laying hens for optimum performance and eggshell quality. Poult. Sci. 1993;72:144–153. [Google Scholar]
- Korver D.R. Modern poultry production and avian bone biology. Proc. Aust. Poult. Sci. Sym. 2004;16:108–111. [Google Scholar]
- Korver D.R., Saunders-Blades J.L., Nadeau K.L. Assessing bone mineral density in vivo: quantitative computed tomography. Poult. Sci. 2004;83:222–229. doi: 10.1093/ps/83.2.222. [DOI] [PubMed] [Google Scholar]
- Leeson S., Summers J.D., Caston L. Response of brown-egg strain layers to dietary calcium or phosphorus. Poult. Sci. 1993;72:1510–1514. [Google Scholar]
- Leyva-Jimenez H., Alsadwi A.M., Gardner K., Voltura E., Bailey C.A. Evaluation of high dietary phytase supplementation on performance, bone mineralization, and apparent ileal digestible energy of growing broilers. Poult. Sci. 2019;98:811–819. doi: 10.3382/ps/pey389. [DOI] [PubMed] [Google Scholar]
- Li J., Bao Q., Chen S., Liu H., Feng J., Qin H., Li A., Liu D., Shen Y., Zhao Y., Zong Z. Different bone remodeling levels of trabecular and cortical bone in response to changes in Wnt/beta-catenin signaling in mice. J. Orthop. Res. 2017;35:812–819. doi: 10.1002/jor.23339. [DOI] [PubMed] [Google Scholar]
- Lohmann Tierzucht . Lohmann Tierzucht GmbH; Cuxhaven, Germany: 2012. Management Guide Layers, Lohmann LSL-Lite North American Edition. [Google Scholar]
- Machal L., Simeonovova J. The relationship of shortening and strength of eggshell to some egg quality indicators and egg production in hens of different initial laying lines. Arch. Tierz.-Arch. Anim. Breed. 2002;45:287–296. [Google Scholar]
- Maenz D.D., Classen H.L. Phytase activity in the small intestinal brush border membrane of the chicken. Poult. Sci. 1998;77:557–563. doi: 10.1093/ps/77.4.557. [DOI] [PubMed] [Google Scholar]
- Martinez Rojas I.Y., Ávila González E., Arce Menocal J., Dos Santos T.T., Rubio Arguello J., López Coello C. Assessment of a phytase included with lactic acid on productive parameters and on deposition of phosphorus, calcium, and zinc in laying hens fed with sorghum-soybean-meal-based diets. J. Appl. Anim. Res. 2018;46:314–321. [Google Scholar]
- Menezes-Blackburn D., Gabler S., Greiner R. Performance of seven commercial phytases in an in vitro simulation of poultry digestive tract. J. Agric. Food Chem. 2015;63:6142–6149. doi: 10.1021/acs.jafc.5b01996. [DOI] [PubMed] [Google Scholar]
- Murakami F.S., Rodrigues P.O., de Campos C.M.T., Silva M.A.S. Physicochemical study of CaCO3 from egg shells. Ciencia Tecnol. Aliment. 2007;27:658–662. [Google Scholar]
- Naves L.D., Rodrigues P.B., Meneghetti C., Bernardino V.M.P., Oliveira D.H., Saldanha M.M., Teixeira L.D., Santos L.M. Efficiency of microbial phytases in diets formulated with different calcium: phosphorus ratios supplied to broilers from 35 to 42 days of age. J. Appl. Anim. Res. 2016;44:446–453. [Google Scholar]
- Nelson T.S., Kirby L.K. The calcium-binding properties of natural phytate in chick diets. Nutr. Rep. Int. 1987;35:949–956. [Google Scholar]
- Nie W., Yang Y., Yuan J., Wang Z., Guo Y. Effect of dietary nonphytate phosphorus on laying performance and small intestinal epithelial phosphate transporter expression in Dwarf pink-shell laying hens. J. Anim. Sci. Biotechnol. 2013;4:34–40. doi: 10.1186/2049-1891-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC . 9th rev. ed. National Academy Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Onyango E.M., Bedford M.R., Adeola O. Phytase activity along the digestive tract of the broiler chick: a comparative study of an Escherichia coli-derived and Peniophora lycii phytase. Can. J. Anim. Sci. 2005;85:61–68. [Google Scholar]
- Pallauf J., Rimbach G. Nutritional significance of phytic acid and phytase. Arch. Anim. Nutr. 1997;50:301–319. doi: 10.1080/17450399709386141. [DOI] [PubMed] [Google Scholar]
- Pelicia K., Garcia E.A., Faitarone A.B.G., Silva A.P., Berto D.A., Molino A.B., Vercese F. Calcium and available phosphorus levels for laying hens in second production cycle. Braz. J. Poult. Sci. 2009;11:39–49. [Google Scholar]
- Ponnuvel P., Narayanankutty K., Jalaludeen A., Anitha P. Economics of phytase enzyme supplementation in low energy-protein layer chicken diet. Int. J. Livest. Prod. 2014;5:113–116. [Google Scholar]
- Punna S., Roland D.A., Sr. Influence of supplemental microbial phytase on first cycle laying hens fed phosphorus-deficient diets from day one of age. Poult. Sci. 1999;78:1407–1411. doi: 10.1093/ps/78.10.1407. [DOI] [PubMed] [Google Scholar]
- Rama Rao S.V., Panda A.K., Raju M.V.L.N., Shyam Sunder G., Praharaj N.K. Requirement of calcium for commercial broilers and White Leghorn layers at low dietary phosphorus levels. Anim. Feed Sci. Technol. 2003;106:199–208. [Google Scholar]
- Rao K.S., Roland D.A., SR Influence of dietary calcium and phosphorus on urinary calcium in commercial Leghorn hens. Poult. Sci. 1990;69:1991–1997. doi: 10.3382/ps.0691991. [DOI] [PubMed] [Google Scholar]
- Ravindran V., Bryden W.L., Kornegay E.T. Phytates: occurrence, bioavailability and implications in poultry nutrition. Poult. Avian Biol. Rev. 1995;6:125–143. [Google Scholar]
- Riczu C.M., Saunders-Blades J.L., Yngvesson A.K., Robinson F.E., Korver D.R. End-of-cycle bone quality in white- and brown-egg laying hens. Poult. Sci. 2004;83:375–383. doi: 10.1093/ps/83.3.375. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Navarro C., Kudlacz K., Cizer O., Ruiz-Agudo E. Formation of amorphous calcium carbonate and its transformation into mesostructured calcite. Cryst. Eng. Comm. 2015;17:58–72. [Google Scholar]
- SAS Institute Inc . SAS Institute Inc.; Cary, NC: 2012. SAS 9.4 Workstation for Windows. [Google Scholar]
- Saunders-Blades J.L., Korver D.R. Effect of hen age and maternal vitamin D source on performance, hatchability, bone mineral density, and progeny in vitro early innate immune function. Poult. Sci. 2015;94:1233–1246. doi: 10.3382/ps/pev002. [DOI] [PubMed] [Google Scholar]
- Saunders-Blades J.L., MacIsaac J.L., Korver D.R., Anderson D.M. The effect of calcium source and particle size on the production performance and bone quality of laying hens. Poult. Sci. 2009;88:338–353. doi: 10.3382/ps.2008-00278. [DOI] [PubMed] [Google Scholar]
- Scott T.A., Boldaji F. Comparison of inert markers chromic oxide or insoluble ash (CeliteTM) for determining apparent metabolizable energy of wheat- or barley-based broiler diets with or without enzymes. Poult. Sci. 1997;76:594–598. doi: 10.1093/ps/76.4.594. [DOI] [PubMed] [Google Scholar]
- Selle P.H., Cowieson A.J., Ravindran V. Consequences of calcium interactions with phytate and phytase for poultry and pigs. Livest. Sci. 2009;124:126–141. [Google Scholar]
- Selle P.H., Ravindran V. Microbial phytase in poultry nutrition. Anim. Feed Sci. Technol. 2007;135:1–41. [Google Scholar]
- Sommerfeld V., Kunzel S., Schollenberger M., Kühn I., Rodehutscord M. Influence of phytase or myo-inositol supplements on performance and phytate degradation products in the crop, ileum, and blood of broiler chickens. Poult. Sci. 2018;97:920–929. doi: 10.3382/ps/pex390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerfeld V., Schollenberger M., Kühn I., Rodehutscord M. Interactive effects of phosphorus, calcium, and phytase supplements on products of phytate degradation in the digestive tract of broiler chickens. Poult. Sci. 2018;97:1177–1188. doi: 10.3382/ps/pex404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan T.N., Wang B., Espinosa H.D., Meyers M.A. Extreme lightweight structures: avian feathers and bones. Mater. Today. 2017;20:377–391. [Google Scholar]
- Tamim N.M., Angel R., Christman M. Influence of dietary calcium and phytase on phytate phosphorus hydrolysis in broiler chickens. Poult. Sci. 2004;83:1358–1367. doi: 10.1093/ps/83.8.1358. [DOI] [PubMed] [Google Scholar]
- Taylor T.G., Moore J.H. Skeletal depletion in hens laying on a low-calcium diet. Br. J. Nutr. 1954;8:112–124. doi: 10.1079/bjn19540020. [DOI] [PubMed] [Google Scholar]
- Van der Klis J., Versteegh H., Simons P., Kies A. The efficacy of phytase in corn-soybean meal-based diets for laying hens. Poult. Sci. 1997;76:1535–1542. doi: 10.1093/ps/76.11.1535. [DOI] [PubMed] [Google Scholar]
- Van Wyhe R.C., Applegate T.J., Lilburn M.S., Karcher D.M. A comparison of long bone development in historical and contemporary ducks. Poult. Sci. 2012;91:2858–2865. doi: 10.3382/ps.2012-02385. [DOI] [PubMed] [Google Scholar]
- Walk C.L., Bedford M.R., McElroy A.P. Influence of limestone and phytase on broiler performance, gastrointestinal pH, and apparent ileal nutrient digestibility. Poult. Sci. 2012;91:1371–1378. doi: 10.3382/ps.2011-01928. [DOI] [PubMed] [Google Scholar]
- Whitehead C.C. Overview of bone biology in the egg-laying hen. Poult. Sci. 2004;83:193–199. doi: 10.1093/ps/83.2.193. [DOI] [PubMed] [Google Scholar]
- Wu G., Bryant M.M., Gunawardana P., Roland D.A. Effect of nutrient density on performance, egg components, egg solids, egg quality, and profits in eight commercial Leghorn strains during phase one. Poult. Sci. 2007;86:691–697. doi: 10.1093/ps/86.4.691. [DOI] [PubMed] [Google Scholar]
- Zyla K., Mika M., Dulinski R., Swiatkiewicz S., Koreleski J., Pustkowiak H., Piironen J. Effects of inositol, inositol-generating phytase B applied alone, and in combination with 6-phytase A to phosphorus-deficient diets on laying performance, eggshell quality, yolk cholesterol, and fatty acid deposition in laying hens. Poult. Sci. 2012;91:1915–1927. doi: 10.3382/ps.2012-02198. [DOI] [PubMed] [Google Scholar]