Abstract
Goblet cells secrete mucin 2 (Muc2), which is a major component of the mucus that lines the intestinal tract and creates a protective barrier between pathogens and the intestinal epithelial cells and thus are important for chick health. The objectives of this study were to determine the age-specific and intestinal segment–specific expression of Muc2 mRNA and changes in the number of goblet cells from late embryogenesis to early after hatch. Small intestinal samples from the duodenum, jejunum, and ileum were collected from Cobb 500 broilers at embryonic day 19 (e19), day of hatch (doh), and day 2 and 4 after hatch. Cells expressing Muc2 mRNA and mucin glycoprotein were detected by in situ hybridization or alcian blue and periodic acid–Schiff staining, respectively. Along the villi, there were many more cells expressing Muc2 mRNA than those stained for mucin glycoprotein. In the crypt, cells expressing Muc2 mRNA did not stain for mucin glycoprotein. There was an increase in the density of goblet cells in the villi and Muc2 mRNA expressing cells in the crypts of the jejunum and ileum from e19 to doh and day 2 to day 4, with no change between doh and day 2. In contrast, in the duodenum, the density of goblet cells in the villi and Muc2 mRNA expressing cells in the crypts remained constant from e19 to day 4. At day 4, the villi in the ileum had a greater density of goblet cells than the duodenum. In the crypt, the ileum had a greater density of Muc2 mRNA expressing cells than the duodenum at doh, and the ileum and jejunum both had greater densities of Muc2 mRNA expressing cells than the duodenum at day 4. These results indicate that the population of goblet cells has reached a steady state by doh in the duodenum, whereas in the jejunum and ileum, a steady-state population was not reached until after hatch.
Key words: goblet cell, mucin 2, villus, crypt, intestine
Introduction
The small intestinal villi are lined with a number of epithelial cells, such as enterocytes and goblet cells, which are derived from stem cells in the crypt and important for nutrient uptake and host defense (Barker, 2014, Carulli et al., 2014). Goblet cells secrete mucins, which consist of a peptide backbone and attached polysaccharide chains. The mucin glycoproteins and water form a mucus layer that not only serves as a physical barrier but also mediates a number of immunological functions to inhibit pathogens from invading the epithelial cells (Birchenough et al., 2015, Johansson and Hansson, 2016).
In chickens, the expression of mucin 2 (Muc2) mRNA and ontogeny of goblet cells in the small intestine have been investigated. Goblet cells have been identified using alcian blue (AB) to stain acidic mucins and periodic acid–Schiff (PAS) to stain neutral mucins. Uni et al. (2003) reported that goblet cells producing acidic but not neutral mucin were detected in the small intestine 3 D before hatch. From day of hatch (doh) until day 7 after hatch, both acidic and neutral mucins were detected in goblet cells of the duodenum, jejunum, and ileum. The density of goblet cells in the duodenum was constant from doh until day 7 after hatch, whereas in the jejunum and ileum, there was an increase in the density of goblet cells. Consistent with the developmental increase in goblet cells in the jejunum, Smirnov et al. (2006) reported that there was a gradual increase in Muc2 mRNA abundance from embryonic day 17 to day 3 after hatch.
Jiang et al. (2013) cloned a full-length, 11,359 bp chicken Muc2 cDNA and showed that Muc2 mRNA was expressed in a spatiotemporal pattern. There was an increase in Muc2 mRNA abundance in the duodenum, jejunum, and ileum from embryonic day 14.5 until day 7 after hatch, with greater expression in the ileum than in the duodenum. There was a tissue-specific change in the expression pattern from doh until day 1 after hatch. At doh, there was a rapid increase in Muc2 mRNA abundance in the duodenum and jejunum followed by a steady increase; whereas in the jejunum, there was a rapid increase in Muc2 mRNA after doh that remained relatively high from day 1 to day 7 after hatch. Zhang et al. (2015) likewise showed that Muc2 mRNA abundance in the ileum and cecum of chicks and Pekin ducks increased from embryonic day 14 to day 21 after hatch. In poults, intestinal Muc2 mRNA abundance increased from doh to day 4 after hatch, with no further increase from day 4 to day 9 after hatch (Hutsko et al., 2016).
The objective of this study was to assess Muc2 mRNA expression using quantitative PCR and in situ hybridization in the duodenum, jejunum and ileum of chicks from late embryogenesis to early after hatch. The combination of qPCR and in situ hybridization allows for not only quantification of Muc2 mRNA but also analysis of the number and distribution of goblet cells in the villi and crypts. A comparison between the in situ hybridization method for detecting Muc2 mRNA and staining for mucin glycoprotein was also examined.
Materials and methods
Animals and Tissue Sampling
Cobb 500 broiler eggs were obtained from a local hatchery and incubated at 37.5°C with 55% relative humidity. All animal procedures were approved by the Institutional Animal Care and Use Committee of Virginia Tech. At embryonic day 19 (e19), 6 eggs were chosen at random and opened. Viable chick embryos were euthanized by cervical dislocation (n = 6). The small intestine was collected and separated into the duodenum, jejunum and ileum. Three equal segments were cut from the center of the duodenum, jejunum, and ileum and rinsed in ice-cold PBS. The middle segment was quickly frozen and stored at −80°C until processed for quantitative PCR. The 2 adjoining segments were placed into 10% neutral-buffered formalin for 24 h, followed by 70% ethanol for 24 h at room temperature. The samples were transferred to new tubes with 70% ethanol and stored at 4°C until being embedded in paraffin (Histo-Scientific Research Labs, Mount Jackson, VA).
At doh, all chicks were weighed and wing banded. Total hatchability was 93%. The duodenum, jejunum and ileum were collected from 6 chicks at doh and processed as described previously. The remainder (n = 30) were placed into 3 battery cages with 10 chicks per cage. The chicks were provided ad libitum access to water and a corn–soybean–based starter diet that contained 22% crude protein and was formulated to meet the National Research Council requirements (National Research Council, 1994). At day 2 and 4 after hatch, 6 chicks were randomly selected (2 per cage) for collection of the duodenum, jejunum, and ileum and processed as described previously.
RNA Extraction and Quantitative PCR
Small intestinal samples (25–35 mg) were homogenized in TriReagent using a tissue lyser, and total RNA was extracted using the Direct-zol RNA MiniPrep kit (Zymo Research, Irvine, CA). RNA concentration and purity were determined using a Nanodrop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA). Complementary DNA was synthesized using 1 mg of total RNA and the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Grand Island, NY). Quantitative PCR (qPCR) reactions consisted of 5 μL Fast SYBR Green Master Mix (Applied Biosystems), 1 μL of forward primer (5 μm), 1 μL of reverse primer (5 μM), and 1.5 μL of cDNA (diluted 1:30). Duplicate qPCR reactions were performed using an Applied Biosystems 7,500 Fast Real-Time PCR system (Thermo Fisher Scientific). The cycling conditions were as follows: 95°C for 20 s, then 40 cycles of 90°C for 3 s and 60°C for 30 s. Primers were designed using Primer Express 3.0 (Applied Biosystems) and are listed in Table 1. Primer efficiency (mean ± SD) was determined using 5 different RNA samples and the ABI 7500 relative standard curve program. Chicken ribosomal protein L4 (RPL4) and ribosomal protein lateral stalk subunit P1 (RPLP1) were used as reference genes. The geometric mean of RPLP1 and RPL4 was subtracted from the Ct value to obtain the ΔCt value for each sample. The average ΔCt value of the duodenum at doh was used as the calibrator to calculate ΔΔCt and fold change using the 2−ΔΔCt method (Livak and Schmittgen, 2001)
Table 1.
Primers for quantitative PCR.
| Gene | Gene name | Forward/reverse primer | Amplicon (bp) | Amplification efficiency1 | Accession no. |
|---|---|---|---|---|---|
| Muc2 | Mucin 2 | CTGATTGTCACTCACGCCTTAATC/GCCGGCCACCTGCAT | 147 | 95.9 ± 4.5% | JX284122.1 |
| RPL4 | Ribosomal protein L4 | TCAAGGCGCCCATTCG/TGCGCAGGTTGGTGTGAA | 55 | 87.9 ± 5.1% | NM_001007479.1 |
| RPLP1 | Ribosomal protein lateral stalk subunit P1 | TCTCCACGACGACGAAGTCA/CCGCCGCCTTGATGAG | 63 | 94.5 ± 5.6% | NM_205322.1 |
Efficiency is shown as mean ± SD of 5 different RNA samples.
Identification of Cells Expressing Muc2 mRNA and Glycoprotein
The formalin-fixed paraffin-embedded tissues (n = 3/time point) were sectioned (5–6 μm) using a microtome and adhered to Superfrost Plus glass slides. Samples were deparaffinized with xylene and washed in 100% ethanol. Cells expressing mucin glycoprotein were identified using a combined AB (MiliporeSigma, St. Louis, MO) and PAS (MilliporeSigma) staining method as described in Layton and Bancroft (2019). Cells expressing Muc2 mRNA were identified using the RNAscope in situ hybridization method (Advanced Cell Diagnostics, ACD, Newark, CA). The Muc2 probe was detected using the RNAscope 2.5 HD Detection Reagent Kit (Brown) and counterstained with 50% Gill's hematoxylin #1 for bright-field images or the RNAscope 2.5 HD Detection reagent kit (Red) and counterstained with the nuclear stain 4′,6-diamidino-2-phenylindole (DAPI, 1:1,000) for fluorescent images. Bright-field microscopy and fluorescent imaging were performed using a Nikon Eclipse 80i microscope with a DS-Ri1 digital camera. As per the manufacturer's protocol, the negative control for the RNAscope in situ hybridization method uses the bacterial gene dapB (Wang et al., 2012). An example of this negative control using chicken jejunum was shown in the study conducted by Zhang et al. (2019).
The number of Muc2 mRNA–expressing (Muc2 mRNA+) cells was counted using the multipoint counter on ImageJ from the National Institutes of Health (Bethesda, MD). Three samples from each intestinal segment and time point were analyzed. Ten villi and crypts, chosen at random from each sample, were counted for cells expressing Muc2 mRNA. The area of the villi and crypts was measured using ImageJ. The number of Muc2 mRNA+ cells was divided by the area to calculate the density of Muc2 mRNA+ cells per unit area.
Statistical Analysis
Goblet cell density and Muc2 mRNA abundance were analyzed with an ANOVA test using JMP v14.0 (SAS Institute, Inc., Cary, NC). The density of goblet cells (number of goblet cells/unit area of intestine) and Muc2 mRNA abundance were analyzed with models that included the main effects of age and intestinal segment and the age × segment interaction. Means were separated using Tukey's test (P ≤ 0.05).
Results
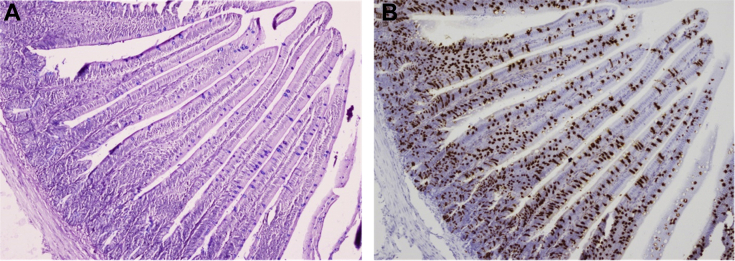
Cells expressing Muc2 mRNA were detected using in situ hybridization, whereas cells expressing mucin glycoprotein were stained with a combined AB-PAS method to detect acidic and neutral mucins, respectively. Figure 1 shows serial sections stained for mucin glycoprotein and Muc2 mRNA in the jejunum of a day-2 chick. Cells expressing mucin glycoprotein and Muc2 mRNA were present along the villi and represent goblet cells. However, the number of cells expressing Muc2 mRNA was much greater than those staining for mucin glycoprotein. Most goblet cells stained with AB and only a few stained with PAS. Cells in the crypt expressed Muc2 mRNA but did not stain for mucin glycoprotein. The same results were seen for the duodenum and ileum (data not shown).
Figure 1.
Identification of cells expressing mucin2 mRNA or mucin glycoprotein. Serial sections were cut from formalin-fixed paraffin-embedded samples of the jejunum of 2-day-old chicks. One section (A) was stained with alcian blue and periodic acid Schiff to detect acidic and neutral mucin glycoproteins, whereas the other section (B) was processed for in situ hybridization using the RNAscope 2.5 HD kit (Brown) and a mucin2 (Muc2) probe. Tissues were counterstained with hematoxylin. Cells expressing acidic mucin glycoprotein stained blue (A), whereas cells expressing Muc2 mRNA stained brown (B). Images were captured at 100X magnification (n = 2).
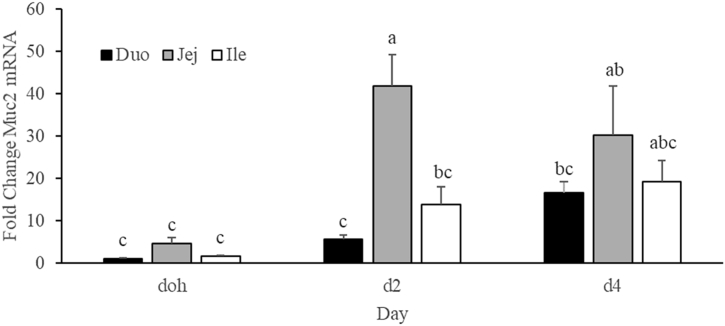
The temporal expression of Muc2 mRNA was examined for the 3 small intestinal segments using qPCR (Figure 2). There was a segment × age interaction. At day 2, Muc2 mRNA was 7.5- and 3.0-fold greater in the jejunum compared with the duodenum and ileum, respectively, whereas at doh and day 4, there was no difference in Muc2 mRNA abundance between the duodenum, jejunum, and ileum. There was an 8.9-fold increase in Muc2 mRNA abundance from doh to d2, which stayed constant from day 2 to day 4. In contrast, the duodenum and ileum showed no difference in Muc2 mRNA abundance between doh, day 2, and day 4.
Figure 2.
Muc2 mRNA expression by qPCR in the small intestine. Quantitative PCR was performed to measure Muc2 mRNA abundance at day of hatch (doh) and days 2 (d2) and 4 (d4) after hatch in the duodenum (Duo), jejunum (Jej), and ileum (Ile). The duodenum at doh was used as the calibrator for calculating fold change. A full factorial model that included 3 intestinal segments and 3 time points was analyzed. Bars with different letters are significantly different (P ≤ 0.05; n = 5 or 6).
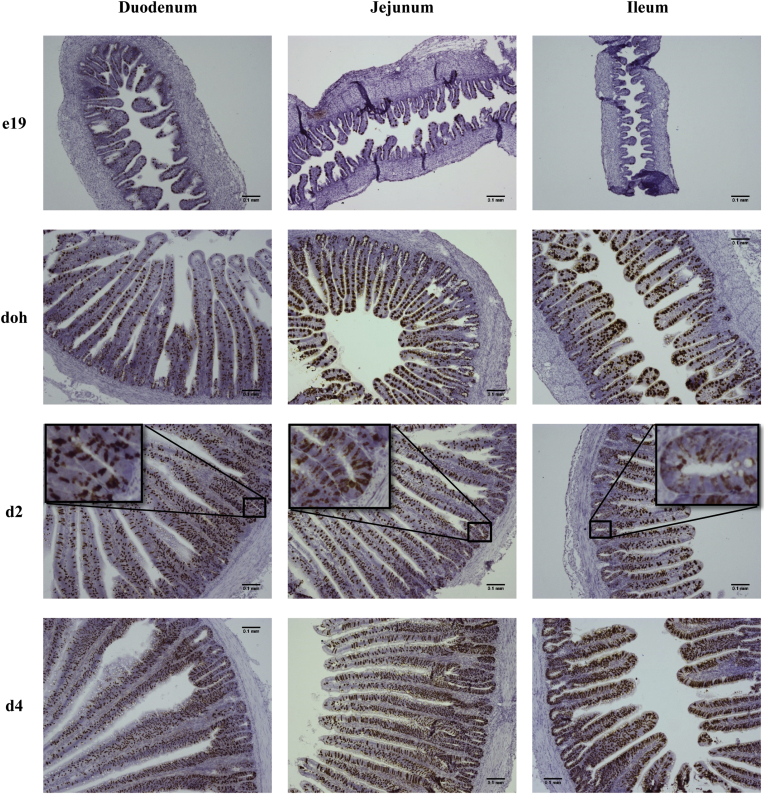
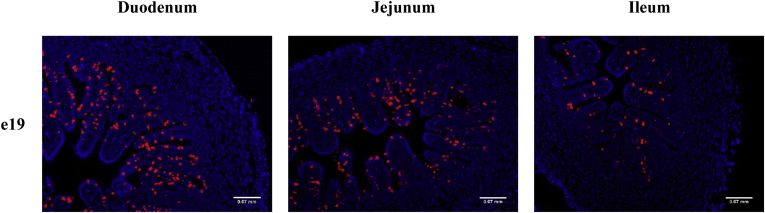
To examine age- and tissue-specific changes, the number and organization of Muc2 mRNA+ cells were examined by in situ hybridization in the villi and crypts of the duodenum, jejunum, and ileum from e19 to day 4 (Figure 3). The number of Muc2 mRNA+ cells was low in the crypts and villi of all 3 small intestinal segments at e19 and increased with age. In the crypt, there was an alternating pattern of Muc2 mRNA+ cells with non–Muc2 mRNA+ cells (Figure 3 insets). To enhance the detection of Muc2 mRNA+ cells at e19, a more sensitive fluorescent red chromogen was used in combination with 4’,6-diamidino-2-phenylindole staining of nuclei (Figure 4).
Figure 3.
Muc2 mRNA expression by in situ hybridization in the small intestine. Cells expressing Muc2 mRNA were detected by in situ hybridization using the RNAscope 2.5 HD kit (Brown) in the duodenum, jejunum, and ileum at embryonic day 19 (e19), day of hatch (doh), and days 2 (d2) and 4 (d4) after hatch. The tissue was counterstained with hematoxylin. Images were captured at 100X magnification (n = 3). Insets in day 2 images are magnifications of the crypt showing the interspersed pattern of Muc2 mRNA expressing cells.
Figure 4.
Muc2 mRNA expression by fluorescence in situ hybridization in the small intestine. Cells expressing Muc2 mRNA were detected by in situ hybridization using the RNAscope 2.5 HD kit (Red) in the duodenum, jejunum, and ileum at embryonic day 19 (e19). The tissue was counter stained with 4’,6-diamidino-2-phenylindole (1:1,000). Images were captured with fluorescence at 200X magnification (n = 3).
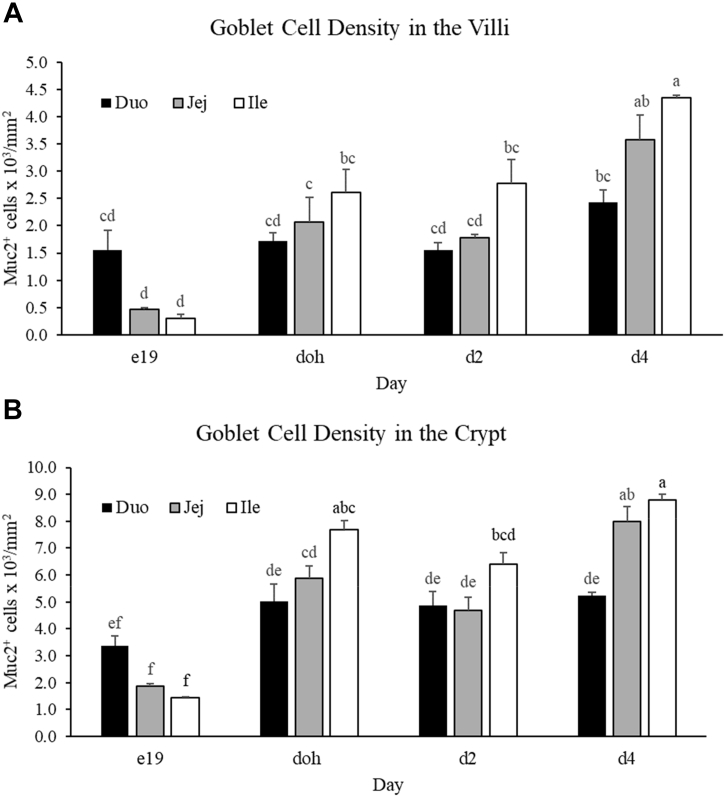
The numbers of goblet cells in the villi and Muc2 mRNA+ cells in the crypts were enumerated, and the density was calculated per unit area of the villus or crypt. There was no change in goblet cell density in the villi of the duodenum from e19 to day 4 (Figure 5A). There was, however, an increase in the density of goblet cells in the villi of the jejunum and ileum from e19 to day 4. From e19 to doh, the density of goblet cells increased 4.4- and 8.9-fold in the jejunum and ileum, respectively. There was no increase in the density of goblet cells in the jejunum and ileum from doh to day 2. From day 2 to day 4, the density of goblet cells increased 2.0- and 1.6-fold in the jejunum and ileum, respectively. At day 4, the ileal villi showed a 1.8-fold greater density of goblet cells compared with the duodenal villi. The age-specific change in density of Muc2 mRNA+ cells in the crypt was similar to the change in density of goblet cells observed in the villi. In the duodenal crypt, the density of Muc2 mRNA+ cells did not change from e19 to day 4 (Figure 5B). There was a 3.1- and 5.4-fold increase in the density of Muc2 mRNA+ cells in the crypts of the jejunum and ileum from e19 to doh, respectively. There was no increase in the density of Muc2 mRNA+ cells in the crypts of the jejunum and ileum from doh to day 2. From day 2 to day 4, the density of Muc2 mRNA+ cells in the crypts of the jejunum and ileum increased 1.7- and 1.4-fold, respectively. At doh, the ileal crypts had a 1.5-fold greater density of Muc2 mRNA+ cells than the duodenal crypts, whereas at day 4, the jejunal and ileal crypts had 1.5- to 1.7-fold greater density of Muc2 mRNA+ cells compared with the duodenal crypts. Overall, the density of Muc2 mRNA+ cells in the crypts was greater than the density of goblet cells along the villi.
Figure 5.
Muc2 mRNA expressing cells in the villi and crypts. The density of cells expressing Mucin2 mRNA (Muc2+) at embryonic day 19 (e19), day of hatch (doh), and days 2 (d2) and 4 (d4) after hatch in the (A) villi and (B) crypts of the duodenum (Duo), jejunum (Jej), and ileum (Ile). A full factorial model that included 3 intestinal segments and 4 time points was analyzed. Bars with different letters are significantly different (P ≤ 0.05; n = 3).
Discussion
We have used in situ hybridization as an alternative method to staining with AB and PAS to detect goblet cells. Our results show that along the villi, there are many more cells expressing Muc2 mRNA than mucin glycoprotein, which suggests that in situ hybridization is a more sensitive method for detecting goblet cells.
Abundance of Muc2 mRNA showed age- and tissue-specific expression. Jiang et al. (2013) showed that chicken Muc2 mRNA abundance increased temporally from e14.5 to day 7 after hatch in all 3 segments of the small intestine and increased spatially from the duodenum to the ileum. A similar temporal increase in Muc2 mRNA abundance was reported for chickens and ducks from e14 to day 21 after hatch (Zhang et al., 2015) and for poults from doh to day 4 and day 9 (Hutsko et al., 2016). Our results are consistent with the temporal increase in Muc2 mRNA, but in contrast to the results of the study by Jiang et al. (2013), our results showed that the jejunum had greater Muc2 mRNA than the duodenum and ileum at day 2.
Goblet cell density also showed age- and tissue-specific patterns. Our results showed that there were increases in goblet cell density in the jejunum and ileum from e19 to doh and from day 2 to day 4, with no change in the duodenum from e19 to day 4, which did not parallel the increase in Muc2 mRNA abundance from doh to day 2 in the jejunum. This would suggest that goblet cell development is not directly related to Muc2 mRNA abundance. The change in goblet cell density was consistent with that previously reported by Uni et al. (2003), who showed that the density of goblet cells showed a slight increase in the villi of the duodenum from e18 to day 7 after hatch and a rapid increase in goblet cell density in the villi of the jejunum and ileum from e18 to day 7 after hatch. These results suggest that at hatch, the duodenum has already reached a steady-state population of goblet cells, whereas the populations of goblet cells in the jejunum and ileum continue to develop after hatch. This may be because the duodenum is the first intestinal segment to encounter pathogens that enter orally, and therefore, a well-formed mucus layer is needed to provide a protective barrier for the newly hatched chick.
The intestine of the newly hatched chick is rapidly colonized with bacteria. Smith (1965) showed that the number of viable Escherichia coli increased rapidly during the first day after hatch, whereas Clostridium, Streptococcus, and Lactobacillus appeared after E. coli. Apajalahti et al. (2004) determined that the bacterial density in the ileum reached a plateau (∼109/g digesta) by day 3 or 4 after hatch. It is likely that the rapid expansion of goblet cells during the early after hatch period is required to produce the protective mucus layer during the establishment of the intestinal microbiome.
Uni et al. (2003) found that goblet cells were present along the villi but not in the crypt, which was observed using the AB-PAS method to stain for mucin glycoproteins. Our results confirm those of Uni et al. (2003) that there were no cells expressing mucin glycoprotein in the crypt; however, our in situ hybridization results clearly showed that there were cells expressing Muc2 mRNA in the intestinal crypts. The Muc2 mRNA+ cells in the crypt may be nascent goblet cells that have started to differentiate but have not yet migrated out of the crypts. Cells expressing Muc2 have also been identified in the intestinal crypts of humans (Chambers et al., 1994, Weiss et al., 1996) and mice (Paulus et al., 1993). Chambers et al. (1994) showed that Muc2 mRNA was detected by 12 wk in both the crypts and villi of the jejunum and ileum in human embryos, with the concentration of Muc2 mRNA+ cells in the crypts greater than along the villi of the jejunum and ileum.
There was an alternating pattern of Muc2 mRNA expressing and nonexpressing cells in the crypts, which was most apparent at day 2 in the 3 intestinal segments. This pattern is similar to the alternating pattern of Paneth cells and crypt base columnar cells in the small intestinal crypt of mammals (Carulli et al., 2014). Crypt base columnar cells are active stem cells that express olfactomedin 4 (Olfm4), whereas Paneth cells do not express Olfm4 and secrete lysozyme, antimicrobials, and factors that maintain the stem cell niche (van Es and Cleavers, 2014). In mice but not humans, Paneth cells also expressed Muc2 (Stahl et al., 2018). The presence of Paneth cells in the intestinal crypts of chickens is controversial. Wang et al. (2016) detected lysozyme mRNA and protein expression in the intestinal crypts of 6-month-old chickens. In contrast, Nile et al. (2004) reported that cells staining for lysozyme were only present along the villi in the small intestine of chickens, and Bar-Shira et al. (2018) showed the presence of a novel rod-shaped cell that stained for lysozyme located along the intestinal villi. Thus, whether the Muc2 mRNA+ cells in the intestinal crypts identified in this study are equivalent to Paneth cells in chickens remains to be determined.
Alternatively, the Muc2 mRNA+ cells in the crypt may represent a novel cell type present in chickens. Zhang and Wong (2018) showed by in situ hybridization that every cell within the intestinal crypt of chickens expressed Olfm4 mRNA. Thus, a subset of crypt cells in this study stained for both Olfm4 and Muc2 mRNA. These Muc2 mRNA+ cells may be a multifunctional cell that not only serves as a stem cell but also protects the crypt by producing mucin in the absence of classical Paneth cells in the crypt.
In conclusion, cells expressing Muc2 mRNA showed the same age-specific pattern along the villi and in the crypts of the chicken small intestine. The density of goblet cells along the villi and Muc2 mRNA+ cells in the crypt stayed constant in the duodenum from e19 to day 4, whereas in the jejunum and ileum, there was an increase in cell density from e19 to day 4. The origin and function of the Muc2 mRNA+ cells in the crypt is yet to be determined. These cells could represent nascent goblet cells that have started to differentiate but have not migrated out of the crypt, Paneth-like cells that express Muc2 mRNA, or a novel multifunctional cell with stem and goblet-cell properties.
Acknowledgments
Funding for this project was provided in part by the Virginia Agricultural Experiment Station, the Hatch Program of the National Institute of Food and Agriculture, U.S. Department of Agriculture, Washington DC and USDA NIFA grant 2017-67015-26588.
Conflict of Interest Statement: The authors declare no conflicts of interest.
References
- Apajalahti J., Kettunen A., Graham H. Characteristics of the gastrointestinal microbial communities, with special reference to the chicken. World’s Poult. Sci. J. 2004;60:223–232. [Google Scholar]
- Bar Shira E., Friedman A. Innate immune functions of avian intestinal epithelial cells: Response to bacterial stimuli and localization of responding cells in the developing avian digestive tract. PLoS One. 2018;13:e0200393. doi: 10.1371/journal.pone.0200393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat. Rev. 2014;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- Birchenough G.M.H., Johansson M.E.V., Gustafsson J.K., Bergstrom J.H., Hansson G.C. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 2015;8:712–719. doi: 10.1038/mi.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carulli A.J., Samuelson L.C., Schnell S. Unraveling intestinal stem cell behavior with models of crypt dynamics. Integr. Biol. 2014;6:243–257. doi: 10.1039/c3ib40163d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers J.A., Hollingsworth M.A., Trezise A.E., Harris A. Developmental expression of mucin genes MUC1 and MUC2. J. Cell Sci. 1994;107:413–424. doi: 10.1242/jcs.107.2.413. [DOI] [PubMed] [Google Scholar]
- Hutsko S.L., Meizlisch K., Wick M., Lilburn M.S. Early intestinal development and mucin transcription in the young poult with probiotic and mannan oligosaccharide prebiotic supplementation. Poult. Sci. 2016;95:1173–1178. doi: 10.3382/ps/pew019. [DOI] [PubMed] [Google Scholar]
- Jiang Z., Applegate T.J., Lossie A.C. Cloning, annotation and developmental expression of the chicken intestinal MUC2 gene. PLoS One. 2013;8:e53781. doi: 10.1371/journal.pone.0053781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M.E.V., Hansson G.C. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 2016;16:639–649. doi: 10.1038/nri.2016.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layton C., Bancroft J.D. Carbohydrates. Ch. 13. In: Suvarna S.K., Layton C.L., Bancroft J.D., editors. Bancroft’s Theory and Practice of Histological Techniques. 8th ed. Elsevier; Amsterdam: 2019. pp. 185–186. [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- National Research Council . 9th rev. edn. The National Academies Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Nile C.J., Townes C.L., Michailidis G., Hirst B.H., Hall J. Identification of chicken lysozyme g2 and its expression in the intestine. Cell. Mol. Life Sci. 2004;61:2760–2766. doi: 10.1007/s00018-004-4345-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus U., Loeffler M., Zeidler J., Owen G., Potten C.S. The differentiation and lineage development of goblet cells in the murine small intestinal crypt: experimental and modelling studies. J. Cell Sci. 1993;106:473–484. doi: 10.1242/jcs.106.2.473. [DOI] [PubMed] [Google Scholar]
- Smirnov A., Tako E., Ferket P.R., Uni Z. Mucin gene expression and mucin content in the chicken intestinal goblet cells are affected by in ovo feeding of carbohydrates. Poult. Sci. 2006;85:669–673. doi: 10.1093/ps/85.4.669. [DOI] [PubMed] [Google Scholar]
- Smith H.W. The development of the flora of the alimentary tract in young animals. J. Path. Bact. 1965;90:495–513. [PubMed] [Google Scholar]
- Stahl M., Tremblay S., Montero M., Vogl W., Xia L., Jacobson K., Menendez A., Vallance B.A. The Muc2 mucin coats murine Paneth cell granules and facilitates their content release and dispersion. Am. J. Gastrointest. Liver Physiol. 2018;315:G195–G205. doi: 10.1152/ajpgi.00264.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uni Z., Smirnov A., Sklan D. Pre- and posthatch development of goblet cells in the broiler small intestine: effect of delayed access to feed. Poult. Sci. 2003;82:320–327. doi: 10.1093/ps/82.2.320. [DOI] [PubMed] [Google Scholar]
- van Es J.H., Clevers H. Paneth cells. Curr. Biol. 2014;24:R547–R548. doi: 10.1016/j.cub.2014.04.049. [DOI] [PubMed] [Google Scholar]
- Wang F., Flanagan J., Su N., Wang L., Bui S., Nielson A., Wu X., Vo H., Ma X., Luo Y. RNAscope A novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J. Mol. Diag. 2012;14:22–29. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Li J., Li J., Jr., Li R.X., Lv C.F., Li S., Mi Y.L., Zhang C.Q. Identification of the Paneth cells in chicken small intestine. Poult. Sci. 2016;95:1631–1635. doi: 10.3382/ps/pew079. [DOI] [PubMed] [Google Scholar]
- Weiss A.A., Babyatsky M.W., Ogata S., Chen A., Itzkowitz S.H. Expression of MUC2 and MUC3 mRNA in human normal, malignant, and inflammatory intestinal tissue. J. Histochem. Cytochem. 1996;44:1161–1166. doi: 10.1177/44.10.8813081. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Eicher S.D., Applegate T.J. Development of intestinal mucin 2, IgA, and polymeric Ig receptor expressions in broiler chickens and Pekin ducks. Poult. Sci. 2015;94:172–180. doi: 10.3382/ps/peu064. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wong E.A. Identification of cells expressing OLFM4 and LGR5 mRNA by in situ hybridization in the yolk sac and small intestine of embryonic and early posthatch chicks. Poult. Sci. 2018;97:628–633. doi: 10.3382/ps/pex328. [DOI] [PubMed] [Google Scholar]
- Zhang H., Li H., Kidrick J., Wong E.A. Localization of cells expressing SGLT1 in the yolk sac and small intestine of broilers. Poult. Sci. 2019;98:984–990. doi: 10.3382/ps/pey343. [DOI] [PubMed] [Google Scholar]