Abstract
Undigested proteins entering the hindgut may favor the proliferation of Clostridium perfringens. Using phytase to eliminate the need for meat and bone meal (MBM) as a P source may reduce potential infection with C. perfringens. A study was conducted to determine the impact of MBM, phytase, and antibiotics (AB) on intestinal permeability and morphology, organ weights, and jejunal gene expression in Ross 308 chickens challenged with subclinical necrotic enteritis (NE). Male Ross 308-day-old chicks (672 each) were randomly allocated to 8 treatments with 6 replicate pens each housing 14 birds. A 2 × 2 × 2 factorial arrangement of treatments was used: MBM (no or yes); AB (no or yes—Zn bacitracin 100 in S and 50 ppm in G/F and salinomycin Na 60 ppm in all phases); phytase (500 or 1,500 FTU/kg, both using 500 FTU matrix values) using wheat-SBM-canola meal diets. Birds were challenged with Eimeria spp on day 9, and C. perfringens strain EHE-NE18 on day 14 and 15. An AB × MBM interaction (P < 0.05) was detected for relative gizzard weight (with contents) being lower in birds fed MBM and AB compared to those fed MBM and no AB. A MBM × AB interaction (P > 0.01) was detected for lymphocyte counts being lower with MBM and AB compared to MBM without AB. A phytase × AB interaction (P < 0.05) was observed for villi length being increased with high phytase and no AB compared to with AB. Inclusion of MBM increased (P < 0.05) blood FICT-d concentration, whereas AB decreased it (P < 0.05). Antibiotics increased RBC (P < 0.05), Hgb (P < 0.05), and PCV (P < 0.05) and expression of Ca-binding protein, CALB1 (P > 0.05). Inclusion of MBM decreased expression of MUC2 (P < 0.05). Results indicate that dietary MBM has a detrimental effect on gut health of broilers but this may be counteracted using AB.
Key words: meat and bone meal, phytase, intestinal health, gene expression and necrotic enteritis
Introduction
Meat and bone meal (MBM) is produced by the rendering industry from slaughterhouse wastes and dead animals that are not suitable for human consumption. It can be produced from the rendering of cattle, sheep, and/or pigs as either an individual or a mixture of species. It is included in poultry feed as a valuable source of protein, energy, P, and Ca (Sell, 1996, Sell and Jeffrey, 1996, Shirley and Parsons, 2001, Sulabo and Stein, 2013, Liu et al., 2016, Munoz et al., 2018). It typically contains 48 to 58% protein, 33 to 35% ash, 8 to 10% Ca, 4 to 5% P, 8 to 12% fat, and 4 to 7% water. Inclusion of MBM at levels around 5% can reduce or eliminate the need for inorganic P and as such may reduce feed cost. Phosphorus in MBM is considered more available than inorganic supplements, and unlike the P present in plants and seeds, there is no association with phytic acid (Selle et al., 2009). High-quality MBM is considered a digestible source of protein and amino acids (Cascarosa et al., 2012, Suloma et al., 2013). Inclusion of a high proportion of MBM may reduce the need for supplemental fat making feeds economical when fat is expensive. There is also the notion of “unidentified growth factors” that may increase palatability and/or growth due to the presence of peptides and/or trace minerals.
Despite its nutritional benefits, MBM is a heat-processed commodity and as such may contain high levels of indigestible protein (Kim et al., 2012). The bone component of MBM contains 25% protein, with 83% being collagen (Eastoe and Long, 1960). Collagen contains almost no tryptophan and is deficient in several other essential amino acids. Keratin from residual wool and hair is not digested by pepsin unless the rendering process includes a hydrolysis pressure cycle. Application of heat and pressure can denature amino acids making them less bioavailable. Lysine, cysteine, and threonine are particularly susceptible. Thus, it is generally accepted that MBM is a variable commodity based on the availability of protein and amino acids.
Clostridium perfringens is a common inhabitant of the gastrointestinal tract and is heavily involved in protein degradation in the hindgut and ceca. The aromatic amino acids tyrosine and tryptophan in MBM (Suloma et al., 2013) are anaerobically fermented by putrefactive C. perfringens leading to the formation of phenols, indoles, ammonia, and amines as a result of deamination and decarboxylation. These metabolites tend to increase the pH providing a favorable environment for the proliferation of the otherwise acid-sensitive C. perfringens (Rinttilä and Apajalahti, 2013). It can thus be hypothesized that high inclusion of dietary MBM would increase the risk of infection from necrotic enteritis (NE). However, little information is available in the literature regarding MBM instigation of NE in broilers.
The most effective control against NE is the routine use of in-feed antibiotics (AB). With current restrictions on the use of in-feed AB, however, alternative additives and improved feeding strategies are being sought as substitutes. One option may be the replacement of MBM (predisposing factor) with phytase enzyme to supply Ca and P. Phytase increases nutrient digestion from plant-based ingredients and limits the quantity of nutrients available for growth of pathogenic bacteria in the gut (Ptak et al., 2015). The effect of MBM and AB on growth performance, intestinal pH, apparent ileal digestibility, cecal microbiota, and bone mineralization during NE are reported in part 1 in this series. The main conclusion of that report was that MBM predisposes birds to NE and that AB protects birds against detrimental effects of NE on performance and livability. Superdosing of phytase (phytase inclusion above recommended dosage) improved performance during NE challenge in the presence of MBM and AB. The objective of the current work was to provide further insight into the mechanisms and interactions of MBM, phytase, and AB on intestinal permeability, organ weights, hematology, intestinal morphology, and jejunal gene expression in broilers during NE challenge.
Materials and methods
Birds and Management
All experimental procedures were reviewed and approved by the University of New England - Australia Animal Ethics Committee (AEC17-009). A total of 672 one-day-old Ross 308 male broiler chicks were obtained from the Darwalla poultry hatchery (Mt. Cotton Queensland 4169, Australia). Details are described in part 1 in this series.
Dietary Treatments
Details of feed analysis, formulations, and feeding are described in part 1 (Zanu et al., 2020) of this series. A 2 × 2 × 2 factorial arrangement of treatments was used. Factors were MBM (no or yes, 6% in starter (S) and 5% in grower (G) and finisher (F), AB (zinc bacitracin, 100 mg/kg in S and 50 mg/kg in G and F, and salinomycin, 60 mg/kg in all phases), phytase (500 or 1,500 FTU/kg) (Quantum Blue, AB Vista, Marlborough, UK). Custom-formulated broiler premixes, as well as salinomycin Na (Sacox 120, Huvepharma), were purchased from BEC Feed Solutions P/L, (Brisbane, QLD, Australia). Zinc bacitracin (Albac 150, Zoetis) was purchased from Ridley AgriProducts (Tamworth, NSW, Australia), and phytase (Quantum Blue, AB Vista, Marlborough, UK) was sourced from RCI Industries (Sydney, Australia).
Necrotic Enteritis Challenge
The NE challenge was performed in accordance with reported procedures (Stanley et al., 2014, Rodgers et al., 2015). All birds were challenged per os with 5,000 field strains of Eimeria acervulina, and Eimeria maxima and 2,500 sporulated oocysts of Eimeria brunetti (Eimeria Pty Ltd., Werribee, VIC, Australia) in 1 mL of 1% (w/v) sterile saline on day 9, and 108 CFU of C. perfringens Strain EHE-NE18 (known to express NetB toxin (CSIRO Livestock, Geelong, VIC, Australia) on day 14 and again on day 15.
Gut Permeability With Fluorescein Isothiocyanate Dextran (FITC-D)
Two birds (of average weight) per pen were gavaged with fluorescein isothiocyanate dextran solution (FITC-d; Sigma-Aldrich, Sweden; average mol weight of 4,000 and FITC-d: glucose of 1:250) (4.17 mg/kg bird dissolved in water) on day 16. After exactly 150 min, blood samples were taken from the jugular vein by decapitation of birds after electrical stunning using a CAT44 Electric Poultry Stunner (Mitchell Engineering Food Equipment Pty Ltd., Clontarf, Qld, Australia). The blood samples were centrifuged at 3,000 × g for 15 m, and the serum samples were collected and stored in −20°C until used. The serum samples (10 mL) were diluted in phosphate buffer saline (10 mL) in a 1:1. The samples were placed into 96-well black holder with transparent bottom plate. Fluorescence was measured at 485 nm excitation and 528 nm emission using SpectraMax M2e Microplate Reader (Molecular Devices, California). Levels of fluorescence in the samples were converted to respective FITC-d microgram per mL of serum based on a calculated standard curve.
Blood Collection and Hematology
On day 16 post-hatch, separate blood samples (other than those for FITC-d determination) were collected from the 2 birds into a plastic blood tube (spray-coated with K2EDTA) for hematology analysis. The blood sample tubes were immediately rolled for 4 min and analyzed for complete blood cell counts using a CELL-DYN 3,700 automated blood analyzer (Abbott Laboratories, Abbott Park). The hematologic variables measured were as follows: leucocytes; white blood cells (WBC), heterotrophils, lymphocytes, monocytes, eosinophils, basophils, and erythrocytes; red blood cells (RBC), hemoglobin (Hgb), packed cell volume (PCV), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and platelets.
Relative Organ Abdominal Fat Pad and Leg Weights
Two birds per pen were weighed individually and euthanized via electric stunning (as described previously) followed by cervical dislocation on day 42. The gizzard plus digesta, liver, abdominal fat pad, breast meat, ileum, and leg were then taken and weighed. Organ weights and abdominal fat were expressed as a percentage of live body weight (BW).
Intestinal Morphology
Ileal samples were collected from the 2 birds from which blood was collected and fixed in 10% buffered formalin until processing. The tissue samples were processed in Leica TP 1,020 45 processor (GMI Inc., Ramsey, MN) according to the program as follows: 30% ethanol for 2 h; 50% ethanol for 2 h; 70% ethanol for 2 h; 80% ethanol for 2 h; 95% ethanol for 1 h; absolute ethanol for 1 h; absolute ethanol for 1 h; 50:50 ethanol:xylol for 1 h; xylol for 1 h; xylol for 1 h; paraplast for 2 h and paraplast + Vac for 2 h. Tissue blocks were prepared and sectioned. The tissue blocks were cut into 6 μm cross-section thickness and mounted on glass slides. The slides were then stained using Harris's hematoxylin and eosin staining method. The cross-sections were viewed by light microscopy (Olympus CX41 microscope) using a 10 × objective and color images captured with the software Analysis 5.0 (Olympus Soft Imaging Solutions GmbH, Münster, Germany). Five villi height, width, and crypt depth per section and 4 sections per sample were measured, resulting in a total of 20 villi and crypts per bird. The apparent villi surface area was calculated as follows: (basal width + apical width)/2 × villus height.
RNA Extraction and Quality
For RNA extraction, the jejunal tissues were carefully flushed with phosphate-buffered saline and immediately placed in RNAlater (Invitrogen, Carlsbad, CA) and stored at −20°C until further use. Approximately 80 mg of the sample tissue was immersed in 1 mL TRIsure, (Bioline, Sydney, Australia) homogenized with an IKA ultra-turrax homogenizer (IKA-Work, Staufen, Germany). Total RNA was extracted as per the manufacturer's instructions. Total RNA was purified using ISOLATE II RNA Mini Kit (Bioline, Sydney, Australia) and a DNase I digestion step was included to remove any remaining genomic DNA as per the manufacturer's instructions. A NanoDrop ND-8000 spectrophotometer (Thermo Fisher Scientific, Waltham) and the Agilent 2,100 Bioanalyzer (Agilent Technologies, Inc., Waldbronn, Germany) were used to determine the concentration and RNA integrity (RNA Integrity Number, or RIN), respectively. Purified total RNA was stored at −80°C until further use.
cDNA Synthesis
The extracted RNA of each sample was reverse-transcribed to cDNA with the SensiFAST (Bioline, Sydney, Australia) cDNA Synthesis Kit as per manufacturer's instructions. A Rotorgene 6,000 real-time PCR instrument (Corbett, Sydney, Australia) was used to convert the RNA into cDNA. Synthesized cDNA samples were diluted 1:10 with nuclease-free water and stored at −20°C until used.
Primer Sources
Primers for qPCR were either sourced from published papers or designed with NCBI primer tool (https://www.ncbi.nlm.nih.gov). Table 1 presents the primers that were used in this study. An Agilent 2,100 Bioanalyzer (Agilent Technologies, Inc., Germany) was used to determine the primer specificity for each pair using an Agilent DNA 1,000 Kit (Agilent Technologies, Inc., Germany). Only the specific primer pairs with high amplification efficiency were used in this study.
Table 1.
Sequences of primers used for quantitative real-time PCR.
| Gene symbol | Group | Gene title | Primer sequence (5ʹ-3ʹ) | Ta °C |
Amplicon size (bp) | Reference | Function |
|---|---|---|---|---|---|---|---|
| CLDN1 | Tight junction protein | Claudin 1 | F-CTTCATCATTGCAGGTCTGTCAG R-AAATCTGGTGTTAACGGGTGTG |
60 | 103 | This study | Maintenance of intestinal barrier function |
| CLDN5 | Tight junction protein | Claudin 5 | F-GCAGGTCGCCAGAGATACAG R-CCACGAAGCCTCTCATAGCC |
61 | 162 | This study | Maintenance of intestinal barrier function |
| JAM2D | Tight junction protein | Junctional adhesion 2 | F-AGACAGGAACAGGCAGTGCTAG R-ATCCAATCCCATTTGAGGCTAC |
60 | 135 | This study | Maintenance of intestinal barrier function |
| TJP1 | Tight junction protein | Tight junction protein | F-GGATGTTTATTTGGGCGGC R-GTCACCGTGTGTTGTTCCCAT |
60 | 187 | This study | Maintenance of intestinal barrier function |
| OCLD | Tight junction protein | occludin | F- ACGGCAGCACCTACCTCAA R- GGGCGAAGAAGCAGATGAG |
60 | 123 | (Du, et al., 2016a) | Maintenance of intestinal barrier function |
| APN | Amino acid transporter | Aminopeptidase N | F-AATACGCGCTCGAGAAAACC R-AGCGGGTACGCCGTGTT |
60 | 70 | (Gilbert, et al., 2007) | Amino acid transporter |
| ASCT | Amino acid transporter | Alanine, serine, cysteine, and threonine transporter | F-TTGGCCGGGAAGGAGAAG R-AGACCATAGTTGCCTCATTGAATG |
60 | 63 | (Paris and Wong, 2013) | Amino acid transporter |
| NaPi-IIb | Phosphorus transporter | Na-dependent Pi cotransporters, type IIb | F- CGGTCCGTTCACTCTGTTGC R- GAAGCCACGTTGCCTTTGTG |
60 | 166 | This study | Mediation of intestinal phosphate uptake |
| VDR | Vitamin D transporter | Vitamin D receptor | F- ACGCAGACATGGACACCGT R- GGACAGGTGAACATCGCTTTC |
60 | 193 | This study | Provides instructions for making vitamin D receptor |
| CALB1 | Calcium transporter | Calbindin 1 | F- GGCAGGCTTGGACTTAACACC R- GTCGGCAACACCTGAGCAAG |
60 | 105 | This study | Transport protein of calcium |
| ATP1A1 | Calcium transporter | ATPase Na+/K+ transporting subunit alpha 1 | F- GTCAACCCGAGGGATGCTAA R- ACTGCTACAATGGCACCCTG |
60 | 179 | (Kheravii, et al., 2018) | Provides instructions for making Na+/K+ ATPase |
| ATP5A1W | Calcium transporter | ATP synthase subunit alpha | F- GGCAATGAAACAGGTGGCAG R- GGGCTCCAGCTTGTCTAAGTGA |
60 | 232 | This study | Synthesis of ATP |
| ATP13A4 | Calcium transporter | ATPase type 13A4 | F-CCAAAGCTCCTGCTAAATGC R-ATGCCTCCTGCTCTGACAGT |
61 | (Lee and Kim, 2018) | Synthesis of ATP | |
| ATP2A1 | Calcium transporter | ATPase, Ca++ transporting, cardiac muscle, fast twitch 1 | F-CGCTGTCAATCAGGACAAGA R-GTCGTTAAAGTGGCCGATGT |
61 | 250 | (Lee and Kim, 2018) | Catalyzes the hydrolysis of ATP and translocation of calcium from the cytosol to the sarcoplasmic reticulum lumen. |
| ATP2B1 | Calcium transporter | ATPase, Ca++ transporting, plasma membrane 1 | F-CTGGGCATGGGAACACTACT R-CACGACGTAATTCTCGCTCA |
60 | 169 | (Lee and Kim, 2018) | Catalyzes the hydrolysis of ATP and maintenance of intracellular calcium homeostasis |
| CASR | Calcium transporter | Calcium-sensing receptor | F-CTGCGTGATTTGGCTCTACA R-GGCAAAGAAGAAGCAGATGG |
60 | 160 | (Lee and Kim, 2018) | Regulator of parathyroid hormone synthesis and secretion and systemic calcium homeostasis |
| SLC8A1 | Calcium transporter | Solute carrier family 8 (sodium/calcium exchanger), member 1 | F-TCGTACCCCCGACAGACTAC R-GCCACCTTACTGGCAAATGT |
60 | 196 | (Lee and Kim, 2018) | Mediates the exchange of one Ca2+ against 3 to 4 Na+ ions across the cell membrane |
| SLC8B1 | Calcium transporter | (sodium/lithium/calcium exchanger), member B1 | F-CTTCGAGCTGAGCAACACTG R-CCCACACCCACCAGAATATC |
60 | 169 | (Lee and Kim, 2018) | Mediates mitochondrial calcium extrusion and mitochondrial calcium homeostasis |
| CACNA1A | Calcium channel | Calcium channel, voltage-dependent, P/Q type alpha 1A subunit | F-GCAGCGGGTCTATAAGCAGT R-GCGATGATGGTGGCTAAAAT |
60 | 198 | (Lee and Kim, 2018) | Provides instructions for making calcium channels |
| CACNB1 | Calcium channel | Calcium channel, voltage-dependent, beta 1 subunit | F-GGCAGCATATGGTGTGTGAC R-GGACAAAATCCTGGTGCTGT |
61 | 206 | (Lee and Kim, 2018) | Regulates the activity of L-type calcium channels that contain CACNA1C as pore-forming subunit |
| CACNG1 | Calcium channel | calcium channel, voltage-dependent, gamma subunit 1 | F-CAGCTACTCCTGGTCCTTCG R-GGAGATCCCGACATCTGAAA |
60 | 216 | (Lee and Kim, 2018) | Plays a role in excitation-contraction coupling |
| TPCN1 | Calcium channel | Two pore segment channel 1 | F-GACGGCCTGTCTCTGAGTTC R-ATCCCACTGCCTGATTTACG |
60 | 186 | (Lee and Kim, 2018) | Very little is known about the physiological functions but believe to form functional Ca2+-permeable channel |
| TPCN2 | Calcium channel | Two pore segment channel 2 | F-AGGTGCTGTGGTTCCTATGG R-GCCCACGGACTTGTGTATCT |
60 | 210 | (Lee and Kim, 2018) | Very little is known about the physiological functions but believe to form functional Ca2+-permeable channel |
| TPCN3 | Calcium channel | Two-pore calcium channel 3 | F-TGGGAATGGGAGTTCAAGAG R-CTGCCTCAAACATACGCTGA |
60 | 183 | (Lee and Kim, 2018) | Very little is known about the physiological functions but believe to form functional Ca2+-permeable channel |
| MUC2 | Inflammatory genes | Mucin 2 | F- CCCTGGAAGTAGAGGTGACTG R- TGACAAGCCATTGAAGGACA |
143 | 60 | (Fan, et al., 2015) | The physical and biological barrier protecting mucousepithelia |
| MUC-5AC | Inflammatory genes | Mucin 5 | F- AAGACGGCATTTATTTCTCCAC R- TCATTACCAACAAGCCAGTGA |
244 | 60 | (Fan, et al., 2015) | The physical and biological barrier protecting mucous epithelia |
| HPRT1 | Housekeeping genes | Hypoxanthine guanine phosphoribosyl transferase 1 | F- ACTGGCTGCTTCTTGTG R- GGTTGGGTTGTGCTGTT |
62 | 245 | (Yang, et al., 2013) | Provides instructions for making hypoxanthine phosphoribosyl transferase 1 |
| GAPDH | Housekeeping genes | Glyceraldehyde-3-phosphate dehydrogenase | F: GAAGCTTACTGGAATGGCTTTCC R: CGGCAGGTCAGGTCAACAA |
61 | 66 | (Kuchipudi, et al., 2012) | Energy metabolism and the synthesis of ATP and pyruvate |
Real-Time Quantitative PCR
Quantitative PCR was performed using a Rotorgene 6,000 real-time PCR machine (Corbett Research, Sydney, Australia). The qBase + version 3.0 (Biogazelle, Zwijnbeke, Belgium) software was used to determine the 2 most stable genes among seven (18S, ACTB, GAPDH, HPRT1, HMBS, TBP, and YWHAZ) different reference genes. Based on the expression stability, GAPDH and HPRT1 were used to normalize the target genes in the jejunum. The relative quantification of genes using the arithmetic mean method in qBase+ was exported to SAS 9.3 package for further analysis.
Statistical Analysis of Data
The data were evaluated as a fixed-effect model using the effects described in the statistical model, as follows:
where Yij is the response expected independent variables, μ = overall mean, Pi = fixed effect of phytase (i = low or high dose), Mj = the fixed effect of MBM (j = inclusion or not in the diet), Ak = the fixed effect of AB (k = inclusion or not in the diet), (PM)ij = interaction between phytase and MBM, (PA)ik = interaction between phytase and antibiotics, (MA)jk = interaction between MBM and AB, (PMA)ijk = is the 3-way interaction, and eij is the random residual error ∼ N (0, s2e).
The data were analyzed in a completely randomized design using a 2 × 2 × 2 factorial arrangement of treatments using PROC GLM (SAS Institute Inc., 2010) to assess the main effects (MBM, no or yes; AB, no or yes; and phytase, 500 or 1,500 FTU/kg) and 2 or 3-way interactions. Tukey's mean separation test was used to make pairwise comparisons between treatment means (P < 0.05). The SAS statistical package (PROC NPAR1WAY WILCOXON) was used to test and confirm normality for the data before analysis.
Results
Gut Permeability, day 16 Post-Hatch
Meat and bone meal increased (P < 0.05) FICT-d uptake from gut into serum across AB and phytase groups. No interactions were detected (P > 0.05). Dietary AB inclusion decreased FITC-d uptake from gut to serum across MBM and phytase groups (P < 0.05). No interactions were detected (P > 0.05). Results of FITC-d uptake are shown in Table 2.
Table 2.
Effect of meat and bone meal, phytase, antibiotics, and necrotic enteritis on FITC-d (ug/mL) concentration in serum of broilers, day 16 post-hatch.
| Effects | MBM | Phy | AB | FITC-d (ug/mL) |
|---|---|---|---|---|
| Main effects | ||||
| MBM | − | 0.236b | ||
| + | 0.348a | |||
| AB | − | 0.352a | ||
| + | 0.231b | |||
| P > f | ||||
| MBM | 0.043 | |||
| Phy | 0.360 | |||
| AB | 0.028 | |||
| MBM × Phy | 0.685 | |||
| MBM × AB | 0.313 | |||
| Phy × AB | 0.711 | |||
| MBM × Phy × AB | 0.138 |
a,b,c Means in the same column within the main effect, 2-way interaction, or treatment means with different superscripts are different (P < 0.05).
2 or 3-way interaction separated by Tukey.
Abbreviations: AB, antibiotics and FITC-d, fluorescein isothiocyanate dextran; AB, salinomycin 60 ppm in S, G, F; Zn bacitracin 100 ppm in S, G and 50 ppm in F; MBM, meat and bone meal; Phy, phytase (Quantum Blue 5G).
Organ, Fat Pad, and Leg Weight, day 42 Post-Hatch
Relative organ and body part weights (as a % of BW) on day 42 are given in Table 3. An AB × MBM interaction was detected (P < 0.05) for relative gizzard weight (including contents) showing lower weight in birds fed MBM and AB compared to those fed MBM and no AB. A phytase × AB interaction was detected (P < 0.05) for relative gizzard weight (including contents) showing higher weight in birds fed the high (superdose) phytase without AB compared to those fed the low phytase dose, regardless of AB presence. Dietary inclusion of MBM (across phytase and AB groups) decreased the relative weight of ileum (P < 0.05). Dietary inclusion of AB (across MBM and phytase groups) decreased relative ileum weight (P < 0.001). No 2-way or 3-way interactions were detected in relative ileum, liver, fat pad, breast, or leg weights (P > 0.05). No effect of MBM, AB, or phytase was observed for the relative weight of liver, fat pad, breast, or leg (P > 0.05).
Table 3.
Effect of meat and bone meal, phytase, antibiotics, and necrotic enteritis on relative organ weight (% of BW), day 42 post-hatch.
| Effects | MBM | Phy | AB | Gizzard + digesta | Liver | Fat pad | Breast | Ileum | Leg |
|---|---|---|---|---|---|---|---|---|---|
| 2-way interactions | |||||||||
| MBM*AB | |||||||||
| − | − | 1.71a,b | 2.31 | 0.71 | 18.78 | 0.90 | 19.12 | ||
| − | + | 1.82a | 2.30 | 0.71 | 19.40 | 0.76 | 20.22 | ||
| + | − | 1.87a | 2.29 | 0.62 | 17.10 | 0.80 | 20.40 | ||
| + | + | 1.58b | 2.25 | 0.73 | 18.42 | 0.70 | 20.31 | ||
| Phy*AB | |||||||||
| 500 | − | 1.52b | 2.25 | 0.69 | 18.43 | 0.84 | 19.18 | ||
| 500 | + | 1.66b | 2.31 | 0.74 | 19.03 | 0.72 | 20.68 | ||
| 1,500 | − | 2.05a | 2.35 | 0.64 | 17.45 | 0.87 | 20.34 | ||
| 1,500 | + | 1.74a,b | 2.25 | 0.70 | 18.81 | 0.73 | 19.18 | ||
| MBM | − | 1.77 | 2.31 | 0.71 | 19.09 | 0.83a | 19.67 | ||
| + | 1.72 | 2.27 | 0.68 | 17.77 | 0.74b | 20.36 | |||
| AB | − | 1.77 | 2.30 | 0.67 | 17.94 | 0.85a | 19.76 | ||
| + | 1.70 | 2.28 | 0.72 | 18.92 | 0.73b | 20.27 | |||
| P > f | |||||||||
| MBM | 0.686 | 0.874 | 0.445 | 0.105 | 0.011 | 0.183 | |||
| Phy | 0.006 | 0.812 | 0.443 | 0.489 | 0.588 | 0.655 | |||
| AB | 0.462 | 0.694 | 0.241 | 0.234 | 0.001 | 0.296 | |||
| MBM × Phy | 0.999 | 0.805 | 0.465 | 0.820 | 0.525 | 0.224 | |||
| MBM × AB | 0.043 | 0.909 | 0.268 | 0.717 | 0.683 | 0.241 | |||
| Phy × AB | 0.033 | 0.346 | 0.964 | 0.759 | 0.732 | 0.066 | |||
| MBM × Phy × AB | 0.893 | 0.276 | 0.371 | 0.756 | 0.938 | 0.373 | |||
a,b,c Means in the same column within the main effect, 2-way interaction, or treatment means with different superscripts are different (P < 0.05).
2- or 3-way interaction separated by Tukey.
Abbreviations: AB, antibiotics; AB, salinomycin 60 ppm in S, G, F; Zn bacitracin 100 ppm in S, G and 50 ppm in F; MBM, meat and bone meal; Phy, phytase (Quantum Blue 5G).
Erythrocytes and Leucocytes Count, day 16 Post-Hatch
The effect of treatment on erythrocytes on day 16 is shown in Table 4 and that on leucocytes on day 16 is shown in Table 5. Dietary inclusion of AB (across phytase and MBM) increased the concentration of RBC (P < 0.05), Hgb (P < 0.05), and PCV (P < 0.05). A 3-way interaction was detected for platelet counts (P > 0.001), where the highest platelet count was observed in the no-MBM, low phytase, and AB group and the lowest platelet count was observed in the MBM, high phytase, and no-AB group (Table 4). A MBM × AB interaction was detected (P < 0.01) for lymphocyte counts, with increased lymphocytes observed in birds fed no MBM and no AB compared to those fed no-MBM with AB. Across MBM and AB, the inclusion of high phytase (main effect) tended to increase the concentration of WBC (P = 0.083).
Table 4.
Effect of meat and bone meal, phytase, antibiotics, and necrotic enteritis on erythrocytes, day 16 post-hatch.
| Effects | MBM | Phy | AB | RBC (109/mL)12.5–3.5 | Hgb (g/dl)17–13 | PCV (%)122–52 | MCV (fL)1 90–140 | MCH (pg)133–47 | MCHC (g/dL)126–35 | Platelets (106/mL) |
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment means | ||||||||||
| 1. | − | 500 | − | 2.01 | 10.9 | 21.5 | 107.1 | 54.4 | 50.8 | 0.74b,c |
| 2. | − | 1,500 | − | 2.25 | 12.3 | 24.6 | 109.5 | 54.8 | 50.0 | 2.72a,b |
| 3. | − | 500 | + | 2.08 | 11.2 | 22.5 | 108.2 | 53.9 | 49.8 | 3.25a |
| 4. | − | 1,500 | + | 2.29 | 12.6 | 24.6 | 107.6 | 55.1 | 51.2 | 1.19a,b,c |
| 5. | + | 500 | − | 2.01 | 11.1 | 21.5 | 107.2 | 57.2 | 53.4 | 1.43a,b,c |
| 6. | + | 1,500 | − | 2.15 | 11.9 | 23.2 | 107.7 | 55.5 | 51.5 | 0.62c |
| 7. | + | 500 | + | 2.15 | 11.7 | 23.0 | 107.1 | 54.3 | 50.8 | 0.95b,c |
| 8. | + | 1,500 | + | 2.15 | 11.8 | 23.3 | 108.3 | 54.6 | 50.4 | 1.09b,c |
| Main effects | ||||||||||
| AB | − | 2.06b | 11.2b | 22.12b | 107.4 | 54.9 | 51.2 | 1.60 | ||
| + | 2.21a | 12.2b | 23.90a | 108.3 | 54.9 | 50.8 | 1.40 | |||
| P > f | ||||||||||
| MBM | 0.548 | 0.702 | 0.441 | 0.392 | 0.350 | 0.214 | 0.006 | |||
| Phy | 0.317 | 0.465 | 0.336 | 0.894 | 0.290 | 0.311 | 0.468 | |||
| AB | 0.027 | 0.010 | 0.014 | 0.182 | 0.963 | 0.650 | 0.564 | |||
| MBM × Phy | 0.901 | 0.867 | 0.835 | 0.616 | 0.327 | 0.254 | 0.458 | |||
| MBM × AB | 0.257 | 0.178 | 0.245 | 0.955 | 0.400 | 0.397 | 0.654 | |||
| Phy × AB | 0.516 | 0.575 | 0.398 | 0.368 | 0.455 | 0.281 | 0.025 | |||
| MBM × Phy × AB | 0.676 | 0.573 | 0.902 | 0.147 | 0.754 | 0.855 | 0.001 | |||
a,b,c Means in the same column within the main effect, 2-way interaction, or treatment means with different superscripts are different (P < 0.05).
2- or 3-way interaction separated by Tukey.
Abbreviations: AB, salinomycin 60 ppm in S, G, F; Zn bacitracin 100 ppm in S, G and 50 ppm in F; Hgb, hemoglobin; MBM, meat and bone meal; MCH, mean corpuscular hemoglobin and MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; PCV, packed cell volume; Phy, phytase (Quantum Blue 5G); RBC, red blood cells.
Table 5.
Effect of meat and bone meal, phytase, antibiotics, and necrotic enteritis on leucocytes, day 16 post-hatch.
| Effects | MBM | Phy | AB | WBC | Heterophils (106/mL) 13–17 |
Lymphocytes (106/mL) 110–30 |
Monocytes (106/mL) 10–5 |
Eosonophils (106/mL) 10–0.5 |
Basophils (106/mL) 20.3–2.5 |
|---|---|---|---|---|---|---|---|---|---|
| 2-way interactions | |||||||||
| MBM1AB | |||||||||
| − | − | 63.96 | 10.56 | 29.03a | 11.54 | 0.67 | 12.16 | ||
| − | + | 67.51 | 28.99 | 17.95b | 14.02 | 2.77 | 14.73 | ||
| + | − | 66.00 | 25.55 | 22.25a,b | 12.47 | 2.01 | 12.30 | ||
| + | + | 70.46 | 34.99 | 25.35a,b | 9.95 | 3.28 | 11.63 | ||
| Main effects | |||||||||
| Phy | 500 | 61.32 | 22.03 | 24.53 | 10.80 | 2.02 | 10.42 | ||
| 1,500 | 72.65 | 28.02 | 23.25 | 13.19 | 2.34 | 14.99 | |||
| P > f | |||||||||
| MBM | 0.697 | 0.419 | 0.726 | 0.630 | 0.460 | 0.643 | |||
| Phy | 0.083 | 0.643 | 0.580 | 0.465 | 0.795 | 0.157 | |||
| AB | 0.532 | 0.284 | 0.137 | 0.995 | 0.180 | 0.767 | |||
| MBM × Phy | 0.439 | 0.429 | 0.585 | 0.993 | 0.228 | 0.448 | |||
| MBM × AB | 0.943 | 0.728 | 0.002 | 0.443 | 0.736 | 0.613 | |||
| Phy × AB | 0.302 | 0.975 | 0.713 | 0.937 | 0.689 | 0.522 | |||
| MBM × Phy × AB | 0.196 | 0.252 | 0.606 | 0.566 | 0.242 | 0.140 |
a,b,cMeans in the same column within the main effect, 2-way interaction or treatment means with different superscripts are different (P < 0.05).
2- or 3-way interaction separated by Tukey.
Abbreviations: AB, salinomycin 60 ppm in S, G, F; Zn bacitracin 100 ppm in S, G and 50 ppm in F; MBM, meat and bone meal; Phy, phytase (Quantum Blue 5G); WBC, white blood cells.
Intestinal Morphology, day 16 Post-Hatch
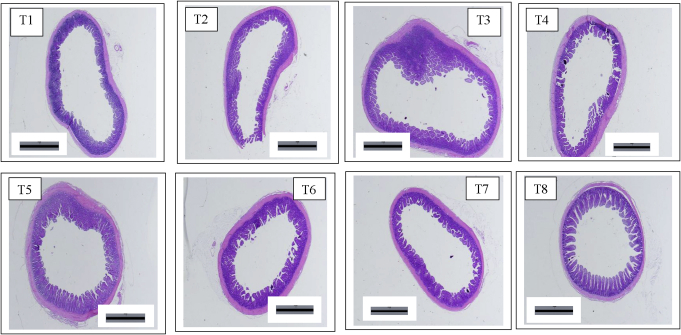
As shown in Table 6, a 3-way interaction was detected for villi apical width (P < 0.05) in which the treatment given MBM, low phytase, and AB had the highest villi apical width, whereas treatments with no MBM, no AB, and high phytase; with MBM, low phytase, and no AB; and with MBM, high phytase, with AB were low and not different from each other. A phytase × AB interaction was observed for villi height (P < 0.05) showing greater villi height in birds fed high phytase and no AB compared to those fed high phytase and with AB. A phytase × AB interaction was detected for apparent villi area (P < 0.05), showing greater surface area in birds fed low phytase, with AB and high phytase, no AB as compared to those fed low phytase, no AB and high phytase, with AB. No interactions were detected in the thickness of muscularis layer (P > 0.05); however, high phytase across MBM and AB increased (P < 0.05) the muscularis layer. No 2-way or 3-way interactions were detected for crypt depth (P > 0.05); however, birds with no AB across MBM and phytase groups had higher crypt depth than those fed AB (P < 0.05). Figure 1 shows that birds fed MBM, high phytase and AB had the healthiest enterocyte compare to the thick, lumpy, and blunt villi observed in other treatments.
Table 6.
Effect of meat and bone meal, phytase, antibiotics, and necrotic enteritis on ileal morphology, day 16 post-hatch.
| Effects | MBM | Phy | AB | Total height (μm) | Muscularis layer (μm) | Crypt depth (μm) | Villi height (μm) | Basal width (μm) | Apical width (μm) | Villi height: crypt depth | Apparent villi area (μm2 × 103) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment means | |||||||||||
| 1 | − | 500 | − | 751.1 | 202.8 | 272.9 | 275.4 | 47.50 | 38.89b | 1.022 | 12.15 |
| 2 | − | 1,500 | − | 842.0 | 226.5 | 250.6 | 365.0 | 45.52 | 33.65c | 1.799 | 14.50 |
| 3 | − | 500 | + | 749.2 | 195.7 | 254.5 | 299.0 | 47.54 | 37.00b | 1.187 | 12.79 |
| 4 | − | 1,500 | + | 726.1 | 247.8 | 254.5 | 223.7 | 44.27 | 37.73b | 0.878 | 9.39 |
| 5 | + | 500 | − | 758.7 | 218.7 | 294.0 | 245.9 | 40.06 | 28.75c | 0.877 | 8.78 |
| 6 | + | 1,500 | − | 849.8 | 228.2 | 285.8 | 335.8 | 49.63 | 38.30b | 1.192 | 14.59 |
| 7 | + | 500 | + | 752.8 | 216.2 | 227.4 | 309.1 | 46.41 | 41.80a | 1.372 | 13.77 |
| 8 | + | 1,500 | + | 783.3 | 266.5 | 248.2 | 268.5 | 42.05 | 34.81b,c | 1.163 | 10.95 |
| 2-way interactions | |||||||||||
| Phy*AB | |||||||||||
| 500 | − | 754.9 | 210.8 | 263.7 | 260.7c | 43.78 | 33.82 | 0.950 | 10.46b | ||
| 500 | + | 751.0 | 206.0 | 260.7 | 304.0b | 46.98 | 39.40 | 1.279 | 13.28a | ||
| 1,500 | − | 845.9 | 227.4 | 252.5 | 350.4a | 47.57 | 35.98 | 1.495 | 14.54a | ||
| 1,500 | + | 754.7 | 257.2 | 267.0 | 246.1d | 43.16 | 36.27 | 1.021 | 10.17c | ||
| Main effects | |||||||||||
| Phy | 500 | 752.9 | 208.4b | 262.2 | 282.3 | 45.38 | 36.61 | 1.258 | 11.87 | ||
| 1,500 | 800.3 | 242.3a | 259.8 | 298.3 | 45.37 | 36.12 | 1.114 | 12.36 | |||
| AB | − | 800.4 | 219.1 | 275.8a | 305.5 | 45.68 | 34.90 | 1.222 | 12.50 | ||
| + | 752.8 | 231.6 | 246.2b | 275.1 | 45.07 | 37.83 | 1.150 | 11.73 | |||
| P > f | |||||||||||
| MBM | 0.529 | 0.338 | 0.675 | 0.976 | 0.528 | 0.710 | 0.741 | 0.904 | |||
| Phy | 0.122 | 0.026 | 0.860 | 0.598 | 0.996 | 0.841 | 0.501 | 0.752 | |||
| AB | 0.121 | 0.398 | 0.035 | 0.315 | 0.817 | 0.231 | 0.734 | 0.614 | |||
| MBM × Phy | 0.656 | 0.786 | 0.526 | 0.771 | 0.325 | 0.468 | 0.671 | 0.514 | |||
| MBM × AB | 0.707 | 0.716 | 0.107 | 0.348 | 0.999 | 0.449 | 0.157 | 0.347 | |||
| Phy × AB | 0.154 | 0.245 | 0.350 | 0.018 | 0.155 | 0.280 | 0.065 | 0.024 | |||
| MBM × Phy × AB | 0.659 | 0.833 | 0.904 | 0.776 | 0.235 | 0.025 | 0.510 | 0.641 | |||
a,b,cMeans in the same column within the main effect, 2-way interaction or treatment means with different superscripts are different (P < 0.05).
2- or 3-way interaction separated by Tukey.
Abbreviations: AB, salinomycin 60 ppm in S, G, F; Zn bacitracin 100 ppm in S, G and 50 ppm in F; MBM, meat and bone meal; Phy, phytase (Quantum Blue 5G).
Figure 1.
Representative photomicrographs of the ileal cross-section in broilers. T1: no-MBM, low phytase, no-AB; T2: no-MBM, high phytase, no AB; T3: no-MBM, low phytase, yes-AB; T4: no-MBM, high phytase, yes AB; T5: yes-MBM, low phytase, no-AB; T6: yes-MBM, high phytase, no AB; T7: yes-MBM, low phytase, yes AB; T8: yes-MBM, high phytase, yes AB (T1, T2, T3, T4, T5, T6, T7 and T8; scale bar = 1,000 μm).
Jejunal Gene Expression, day 16 Post-Hatch
Jejunal gene expression results measured from birds on day 16 are given in Table 7. Meat and bone meal (across phytase level and AB inclusion) as a main effect decreased the expression of MUC2 (P < 0.05), while MBM tended to decrease the expression of MUC5 (P = 0.070) and ATP1A1 (P = 0.073). Dietary inclusion of AB (across MBM and phytase groups) increased the expression of CALB1 (P < 0.05). A tendency (P = 0.1) for a MBM × phytase interaction was detected for ATP13A4. Birds fed diets with no MBM had expression upregulated only with high phytase, whereas with MBM, the expression was upregulated with low phytase. A tendency (P < 0.1) for a MBM × phytase × AB interaction was observed for CACNA. The highest expression was detected with high phytase, with MBM and with AB, whereas those fed diets with MBM, high phytase, and without AB resulted in the downregulation of CACNA. Also, in birds fed diets without MBM, low phytase and with AB had upregulated CACNA while the presence of MBM led to a downregulation. A tendency for a phytase × AB interaction was detected (P < 0.1) for CACNB and TPCN3 where expressions of both genes were upregulated in the absence of AB with low phytase; however, in those fed high phytase, the expression of the gene was downregulated. As no significant effects of the dietary treatments or interactions (P > 0.1) on expression of CLDN1, CLDN5, JAM2D, TJP1, OCLD, APN, ASCT, NaPi-IIb, VDR, ATP5A1W, ATP2A1, ATP2B1, CACNG, CASR, SLC8A1, SLC8B1, TPCN1, and TPCN2 were detected, these results have been presented as a Supplementary Tables. Only target genes which showed significant difference or tendencies are discussed.
Table 7.
Effect of meat and bone meal, phytase, antibiotics, and necrotic enteritis on jejunal gene expression, day 16 post-hatch.
| Effects | MBM | Phy | AB | CALB1 | MUC2 | MUC-5AC | ATP1A1 | ATP13A4 | CACNA1 | CACNB1 | TPCN3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment means | |||||||||||
| 1 | − | 500 | − | 1.111 | 1.088 | 0.989 | 1.065 | 1.146 | 1.221b,c | 1.202 | 1.098 |
| 2 | − | 1,500 | − | 1.295 | 1.564 | 1.226 | 1.339 | 1.227 | 1.964a | 1.130 | 0.947 |
| 3 | − | 500 | + | 2.329 | 1.113 | 1.467 | 1.353 | 0.849 | 1.557a,b | 0.919 | 1.068 |
| 4 | − | 1,500 | + | 1.938 | 1.354 | 1.450 | 1.275 | 1.283 | 1.549a,b | 0.961 | 1.050 |
| 5 | + | 500 | − | 0.780 | 1.120 | 0.976 | 1.162 | 1.241 | 1.347b | 1.422 | 1.409 |
| 6 | + | 1,500 | − | 1.071 | 0.901 | 0.969 | 0.809 | 0.924 | 0.885c | 0.974 | 0.968 |
| 7 | + | 500 | + | 1.171 | 0.961 | 1.017 | 1.079 | 1.025 | 0.762c,d | 0.965 | 0.876 |
| 8 | + | 1,500 | + | 1.41 | 1.112 | 1.155 | 1.090 | 1.007 | 1.502b | 1.280 | 1.083 |
| 2-way interactions | |||||||||||
| MBM*Phy | − | 500 | 1.720 | 1.101 | 1.228 | 1.209 | 0.998b | 1.389 | 1.060 | 1.083 | |
| − | 1,500 | 1.617 | 1.459 | 1.338 | 1.307 | 1.255a | 1.757 | 1.045 | 0.998 | ||
| + | 500 | 0.975 | 1.040 | 0.997 | 1.121 | 1.133a | 1.055 | 1.194 | 1.142 | ||
| + | 1,500 | 1.242 | 1.007 | 1.062 | 0.949 | 0.966b | 1.757 | 1.127 | 1.026 | ||
| Phy*AB | |||||||||||
| 500 | − | 0.946 | 1.104 | 0.983 | 1.114 | 1.193 | 1.284 | 1.312a | 1.253a | ||
| 500 | + | 1.750 | 1.037 | 1.242 | 1.216 | 0.937 | 1.159 | 0.942b | 0.972b | ||
| 1,500 | − | 1.183 | 1.233 | 1.098 | 1.074 | 1.075 | 1.425 | 1.052b | 0.957b | ||
| 1,500 | + | 1.675 | 1.233 | 1.302 | 1.182 | 1.145 | 1.526 | 1.120a,b | 1.067a,b | ||
| Main effects | |||||||||||
| MBM | − | 1.668a | 1.280a | 1.283 | 1.258 | 1.126 | 1.573 | 1.053 | 1.041 | ||
| + | 1.109b | 1.024b | 1.029 | 1.035 | 1.049 | 1.124 | 1.160 | 1.084 | |||
| AB | − | 1.064b | 1.168 | 1.040 | 1.094 | 1.134 | 1.355 | 1.182 | 1.105 | ||
| + | 1.713a | 1.135 | 1.272 | 1.199 | 1.041 | 1.343 | 1.031 | 1.019 | |||
| P > f | |||||||||||
| MBM | 0.044 | 0.044 | 0.070 | 0.073 | 0.495 | 0.112 | 0.405 | 0.703 | |||
| Phy | 0.764 | 0.194 | 0.523 | 0.764 | 0.689 | 0.364 | 0.751 | 0.378 | |||
| AB | 0.021 | 0.788 | 0.096 | 0.390 | 0.408 | 0.965 | 0.244 | 0.449 | |||
| MBM × Phy | 0.497 | 0.118 | 0.868 | 0.272 | 0.065 | 0.682 | 0.841 | 0.886 | |||
| MBM × AB | 0.302 | 0.632 | 0.389 | 0.959 | 0.809 | 0.920 | 0.559 | 0.091 | |||
| Phy × AB | 0.566 | 0.788 | 0.842 | 0.979 | 0.152 | 0.684 | 0.093 | 0.283 | |||
| MBM × Phy × AB | 0.628 | 0.225 | 0.467 | 0.147 | 0.904 | 0.085 | 0.212 | 0.258 |
a,b,c Means in the same column within the main effect, 2-way interaction or treatment means with different superscripts are different (P < 0.05).
2-way interaction separated by least square difference; 3-way interaction by Tukey.
Abbreviations: AB, salinomycin 60 ppm in S, G, F; Zn bacitracin 100 ppm in S, G and 50 ppm in F; ATP13A4, ATPase type 13A4; ATP1A1, ATPase Na+/K+ transporting subunit alpha 1; CALB1, calbindin 1; CACNA1, calcium channel; CACNB1, calcium channel, voltage-dependent, beta 1 subunit; MBM, meat and bone meal; MUC2, mucin 2; MUC-5AC, mucin 5; Phy, phytase (Quantum Blue 5G); TPCN3, two-pore calcium channel 3.
Discussion
This study investigated the effects of dietary MBM, level of phytase, and AB on the intestinal permeability, organ weights, hematology, intestinal morphology, and jejunal gene expression of broilers during NE.
In a healthy functioning gut, FITC-d is well contained in the intestine because of its high molecular weight (3–5 kDa) and low permeability in the gut. For this reason, a compromise in the tight junctions (TJ) between the enterocytes leads to the detection of FITC-d in serum, indicating a change in paracellular permeability following the oral administration of molecule. The results showed that on day 16, soon after infection with NE, there was higher plasma FITC-d in birds fed MBM compared to those not fed MBM. This suggests MBM may have provided a favorable environment for pathogenic bacteria and/or Eimeria to colonize the gut lining. Previous work has shown that secretions of C. perfringens toxins affect the intestinal TJ barrier leading to the loss of intestinal epithelial homeostasis and cause destruction in the epithelial cells (Field, 2003, Navarro et al., 2018). Though translocation of C. perfringens was not measured in this study, an increased intestinal leakage as a result of MBM inclusion could have led to bacterial translocation from the gut lumen into circulating blood. Migration of bacteria via portal circulation into the liver and blood is known (Cooper et al., 2013). In the present study, the inclusion of AB increased RBC and reduced FITC-d transfer from the gut lumen to circulating blood indicating that AB may have alleviated possible anemia caused by NE. The presence of AB might have decreased the presence of C. perfringens thereby protecting the epithelial layer as reported by Crisol-Martínez et al. (2017). It has been demonstrated that when ingredient quality is poor, there is reduced intestinal absorption of nutrients but increased bacterial translocation and intestinal leakage (Tellez et al., 2014). A reduction of C. perfringens in intestinal contents of chicks fed diets containing AB compared to those fed unmedicated feed has also been reported (Gaucher et al., 2015). It is unknown what impact the presence of NetB toxin from C. perfringens might be on RBC in birds not fed AB. NetB appears to be the causative agent in pore formation in cellular membranes resulting in an influx of ions into the cytoplasm eventually leading to osmotic lysis of enterocytes (Keyburn et al., 2010).
Furthermore, the high lymphocyte concentrations observed in this study are likely associated with an inflammatory response as a result of intestinal epithelial damage as suggested in previous studies (Ross, 1994). Birds fed diets without MBM but with AB recorded the lowest lymphocyte count, confirming the positive effect of AB on host immunity as previously observed (Kumar et al., 2018). The main function of lymphocytes is an immune response. Somewhat paradoxically, no significant effect of the treatments on molecular microcellular intestinal lesions was observed (Supplementary Tables). This might be because the challenge is subclinical and only limited inflammation-related score was recorded.
The weight of the gizzard plus digesta was used in this study to indicate the rate of feed passage. High relative gizzard plus digesta weight indicates slower feed passage and lower relative gizzard plus digesta weight indicates higher passage rate as reported by Svihus et al. (2002) and is related to diet composition. In the current experiment, birds fed MBM with AB had lower relative gizzard plus digesta weights compared to those fed MBM without AB or birds fed no MBM across AB. Similarly, birds fed high phytase without AB had higher gizzard plus digesta weights than those fed low phytase with or without AB. This indicates that MBM, AB, and phytase in various combinations affect feed passage rate through the gizzard. Transit time in the gizzard according to Zhang and Coon (1997) is associated with dietary Ca levels. These authors reported decreased gizzard retention times in birds fed higher percentages of Ca and this resulted in decreased Ca solubilization. In the current experiment, dietary inclusion of AB may have had a greater influence on passage rate in birds fed MBM due to the reduction of stress caused by MBM. During NE infection, birds consume less feed than uninfected controls (AB-fed birds in this study), hence a slower feed passage. Ravindran (2013) believes that a less developed gizzard serves as transit organ rather than a grinding site, with implications for reduced retention time. Feed passage rate in broilers is known to be influenced by feed intake (FI) (Watson et al., 2006, Mendes et al., 2013). Not only was increased feed passage observed in birds fed MBM and phytase, but also in birds fed 1,500 FTU phytase with AB. Though phytase has been reported to increase feed passage rate in chickens (Watson et al., 2006), it appears in this study that it depended on AB to do so. Birds fed AB showed greater FI than those not fed AB (manuscript 1 in series) and by implication greater feed passage.
Dietary inclusion of AB has been shown to have an intestinal thinning effect in birds (Jukes et al., 1956, Miles et al., 2006) and this was observed in the present study (as lower relative ileum weight). One of the benefits of thin ileum is that it spares energy from tissue maintenance that can be used instead for growth. In addition, dietary inclusion of MBM decreased relative ileal weight in this study. But this observation might be rather due to NE. One of the clinical signs of clostridial enteric diseases includes thin-walled and friable intestines (Timbermont et al., 2011, Cooper et al., 2013).
Intestinal morphology is an indicator of intestinal health and integrity. An ideal intestinal morphology has been suggested to have longer and wider intestinal villi as this is indicative of a larger absorptive surface area and higher consequent digestive enzyme activity to enhance uptake of nutrients (Laudadio et al., 2012). The tip of the villi is an integral part of the brush border, which aids the absorption of nutrients. It has been reported that in subclinical cases of NE, the intestinal necrosis is usually observed at the villous tips (Kaldhusdal and Hofshagen, 1992). In the present study, villi height was decreased by AB in birds fed high phytase but increased in birds fed low phytase diets. This inconsistency could be possibly due to the fact the low dose released nutrients more slowly so lower gut morphology had to develop to recover these nutrients. Effective nutrient absorption is dependent on the surface area of the villi. In this study, there was no consistency of the effect of phytase and AB interaction on the apparent villi area. It was evident however that without AB there was an increase in the villi surface area of birds on high-dose phytase than when AB was present. It is likely that the increased villi area is a sign of villi atrophy, which Awad et al. (2015) had reported in 21-day-old chickens infected with Campylobacter jejuni. A similar observation was also reported by Zhang et al. (2018). In this study, birds fed AB and a superdose of phytase had increased gain and reduced feed conversion ratio during the postchallenge era and increased the count of Bacillus while these traits were decreased in birds fed high-dose phytase without AB. Therefore, a reduced villus surface in birds fed AB in the present study was surprising.
Deep crypts are an indication of faster cellular turnover, which allows the renewal of villus to replace sloughed or inflammatory villi (Jayaraman et al., 2013). A longer villi length and deeper crypts have been reported as a result of NE challenge, and this is indicative of decreased nutrient absorption due to reduced turnover rate (Guo and Guo, 2012, Du et al., 2016a, Kiess et al., 2018). In the present study, the absence of AB led to a deeper crypt in the ileum. It is possible that the proliferation of C. perfringens eliminated the absorptive villi and resulted in the crypts between them to deepen thus making the intestinal lining non-absorptive, thick, and lumpy.
Previous studies have implicated Eimeria infection to cause intestinal damage including a reduction in villus height with a consequent decrease in growth performance and an increased potential for C. perfringens to colonize (Wu et al., 2014, Wu et al., 2016, Rodgers et al., 2015).
The secretory MUC2 is a gel form of mucin and the major product of the goblet cells. It is in direct contact with intestinal bacteria and provides a barrier to infection. Mucin also facilitates the formation of secretory IgA-mediated immune defense, which prevents the invasion of the intestinal epithelial cell by gut bacteria and promotes adherent growth of normal gut flora. In this study, MBM downregulated the expression of MUC 2. This finding is not surprising as MBM exacerbated NE and as such decreased the expression of MUC2. In other challenge studies, high mucin secretion due to Eimeria infection was reported to have increased the incidence of C. perfringens colonization (Collier et al., 2008). In addition, Collier et al. (2003) had earlier confirmed the hypothesis that intestinal conditions that favor enhanced mucus production can provide a selective advantage for C. perfringens. The infected mucous layer becomes a nutritional source for enteric pathogens and a myriad of reports show that such pathogens then downregulated the expression of MUC2 in chickens (Forder et al., 2012, Awad et al., 2017, Lee et al., 2018). Antibiotics increase the secretion of mucin and improve the general health of the intestines (Broom, 2018). In the present study, however, AB tended to increase the expression of MUC5 genes. According to Smirnov et al. (2004), MUC2 is the primary mucin in the small intestine and colon of poultry, whereas MUC5 is more largely expressed in the proventriculus. Jejunal tissues were used in this study.
The main Ca2+ transport proteins are mostly calcium-binding protein calbindin-D28 K, plasma membrane Ca2+ ATPase (PMCA), epithelial calcium channels, and Na+/Ca2+ exchangers (NCX1). Briefly, the process involves the transport of Ca2+ from the lumen of the intestine across the epithelia into the cell through specific epithelial calcium channels, then moves throughout the cytoplasm bound to calcium-binding proteins, such as calbindin-D28 K and is extruded to the extracellular medium by the action of PMCA1b and NCX1 (Proszkowiec-Weglarz and Angel, 2013). Ca-binding protein (CaBP) was downregulated by MBM in the present study. This protein plays an essential role in the absorption of Ca across the enterocytes of proximal intestines (duodenum and jejunum). Free Ca2+, after having entered the Ca channels located in the brush border membranes, binds to Ca-binding proteins. Calcium exits from the cell with the help of ATPase (an enzyme that releases ATP). Thus, the results of the present study are in agreement with the report by González-Vega and Stein (2016). That is, at high dietary concentrations of Ca, mRNA expression of Ca transporters involved in the transcellular route is downregulated in the jejunum. Although the calculated diet Ca level was not different in any of the treatments in the present study, there was likely additional unaccounted Ca resulting from the high (superdose) phytase supplementation without a nutrient matrix used. A similar downregulation was also observed for ATP13A4 in the presence of MBM and phytase. A recent report by Lee and Kim (2018) revealed that ATP13A4 and voltage-dependent calcium channels (CACNA, CACNB, and TPCN3) might play a role in Ca2+ transport in broilers. An earlier study had demonstrated that ATP13A4 was involved in Ca regulation (Vallipuram et al., 2010). In the present study, superdosing phytase increased the expression of ATP13A4 without MBM, while with MBM, superdosing phytase downregulated the expression of this transport protein. This observation is logical, in that, the Ca concentration in the intestine would increase with the addition of MBM and phytase as they both release Ca and lead to low expression. It also suggests that the expression of this transporter is greatest in a state of low dietary Ca or during increased Ca need. Other authors have confirmed that low intake of Ca leads to an upregulation of the active transcellular calcium transport (Auchère et al., 1998, Bronner and Pansu, 1999, Li et al., 2012, Shet et al., 2018). But, also involved in the active intestinal Ca transport across the cell membrane are voltage-gated Ca channels CACNA1A, CACNB, and TPCN3. Little is known about these genes and their role in Ca transport in chickens. However, in the present study, a likely high Ca concentration resulting from the combination of MBM, superdosing phytase (without taking additional Ca release into consideration), and without AB led to a downregulation of CACNA1A. However, a higher regulation was seen in birds fed the same diet but with no MBM confirming the relationship between Ca density and regulation of Ca transporters. It also appeared that phytase supplementation at a lower concentration in the absence of AB upregulated the expression of CACNB1 and TPCN3. The CACNB1 has also been reported to be associated with growth (Chen et al., 2011, Tarsani et al., 2019).
Conclusion
This study reported a number of interactions including 3-way factorial and the following findings were associated with these interactive effects of the main experimental factors. The results indicate that MBM has a detrimental effect on intestinal health by promoting gut leakage and reducing immunity. The findings also suggest that birds fed low phytase and treated with AB or fed high phytase without AB may have improved intestinal morphometric traits. The inclusion of AB reduced the negative effect of MBM by enhancing the defense mechanism of the gut against C. perfringens and improving nutrients transport in the presence of high phytase. Thus, the overall study supports the hypothesis that MBM is indeed a predisposing factor of NE and can be replaced with high phytase in combination with AB for optimal growth performance and sound gut health.
Acknowledgments
The authors hereby acknowledge AB Vista Feed Ingredient, Marlborough, UK, for funding this research. The authors also do acknowledge Brett Ruth of Ruth Consolidated Industries Pty. Ltd. for the input he made in the design of this study. Also, the University of New England, Armidale (Australia), is acknowledged for providing the international postgraduate research award (IPRA) to the lead author for his PhD.
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2019.12.049.
Supplementary data
References
- Auchère D., Tardivel S., Gounelle J.-C., Drüeke T., Lacour B. Role of transcellular pathway in ileal Ca2+ absorption: stimulation by low-Ca2+ diet. Am. J. Physiol. Gastrointest. Liver Physiol. 1998;275:951–956. doi: 10.1152/ajpgi.1998.275.5.G951. [DOI] [PubMed] [Google Scholar]
- Awad W.A., Hess C., Hess M. Enteric pathogens and their toxin-induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins (Basel) 2017;9:60. doi: 10.3390/toxins9020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad W.A., Molnar A., Aschenbach J.R., Ghareeb K., Khayal B., Hess C., Liebhart D., Dublecz K., Hess M. Campylobacter infection in chickens modulates the intestinal epithelial barrier function. J. Innate. Immun. 2015;21:151–160. doi: 10.1177/1753425914521648. [DOI] [PubMed] [Google Scholar]
- Bronner F., Pansu D. Nutritional aspects of calcium absorption. J. Nutr. 1999;129:9–12. doi: 10.1093/jn/129.1.9. [DOI] [PubMed] [Google Scholar]
- Broom L.J. Gut barrier function: effects of (antibiotic) growth promoters on key barrier components and associations with growth performance. Poult. Sci. 2018;97:1572–1578. doi: 10.3382/ps/pey021. [DOI] [PubMed] [Google Scholar]
- Cascarosa E., Gea G., Arauzo J. Thermochemical processing of meat and bone meal: a review. Renew. Sust. Energ. Rev. 2012;16:942–957. [Google Scholar]
- Chen F., Liu Y., Sugiura Y., Allen P.D., Gregg R.G., Lin W. Neuromuscular synaptic patterning requires the function of skeletal muscle dihydropyridine receptors. Nat. Neurosci. 2011;14:570. doi: 10.1038/nn.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier C.T., Hofacre C.L., Payne A.M., Anderson D.B., Kaiser P., Mackie R.I., Gaskins H.R. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet. Immunol. Immunopathol. 2008;122:104–115. doi: 10.1016/j.vetimm.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Collier C.T., van der Klis J.D., Deplancke B., Anderson D.B., Gaskins H.R. Effects of tylosin on bacterial mucolysis, Clostridium perfringens colonization, and intestinal barrier function in a chick model of necrotic enteritis. Antimicrob. Agents Chemother. 2003;47:3311–3317. doi: 10.1128/AAC.47.10.3311-3317.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K.K., Songer J.G., Uzal F.A. Diagnosing clostridial enteric disease in poultry. J. Vet. Diagn. Invest. 2013;25:314–327. doi: 10.1177/1040638713483468. [DOI] [PubMed] [Google Scholar]
- Crisol-Martínez E., Stanley D., Geier M.S., Hughes R.J., Moore R.J. Understanding the mechanisms of zinc bacitracin and avilamycin on animal production: linking gut microbiota and growth performance in chickens. Appl. Microbiol. Biotechnol. 2017;101:4547–4559. doi: 10.1007/s00253-017-8193-9. [DOI] [PubMed] [Google Scholar]
- Douglas J. Schalm’s Veterinary Hematology. 2000. In: Bernard V., editor. 6th ed. Feldman Lippincott Williams and Wilkins Publishers; Baltimore, MD, USA: 2000. p. 965. [Google Scholar]
- Du E., Wang W., Gan L., Li Z., Guo S., Guo Y. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2016;7:19. doi: 10.1186/s40104-016-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastoe J.E., Long J.E. The amino-acid composition of processed bones and meat. J. Sci. Food Agric. 1960;11:87–92. [Google Scholar]
- Fan S.P., Liberman Z., Keysar B., Kinzler K.D. The exposure advantage: early exposure to a multilingual environment promotes effective communication. Psychol. Sci. 2015;26:1090–1097. doi: 10.1177/0956797615574699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M. Intestinal ion transport and the pathophysiology of diarrhea. J. Clin. Invest. 2003;111:931–943. doi: 10.1172/JCI18326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forder R.E.A., Nattrass G.S., Geier M.S., Hughes R.J., Hynd P.I. Quantitative analyses of genes associated with mucin synthesis of broiler chickens with induced necrotic enteritis. Poult. Sci. 2012;91:1335–1341. doi: 10.3382/ps.2011-02062. [DOI] [PubMed] [Google Scholar]
- Frazer C.M., Bergeron J.A., Mays A., Aiello S.E. 7th ed. Merck Inc; New Jersey: 1991. Chemotherapeutics. The Merck Veterinary Manual; p. 968. [Google Scholar]
- Gaucher M.-L., Quessy S., Letellier A., Arsenault J., Boulianne M. Impact of a drug-free program on broiler chicken growth performances, gut health, Clostridium perfringens and Campylobacter jejuni occurrences at the farm level. Poult. Sci. 2015;94:1791–1801. doi: 10.3382/ps/pev142. [DOI] [PubMed] [Google Scholar]
- Gilbert E.R., Li H., Emmerson D.A., Webb K.E., Jr., Wong E.A. Developmental regulation of nutrient transporter and enzyme mrna abundance in the small intestine of broilers. Poult. Sci. 2007;86:1739–1753. doi: 10.1093/ps/86.8.1739. [DOI] [PubMed] [Google Scholar]
- González-Vega J.C., Stein H.H. Chapter 14 calcium transporters and gene expression and absorption of calcium in pigs. In: Walk C.L., Kühn I., Stein H.H., Kidd M.T., Rodehutscord M., editors. Phytate Destruction - Consequences for Precision Animal Nutrition. 2016. pp. 217–224. [Google Scholar]
- Guo S., Guo Y. Xylanase supplementation to a wheat-based diet alleviated the intestinal mucosal barrier impairment of broiler chickens challenged by Clostridium perfringens. Avian Pathol. 2012;41:291–298. doi: 10.1080/03079457.2012.684089. [DOI] [PubMed] [Google Scholar]
- Jukes T.H., Hill D.C., Branion H.D. Effect of feeding antibiotics on the intestinal tract of the chick. Poult. Sci. 1956;35:716–723. [Google Scholar]
- Jayaraman S., Thangavel G., Kurian H., Mani R., Mukkalil R., Chirakkal H. Bacillus subtilis PB6 improves intestinal health of broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis. Poult. Sci. 2013;92:370–374. doi: 10.3382/ps.2012-02528. [DOI] [PubMed] [Google Scholar]
- Kaldhusdal M., Hofshagen M. Barley inclusion and avoparcin supplementation in broiler diets.: 2. Clinical, pathological, and bacteriological findings in a mild form of necrotic enteritis. Poult. Sci. 1992;71:1145–1153. doi: 10.3382/ps.0711145. [DOI] [PubMed] [Google Scholar]
- Keyburn A.L., Bannam T.L., Moore R.J., Rood J.I. NetB, a pore-forming toxin from necrotic enteritis strains of Clostridium perfringens. Toxins (Basel) 2010;2:1913–1927. doi: 10.3390/toxins2071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheravii S.K., Swick R.A., Choct M., Wu S.-B. Upregulation of genes encoding digestive enzymes and nutrient transporters in the digestive system of broiler chickens by dietary supplementation of fiber and inclusion of coarse particle size corn. BMC Genomics. 2018;19:208. doi: 10.1186/s12864-018-4592-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiess A.S., Peebles E.D., Wamsley K.G.S., Wang X., Zhai W. Effects of Bacillus subtilis and zinc on the growth performance, internal organ development, and intestinal morphology of male broilers with or without subclinical coccidia challenge. Poult. Sci. 2018;97:3947–3956. doi: 10.3382/ps/pey262. [DOI] [PubMed] [Google Scholar]
- Kim E.J., Utterback P.L., Parsons C.M. Comparison of amino acid digestibility coefficients for soybean meal, canola meal, fish meal, and meat and bone meal among 3 different bioassays. Poult. Sci. 2012;91:1350–1355. doi: 10.3382/ps.2011-01861. [DOI] [PubMed] [Google Scholar]
- Kuchipudi S.V., Tellabati M., Nelli R.K., White G.A., Perez B.B., Sebastian S., Slomka M.J., Brookes S.M., Brown I.H., Dunham S.P., Chang K.-C. 18S rRNA is a reliable normalisation gene for real time PCR based on influenza virus infected cells. J. Virol. 2012;9 doi: 10.1186/1743-422X-9-230. 230-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Chen C., Indugu N., Werlang G.O., Singh M., Kim W.K., Thippareddi H. Effect of antibiotic withdrawal in feed on chicken gut microbial dynamics, immunity, growth performance and prevalence of foodborne pathogens. PLoS One. 2018;13(2):e0192450. doi: 10.1371/journal.pone.0192450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudadio V., Passantino L., Perillo A., Lopresti G., Passantino A., Khan R.U., Tufarelli V. Productive performance and histological features of intestinal mucosa of broiler chickens fed different dietary protein levels. Poult. Sci. 2012;91:265–270. doi: 10.3382/ps.2011-01675. [DOI] [PubMed] [Google Scholar]
- Lee S.I., Kim I.H. Difructose dianhydride improves intestinal calcium absorption, wound healing, and barrier function. Sci. Rep. 2018;8:7813. doi: 10.1038/s41598-018-26295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.S., Lee S.H., Gadde U.D., Oh S.T., Lee S.J., Lillehoj H.S. Allium hookeri supplementation improves intestinal immune response against necrotic enteritis in young broiler chickens. Poult. Sci. 2018;97:1899–1908. doi: 10.3382/ps/pey031. [DOI] [PubMed] [Google Scholar]
- Li J., Yuan J., Guo Y., Sun Q., Hu X. The influence of dietary calcium and phosphorus imbalance on intestinal NaPi-IIb and calbindin mRNA expression and tibia parameters of broilers. Asian Austral. J. Anim. Sci. 2012;25:552–558. doi: 10.5713/ajas.2011.11266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.Y., Cowieson A.J., Selle P.H. The influence of meat and bone meal and exogenous phytase on growth performance, bone mineralisation and digestibility coefficients of protein (N), amino acids and starch in broiler chickens. Anim. Nutr. 2016;2:86–92. doi: 10.1016/j.aninu.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes A.R., Ribeiro T., Correia B.A., Bule P., Maçãs B., Falcão L., Freire J.P.B., Ferreira L.M.A., Fontes C.M.G.A., Lordelo M.M. Low doses of exogenous xylanase improve the nutritive value of triticale-based diets for broilers. J. Appl. Poult. Res. 2013;22:92–99. [Google Scholar]
- Miles R.D., Butcher G.D., Henry P.R., Littell R.C. Effect of antibiotic growth promoters on broiler performance, intestinal growth parameters, and quantitative morphology. Poult. Sci. 2006;85:476–485. doi: 10.1093/ps/85.3.476. [DOI] [PubMed] [Google Scholar]
- Munoz J.A., Hanna C.D., Utterback P.L., Parsons C.M. Phosphorus retention in corn, spray dried plasma protein, soybean meal, meat and bone meal, and canola meal using a precision-fed rooster assay. Poult. Sci. 2018;97:4324–4329. doi: 10.3382/ps/pey322. [DOI] [PubMed] [Google Scholar]
- Navarro M.A., McClane B.A., Uzal F.A. Mechanisms of action and cell death associated with Clostridium perfringens toxins. Toxins (Basel) 2018;10:212. doi: 10.3390/toxins10050212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris N.E., Wong E.A. Expression of digestive enzymes and nutrient transporters in the intestine of Eimeria maxima-infected chickens. Poult. Sci. 2013;92:1331–1335. doi: 10.3382/ps.2012-02966. [DOI] [PubMed] [Google Scholar]
- Proszkowiec-Weglarz M., Angel R. Calcium and phosphorus metabolism in broilers: effect of homeostatic mechanism on calcium and phosphorus digestibility. J. Appl. Poult. Res. 2013;22:609–627. [Google Scholar]
- Ptak A., Bedford M.R., Świątkiewicz S., Żyła K., Józefiak D. Phytase modulates ileal microbiota and enhances growth performance of the broiler chickens. PLoS One. 2015;10:e0119770. doi: 10.1371/journal.pone.0119770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran V. Feed enzymes: the science, practice, and metabolic realities. J. Appl. Poult. Res. 2013;22:628–636. [Google Scholar]
- Rinttilä T., Apajalahti J. Intestinal microbiota and metabolites—implications for broiler chicken health and performance. J. Appl. Poult. Res. 2013;22:647–658. [Google Scholar]
- Rodgers N.J., Swick R.A., Geier M.S., Moore R.J., Choct M., Wu S.-B. A multifactorial analysis of the extent to which Eimeria and fishmeal predispose broiler chickens to necrotic enteritis. Avian Dis. 2015;59:38–45. doi: 10.1637/10774-011614-reg.1. [DOI] [PubMed] [Google Scholar]
- Ross R. The role of T lymphocytes in inflammation. Proc. Natl. Acad. Sci. U. S. A. 1994;91:2879. doi: 10.1073/pnas.91.8.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS . SAS Inst. Inc.; Cary, NC: 2010. SAS User’s Guide: Statistics. Version 9.3. [Google Scholar]
- Sell J.L. Influence of dietary concentration and source of meat and bone meal on performance of turkeys. Poult. Sci. 1996;75:1076–1079. doi: 10.3382/ps.0751076. [DOI] [PubMed] [Google Scholar]
- Sell J.L., Jeffrey M.J. Availability for poults of phosphorus from meat and bone meals of different particle sizes. Poult. Sci. 1996;75:232–239. doi: 10.3382/ps.0750232. [DOI] [PubMed] [Google Scholar]
- Selle P.H., Cowieson A.J., Ravindran V. Consequences of calcium interactions with phytate and phytase for poultry and pigs. Liv. Sci. 2009;124:126–141. [Google Scholar]
- Shet D., Ghosh J., Ajith S., Awachat V.B., Elangovan A.V. Efficacy of dietary phytase supplementation on laying performance and expression of osteopontin and calbindin genes in eggshell gland. Anim. Nutr. 2018;4:52–58. doi: 10.1016/j.aninu.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley R.B., Parsons C.M. Effect of ash content on protein quality of meat and bone meal. Poult. Sci. 2001;80:626–632. doi: 10.1093/ps/80.5.626. [DOI] [PubMed] [Google Scholar]
- Smirnov A., Sklan D., Uni Z. Mucin dynamics in the chick small intestine are altered by starvation. J. Nutr. 2004;134:736–742. doi: 10.1093/jn/134.4.736. [DOI] [PubMed] [Google Scholar]
- Stanley D., Wu S.-B., Rodgers N., Swick R.A., Moore R.J. Differential responses of cecal microbiota to fishmeal, Eimeria and Clostridium perfringens in a necrotic enteritis challenge model in chickens. PLoS One. 2014;9:e104739. doi: 10.1371/journal.pone.0104739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulabo R.C., Stein H.H. Digestibility of phosphorus and calcium in meat and bone meal fed to growing pigs. J. Anim. Sci. 2013;91:1285–1294. doi: 10.2527/jas.2011-4632. [DOI] [PubMed] [Google Scholar]
- Suloma A., Mabroke R.S., El-Haroun E.R. Meat and bone meal as a potential source of phosphorus in plant-protein-based diets for Nile tilapia (Oreochromis niloticus) Aquault. Int. 2013;21:375–385. [Google Scholar]
- Svihus B., Hetland H., Choct M., Sundby F. Passage rate through the anterior digestive tract of broiler chickens fed on diets with ground and whole wheat. Br. Poult. Sci. 2002;43:662–668. doi: 10.1080/0007166021000025037. [DOI] [PubMed] [Google Scholar]
- Tarsani E., Kranis A., Maniatis G., Avendano S., Hager-Theodorides A.L., Kominakis A. Discovery and characterization of functional modules associated with body weight in broilers. Sci. Rep. 2019;9:9125. doi: 10.1038/s41598-019-45520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez G., Latorre J.D., Kuttappan V.A., Kogut M.H., Wolfenden A., Hernandez-Velasco X., Hargis B.M., Bottje W.G., Bielke L.R., Faulkner O.B. Utilization of rye as energy source affects bacterial translocation, intestinal viscosity, microbiota composition, and bone mineralization in broiler chickens. Front. Genet. 2014;5:339. doi: 10.3389/fgene.2014.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbermont L., Haesebrouck F., Ducatelle R., Van Immerseel F. Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathol. 2011;40:341–347. doi: 10.1080/03079457.2011.590967. [DOI] [PubMed] [Google Scholar]
- Vallipuram J., Grenville J., Crawford D.A. The E646D-ATP13A4 mutation associated with autism reveals a defect in calcium regulation. Cell. Mol. Neurobiol. 2010;30:233–246. doi: 10.1007/s10571-009-9445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson B.C., Matthews J.O., Southern L.L., Shelton J.L. The effects of phytase on growth performance and intestinal transit time of broilers fed nutritionally adequate diets and diets deficient in calcium and phosphorus. Poult. Sci. 2006;85:493–497. doi: 10.1093/ps/85.3.493. [DOI] [PubMed] [Google Scholar]
- Wu S.-B., Stanley D., Rodgers N., Swick R.A., Moore R.J. Two necrotic enteritis predisposing factors, dietary fishmeal and Eimeria infection, induce large changes in the caecal microbiota of broiler chickens. Vet. Microbiol. 2014;169:188–197. doi: 10.1016/j.vetmic.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Wu S.B., Rodgers N.J., Cui G., Sun Y., Choct M. Dynamics of intestinal metabolites and morphology in response to necrotic enteritis challenge in broiler chickens. Avian Pathol. 2016;45:346–356. doi: 10.1080/03079457.2016.1140896. [DOI] [PubMed] [Google Scholar]
- Yang F., Lei X., Rodriguez-Palacios A., Tang C., Yue H. Selection of reference genes for quantitative real-time PCR analysis in chicken embryo fibroblasts infected with avian leukosis virus subgroup. BMC Res. Notes. 2013;6:402. doi: 10.1186/1756-0500-6-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Coon C. The relationship of calcium intake, source, size, solubility in vitro and in vivo, and gizzard limestone retention in laying hens. Poult. Sci. 1997;76:1702–1706. doi: 10.1093/ps/76.12.1702. [DOI] [PubMed] [Google Scholar]
- Zhang B., Lv Z., Li Z., Wang W., Li G., Guo Y. Dietary l-arginine supplementation alleviates the intestinal injury and modulates the gut microbiota in broiler chickens challenged by Clostridium perfringens. Front. Microbiol. 2018;9:1716. doi: 10.3389/fmicb.2018.01716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanu H.K., Keerqin C., Kheravii S.K., Morgan N.K., Wu S.-B., Bedford M.R., Swick R.A. Influence of meat and bone meal, phytase, and antibiotics on broiler chickens challenged with subclinical necrotic enteritis: 1. growth performance, intestinal pH, apparent ileal digestibility, cecal microbiota, and tibial mineralization. Poult. Sci. 2020;99:1540–1550. doi: 10.1016/j.psj.2019.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.